Abstract
Background:
Per- and polyfluoroalkyl substances (PFAS) have been previously linked to polycystic ovarian syndrome (PCOS), but only a few legacy PFAS were examined.
Objectives:
This study aimed to explore this association with a variety of PFAS, including legacy, branched-chain isomers, and emerging alternatives, as well as a PFAS mixture.
Methods:
From 2014 to 2016, we conducted a multicenter, hospital-based case–control study on environmental endocrine disruptors and infertility in China. Three hundred sixty-six women with PCOS-related infertility and 577 control participants without PCOS were included in the current analysis. Twenty-three PFAS, including 3 emerging PFAS alternatives, 6 linear and branched PFAS isomers, 6 short-chain PFAS, and 8 legacy PFAS, were quantified in the plasma. Logistic regression and two multipollutant models [quantile-based g-computation (QGC) and Bayesian kernel machine regression (BKMR) methods] were used to assess the association of individual PFAS and PFAS mixture with PCOS, as well as the potential interactions among the congeners.
Results:
After adjusting for potential confounders, Each 1-standard deviation higher difference in ln-transformed 6:2 chlorinated perfluoroalkyl ether sulfonic acid (6:2 Cl-PFESA) and hexafluoropropylene oxide dimer acid (HFPO-DA) level was significantly associated with a 29% (95% CI: 1.11, 1.52) and 39% (95% CI:1.16, 1.68) higher odds of PCOS, respectively. Meanwhile, branched isomers of perfluorooctane sulfonate (PFOS) and perfluorohexane sulfonate (PFHxS) (i.e., br-PFHxS, n-PFOS, , ), short-chain PFAS (i.e., PFPeS and PFHxA) and other legacy PFAS [i.e., total concentrations of PFOS (T-PFOS), and perfluorododecanoic acid (PFDoA)] were significantly associated with increased odds of PCOS. The PFAS mixture was positively related to PCOS in the BKMR model. A similar trend was observed in QGC model, a ln-unit increase in the PFAS mixture was associated with a 20% increased risk of PCOS [ (95% CI: 1.06, 1.37)]. After controlling for other PFAS homologs, 6:2 Cl-PFESA, HFPO-DA, , and PFDoA were the major contributors based on the QGC and BKMR models. The associations were more pronounced in overweight/obese women.
Conclusions:
In this group of women, environmental exposure to a PFAS mixture was associated with an elevated odds of PCOS, with 6:2 Cl-PFESA, HFPO-DA, , and PFDoA being the major contributors, especially in overweight/obese women. https://doi.org/10.1289/EHP11814
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a large class of synthetic aliphatic hydrocarbons containing at least one carbon atom, and all hydrogen atoms on the carbon chain are replaced by fluorine atoms to form perfluoroalkyl.1 PFAS have excellent stability in the environment and the human body owing to its extremely stable carbon–fluorine () bond.1 Specifically, traditional long-chain PFAS have long half-lives in humans. For example, perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA), the two most common PFAS, have half-lives of 5.4 and 3.8 y, respectively.2,3 Because of rising health-risk concerns, the use of these long-chain PFAS has been restricted or banned in Europe and North America.4,5 This move has indirectly steered PFAS manufacturing toward new alternatives, such as short-chain congeners [e.g., perfluorobutyric acid (PFBA) and perfluorobutanesulfonic acid (PFBS)],6 and chlorinated polyfluoroether sulfonates (Cl-PFESA, trade name F-53B).7 At the same time, the manufacturing of these chemicals has also relocated to developing countries, such as China.8 Considerable evidence from epidemiological and toxicological studies suggests that legacy PFAS have endocrine-disrupting properties and potential reproductive and developmental toxicity, but the knowledge on PFAS alternatives is limited and inconsistent.9–11
Polycystic ovarian syndrome (PCOS) is a common endocrine disorder in women of reproductive age.12 It is among the most common causes of infertility, resulting in 80% of female anovulatory infertility.13 Although the etiology of PCOS is unclear, endocrine-disrupting chemicals (EDCs) could be one of the most important environmental drivers.14 Experimental research in human cell lines indicates that some legacy PFAS and alternatives (e.g., Cl-PFESA) can cause follicle-stimulating hormone (FSH)–stimulated down-regulation of insulin-like growth factor 1 (IGF-1), steroidogenic factor-1 (SF-1), zinc finger DNA-binding protein 4 (GATA4), aromatase, and estrogen via induction of peroxisome proliferator-activated receptor- () at higher concentraitons.15 Studies in mice have shown exposure can also reduce the expression of steroidogenic acute regulatory protein (StAR) and cholesterol side chain decomposition enzyme (P450scc) mRNA, which impairs the production of FSH, thereby further increasing the prevalence of PCOS.16,17 However, to our knowledge, only two human studies have assessed the association between PFAS exposure and PCOS,11,18 and these studies were limited to legacy PFAS. Thus, it is unknown whether emerging PFAS alternatives are linked to PCOS.
PFAS are primarily produced via telomerization and electrochemical fluorination processes, which typically involve the scission and reorganization of carbon chains.19 Humans are exposed to both branched-chain and linear PFAS. Previous studies have suggested that, unlike linear isomers, branched-chain isomers may pose different risks to human health (e.g., adverse cardiometabolic and birth outcomes).20–22 However, the reproductive toxicity of branched-chain PFAS isomers has received little attention. Furthermore, rather than being exposed to PFAS in isolation, people are typically exposed to numerous PFAS at the same time. Yet, previous studies examined only the associations with individual PFAS, and the effects of PFAS mixture as a whole have not been thoroughly investigated.11,18 Given that some PFAS are highly correlated owing to similar sources,23 ignoring PFAS mixtures may lead to biased estimates of individual PFAS. At the same time, different degrees of insulin resistance are common in patients with PCOS. Obesity amplifies insulin resistance in patients with PCOS and promotes PCOS development.24 We hypothesized that obesity status in women of reproductive age may modify the health effects of PFAS exposure. In addition to legacy PFAS, the present study aimed to further explore the relationship between emerging PFAS alternatives and branched-chain isomers and PCOS, the impact of a joint mixture exposure, and the interaction between PFAS and body mass index (BMI) on PCOS.
Methods and Materials
Study Population
From May 2014 to December 2016, we conducted a multicenter, hospital-based case–control study on environmental endocrine disruptors and female infertility in Shanghai, Shandong, and Zhejiang provinces, China. Infertility was defined as couples who had regular intercourse without using any contraceptives for 1 y and failed to conceive.25 Women were considered potentially eligible if they were 20–40 years of age; had no severe underlying diseases, such as cancer, cerebrovascular disease, trauma, severe liver, kidney, heart, or respiratory diseases or chromosomal abnormalities; and were first diagnosed as infertile at the participating hospitals (including Shandong University Fertility Center, Renji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and Women’s Hospital affiliated to Zhejiang University School of Medicine). Participants were interviewed by trained research assistants using a standardized questionnaire on demographic characteristics (e.g., date of birth, household income, education, occupation, height, weight), menstrual history (e.g., age of menarche, menstrual cycle, menstrual duration), reproductive characteristics (e.g., gravidity, parity), behavior and lifestyle (e.g., smoking and drinking status, physical activities, sleep quality), family history, medical history (e.g., PCOS), and medication in the past 3 months (e.g., contraceptives, estrogen and progesterone, ovulation drugs). Medical records were reviewed and information on infertility testing was abstracted by trained researchers. All participants provided their informed consent prior to the study. The study protocol was approved by the ethics committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (approval no. XHEC-C-2015-046) and participating hospitals.
For this cross-sectional case–control study, we selected the cases as women whose infertility was due to PCOS, defined according to the National Institutes of Health (NIH) criteria, including biochemical or clinical androgen hypertrophy and ovulatory dysfunction, with the exclusion of other specific disorders.26 The control participants consisted of healthy women with no endocrine disorders who were planning artificial insemination with donor sperm. There were no specific matching criteria for the controls. After further consideration of the availability of blood samples and missing values on key covariates, 366 PCOS cases and 577 controls with complete data were included in the final analyses (Figure S1).
PFAS Measurements
Blood and urine samples were collected at enrollment, processed immediately, and stored at until testing. Thirty-five target PFAS congeners were measured in of plasma, including perfluoroalkyl carboxylic acids (PFCAs) [; PFBA, perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), perfluoroheptanoic acid (PFHpA), PFOA, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), perfluorododecanoic acid (PFDoA), perfluorotridecanoic acid (PFTrDA), perfluorotetradecanoic acid (PFTeDA)]; perfluoroalkyl sulfonates (PFSAs) [; PFBS, perfluoropentane sulfonic acid (PFPeS), n-perfluorohexane sulfonate (n-PFHxS), branched PFHxS (br-PFHxS), perfluorohexane sulfonate (PFHpS), n-PFOS, perfluoro-6-methylheptanesulfonate (), , , , , , perfluorononanesulfonic acid (PFNS), perfluorodecane sulfonic acid (PFDS)]; perfluoroalkyl acid (PFAA) precursors [; perfluorooctanesulfonamide (FOSA), -methylperfluorooctane sulfonamido acetic acid (N-MeFOSAA), -ethylperfluorooctane sulfonamido acetic acid (N-EtFOSAA), 1H,1H,2H,2H-perfluorohexane sulfonic acid (4:2FTS), 1H,1H,2H,2H-perfluorooctane sulfonic acid (6:2FTS), 1H,1H,2H,2H-perfluorodecane sulfonic acid (8:2FTS)]; and PFAS alternatives [; hexafluoropropylene oxide dimer acid (HFPO-DA), ammonium 4,8-dioxa-3H-perfluorononanoate (ADONA), 6:2 chlorinated perfluoroalkyl ether sulfonic acid (6:2 Cl-PFESA), 8:2 chlorinated perfluoroalkyl ether sulfonic acid (8:2 Cl-PFESA)]. PFAS were measured using ultra-performance liquid chromatography (UPLC; Agilent 1290) coupled with an Agilent 6495C triple quadrupole tandem mass spectrometry (MS/MS; Agilent Technologies). PFAS standards () were purchased from the Wellington Laboratories. Table 1 contains a list of the reference standards, chemical names, and acronyms for the selected PFAS investigated in the present study.
Table 1.
Names, acronyms, and detection limits of selected PFAS.
| Acronym | Chemical full name | Quantification transitions | Surrogate standards | LOD () |
|---|---|---|---|---|
| Legacy PFAS | ||||
| PFOA | Perfluorooctanoic acid | 413/369 | M8PFOA | 0.0083 |
| T-PFOSa | Total concentrations of perfluorooctane sulfonate | — | — | — |
| PFNA | Perfluorononanoic acid | 463/419 | M9PFNA | 0.0037 |
| PFDA | Perfluorodecanoic acid | 513/469 | M6PFDA | 0.0017 |
| T-PFHxSb | Total concentrations of perfluorohexane sulfonate | — | — | — |
| PFHpS | Perfluorohexane sulfonate | 449/80 | M3PFHxS | 0.0051 |
| PFDoA | Perfluorododecanoic acid | 613/569 | MPFDoA | 0.0038 |
| PFUnDA | Perfluoroundecanoic acid | 563/519 | M7PFUdA | 0.0072 |
| PFAS isomers | ||||
| n-PFHxS | Linear perfluorohexane sulfonate | 399/80 | M3PFHxS | 0.0066 |
| br-PFHxS | Branched perfluorohexane sulfonate | 399/80 | M3PFHxS | 0.0025 |
| n-PFOS | Linear perfluorooctane sulfonate | 499/80 | M8PFOS | 0.0044 |
| Perfluoro-1-methylheptanesulfonate | 499/419 | M8PFOS | 0.0044 | |
| Perfluoro-6-methylheptanesulfonate | 499/80 | M8PFOS | 0.0013 | |
| Perfluoro-3/4/5-methylheptanesulfonate | 499/80 | M8PFOS | 0.0005 | |
| Emerging PFAS alternatives | ||||
| 6:2 Cl-PFESA | 6:2 Chlorinated perfluoroalkyl ether sulfonic acid | 531/361 | M8PFOS | 0.0027 |
| 8:2 Cl-PFESA | 8:2 Chlorinated perfluoroalkyl ether sulfonic acid | 631/451 | M8PFOS | 0.0054 |
| HFPO-DA | Hexafluoropropylene oxide dimer acid | 285/185 | M8PFOA | 0.0064 |
| Short-chain PFAS | ||||
| PFBA | Perfluorobutanoic acid | 213/169 | MPFBA | 0.0060 |
| PFBS | Perfluorobutane sulfonate | 299/80 | M3PFBS | 0.0030 |
| PFPeA | Perfluoropentanoic acid | 263/219 | M5PFPeA | 0.0017 |
| PFPeS | Perfluoropentane sulfonate | 349/80 | M3PFHxS | 0.0010 |
| PFHxA | Perfluorohexanoic acid | 313/269 | M5PFHxA | 0.0020 |
| PFHpA | Perfluoroheptanoic acid | 363/319 | M4PFHpA | 0.0020 |
Note: —, not applicable; LOD, limit of detection; PFAS, per- and polyfluoroalkyl substances.
Sum of dimethyl isomers of PFOS.
Sum of branched isomers of PFHxS.
Pretreatment of PFAS.
For the determination of PFAS, an aliquot of of plasma was cleaned up by solid phase extraction (SPE) as described in our previous study.27 In brief, after thawing and centrifuging, plasma was buffered with of formic acid after internal standards were spiked. The mixture was loaded onto an Oasis HLB cartridge (; Waters Corp.), which was preconditioned with of methanol (MeOH) and of formic acid. Sequentially, the cartridge was washed with of formic acid, of formic acid (1:1, vol/vol), and of 1% ammonium hydroxide to remove matrix interference. After the cartridge was completely dried under vacuum for 20 min, the retained analytes were eluted with of acetonitrile containing 1% ammonium hydroxide. The eluent was concentrated to near dryness by an SPD121P SpeedVac (Thermo Scientific). Finally, the extract was reconstituted in of ammonium acetate (6:4, vol/vol) and then transferred into UPLC-MS/MS analysis. Calibration curves ranged from 0.01 to and exhibited excellent linearity, with .
Instrument analysis.
UPLC-MS/MS analysis was conducted using a 1290 Infinity Series high-performance LC (HPLC) system (Agilent), coupled to a 6495 C triple quadrupole mass spectrometer (Agilent) that was operated under the electrospray ionization (ESI) negative ion mode. A ZORBAX RRHD Eclipse Plus C18 column (, ; Agilent) equipped with a ZORBAX Eclipse Plus C18 guard column (, ; Agilent) maintained at 40°C. A ZORBAX Eclipse Plus C18 column (, ; Agilent) was placed between the pump and the injector valve to remove interferences from the mobile phase. The injection volume was . The separation was achieved by gradient elution of mobile phase A (milli-Q water with ammonium acetate) and mobile phase B (MeOH) at a flow rate of . A gradient program was used as follows: 0–0.75 min, 40% B; 2.75 min, 60% B; 6.75 min, 65% B; 9.70 min, 90% B; 13.70 min, 98% B with a final hold of 1.3 min (15.0 min). The mass spectrometer was operated in multiple reaction monitoring (MRM) modes. The temperature and flow of the sheath gas were set at 375°C and , respectively. The nebulizer gas was 35 psi and the capillary voltage was .
Quality assurance and quality control.
To monitor the impurity from the instrument, one solvent blank (MeOH) was injected before the operation of each batch and after every 10 samples. One blank control (sheep plasma or newborn calf serum) was analyzed every 21 samples to monitor the background contamination from the whole process. To ensure the stability of the method, we analyzed two quality control samples (QCs) every 21 samples, including a QC of low spiked level (QClow; ) and a QC of high spiked level (QChigh; ). All the polypropylene tubes and the tips of the pipette used in the experiments were soaked in MeOH for and dried to avoid background contamination. All the repeatedly used glassware was rinsed consecutively with MeOH/water (1:1, vol/vol) and milli-Q water before use. MeOH was injected into the instrument continuously before the analysis until the instrumental background baseline was stable.
A total of 23 PFAS had a detection rate of all participants and were included in the data analysis. The limit of detection (LOD) for each PFAS was set at a signal-to-noise ratio of 3 (), ranging from 0.0005 to (Table 1). PFAS concentrations below the LOD were estimated as the LOD divided by the square root of 2.28
Covariates
Previous research and a directed acyclic graph were used to generate a set of minimal sufficient adjustment variables for confounding control (Figure S2).11,18 They included age (linear), age at menarche (linear), BMI (, 18.5–23.9, 24.0–27.9, and ), educational level (less than high school, high school graduate, and college graduate or higher), annual household income (30,000, 30,000–50,000, 50,000–100,000, and RMB/person), study site (Zhejiang, Shanghai, and Shandong), and menstrual volume (normal, menorrhagia, and hypomenorrhea). The height and weight of the participants were measured by trained researchers and BMI (in kilograms per meter squared) was calculated. Menstrual volume was assessed by asking participants to use the pictorial blood loss assessment chart (PBAC). Participants rated the degree of blood staining of each tampon according to the Higham criteria, which reflect the degree of staining of tampons and cotton tampons during the menstrual cycle.29,30
Statistical Analysis
In this study, continuous variables were reported as medians and interquartile ranges, whereas categorical covariates were reported as numbers and percentages. For further model analysis, all PFAS concentrations were ln-transformed. The Wilcoxon rank-sum test (for skewed distributions) or the chi-square test (for categorical variables) was used to test differences in PFAS and characteristics between the cases and controls. To estimate pairwise correlations among ln-transformed PFAS, Spearman correlation coefficients were calculated.
We applied logistic regression to explore the association between PFAS and PCOS prevalence. To evaluate covariate multicollinearity, tolerance and variance inflation factor (VIF) values were determined; a tolerance of and VIF were regarded as an indication of multicollinearity. All models were adjusted for age (continuous), BMI (continuous), annual household income (categorical), educational level (categorical), study site (categorical), age at menarche (continuous), and menstrual volume (categorical). We then used a restricted cubic spline (RCS) with three nodes (10th, 50th, and 90th percentiles) to explore the potential nonmonotonic response between PFAS exposure and the prevalence of PCOS.
Because of the close correlations among some PFAS, Bayesian kernel machine regression (BKMR) was employed to assess the individual and cumulative effects of PFAS on PCOS prevalence. BKMR is an analytical model specifically designed to assess the health effects of mixture exposure to multiple environmental pollutants.31 The basic principle of this method is to estimate the multipollutant exposure–response relationship based on the covariance (inner product) between individual pollutant exposure characteristics by introducing a kernel function.31 At present, simulation studies have demonstrated that BKMR can fully fit the potential complex nonlinear relationship and estimate the difference of study outcomes under different exposure levels (e.g., the 75th vs. 25th quantile) and possible interactions between pollutants. Here, to reduce the influence of the variability of different PFAS concentrations, prior to the BKMR analysis, all PFAS concentrations were further normalized after ln-transformation using the -score method. The following formula was used to scale a specific PFAS concentration:
where represents a ln-transformed PFAS, refers to the mean value of ln-transformed PFAS, and is the standard deviation of ln-transformed PFAS. Here, PFAS can be any individual PFAS congener, rather than the total or sum of PFAS. Then PFAS were screened using a hierarchical variable screening method.32 The 21 PFAS were divided into branched-chain and straight-chain PFAS. The relative importance of individual PFAS was assessed primarily in two perspectives: the exposure–response relationship and the importance of each PFAS in association with a specific outcome using the posterior inclusion probability (PIP). A grouping level PIP (groupPIP) of indicates that the overall PFAS of the group may be more important, whereas the relative importance of PFAS within a group is more dependent on the relative size of the conditional PIP (condPIP) within the group. The combined exposure effect of PFAS is defined as the difference from the predicted value of the outcome variable at a specific quantile (e.g., the 75th quantile) of PFAS when all PFAS component concentrations are at their median concentrations. The effect of a single PFAS was defined as the mean change in the corresponding outcome variable as the target PFAS concentration increased from the 25th quantile to the 75th quantile when other coexisting PFAS concentrations were fixed at the median concentration. Estimates of BKMR were generated after 20,000 iterations.
We also used parametric estimation to evaluate the combined effect by the recently developed quantile-based g-computation (QGC) method.33 The basic principle of QGC is to reflect the mixed exposure of multiple pollutants by constructing a weighted index, and the weight of each pollutant represents the corresponding relative importance. When g-computation constructs the weighted quantile sum (WQS) index of exposure to multiple pollutants, it allows different components to be associated with target outcomes in different directions, and at the same time, it can also consider mixed exposures and the nonlinear relationship between single pollutants and outcomes to a certain extent. Before analysis, the PFAS was subjected to quantile processing (quartiles were used in this study). Given that the constructed WQS index does not satisfy the parametric distribution, the corresponding regression coefficients and statistical inference are based on 200 bootstrap sampling. Furthermore, because PFAS exposure may be associated with weight gain in women of reproductive age, obesity may increase the risk of PCOS, stratified analyses by BMI ( or ) were performed in the BKMR and QGC models.34
Furthermore, to assess the robustness of our results, several sensitivity analyses were performed. a) We further classified plasma PFAS concentrations of the controls into tertiles, with the first tertile serving as a reference, and applied logistic regression to examine the association between PFAS in tertiles and the prevalence of PCOS, adjusting for the above covariates. b) Menstrual bleeding is thought to be a significant pathway of PFAS excretion in premenopausal women.35 Simultaneous expulsion of the placenta, fetus, and other tissues when giving birth and breastfeeding are the primary pathways of PFAS clearance in women of reproductive age.36 Therefore, we performed a sensitivity analysis in women with normal menstrual bleeding and in nulliparous women.
All statistical analyses were conducted in R (version 3.6.1; R Development Core Team). The RCS, BKMR, and QGC models were implemented using the R package “rcs,” “bkmr,” and “QGC,” respectively. The threshold for statistical significance was set at (two-tailed).
Results
Population Characteristics
Demographic characteristics of the study population are presented in Table 2. The average age of the participants was 29.3 y. The mean BMI was . Almost a third of the participants () were overweight or obese. The PCOS group had a higher BMI than the controls (). In both groups, most participants were nulliparous (). Most women had normal menstrual volume (81.7% of women with PCOS and 92% of controls). Compared with the controls, the cases had longer menstrual cycles () and menstruation (), lower annual household income (), and a higher prevalence of hypomenorrhea ().
Table 2.
Characteristics of women diagnosed with infertility, by polycystic ovarian syndrome (PCOS) status (), from a hospital-based study in Shanghai, Shandong, and Zhejiang provinces, China, 2014–2016.
| Characteristicsa | PCOS () | Non-PCOS () | -Valueb |
|---|---|---|---|
| Age [y, median (Q1–Q3)] | 28.0 (26.0–32.0) | 28.0 (26.0–32.0) | 0.81 |
| BMI () [ (%)] | 0.002 | ||
| 15 (4.1) | 70 (12.1) | ||
| 18.5–23.9 | 166 (45.4) | 371 (64.3) | |
| 24.0–27.9 | 95 (26.0) | 100 (17.3) | |
| 90 (24.5) | 36 (6.3) | ||
| Educational level [ (%)] | 0.21 | ||
| Less than high school | 188 (51.4) | 266 (46.1) | |
| High school graduate | 101 (27.6) | 188 (32.6) | |
| College graduate or higher | 77 (21.0) | 123 (21.3) | |
| Annual household income (RMB/person) [ (%)] | 0.007 | ||
| 250 (68.3) | 280 (48.5) | ||
| 30,000–50,000 | 61 (16.7) | 157 (27.2) | |
| 50,000–100,000 | 29 (7.9) | 84 (14.6) | |
| 26 (7.1) | 56 (9.7) | ||
| Parity [ (%)] | 0.18 | ||
| Nulliparous | 300 (82.0) | 492 (85.3) | |
| Multipara | 66 (18.0) | 85 (14.7) | |
| Study site | 0.002 | ||
| Zhejiang | 34 (9.3) | 159 (27.6) | |
| Shanghai | 33 (9.0) | 165 (28.6) | |
| Shandong | 299 (81.7) | 253 (43.8) | |
| Menstrual cycle length [d, median (Q1–Q3)] | 33.0 (30.0–40.0) | 30.0 (28.0–30.0) | 0.009 |
| Menstrual period length [d, median (Q1–Q3)] | 6.0 (5.0–7.0) | 5.0 (4.0–5.0) | 0.005 |
| Age at menarche [y, median (Q1–Q3)] | 14.0 (13.0–15.0) | 14.0 (13.0–15.0) | 0.48 |
| Menstrual volume [ (%)] | 0.0002 | ||
| Normal | 299 (81.7) | 531 (92.0) | |
| Menorrhagia | 22 (6.0) | 26 (4.5) | |
| Hypomenorrhea | 45 (12.3) | 20 (3.5) |
Note: BMI, body mass index; menstrual cycle length, duration of the first day of two consecutive menstrual periods; menstrual period length, duration of each menstrual period; Q, quartile.
Data are complete for all characteristics.
-Values were calculated using the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables.
Plasma PFAS Concentrations
Table 3 summarizes the distribution and detection rate of plasma PFAS concentrations. All PFAS congeners were highly detectable (88%–100%) in the plasma samples. In general, the cases had significantly higher levels of emerging PFAS alternatives (6:2 Cl-PFESA, 8:2 Cl-PFESA, and HFPO-DA), PFAS isomers (n-PFHxS, br-PFHxS, n-PFOS, , , ), short-chain PFAS (PFBA, PFPeS, PFPeA, PFHxA, and PFHpA) and other legacy PFAS (PFOA, T-PFOS, PFNA, PFDA, T-PFHxS, PFHpS, PFDoA and PFUnDA) than the controls. Some PFAS concentrations were highly correlated with each other, with Spearman correlation coefficients ranging from 0.01 to 0.92 (Figure S3; Excel Table S1). We further evaluated the association of PFAS separately in the PCOS case group and the control group and found similar associations between the two groups (Figure S4; Excel Tables S2 and S3). In addition to the strong correlations observed for traditional PFAS (i.e., PFDoA, PFNA, PFDA, and PFUnDA) (), PFAS alternatives (i.e., 6:2 Cl-PFESA) and branched PFAS (i.e., n-PFOS) were also observed to have significant correlations (Figure S4; Excel Tables S2 and S3).
Table 3.
Distribution of plasma PFAS in polycystic ovarian syndrome (PCOS) cases and controls among women diagnosed with infertility in Shanghai, Shandong, and Zhejiang provinces, China, 2014–2016.
| PFAS (ng/mL)a | PCOS cases () | Controls () | -Valueb | ||
|---|---|---|---|---|---|
| (%) | Median (P25–P75) | (%) | Median (P25–P75) | ||
| Legacy PFAS | |||||
| PFOA | 100 | 8.52 (5.37–14.75) | 100 | 7.18 (3.70–12.61) | 0.006 |
| T-PFOSc | 100 | 5.07 (3.73–7.61) | 100 | 3.91 (2.71–6.66) | 0.009 |
| PFNA | 100 | 0.92 (0.57–1.48) | 100 | 0.77 (0.41–1.44) | 0.007 |
| PFDA | 100 | 0.92 (0.59–1.56) | 100 | 0.69 (0.34–1.44) | 0.005 |
| T-PFHxSd | 100 | 0.31 (0.17–0.65) | 100 | 0.22 (0.10–0.52) | 0.0006 |
| PFHpS | 98.4 | 0.09 (0.06–0.14) | 98.3 | 0.08 (0.05–0.14) | 0.002 |
| PFDoA | 98.6 | 0.14 (0.11–0.20) | 99.8 | 0.11 (0.08–0.17) | 0.0002 |
| PFUnDA | 100 | 0.66 (0.43–1.19) | 100 | 0.49 (0.23–1.14) | 0.003 |
| PFAS isomers | |||||
| n-PFHxS | 99.2 | 0.30 (0.21–0.50) | 99.7 | 0.22 (0.12–0.38) | 0.008 |
| br-PFHxS | 89.6 | 0.03 (0.02–0.04) | 92.0 | 0.02 (0.01–0.02) | 0.001 |
| n-PFOS | 100 | 4.26 (2.59–7.42) | 100 | 3.29 (1.72–6.69) | 0.003 |
| 89.3 | 0.39 (0.21–0.63) | 91.7 | 0.31 (0.17–0.48) | 0.009 | |
| 98.6 | 0.16 (0.11–0.22) | 99.1 | 0.11 (0.08–0.20) | 0.004 | |
| 99.2 | 0.58 (0.38–1.11) | 99.3 | 0.47 (0.23–0.87) | 0.0007 | |
| PFAS alternatives | |||||
| 6:2 Cl-PFESA | 100 | 3.72 (2.55–6.07) | 100.0 | 2.96 (1.57–6.46) | 0.0001 |
| 8:2 Cl-PFESA | 92.1 | 0.10 (0.05–0.15) | 91.7 | 0.06 (0.03–0.14) | 0.001 |
| HFPO-DA | 94.3 | 0.04 (0.03–0.07) | 89.9 | 0.02 (0.01–0.04) | 0.0009 |
| Short-chain PFAS | |||||
| PFBS | 89.3 | 0.06 (0.03–0.10) | 89.4 | 0.05 (0.03–0.10) | 0.06 |
| PFBA | 99.5 | 0.09 (0.06–0.13) | 99.1 | 0.08 (0.06–0.11) | 0.008 |
| PFPeS | 95.1 | 0.01 (0.01–0.01) | 95.5 | 0.01 (0.00–0.01) | 0.03 |
| PFPeA | 88 | 0.04 (0.02–0.10) | 87.5 | 0.02 (0.01–0.08) | 0.007 |
| PFHxA | 94.5 | 0.02 (0.02–0.03) | 94.5 | 0.01 (0.01–0.01) | 0.0003 |
| PFHpA | 98.6 | 0.07 (0.05–0.11) | 99.3 | 0.05 (0.03–0.08) | 0.006 |
Note: LOD, limit of detection; P, percentile; PFAS, per- and polyfluoroalkyl substances.
For full chemical names see Table 1.
Wilcoxon rank-sum test for PFAS level between PCOS cases and controls.
.
.
Associations between PFAS Concentrations in Plasma and the Prevalence of PCOS
Table 4 presents the association between individual PFAS exposure and PCOS prevalence before and after controlling for potential confounders. Two long-chain PFAS (T-PFOS and PFDoA), four branched PFAS isomers (br-PFHxS, n-PFOS, , ), two emerging PFAS alternatives (6:2 Cl-PFESA and HFPO-DA) and two short-chain PFAS (PFPeS and PFHxA) were significantly positively associated with PCOS (all ). RCS analysis indicated that exposure to most PFAS congeners showed a significant nonmonotonic response with PCOS prevalence (Figure S5; Excel Table S4).
Table 4.
Associations of ln-transformed plasma PFAS concentrations with PCOS-related infertility in logistic regression model for women from Shanghai, Shandong, and Zhejiang provinces, China (), 2014–2016.
| PFASa | Crude OR (95% CI)b | -value | Adjusted OR (95% CI)c | -Value |
|---|---|---|---|---|
| Legacy PFAS | ||||
| PFOA | 1.05 (0.86, 1.25) | 0.86 | 1.07 (0.83, 1.36) | 0.61 |
| T-PFOS | 1.16 (1.05, 1.32) | 0.01 | 1.27 (1.08, 1.45) | 0.007 |
| PFNA | 1.02 (0.85, 1.20) | 0.85 | 1.13 (0.89, 1.44) | 0.42 |
| PFDA | 1.02 (0.76, 1.28) | 0.62 | 1.08 (0.85, 1.32) | 0.27 |
| T-PFHxS | 1.20 (0.92, 1.54) | 0.07 | 1.26 (0.98, 1.65) | 0.22 |
| PFHpS | 1.01 (0.81, 1.23) | 0.08 | 1.06 (0.85, 1.28) | 0.16 |
| PFDoA | 1.21 (1.10, 1.36) | 0.01 | 1.32 (1.17, 1.49) | 0.005 |
| PFUnDA | 1.06 (0.86, 1.27) | 0.36 | 1.15 (0.89, 1.46) | 0.28 |
| PFAS isomers | ||||
| n-PFHxS | 1.02 (0.92, 1.15) | 0.16 | 1.08 (0.96, 1.22) | 0.07 |
| br-PFHxS | 1.12 (1.02, 1.25) | 0.03 | 1.18 (1.05, 1.34) | 0.02 |
| n-PFOS | 1.12 (1.01, 1.32) | 0.06 | 1.22 (1.07, 1.41) | 0.01 |
| 1.16 (0.91, 1.42) | 0.16 | 1.25 (0.98, 1.53) | 0.08 | |
| 1.17 (1.02, 1.34) | 0.02 | 1.27 (1.09, 1.49) | 0.009 | |
| 1.26 (1.08, 1.45) | 0.04 | 1.35 (1.12, 1.57) | 0.002 | |
| PFAS alternatives | ||||
| 6:2 Cl-PFESA | 1.21 (1.08, 1.36) | 0.0009 | 1.29 (1.11, 1.52) | 0.0007 |
| 8:2 Cl-PFESA | 1.14 (0.86, 1.42) | 0.36 | 1.18 (0.91, 1.47) | 0.26 |
| HFPO-DA | 1.26 (1.11, 1.47) | 0.01 | 1.39 (1.16, 1.68) | 0.006 |
| Short-chain PFAS | ||||
| PFBS | 1.15 (0.81, 1.49) | 0.42 | 1.26 (0.91, 1.60) | 0.25 |
| PFBA | 0.78 (0.65, 0.96) | 0.02 | 0.86 (0.69, 1.07) | 0.07 |
| PFPeS | 1.17 (1.02, 1.31) | 0.03 | 1.19 (1.04, 1.37) | 0.04 |
| PFPeA | 1.05 (0.82, 1.28) | 0.12 | 1.11 (0.91, 1.31) | 0.85 |
| PFHxA | 1.20 (1.04, 1.35) | 0.02 | 1.21 (1.06, 1.39) | 0.01 |
| PFHpA | 1.19 (0.90, 1.47) | 0.22 | 1.16 (0.74, 1.57) | 0.52 |
Note: BMI, body mass index; CI, confidence interval; OR, odds ratio; PCOS, polycystic ovarian syndrome; PFAS, per- and polyfluoroalkyl substances.
For full chemical names see Table 1.
Logistic regression models were carried out to assess the OR and 95% CI of PCOS, which were estimated by 1-standard deviation higher difference in ln-transformed PFAS as continuous variables.
Adjusting for age (linear), BMI (categorical), annual household income (categorical), educational level (categorical), study site (categorical), age at menarche (linear), and menstrual volume (categorical).
Results of PFAS Joint and Individual Exposure in BKMR Analyses
Figure 1 shows that the PFAS mixture was positively associated with PCOS after adjusting for potential confounders (Excel Table S5). The BKMR model also found 6:2 Cl-PFESA (), HFPO-DA (), PFDoA (), and () as significant contributors to the overall association (Figure 2; Table S1; Excel Table S6). There was no significant interaction between PFAS homologs (Figure S6; Excel Table S7).
Figure 1.
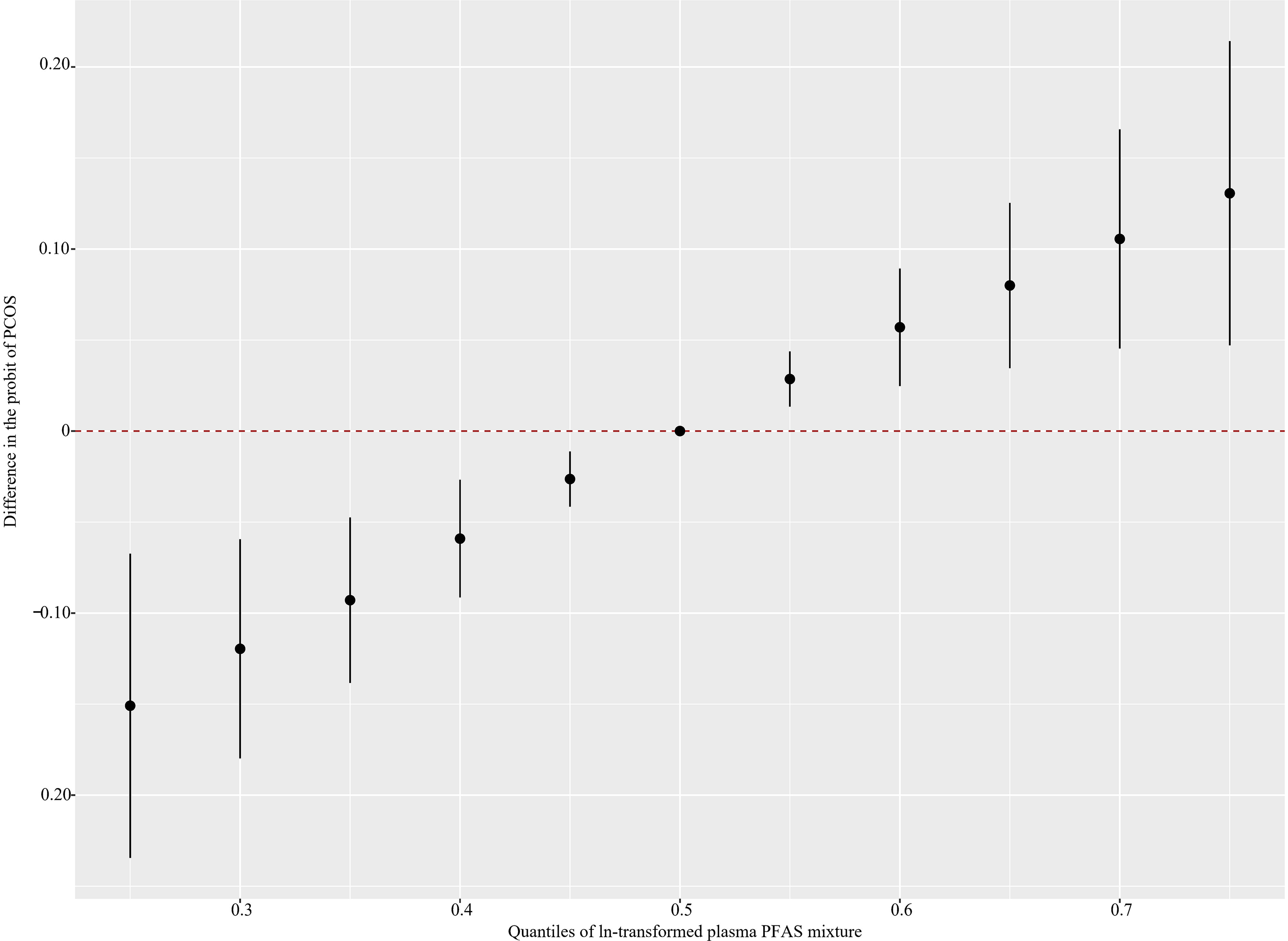
Overall joint associations of PFAS mixture (estimates and 95% confidence intervals) with the PCOS prevalence in Chinese women diagnosed with infertility estimated by Bayesian kernel machine regression (BKMR) () (see Excel Table S5 for corresponding numeric data). All estimates were adjusted for age (continuous), BMI (categorical), annual household income (categorical), educational level (categorical), study site (categorical), age at menarche (continuous), and menstrual volume (categorical). This figure plots the estimated difference in the probit of PCOS when exposures are at a particular percentile (x-axis) in comparison with when exposures are all at the 50th percentile (). Note: BMI, body mass index; PCOS, polycystic ovarian syndrome; PFAS, per- and polyfluoroalkyl substances.
Figure 2.
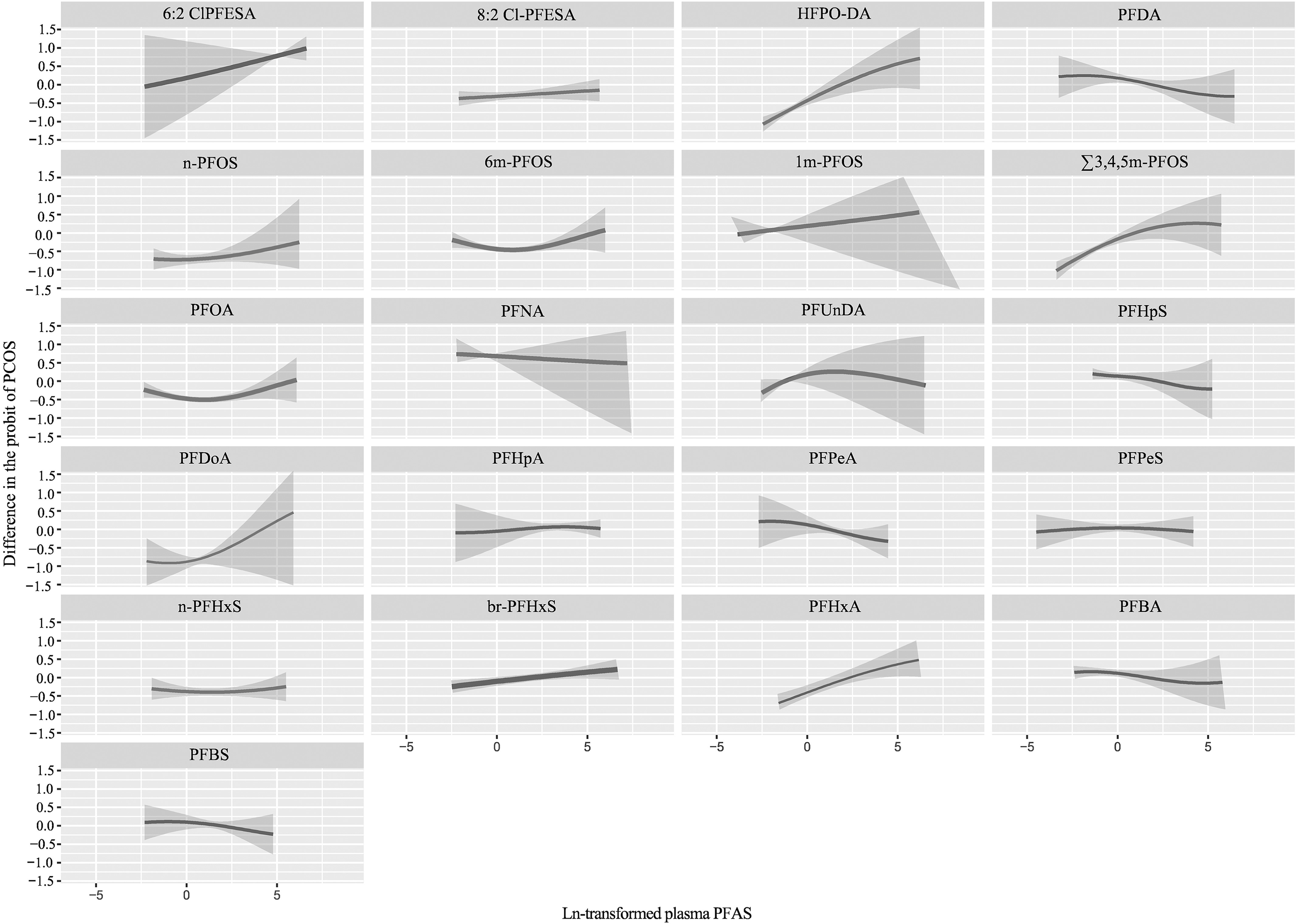
Univariate exposure–response relationship of individual plasma PFAS concentrations (estimates and 95% CIs) with the prevalence of PCOS, in Chinese women diagnosed with infertility estimated by Bayesian kernel machine regression (BKMR) for each PFAS, with the other pollutants fixed at the median () (see Excel Table S6 for corresponding numeric data). All estimates were adjusted for age (continuous), BMI (categorical), annual household income (categorical), educational level (categorical), study site (categorical), age at menarche (continuous), and menstrual volume (categorical). The boundaries of the gray areas represent the 95% CIs of the exposure–response relationship. For full chemical names see Table 1. Note: CI, confidence interval; BMI, body mass index; PCOS, polycystic ovarian syndrome; PFAS, per- and polyfluoroalkyl substances.
When stratified by BMI, a significant positive association of PFAS mixture with the prevalence of PCOS was more pronounced in overweight/obese women and attenuated in normal-weight participants (Figure S7; Excel Tables S8 and S9). Among normal-weight participants, only 6:2 Cl-PFESA () and HFPO-DA () showed relatively greater importance. In the overweight/obese group, the major contributors (6:2 Cl-PFESA, HFPO-DA, PFDoA, and ) were consistent with the main results (Table S1).
Results of Mixture Analyses Based on the QGC Model
The results of the QGC analysis are shown in Table 5. Overall, PFAS were associated with an increased prevalence of PCOS, with an odds ratio of 1.20 [95% confidence interval (CI): 1.06, 1.37] for each quantile of PFAS concentration. 6:2 Cl-PFESA, HFPO-DA, PFDoA, and were more strongly associated with PCOS prevalence (). In addition, estimated weights of individual PFAS homologs showed that 6:2 Cl-PFESA had the highest weight among PFAS that were significantly associated with PCOS. This is consistent with the BKMR results. Likewise, the associations between PFAS mixture and individual PFAS (6:2 Cl-PFESA, HFPO-DA, PFDoA, and ) and PCOS prevalence were stronger in overweight/obese women.
Table 5.
Associations of plasma PFAS mixture and individual plasma PFAS concentrations with PCOS after controlling for other PFAS congeners based on quantile-based g-computation model, in women Shanghai, Shandong, and Zhejiang provinces, China ().
| Itemsa | All participants ()b | Normal-weight participants ()c | Overweight or obese participants ()c | |||
|---|---|---|---|---|---|---|
| Weightd | OR (95% CI)e | Weightd | OR (95% CI)e | Weightd | OR (95% CI)e | |
| PFAS mixture | — | 1.20 (1.06, 1.37) | — | 1.08 (0.92, 1.25) | — | 1.28 (1.09, 1.42) |
| Legacy PFAS | ||||||
| PFOA | 0.009 | 1.06 (0.85, 1.32) | 0.006 | 1.05 (0.90, 1.24) | 0.008 | 1.14 (0.87, 1.26) |
| PFNA | 0.01 | 1.01 (0.81, 1.29) | 0.01 | 1.09 (0.98, 1.30) | 0.009 | 1.06 (0.75, 1.21) |
| PFDA | 0.96 (0.79, 1.20) | 0.95 (0.81, 1.19) | 0.95 (0.70, 1.07) | |||
| PFHpS | 0.009 | 1.07 (0.95, 1.26) | 0.06 | 1.04 (0.89, 1.21) | 0.01 | 1.09 (0.89, 1.28) |
| PFDoA | 0.19 | 1.18 (1.09, 1.31) | 0.15 | 1.12 (0.94, 1.25) | 0.21 | 1.21 (1.11, 1.35) |
| PFUnDA | 0.08 | 1.05 (0.86, 1.27) | 0.04 | 1.02 (0.86, 1.17) | 0.05 | 1.08 (0.86, 1.20) |
| PFAS isomers | ||||||
| n-PFHxS | 0.84 (0.62, 1.08) | 0.82 (0.71, 1.14) | 0.87 (0.75, 1.16) | |||
| br-PFHxS | 0.07 | 1.06 (0.92, 1.25) | 0.006 | 1.06 (0.89, 1.22) | 0.06 | 1.08 (0.92, 1.24) |
| n-PFOS | 0.01 | 1.03 (0.82, 1.17) | 0.01 | 1.08 (0.91, 1.26) | 0.007 | 1.06 (0.91, 1.15) |
| 0.007 | 1.06 (0.92, 1.11) | 0.005 | 1.00 (0.85, 1.09) | 0.01 | 1.04 (0.82, 1.12) | |
| 0.04 | 1.09 (0.97, 1.18) | 0.02 | 1.04 (0.90, 1.16) | 0.05 | 1.07 (0.94, 1.19) | |
| 0.17 | 1.15 (1.04, 1.29) | 0.11 | 1.10 (0.94, 1.25) | 0.16 | 1.19 (1.06, 1.32) | |
| PFAS alternatives | ||||||
| 6:2 Cl-PFESA | 0.22 | 1.19 (1.07, 1.34) | 0.19 | 1.11 (0.98, 1.21) | 0.20 | 1.22 (1.09, 1.37) |
| 8:2 Cl-PFESA | 0.07 | 1.04 (0.81, 1.26) | 0.06 | 1.07 (0.87, 1.29) | 0.09 | 1.09 (0.95, 1.26) |
| HFPO-DA | 0.15 | 1.09 (1.02, 1.22) | 0.14 | 1.07 (0.95, 1.19) | 0.17 | 1.14 (1.05, 1.29) |
| Short-chain PFAS | ||||||
| PFBS | 0.97 (0.85, 1.10) | 0.95 (0.83, 1.10) | 0.97 (0.86, 1.12). | |||
| PFBA | 0.85 (0.67, 1.06) | 0.84 (0.70, 1.05) | 0.84 (0.66, 1.03) | |||
| PFPeS | 0.002 | 1.06 (0.84, 1.24) | 0.007 | 1.09 (0.94, 1.26) | 0.005 | 1.07 (0.96, 1.21) |
| PFPeA | 0.005 | 1.01 (0.79, 1.19) | 0.001 | 1.07 (0.91, 1.22) | 0.007 | 1.06 (0.91, 1.17) |
| PFHxA | 0.06 | 1.15 (0.98, 1.27) | 0.12 | 1.02 (0.90, 1.18) | 0.15 | 1.12 (1.04, 1.26) |
| PFHpA | 0.007 | 1.02 (0.89, 1.22) | 0.006 | 1.01 (0.85, 1.16) | 0.02 | 1.09 (0.95, 1.32) |
Note: Overweight/obesity was defined by BMI . —, Not applicable; BMI, body mass index; CI, confidence interval; OR, odds ratio; PCOS, polycystic ovarian syndrome; PFAS, per- and polyfluoroalkyl substances.
For full chemical names see Table 1.
Quantile-based g-computation model adjusting for age (linear), BMI (categorical), annual household income (categorical), educational level (categorical), study site (categorical), age at menarche (linear), and menstrual volume (categorical).
Quantile-based g-computation model adjusting for age (linear), annual household income (categorical), educational level (categorical), study site (categorical), age at menarche (linear), and menstrual volume (categorical).
Weights indicated the contribution of individual PFAS in the associations between PFAS mixture with PCOS.
Quantile-based g-computation models were carried out to assess the OR and 95% CI of PCOS, which were estimated by 1-standard deviation higher difference in quartiles of ln-transformed PFAS and PFAS mixture.
Results of Sensitivity Analyses
The sensitivity analyses showed that the associations of PFAS with PCOS did not change substantially after the analyses were restricted to women with normal menstrual volume and in nulliparas (Tables S2 and S3, respectively). When PFAS concentrations were in tertiles, the results were consistent with Table 4. The highest tertiles of T-PFOS, T-PFHxS, Br-PFHxS, n-PFOS, and PFPeS exposure were positively associated with PCOS prevalence (), whereas exposures in both the second and third tertiles of 6:2 Cl-PFESA, HFPO-DA, PFDoA, , and PFHxA were associated with an increased prevalence of PCOS (Tables S4–S7).
Discussion
This multicenter case–control study showed significant associations of exposure to legacy PFAS, emerging substitutes, and PFAS isomers with PCOS in women of reproductive age with infertility. The combined effect of multiple PFAS homologs was more pronounced in overweight/obese women. More specifically, 6:2 Cl-PFESA, HFPO-DA, PFDoA, and were the main contributors.
Recently, 6:2 Cl-PFESA was recognized as the most persistent PFAS in humans with the longest median elimination half-life (15.3 y); that is, it may be more important than PFOS when considering human bioaccumulation (half-life: 6.7 y).37 In addition, 6:2 Cl-PFESA has a higher placental transfer efficiency than PFOS, raising more concerns of increased fetal toxicity.38 Limited epidemiological studies have shown that 6:2 Cl-PFESA had similar or more serious effects on neonatal birth weight,39 preterm birth,39 and estradiol levels40 compared with PFOS. Studies based on zebrafish zygotes have shown that 6:2 Cl-PFESA exposure can enhance the expression of ,36 compete with estradiol for binding to the estrogen receptor (), and up-regulate the expression of the gene,41 but it inhibits the expression of the androgen receptor (AR) gene, thereby interfering with the ER and downstream AR receptors.42 Normal regulation of signaling is closely related to negative feedback regulation of gonadotropin-releasing hormone (GnRH) in the hypothalamus and the normal process of oogenesis.43 6:2 Cl-PFESA and HFPO-DA may interfere with the expression of key hormones and receptors in the regulation of hypothalamic–pituitary–ovarian axis (HPO) signaling and become one of the potential mechanisms through which the two PFAS exposures increase the risk of PCOS.44,45 In addition to directly affecting endocrine organs, PFAS can alter the normal function of the hypothalamic–pituitary–gonadal (HPG) axis, disrupting hormone secretion and the menstrual cycle, further producing reproductive dysfunction.46 Meanwhile, PFAS can also alter the levels of prolactin and human chorionic gonadotropin (hCG).46
Meanwhile, several in vivo and in vitro experiments have demonstrated that 6:2 Cl-PFESA and HFPO-DA can significantly inhibit the synthesis of androgen and stimulate the synthesis of estrogen, resulting in an imbalance in the ratio of estrogen and androgen in the body.41,47 Blake et al. used a high-throughput toxicity screening (HTTS) technique to evaluate the effect of PFAS on gene expression in human placental JEG-3 trophoblast cells, and the results suggested that placental dysfunction is accompanied by altered steroid hormone exposure associated with HFPO-DA.48 The disturbance of hormone homeostasis may affect follicle maturation.49 Nonetheless, the reproductive effects of PFAS alternatives remain to be unveiled.
We also observed that legacy PFAS and their isoforms were associated with an increased prevalence of PCOS. Our findings were, in general, similar to those of previous research. Vagi et al. found that PFOA and PFOS had a significant positive association with PCOS.18 Meanwhile, a case–control study in China observed that with increasing PFDoA concentration, the risk of PCOS increased significantly (). However, no association was found with PFBS, PFHpA, PFHxS, PFOA, PFOS, PFNA, and PFDA.11 In another study, the patients with PCOS had higher serum PFOS levels than the controls.50 In both women with PCOS and normal controls, high serum PFOS levels were associated with menstrual irregularities. These results suggested that PFAS might increase the prevalence of PCOS and PCOS-related disorders in women of reproductive age.
On the other hand, among the six branched PFAS isomers examined (4 branched PFOS isomers and 2 branched PFHxS isomers), only was found to be positively associated with PCOS prevalence. We have no prior study to compare with at the moment. Given the high detection rate,20 possible biomagnifications,51 and well-documented threats to other systems (e.g., birth outcomes, cardiometabolic profiles),19,52 more research is warranted to confirm the association and elucidate the underlying mechanisms.
We observed a significant positive association between PFHxA, one of the new short-chain alternatives, and PCOS. Despite that epidemiological research in humans is still lacking, PFHxA has been shown to be harmful to reproductive systems in various laboratory experiments. Specifically, animal models have suggested the potential association between PFHxA and PCOS.15,53 For example, in a previous study, female offspring from pregnant mice exposed to PFHxA exhibited symptoms of impaired ovarian functions, such as ovarian size, decreased ovarian follicle numbers (all stages),54 delayed vaginal opening, delayed estrus,55 prolonged interestrus, and decreased serum estradiol levels.56 In vitro experiments have also shown that short-chain PFAS can activate peroxisome proliferator-activated receptor- () and -.57 The activation of these receptors, especially the subtype, can inhibit the conversion of androgens to estrogens by interfering with the nuclear factor kappa light chain enhancer of activated B cells (NF-kB) pathway and inhibiting the activity of aromatase.58 A study on porcine follicles found that activation of significantly inhibits CYP17 and dehydrogenase () enzymatic activity, decreases the expression of related genes, reduces progesterone levels, and results in delayed ovulation.59 Whether these mechanisms mediate the association between PFHxA and PCOS prevalence in women remains to be confirmed.
The association between PFAS exposure and PCOS varied in previous observational studies, probably owing to differences in PFAS exposure levels, demographics, and exposure routes. For example, our research showed that this relationship is nonmonotonic. At certain exposure levels, no association or even a negative association was observed. A significant positive association was noted when the exposure levels reached or exceeded a certain level (Figure S4). Furthermore, because previous studies used traditional logistic regression models to associate a single pollutant with PCOS risk, the association between PFAS was not fully considered.11,18 To account for correlations among PFAS, we employed two flexible multipollutant models. Notably, when the menstrual features and parity history were taken into account, these associations of PFAS with PCOS-related infertility remained constant. Further research on the possible link and the underlying mechanisms are warranted.
Our study also revealed that the association of PFAS exposure with PCOS prevalence is more pronounced in overweight/obese women than in normal-weight women; that is, BMI may play a moderating role in the association. Previous studies adjusted for BMI as a covariate in regression models but did not explore its possible moderating role in different BMI subgroups.11,18 Insulin resistance is common in patients with PCOS, and PFAS may directly interfere with glucose homeostasis, further increasing the risk of PCOS.47,60 Animal experiments revealed that PFAS increase the apoptosis rate of pancreatic cells in nonobese diabetic mice, significantly reduce the normal immune function of macrophages, and promote the progression of insulin resistance.61 Obesity leads to insulin resistance by inducing the levels of free fatty acids, inflammation, and oxidative stress in the body.62 Therefore, obesity may promote the development of PCOS by further amplifying the disturbing effect of PFAS on insulin homeostasis.63 This may be a potential explanation as to why the association of PFAS with PCOS prevalence is more pronounced in overweight/obese women than in normal-weight women, but the role of BMI in the PFAS exposure–PCOS prevalence relationship requires further evaluation.
Our study has several strengths. First, we applied strict eligibility criteria in selecting PCOS cases. Women with chromosomal, other endocrine, anatomical, alloimmune, autoimmune, or infectious factors were excluded. Second, our study has a much larger sample size than previous ones, allowing us to conduct subgroup analyses. Third, our observation on the association between PFAS alternatives and isomers and PCOS may prompt further investigation. Last, the BKMR and QGC methods allow joint marginal structural models to estimate mixed dose–response parameters. With regard to the nonlinear relationship between PFAS mixture exposure and PCOS prevalence, the BKMR model serves as a good predictor of key pollutants and the QGC model can generate parametric estimates of the mixture effects.
The present study also has several limitations. First, owing to the nature of a case–control study design, blood samples were collected after PCOS had occurred, which limited our ability to make a causal inference between PFAS levels and PCOS. Given the possible reverse causation,42 it would be ideal to evaluate PFAS exposure before PCOS has occurred in a large prospective cohort study. However, most PFAS are steadily persistent chemicals with long half-lives, indicating that PFAS levels are stable in months. Second, evidence shows that a history of hormonal use (e.g., contraceptive use) is associated with the risk of PCOS.64,65 Because our cases and controls were seeking fertility treatment, it was unlikely that they were using any form of contraceptives. What is more, owing to the hydrophobic and hydrophilic properties of PFAS, they are widely used in daily home products, such as greaseproof paper, fast food containers, and wrapping paper.1 Studies have shown an association between female exposure to PFAS and an increased risk of being overweight and obese.66 At the same time, overweight/obese women may eat more fast foods, which may cause higher levels of PFAS exposure.67 Therefore, the causality of the association between PFAS exposure and high BMI remains uncertain. However, our study did not collect information on dietary habits, which might lead to residual confounding in our results. Furthermore, we cannot rule out another potential residual confounding from unmeasured environmental pollutants. Third, even if the PBAC-based assessment of menstrual volume is considered accurate, it is still a semi-quantitative method.28,29 Fourth, for short-chain PFAS with short half-lives (less than a month) and easy renal excretion, urine may be a more suitable matrix than blood for measuring concentrations of short-chain PFAS.68 Therefore, in future studies, we will add short-chain PFAS concentrations in urine to accurately assess their reproductive toxicity. Last, our study participants were recruited at infertility clinics, which might have resulted in selection bias. Such bias could limit the generalizability of our findings in other populations.
Conclusion
Our study showed that exposure to a PFAS mixture was associated with an increased prevalence of PCOS in women of reproductive age. 6:2 Cl-PFESA, HFPO-DA, PFDoA, and may be the main contributors. The association of PFAS with PCOS was more pronounced in overweight/obese women. These findings expand our knowledge of the reproductive toxicity of emerging PFAS alternatives and isomers and may have important public health implications.
Supplementary Material
Acknowledgments
This work was supported by funding from the National Natural Science Foundation of China (41991314, to J.Z.), the Science and Technology Commission of Shanghai Municipality (21410713500, to J.Z.), and the National Key Research and Development Program of China (2021YFC2700400, to H.Z.).
References
- 1.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, et al. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7(4):513–541, PMID: , 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng CA, Hungerbühler K. 2014. Bioaccumulation of perfluorinated alkyl acids: observations and models. Environ Sci Technol 48(9):4637–4648, PMID: , 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- 3.Dhore R, Murthy GS. 2021. Per/polyfluoroalkyl substances production, applications and environmental impacts. Bioresour Technol 341:125808, PMID: , 10.1016/j.biortech.2021.125808. [DOI] [PubMed] [Google Scholar]
- 4.Fenton S, Ducatman A, Boobis A, DeWitt J, Lau C, Ng C, et al. 2021. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ Toxicol Chem 40(3):606–630, PMID: , 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFAS) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: , 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ateia M, Maroli A, Tharayil N, Karanfil T. 2019. The overlooked short- and ultrashort-chain poly-and perfluorinated substances: a review. Chemosphere 220:866–882, PMID: , 10.1016/j.chemosphere.2018.12.186. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Huang J, Yang Y, Hui Y, Ge Y, Larssen T, et al. 2013. First report of a Chinese PFOS alternative overlooked for 30 years: its toxicity, persistence, and presence in the environment. Environ Sci Technol 47(18):10163–10170, PMID: , 10.1021/es401525n. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Vestergren R, Herzke D, Yu J, Cousins IT. 2016. Levels, isomer profiles, and estimated riverine mass discharges of perfluoroalkyl acids and fluorinated alternatives at the mouths of Chinese rivers. Environ Sci Technol 50(21):11584–11592, PMID: , 10.1021/acs.est.6b03752. [DOI] [PubMed] [Google Scholar]
- 9.Di Nisio A, Foresta C. 2019. Water and soil pollution as determinant of water and food quality/contamination and its impact on male fertility. Reprod Biol Endocrinol 17(1):4, PMID: , 10.1186/s12958-018-0449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YR, White N, Bräunig J, Vijayasarathy S, Mueller JF, Knox CL, et al. 2020. Per-and poly-fluoroalkyl substances (PFAS) in follicular fluid from women experiencing infertility in Australia. Environ Res 190:109963, PMID: , 10.1016/j.envres.2020.109963. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Zhou W, Wu S, Liang F, Li Y, Zhang J, et al. 2019. Perfluoroalkyl substances exposure and risk of polycystic ovarian syndrome related infertility in Chinese women. Environ Pollut 247:824–831, PMID: , 10.1016/j.envpol.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. 2016. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 22(6):687–708, PMID: , 10.1093/humupd/dmw025. [DOI] [PubMed] [Google Scholar]
- 13.Escobar-Morreale HF. 2018. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 14(5):270–284, PMID: , 10.1038/nrendo.2018.24. [DOI] [PubMed] [Google Scholar]
- 14.Palioura E, Diamanti-Kandarakis E. 2015. Polycystic ovary syndrome (PCOS) and endocrine disrupting chemicals (EDCs). Rev Endocr Metab Disord 16(4):365–371, PMID: , 10.1007/s11154-016-9326-7. [DOI] [PubMed] [Google Scholar]
- 15.Behr AC, Lichtenstein D, Braeuning A, Lampen A, Buhrke T. 2018. Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicol Lett 291:51–60, PMID: , 10.1016/j.toxlet.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 16.Feng X, Wang X, Cao X, Xia Y, Zhou R, Chen L. 2015. Chronic exposure of female mice to an environmental level of perfluorooctane sulfonate suppresses estrogen synthesis through reduced histone H3K14 acetylation of the StAR promoter leading to deficits in follicular development and ovulation. Toxicol Sci 148(2):368–379, PMID: , 10.1093/toxsci/kfv197. [DOI] [PubMed] [Google Scholar]
- 17.Szilagyi JT, Avula V, Fry RC. 2020. Perfluoroalkyl substances (PFAS) and their effects on the placenta, pregnancy, and child development: a potential mechanistic role for placental peroxisome proliferator–activated receptors (PPARs). Curr Environ Health Rep 7(3):222–230, PMID: , 10.1007/s40572-020-00279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vagi SJ, Azziz-Baumgartner E, Sjödin A, Calafat AM, Dumesic D, Gonzalez L, et al. 2014. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: a case–control study. BMC Endocr Disord 14:86, PMID: , 10.1186/1472-6823-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz K, Silva MR, Klaper R. 2020. Distribution and effects of branched versus linear isomers of PFOA, PFOS, and PFHxS: a review of recent literature. Sci Total Environ 733:139186, PMID: , 10.1016/j.scitotenv.2020.139186. [DOI] [PubMed] [Google Scholar]
- 20.Bao WW, Qian ZM, Geiger SD, Liu E, Liu Y, Wang SQ, et al. 2017. Gender-specific associations between serum isomers of perfluoroalkyl substances and blood pressure among Chinese: Isomers of C8 Health Project in China. Sci Total Environ 607–608:1304–1312, PMID: , 10.1016/j.scitotenv.2017.07.124. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Zeng XW, Qian ZM, Vaughn MG, Sauvé S, Paul G, et al. 2017. Isomers of perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environ Int 102:1–8, PMID: , 10.1016/j.envint.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Tian YP, Zeng XW, Bloom MS, Lin S, Wang SQ, Yim SHL, et al. 2019. Isomers of perfluoroalkyl substances and overweight status among Chinese by sex status: Isomers of C8 Health Project in China. Environ Int 124:130–138, PMID: , 10.1016/j.envint.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Luo K, Huang W, Zhang Q, Liu X, Nian M, Wei M, et al. 2022. Environmental exposure to legacy poly/perfluoroalkyl substances, emerging alternatives and isomers and semen quality in men: a mixture analysis. Sci Total Environ 833:155158, PMID: , 10.1016/j.scitotenv.2022.155158. [DOI] [PubMed] [Google Scholar]
- 24.Ezeh U, Chen IYD, Chen YH, Azziz R. 2020. Adipocyte insulin resistance in PCOS: relationship with GLUT-4 expression and whole-body glucose disposal and β-cell function. J Clin Endocrinol Metab 105(7):e2408–e2420, PMID: , 10.1210/clinem/dgaa235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vander Borght M, Wyns C. 2018. Fertility and infertility: definition and epidemiology. Clin Biochem 62:2–10, PMID: , 10.1016/j.clinbiochem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. 2004. Revised 2003 consensus on diagnostic criteria and long‐term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19(1):41–47, PMID: , 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 27.Nian M, Huo X, Zhang J, Mao Y, Jin F, Shi Y, et al. 2022. Association of emerging and legacy per- and polyfluoroalkyl substances with unexplained recurrent spontaneous abortion. Ecotoxicol Environ Saf 239:113691, PMID: , 10.1016/j.ecoenv.2022.113691. [DOI] [PubMed] [Google Scholar]
- 28.Hornung RW, Reed LD. 1990. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg 5(1):48–51, 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 29.Zakherah MS, Sayed GH, El-Nashar SA, Shaaban MM. 2011. Pictorial blood loss assessment chart in the evaluation of heavy menstrual bleeding: diagnostic accuracy compared to alkaline hematin. Gynecol Obstet Invest 71(4):281–284, PMID: , 10.1159/000320336. [DOI] [PubMed] [Google Scholar]
- 30.Higham JM, O’Brien PM, Shaw RW. 1990. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol 97(8):734–739, PMID: , 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- 31.Bobb JF, Claus Henn B, Valeri L, Coull BA. 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1):67, PMID: , 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebeaux RM, Doherty BT, Gallagher LG, Zoeller RT, Hoofnagle AN, Calafat AM, et al. 2020. Maternal serum perfluoroalkyl substance mixtures and thyroid hormone concentrations in maternal and cord sera: the HOME Study. Environ Res 185:109395, PMID: , 10.1016/j.envres.2020.109395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. 2020. A quantile-based g-computation approach to addressing the effects of exposure mixtures. Environ Health Perspect 128(4):47004, PMID: , 10.1289/EHP5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia YT, Yan HM, Wang LM, Liu SB, Xu TL, Shen T, et al. 2019. A study regarding the control attempts on body weight and related factors among overweight and obese adults in China, 2013 [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 40(6):621–626, PMID: , 10.3760/cma.j.issn.0254-6450.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Upson K, Shearston JA, Kioumourtzoglou MA. 2022. An epidemiologic review of menstrual blood loss as an excretion route for per- and polyfluoroalkyl substances. Curr Environ Health Rep 9(1):29–37, PMID: , 10.1007/s40572-022-00332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olsen GW, Butenhoff JL, Zobel LR. 2009. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod Toxicol 27(3–4):212–230, PMID: , 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, Vestergren R, Xu L, Zhou Z, Li C, Liang Y, et al. 2016. Human exposure and elimination kinetics of chlorinated polyfluoroalkyl ether sulfonic acids (Cl-PFESAs). Environ Sci Technol 50(5):2396–2404, PMID: , 10.1021/acs.est.5b05849. [DOI] [PubMed] [Google Scholar]
- 38.Gao K, Zhuang T, Liu X, Fu J, Zhang J, Fu J, et al. 2019. Prenatal exposure to per-and polyfluoroalkyl substances (PFAS) and association between the placental transfer efficiencies and dissociation constant of serum proteins–PFAS complexes. Environ Sci Technol 53(11):6529–6538, PMID: , 10.1021/acs.est.9b00715. [DOI] [PubMed] [Google Scholar]
- 39.Chu C, Zhou Y, Li QQ, Bloom MS, Lin S, Yu YJ, et al. 2020. Are perfluorooctane sulfonate alternatives safer? New insights from a birth cohort study. Environ Int 135:105365, PMID: , 10.1016/j.envint.2019.105365. [DOI] [PubMed] [Google Scholar]
- 40.Liu H, Pan Y, Jin S, Sun X, Jiang Y, Wang Y, et al. 2021. Associations between six common per-and polyfluoroalkyl substances and estrogens in neonates of China. J Hazard Mater 407:124378, PMID: , 10.1016/j.jhazmat.2020.124378. [DOI] [PubMed] [Google Scholar]
- 41.Xin Y, Wan B, Yu B, Fan Y, Chen D, Guo LH. 2020. Chlorinated polyfluoroalkylether sulfonic acids exhibit stronger estrogenic effects than perfluorooctane sulfonate by activating nuclear estrogen receptor pathways. Environ Sci Technol 54(6):3455–3464, PMID: , 10.1021/acs.est.9b07708. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Zhou X, Sheng N, Cui R, Cui Q, Guo H, et al. 2018. Subchronic hepatotoxicity effects of 6:2 chlorinated polyfluorinated ether sulfonate (6:2 Cl-PFESA), a novel perfluorooctanesulfonate (PFOS) alternative, on adult male mice. Environ Sci Technol 52(21):12809–12818, PMID: , 10.1021/acs.est.8b04368. [DOI] [PubMed] [Google Scholar]
- 43.Levavi-Sivan B, Biran J, Fireman E. 2006. Sex steroids are involved in the regulation of gonadotropin-releasing hormone and dopamine D2 receptors in female tilapia pituitary. Biol Reprod 75(4):642–650, PMID: , 10.1095/biolreprod.106.051540. [DOI] [PubMed] [Google Scholar]
- 44.Haverinen E, Fernandez MF, Mustieles V, Tolonen H. 2021. Metabolic syndrome and endocrine disrupting chemicals: an overview of exposure and health effects. Int J Environ Res Public Health 18(24):13047, PMID: , 10.3390/ijerph182413047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li CH, Ren XM, Guo LH. 2019. Adipogenic activity of oligomeric hexafluoropropylene oxide (perfluorooctanoic acid alternative) through peroxisome proliferator-activated receptor γ pathway. Environ Sci Technol 53(6):3287–3295, PMID: , 10.1021/acs.est.8b06978. [DOI] [PubMed] [Google Scholar]
- 46.Rickard BP, Rizvi I, Fenton SE. 2022. Per- and poly-fluoroalkyl substances (PFAS) and female reproductive outcomes: PFAS elimination, endocrine-mediated effects, and disease. Toxicology 465:153031, PMID: , 10.1016/j.tox.2021.153031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xin Y, Ren XM, Wan B, Guo LH. 2019. Comparative in vitro and in vivo evaluation of the estrogenic effect of hexafluoropropylene oxide homologues. Environ Sci Technol 53(14):8371–8380, PMID: , 10.1021/acs.est.9b01579. [DOI] [PubMed] [Google Scholar]
- 48.Blake BE, Rickard BP, Fenton SE. 2022. A high-throughput toxicity screen of 42 per- and polyfluoroalkyl substances (PFAS) and functional assessment of migration and gene expression in human placental trophoblast cells. Front Toxicol 4:881347, PMID: , 10.3389/ftox.2022.881347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giacobini P, Prevot V. 2013. Semaphorins in the development, homeostasis and disease of hormone systems. Semin Cell Dev Biol 24(3):190–198, PMID: , 10.1016/j.semcdb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Heffernan AL, Cunningham TK, Drage DS, Aylward LL, Thompson K, Vijayasarathy S, et al. 2018. Perfluorinated alkyl acids in the serum and follicular fluid of UK women with and without polycystic ovarian syndrome undergoing fertility treatment and associations with hormonal and metabolic parameters. Int J Hyg Environ Health 221(7):1068–1075, PMID: , 10.1016/j.ijheh.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Jin H, Zhang Y, Jiang W, Zhu L, Martin JW. 2016. Isomer–specific distribution of perfluoroalkyl substances in blood. Environ Sci Technol 50(14):7808–7815, PMID: , 10.1021/acs.est.6b01698. [DOI] [PubMed] [Google Scholar]
- 52.Zeng XW, Lodge CJ, Dharmage SC, Bloom MS, Yu Y, Yang M, et al. 2019. Isomers of per-and polyfluoroalkyl substances and uric acid in adults: Isomers of C8 Health Project in China. Environ Int 133(pt A):105160, PMID: , 10.1016/j.envint.2019.105160. [DOI] [PubMed] [Google Scholar]
- 53.Loveless SE, Slezak B, Serex T, Lewis J, Mukerji P, O’Connor JC, et al. 2009. Toxicological evaluation of sodium perfluorohexanoate. Toxicology 264(1–2):32–44, PMID: , 10.1016/j.tox.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Borghoff SJ, Fitch S, Rager JE, Huggett D. 2018. A hypothesis-driven weight-of-evidence analysis to evaluate potential endocrine activity of perfluorohexanoic acid. Regul Toxicol Pharmacol 99:168–181, PMID: , 10.1016/j.yrtph.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Butenhoff JL, Chang SC, Ehresman DJ, York RG. 2009. Evaluation of potential reproductive and developmental toxicity of potassium perfluorohexanesulfonate in Sprague Dawley rats. Reprod Toxicol 27(3–4):331–341, PMID: , 10.1016/j.reprotox.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Rice PA, Aungst J, Cooper J, Bandele O, Kabadi SV. 2020. Comparative analysis of the toxicological databases for 6:2 fluorotelomer alcohol (6:2 FTOH) and perfluorohexanoic acid (PFHxA). Food Chem Toxicol 138:111210, PMID: , 10.1016/j.fct.2020.111210. [DOI] [PubMed] [Google Scholar]
- 57.Jiang L, Hong Y, Xie G, Zhang J, Zhang H, Cai Z. 2021. Comprehensive multi-omics approaches reveal the hepatotoxic mechanism of perfluorohexanoic acid (PFHxA) in mice. Sci Total Environ 790:148160, PMID: , 10.1016/j.scitotenv.2021.148160. [DOI] [PubMed] [Google Scholar]
- 58.Lin CY, Lee HL, Hwang YT, Su TC. 2020. The association between total serum isomers of per-and polyfluoroalkyl substances, lipid profiles, and the DNA oxidative/nitrative stress biomarkers in middle-aged Taiwanese adults. Environ Res 182:109064, PMID: , 10.1016/j.envres.2019.109064. [DOI] [PubMed] [Google Scholar]
- 59.Abdel‐Raheem IT, Omran GA, Katary MA. 2015. Irbesartan, an angiotensin II receptor antagonist, with selective PPAR‐gamma‐modulating activity improves function and structure of chemotherapy‐damaged ovaries in rats. Fundam Clin Pharmacol 29(3):286–298, PMID: , 10.1111/fcp.12119. [DOI] [PubMed] [Google Scholar]
- 60.Faulds G, Rydén M, Ek I, Wahrenberg H, Arner P. 2003. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovarian syndrome. J Clin Endocrinol Metab 88(5):2269–2273, PMID: , 10.1210/jc.2002-021573. [DOI] [PubMed] [Google Scholar]
- 61.Bodin J, Groeng EC, Andreassen M, Dirven H, Nygaard UC. 2016. Exposure to perfluoroundecanoic acid (PFUnDA) accelerates insulitis development in a mouse model of type 1 diabetes. Toxicol Rep 3:664–672, PMID: , 10.1016/j.toxrep.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Valenzuela R, Videla LA. 2011. The importance of the long-chain polyunsaturated fatty acid n-6/n-3 ratio in development of non-alcoholic fatty liver associated with obesity. Food Funct 2(11):644–648, PMID: , 10.1039/c1fo10133a. [DOI] [PubMed] [Google Scholar]
- 63.Bonato M, Corrà F, Bellio M, Guidolin L, Tallandini L, Irato P, et al. 2020. PFAS environmental pollution and antioxidant responses: an overview of the impact on human field. Int J Environ Res Public Health 17(21):8020, PMID: , 10.3390/ijerph17218020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Y, Su J, van Dam RM, Prem K, Hoong JY, Zou L, et al. 2017. Dietary predictors and plasma concentrations of perfluorinated alkyl acids in a Singapore population. Chemosphere 171:617–624, PMID: , 10.1016/j.chemosphere.2016.12.107. [DOI] [PubMed] [Google Scholar]
- 65.Maybin JA, Critchley HOD. 2016. Medical management of heavy menstrual bleeding. Womens Health (Lond) 12(1):27–34, PMID: , 10.2217/whe.15.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wielsøe M, Long M, Bossi R, Vorkamp K, Bonefeld-Jørgensen EC. 2022. Persistent organic pollutant exposures among Greenlandic adults in relation to lifestyle and diet: new data from the ACCEPT cohort. Sci Total Environ 827:154270, PMID: , 10.1016/j.scitotenv.2022.154270. [DOI] [PubMed] [Google Scholar]
- 67.Liu G, Dhana K, Furtado J, Rood J, Zong G, Liang L, et al. 2018. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: a prospective study. PLoS Med 15(2):e1002502, PMID: , 10.1371/journal.pmed.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J, Li J, Ma Y, Chen B, Wang X, Jiao X, et al. 2021. Urine concentrations of perfluoroalkyl acids in children and contributions of dietary factors: a cross-sectional study from Shanghai, China. Environ Sci Pollut Res Int 28(16):20440–20450, PMID: , 10.1007/s11356-020-12293-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


