Abstract
Background
Cabotegravir (CAB) + rilpivirine (RPV) dosed intramuscularly monthly or every 2 months is a complete, long-acting (LA) regimen for the maintenance of HIV-1 virologic suppression. Here, we report the antiretroviral therapy as long acting suppression (ATLAS)-2M study week 152 results.
Methods
ATLAS-2M is a phase 3b, randomized, multicenter study assessing the efficacy and safety of CAB+RPV LA every 8 weeks (Q8W) versus every 4 weeks (Q4W). Virologically suppressed (HIV-1 RNA <50 copies/mL) individuals were randomized to receive CAB+RPV LA Q8W or Q4W. Endpoints included the proportion of participants with plasma HIV-1 RNA ≥50 copies/mL and <50 copies/mL, incidence of confirmed virologic failure (CVF; 2 consecutive measurements ≥200 copies/mL), safety, and tolerability.
Results
A total of 1045 participants received CAB+RPV LA (Q8W, n = 522; Q4W, n = 523). CAB+RPV LA Q8W demonstrated noninferior efficacy versus Q4W dosing, with 2.7% (n = 14) and 1.0% (n = 5) of participants having HIV-1 RNA ≥50 copies/mL, respectively, with adjusted treatment difference being 1.7% (95% CI: 0.1–3.3%), meeting the 4% noninferiority threshold. At week 152, 87% of participants maintained HIV-1 RNA <50 copies/mL (Q8W, 87% [n = 456]; Q4W, 86% [n = 449]). Overall, 12 (2.3%) participants in the Q8W arm and 2 (0.4%) in the Q4W arm had CVF. Eight and 10 participants with CVF had treatment-emergent, resistance-associated mutations to RPV and integrase inhibitors, respectively. Safety profiles were comparable, with no new safety signals observed since week 48.
Conclusions
These data demonstrate virologic suppression durability with CAB+RPV LA Q8W or Q4W for ∼3 years and confirm long-term efficacy, safety, and tolerability of CAB+RPV LA as a complete regimen to maintain HIV-1 virologic suppression.
Keywords: antiretroviral therapy, cabotegravir, HIV-1, long-acting, rilpivirine
Cabotegravir+rilpivirine long-acting dosed every 8 weeks continued to be noninferior to every-4-week dosing over 152 weeks, with comparable safety profiles and no new safety signals since week 96. These data demonstrate durability of virologic suppression with cabotegravir+rilpivirine long-acting for nearly 3 years.
(See the Editorial Commentary by Llibre and Kuritzkes on pages 1655–7.)
Advances in antiretroviral therapy (ART) have improved durable virologic suppression and significantly reduced the morbidity and mortality associated with human immunodeficiency virus (HIV) infection [1, 2], transforming HIV from a fatal condition to a manageable chronic disease. Although effective, current oral ART regimens require high levels of adherence to maintain virologic suppression [3]. Treatment interruption is associated with rebound viremia, increased risk of transmission, and increased morbidity and mortality [3, 4]. In addition, daily oral ART regimens have several inherent challenges for some people with HIV (PWH), including fear of disclosure, stigmatization, anxiety related to staying adherent, and the daily reminder of HIV status [5, 6]. Therefore, patients and providers have expressed interest in long-acting (LA) ART treatments with reduced dosing frequencies [7, 8].
Cabotegravir (CAB), an integrase strand transfer inhibitor (INSTI), and rilpivirine (RPV), a nonnucleoside reverse transcriptase inhibitor, have been developed for LA administration as intramuscular injections [9–11]. CAB+RPV LA has been approved for monthly dosing and every-2-month (Q2M) dosing in Australia, Canada, the European Union, and the United States [12–15]. Q2M dosing is commercially available in Australia, Austria, Belgium, Canada, Denmark, Finland, France, Germany, Hong Kong, Ireland, Italy, Japan, Norway, Poland, Sweden, Switzerland, Taiwan, the Netherlands, the United Kingdom, and the United States. Approval of monthly dosing was based on the ongoing phase 3 antiretroviral therapy as long acting suppression (ATLAS) (NCT02951052) and first long-acting injectable regimen (FLAIR) (NCT02938520) studies, which demonstrated CAB+RPV LA dosed every 4 weeks (Q4W) was noninferior to daily oral ART over 48 weeks, with continued high rates of virologic suppression through 96 weeks of treatment [9, 11, 16, 17]. Approval of Q2M dosing was based on the ongoing phase 3b ATLAS-2M (NCT03299049) study [10, 18], which demonstrated CAB+RPV LA dosed every 8 weeks (Q8W) was noninferior to Q4W dosing over 48 and 96 weeks, with similar safety profiles. Additionally, most participants preferred Q8W dosing over Q4W dosing and oral ART [10].
Here, we report the efficacy, safety, tolerability, and patient-reported outcomes through 152 weeks of CAB+RPV LA treatment dosed Q8W and Q4W from the phase 3b ATLAS-2M study.
METHODS
Study Design
ATLAS-2M is a phase 3b randomized, open-label, active-controlled, multicenter, parallel-group noninferiority study to evaluate the efficacy, safety, and tolerability of CAB+RPV LA administered Q8W versus Q4W in virologically suppressed PWH. The full inclusion and exclusion criteria, study design, and procedures have been previously published [10]. The full study protocol is available online [19].
The ATLAS-2M study was conducted following the Declaration of Helsinki [20]. All participants provided written informed consent. The study protocol, amendments, informed consent, and other information that required preapproval were reviewed and approved by a national, regional, or investigational center ethics committee or institutional review board.
Randomization
Full details of randomization have been published previously [10]. Participants were randomly assigned (unblinded) 1:1 to receive CAB+RPV LA Q8W or Q4W. Randomization was stratified by previous CAB+RPV exposure (0 weeks, 1–24 weeks, >24 weeks) to account for individuals who transitioned from the ATLAS study having received CAB+RPV LA [10].
Outcomes
The primary endpoint was the proportion of participants with plasma HIV-1 RNA of 50 copies/mL or greater at week 48 using the US Food and Drug Administration Snapshot algorithm [21], as previously published [10]. Endpoints assessed at week 152 included the following: the proportion of participants with plasma HIV-1 RNA less than 50 copies/mL and HIV-1 RNA of 50 copies/mL or greater, incidence of confirmed virologic failure (CVF; 2 consecutive plasma HIV-1 RNA measurements ≥200 copies/mL), treatment-emergent genotypic and phenotypic resistance, incidence and severity of adverse events (AEs), proportion of discontinuations due to AEs, CAB+RPV pharmacokinetics, and treatment satisfaction measured using the HIV Treatment Satisfaction Questionnaire status version (HIVTSQs) [22].
Statistical Analysis
The efficacy analyses performed at week 152 included the proportion of participants with plasma HIV-1 RNA less than 50 copies/mL and 50 copies/mL or greater, per the Snapshot algorithm at week 152, based on the intention-to-treat exposed (ITT-E) population and per-protocol population. The analysis method and stratification factors from the primary analyses [10] were used, with modifications to include coronavirus disease 2019 (COVID-19)–related reasons for participants having no virologic data.
RESULTS
Participant Characteristics and Disposition
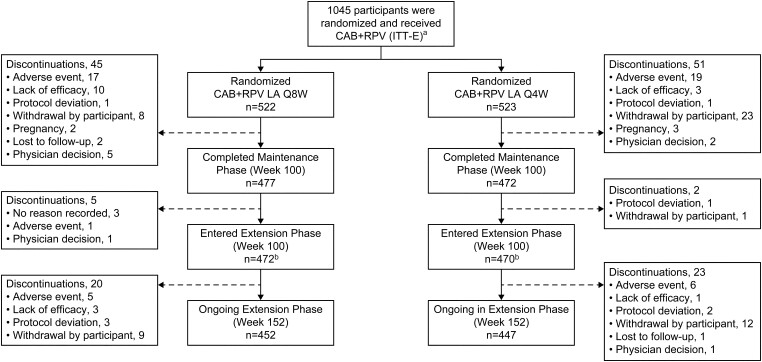
Overall, 1045 participants were included in the ITT-E population (Q8W, n = 522; Q4W, n = 523); 391 (37%) participants had prior CAB+RPV exposure. Baseline characteristics were previously published [10]. The number of participants in the study at the week 152 data cutoff was similar in both arms (Q8W, n = 452 [87%]; Q4W, n = 447 [85%]) (Figure 1). Overall, there were 139 treatment withdrawals (Q8W, n = 65 [12%]; Q4W, n = 74 [14%]), including 20 participants in the Q8W arm and 24 participants in the Q4W arm since the 96-week analysis [18]. The most common reason for withdrawal was participant decision, which occurred less frequently in the Q8W arm than in the Q4W arm (3% vs 7%). The most common reasons for withdrawal by a participant included frequency of visits (Q8W, n = 4 [<1%]; Q4W, n = 10 [2%]), participant relocation (Q8W, n = 1 [<1%]; Q4W, n = 6 [1%]), and injection intolerability (Q8W, n = 1 [<1%]; Q4W, n = 8 [2%]).
Figure 1.
Participant disposition. No study withdrawals were due to COVID-19 adverse events; however, 2 participants withdrew for COVID-19–related reasons (travel restrictions, n = 1; participant did not want to return to the clinic due to the pandemic, n = 1). aA total of 1049 participants were randomized. However, 4 participants did not receive the study drug and therefore were not part of the ITT-E population. bSeven participants completed the maintenance phase but did not enter the extension phase (Q8W, n = 5; Q4W, n = 2). Abbreviations: CAB, cabotegravir; COVID-19, coronavirus disease 2019; ITT-E, intention-to-treat exposed; LA, long-acting; Q4W, every 4 weeks; Q8W, every 8 weeks; RPV, rilpivirine.
Most injection visits occurred within the 7-day window (Q8W, n = 9509/9809 [97%]; Q4W, n = 18 829/19 321 [97%]) (Supplementary Figure 1). Overall, 44 (<1%) injection visits in the Q8W group and 98 (<1%) injection visits in the Q4W group were missed (outside the ±7-day window with oral therapy), and participants were administered CAB+RPV oral therapy (Q8W, n = 37/9809 [<1%]; Q4W, n = 90/19 321 [<1%]) or an alternative oral ART regimen (Q8W, n = 7/9809 [<1%]; Q4W, n = 8/19 321 [<1%]) until resuming injectable therapy. Most interruptions occurred after week 96 (Q8W, n = 31/37 [84%]; Q4W, n = 65/90 [72%]). In total, 38 (<1%) injection visits in the Q8W group and 69 (<1%) injection visits in the Q4W group were missed due to COVID-19 infection or clinic disruption. One injection visit (Q8W; week 112) was missed without oral coverage (COVID-19 related); this participant resumed CAB+RPV LA at week 128 and maintained virologic suppression.
Efficacy
After 152 weeks of therapy, Q8W dosing of CAB+RPV LA continued to demonstrate similar antiviral activity compared with Q4W dosing (Table 1). At week 152, 14 (3%) participants in the Q8W arm and 5 (1%) in the Q4W arm had HIV-1 RNA of 50 copies/mL or greater. The adjusted treatment difference in proportions was 1.7% (95% confidence interval [CI]: .1–3.3%), meeting the prespecified 4% noninferiority threshold. At week 152, 87% (n = 456/522) of participants in the Q8W arm and 86% (n = 449/523) in the Q4W arm had HIV-1 RNA of less than 50 copies/mL. The adjusted treatment difference (Q8W – Q4W arm) in proportions was 1.5% (95% CI: −2.6% to 5.6%), meeting the prespecified −10% noninferiority threshold. Tests for homogeneity were not significantly different by prior CAB+RPV exposure strata (HIV-1 RNA: <50 copies/mL [P = .441]; ≥50 copies/mL [P = .146]). No treatment differences between Q8W and Q4W dosing regimens for the proportion of participants with HIV-1 RNA of 50 copies/mL or greater and less than 50 copies/mL were observed within most of the demographic and baseline characteristic subgroups (Supplementary Figures 2 and 3).
Table 1.
Efficacy Outcomes at Week 152 (FDA Snapshot Algorithm)
| Outcome at Week 152 | Q8W (n = 522), n (%) |
Q4W (n = 523), n (%) |
Adjusteda Difference (95% CI) |
|---|---|---|---|
| ITT-E analysis | |||
| HIV-1 RNA ≥50 copies/mLb | 14 (2.7) | 5 (1.0) | 1.7 (.1, 3.3) |
| Data in window not below threshold | 1 (0.2) | 0 | … |
| Discontinued for lack of efficacy | 12 (2.3) | 4 (0.8) | … |
| Discontinued for other reason while not below threshold | 1 (0.2) | 1 (0.2) | … |
| Change in background therapy | 0 | 0 | … |
| HIV-1 RNA <50 copies/mLc | 456 (87.4) | 449 (85.9) | 1.5 (−2.6, 5.6) |
| No virologic data | 52 (10.0) | 69 (13.2) | … |
| Discontinued study due to AE or deathd | 23 (4.4) | 24 (4.6) | … |
| Discontinued study for other reasone | 28 (5.4) | 44 (8.4) | … |
| On study but missing data in window | 1 (0.2)f | 1 (0.2) | … |
| Test for homogeneity by strata for HIV-1 RNA ≥50 copies/mL | |||
| Prior exposure to CAB+RPV | |||
| 0 weeks | 10/327 (3.1) | 5/327 (1.5) | 1.5 (−.9, 4.2) |
| 1–24 weeks | 4/69 (5.8) | 0/68 (0) | 5.8 (.2, 14.2) |
| >24 weeks | 0/126 (0) | 0/128 (0) | 0 |
| P-value test of homogeneityg | .146 | ||
| Test for homogeneity by strata for HIV-1 RNA <50 copies/mL | |||
| Prior exposure to CAB+RPV | |||
| 0 weeks | 279/327 (85.3) | 271/327 (82.9) | 2.4 (−3.2, 8.1) |
| 1–24 weeks | 62/69 (89.9) | 64/68 (94.1) | −4.3 (−14.8, 5.6) |
| >24 weeks | 115/126 (91.3) | 114/128 (89.1) | −2.2 (−5.6, 10.0) |
| P-value test of homogeneityg | .441 | ||
| Per-protocol analysis | |||
| HIV-1 RNA ≥50 copies/mL | 12/510 (2.4) | 5/513 (1.0) | 1.4 (−0.2, 2.9) |
| HIV-1 RNA <50 copies/mL | 450/510 (88.2) | 446/513 (86.9) | 1.3 (−2.7, 5.3) |
| CVF (ITT-E population) | |||
| CVF between week 96 and 152 analyses | 3 (0.6)h | 0 | … |
| Total CVFs through week 152 | 12 (2.3) | 2 (0.4) | … |
Abbreviations: AE, adverse event; CAB, cabotegravir; CI, confidence interval; COVID-19, coronavirus disease 2019; CVF, confirmed virologic failure; FDA, US Food and Drug Administration; HIV-1, human immunodeficiency virus type 1; ITT-E, intention-to-treat exposed; LA, long-acting; Q4W, every 4 weeks; Q8W, every 8 weeks; RPV, rilpivirine; SVF, suspected virologic failure.
Cochran–Mantel–Haenszel stratified analysis adjusting for prior CAB+RPV LA exposure (0, 1–24, or >24 weeks).
Noninferiority was determined if the upper bound of the 95% CI about the adjusted Q8W–Q4W difference was below 4%.
Noninferiority was determined if the lower bound of the 95% CI about the adjusted Q8W–Q4W difference was above −10%.
Four deaths occurred since the 96-week analysis (Q8W, n = 2; Q4W, n = 2).
Other reasons included: Q8W—withdrawal by participant (n = 16), physician decision (n = 5), protocol deviation (n = 2), protocol-specified withdrawal criterion met (n = 2), lost to follow-up (n = 2), lack of efficacy (n = 1); Q4W—withdrawal by participant (n = 33), protocol deviation (n = 4), physician decision (n = 3), protocol-specified withdrawal criterion met (n = 3), lost to follow-up (n = 1).
Missing data were related to COVID-19.
One-sided P value from weighted least-squares chi-square statistic. A P value <.10 indicates statistically significant evidence of heterogeneity in the difference in proportions across levels of each analysis stratum.
One participant had a non–protocol-defined virologic failure. This participant met the SVF criterion at week 48 with an HIV-1 RNA value of 918 copies/mL; however, virologic failure was not confirmed at the week 48 retest result (39 copies/mL). At week 56, HIV-1 RNA was elevated again at 1038 copies/mL.
Virologic Failure
Overall, there were numerically more virologic failures in the Q8W arm (2% [n = 12/522]), including 2 participants since week 96 [18], than in the Q4W arm (<1% [n = 2/523]; no new CVFs since week 48). An additional participant in the Q8W arm experienced a non–protocol-defined virologic failure at week 48 and is included in the total (Supplementary Table 1). This participant was classified as having virologic failure after the week 96 publication based on an exploratory viral load assay. Excluding the participant with non–protocol-defined virologic failure, 5 (45%) of the 11 participants with CVF through week 96 developed RPV resistance-associated mutations (RAMs) (Y188L, K101E, K101E + E138A, E138E/K, K101E + M230L) in combination with INSTI RAMs (Q148Q/R + N155N/H, Q148R, N155H, Q148R + E138E/K). Both participants with CVF since week 96 had treatment-emergent RAMs to RPV (E138A + M230M/L, E138A + Y181Y/C) and INSTIs (Q148R) at suspected virologic failure (SVF) (Supplementary Table 1). The additional participant who had non–protocol-defined virologic failure at week 48 (Q8W) had no RAMs to RPV or INSTIs present from baseline peripheral blood mononuclear cells; the RPV RAM E138K and the integrase inhibitor mutation S230S/R were detected at week 56. Most cases of CVF occurred by or at week 48 (n = 11/14 [79%]).
Of the 14 participants with CVF, 6 (43%) had 2 or more of the baseline factors associated with increased risk of CVF with this regimen (pro-viral RPV RAMs, HIV-1 subtype A6/A1, body mass index [BMI] ≥30 kg/m2) [23]. One of the 2 participants with CVF since week 96 had an associated baseline factor (HIV-1 subtype A6/A1). All participants with CVF through 152 weeks received injections within 7 days of the scheduled visit. Overall, 13 of 14 (93%) participants with CVF achieved virologic re-suppression on subsequent oral ART regimens (protease inhibitor-based regimen [71%, n = 10/14] or dolutegravir [DTG]-based regimen [21%, n = 3/14]); the participant who did not re-suppress was nonadherent to the subsequent oral therapy, as reported previously [10].
Safety
CAB+RPV LA was well tolerated, with similar AE profiles between arms (Table 2). Drug-related AEs (per investigator assessment) were common and comparable across arms (Q8W, n = 427 [82%]; Q4W, n = 427 [82%]). This finding is largely attributable to injection-site reactions (ISRs), which represent 64% (n = 545/854) of all participants with drug-related AEs. Excluding ISRs, 27% (n = 142) of participants in the Q8W arm and 32% (n = 167) in the Q4W arm experienced a drug-related AE. The most commonly occurring AEs and drug-related AEs, excluding ISRs, are listed in Table 2. Most AEs were grade 1 or 2 (n = 848/1011 [84%]). A total of 23 (4%) participants in the Q8W arm and 25 (5%) participants in the Q4W arm had AEs leading to withdrawal. Of these participants, 5 withdrew from the Q8W arm and 6 withdrew from the Q4W arm after week 96. No drug-related serious AEs (SAEs) were reported after week 96 [10, 18]. Since the week 96 analyses, 4 deaths occurred, as detailed in Table 2; none were considered by the investigator to be related to the study drugs. No additional safety signals were identified since the week 48 analysis [10].
Table 2.
Adverse Event Summary
| Cumulative Week 152 Data Analysis,a n (%) | Participants With Incident AEs Between Week 96 and Week 152 Data Analyses, n (%) | |||
|---|---|---|---|---|
| Q8W (n = 522) | Q4W (n = 523) | Q8W (n = 522) | Q4W (n = 523) | |
| Any AE | 503 (96) | 508 (97) | 15 (3) | 9 (2) |
| Drug-related AEs | 427 (82) | 427 (82) | 12 (2) | 14 (3) |
| Excluding ISRs | 142 (27) | 167 (32) | 20 (4) | 21 (4) |
| Any grade 2 to 5 AE | 367 (70) | 377 (72) | 42 (8) | 44 (8) |
| Drug-related | 195 (37) | 210 (40) | 17 (3) | 23 (4) |
| Leading to withdrawal | 23 (4) | 25 (5) | 5 (1) | 6 (1) |
| Drug-related | 12 (2) | 18 (3) | 0 | 3 (<1) |
| Any SAE | 49 (9) | 44 (8) | 16 (3) | 16 (3) |
| Drug-relatedb | 4 (<1) | 3 (<1) | 0 | 0 |
| Fatal SAEs | 3 (<1) | 3 (<1)c | 2 (<1)d | 2 (<1)e |
| Drug-related | 0 | 0 | 0 | 0 |
| Common non-ISR AEs (≥10% in either treatment group) | ||||
| Nasopharyngitis | 97 (19) | 105 (20) | 7 (1) | 9 (2) |
| Upper respiratory tract infection | 80 (15) | 98 (19) | 8 (2) | 4 (<1) |
| Headache | 66 (13) | 82 (16) | 14 (3) | 35 (7) |
| Back pain | 64 (12) | 77 (15) | 19 (4) | 29 (6) |
| Arthralgia | 62 (12) | 65 (12) | 31 (6) | 29 (6) |
| Diarrhea | 56 (11) | 66 (13) | 12 (2) | 13 (2) |
| Pyrexia | 48 (9) | 73 (14) | 10 (2) | 26 (5) |
| Cough | 37 (7) | 59 (11) | 4 (<1) | 17 (3) |
| Common non-ISR drug-related AEs (≥3% in either treatment group) | ||||
| Pyrexia | 23 (4) | 33 (6) | 3 (<1) | 8 (2) |
| Fatigue | 11 (2) | 23 (4) | 0 | 2 (<1) |
Abbreviations: AE, adverse event; ISR, injection-site reaction; Q4W, every 4 weeks; Q8W, every 8 weeks; RPV, rilpivirine; SAE, serious adverse event.
Drug-related SAEs (per investigator assessment) were injection-site abscess (n = 1), osteonecrosis (n = 1), presyncope (n = 1), and acute pancreatitis and sepsis (n = 1) in the Q8W arm, and hypersensitivity and suspected (partial) intravenous administration of RPV (n = 1), drug hypersensitivity and suspected postinjection reaction (n = 1), and myocardial infarction (n = 1) in the Q4W group.
An additional participant died 2 months after completing the long-term follow-up. This was due to respiratory failure secondary to cardiac arrest.
Suicide (n = 1) and pancreatic cancer (n = 1).
Cardiac arrest (n = 1) and chronic obstructive pulmonary disease and chronic renal failure (n = 1).
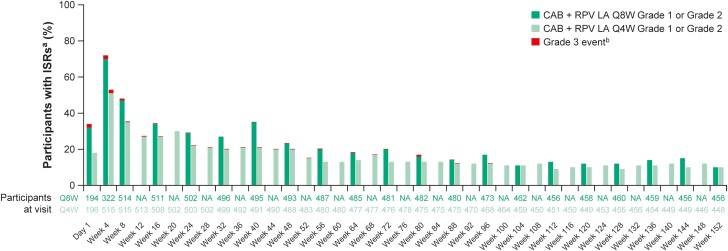
Overall, 60 041 injections were administered with 9662 ISRs (Table 3). Most ISRs were grade 1 or 2 (n = 9558/9662 [99%]), with a median duration of 3 days (interquartile range [IQR]: 2–5 days) for both treatment arms, and 84% (n = 8071/9663) resolved within 7 days. There were no grade 4 or 5 ISRs. Injection-site pain was the most frequent ISR in both treatment groups (Q8W, 77% of ISRs [n = 3189/4168]; Q4W, 76% of ISRs [n = 4180/5494]). The types and severity of ISR events were similar between treatment arms, except for injection-site nodules, which occurred more often in the Q4W group. The number of participants reporting ISRs at each visit decreased over the first 48 weeks and remained stable thereafter (Figure 2). Four participants withdrew due to injection-related reasons since the week 96 analysis, citing injection intolerability.
Table 3.
Injection-Site Reactions
| Q8W (n = 522) | Q4W (n = 523) | |
|---|---|---|
| Number of injections | 20 563 | 39 478 |
| Number of ISR eventsa | 4168 | 5494 |
| Grade or intensityb | ||
| Grade 1c | 3343 (80) | 4571 (83) |
| Grade 2c | 771 (18) | 873 (16) |
| Grade 3c | 54 (1) | 50 (<1) |
| Type of ISR adverse events (≥1% of injections as reported) | ||
| Injection-site paind,e | 3189 (16) | 4180 (11) |
| Injection-site noduled,f | 259 (1) | 457 (1) |
| Duration (days) | ||
| 1–7c | 3477 (83) | 4594 (84) |
| 8–14c | 368 (9) | 435 (8) |
| >14c | 292 (7) | 425 (8) |
| Median (IQR), days | 3 (2–5) | 3 (2–5) |
| Participants withdrawing for injection-related reasonsg | 8 (2) | 14 (3) |
Data are presented as n (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; ISR, injection-site reaction; Q4W, every 4 weeks; Q8W, every 8 weeks.
Each ISR event was counted separately. A participant may have had multiple ISR events following a single injection.
There were no grade 4 or grade 5 ISRs.
Percentages are calculated from the total number of ISR events.
Percentages are calculated from the total number of injections.
Q8W: 3189 injection-site pain events occurred in 398/522 (76%) participants; Q4W: 4180 injection-site pain events occurred in 387/523 (74%) participants.
Q8W: 259 injection-site nodule events occurred in 91/522 (17%) participants; Q4W: 457 injection-site nodule events occurred in 141/523 (27%) participants.
Percentages are calculated from the total number of participants with injections (Q8W, n = 516/522 [99%]; Q4W, n = 517/523 [99%]).
Figure 2.
ISRs over time. aAE grade is the maximum grade reported by the participant at each visit. bThere were no grade 4 or 5 ISRs. Abbreviations: AE, adverse event; CAB, cabotegravir; ISR, injection-site reaction; LA, long-acting; NA, not applicable; Q4W, every 4 weeks; Q8W, every 8 weeks; RPV, rilpivirine.
Median (IQR) weight gain from baseline to week 152 was +2.0 kg (−0.6 to 6.0 kg) in the Q8W arm and +1.7 kg (−1.2 to 5.0 kg) in the Q4W arm, of which +0.2 kg and +0.35 kg was gained between week 96 and week 152, respectively. Most participants remained in the same BMI category since baseline (Q8W, n = 332/522 [64%]; Q4W, n = 343/523 [66%]) (Supplementary Figure 4).
Since the week 96 analysis, no clinically meaningful differences were observed between the Q8W and Q4W arms for any electrocardiogram parameters or vital signs. Six participants (Q8W, n = 4 [1%]; Q4W, n = 2 [<1%]) had alanine aminotransferase elevations at least 3 times the upper limit of the normal range since week 96, 4 (<1%) of whom continued treatment. The remaining 2 (<1%) participants met the liver-stopping criteria (ie, abnormal alanine aminotransferase and bilirubin concentrations). One event was associated with fatal pancreatic cancer. The other participant had chronic HIV and hepatitis C coinfection with a history of binge alcohol abuse and continued receiving CAB+RPV LA dosing. Both were deemed not drug-related per investigator assessment.
Pharmacokinetics
The median CAB and RPV pre-dose concentrations for both dosing regimens are shown in Supplementary Figure 5. At week 152, geometric mean CAB pre-dose concentrations ranged from 1.45 μg/mL to 1.98 μg/mL for the Q8W arm and 2.75 μg/mL to 2.83 μg/mL for the Q4W arm across the 3 previous CAB+RPV exposure strata and were comparable to the week 48 and week 96 results. Geometric mean RPV pre-dose concentrations at week 152 ranged from 95.7 ng/mL to 105 ng/mL for the Q8W arm and 132 ng/mL to 153 ng/mL for the Q4W arm across the 3 previous CAB+RPV exposure strata. Geometric mean RPV pre-dose concentration in participants in the Q8W arm increased from 65.4 ng/mL at week 48 to 95.7 ng/mL at week 152 for participants with no prior exposure, and from 77.6 ng/mL at week 48 to 105 ng/mL at week 152 for participants with 1–24 weeks of previous exposure. These findings are consistent with the 28-week half-life of RPV LA, resulting in ongoing accumulation beyond the first year of treatment. In participants with more than 24 weeks of prior exposure, apparent steady state for RPV was achieved by week 48, with little further accumulation at week 152.
Plasma drug concentrations for participants with CVF (Supplementary Table 1) were generally in the first quartile of the overall study geometric mean values, but above the protein-adjusted concentration required for 90% inhibition (PA-IC90) values for both agents.
Plasma CAB and RPV concentrations were available for 95 participants (Q8W, n = 48/522 [9%]; Q4W, n = 47/523 [9%]) who stopped injectable therapy and entered the long-term follow-up phase, with concentrations shown in Supplementary Table 2. Notably, the geometric mean value remained above the PA-IC90 value 6 months after the last injection for CAB and 12 months for RPV.
Treatment Satisfaction
In participants without prior CAB+RPV exposure, HIVTSQs mean scores markedly increased from baseline to week 152 for both treatment arms, and significantly favored Q8W dosing at all 3 time points (Supplementary Figure 6).
DISCUSSION
The noninferior efficacy of CAB+RPV LA Q8W dosing compared with Q4W dosing at week 152 of the phase 3b ATLAS-2M study confirms and extends the 48-week and 96-week results, demonstrating that CAB+RPV LA dosed Q8W is a durable and effective therapy for the maintenance of HIV-1 virologic suppression in PWH. At week 152, 2.7% of participants receiving CAB+RPV LA Q8W and 1.0% receiving Q4W had HIV-1 RNA of 50 copies/mL or greater. Overall, 87% of participants maintained virologic suppression after 152 weeks of LA therapy (Q8W or Q4W), supporting the durability of CAB+RPV LA over approximately 3 years. Virologic failure through week 152 occurred more frequently in the CAB+RPV LA Q8W arm (2%) than in the Q4W arm (<1%). Similar rates have been reported in switch studies of dual oral therapy with DTG-RPV, with 1% of participants meeting the protocol-defined confirmed virologic withdrawal criteria (HIV-1 RNA ≥50 copies/mL and a confirmatory measurement of HIV-1 RNA ≥200 copies/mL) at week 148 of the SWORD-1 and SWORD-2 studies (Regimen Switch to Dolutegravir + Rilpivirine From Current Antiretroviral Regimen in Human Immunodeficiency Virus Type 1 Infected and Virologically Suppressed Adults) [24]. Participant retention was high through week 152, with 87% (n = 452) of Q8W and 85% (n = 447) of Q4W participants remaining in the study, demonstrating that most participants receiving injectable therapy wanted to continue injections after approximately 3 years of CAB+RPV. These Q8W data have recently been replicated in the CARISEL study [25].
Treatment compliance was high, with 97% (n = 28 338/29 130) of injections received within the allowed dosing window and only 1 injection missed without a planned alternative oral therapy (COVID-19 related). The use of oral therapy to cover missed doses increased during the extension phase (post–week 100), mainly attributable to COVID-19–related clinic disruption. Missed injections were more frequent in the Q4W arm than in the Q8W arm, suggesting that the longer dosing intervals may offer additional flexibility to better accommodate participants’ schedules or an emergent pandemic. There were fewer withdrawals due to participant choice in the Q8W arm (n = 17 [3%]) compared with the Q4W arm (n = 36 [7%]), which may reflect this increased convenience of Q8W dosing.
Since the week 96 analysis, 2 additional participants in the Q8W arm had CVF. No additional CVFs occurred in the Q4W arm. One non–protocol-defined failure (<1%, n = 1/1045) was identified at week 48 and is also reported. Except for 1 participant who was nonadherent to the subsequent oral therapy, these (n = 13/14) participants achieved re-suppression on an alternative oral treatment regimen (protease inhibitor–based regimen [71%, n = 10/14] or DTG-based regimen [21%, n = 3/14]). Seven participants with CVF in the Q8W arm and 1 participant in the Q4W arm developed RPV RAMs through week 152. The 2 participants with CVF since the week 96 analysis had no RPV or INSTI RAMs at baseline; however, both had treatment-emergent RAMs in SVF samples (Supplementary Table 1). The RPV RAMs were detected in previous ATLAS-2M participants with CVF [10, 18].
Multivariable analyses of phase 3 studies, including ATLAS-2M, identified 3 baseline factors associated with increased risk of CVF when present in combination of 2 or more factors (pro-viral RPV RAMs, HIV-1 subtype A6/A1, and/or BMI ≥30 kg/m2) during the first year of CAB+RPV initiation [23]. Dosing regimen (Q8W vs Q4W) was not identified as a significant risk factor [23]. Although 6 of 14 (43%) participants with CVF had 2 or more of these associated factors at baseline, only 1 of the 2 participants with CVF since week 96 had an associated baseline factor (HIV-1 subtype A6/A1). Expanded multivariable analyses of predictors of CVF are ongoing.
CAB+RPV LA was well tolerated, with a comparable safety profile between the treatment arms. Specifically, the proportions of participants reporting SAEs, AEs, and AEs leading to withdrawal were similar for Q8W and Q4W dosing, consistent with previous analyses [10, 18]. No new safety signals were identified since the week 48 analysis. The number of ISRs experienced across both treatment arms was similar and consistent with previous analyses from ATLAS [17], FLAIR [16], and ATLAS-2M [10, 18], with most ISRs being mild to moderate and short lived, with few discontinuations. The frequency of ISRs at each visit decreased over the first 48 weeks and remained consistent thereafter, as demonstrated in the FLAIR 96-week analysis [16]. The median weight change at week 152 increased since the week 96 analysis [18] but remained similar to that reported in a pooled analysis of clinical trials for other INSTIs [26].
CAB+RPV LA demonstrated high satisfaction levels in both treatment arms, with participants favoring the Q8W dosing regimen at all time points. This aligns with the high satisfaction and preference for CAB+RPV LA in patient-reported outcome analyses [17, 27].
The observed CAB and RPV plasma concentrations were consistent with previous observations. Mean CAB and RPV concentrations were lower in participants receiving Q8W dosing compared with Q4W dosing. For both regimens, the concentrations were above the respective PA-IC90 values, and virologic suppression was similar for both dosing regimens. For both regimens, RPV appeared to reach near steady state by week 96, and steady state was confirmed for CAB by week 48. This is consistent with the longer half-life for RPV (13–28 weeks) compared with CAB (5.6–11.5 weeks) [14]. CAB and RPV concentrations following Q4W dosing recapitulated those observed with Q4W dosing in FLAIR, and concentrations for Q8W dosing were consistent with those observed with Q8W dosing in LATTE-2 (long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection) and ATLAS [9, 16, 17, 28].
Variable RPV concentrations have been observed in real-world evidence studies and are potentially associated with therapeutic failure or toxicity; however, the majority of participants with CVF in the present study had RPV concentrations 3-fold greater than the PA-IC90 [29].
Limitations
The current study has several limitations. The lack of blinding may have caused participants to anticipate and report more AEs due to the administration of CAB+RPV LA. Due to the difference in dosing frequency, more frequent safety assessments were performed for the Q4W arm than the Q8W arm, which may have increased the number of AEs reported in the Q4W arm. A direct comparison with oral treatments was not possible due to the lack of an oral ART comparator arm. While a post hoc indirect analysis showed comparability of Q8W dosing with oral therapy [30], definitive conclusions cannot be made regarding adherence benefits with CAB+RPV LA versus oral ART from these data.
Conclusions
In summary, CAB+RPV LA dosed Q8W continued to be noninferior to Q4W therapy at 152 weeks. These long-term data demonstrate that CAB+RPV LA administered monthly or Q2M is an effective, durable, and well-tolerated treatment for the maintenance of HIV-1 virologic suppression.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Edgar T Overton, Division of Infectious Diseases, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Gary Richmond, Department of Medicine, Broward Health Medical Center, Fort Lauderdale, Florida, USA.
Giuliano Rizzardini, Department of Infectious Diseases, Fatebenefratelli Sacco Hospital, Milan, Italy; School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Anders Thalme, Department of Infectious Diseases, Karolinska University Hospital, Stockholm, Sweden.
Pierre-Marie Girard, Department of Infectious and Tropical Diseases and Inserm, University of Paris, St-Antoine Hospital, Paris, France.
Alexander Wong, Department of Medicine, University of Saskatchewan, Regina, Canada.
Norma Porteiro, Fundación IDEAA, Buenos Aires, Argentina.
Susan Swindells, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Jacques Reynes, Department of Infectious Diseases, Montpellier University Hospital, Montpellier, France; Inserm, University of Montpellier, Montpellier, France.
Sebastian Noe, HIV Research and Clinical Care Center, MVZ München Am Goetheplatz, Munich, Germany.
Conn Harrington, Clinical Development, ViiV Healthcare, Durham, North Carolina, USA.
Carlos Martín Español, GSK, Brentford, United Kingdom.
Carolina Acuipil, Research and Development, ViiV Healthcare, Durham, North Carolina, USA.
Asma Aksar, GSK, Manchester, United Kingdom.
Yuanyuan Wang, Development Biostatistics, GSK, Collegeville, Pennsylvania, USA.
Susan L Ford, Clinical Pharmacology Modeling and Simulation, GSK, Durham, North Carolina, USA.
Herta Crauwels, Clinical Pharmacology, Janssen Research and Development, Beerse, Belgium.
Veerle van Eygen, Clinical Microbiology and Immunology, Janssen Research and Development, Beerse, Belgium.
Rodica Van Solingen-Ristea, Medical Department, Janssen Research and Development, Beerse, Belgium.
Christine L Latham, Translational Medicine Research, ViiV Healthcare, Durham, North Carolina, USA.
Shanker Thiagarajah, Pharma Safety, GSK, Brentford, United Kingdom.
Ronald D’Amico, Research and Development, ViiV Healthcare, Durham, North Carolina, USA.
Kimberly Y Smith, Research and Development, ViiV Healthcare, Durham, North Carolina, USA.
Kati Vandermeulen, Department of Infectious Diseases, Janssen Research and Development, Beerse, Belgium.
William R Spreen, Research and Development, ViiV Healthcare, Durham, North Carolina, USA.
Notes
Author Contributions. E. T. O., G. Richmond, G. Rizzardini, A. T., P.-M. G., A. W., N. P., S. S., J. R., S. N., and C. M. E. were study investigators and/or participated in the conduct of the study, including recruitment and follow-up of participants. C. H., C. A., A. A., Y. W., S. L. F., H. C., V. v. E., R. V. S.-R., C. L. L., S. T., R. D., K. Y. S., K. V., and W. R. S. participated in the analysis of the study data and the conceptualization and design of the studies. All authors were involved in the drafting and review of the manuscript and approved the final version.
Acknowledgments. The authors thank everyone who has contributed to the success of the study: all study participants and their families and the ATLAS-2M clinical investigators and their staff. Professional medical writing and editorial assistance was provided by Poppie Cooper, MSc, at Scimentum (Nucleus Global), funded by ViiV Healthcare.
Data sharing. Data sharing requests will be considered by the management group upon written request to the corresponding author. Deidentified participant data or other prespecified data will be available subject to a written proposal and a signed data sharing agreement.
Financial support. This work was supported by ViiV Healthcare and Janssen Research and Development. The funders participated in the collection, analysis, interpretation of data, in the writing of the report, and in the decision to submit the paper for publication. All authors vouch for the accuracy and completeness of the data, data analyses, and interpretation and fidelity to the protocol, and all have approved the final manuscript for submission. C. M. E. and C. H. also report support for this work from GSK (authors were employees of GSK, the company delegated to operationalize 207966 ATLAS-2M study). S. L. F. reports support for this work from GSK in the form of a salary received from GSK for the author's employment. A. T. reports that the study was funded by GSK and all costs for the study was covered by GSK and paid to the institution. S. T. reports support for this work from GSK (author's employer; author provided services on behalf of employer to ViiV Healthcare) and reports that SciMentum provided medical writing support for the manuscript. K. V. reports support for this work as an employee of Johnson and Johnson (Infectious Diseases R&D). Y. W. reports support for this work from GSK (employee).
References
- 1. Lesko CR, Cole SR, Hall HI, et al. The effect of antiretroviral therapy on all-cause mortality, generalized to persons diagnosed with HIV in the USA, 2009-11. Int J Epidemiol 2016; 45:140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med 2001; 135:17–26. [DOI] [PubMed] [Google Scholar]
- 3. US Department of Health and Human Services . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at:https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new-guidelines. Accessed 1 September 2022.
- 4. Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence 2019; 13:475–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Los Rios P, Young B, Marcotullio S, et al. 1329. Experiences and emotional challenges of antiretroviral treatment (ART)—findings from the positive perspectives study. Open Forum Infect Dis 2019; 6(Suppl 2):S481. [Google Scholar]
- 6. Rueda S, Mitra S, Chen S, et al. Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: a series of meta-analyses. BMJ Open 2016; 6:e011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dandachi D, Dang BN, Lucari B, Swindells S, Giordano TP. Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care 2021; 33:801–9. [DOI] [PubMed] [Google Scholar]
- 8. Akinwunmi B, Buchenberger D, Scherzer J, et al. Factors associated with interest in a long-acting HIV regimen: perspectives of people living with HIV and healthcare providers in four European countries. Sex Transm Infect 2021; 97:566–73. [DOI] [PubMed] [Google Scholar]
- 9. Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–35. [DOI] [PubMed] [Google Scholar]
- 10. Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2021; 396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 11. Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–23. [DOI] [PubMed] [Google Scholar]
- 12. European Medicines Agency . Vocabria product information. Available at:https://www.ema.europa.eu/en/documents/product-information/vocabria-epar-product-information_en.pdf. Accessed 24 February 2022.
- 13. ViiV Healthcare . Prescribing information. Available at:https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212888s005s006lbl.pdf. Accessed 1 October 2022.
- 14. ViiV Healthcare . Vocabria (cabotegravir tablets) and Cabenuva (cabotegravir and rilpivirine extended release injectable suspensions) product monograph. Canada, March 2020. Available at: https://viivhealthcare.com/content/dam/cf-viiv/viiv-healthcare/en_GB/medicines/CABENUVA-VOCABRIA-PM-26-Mar-2021.pdf. Accessed 1 September 2022.
- 15.Australian Department of Health. Australian Public Assessment Report for cabotegravir sodium and cabotegravir/rilpivirine. Therapeutic Goods Administration, Department of Health, Commonwealth of Australia, May 2021.
- 16. Orkin C, Oka S, Philibert P, et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 2021; 8:e185–96. [DOI] [PubMed] [Google Scholar]
- 17. Swindells S, Lutz T, Van Zyl L, et al. Week 96 extension results of a phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment. AIDS (London, England) 2022; 36:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jaeger H, Overton ET, Richmond G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV 2021; 8:e679–89. [DOI] [PubMed] [Google Scholar]
- 19. ClinicalTrials.gov . Efficacy, safety and tolerability study of long-acting cabotegravir plus long-acting rilpivirine (CAB LA + RPV LA) in human-immunodeficiency virus-1 (HIV-1) infected adults. Available at:https://www.clinicaltrials.gov/ct2/show/NCT03299049. Accessed 30 March 2021.
- 20. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–4. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration. Human immunodeficiency virus-1 infection: developing antiretroviral drugs for treatment. Guidance for industry 2015. Available at:https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm355128.pdf. Accessed 17 October 2019.
- 22. Woodcock A, Bradley C. Validation of the revised 10-item HIV treatment satisfaction questionnaire status version and new change version. Value Health 2006; 9:320–33. [DOI] [PubMed] [Google Scholar]
- 23. Cutrell AG, Schapiro JM, Perno CF, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS (London, England) 2021; 35:1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Wyk J, Orkin C, Rubio R, et al. Brief report: durable suppression and low rate of virologic failure 3 years after switch to dolutegravir + rilpivirine 2-drug regimen: 148-week results from the SWORD-1 and SWORD-2 randomized clinical trials. JAIDS J Acquir Immune Defic Syndr 2020; 85:325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jonsson-Oldenbüttel C, Ghosn J, van der valk M, et al. Safety and effectiveness from the CARISEL study: phase 3b hybrid-III implementation study integrating cabotegravir + rilpivirine long-acting into European clinical settings. Poster presented at: 24th International AIDS Conference; 29 July–2 August 2022; Montreal, Canada, and virtual. Poster EPLBB05. [Google Scholar]
- 26. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murray M, Antela A, Mills A, et al. Patient-reported outcomes in ATLAS and FLAIR participants on long-acting regimens of cabotegravir and rilpivirine over 48 weeks. AIDS Behav 2020; 24:3533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390:1499–510. [DOI] [PubMed] [Google Scholar]
- 29. Thoueille P, Alves Saldanha S, Schaller F, et al. Real-life therapeutic concentration monitoring of long-acting cabotegravir and rilpivirine: preliminary results of an ongoing prospective observational study in Switzerland. Pharmaceutics 2022; 14:1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chounta V, Snedecor S, Wu S, Van de Velde N. Comparability of 48-week efficacy and safety of cabotegravir and rilpivirine long-acting every 8 weeks to standard of care in suppressed people living with HIV-1. Poster presented at: HIV Glasgow; 5–8 October 2020; virtual. Poster P011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.