Abstract
Documenting trends of stream macroinvertebrate biodiversity is challenging because biomonitoring often has limited spatial, temporal, and taxonomic scopes. We analyzed biodiversity and composition of assemblages of >500 genera, spanning 27 years, and 6131 stream sites across forested, grassland, urban, and agricultural land uses throughout the United States. In this dataset, macroinvertebrate density declined by 11% and richness increased by 12.2%, and insect density and richness declined by 23.3 and 6.8%, respectively, over 27 years. In addition, differences in richness and composition between urban and agricultural versus forested and grassland streams have increased over time. Urban and agricultural streams lost the few disturbance-sensitive taxa they once had and gained disturbance-tolerant taxa. These results suggest that current efforts to protect and restore streams are not sufficient to mitigate anthropogenic effects.
In streams macroinvertebrate density and disturbance-sensitive taxa have decreased, and disturbance-tolerant taxa have increased.
INTRODUCTION
Freshwater systems support an immense fraction of global biodiversity (1) and provide critical ecosystem services to support human livelihoods and well-being (2). However, despite strong evidence of declines in freshwater vertebrates (3), little is known about broadscale temporal trends in biodiversity of freshwater invertebrate communities, including insects, and how these patterns vary with environmental conditions, such as land use. Several gaps in the literature exist. For example, several studies have documented broadscale declines in terrestrial but increases in freshwater, insect abundance, biomass, and occupancy (4–8). However, most of these studies have not conducted community composition analyses to determine which taxa are changing. Composition analyses could influence how these increases and decreases are interpreted. For instance, overall abundance or biomass increases could reflect proliferation of disturbance-tolerant taxa and actual declines of disturbance-sensitive taxa (9, 10). In this case, the overall increase in abundance or biomass should not be interpreted as freshwater communities recovering from disturbance. Another gap is that studies proclaiming evidence of an “insect apocalypse” or “insect Armageddon” have often only shown marked local- or regional-scale declines in populations of select taxonomic groups of insects and other invertebrates (11–13). Their limited scopes have made it challenging to determine the spatial and taxonomic extents of these declines. Last, some studies have attempted to examine temporal trends of insects and arthropods over broad temporal, spatial, and/or taxonomic scopes (14, 15), but these studies present inconclusive results because they fail to account for complex sampling histories inherent to long-term monitoring, including differences in sampling effort and changes in the abilities of taxonomists to make identifications over time (16, 17).
Overall, few studies have attempted to use robust approaches to holistically examine the multiple facets of biodiversity, including abundance, richness (at sites and within regions), spatial turnover (β diversity), and composition (18–20) over large temporal, geographic, and taxonomic scopes while also controlling for sampling effort and complex sampling histories, and even fewer have examined how these temporal trends vary across land use (21). Addressing these gaps is a critical first step to gain a nuanced understanding of how freshwater communities are changing across space and time in the face of immense human-induced alterations to environmental conditions, which could inform policy aimed at conserving and restoring freshwater systems.
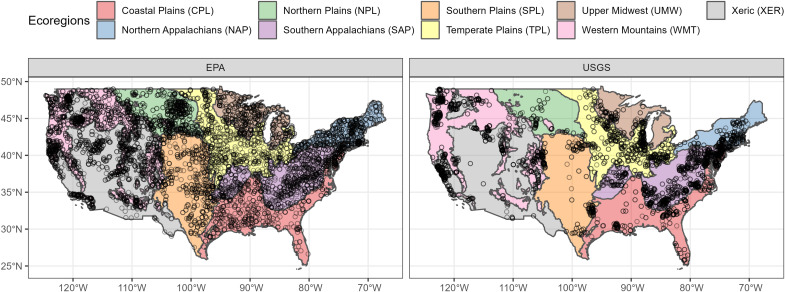
With thousands of sites across the contiguous United States and standardized methods used for nearly three decades (1993 to 2019), U.S. federal biomonitoring programs of stream macroinvertebrate assemblages provide unique datasets capable of addressing these gaps (22, 23). Monitoring programs included six U.S. Environmental Protection Agency (EPA) national projects and 64 U.S. Geological Survey (USGS) national and regional projects (table S1). Overall, we compiled data from 3914 unique EPA and 2217 unique USGS wadeable stream sites, which included >500 macroinvertebrate genera. Using these data, we derived site-level total density and α (the number of genera at sites), region-level ᾱ (the average number of genera at sites within ecoregions), γ (the total number of genera within ecoregions), and β (the spatial turnover of genera among sites within ecoregions) diversity metrics for macroinvertebrate communities, identified to genus (Fig. 1 and figs. S1 and S2). Steps were taken to account for differences in sampling locations and methods, differences in identification methods of specimens, changes in taxonomy through time, and abilities of taxonomists to make genera-level identifications, among other factors (see Materials and Methods and fig. S2). Given the pivotal role that insects play in ecosystem services of freshwater systems (24, 25), we also conducted analyses on insects alone to determine whether insects showed similar or different trends compared to all macroinvertebrates.
Fig. 1. The spatial distribution of sites within ecoregions that were used to calculate site-level biodiversity metrics (total densities and α diversity) and regional biodiversity metrics (, γest, and β diversities).
We used a newly derived dataset that combines 27 years of standardized stream macroinvertebrate monitoring conducted by the EPA and USGS. For an additional detailed map, see fig. S1.
Our objectives were as follows: (i) to assess how total densities and α as well as ᾱ, γest, and β diversities changed over time; (ii) to evaluate how these trends in biodiversity metrics varied across dominant land uses; and (iii) to determine the taxa driving the biodiversity patterns by examining how community composition changed with land use through time. Given the large-scale benefits to water quality initiated by the Clean Water Act in the United States beginning in 1972 (26) and increases in freshwater insect abundance, biomass, and occupancy found in North America and elsewhere (4, 8, 27, 28), a reasonable prediction is recovery of communities from historic disturbance and pollution, namely, differentiation of communities and increases in total densities; α, ᾱ, and γest diversity; and genera sensitive to habitat disturbance. Alternatively, given sustained anthropogenic modification of freshwater habitats, increases in the U.S. human population, and climate change, a defensible alternative prediction is continued degradation of stream ecosystems, which could include homogenization of communities and decreases in total densities; α, ᾱ, and γest diversity; and genera sensitive to habitat disturbance. Regardless of whether we observed recovery or degradation of communities, we expected these temporal trends to vary with land use; we expected improvements or less severe degradation for forest/wetland and grassland/shrub streams, compared to agricultural and urban streams. Furthermore, given that the Clean Water Act targets urban point sources of pollution and categorically excludes agricultural streams, we expected more improvements or less severe degradation of communities in urban than agricultural streams.
RESULTS AND DISCUSSION
Overall, our study supports two major conclusions. First, over the past 27 years, streams across the United States have seen a decrease in the total density of macroinvertebrates and an increase in α diversity, while other diversity metrics have not appreciably changed. Second, existing differences in richness and composition between streams draining forest/wetland and grassland/shrub versus urban and agriculture have gotten larger over time. Urban and agricultural streams have lost the few disturbance-sensitive taxa they once had and have gained disturbance-tolerant taxa, which have led to urban stream communities becoming more similar to each other through time. These conclusions are explored in additional detail below.
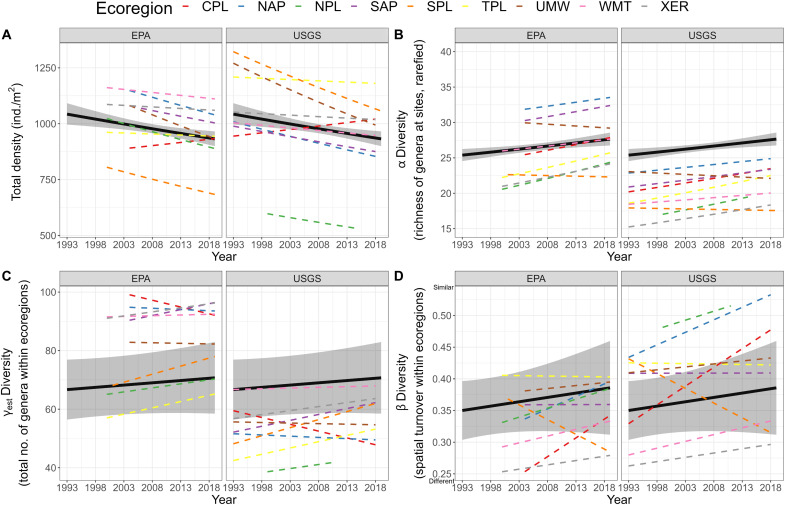
In streams across the United States, the total density of macroinvertebrates decreased and α diversity at sites increased, while ᾱ diversity, γest diversity, and β diversity did not change significantly (Fig. 2, fig. S7, and table S2). At the site-level, macroinvertebrate density declined by 11.0% and richness (α) increased by 12.2% over 27 years. To qualitatively evaluate how much change in α was a product of changes in density (29), we also generated α without rarefication, which also increased (fig. S7 and table S2). Together, decreasing density and increasing rarefied and nonrarefied richness suggest that changes in α through time are a function of changes in the shape of genera abundance distributions within ecoregions and increasing evenness of communities through time. Insects alone decreased by 23.3% in density and 6.8% in richness (α) over 27 years, while γest and β diversities did not change (table S3 and fig. S8). Increases in macroinvertebrate and decreases in insect richness (α) indicate that increases in macroinvertebrate richness are due to a proliferation of noninsect macroinvertebrates. Decreases in densities of freshwater macroinvertebrates and insects and decreases in insect richness align with other studies that suggest widespread declines of insects in terrestrial ecosystems (4–7). These trends could support either declines in most genera or shifts in composition with some genera increasing and others decreasing, as shown in other systems (30–33). Decreasing densities and no change in γ diversity stand in contrast to other recent analyses, suggesting increases in biomass and abundance of freshwater insects (in North America from 1990 to 2018) (4) and increases in occupancy of freshwater macroinvertebrates (in the United Kingdom from 1990 to 2015) (8). To make our results more comparable with previous studies, we also examined trends in density of all macroinvertebrates, not only those identified to genera; these results showed no change in density across time (fig. S8 and table S2). Contrasting results could be due to the following reasons. Previous studies either had relatively limited spatial coverage (8) or relatively few or spatially clustered sites (4, 8), which suggests that their results might not truly be representative of their focal region. In contrast, EPA sites, which make up most of our sites, are randomly selected using a probability-based sampling design to ensure that they are representative of streams across the United States. Alternatively, some studies (8) are focused on an entirely different region of the world, where environmental conditions or differences in assemblages could result in different trends. In addition, previous studies included a wide range of freshwater habitats (4, 8), while we only examined wadable streams, which could have selected for different environmental conditions or assemblages. Whereas our study included entire macroinvertebrate assemblages, previous studies (4) also included trend estimates from population-level studies, which can lead to poor coverage of the entire assemblage and introduce variation in trend estimates (30). Some previous studies (4) also included trends following restoration efforts, which could have led to bias in finding increasing temporal trends. In addition, previous studies (4, 8) combining datasets fail to account for differences in complexities in sampling methodologies (e.g., sampling area and proportion of sample identified) or the ability of taxonomists to make identifications. We have shown that the sampling area and proportion of the sample identified can change through time even in standardized biomonitoring program and are correlated with total density (figs. S4 and S5), and controlling for improvements in taxonomic identifications can even flip the macroinvertebrate density trends from increasing to declining (fig. S13). Moreover, while extracting slopes from individual studies accounts for variation in these factors across studies (4), it fails to account for variation of these factors within studies across time, and combining slopes or effect sizes from individual studies relies on the potentially invalid assumption of equal weight given that some datasets might be more or less prone to finding temporal changes.
Fig. 2. Temporal biodiversity trends of macroinvertebrate communities.
Trends show (A) decreases in total density of macroinvertebrates identified to genus [conditional coefficient of determination (R2), 0.81; marginal R2, 0.77; F(Year), 8.4; P(Year), 0.004], (B) increases in α diversity [conditional R2, 0.68; marginal R2, 0.34; χ2 (Year), 68.0; P(Year), <0.0001], and no change in (C) γest diversity [conditional R2, 0.82; marginal R2, 0.05; F(Year), 0.7; P(Year), 0.424] or (D) β diversity [conditional R2, 0.72; marginal R2, 0.03; F(Year), 1.0; P(Year), 0.344]. Here, greater values of β diversity specify increasingly similar communities. Additional statistical output provided in table S2. Ecoregion abbreviations provided in Fig. 1.
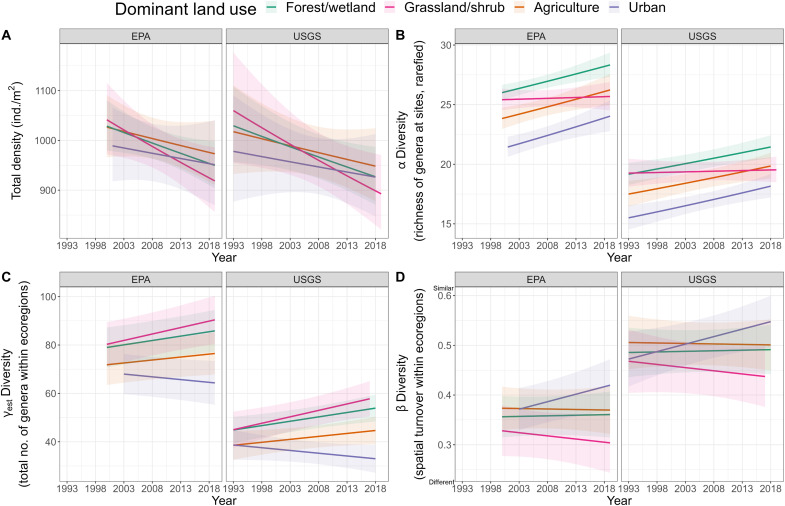
These changes in quantities of biodiversity and composition vary with land use in two ways. First, regardless of time, urban and agricultural streams generally have less biodiversity and fewer disturbance-sensitive and more disturbance-tolerant taxa compared to forest/wetland and grassland/shrub streams. Urban and agricultural stream sites, on average, had fewer genera compared to all other land use types (urban, 19.1; agriculture, 21.3, versus forest/wetland, 23.2; grassland/shrub, 22.0 genera; pairwise comparisons of urban versus other land use types, t ratio ≥ 12.658, P ≤ 0.001; Fig. 3B). Regardless of time, forest/wetland and grassland/shrub streams had more disturbance-sensitive taxa [Ephemeroptera (mayflies), Trichoptera (caddisflies), Plecoptera (stoneflies), and Megaloptera (alderflies, dobsonflies, and fishflies)] and fewer taxa tolerant to disturbance (e.g., Chironominae and Orthocladiinae flies) than agricultural and urban streams (Fig. 4) (10, 34). Here, we refer to tolerance to a range of conditions including water quality, pollution, water temperature, and stream flow.
Fig. 3. Temporal biodiversity trends of macroinvertebrate communities across dominant land use types.
There was (A) no variation in total density across land use types [conditional R2, 0.81; marginal R2, 0.77; F(Year), 0.7; P(Year), 0.418; F(Land use), 0.4; P(Land use), 0.748; F(Year*Land use), 0.4; P(Year*Land use), 0.717], (B) lower α diversity in urban streams than all other land use types [conditional R2, 0.63; marginal R2, 0.36; χ2 (Year), 6.5; P(Year), 0.011; χ2 (Land use), 65.7; P(Land use), <0.0001; χ2 (Year*Land use), 9.4; P(Year*Land use), similar different 0.024], (C) decreases in γest diversity for urban streams, no change for agricultural streams, and increases for forest/wetland and grassland/shrub [conditional R2, 0.74; marginal R2, 0.68; F(Year), 2.7; P(Year), 0.103; F(Land use), 1.3; P(Land use), 0.299; F(Year*Land use), 4.7; P(Year*Land use), 0.003], and (D) urban stream communities homogenize through time, although these trends did not vary significantly in comparison to other land use types [conditional R2, 0.43; marginal R2, 0.28; F(Year), 0.02; P(Year), 0.878; F(Land use), 0.5; P(Land use), 0.655; F(Year*Land use), 2.0; P(Year*Land use), 0.111]. Additional statistical output is provided in table S4.
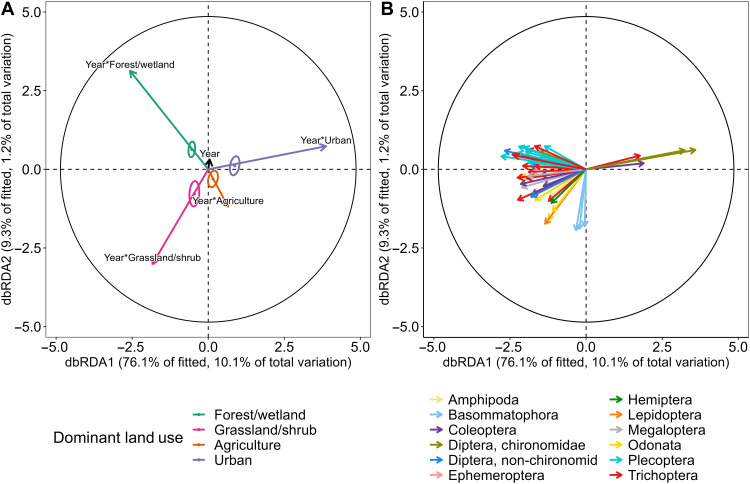
Fig. 4. Partial distance-based redundancy analysis (accounting for agency, ecoregion, and improvements in taxonomic identification) demonstrating that composition of macroinvertebrate communities has changed differentially through time according to land use.
(A) Plot of model predictors showing the additive and interactive effects of land use and year. Individual points and circles are the centroids and 95% confidence intervals of ecoregion-year combinations according to dominant land use. (B) Corresponding vector overlay of model responses. Individual vectors are either families for all non-chironomid macroinvertebrates or subfamilies for chironomids. As shown in the legend, non-chironomid macroinvertebrate vectors are colored by order, and chironomid vectors are colored by family. Vectors with lengths less than 0.3 have been excluded. In both plots, the black circles correspond to vector lengths that would have a correlation coefficient of one with each axis. The entire dbRDA model explains 44.6% of the variance. Additional statistical output included in table S6.
Second, through time, these existing differences in richness and composition have grown substantially larger. Macroinvertebrate γest diversity in urban streams tends to decrease through time, while, in contrast, γest diversity of all other land use types increases (pairwise comparisons of slopes of urban versus all other land use types, t ratio ≥ 2.39, P ≤ 0.02; Fig. 3C and table S4). Within ecoregions, urban lost 0.23 genera per year (6.21 genera over 27 years), respectively, while agricultural, forest/wetland, and grassland/shrub streams gained 0.24, 0.36, and 0.53 genera per year (6.48, 9.72, and 14.31 genera over 27 years), respectively.
These differences in richness within ecoregions between streams draining less human-modified land uses and more human-modified land uses were driven by the loss of the few existing sensitive taxa present in early years and the proliferation of more tolerant taxa in urban and agricultural streams. Genera in sensitive groups (Ephemeroptera, Trichoptera, Plecoptera, and Megaloptera) likely decreased, while genera within tolerant groups (Chironominae and Orthocladiinae) likely increased (Fig. 3C). For urban streams, these changes led to homogenization of communities (Fig. 3D). The largest differences in β diversity trends occurred between communities in grassland/shrub streams and in urban streams (pairwise comparison of slopes of grassland/shrub versus urban, t ratio = −2.201, P = 0.03). Similarly, analyses on insects alone showed that insect communities diverged through time according to land use (tables S5 and S7 and figs. S9 and S10).
Interesting questions emerge concerning the drivers of decreases in total densities of macroinvertebrates and the divergence in biodiversity and composition among streams with more and less anthropogenic modification. We hypothesize that decreases in total densities in all streams may be in response to rising water temperatures (35) and more frequent hydrologic disturbances (e.g., flashier hydrograph and altered channel morphology) (36) that are observed across North America as a result of climate change. We hypothesize that divergence in community composition among streams with less and more anthropogenic modification could be caused by additive or synergistic effects among rising temperatures, hydrologic disturbances, and poor water quality, including high nutrients, salts, suspended solids, and anthropogenic chemical contamination (37, 38). While rising temperatures and hydrologic disturbance are likely increasing in all streams across the nation, poor water quality and chemical contamination are more likely at streams within urban and agricultural regions compared to forest/wetland and grassland/shrub regions. Future studies will test these hypotheses.
Despite decades of regulatory and management actions aimed at reducing freshwater contaminants and restoring freshwater habitat, we observe continued degradation of the communities in urban and agricultural streams and no evidence of widespread recovery of sensitive aquatic taxa across U.S. stream networks. Our results suggest that current legislation and conservation and restoration land use practices (39) have not been enough to stem the pressures on these communities. Without protections for stream communities, especially those in anthropogenically modified habitats, they likely will continue to degrade and perhaps lead to a loss of ecosystem functions and services.
MATERIALS AND METHODS
Data management
We derived site-level density and α and region-level ᾱ, γ, and β diversity metrics for macroinvertebrate communities, within the orders Arthropoda, Mollusca, and Annelida, using data from federal biomonitoring programs in the United States that spanned 27 years, from 1993 until 2019 (Fig. 1 and figs. S1 and S2). These programs included six U.S. EPA federal projects and 64 USGS federal and regional projects (table S1). We refer to EPA and USGS as two agencies. Overall, the dataset was based on 3914 unique EPA sites and 2217 unique USGS sites. Regions used in the present analyses are ecoregions, which are areas of grossly similar environmental characteristics, such as climate, vegetation, soil type, and geology, as defined by the EPA National Aquatic Resource Surveys.
Before the calculation of diversity metrics, reproducible site by genera matrices was generated using data management techniques to account for methodological variation among programs and across space and time. The focus of data management was to account for differences in sampling methods (e.g., habitat types, sampling techniques, and equipment) according to program, differences in identification methods of specimen according to program, changes in taxonomy across time, and ambiguously identified genera (e.g., specimen identified as genera A/genera B, or not identified to genera). In addition, we accounted for improvements in the abilities of taxonomists to make identifications of macroinvertebrate genera within our statistical models (see the “Statistical analyses” section).
To account for differences in sampling methodologies, first, we selected only sites that occurred in wadeable streams so that habitat type was comparable across sites. For USGS sites, only “main-body component” samples were included. Main-body component samples are a portion of the total field sample defined by USGS methods (40). In addition, to account for differences in sampling techniques and equipment, the following method codes for sampling events were selected: BERW, IRTH, SWAMP, EMAP, CDPHE, and PNAMP. Previous studies have suggested that these methods, which use similar mesh sizes, are comparable in their ability to detect the presence and absence of taxa (41–43). Only area-limited sample data were used. Next, given that differences in methodologies or personnel in the identification of specimens by EPA and USGS could still have led to differences in the number of genera identified within families, only genera that were identified by both EPA and USGS programs were included in analyses.
Across the time span of our dataset, the taxonomy of many species changed. In cases in which a specimen was identified to species, we were able to update the genus designation from the bench identification to the now used genus names. In other cases, a given specimen was only identified to genus at the bench, and only some species within the given genus were moved into a new genus. This scenario prevented us from being confident in the identity of the current genus of the given specimen. In those cases, we created complexes of genera by combining all possible current genera of the given specimen. This approach prioritized retaining observations of specimens by giving a unified name (e.g., genus1/genus2/genus3) to a complex of genera that have been linked through changes in taxonomy through time. There was no inflation of the number of genera with this approach given that individual genera (e.g., genus1) never appeared outside of the unified genera name (e.g., genus1/genus2/genus3). Within the combined EPA and USGS dataset, 98 genera existed that were linked through changes in taxonomy through time. The approach described above reduced these 98 genera to 13 complexes of genera. All but two complexes of genera were composed of two individual genera. A single complex of genera within the Ephemeroptera order included 70 individual genera, and a second complex of Ephemeroptera genera included six individual genera. There were a total of 528 genera included in the dataset after groupings were applied.
Other specimens were not identified to genera or were ambiguously identified. In the cases in which a specimen was identified to family or another high level of biological organization, the specimen was dropped from further analyses. In other cases, a specimen was identified at the bench with multiple species or genera names (e.g., species1/species2 or genus1/genus2). In cases in which the combined species name was in the same genus, the classification was simply rolled up to genus. Some instances of combined genera names were not consistent between EPA and USGS. Therefore, USGS could contain a specimen identified as genus1/genus2, while EPA included instances of separate identifications of genus1 and genus2. To ensure consistency between the programs, we renamed all single instances of genus1 and genus2 to genus1/genus2 throughout the combined EPA and USGS dataset.
Once these data management steps were taken, we calculated total densities of macroinvertebrates at sites, α diversity, the number of genera at sites, and region-level biodiversity metrics including ᾱ diversity, the average number of genera across sites within a given ecoregion; γest diversity, the total number of genera estimated between 70 and 80% of sample coverage; and β diversity, a metric of homogenization defined as 1 − proportional β diversity (1 – ᾱ/γest), which simplifies to ᾱ/γest. β diversity is the proportion of genera in an ecoregion that is contained within an average site (fig. S2). We chose this metric of β diversity because it is independent of ᾱ diversity (44), meaning that the value of β diversity for a given ecoregion was not influenced by the ᾱ diversity within that same ecoregion. We chose 70 to 80% sample coverage to balance the exclusion of experimental units that could not generate an estimate between 70 and 80% with completeness of the sample coverage estimate.
Some sites were spatially subsampled at multiple stream reaches, and some sites were sampled multiple times in a given year. In calculating total density, we randomly subsampled a single stream reach for each site. Statistical models of density included a noninteger predictor of time, including year, month, and day (see the “Statistical analyses” section), so temporal samples were kept independent. In calculating region-level diversity metrics, we combined the presence and absence of genera at multiple stream reaches and multiple sampling events in a given year within a given site. Therefore, each observation in the dataset before region-level diversity metrics were calculated corresponded to a single site per year. Statistical models of region-level diversity metrics included an integer predictor of year (see the “Statistical analyses” section).
To calculate total density of macroinvertebrates at all sites, we calculated sample abundance and then divided sample abundance by total area sampled. Sample abundance was calculated as the number of individual organisms identified divided by the proportion of the total sample identified. For EPA samples, the proportion of the total sample identified was provided. For USGS sites, the proportion of the total sample identified was calculated as the laboratory subsampling ratio multiplied by the field split ratio. The laboratory subsampling ratio was the proportion of the laboratory samples that had all individuals identified. The field split ratio was the proportion of the field sample that was brought to the laboratory. We dropped sites with a very low proportion of the sample identified (<0.055) and very low or high area sampled (<0.05 and >5 m2) because of high error associated with scaling these subsamples to whole samples and densities.
Sampling effort varied at two spatial scales, which influenced how diversity metrics were calculated. First, the number of specimens identified in each sampling event varied across sites. In addition, we lacked information on a standard target of individuals within sampling events for some USGS sites, so it was not possible to know whether a given sample had few individuals because the site had poor total abundance or because the sampling event used a low target number of organisms. Target counts for EPA sites were typically 500. Given the common relationship between the number of genera and the number of organisms sampled within sites, we accounted for differences in the number of specimens identified in the calculation of ᾱ and γest diversity metrics using rarefaction. After accounting for laboratory subsampling ratios, we dropped sampling events that included fewer than 300 individuals (21.2% of all sampling events), and we randomly subsampled sampling events to 300 individuals. We set this rarefaction threshold at 300 to balance the number of samples retained in the dataset with the average number of genera added to sites (fig. S3). We did not rarefy samples at the site level in the calculation of total densities because of the likelihood that rarefying would exclude sites with low total densities that were a product of disturbance or natural variation.
Second, the number of sites varied across ecoregions and years. Given the relationship between total number of genera within ecoregions and number of sites sampled, we accounted for these differences in the number of sites within ecoregions and years using sample-based coverage estimates of γ diversity (45). The γest diversity was estimated using sample coverage, a measure of sample completeness from genera-accumulation curves, with the estimateD function in the iNEXT package in R (46). Initially, we set a target of 75% sample coverage. However, most estimates fell within 70 and 80% of sample coverage, so in an effort to include as many ecoregion-year combinations as possible and to balance concerns about the accuracy of γest diversity, we excluded ecoregion-year combinations that had too few sites to estimate γest within 70 to 80% of sample coverage, and we extracted 95% confidence intervals for each γest and used these error measurements as weights in statistical models (see next section).
Statistical analyses
We first sought to evaluate how site-level total density; site-level α diversity; and region-level ᾱ, γ, and β diversities of macroinvertebrate communities changed over time using linear mixed-effects models. In the models of site-level total density and α diversity, the response was log-transformed total density of macroinvertebrates or number of genera, and the predictors were proportion of the sample identified, proportion of specimen within samples identified to genus, area of the sample, year (as a noninteger, incorporating year, month, and day of sampling), ecoregion, agency, the interaction of year and ecoregion, the interaction of ecoregion and agency, and a random effect of site. The proportion of the sample identified and the area of the sample were covariates to account for residual correlation with time and total density (figs. S4 and S5). The proportion of specimen within samples identified to genus accounted for improvements in the ability of taxonomists to identify specimen through time (fig. S6). The interaction of year and ecoregion allowed the effects of time to vary across ecoregions but remain consistent among agencies. The interaction of ecoregion and agency allowed the intercepts of ecoregion-year trends to vary. Our impetus was to generate ecoregion-level trends across agencies to test the hypothesis that densities change through time. At the same time, we wanted the intercepts to vary to account for agency-level differences in densities.
In region-level analyses of temporal changes in ᾱ diversity, the response was ᾱ diversity, the average number of genera across sites within ecoregions, and the predictors were a fixed effect of year (as an integer), proportion of specimen within samples identified to genus, a random slope of year across ecoregions, and a random intercept term for ecoregion-agency combinations. Similar to the density model, our impetus was to generate ecoregion-level trends across agencies to test the hypothesis that ᾱ diversity changed through time. At the same time, we wanted the intercepts to vary to account for agency-level differences in ᾱ diversity. To evaluate temporal changes in γest and β diversity, models had identical predictors compared to the ᾱ diversity model. In addition, models of γest and β diversity included weights for error estimate of γest. Weights were the inverse of the 95% confidence interval for γest, standardized to the mean.
Next, we evaluated how temporal trends in site-level total density; α diversity; and region-level ᾱ, γ, and β diversities of macroinvertebrate communities varied according to dominant land use using mixed-effects models. First, we joined site-level macroinvertebrate community data to proportion land use within catchments from NLCD using EPA StreamCat data in R (47). Sites were temporally matched to the closest year of available NLCD data. Several similar NLCD land use categories were combined to create four aggregate categories: (i) forest/wetland, (ii) grassland/shrub, (iii) agriculture, and (iv) urban. We combined forest and wetland land uses to represent relatively less disturbed, highly moist habitats. We combined grassland and shrub land uses to represent relatively less disturbed, arid habitats. Agriculture and urban land uses represented relatively disturbed habitats. Forest/wetland included deciduous forest, coniferous forest, mixed forest, woody wetlands, emergent herbaceous wetlands, and open water. Grassland/shrub was barren land, shrub/scrub, and grassland/herbaceous. Agriculture included pasture/hay and cultivated crops, and, lastly, urban was open-space–, low-intensity–, medium-intensity–, and high-intensity–developed. Then, we assigned a dominant land use category to each site, which was the maximum proportion land use type within catchments. Sites assigned forest/wetland, grassland/shrub, agriculture, and urban were between 31.2 and 100%, 35.4 and 100%, 32.4 and 99.3%, and 28.8 and 100% of their respective land use type within the catchment. Last, we recalculated ᾱ, γest, and β diversities within ecoregions, agencies, and dominant land use categories, as previously described. We dropped ecoregion, agency, land use categories with too few sites to estimate γ between 70 and 80% sample coverage.
To evaluate how temporal trends in site-level α diversity and density vary with dominant land use, we fit a model with log-transformed total density as the response, and the predictors were proportion of the sample identified, proportion of specimen within samples identified to genus, area of the sample, year (as a noninteger, incorporating year, month, and day of sampling), ecoregion, agency, land use, the interaction of year and ecoregion, the interaction of ecoregion and agency, the interaction of year and land use, and a random effect of site. In region-level analyses of temporal changes in ᾱ diversity by dominant land use, the response was ᾱ diversity, and the predictors were the fixed effects of year, proportion of specimen within samples identified to genus, land use, agency, and the interaction of year and land use; the random effects included a random intercept of year across ecoregion and land use combinations and a random slope of agency across ecoregions. To evaluate how temporal changes in γest and β diversity varied with land use, we fit models with the same predictors compared to the ᾱ diversity model. In addition, models of γest and β diversity included weights for error estimate of γest. Weights were the inverse of the 95% confidence interval for γest, standardized to the mean. Preliminary analyses with generalized additive models revealed that temporal trends in biodiversity metrics both with and without land use were not significantly nonlinear. We checked plots of the relationship between observed and model predicted values, which indicated a good correlation for both the region-level analyses (fig. S11) and the land-use analyses (fig. S12). We also checked model assumptions via residual and q-q plots, which indicated no major violations of model assumptions.
Next, we sought to determine which taxa were responsible for differences in temporal region-level biodiversity trends among land use categories. We used a partial distance-based redundancy analysis (partial dbRDA), a constrained ordination technique on a community similarity matrix that accounts for variation in the community matrix attributed to one or more conditional variables before performing the dbRDA (21). The multivariate response was a presence/absence matrix of either families for all non-chironomid macroinvertebrates within ecoregions or subfamilies for all chironomids within ecoregions. We dropped taxa that occurred in only one or two land use–ecoregion–year combinations. Chironomids were assessed at the subfamily level because of their immense diversity relative to other macroinvertebrate families. We omitted the mayfly families Baetidae, Caenidae, Potamanthidae, Ephemerellidae, Baetiscidae, Polymitarcyidae, Leptophlebiidae, Heptageniidae, and Ephemeridae from analyses because of changes in taxonomy (see above). Therefore, our approach to lumping genera with linked taxonomy prevented accurate estimation of the presence and absence of these mayfly families within ecoregions. Because of this exclusion, we cannot discern how these mayfly families are contributing to changes in composition. The response in the partial dbRDA was based on Sorenson similarities of this presence-absence matrix. We modeled the community response as a function of year (as a continuous variable), land use, and their interaction after accounting for the conditional variables of agency, ecoregion, and a categorical variable that was early (1993 to 2004) and late (2005 to 2019) time within our dataset. Because genera within the orders Trombidiformes and Arhynchobdellida only appeared in our dataset after 2004 because of improvements in taxonomic identifications (fig. S6), we accounted for this artifact by including the conditional variable of early/late time. Significance of year, land use, and their interaction was determined using a permutation test, with 9999 permutations of the residuals after the effects of agency and ecoregion removed.
Last, all analyses were run with insects alone to determine whether benthic insects showed similar or different trends compared to benthic macroinvertebrates. We examined (i) how trends in biodiversity (total density and ᾱ, γest, and β diversity) differed across time, (ii) how temporal trends in biodiversity varied with land use, and (iii) how composition varied across time and land use. Statistical methods for these analyses were identical to those described above for all benthic macroinvertebrates.
Acknowledgments
Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. government. The findings and conclusions here do not necessarily represent the views or policies of the U.S. Environmental Protection Agency or the U.S. Geological Survey.
Funding: This work was conducted as part of the Analyses of Contaminant Effects in Freshwater Systems: Synthesizing Abiotic and Biotic Stream Datasets for Long-Term Ecological Research Working Group supported by the John Wesley Powell Center for Analysis and Synthesis, funded by the U.S. Geological Survey.
Author contributions: Conceptualization: S.L.R., M.B.M., D.K.J., and J.R.R. Data curation: S.L.R., M.B.M., D.K.J., and R.H. Investigation: S.L.R., M.B.M., and D.K.J. Methodology: S.L.R., M.B.M., D.K.J., W.B., J.B., E.S.B., P.B., F.D.L., R.H., E.R., R.S., T.S.S., M.S., and J.R.R. Funding acquisition: D.K.J., W.B., T.S.S., and J.R.R. Project administration: S.L.R. Supervision: J.R.R. Validation: S.L.R. and M.B.M. Writing—original draft: S.L.R. and M.B.M. Writing—reviewing and editing: S.L.R., M.B.M., D.K.J., W.B., J.B., E.S.B., P.B., E.B., F.D.L., R.H., S.K., S.L., E.R., R.S., T.S.S., M.S., K.S., K.V., and J.R.R.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Datasets and code for analyses and generation of figures are available via FigShare (doi: 10.6084/m9.figshare.22266046) and a public GitHub repository (https://github.com/rumschsl/MacroBiodivTrends).
Supplementary Materials
This PDF file includes:
Tables S1 to S7
Figs. S1 to S13
REFERENCES AND NOTES
- 1.Strayer D. L., Dudgeon D., Freshwater biodiversity conservation: Recent progress and future challenges. J. North Am. Benth. Soc. 29, 344–358 (2010). [Google Scholar]
- 2.Baron J. S., Poff N. L. R., Angermeier P. L., Dahm C. N., Gleick P. H., Hairston N. G. Jr., Jackson R. B., Johnston C. A., Richter B. D., Steinman A. D., Meeting ecological and societal needs for freshwater. Ecol. Appl. 12, 1247–1260 (2002). [Google Scholar]
- 3.He F., Zarfl C., Bremerich V., David J. N. W., Hogan Z., Kalinkat G., Tockner K., Jähnig S. C., The global decline of freshwater megafauna. Glob. Chang. Biol. 25, 3883–3892 (2019). [DOI] [PubMed] [Google Scholar]
- 4.van Klink R., Bowler D. E., Gongalsky K. B., Swengel A. B., Gentile A., Chase J. M., Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 368, 417–420 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Dirzo R., Young H. S., Galetti M., Ceballos G., Isaac N. J. B., Collen B., Defaunation in the Anthropocene. Science 345, 401–406 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Schuch S., Wesche K., Schaefer M., Long-term decline in the abundance of leafhoppers and planthoppers (Auchenorrhyncha) in Central European protected dry grasslands. Biol. Conserv. 149, 75–83 (2012). [Google Scholar]
- 7.Hallmann C. A., Sorg M., Jongejans E., Siepel H., Hofland N., Schwan H., Stenmans W., Müller A., Sumser H., Hörren T., Goulson D., de Kroon H., More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLOS ONE 12, e0185809 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Outhwaite C. L., Gregory R. D., Chandler R. E., Collen B., Isaac N. J. B., Complex long-term biodiversity change among invertebrates, bryophytes and lichens. Nat. Ecol. Evol. 4, 384–392 (2020). [DOI] [PubMed] [Google Scholar]
- 9.Jähnig S. C., Baranov V., Altermatt F., Cranston P., Friedrichs-Manthey M., Geist J., He F., Heino J., Hering D., Hölker F., Jourdan J., Kalinkat G., Kiesel J., Leese F., Maasri A., Monaghan M. T., Schäfer R. B., Tockner K., Tonkin J. D., Domisch S., Revisiting global trends in freshwater insect biodiversity. Wires Water 8, e1506 (2021). [Google Scholar]
- 10.Kerans B. L., Karr J. R., A benthic index of biotic integrity (B-Ibi) for rivers of the Tennessee Valley. Ecol. Appl. 4, 768–785 (1994). [Google Scholar]
- 11.Thomas J. A., Telfer M. G., Roy D. B., Preston C. D., Greenwood J. J. D., Asher J., Fox R., Clarke R. T., Lawton J. H., Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303, 1879–1881 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Stepanian P. M., Entrekin S. A., Wainwright C. E., Mirkovic D., Tank J. L., Kelly J. F., Declines in an abundant aquatic insect, the burrowing mayfly, across major North American waterways. Proc. Natl. Acad. Sci. U.S.A. 117, 2987–2992 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lydeard C., Cowie R. H., Ponder W. F., Bogan A. E., Bouchet P., Clark S. A., Cummings K. S., Frest T. J., Gargominy O., Herbert D. G., Hershler R., Perez K. E., Roth B., Seddon M., Strong E. E., Thompson F. G., The global decline of nonmarine mollusks. BioScience 54, 321–330 (2004). [Google Scholar]
- 14.Crossley M. S., Meier A. R., Baldwin E. M., Berry L. L., Crenshaw L. C., Hartman G. L., Lagos-Kutz D., Nichols D. H., Patel K., Varriano S., Snyder W. E., Moran M. D., No net insect abundance and diversity declines across U.S. long term ecological research sites. Nat. Ecol. Evol. 4, 1368–1376 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Lister B. C., Garcia A., Climate-driven declines in arthropod abundance restructure a rainforest food web. Proc. Natl Acad. Sci U.S.A. 115, E10397–E10406 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welti E. A. R., Joern A., Ellison A. M., Lightfoot D. C., Record S., Rodenhouse N., Stanley E. H., Kaspari M., Studies of insect temporal trends must account for the complex sampling histories inherent to many long-term monitoring efforts. Nat. Ecol. Evol. 5, 589–591 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Willig M. R., Woolbright L., Presley S. J., Schowalter T. D., Waide R. B., Heartsill Scalley T., Zimmerman J. K., González G., Lugo A. E., Populations are not declining and food webs are not collapsing at the Luquillo Experimental Forest. Proc. Natl. Acad. Sci. U.S.A. 116, 12143–12144 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E., Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Biesmeijer J. C., Roberts S. P. M., Reemer M., Ohlemüller R., Edwards M., Peeters T., Schaffers A. P., Potts S. G., Kleukers R., Thomas C. D., Settele J., Kunin W. E., Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Habel J. C., Segerer A., Ulrich W., Torchyk O., Weisser W. W., Schmitt T., Butterfly community shifts over two centuries. Conserv. Biol. 30, 754–762 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Seibold S., Gossner M. M., Simons N. K., Blüthgen N., Müller J., Ambarlı D., Ammer C., Bauhus J., Fischer M., Habel J. C., Linsenmair K. E., Nauss T., Penone C., Prati D., Schall P., Schulze E. D., Vogt J., Wöllauer S., Weisser W. W., Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature 574, 671–674 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Bini L. M., Landeiro V. L., Padial A. A., Siqueira T., Heino J., Nutrient enrichment is related to two facets of beta diversity for stream invertebrates across the United States. Ecology 95, 1569–1578 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Patrick C. J., Yuan L. L., The challenges that spatial context present for synthesizing community ecology across scales. Oikos 128, 297–308 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chagnon M., Kreutzweiser D., Mitchell E. A. D., Morrissey C. A., Noome D. A., van der Sluijs J. P., Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ. Sci. Pollut. Res. Int. 22, 119–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raitif J., Plantegenest M., Roussel J.-M., From stream to land: Ecosystem services provided by stream insects to agriculture. Agr. Ecosyst. Environ. 270-271, 32–40 (2019). [Google Scholar]
- 26.Keiser D. A., Shapiro J. S., Consequences of the Clean Water Act and the demand for water quality. Q. J. Econ. 134, 349–396 (2019). [Google Scholar]
- 27.Rochlin I., Faraji A., Ninivaggi D. V., Barker C. M., Kilpatrick A. M., Anthropogenic impacts on mosquito populations in North America over the past century. Nat. Commun. 7, 13604 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Strien A. J., Meyling A. W. G., Herder J. E., Hollander H., Kalkman V. J., Poot M. J. M., Turnhout S., van der Hoorn B., van Strien-van Liempt W. T. F. H., van Swaay C. A. M., van Turnhout C. A. M., Verweij R. J. T., Oerlemans N. J., Modest recovery of biodiversity in a western European country: The Living Planet Index for the Netherlands. Biol. Conserv. 200, 44–50 (2016). [Google Scholar]
- 29.Blowes S. A., Daskalova G. N., Dornelas M., Engel T., Gotelli N. J., Magurran A. E., Martins I. S., McGill B., McGlinn D. J., Sagouis A., Shimadzu H., Supp S. R., Chase J. M., Local biodiversity change reflects interactions among changing abundance, evenness, and richness. Ecology 103, e3820 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Dornelas M., Gotelli N. J., McGill B., Shimadzu H., Moyes F., Sievers C., Magurran A. E., Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Dornelas M., Gotelli N. J., Shimadzu H., Moyes F., Magurran A. E., McGill B. J., A balance of winners and losers in the Anthropocene. Ecol. Lett. 22, 847–854 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Hillebrand H., Blasius B., Borer E. T., Chase J. M., Downing J. A., Eriksson B. K., Filstrup C. T., Harpole W. S., Hodapp D., Larsen S., Lewandowska A. M., Seabloom E. W., van de Waal D. B., Ryabov A. B., Biodiversity change is uncoupled from species richness trends: Consequences for conservation and monitoring. J. Appl. Ecol. 55, 169–184 (2018). [Google Scholar]
- 33.Supp S. R., Ernest S. K. M., Species-level and community-level responses to disturbance: A cross-community analysis. Ecology 95, 1717–1723 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Freshwater Biological Traits Database (Final Report) (EPA/600/R-11/038F, 2012). [Google Scholar]
- 35.Nelson K. C., Palmer M. A., Stream temperature surges under urbanization and climate change: Data, models, and responses. J. Am. Water Resour. As. 43, 440–452 (2007). [Google Scholar]
- 36.Palmer M., Ruhi A., Linkages between flow regime, biota, and ecosystem processes: Implications for river restoration. Science 365, eaaw2087 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Fruh D., Stoll S., Haase P., Physicochemical and morphological degradation of stream and river habitats increases invasion risk. Biol. Invasions 14, 2243–2253 (2012). [Google Scholar]
- 38.Jourdan J., O'Hara R. B., Bottarin R., Huttunen K.-L., Kuemmerlen M., Monteith D., Muotka T., Ozoliņš D., Paavola R., Pilotto F., Springe G., Skuja A., Sundermann A., Tonkin J. D., Haase P., Effects of changing climate on European stream invertebrate communities: A long-term data analysis. Sci. Total Environ. 621, 588–599 (2018). [DOI] [PubMed] [Google Scholar]
- 39.Rohr J. R., Bernhardt E. S., Cadotte M. W., Clements W. H., The ecology and economics of restoration: When, what, where, and how to restore ecosystems. Ecol. Soc. 23, 15 (2018). [Google Scholar]
- 40.T. F. Cuffney, M. E. Gurtz, M. R. Meador, “Methods for collecting benthic invertebrate samples as part of the National Water-Quality Assessment Program,” Open-File Report (1993).
- 41.D. A. Peterson, J. R. Zumberge, “Comparison of macroinvertebrate community structure between two riffle-based sampling protocols in Wyoming, Colorado, and Montana, 2000–2001,” Scientific Investigations Report (2006).
- 42.J. F. Bruce, J. J. Roberts, R. E. Zuellig, “Comparability among four invertebrate sampling methods and two multimetric indexes, Fountain Creek Basin, Colorado, 2010–2012,” Scientific Investigations Report (Reston, VA, 2018). [Google Scholar]
- 43.R. E. Zuellig, J. F. Bruce, S. R. W. Stogner, K. D. Brown, “Comparability among four invertebrate sampling methods, Fountain Creek Basin, Colorado, 2010–2012,” Scientific Investigations Report (Reston, VA, 2014). [Google Scholar]
- 44.Tuomisto H., A diversity of beta diversities: Straightening up a concept gone awry. Part 1. Defining beta diversity as a function of alpha and gamma diversity. Ecography 33, 2–22 (2010). [Google Scholar]
- 45.Chao A., Jost L., Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 93, 2533–2547 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Chao A., Gotelli N. J., Hsieh T. C., Sander E. L., Ma K. H., Colwell R. K., Ellison A. M., Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 84, 45–67 (2014). [Google Scholar]
- 47.Hill R. A., Weber M. H., Leibowitz S. G., Olsen A. R., Thornbrugh D. J., The stream-catchment (Streamcat) dataset: A database of watershed metrics for the conterminous United States. J. Am. Water Resour. As. 52, 120–128 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S7
Figs. S1 to S13