Summary
Cells respond to environmental cues by remodeling their inventories of multiprotein complexes. Cellular repertoires of SCF (SKP1-CUL1-F box protein) ubiquitin ligase complexes, which mediate much protein degradation, require CAND1 to distribute the limiting CUL1 subunit across the family of ∼70 different F box proteins. Yet, how a single factor coordinately assembles numerous distinct multiprotein complexes remains unknown. We obtained cryo-EM structures of CAND1-bound SCF complexes in multiple states and correlated mutational effects on structures, biochemistry, and cellular assays. The data suggest that CAND1 clasps idling catalytic domains of an inactive SCF, rolls around, and allosterically rocks and destabilizes the SCF. New SCF production proceeds in reverse, through SKP1-F box allosterically destabilizing CAND1. The CAND1-SCF conformational ensemble recycles CUL1 from inactive complexes, fueling mixing and matching of SCF parts for E3 activation in response to substrate availability. Our data reveal biogenesis of a predominant family of E3 ligases, and the molecular basis for systemwide multiprotein complex assembly.
Keywords: ubiquitin, E3 ligase, SCF, cullin-RING ligase, CRL, protein complex assembly, CAND1, cryo-EM, proteomics, NEDD8
Graphical abstract
Highlights
-
•
47 cryo-EM structures show CAND1-SCF complexes in multiple destabilized conformations
-
•
Mutations suggest CAND1 rolls around/off idling SCFs as F box proteins rock off/on
-
•
Rock-and-roll mechanism recycles limiting CUL1 to form new SCF complexes
-
•
CAND1 action on SCFs determines cellular E3 ligase repertoire and response to signal
How does a single regulatory factor control the assembly and disassembly of E3 ligases containing the ∼70 different F box proteins?
Introduction
The biogenesis of many multi-subunit complexes depends on dedicated assembly factors guiding inter-subunit interactions.1,2,3,4,5 In contrast, the modular SCF (SKP1-CUL1-F box protein) E3 ubiquitin (UB) ligases are both dynamically formed and dismantled on a systemwide level: the collection of unique complexes with ∼70 different F box proteins (Fbps) depends on the single assembly/disassembly factor CAND1 (Cullin-associated NEDD8-dissociated protein 1).6,7,8,9 CAND1 has been proposed to sense and destabilize inactive SCFs while ignoring those that are ubiquitylating substrates.6,7,8,9,10,11,12,13,14 Fundamental E3 ligase-dependent biological regulation—encompassing metabolic signaling in fungi, phytohormone signaling in plants, and responses to cytokines, redox stresses, DNA-damaging agents, and “degrader” drugs in humans—relies on CAND1.7,9,10,11,15,16,17,18,19
SCFs are founding members of the cullin-RING ligase (CRL) superfamily of E3 enzymes.20,21,22 SCFs are assembled from two pre-formed subcomplexes: the CUL1-RBX1 subcomplex promotes ubiquitylation of substrates recruited to interchangeable SKP1-Fbp subcomplexes. Fbps recognize unique substrate degron motifs.23,24,25,26
SCF holoenzyme complexes form upon CUL1-RBX1 association with a SKP1-Fbp in vitro by mixing the two purified subcomplexes. In cells, however, SCF-dependent ubiquitylation is regulated by a multimodal mechanism that both activates and marks substrate-bound complexes. Substrate binding is typically controlled through conditional formation of degron motifs, for example, by phosphorylation.27,28,29,30 E3 ligase activity also depends on modification of CUL1’s C-terminal WHB domain by the ubiquitin-like protein NEDD8, which occurs through a multi-enzyme neddylation cascade similar to ubiquitylation.31,32 SCFs are deactivated by deconjugation of NEDD8 from CUL1 by the CSN (COP9 Signalosome) deneddylase.33 The deneddylation machinery senses whether the SCF complex is bound to a protein substrate and thus should not be disturbed.12,13,14,34,35,36,37 Substrate-bound SCFs retain NEDD8 and E3 ligase activity, whereas substrate-free complexes are subject to deneddylation. Unneddylated CUL1-RBX1 binds CAND1, which inhibits neddylation.38,39,40 Seemingly paradoxically, CSN and CAND1 are required in cells for timely degradation of many SCF substrates despite their counteracting neddylation, which is crucial for ubiquitylation.6,7,8,9,10,15,16,17,34,41,42,43
The central reason why cells require CAND1 and CSN for proper SCF function is that they solve a supply chain problem preventing simultaneous production of all possible SCFs: cellular concentrations of SKP1-Fbps are in substantial excess of CUL1-RBX1.9,44 Furthermore, purified SCFs are biochemically stable, with half-lives of days for the dissociation of an SCF complex into its CUL1-RBX1 and SKP1-Fbp substituents.6,45,46 Thus, pre-formed SCFs seemingly would sequester their constituent CUL1-RBX1 and prevent formation of alternative SCFs needed under distinct cellular conditions. However, biochemical experiments suggested CAND1 provides a solution to how excess Fbps can gain access to limiting CUL1-RBX1.6,10 Unneddylated SCFs at least partially release their SKP1-Fbp subcomplex if supplied with CAND1.6 Conversely, CAND1 at least partly dissociates from the otherwise stable CAND1-CUL1-RBX1 complex upon addition of a SKP1-Fbp complex. Furthermore, in vitro ubiquitylation assays implied that CAND1 allowed an isolated SKP1-Fbp subcomplex to form an SCF with CUL1-RBX1 only attainable through dismantling of another SCF.6 Finally, the proteomic surveying of more than 40 human SCF complexes showed that the cellular cohorts of Fbps bound to CUL1 depend on CAND1 and the neddylating and deneddylating machineries.9
While biochemical and cell biological results are united in support of CAND1-mediated SCF assembly and disassembly,6,7,8,9 the structures of SCF and CAND1-CUL1-RBX1 complexes are at odds with such a role.40,45,47,48 For instance, CAND1-dependent destabilization of an SCF, and the mutual destabilization of CAND1-CUL1-RBX1 by SKP1-Fbp, were hypothesized to proceed through a singular unstable CAND1-SCF intermediate.6,9,10 However, such a CAND1-SCF complex has never been detected, and it is incompatible with existing structures wherein CUL1-RBX1 binding to CAND1 or a SKP1-Fbp are mutually exclusive.40,45,47,48 The CAND1-CUL1-RBX1 crystal structure showed CAND1 comprises three arches forming a continuous sinusoid that clamps around CUL1-RBX1. CAND1’s central arch curls back-and-forth around the middle of CUL1, securing N- and C-terminal arches against the two ends of CUL1-RBX1.40 CAND1’s N-terminal arch (henceforth termed “anti-neddylation domain”) clasps CUL1’s unmodified neddylation site together with RBX1’s RING domain and opposes neddylation.40 CAND1’s C-terminal arch (henceforth “anti-SKP1 domain”) displays a β-hairpin that grips a CUL1 groove that otherwise binds SKP1 in an SCF. Indeed, prior SCF structures showed a continuous surface from both SKP1 and the Fbp binding to CUL1,45 obstructing CUL1 access to CAND1’s β-hairpin. Thus, it remains unclear how nascent SCF complexes are generated in a CAND1-dependent manner.
To address this problem, we performed structural, biochemical, and cell biological studies that collectively reveal the mechanism by which CAND1 shapes the cellular repertoire of SCF complexes. Overall, the data illuminate recycling the limiting CUL1-RBX1 component from idling SCF complexes for reuse in other complexes needed for dynamic cellular regulation.
Results
CAND1-SCF conformational ensembles reduce CUL1 contacts to SKP1-Fbps and CAND1
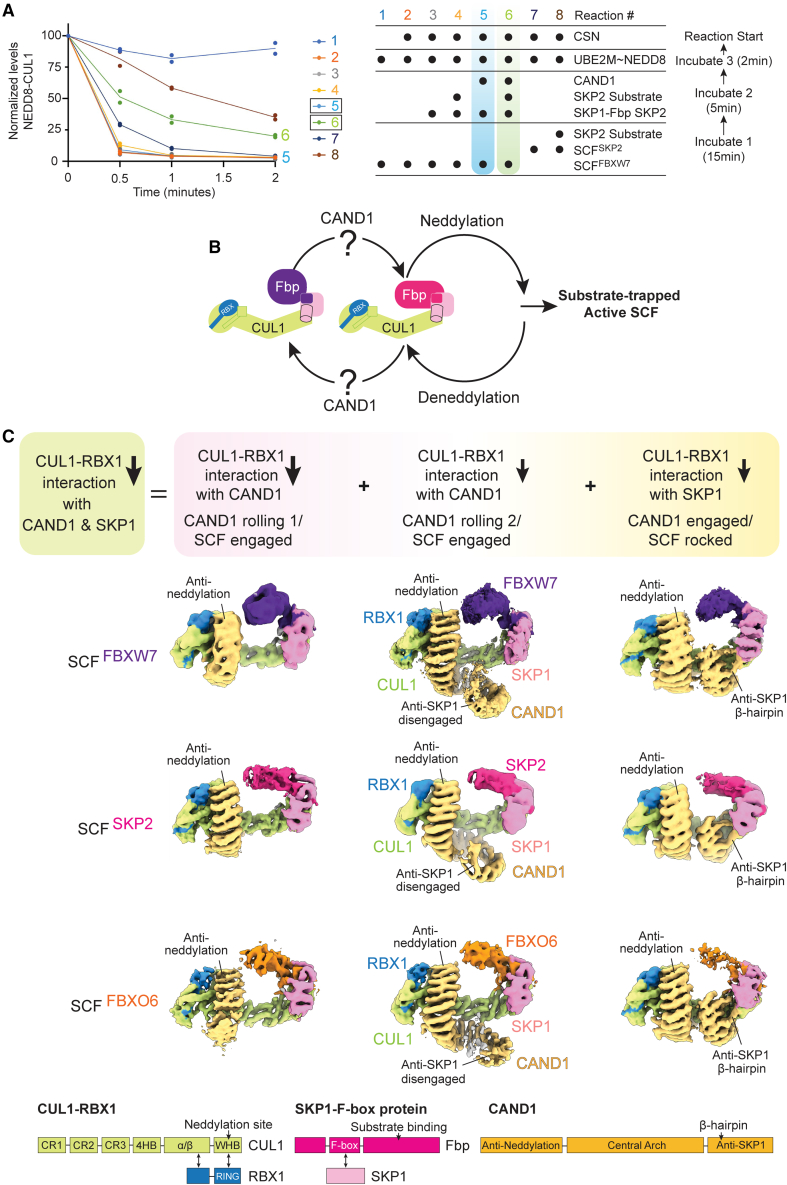
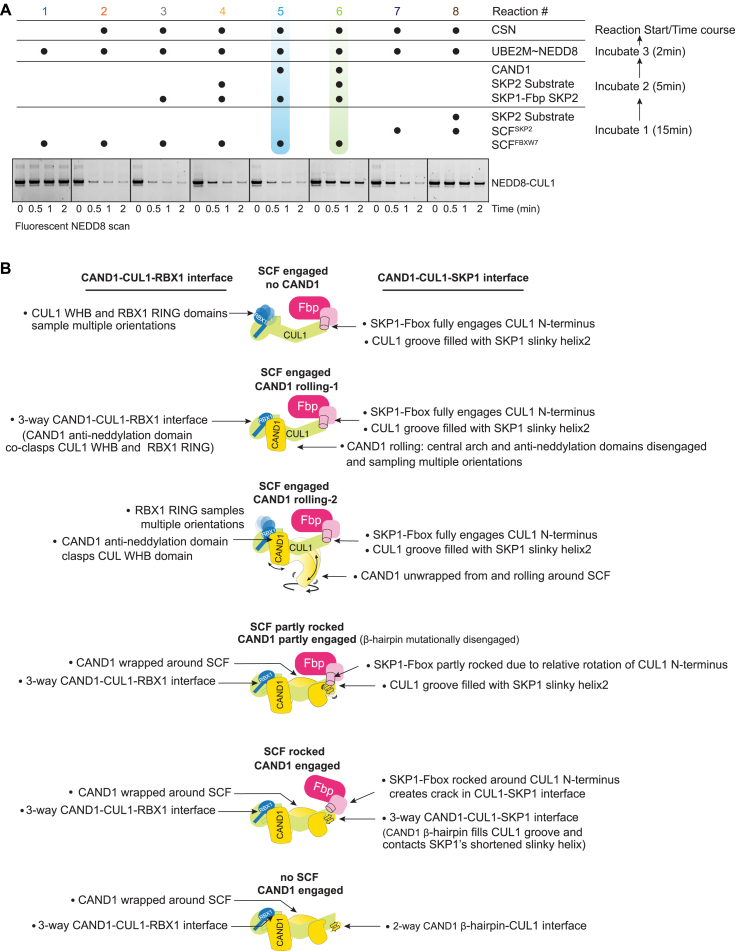
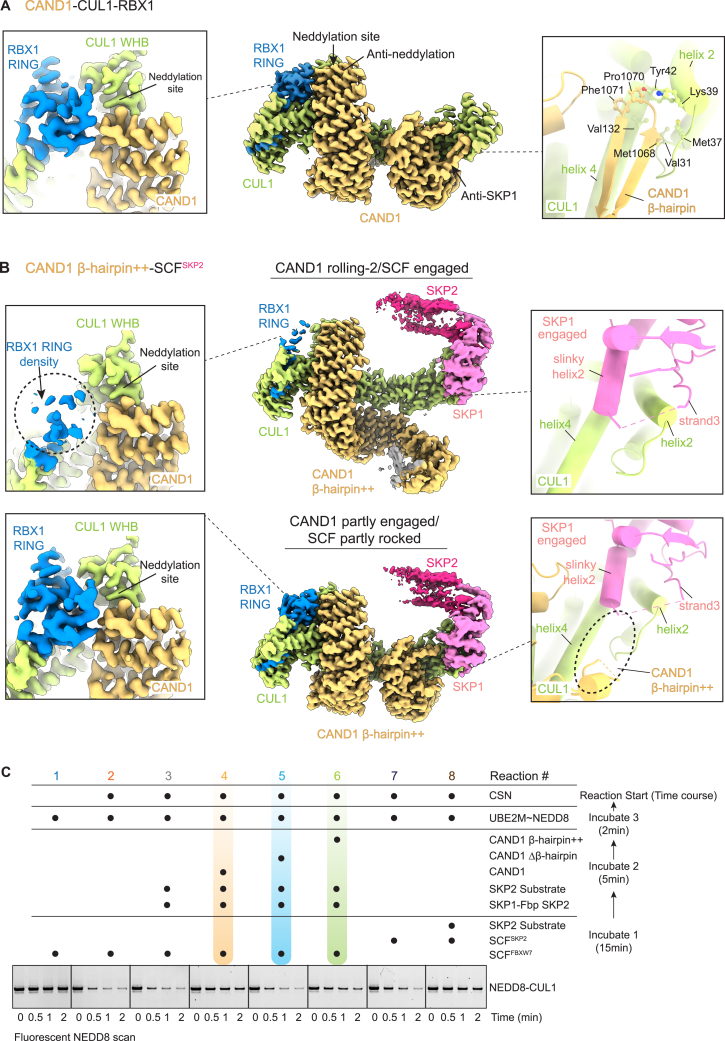
As a first step toward examining the cyclical regulation of systemwide SCF complex disassembly and assembly, we reconstituted simultaneous CUL1 neddylation, deneddylation, and CAND1-dependent SKP1-Fbp exchange with purified components (Figures 1A and S1A). Production of an active SCF was assayed by monitoring transfer of NEDD8 from its E2 enzyme (UBE2M) to CUL1 supplied within a pre-assembled SCF. Initiating reactions with SCFFBXW7 resulted in a burst of CUL1 neddylation, rapidly followed by CSN-catalyzed deneddylation. The same is true if the reaction is initiated with SCFSKP2, except deneddylation was significantly slowed for its substrate-bound form49 (recall CSN is most active when Fbps are not bound to their substrates12,13). Importantly, adding the Fbp SKP2's substrate to the reaction initiated with SCFFBXW7 also slowed deneddylation for reactions concomitantly supplied with both its cognate SKP1-SKP2 and CAND1 (Figure 1A). The results suggest CAND1 allowed disassembly of deneddylated SCFFBXW7, and formation and neddylation of SCFSKP2, whose substrate impeded its deneddylation (Figure 1B). Thus, CAND1 is required when the system limits SKP1-Fbps from accessing CUL1-RBX1 to form substrate-bound, activated SCF complexes.
Figure 1.
CAND1-SCF complexes adopt multiple conformations that collectively reduce CUL1 contacts to SKP1-Fbps and to CAND1
(A) CAND1, neddylation and deneddylation machineries, SKP1-SKP2, and/or the SKP2 substrate complex (phosphorylated p27-CKSHS1-CyclinA-CDK2) were added to a pre-formed SCFFBXW7 or SCFSKP2, as indicated. Systemwide SCF complex formation was read out by CAND1- and SKP2-substrate-dependent retention of NEDD8 linked to CUL1 initially provided in SCFFBXW7. n = 2 dots are plotted.
(B) Cartoon representation of CAND1- and substrate-regulated switching of SKP1-Fbps incorporated into activated SCFs. The structural basis for neddylation, deneddylation, and substrate is well understood.12,27,30,32,35,36,37,48,50 How CAND1 can structurally promote SKP1-Fbp switching in SCFs remains elusive, as indicated by “?”.
(C) Cryo-EM maps showing CAND1-SCF complexes for the Fbps FBXW7, SKP2, and FBXO6. CAND1-SCF complexes form a collection of structures in which CUL1-RBX1 interactions with CAND1 or the SKP1-Fbp are reduced. This would overall lower the barrier to both dissociating: summing relative affinities across individual CAND1-SCF complexes within the ensemble would lower CUL1-RBX1 affinity for both CAND1 and the SKP1-Fbp. Schematics for proteins and their domains are shown below.
See also Figure S1.
Figure S1.
Systemwide SCF disassembly and assembly, related to Figure 1
(A) Representative gels of the in vitro reconstitution assay shown in Figure 1A.
(B) Cartoon schematics of CAND1-SCF conformations, with details of the CAND1-CUL1-RBX1 and CAND1-CUL1-SKP1 interfaces.
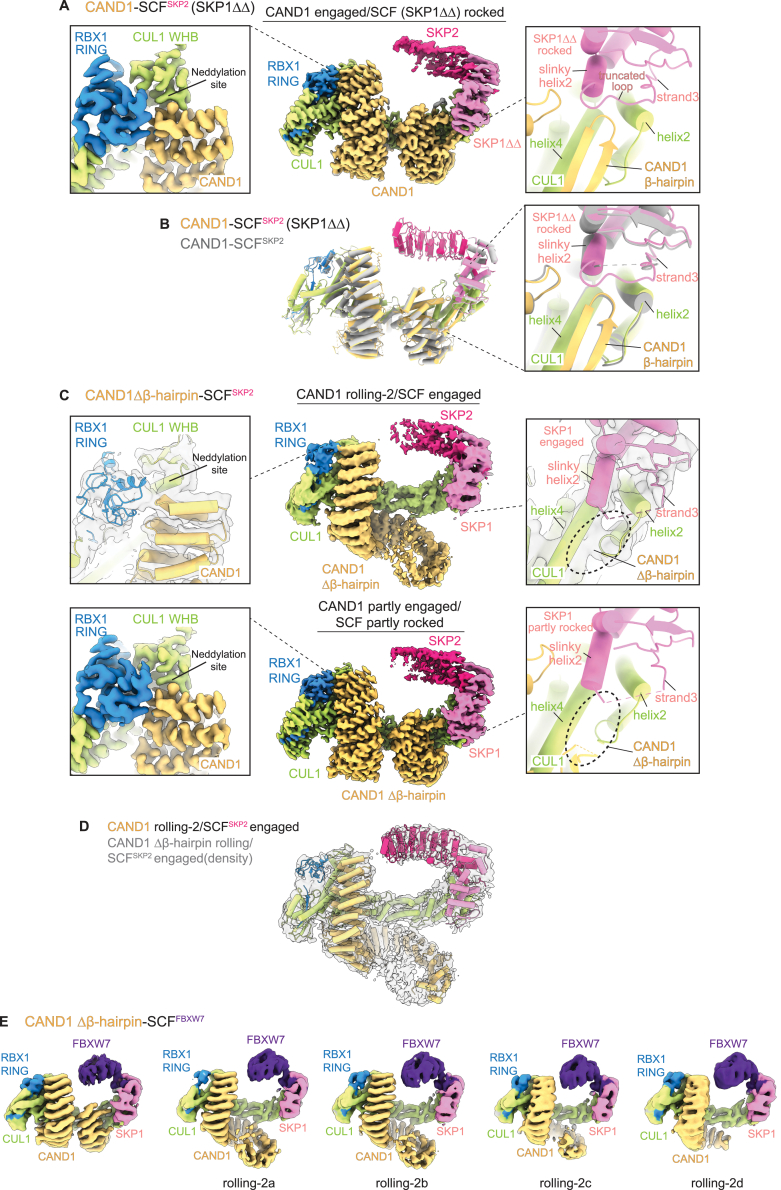
Understanding this systemwide multiprotein complex formation requires structural knowledge of the effects of CAND1 on SCFs. Thus, we mixed CAND1, CUL1-RBX1, and SKP1-Fbp complexes and applied cryo-EM. To survey the SCF system, we screened representative members of each Fbp structural family,51 in some cases with substrates: FBXW (with FBXW7, a tumor suppressor protein whose WD40 domain recognizes substrates including phosphorylated Cyclin E, MYC, and Notch30,52), FBXL (with SKP2, which together with CKSHS1 regulates the cell cycle by its leucine-rich repeat, or LRR, domain recognizing substrates including p2727,49), and FBXO (with FBXO6, which regulates protein quality control by its “other”—lectin-like—domain recognizing Man3GlcNAc2-modifications in misfolded substrates53,54) (Figure 1C; Tables S1 and S2; Methods S1). The cryo-EM maps revealed a collection of complexes including those corresponding to CAND1-CUL1-RBX1 and SCFs alone. Remarkably, the cryo-EM data also revealed three CAND1-SCF assemblies common among the Fbps, albeit in varying proportions for the different Fbps and their protein partners (Methods S1; Tables S1 and S2).
The collection of CAND1-SCF maps at moderate resolution showed that, similar to existing structures of SCF complexes, CUL1 forms an elongated scaffold, with one end binding RBX1 and the other a SKP1-Fbp (Figures 1C and S1B). Moreover, CAND1 retains its sinusoidal structure, and its N-terminal anti-neddylation domain grips CUL1’s C-terminal WHB domain surface containing the neddylation site. Thus, a requirement for deneddylation prior to CAND1-mediated SCF disassembly and assembly is explained by CAND1 reliance on the surface that is occluded by NEDD8 linkage to CUL1.
Despite these apparent similarities to previous SCF and CAND1-CUL1-RBX1 assemblies, the CAND1-SCF complexes adopt multiple conformations that differ significantly from the existing structures (Figures 1C and S1B). Two conformations show drastic reduction in CAND1 contacts compared with the structure of CAND1 bound to CUL1-RBX1 alone.40 These maps vary in extent of density for CAND1. We surmise that the invisible regions adopt multiple orientations.
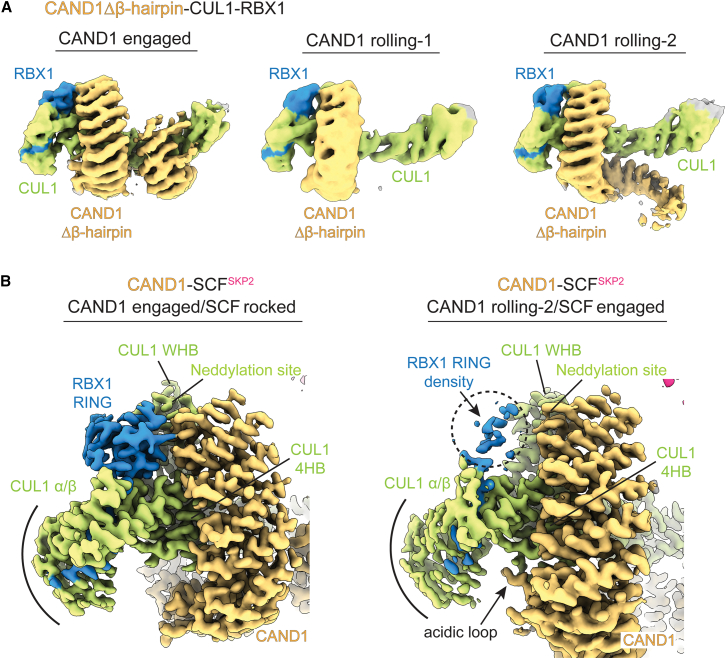
In one CAND1 conformation, which we term “rolling-1,” only the anti-neddylation domain is visible bound to SCFs. The second category, which we term “rolling-2,” also shows CAND1’s central arch, spiraling away from rather than curling around the SCF. This directs CAND1’s anti-SKP1 domain away from the SKP1-Fbp. Yet a third category showed a strikingly distinct alternative assembly: fully visible CAND1 engaged with CUL1-RBX1 in a similar fashion as in CAND1-CUL1-RBX1 alone, enabled by “rocking” of the CUL1-SKP1-F box interface (described in detail below).
The varying configurations suggest that CAND1 lowers the barrier for SKP1-Fbp dissociation from CUL1-RBX1 and vice versa by allosteric remodeling into a collection of conformations. Within the total population of CAND1-bound SCF complexes, some CAND1 proteins make fewer contacts than in CAND1-CUL1-RBX1 alone, while a different subset of complexes display destabilized SCF interfaces. Thus, CAND1-SCF complex formation parses relatively homogeneous SCF and CAND1-CUL1-RBX1 entities into collections of structures in which either SKP1-Fbp or CAND1 interactions with CUL1-RBX1 are impacted. While any entity may retain stable CUL1-RBX1 interactions with CAND1 or a SKP1-Fbp, the propensity for dissociation is increased across the pool of CAND1-SCF complexes.
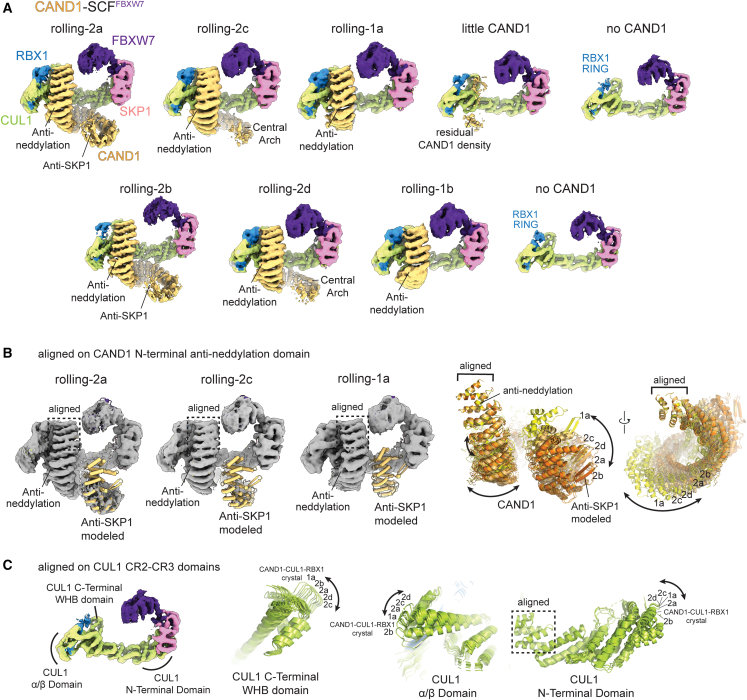
Cycling through CAND1-SCFFBXW7 structures suggests CAND1 rolls around and off an SCF
Cryo-EM samples for CAND1 complexes with SCFFBXW7 predominantly showed classes with CAND1’s anti-SKP1 domain disengaged from CUL1 (Table S1; Methods S1). Seven such classes were visualized from a single dataset obtained upon equilibrating equimolar CAND1, CUL1-RBX1, and SKP1-FBXW7 (Figure 2A). None of the maps showed CAND1’s β-hairpin even at low contour, although some allowed docking the remainder of CAND1’s anti-SKP1 domain (Figure 2B). On the other hand, the CUL1-SKP1-F box interface resembles that in prior SCF structures30,48 without CAND1 (Figures S2A–S2C). We thus refer to these conformations as having the SCF “engaged.” Here, CAND1 cannot engage the SCF because it is blocked by SKP1.
Figure 2.
Cryo-EM maps of CAND1-SCFFBXW7 suggest that CAND1 rolls around an SCF
(A) Cryo-EM maps show CAND1 adopts multiple conformations with its anti-neddylation domain bound, and its anti-SKP1 domain disengaged from SCFFBXW7. Four maps (a–d) adopt rolling-2 conformations, with CAND1's central arch spiraling away from rather than curling around the SCF. Two maps (a and b) adopt rolling-1 conformations, where only CAND1’s anti-neddylation domain is substantially visible in the density. Other maps show little to no CAND1.
(B) To model the conformations and trajectories of CAND1 bound to SCFFBXW7, CAND1 domains40 were docked into maps for rolling-2 conformations. The full-length CAND1 structure was docked into the density for the anti-neddylation domain in rolling-1a conformation. Alignment of the CAND1 models over the anti-neddylation domain shows twisting and turning of CAND1 HEAT repeats in the different conformations.
(C) To model the conformations and trajectories of CUL1 in CAND1-SCFFBXW7 complexes, structures CUL1 domains were docked to the maps. Alignment over CUL1’s CR2-CR3 domains shows that the CUL1 WHB, ⍺/β, and CR1 domains assume multiple relative orientations. The orientations in the crystal structure of CAND1-CUL1-RBX140 are shown for reference.
See also Figure S2.
Figure S2.
Conformational overlays of CAND1-SCFFBXW7 show CAND1 rolling, related to Figure 2
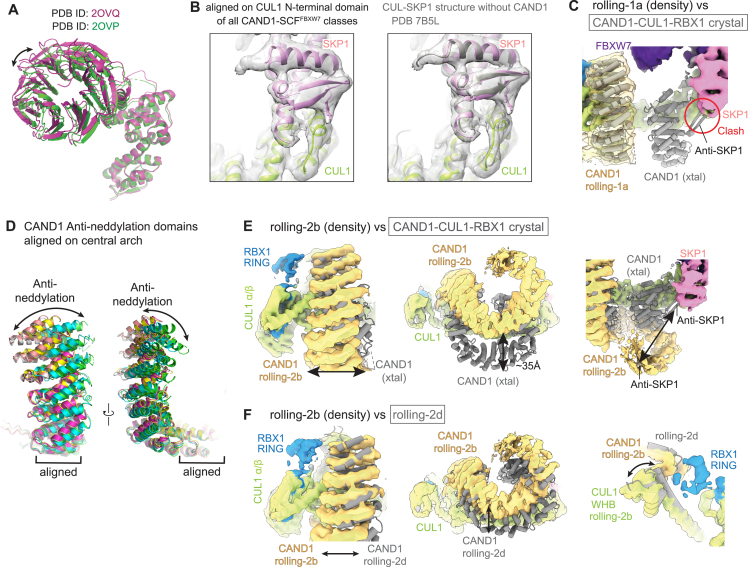
(A) Superposition of two SKP1-FBXW7 crystal structures30 over SKP1 shows intrinsic conformational variability.
(B) Superposition of CAND1-SCFFBXW7 maps over CUL1’s N-terminal CR1 domain (left) shows SKP1 bound to CUL1 as in prior SCF cryo-EM structure48 (right).
(C) Comparison of cryo-EM map for CAND1-SCFFBXW7 rolling-1a class with CAND1-CUL1-RBX1 crystal (xtal) structure.40 In the CAND1-SCF rolling-1a map, the only portion of CAND1 substantially visible in the density is the anti-neddylation domain. Docking CAND1 from the crystal structure into this density shows that if CAND1 adopted the same conformation in the rolling-1a complex, the anti-SKP1 domain clashes with SKP1.
(D) Comparison of CAND1 anti-neddylation domains from CAND1-SCFFBXW7 aligned over CAND1 central arch shows variation in twist, compaction, and curvature of HEAT repeats. The models were generated by fitting each HEAT repeat into the maps.
(E) Comparison of CAND1-SCFFBXW7 rolling-2b map (colored density) with prior CAND1 crystal structure40 over CUL1’s CR2 and CR3 domains shows the rolling-2b conformation shifts the CAND1 anti-neddylation domain toward CUL1’s α/β domain. A ∼35 Å shift of CAND1’s central arch results in its spiraling away from CUL1’s CR domains, and disengagement of its anti-SKP1 domain.
(F) Comparison of cryo-EM maps for CAND1-SCFFBXW7 rolling-2b (colored density) and rolling-2d (gray, model from docked coordinates) classes. While both conformations show CAND1 rolling, they differ in the relative orientations of the anti-neddylation domain, central arch, and CUL1 WHB domain. In both classes, CAND1’s central arch approaches the center of CUL1 in a direction that points the anti-SKP1 domain away from CUL1’s interface with SKP1. Although the arc of CAND1’s anti-neddylation domain in the rolling-2b conformation is more toward the position in CAND1-CUL1-RBX1 alone, the distinct bending angles between CUL1’s N-terminal and penultimate regions prevent CAND1’s central arch from curling around CUL1.
Comparing cryo-EM maps provided insights into the distinct conformations of CAND1 when bound to an SCF. The six maps that clearly show at least CAND1’s anti-neddylation domain differ from each other and from prior structures by the sinusoidal twist and compaction of CAND1 HEAT repeats (Figures 2B and S2D) and the angles between CUL1-RBX1 subdomains (Figure 2C). These same features differ among prior structures of CAND1-CUL-RBX1 and SCF complexes, reflecting propensity for conformational variability.14,40,45,48,50 Fitting prior structures of CAND1 and CUL1 domains into the maps show how subtly varying conformations impact the relative positions of CAND1. Superimposing the maps over CAND1’s N-terminal anti-neddylation domain suggests accordion-like motions within CAND1 itself (Figures 2B, S2E, and S2F). Meanwhile, superimposing over CUL1’s central subdomains shows relative rotations of CUL1’s CAND1-bound WHB domain, and of its N-terminal and penultimate regions varying across the CAND1-SCFFBXW7 conformations (Figure 2C). Notably, the three classes with little to no CAND1 density, and the rolling-2b conformation of CAND1-SCFFBXW7, share in common weak density for RBX1’s RING domain, highlighting its conformational heterogeneity as well (Figure 2A).
Cycling through the maps as movie frames shows that if the assorted conformations were accessible to a single CAND1-SCF complex, then CAND1 clings to CUL1's WHB domain (Video S1). With the SCF engaged and blocking CUL1's N-terminal CR1 domain, CAND1 appears to roll around CUL1 within the SCF. Although there is no obvious linear trajectory across the CAND1-SCFFBXW7 classes, they all show substantially reduced CAND1 contacts compared with the prior CAND1-CUL1-RBX1 structure.40 Thus, CAND1 rolling provides a structural rationale for a SKP1-Fbp increasing propensity for CAND1 dissociation.
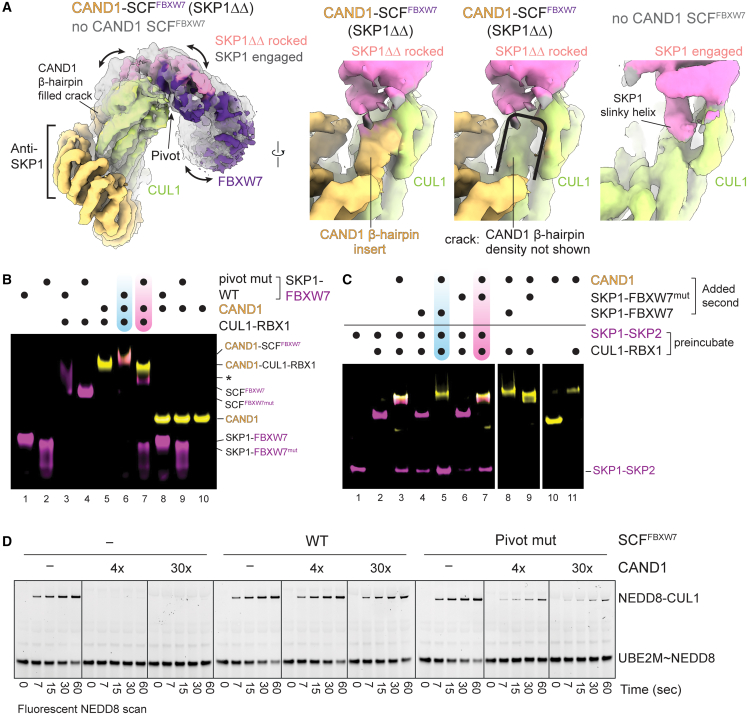
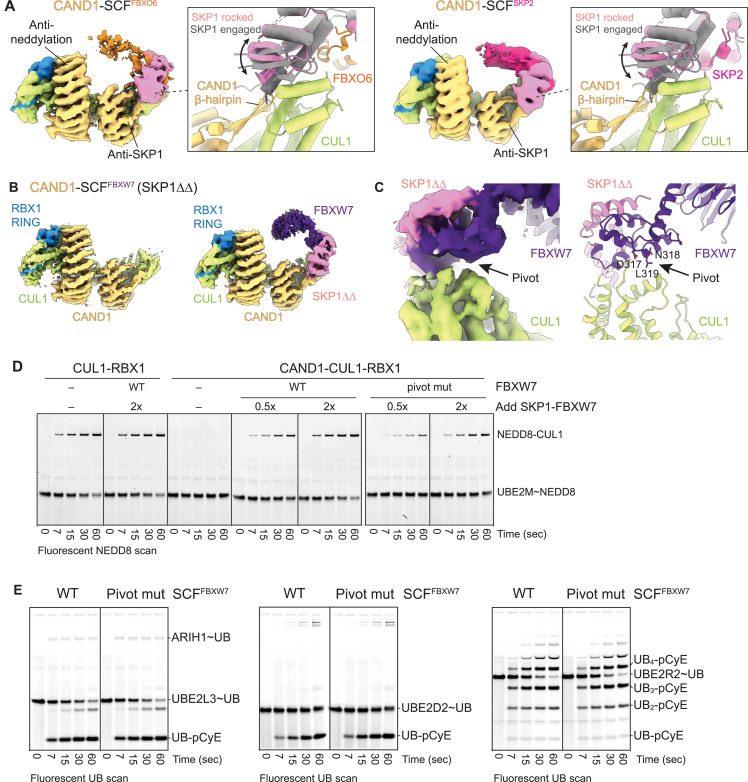
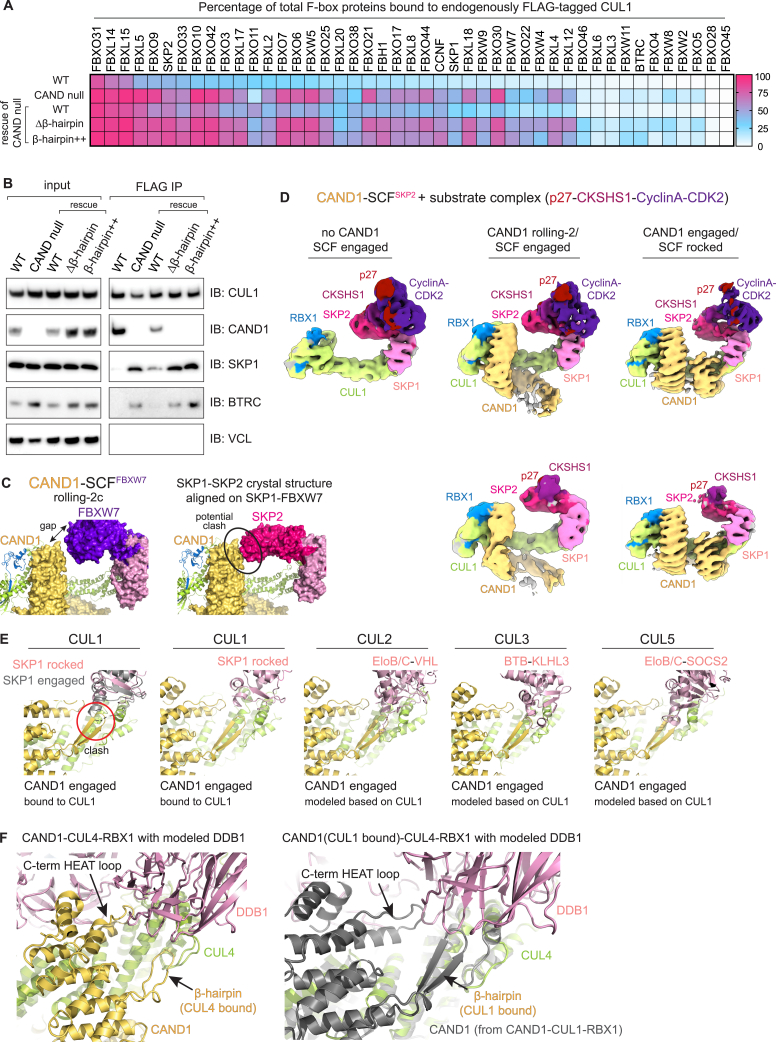
CAND1 engages a rocked SCF with a crack in the CUL1-SKP1 interface
The alternative CAND1 conformation (observed in all samples with SCFSKP2 and SCFFBXO6, and one with WT SCFFBXW7; Table S1; Methods S1) is reminiscent of the CAND1-CUL1-RBX1 crystal structure: CAND1’s anti-neddylation domain secures CUL1's WHB domain and RBX1's RING; its central arch curls around CUL1; and its anti-SKP1 domain engages CUL1's N-terminal CR1 domain (Figures 1C, 3A, S1B, and S3A). We refer to this conformation as “CAND1 engaged.” Notably, use of the so-called “ΔΔ” SKP1 mutant lacking two disordered loops46 increased the proportion CAND1-SCFFBXW7 complexes in the CAND1 engaged conformation (Figure S3B; Table S2; Methods S1). Prior studies showed SKP1ΔΔ-FBXW7 fails to displace CAND1 from CUL1-RBX1.6,10 Thus, CAND1 is likely more stably bound in the engaged conformation due to its more extensive contacts with the SCF.
Figure 3.
CAND1 engages a rocked SCF conformation with a crack in the CUL1-SKP1 interface
(A) Left, overlay of cryo-EM maps with CAND1-engaged SCFFBXW7 (with SKP1ΔΔ) or SCFFBXW7 without CAND1 shows rocking of SKP1 when CAND1 is engaged and its β-hairpin inserted into the crack between CUL1 and SKP1. Right, side-by-side comparison of cryo-EM maps over the CUL1 groove when occupied by CAND1’s β-hairpin in CAND1-engaged SCFFBXW7, or the same map excluding density for CAND1’s β-hairpin to highlight the crack between SKP1 and CUL1, or when occupied by SKP1’s slinky helix with the SCF interface engaged.
(B) Nondenaturing gel assaying CAND1 complex formation with WT SCFFBXW7, or with an FBXW7 pivot mutant (D317M N318P L319D). CAND1 is labeled with TAMRA (yellow), FBXW7 with fluorescein (magenta) for visualization. FBXW7 samples include Cyclin E phosphopeptide to improve homogeneity of migration. ∗Unknown band. See STAR Methods.
(C) Nondenaturing gel assaying role of F box pivot in CAND1-dependent Fbp competition. WT or pivot mutant SKP1-FBXW7 were compared for displacement of SKP1-SKP2 from SCFSKP2, mediated by CAND1. CAND1 is labeled with TAMRA (yellow), SKP2 with Cy5 (magenta) for visualization. FBXW7 samples include Cyclin E phosphopeptide to improve homogeneity of migration.
(D) WT or pivot mutant SCFFBXW7 were tested for resistance to 4- or 30-fold excess CAND1 inhibiting neddylation, monitored by fluorescent NEDD8 transfer from its E2 UBE2M to CUL1 within the SCF.
See also Figure S3.
Figure S3.
CAND1 engagement induces SCF rocking around an F box pivot, related to Figure 3
(A) For both CAND1-SCFFBXO6 and CAND1-SCFSKP2, cryo-EM density is shown on the left for CAND1 engaged/SCF rocked conformations. Close ups show prior active SCFSKP2 E3 ligase structure48 in gray superimposed with colored models of the CAND1-SCF complex based on docking prior structures into the density. The data show that when CAND1’s β-hairpin is engaged, the CUL1-SKP1-F box interface is rocked around CUL1.
(B) Cryo-EM maps for CAND1-SCFFBXW7 (SKP1ΔΔ). CAND1 rolling was not detected. The two major classes instead showed CAND1-CUL1-RBX1 alone (no SKP1-FBXW7), and CAND1 engaged/SCFFBXW7 rocked.
(C) Close up of the CUL1-FBXW7 interface in the cryo-EM map of CAND1-SCFFBXW7 (SKP1ΔΔ), showing the FBXW7 F box pivot residues (D317, N318, and L319).
(D) Assays testing WT or SKP1-FBXW7 pivot mutant for ability to overcome CAND1 inhibition of neddylation of CUL1-RBX1. The assays were performed in pulse-chase format and detect transfer of fluorescent NEDD8 from is E2 (UBE2M) to CUL1-RBX1 either alone or with WT or mutant SKP1-FBXW7 added at the indicated ratios.
(E) Control experiments performed without CAND1 showed the FBXW7 F box pivot mutant readily forms an SCFFBXW7 complex with WT substrate ubiquitylation activities. The assays were performed in pulse-chase format, detecting fluorescent ubiquitin (UB) transfer from each of three distinct types of ubiquitin carrying enzyme partners of SCFs (from left to right, UBE2L3/ARIH1, UBE2D2, and UBE2R2). The substrate is a phosphopeptide derived from Cyclin E.
Engagement of CAND1’s β-hairpin is accommodated by “rocking” of the SKP1-F box unit around CUL1's CR1 domain (Figure 3A). At one edge of the CUL1-SKP1-F box assembly, CAND1’s β-hairpin inserts into a crack produced through rocking liberating CUL1 from one edge of SKP1. At the other edge, CUL1-F box interactions are maintained at the rocking pivot point (Figures 3A and S3C).
We tested roles of this F box interaction by mutating the rocking pivot (FBXW7 D317M N318P L319D). WT SCFFBXW7-CAND1 complex formation was detected by co-migration of fluorescein-FBXW7 and TAMRA-CAND1 in nondenaturing gel electrophoresis. Adding CAND1 to the F box pivot mutant FBXW7 resulted in its substantial dissociation from the SCF and formation of CAND1-CUL1-RBX1 (Figure 3B, lane 6 versus 7). Effects of the pivot mutation were also investigated in the context of other components of systemwide SCF complex formation through CAND1-mediated exchange of SKP1-Fbp modules bound to CUL1. Considering the cryo-EM data, which showed CAND1-SCFSKP2 complexes have greater propensity for the CAND1 engaged/SCF rocked conformation, it seemed SKP1-SKP2 would be more susceptible to displacement from a CAND1-SCF than SKP1-FBXW7 (with substrate phosphopeptide included for technical reasons described in STAR Methods). Consistent with prior results,6 monitoring Cy5-labeled SKP2 showed that adding WT SKP1-FBXW7 results in loss of the CAND1-SCFSKP2 complex and concomitant increase in SKP1-SKP2 alone (Figure 3C, lane 5 versus 3). The F box pivot mutant is impaired (Figure 3C, lane 7 versus 5). We also considered that CAND1 binding is mutually exclusive with formation of an active E3 ligase through neddylation. While a CAND1-CUL1-RBX1 complex is resistant to neddylation, this is counteracted by a SKP1-Fbp promoting dissociation of CAND1.34 Indeed, adding WT SKP1-FBXW7 thwarts this inhibition, allowing neddylation in the presence of CAND1. However, the F box pivot mutant was compromised for this activity (Figures 3D and S3D). Importantly, the defects caused by the F box pivot mutant are specific to CAND1, since it forms a viable SCF promoting ubiquitylation (Figure S3E). Overall, the data suggest CAND1 engagement rocks and destabilizes the SCF interface.
A trajectory for CAND1-assisted SCF disassembly
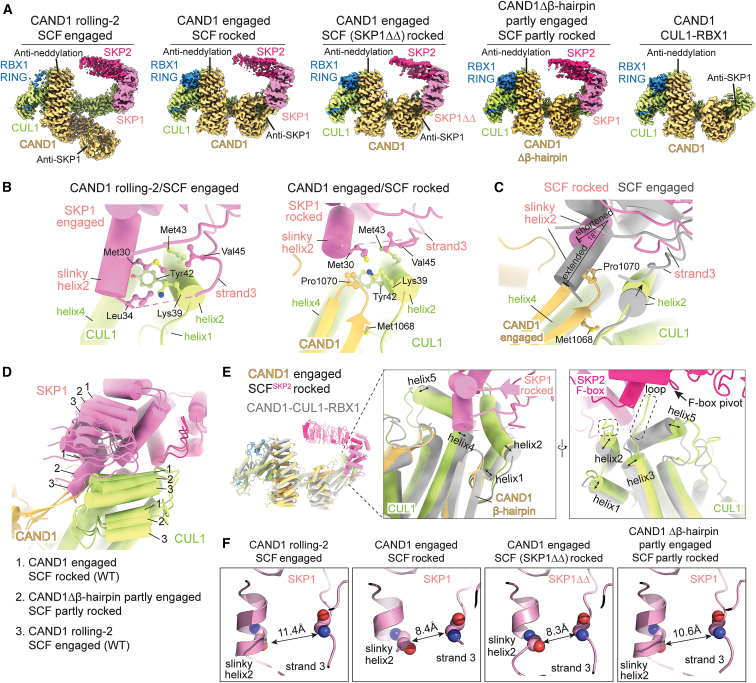
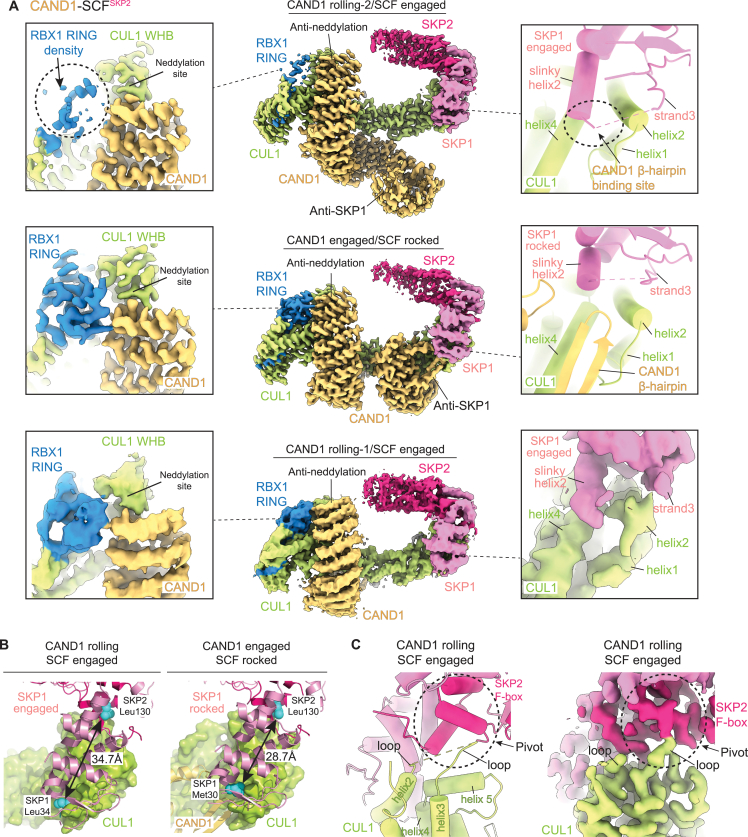
We sought to visualize molecular details and assess functions of specific elements in destabilizing CAND1-SCF complexes. The relative homogeneity of CAND1-SCFSKP2 assemblies, predominantly forming two classes, allowed determining structures of WT proteins in “CAND1 rolling-2/SCF engaged” and “CAND1 engaged/SCF rocked” conformations at 3.5 and 3.0 Å resolution, respectively (Figures 4A and S4; Table S3; Methods S1). We also determined structures of CAND1-SCFSKP2 with SKP1ΔΔ, SCFSKP2 with a mutant CAND1 deleted for the tip of its β-hairpin (Δβ-hairpin), and CAND1-CUL1-RBX1 alone (Figures 4A, S5, and S6). Comparing structures for CUL1 interactions with SKP1 and the Fbp (SKP2) suggests a trajectory toward CAND1-mediated SCF rocking and dissociation.
Figure 4.
Structural steps in CAND1-mediated SKP1-SKP2 dissociation from SCFSKP2
(A) Cryo-EM maps of CAND1-SCFSKP2, CAND1-SCFSKP2 (SKP1ΔΔ), CAND1Δβ-hairpin-SCFSKP2, and CAND1-CUL1-RBX1 in the indicated conformations.
(B) Close up of CUL1-SKP1 interface in the WT CAND1 rolling-2/SCFSKP2 engaged structure (left), and the three-way interface including CAND1 β-hairpin in the CAND1 engaged/SCFSKP2 rocked structure (right).
(C) Overlay of the engaged CUL1-SKP1 and rocked CAND1-CUL1-SKP1 interfaces from WT CAND1-SCFSKP2 structures. The structures were aligned over CUL1’s CR2 and CR3 domains.
(D) Overlay of CUL1-SKP1 interfaces from the engaged and rocked WT CAND1/SCFSKP2 structures and the unique partly rocked conformation of CAND1Δβ-hairpin/SCFSKP2. The structures were aligned over CUL1’s CR2 and CR3 domains.
(E) Overlay of CAND1 engaged/SCFSKP2 rocked (colored) with cryo-EM structure of CAND1-CUL1-RBX1 (gray). Without a bound SKP1-Fbp, the positions of CUL1’s N-terminal helices, and loops are shifted from the conformation in an SCF. Dotted outline: visible parts of loop between CUL1 helices2 and 3.
(F) Close ups showing arrangements of SKP1’s slinky helix2 and ensuing strand3 in the indicated WT or mutant CAND1-SCFSKP2 assemblies.
See also Figure S4.
Figure S4.
High-resolution structures of CAND1-SCFSKP2, related to Figure 4
(A) Cryo-EM maps (center) show various conformations of CAND1-SCFSKP2: CAND1 rolling-2/SCF engaged, CAND1 engaged/SCF rocked, and CAND1 rolling-1/SCF engaged from top to bottom. CAND1-CUL1-RBX1 interface density is shown on the left, and the right shows structures from coordinates for the corresponding CUL1-SKP1 interfaces or density for the lower resolution map.
(B) Distance comparison of the interfaces spanning CUL1 and its bound SKP1-SKP2 F box between CAND1 rolling/SCF engaged and CAND1 engaged/SCF rocked conformations.
(C) Cartoon and density representations of the CUL1-Fbp interface in the CAND1 rolling/SCFSKP2 engaged structure. "Loop" refers to loop between CUL1 helices2 and 3.
Figure S5.
High-resolution structures of mutant CAND1-SCFSKP2, related to Figure 5
(A) Cryo-EM map of CAND1-SCFSKP2 (SKP1ΔΔ) shows predominantly the CAND1 engaged/SCF rocked conformation (center panel). Density corresponding to the CAND1-CUL1-RBX1 interface is shown on the left, and the right shows in cartoon the CAND1-CUL1-SKP1 interface.
(B) Overlay of WT CAND1 engaged/SCFSKP2 rocked (gray) and CAND1-SCFSKP2 (SKP1ΔΔ) (colored) cryo-EM structures.
(C) Cryo-EM maps of CAND1Δβ-hairpin-SCFSKP2 (center panels). One map shows a CAND1 rolling-2/SCF engaged conformation similar to that with WT proteins. The other structure was unique, and intermediate between CAND1 rolling/SCF engaged and CAND1 engaged/SCF rocked conformations. CAND1 is wrapped around the SCF, but without engagement of its deleted β-hairpin, CAND1 is partly engaged. In the absence of CAND1’s β-hairpin, the SCF interface is partly rocked: SKP1’s slinky helix2 is extended into CUL1’s groove (right panel), and CUL1’s CR1 domain is partly rocked about its junction to the CR2 domain.
(D) WT CAND1 rolling-2/SCFSKP2 engaged structure is docked into the cryo-EM map for the corresponding conformation with the CAND1Δβ-hairpin mutant.
(E) Cryo-EM of CAND1Δβ-hairpin-SCFFBXW7 shows CAND1 curled around the SCF, and also various CAND1 rolling conformations.
Figure S6.
High-resolution structures of CAND1-CUL1-RBX1 and CAND1β-hairpin++-SCFSKP2, related to Figure 6
(A) Cryo-EM data for CAND1-CUL1-RBX1 showed only the CAND1 engaged conformation. CAND1-CUL1-RBX1 interface density is shown on the left, and right in cartoon shows the CUL1-CAND1 β-hairpin interface.
(B) Cryo-EM density for CAND1β-hairpin++-SCFSKP2 shows CAND1 rolling-2/SCF engaged and CAND1 partly engaged/SCF partly rocked conformations. CAND1-CUL1-RBX1 interface density is shown on the left, and the right shows in cartoon the CUL1-SKP1 interface. These maps superimpose with those for CAND1Δβ-hairpin-SCFSKP2 shown in Figure S5C. Dotted oval in bottom right panel highlights where density is missing for CAND1 β-hairpin++ mutant.
(C) Representative gels of the in vitro reconstitution assay shown in Figure 6F.
SCF engaged
The CUL1-SKP1-SKP2 interface from the CAND1 rolling-2/SCFSKP2 engaged structure superimposes with that in a prior active SCFSKP2 complex.48 The fully engaged SCF interface traverses ∼35 Å across CUL1's N-terminal CR1 domain (Figure S4B). At one end, SKP1’s helix2 and strand3 straddle CUL1’s helix2 through formation of an intermolecular CUL1-SKP1 hydrophobic core. SKP1's helix2 resembles a “slinky,” differing in number of turns and length between the SCF engaged and rocked conformations (Figures 4B–4D). When engaged, SKP1's slinky helix2 extends 2 1/2 turns and penetrates the groove between CUL1’s helices2 and 4 (which otherwise binds CAND1’s β-hairpin).
In the middle of the SCF interface is a three-way CUL1-SKP1-F box interaction (Figure S4C). Here, side chains from the N terminus of CUL1's helix5 insert between the C terminus of SKP1 and the N terminus of the F box. The interactions with CUL1 continue across the adjacent face of the F box. Residues at the extreme C terminus of the F box domain contact a surface comprising the flexible loop between CUL1’s helices2 and 3 and two turns of CUL1’s helix5 (Figure 4E). While density for this CUL1 loop is limited,14,45,48,50,55,56 it is clearly posed to contact the edge of the F box at the pivot for rocking of the SCF interface (Figure S4C).
SCF rocked
CAND1 fully engages through remodeling of CUL1-SKP1 interactions into a three-way interface including the tip of CAND1's β-hairpin. The groove between CUL1’s helices2 and 4 cradles CAND1’s β-hairpin, not SKP1. SKP1’s slinky helix2 is shortened by a turn, tilted 18°, and perched above rather than inside this CUL1 groove (Figures 4B–4D). C-terminal residues from SKP1’s shortened slinky helix2 contact CAND1's β-hairpin and the extended loop in CAND1's C-terminal HEAT repeat. The N terminus of SKP1's strand3 is translated by ∼3 Å toward the slinky helix to participate in a new hydrophobic core with CUL1 and Pro1070 at the tip of CAND1's β-hairpin (Figures 4B and 4F). Insertion of Met1068 from the opposite side of CAND1's β-hairpin into a snug pocket further shapes the CUL1 groove, which is widened to accommodate CAND1 along with SKP1. Notably, only the CAND1 engaged/SCFSKP2 rocked conformation was observed for the SKP1ΔΔ mutation, suggesting that SKP1 loop removal favors formation of this three-way hydrophobic core with CUL1 and CAND1 (Figures 4F, S5A, and S5B).
The restructuring of CUL1 and SKP1 elements effectively rocks the SKP1-F box surface around the CUL1's N-terminal (CR1 and CR2) domains and places the edge of the F box toward the edge of CUL1 (specifically, helix5 and the loop between helices2 and 3). Rocking decreases contacts between CUL1 and the SKP1-Fbp complex (Figures 4C–4E).
SCF partly rocked with CAND1 partly engaged
Two conformations were observed for CAND1 Δβ-hairpin-bound SCFSKP2 (Figures S5C and S5D). One at 6.8 Å resolution superimposes with the CAND1 rolling-2/SCF engaged conformation. The other, at 3.1 Å resolution, displays features intermediate between the WT conformations. CAND1 Δβ-hairpin curls around the SCF while its mutated anti-SCF domain binds CUL1. Yet, the absence of CAND1's truncated β-hairpin enables SKP1's slinky helix2 to protrude into the CUL1 CR1 domain groove much like when CAND1 is rolling. Nonetheless, the angles between CUL1 domains are altered by CAND1 curling around, as CAND1’s C-terminal HEAT repeat still nestles between CUL1's N-terminal-most domains. As a result, CUL1’s CR1 domain is rotated along the trajectory from the SCF engaged to the SCF rocked conformation (Figure 4D). SKP1’s extended slinky helix2 is also subtly repositioned toward strand3, through slight contraction of SKP1’s hydrophobic core along the progression from an engaged to a rocked SCF (Figure 4F). This SCF configuration is termed “partly rocked.”
CUL1-CAND1 without a bound SKP1-Fbp
The 2.7 Å resolution cryo-EM structure of CAND1-CUL1-RBX1 alone, which mostly superimposed with the crystal structure,40 allowed direct comparison with the CAND1 engaged/SCFSKP2 rocked structure and visualization of conformational differences accompanying dissociation of the SKP1-Fbp (Figures 4A and S6). In CAND1-CUL1-RBX1 alone, the groove between CUL1 helices2 and 4 is relatively narrowed and hydrophobic contacts are condensed around the tip of CAND1’s β-hairpin. Subtle helix rotations propagate across CUL1’s CR1 domain. The greatest differences appear at the distal edge of CUL1, which in CAND1-SCF complexes engages the F box pivot point. In the absence of a SKP1-Fbp, the C terminus of CUL1's helix5 is shifted by 4 Å, and density for CUL1’s F box-binding loop between helices2 and 3 is decreased.
Structural morphing to visualize a trajectory for CAND1-mediated SCF disassembly
Morphing between the high-resolution structures allowed visualizing a potential structural progression for CAND1-dependent destabilization of an SCF (Video S2). Morphing from the WT CAND1 rolling-2 to the unique CAND1 Δβ-hairpin structure showed that CAND1 curling around the SCF partially rocks the SCF region comprising CUL1’s CR1 domain, SKP1, and the F box. Meanwhile, morphing to the WT CAND1 engaged structure showed that the SCF is substantially further rocked by insertion of CAND1's β-hairpin into the CUL1-SKP1 interface. The conformational trajectory increases contacts between CUL1 and CAND1 and commensurately reduces CUL1 contacts to SKP1. At the same time, a new CAND1-CUL1-SKP1 interface is established, and interactions between CUL1 and the F box are maintained. Morphing between the CAND1-SCF rocked structure to that of CAND1-CUL1-RBX1 alone shows subtle rearrangement of CAND1 and CUL1 surfaces upon release of the SKP1-Fbp. It seems this final step would derive entropic benefit from liberation of a SKP1-Fbp. A SKP1-Fbp would form a new SCF interface in reverse, upon encountering CAND1-CUL1-RBX1.
Mechanism of SCF-assisted CAND1 dissociation
Ultimately, production of an active SCF requires CAND1 dissociation. Thus, we compared high-resolution structures to identify determinants shifting the equilibrium from CAND1 engaged to rolling conformations. Comparing structures with WT CAND1 and the Δβ-hairpin mutant suggested a key role for disengagement of CAND1’s β-hairpin. First, since the CAND1 Δβ-hairpin mutant does not oppose the SCF interface, it was unexpected that this would adopt the rolling conformation at all (Figure S5C). Rolling by the CAND1 β-hairpin mutant was even more surprising in light of WT CAND1 exclusively forming the engaged conformation when its β-hairpin was unencumbered, in CAND1-CUL1-RBX1 alone and CAND1-SCFSKP2 with the SKP1ΔΔmutant (Figure S5A). Second, even with CAND1 Δβ-hairpin curled around SCFSKP2, its contacts to SKP1 and CUL1 were diminished compared with WT CAND1. Reexamination of the WT CAND1-CUL1-RBX1 structure revealed that interactions between CAND1’s β-hairpin and CUL1 bury substantial surface area (∼900 Å2). Finally, cryo-EM data for the CAND1 Δβ-hairpin mutant bound to CUL1-RBX1 alone showed rolling conformations even in the absence of a SKP1-Fbp (Figure 5A). Thus, loss of interactions with the β-hairpin triggers CAND1 rolling around CUL1-RBX1.
Figure 5.
Mechanisms underlying CAND1 dissociation from CUL1-RBX1
(A) Cryo-EM maps showing conformations of CAND1Δβ-hairpin-CUL1-RBX1.
(B) Comparing CAND1 engaged and rolling-2 conformations shows shifts in contacts between CAND1’s anti-neddylation domain, RBX1’s RING domain, and CUL1’s WHB, ⍺/β, and 4HB domains.
See also Figure S5.
We also compared the opposite end of the structures, which revealed shifts in contacts between CAND1’s anti-neddylation domain, CUL1, and RBX1. When CAND1 is engaged, its anti-neddylation domain co-clasps four domains of CUL1-RBX1: RBX1's RING, and CUL1's WHB, α/β, and 4HB domains (Figure 5B). In striking contrast, CAND1 in the rolling-2 conformation has released its grip on RBX1’s RING domain, which is poorly visible in the density and presumably relatively dynamic. Here, RBX1’s RING domain contacts to CUL1 are also lost, thereby shifting orientations of CUL1's WHB, α/β, and 4HB domains. Accordingly, their contacts with CAND1’s anti-neddylation domain also shift. In the rolling-2 conformation, the so-called “claw” in CUL1’s α/β-domain ensnares a largely disordered acidic loop between CAND1’s anti-neddylation and central arches (Figure 5B). These contacts are incompatible with the CAND1 rolling-1 orientation, where instead density for RBX1’s RING domain is visible despite the low resolution of the maps. While morphing between maps (shown for SCFFBXW7 in Video S1) suggested that CAND1 rolls around an SCF when its anti-SKP1 domain is obstructed, it seems the rolling-1 and rolling-2 states differently achieve sufficient contact to allow their structural observation. We propose that further rolling reduces these remaining contacts—for example, those with RBX1 since the RING domain is known to rotate to achieve neddylation and ubiquitin E3 ligase activities32,48,50,57,58,59—to ultimately promote dissociation.
The CAND1-CUL1-SKP1 interface mediates systemwide SCF assembly
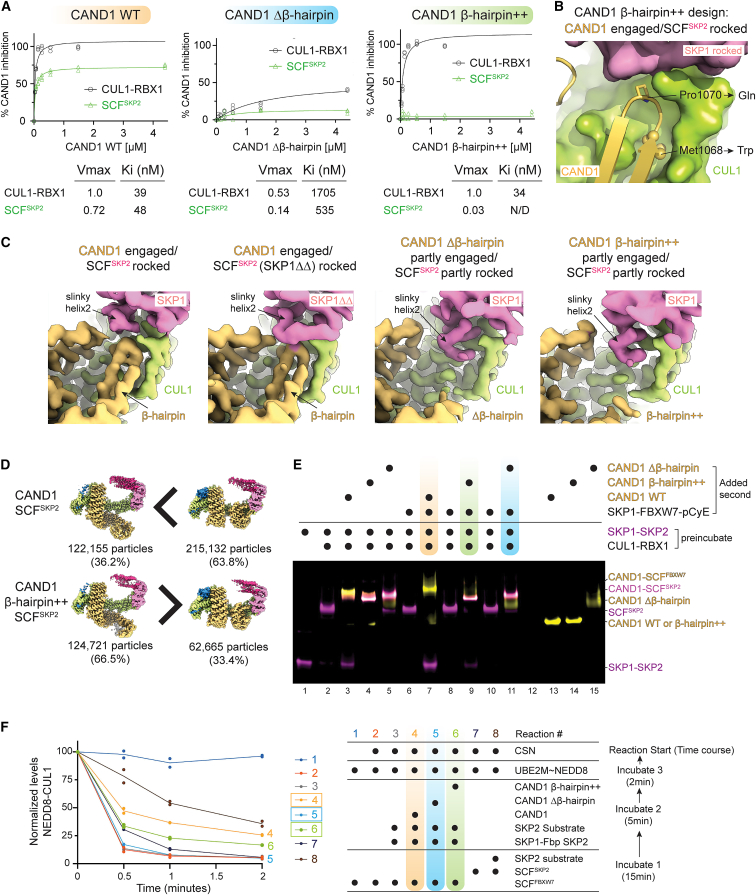
The structural data thus far suggested that CAND1 β-hairpin engagement couples to SCF rocking and destabilization, whereas its disengagement couples CAND1 rolling, dissociation, and SCF E3 ligase activation. These observations led to the prediction that the CAND1Δβ-hairpin mutant should be impaired not only toward an SCF10,40 but also toward CUL1-RBX1 alone. We tested this by quantifying CAND1 inhibition of CUL1-RBX1 neddylation. The CAND1Δβ-hairpin mutant was indeed impaired toward CUL1-RBX1 alone, as well as toward an SCF (Figure 6A). Furthermore, it should be possible to selectively impair CAND1 toward an SCF by impeding the three-way CAND1-CUL1-SKP1 interface. Examining the structures suggested a suitable double mutant: replacing Pro1070 at the tip of CAND1’s β-hairpin with Gln would preserve the key contacts with CUL1 alone but would hinder formation of the interface rocking an SCF. Also, the CAND1-CUL1-SKP1 interface limits the size of the CUL1 pocket accessible to CAND1 M1068; modeling suggested that a M1068W substitution could hamper insertion of CAND1’s β-hairpin between SKP1 and CUL1 (Figure 6B).
Figure 6.
Roles of the CAND1-CUL1-SKP1 interface in SCF disassembly
(A) Role of CAND1 mutants in SCF activation, measured by Michaelis-Menten kinetics. Plots show concentration-dependence of indicated CAND1 variant inhibiting neddylation of CUL1 within either CUL1-RBX1 or an SCFSKP2 complex. n = 3 dots are plotted. Rates were normalized to 100 for CUL1-RBX1 without SKP1-Fbp. Kiapp and Vmax values are listed.
(B) Close up of the WT CAND1-CUL1-SKP1 interface, highlighting the sites of the β-hairpin++ mutations (M1068W and P1070Q).
(C) Close ups showing CAND1-CUL1-SKP1 interfaces in cryo-EM maps of indicated complexes.
(D) Compared with the WT complex, cryo-EM data for CAND1β-hairpin++-SCFSKP2 shows relatively more particles with CAND1 in the rolling conformation.
(E) Nondenaturing gel shift assay monitoring SKP1-FBXW7 displacement of SKP1-SKP2 from SCFSKP2, mediated by WT or β-hairpin mutant versions of CAND1. CAND1 is labeled with TAMRA (yellow), SKP2 by Cy5 (magenta) for visualization. FBXW7 samples include Cyclin E phosphopeptide to improve homogeneity of migration.
(F) Effect of CAND1 β-hairpin mutations on assay for CAND1- and substrate-dependent generation and stabilization of an activated SCF in vitro. The assay reflects a CAND1-dependent switch from SCFFBXW7 to SCFSKP2, for which substrate complex protects CUL1 from deneddylation. SKP2 substrate complex is phosphorylated p27-CKSHS1-CyclinA-CDK2. n = 2 dots are plotted.
See also Figure S6.
Indeed, the CAND1 M1068W P1070Q mutant, which we term “β-hairpin++” retains WT capacity to inhibit neddylation of CUL1-RBX1 alone and is specifically defective toward an SCF (Figure 6A). Cryo-EM structures of CAND1β-hairpin++/SCFSKP2 superimposed with those harboring the CAND1Δβ-hairpin mutant (Figure S6B). CAND1's β-hairpin is not observed, and SKP1’s slinky helix2 extends into CUL1's CR1 domain groove (Figure 6C). We interpret the structures as confirming that insertion of the mutant β-hairpin++ into the CUL1-SKP1 interface is unfavorable. Accordingly, the distribution of particles was also dramatically shifted, from a minority to a majority showing CAND1 rolling (Figure 6D).
To query the role of CAND1’s β-hairpin on complex production in vitro, we first examined SCF-Fbp exchange from CUL1-RBX1 using our nondenaturing gel-based assay (Figure 6E). The CAND1Δβ-hairpin++ mutant was impaired at liberating SKP1-SKP2 from the pre-formed SCFSKP2, and in conversion from a CAND1-SCF complex with SKP2 to a new complex with FBXW7 (Figure 6E, lanes 3 versus 4, 7 versus 9). These activities were not observed at all for the CAND1 Δβ-hairpin mutant (Figure 6E, lanes 5, 11). The effects of the CAND1 mutants showed the same trend in our in vitro assay for “systemwide production” of an active (i.e., neddylated) SCF complex (Figures 6F and S6C). As in Figure 1A, an initial SCF is subject to deneddylation, whereas CAND1-mediated swapping between CUL1-RBX1-bound SKP1-Fbp modules is required for substrate to delay deneddylation of the second. Taken together, the data suggest that CAND1 engaging a rocked SCF is crucial for substrate-regulated switching between which SCFs are assembled or disassembled.
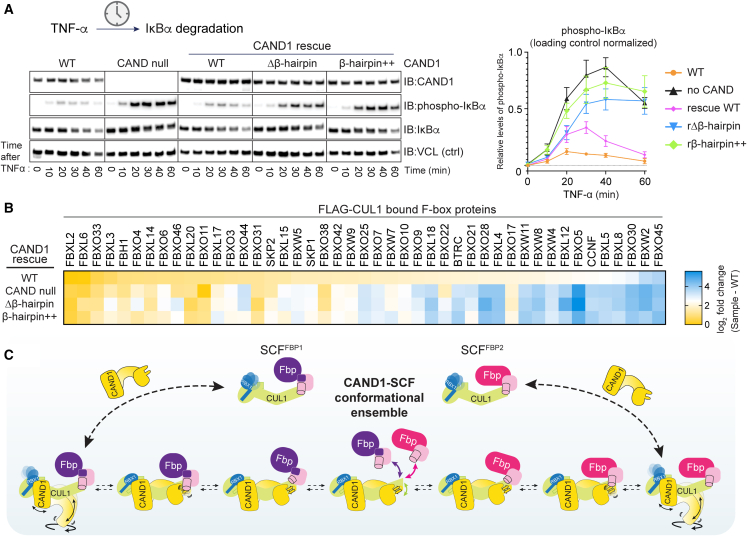
To determine whether the structural mechanism applies to regulation by SCF E3 ligases in cells, TNF-α-stimulated degradation of phosphorylated IκBα, which depends on SCFβ-TRCP, was examined (Figure 7A). A key role of CAND1 in this SCF function was previously established using cells deleted for CAND1 (and the compensatory homolog CAND2), which showed greatly impaired TNF-α-induced reduction of phosphorylated IκBα.10 Full degradation efficiency could be restored in those cells by expressing WT CAND1,10 but we found that the CAND1Δβ-hairpin mutant failed to rescue. Importantly, the β-hairpin++ mutant that specifically probes CAND1 interactions with an SCF is also deficient in TNF-α-stimulated degradation of phosphorylated IκBα.
Figure 7.
CAND1-CUL1-SKP1 interface mediates systemwide SCF assembly
(A) Expression of CAND1 β-hairpin mutants in CAND null 293 cells fails to rescue TNF-α-stimulated degradation of phosphorylated IκBα. Immunoblots (IBs) detect phospho- or total IκBα, CAND1, or vinculin (VCL) loading control at indicated time points after TNF-α treatment. Chemiluminescent signal with adjusted brightness and contrast is shown. Relative levels of phosphorylated IκBα (normalized by loading control) are plotted on the right (n = 3, error bars SEM).
(B) CAND1 β-hairpin mutants fail to rescue WT CAND1-dependent SCF steady-state repertoire in cells. Endogenous FLAG-tagged CUL1 was immunoprecipitated in the presence of recombinant GST-RBX1-CUL1 (“sponge” soaking up free CAND1 and Fbps to suppress exchange), MLN4924 and CSN5i-3, followed by mass spectrometry to monitor the SCF proteome in CAND-null 293 cells stably expressing WT CAND1 (top row) or the indicated CAND1 mutants (bottom two rows). Heatmap shows log2-fold difference for CUL1 association of each Fbp relative to WT cells.
(C) Model of conformational ensemble mechanism of CAND1-mediated SCF disassembly and assembly.
See also Figure S7.
We further tested effects of the mutations on CAND1’s role in determining the cellular repertoire of SCF complexes at steady state. In a previous study, SCF complexes were assessed in a cell line containing endogenously tagged 3xFLAG-CUL1.9 Deshaies and colleagues showed that endogenous CRL repertoires can be queried when cells are lysed in a manner preventing post-lysis CAND1-mediated exchange.9,11 We quantified CUL1-associated proteins upon WT or mutant CAND1 rescue of the same parental cell line through anti-FLAG immunoprecipitation followed by mass spectrometry (Figure 7B; Table S4). This allowed assessing effects of the CAND1 mutants on CUL1 occupancy levels for 44 Fbps and SKP1. Expression of WT CAND1 restored the cellular SCF repertoire of the CAND knockout cells to resemble that of the corresponding parental cells. Although incomplete recovery was thought to result from adaptive changes in Fbp expression levels in CAND-null cells,9 our results agree with the prior work indicating CAND1-dependent SCF complex formation (Figure S7A). Unlike WT CAND1, expression of the β-hairpin mutants had little impact on the cellular SCF repertoire (Figures 7B, S7A, and S7B). Overall, the dramatic impact of the β-hairpin++ mutations is consistent with the structurally observed CAND1 engagement of SCFs—and CAND1 promoting rocking of the SCF interface—regulating SCF complex formation at a systemwide level in cells.
Figure S7.
Roles of CAND1 across cullin-RING ligase systems, related to Figure 7
(A) Heatmap showing percentages of FLAG-CUL1-associated versus total Fbps at steady state from WT or CAND-null cells endogenously expressing FLAG-tagged CUL1,9 or the CAND-null cells stably expressing WT CAND1 or the indicated β-hairpin mutants.
(B) Immunoblotting blotting for components of SCFBTRC at steady state, which align with the proteomics results shown in Figure 7B. Chemiluminescent signal with adjusted brightness and contrast is shown.
(C) Docking of SKP1-SKP2 crystal structure46 onto CAND1-SCFFBXW7 rolling-2c conformation shows potential incompatibility between SKP2 and CAND1’s anti-neddylation domain in this orientation.
(D) Cryo-EM structures show addition of substrate complex (phosphorylated p27-CKSHS1-CyclinA-CDK2) alters distribution of CAND1-SCFSKP2 conformations, including SCFSKP2 complexes free of CAND1.
(E) Overlay of various crystal structures of CUL-substrate receptor complexes with the CAND1 engaged/SCFSKP2 rocked structure. Structures were superimposed over CUL CR1 domains. The modeling shows clashing of CAND1’s β-hairpin with CUL2,56 CUL3,62 and CUL563 substrate receptors, suggesting that their disassembly and assembly mechanisms may parallel that described herein for SCFs.
(F) Alignment of CAND1-CUL4-RBX114 (left) and cryo-EM structure of CAND1-CUL1-RBX1 (right) on CUL4-bound DDB1,55 superimposed over the CUL N-terminal CR1 domains, highlighting positions of CAND1’s β-hairpin and C-terminal HEAT repeat loop.
Discussion
Although CAND1 was shown to impart plasticity to the SCF system nearly a decade ago,6,7,8,9 how CAND1 could destabilize an SCF, and how a SKP1-Fbp could destabilize CAND1 bound to CUL1-RBX1 remained unknown, as these biochemical properties conflicted with the published structures.40,45,47,48 The cryo-EM structures of CAND1-bound complexes presented herein reveal the structural trajectory for CAND1-stimulated disassembly of one SCF and reciprocal formation of a new one, while also suggesting how and why SCF complex formation occurs at a systemwide level in cells.
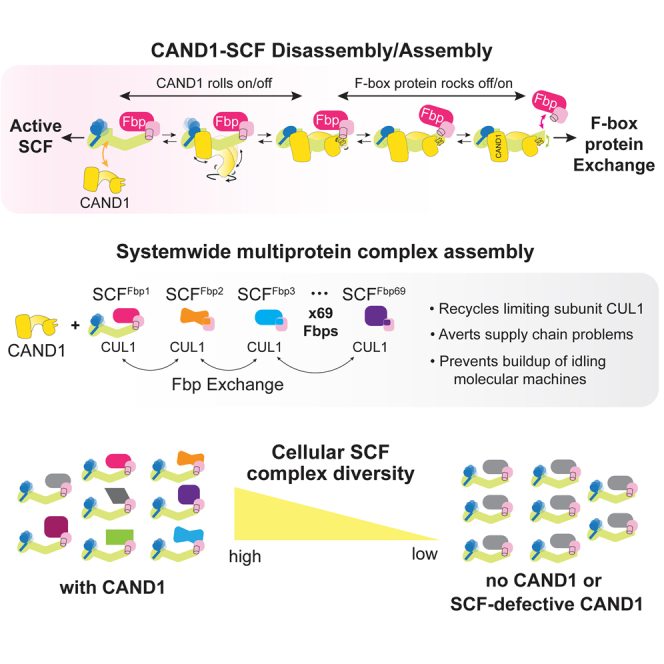
The structural data show that CAND1-SCF complexes do not form a singular high-energy intermediate as previously hypothesized.6 Instead, CAND1 and SCFs bind each other in an ensemble of conformations. Ordering the conformations based on mutational effects suggests a rock-and-roll mechanism of CAND1-mediated SCF disassembly and assembly: at one extreme, CAND1 rocks and dissociates the interface between CUL1 and a SKP1-Fbp; meanwhile, reverse formation of a stable SCF interface causes CAND1 to roll around and ultimately dissociate from an SCF (Videos S1 and S2).
Furthermore, our data collectively indicate that CAND1 exerts regulation through binding SCFs rather than binding a cullin-RING complex alone. The mutant CAND1 specifically impaired at rocking the SCF interface is correspondingly impaired at promoting SKP1-Fbp dissociation (Figures 6 and S6), substrate-dependent switching of Fbps incorporated into active SCFs in vitro (Figure 6F), a cellular SCF degradation response to a cytokine signal (Figure 7A), and establishing the cellular steady-state repertoire of SCF complexes (Figure 7B).
The model that emerges is that within the population of CAND1-SCF complexes, some allosterically decrease CUL1-RBX1 binding to CAND1 while others decrease CUL1-RBX1 binding to the SKP1-Fbp (Figure 7C). No individual destabilized CAND1-SCF structure is sufficient; it is the collection that allows back-and-forth switching of SKP1-Fbps assembled into SCF complexes. From an energetic perspective, the collection of affinities across individual complexes within the ensemble lowers CUL1-RBX1 affinity for both CAND1 and the SKP1-Fbp (Figure 1C). Furthermore, pairwise comparisons between different CAND1-SCF states show that they vary both by loss as well as gain of intermolecular interactions (Figures 4 and 5). Thus, the energetic barrier to transitions between the conformations seems lower than for wholesale dissociation of an SCF or a CAND1-CUL1-RBX1 complex.
The CAND1-SCF conformational ensemble is remarkable both in its targeting of SKP1-CUL1 interactions common to all SCFs, and its malleability allowing biasing by different Fbps and substrates (Tables S1–S3; Methods S1), and presumably other factors. The role of the F box explains previous findings that SKP1 alone is insufficient to trigger CUL1-RBX1 release from CAND1 and allow neddylation; these require a SKP1-Fbp.6,7,8,34,40 Different F box sequences may influence inherent preference to form the engaged or rocked SCF interface in the presence of CAND1, which likely contributes to variation among Fbps in their reliance on CAND1 to access CUL1 in vivo.9,42 This may be particularly important for Fbps present at low levels, such as FBXW7, for which we observed a preponderance of SCF engaged conformations (Figure 2A). The Fbp’s substrate-binding domain could also influence the conformational equilibrium; docking the SKP1-SKP2 crystal structure showed incompatibility with some conformations observed for CAND1-SCFFBXW7 (Figure S7C). Finally, although it is currently thought that substrate predominantly exerts regulation by maintaining neddylation through inhibiting CSN24,25 (Figures 1A and 1B), our data raise the possibility that substrate binding could also stabilize an unneddylated SCF encountering CAND1. Substrate-bound SCFSKP2 assemblies with CAND1 showed a distinct distribution of structures (Figure S7D; Table S2; Methods S1), presumably due to interaction partners influencing conformational preferences of the CUL1-SKP1-F box portion of the complex. The relatively decreased proportion of complexes in the CAND1 engaged/SCF rocked conformation, and the observation of CAND1-free SCFSKP2 complexes suggests yet an additional means by which substrates may stabilize their SCFs.
It seems likely that similar mechanisms generally underlie CAND-mediated reshuffling of CRLs (Figure S7E). We speculate that the remarkable structural plasticity of CAND1 observed herein, together with inherent malleability of CRL structures, could enable a plethora of conformations, perhaps specific for distinct cullins. Interestingly, modeling CAND1 bound to CUL4-RBX1 and its associated DDB1-DCAF substrate-binding module14,55 raises the possibility that the extended loop in CAND1’s C-terminal HEAT repeat could play roles like the β-hairpin in CRL disassembly and assembly (Figure S7F). CAND1’s ortholog CAND2 likely exerts regulation in a structurally similar manner, perhaps in distinct cell types.
What are the advantages of a conformational ensemble mechanism of subunit exchange? When combined with the other components of the system—ongoing neddylation and deneddylation—the CAND1-SCF conformational ensemble transforms a massive number of architecturally related pre-assembled SCF molecular machines into a dynamic system: some SCFs dissociating and others associating (Figure 7C) and subjected to ongoing neddylation and deneddylation until shielded by substrate from deneddylation and disassembly. Thus, this systemwide multiprotein complex assembly recycles a limiting component from idling complexes to fuel mixing and matching of parts and transient stabilization of the subset of complexes needed at any given time. Our data also raise the question of whether similar mechanistic principles apply to other systems wherein a common core binds interchangeable specificity factors. Such complexes function across the cell, from the surface to the nucleus. Examples beyond the massive CRL superfamily include the emerging cohort of solute carriers comprising a variable transport module and a common heavy-chain module (SLC3A1 or SLC3A2),60 and the family of heteromeric nuclear hormone receptors containing the common RXR (retinoid X receptor) subunit.61 It is conceptually appealing to consider potential for their systemwide disassembly and assembly, as this could contribute to metabolic crosstalk, although future studies will be required to determine if such regulation controls these or other multiprotein complexes. Nonetheless, our studies of SCF E3 ligases provide a mechanism for systemwide multiprotein complex assembly that averts supply chain problems, obviates need for producing new parts, prevents buildup of idling and potentially detrimental molecular machines, and rapidly establishes degradation pathways needed for cellular regulation.
Limitations of the study
One limitation of cryo-EM is that the collection of structures presumably represents the lower energy states in a continuum. However, it seems likely that higher energy intermediates would be too conformationally heterogeneous or poorly populated for structure elucidation. Moreover, smaller and dynamic components, such as isolated CAND1 or SKP1-Fbps used herein, evaded detection. Thus, although the structural snapshots described here, together with mutational effects in biochemical and cellular assays, suggest a trajectory for CAND1-mediated SCF disassembly and assembly, visualizing intervening steps will require novel methods.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| NFKBIA | Cell Signaling Technology | Cat# 4814S, RRID: AB_390781 |
| Phospho-NFKBIA (Ser32/36) | Cell Signaling Technology | Cat# 9246S, RRID: AB_2267145 |
| CAND1 | Cell Signaling Technology | Cat# 8759S, RRID: AB_11178669 |
| Vinculin | Abcam | Cat# ab129002, RRID: AB_11144129 |
| CUL1 | Santa Cruz Biotechnology | Cat #sc–17775, RRID: AB_627325 |
| SKP1 | Cell Signaling Technology | Cat #2156, RRID: AB_2270271 |
| BTRC | Cell Signaling Technology | Cat #4394, RRID: AB_10545763 |
| goat-anti-rabbit-HRP secondary antibody | Invitrogen | Cat# 31460, RRID: AB_228341 |
| goat-anti-mouse-HRP secondary antibody | Invitrogen | Cat# 31430, RRID: AB_228307 |
| Anti-FLAG® M2 Magnetic Beads | Sigma-Aldrich | Cat# M8823, RRID: AB_2637089 |
| PureCube Glutathione MagBeads | Cube Biotech | Cat# 32205 |
| Chemicals, peptides, and recombinant proteins | ||
| cOmplete™, EDTA-free Protease Inhibitor Cocktail | Roche | Cat# 11873580001 |
| Puromycin | Alfa Aesar | Cat#J67236.XF |
| Hygromycin B | GIBCO | Cat# 10687010 |
| MLN4924 | MedChemExpress | Cat# HY-70062 |
| CSN5i-3 | MedChemExpress | Cat# HY-112134 |
| Recombinant Human TNF-α | PeproTech | Cat# 300-01A |
| Fluorescein-5-Maleimide | Thermo Fisher | Cat#F150 |
| Alexa Fluor 674 Maleimide C2 | Thermo Fisher | Cat#A20347 |
| Cop9 Signalosome | Enzo | Cat#BML-PW-9425-0020 |
| Fetal Bovine Serum | GIBCO | Cat#10437028 |
| DMEM (1x), high glucose | GIBCO | Cat#11960044 |
| Sodium pyruvate | GIBCO | Cat#11360039 |
| Penicillin Streptomycin | GIBCO | Cat#15140122 |
| GlutaMAX™ | GIBCO | Cat#35050038 |
| Fetal Bovine Serum | GIBCO | Cat#10437028 |
| Lipofectamine 3000 | Invitrogen | Cat# L3000015 |
| ReproSil-Pur C18-AQ 1.9 μm resin | Dr. Maisch GmbH | Cat# r119.aq. |
| Lenti-X GoStix Plus | Takara | Cat# 631280 |
| DMEM (1x), high glucose | GIBCO | Cat#11960044 |
| Sodium pyruvate | GIBCO | Cat#11360039 |
| Trypsin, porcine, Proteomics Grade | Sigma Aldrich | T6567 |
| Lysyl Endopeptidase®, Mass Spectrometry Grade (Lys-C) | FUJIFILM Wako | 125-05061 |
| Ex-cell 420 medium | Sigma Aldrich | Cat#14420C |
| Deposited data | ||
| CAND1-CUL1-RBX1 | This study | EMD-14561, PDB:7Z8R |
| CAND1-SCFSKP2 CAND1 engaged SCF rocked |
This study | EMD-14563, PDB:7Z8T |
| CAND1-SCFSKP2 CAND1 rolling-2 SCF engaged |
This study | EMD-14594, PDB:7ZBW |
| CAND1-SCFSKP2 CAND1 rolling-1 SCF engaged |
This study | EMD-16582 |
| CAND1-SCFSKP2 (SKP1ΔΔ) CAND1 engaged SCF rocked |
This study | EMD-14595, PDB:7Z8V |
| CAND1 Δβ-hairpin-SCFSKP2 CAND1 partly engaged SCF partly rocked |
This study | EMD-14597, PDB:7ZBZ |
| CAND1 Δβ-hairpin-SCFSKP2 CAND1 rolling SCF engaged |
This study | EMD-14598 |
| CAND1 β-hairpin++-SCFSKP2 CAND1 partly engaged SCF partly rocked |
This study | EMD-16576, PDB:8CDK |
| CAND1 β-hairpin++-SCFSKP2 CAND1 rolling SCF engaged |
This study | EMD-16575, PDB:8CDJ |
| CAND1-SCFFBXW7 No CAND1 SCF engaged |
This study | EMD-16579 |
| CAND1-SCFFBXW7 CAND1 rolling-1 SCF engaged |
This study | EMD-16580 |
| CAND1-SCFFBXW7 CAND1 rolling-2 SCF engaged |
This study | EMD-16581 |
| CAND1-SCFSKP2 CAND1 rolling-2 SCF engaged |
This study | EMD-16583 |
| CAND1-SCFSKP2 CAND1 engaged SCF rocked |
This study | EMD-16584 |
| CAND1-SCFFBXO6 No CAND1 SCF engaged |
This study | EMD-16585 |
| CAND1-SCFFBXO6 CAND1 rolling-1 SCF engaged |
This study | EMD-16586 |
| CAND1-SCFFBXO6 CAND1 rolling-2 SCF engaged |
This study | EMD-16587 |
| CAND1-SCFFBXO6 CAND1 engaged SCF rocked |
This study | EMD-16588 |
| CAND1-SCFFBXO6 CAND1 engaged no SKP1-Fbp |
This study | EMD-16589 |
| CAND1(2x)-SCFFBXW7 No CAND1 SCF engaged |
This study | EMD-14615 |
| CAND1(2x)-SCFFBXW7 CAND1 rolling-1 SCF engaged |
This study | EMD-14616 |
| CAND1(2x)-SCFFBXW7 CAND1 rolling-2 SCF engaged |
This study | EMD-16577 |
| CAND1(2x)-SCFFBXW7 CAND1 engaged SCF rocked |
This study | EMD-16578 |
| CAND1-SCFFBXW7 CAND1 rolling-2a SCF engaged |
This study | EMD-14603 |
| CAND1-SCFFBXW7 CAND1 rolling-2b SCF engaged |
This study | EMD-14604 |
| CAND1-SCFFBXW7 CAND1 rolling-2c SCF engaged |
This study | EMD-14601 |
| CAND1-SCFFBXW7 CAND1 rolling-2d SCF engaged |
This study | EMD-14602 |
| CAND1-SCFFBXW7 CAND1 rolling-1a SCF engaged |
This study | EMD-14599 |
| CAND1-SCFFBXW7 CAND1 rolling-1b SCF engaged |
This study | EMD-14600 |
| CAND1-SCFFBXW7 Little CAND1 SCF engaged |
This study | EMD-14605 |
| CAND1-SCFFBXW7 no CAND1-1 SCF engaged |
This study | EMD-14606 |
| CAND1-SCFFBXW7 no CAND1-2 SCF engaged |
This study | EMD-14607 |
| CAND1-SCFFBXW7 (SKP1ΔΔ) CAND1 engaged no SKP1-Fbp |
This study | EMD-14608 |
| CAND1-SCFFBXW7 (SKP1ΔΔ) CAND1 engaged SCF rocked |
This study | EMD-14609 |
| CAND1 Δβ-hairpin-SCFFBXW7 CAND1 partly engaged SCF partly rocked |
This study | EMD-14610 |
| CAND1 Δβ-hairpin-SCFFBXW7 CAND1 rolling-2a SCF engaged |
This study | EMD-14614 |
| CAND1 Δβ-hairpin-SCFFBXW7 CAND1 rolling-2b SCF engaged |
This study | EMD-14613 |
| CAND1 Δβ-hairpin-SCFFBXW7 CAND1 rolling-2c SCF engaged |
This study | EMD-14612 |
| CAND1 Δβ-hairpin-SCFFBXW7 CAND1 rolling-2d SCF engaged |
This study | EMD-14611 |
| CAND1-SCFSKP2+p27-CKSHS1-CycA-CDK2 CAND1 engaged SCF rocked |
This study | EMD-16617 |
| CAND1-SCFSKP2+p27-CKSHS1-CycA-CDK2 CAND1 rolling SCF engaged |
This study | EMD-16621 |
| CAND1-SCFSKP2+p27-CKSHS1 CAND1 engaged SCF rocked |
This study | EMD-16622 |
| CAND1-SCFSKP2+p27-CKSHS1 CAND1 rolling SCF engaged |
This study | EMD-16623 |
| CAND1-SCFSKP2+p27-CKSHS1-CycA-CDK2 No CAND1 SCF engaged |
This study | EMD-16625 |
| CAND1 Δβ-hairpin-CUL1-RBX1, CAND1 partly engaged | This study | EMD-16764 |
| CAND1 Δβ-hairpin-CUL1-RBX1, CAND1 rolling-1 | This study | EMD-16765 |
| CAND1 Δβ-hairpin-CUL1-RBX1, CAND1 rolling-2 | This study | EMD-16766 |
| Mass spectrometry data | This study | Pride database: PXD038661 |
| Raw image data | This study | Mendeley Data: https://doi.org/10.17632/9c6zfpsxfh.1 |
| Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF Ubiquitin Ligase Complex | Zheng et al.45 | PDB: 1LDK |
| Crystal Structure of The Cand1-Cul1-Roc1 Complex | Goldenberg et al.40 | PDB: 1U6G |
| Ubiquitin Ligation to substrate by a cullin-RING E3 ligase at 3.7A resolution: NEDD8-CUL1-RBX1 N98R-SKP1-monomeric b-TRCP1dD-IkBa-UB∼UBE2D2 | Baek et al.50 | PDB: 6TTU |
| Ubiquitin ligation to F-box protein substrates by SCF-RBR E3-E3 super-assembly: NEDD8-CUL1-RBX1-SKP1-SKP2-CKSHS1-Cyclin A-CDK2-p27-UBE2L3∼Ub∼ARIH1. Transition State 1 | Horn-Ghetko et al.48 | PDB: 7B5L |
| Crystal structure of the Cul2-Rbx1-EloBC-VHL ubiquitin ligase complex | Cardote et al.56 | PDB: 5N4W |
| Crystal structure of KLHL3/Cul3 complex | Ji and Privé62 | PDB: 4HXI |
| Structure of the SOCS2-Elongin BC complex bound to an N-terminal fragment of Cullin5 | Kim et al.63 | PDB: 4JGH |
| Structure of the CAND1-CUL4B-RBX1 complex | Fischer et al.14 | PDB: 4A0C |
| Structure of DDB1-DDB2-CUL4A-RBX1 bound to A 12 BP abasic site containing DNA-duplex | Fischer et al.14 | PDB: 4A0K |
| Experimental models: Cell lines | ||
| Human: HEK 293T | DSMZ | 293T ACC 635 |
| Human: HEK293 Flp-In T-Rex 3xFLAGCUL1 | Liu et al.10 | N/A |
| Human: HEK293 Flp-In T-Rex – Cand1/2 double knockout & 3xFLAGCUL1 | Liu et al.10 | N/A |
| Human: HEK293 Flp-In T-Rex – Cand1/2 double knockout & 3xFLAGCUL1, exogenous CAND1 WT | This study | N/A |
| Human: HEK293 Flp-In T-Rex – Cand1/2 double knockout & 3xFLAGCUL1, exogenous CAND1 Δβ-hairpin | This study | N/A |
| Human: HEK293 Flp-In T-Rex – Cand1/2 double knockout & 3xFLAGCUL1, exogenous CAND1 β-hairpin++ | This study | N/A |
| Experimental models: Organisms/strains | ||
| E. coli BL21 Gold (DE3) | Thermo Fisher | Cat#50-125-348 |
| Sf9 Insect cells | Thermo Fisher | Cat#11496015 |
| High-Five Insect cells | Thermo Fisher | Cat#B85502 |
| Recombinant DNA | ||
| pGEX4T1 GST-Thrombin-UBE2D2 | Duda et al.57 | N/A |
| pGEX4T1 GST-Thrombin-UBE2M | Duda et al.57 | N/A |
| pGEX4T1 GST-Thrombin-APPBP1-UBA3 | Duda et al.57 | N/A |
| pGEX4T1 GST-Thrombin-NEDD8 (S>C) | Scott et al.32 | N/A |
| pGEX2TK GST-Thrombin-UB (S>C) | Scott et al.32 | N/A |
| pGEX4T1 GST-TEV-UBE2R | Duda et al.57 | N/A |
| pGEX4T1 GST-TEV-UBE2L3 | Duda et al.64 | N/A |
| pGEX4T1 GST-TEV-ARIH1 | Duda et al.64 | N/A |
| pGEX4T1 GST-TEV-CAND1 | Duda et al.57 | N/A |
| pGEX4T1 GST-TEV-CAND1 (M1068W P1070Q) | This study | N/A |
| pGEX4T1 GST-TEV-CAND1 (Δ1067-1072+Gly) | This study | N/A |
| pGEX4T1 GST-TEV-FBXW7 (263-C)-SKP1 | Duda et al.57 | N/A |
| pGEX4T1 GST-TEV-FBXW7 (263-C)-SKP1ΔΔ | Duda et al.57 | N/A |
| pGEX4T1 GST-TEV-FBXW7 (263-C, pivot mutant D317M N318P L319D)-SKP1 | This study | N/A |
| pGEX4T1 GST-TEV-SKP2-SKP1 | Duda et al.57 | N/A |
| pGEX4T1 GST-TEV-SKP2-SKP1ΔΔ | Duda et al.57 | N/A |
| pACEBacDual SKP1 2xStrep-TEV-FBXO6 | This study | N/A |
| pGEX4T1 GST-TEV-CyclinA | Scott et al.65 | N/A |
| pGEX4T1 GST-TEV-CDK2/Civ1 | Scott et al.65 | N/A |
| pGEX4T1 GST-Thrombin CKSHS1 (5-73) | Scott et al.65 | N/A |
| pGEX4T1 GST-TEV-p27 (22-106) | Horn-Ghetko et al.48 | N/A |
| pRSFDUET His-TEV-p27 (S10A K-) | Scott et al.65 | N/A |
| pLIB | Weissmann et al.66 | N/A |
| pCDNA5 FRT | Thermo Fisher | Cat# V601020 |
| pSPAX.2 | Didier Trono | RRID: Addgene_12260 |
| pMD2.G | Didier Trono | RRID: Addgene_12259 |
| pHR’CMV | Xu and Blackburn67 | RRID: Addgene_23135 |
| pLIB GST-TEV-UBA1 | Baek et al.50 | N/A |
| pLIB CUL1 | Baek et al.50 | N/A |
| pLIB GST-TEV-RBX1 (5-C) | Baek et al.50 | N/A |
| pFastbac SKP1 | Scott et al.65 | N/A |
| pFastbac His-TEV-FBXW11 | Scott et al.65 | N/A |
| pFastbac His-TEV-CyclinE | Scott et al.65 | N/A |
| pFastbac CDK2 | Scott et al.65 | N/A |
| pHR’CMV CAND1 | This study | N/A |
| pHR’CMV CAND1 (M1068W P1070Q) | This study | N/A |
| pHR’CMV CAND1 (Δ1067-1072+Gly) | This study | N/A |
| Software and algorithms | ||
| Serial-EM v3.8.0-b5 | N/A | https://bio3d.colorado.edu/SerialEM/ |
| FEI EPU v2.7.0 | Thermo Scientific | https://www.thermofisher.com/ |
| Typhoon FLA Phosphoimager | GE | https://www.cytivalifesciences.com/ |
| ImageQuant | Cytiva | https://www.cytivalifesciences.com/ |
| ImageJ (Fiji) 1.53t | Schindelin et al.68 | https://imagej.net |
| Prism v5 and 9; | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| RELION v3.1 | Zivanov et al.69 | https://www3.mrc-lmb.cam.ac.uk/relion |
| Gautomatch v0.56 | Kai Zhang | https://www2.mrc-lmb.cam.ac.uk/download/gautomatch-056/ |
| CTFFIND v4.1 | Rohou and Grigorieff70 | https://grigoriefflab.umassmed.edu/ctffind4 |
| GCTF v1.06 | Zhang71 | https://www2.mrc-lmb.cam.ac.uk/download/gctf/ |
| MotionCor2 v1.1 | Zheng et al.72 | https://msg.ucsf.edu/em/software/index.html |
| DeepEMhancer | Sanchez-Garcia et al.73 | https://github.com/rsanchezgarc/deepEMhancer |
| Focus | Biyani et al.74 | https://lbem-focus.epfl.ch/documentation.php |
| Chimera v1.11.2 | Pettersen et al.75 | https://www.cgl.ucsf.edu/chimera/ |
| ChimeraX v1.2 | Goddard et al.76 | https://www.rbvi.ucsf.edu/chimerax/ |
| PyMol v2.3.3 | Schrodinger, LLC | https://pymol.org/2/ |
| COOT v0.8.9.1 | Emsley et al.77 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| Phenix.refine v1.19.2 | Afonine et al.78 | https://www.phenix-online.org/ |
| Spectronaut® v16.1.220730.53000 | Biognosys | https://biognosys.com/software/spectronaut/ |
| Perseus V1.6.7.0 | Tyanova | https://maxquant.net/perseus/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Brenda A. Schulman (Schulman@biochem.mpg.de).
Materials availability
All unique reagents generated in this study will be available from the lead contact upon request.
Experimental model and subject details
Cell culture conditions
Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum, 4 mM GlutaMAX™, 1 mM sodium pyruvate, 100 units/ml penicillin and 100 μg/ml streptomycin at 37˚C and 5% CO2. All cells were periodically tested for mycoplasma using MycoAlert® kits. Trichoplusnia ni High-Five and Sf9 insect cells were cultured in Ex-cell 420 medium. BL21 Gold (DE3) E. coli bacterial cells were cultured in LB or TB medium.
Generation of stable cell lines
All cells used in this study were ultimately derived from HEK-293 cells, which are of human origin and female. Flp-In™ T-REx™ 293 3xFLAGCul1 WT and CAND null cells (CAND1 and CAND2 knockout) were a gift from Xing Liu.9,10 Lentivirus for WT or mutant CAND1 constructs was generated by co-transfection with packaging (psPAX2) and enveloping (pMD.2G) plasmids into HEK293T cells using Lipofectamine 3000. Supernatants containing viral particles were harvested after 72h and the relative viral titer determined using Lenti-X GoStix. Flp-In™ T-REx™ 293 3xFLAGCUL1 CAND null cells were infected with 5 μl of virus and transferred to T75 flasks in media containing 100 μg/ml hygromycin B after 48h. After 10 days, hygromycin B resistant colonies were isolated and screened for expression of WT or mutant CAND1 by immunoblot. Cells showing CAND1 expression approaching endogenous levels were chosen for further experiments.
Method details
Cloning, Protein Expression and Purification
All proteins are of human origin. Wild type CUL1, RBX15-C, SKP1, FBXW11, FBXW7, and UBA1 were cloned into pFastBac vectors. GST-TEV-UBA1, GST-TEV-RBX15-C and CUL1, His-TEV-FBXW11 and SKP1, and His-TEV-CyclinE and CDK2 were expressed individually or co-expressed in Trichoplusnia ni High-Five insect cells by coinfection with baculoviruses prepared using SF9 insect cells. GST-TEV-CAND1 and CAND1 mutants β-hairpin++ (M1068W P1070Q), and Δβ-hairpin (Δ1067-1072+Gly) were expressed in BL21 Gold (DE3) E. coli. These proteins were purified by either glutathione or nickel affinity chromatography, cleaved with TEV protease, and further purified by ion-exchange and size exclusion chromatography. SKP1-SKP2, SKP1ΔΔ-SKP2 (SKP1 with two internal truncations in residues 38-43 and 71-82), SKP1-FBXW7 (263-C), SKP1ΔΔ-FBXW7, SKP1-FBXW7 (pivot mutant, D317M N318P L319D, on 263-C) were co-expressed in BL21 Gold (DE3) E. coli as GST-TEV-F-box protein and untagged SKP1. Proteins were purified by glutathione chromatography, cleaved with TEV protease, and further purified by ion-exchange and size exclusion chromatography. Ubiquitin (UB), UBE2R2, UBE2D2, UBE2L3, ARIH1, NEDD8, APPBP1-UBA3, and CKSHS1 were expressed in BL21 Gold (DE3) E. coli as GST-Thrombin (UB, NEDD8, APPBP1-UBA3, and CKSHS15-73) or GST-TEV (UBE2R2, UBE2D2, UBE2L3, ARIH1) fusion proteins. UB, NEDD8, and CKSHS15-73 were purified by glutathione chromatography, cleaved with Thrombin protease, and further purified by size exclusion chromatography. APPBP1-UBA3, UBE2R2, UBE2D2, UBE2L3, and ARIH1) were purified by glutathione chromatography, cleaved with Thrombin or TEV protease, and further purified by ion-exchange and size exclusion chromatography. UBE2M-His was expressed in Trichoplusnia ni High-Five insect cells. Protein was purified by nickel affinity chromatography followed by ion-exchange and size exclusion chromatography. CyclinA-CDK2 and phosphop27 used in biochemical assays, and CyclinA-CDK2 and p27 N-terminal kinase inhibitory domain (residues 22-106) used in cryo-EM were purified as previously described.48,65 Briefly, CyclinA and CDK2 (phosphorylated by co-expressed S. cerevisiae Cak1p) were expressed separately in E. coli BL21 Gold (DE3) cells as GST-TEV fusion proteins. Fusion proteins were purified by glutathione affinity chromatography. Stoichiometric amounts of GST-TEV-CyclinA and GST-TEV-CDK2 were mixed and liberated from GST by TEV cleavage overnight during extensive dialysis at 4°C. Cleavage reactions were passed back over a glutathione affinity resin to remove free GST and any remaining uncleaved GST-fusion protein. The CyclinA-CDK2 complex collected in the flow fraction was further purified by ion exchange and size exclusion chromatography. His-p27 (containing additional S10A/K- mutations that can be phosphorylated on T187 by CDK2) was expressed in E. coli BL21 Gold (DE3) cells. Protein was purified from cell lysates by Ni affinity chromatography. Bound proteins were eluted from the Ni resin with 250mM imidazole and immediately subjected to desalting over a PD-10 column (GE Healthcare) into 25 mM HEPES, 200 mM NaCl, 1 mM DTT, pH 7.5 as long term exposure to high imidazole levels caused the protein to precipitate from solution. The 6X His tag was liberated by cleavage with TEV overnight at 4°C. Cleavage products were further purified by size exclusion chromatography. WT and mutant CAND1 plasmids for generating rescue cell lines were cloned into a pHR’CMV vector obtained from Addgene plasmid#23135.
CyclinE phosphopeptide sequence used for cryo-EM and native gel electrophoresis: KAMLSEQNRASPLPSGLL(pT)PPQ(pS)GRRASY
p27 C-terminal phosphopeptide sequence used for cryo-EM: CNKRANRTEENVSDGSPNAGSVEQ(pT)PKKPGLDYKDDDDK
Biochemical assays
Neddylation of CUL1 was monitored by the use of pulse-chase assays which isolate transfer of NEDD8 from UBE2M to CUL1.32 Briefly, NEDD8 was labeled with Fluorescein-5-maleimide on an N-terminal Cys-containing tag as described.79 Fluorescent NEDD8 was thioester linked to UBE2M in a “pulse” reaction with 10 μM UBE2M, 15 μM FAMNEDD8, and 400 nM APPBP1-UBA3 in 25 mM HEPES, 200 mM NaCl, 10 mM MgCl2, 2 mM ATP, pH 7.5 for 15 minutes at room temperature. The pulse reaction was quenched for 5 min on ice by the addition of EDTA to 50 mM. “Chase” reactions were performed by diluting the thioester-linked UBE2M∼NEDD8 conjugate to 75 nM in 25 mM HEPES, 100 mM NaCl, 50 mM EDTA, 0.5 mg/ml BSA, pH 7.5. Chase reactions were initiated by the addition of CUL1-RBX1 or pre-formed SCF complexes. SCF complexes were generated by mixing SKP1-Fbp: CUL1-RBX1 (120 nM: 100 nM) and equilibrating for 5 min at RT prior to initiating neddylation reactions. Indicated CAND1 (or its mutants) concentrations were added to the UBE2M∼NEDD8 conjugate at the start of neddylation. Aliquots were removed at the specified times and quenched with 2X SDS-PAGE sample buffer. Reaction products were separated on 4-12% NuPAGE gels (Invitrogen). Fluorescent signal was visualized by scanning on a Typhoon imager (GE), and quantified with ImageQuant (GE). Reaction rates and Michaelis-Menten plots were generated in GraphPad Prism software.
In vitro ubiquitylation assays utilized a pulse-chase format.79 Briefly, Alexa Fluor 647-labeled ubiquitin (∗UB) was thioester-linked to UBE2L3, UBE2D2, or UBE2R2 in a “pulse” reaction incubating 10 μM E2, 15 μM ∗UB, and 400 nM UBA1 in 25 mM HEPES, 200 mM NaCl, 2.5 mM MgCl2, 1 mM ATP, pH 7.5 for 13 minutes at room temperature. The pulse reaction was quenched for 5 min on ice with 50 mM EDTA, and ∗UB was chased from E2 to SCF substrates. Chase reactions consisted of mixing the E2∼∗UB thioester conjugate (400 nM final concentration) with neddylated SCFFBXW7 (400 nM final concentration) pre-equilibrated with phospho-CyE peptide (2 μM final concentration) and, where indicated ARIH1 (300 nM final concentration). Chase reactions were performed on ice in 25 mM MES, 100mM NaCl, pH 6.5. Reactions were quenched at the indicated times by mixing with SDS sample buffer, separated by SDS-PAGE, and analyzed based on fluorescent signal of ∗UB using a Typhoon FLA9500 Phosphoimager (GE Healthcare).
Systemwide SCF complex assays were performed by pre-equilibrating indicated SCF complex (200 nM CUL1-RBX1, 220 nM SKP1-Fbp, and where indicated 600 nM SKP2 substrate complex consisting of phospho-p27-CKSHS1-CyclinA-CDK2 prepared as described previously65) for 15 min at room temperature in 25 mM HEPES, 100 mM NaCl, 0.5 mg/ml BSA, pH=7.5. The second SKP1-Fbp pair was then added to 220 nM and, when indicated included 600 nM SKP2 substate complex, with or without the addition of 200 nM CAND1. Mixtures were further incubated for 5 min at room temperature. Reaction mixtures were then pulse neddylated for 2 min at room temperature by the addition of thioester-linked UBE2M∼∗NEDD8 thioester to 250 nM (∗ refers to fluorescently-labeled). An aliquot was removed and quenched (zero-timepoint) and deneddylation was initiated by the addition of CSN to 15 nM. Aliquots were quenched at the indicated timepoints and reactions were separated on 4-12% Bis-Tris gradient gels, scanned on a Typhoon imager to visualize ∗NEDD8 conjugates, and quantified in ImageQuant (GE).
For all biochemical assays, gels were scanned on a Typhoon imager using the FAM or Alexa Fluor 647 channel at 700V or 500V PMT setting, respectively. The resulting .gel file was opened in ImageQuant, and after adjusting the contrast as desired uniformly across the entire image, the files were exported as a .bmp. The .bmp was opened in Adobe Photoshop (version 23.2.2) and resolution set to 300 dpi. The relevant regions were copied and then pasted into a new layer, and regions to the sides and top and below those not shown in the figure were not included. The resulting layer was saved as a .jpg. The .jpg was placed into Adobe Illustrator (version 26.1) and labeled accordingly.
Protein fluorescent labeling
Reactions to label the amino-terminus CAND1 with TAMRA contained 50 μM CAND1, 2 μM Sortase, 200 μM TAMRA-(PEG)5-LPETGG peptide (synthesized by St. Jude macromolecular synthesis core) in 50 mM Tris, 150 mM NaCl, 10 mM CaCl2. After a two-hour incubation at room temperature the sample was buffer exchanged over a NAP-5 column into 25 mM HEPES, 200 mM NaCl, 1 mM DTT, pH 7.5 (Buffer A) to remove unconjugated fluorescent peptide. Following concentration, the sample was further purified over a Superdex SD200 sizing column in Buffer A. To label the amino-terminus of SKP2 with Cy5, reactions contained 25 μM SKP1-SKP2, 1 μM Sortase, 150 μM Cy5-(PEG)5-LPETGG peptide (synthesized by St. Jude macromolecular synthesis core) in 50 mM Tris, 150 mM NaCl, 10 mM CaCl2. After a forty-five-minute incubation at room temperature the sample was buffer exchanged over a NAP-5 column into Buffer A to remove unconjugated fluorescent peptide. Following concentration, the sample was further purified over a Superdex SD200 sizing column in Buffer A. Extent of labeling was calculated through the ratio of protein concentration by absorbance at 280 nM to the concentration of fluorescent probe. In all cases labeling was estimated to be >85%.
Protein complex evaluation on native gels
To evaluate complexes by native gel electrophoresis, the indicated SCF complexes were pre-formed on ice for 30 min by mixing stoichiometric amounts of all indicated components (final concentration of 3 μM) in 25 mM HEPES, 150 mM NaCl, 2.5% glycerol, 1 mM DTT, pH 7.5. All samples with FBXW7 included a phosphopeptide derived from CyclinE to improve homogeneity of migration in the gel. CAND1 WT or mutant and SKP1-FBXW7 in the case of SKP1-Fbp exchange experiments were added to a final concentration of 3 μM and samples were immediately loaded onto 4-20% Tris-Glycine gels. Samples were electrophoresed at 130V for 180 minutes at 4°C. Fluorescent signal was visualized by scanning on a Typhoon imager (GE) followed by Coomassie staining to visualize all protein components. CAND1 was labeled with TAMRA, SKP2 was labeled with Cy5, and FBXW7 was labeled with fluorescein for visualization. In figures, the two channels were colored yellow and magenta and merged. The ∗ in Figure 3B indicates a band of uncertain composition. Unlike the other bands, that are readily identified based on migrations of the inputs or the two colors, we cannot say with 100% certainty what this band is, although its “magenta” fluorescence suggests that it is some form of SKP1-FBXW7. Given that protein complexes undergo dissociation and association in equilibrium, and we cannot quench these binding events during the course electrophoresis, comigration in nondenaturing gels depends on reassociation of any dissociated parts at a rate that is faster than electrophoretic separation of the parts. Based on the structures, and relative susceptibility of the pivot mutant to CAND1 in the experiment shown in Figure 3D, it seems likely that this band corresponds to a fraction of total SKP1-FBXW7 that dissociates from the CAND1-SCFFBXW7 (pivot mutant) complex during electrophoresis. This would also explain why the SKP1-FBXW7 pivot mutant band in lane 7 does not match the intensity of SKP1-FBXW7 pivot mutant alone (lane 2).
Migration in these non-denaturing gels (4-20%, Tris-Glycine) depends on size and structure (including charge distribution, hydrodynamic radius, conformational ensemble, and dynamics), so migration is determined empirically.
pI values are: CUL1-RBX1: 7.91, CAND1-CUL1-RBX1: 6.09, CUL1-RBX1-SKP1-SKP2: 6.14, CAND1-CUL1-RBX1-SKP1-SKP2: 5.85, SKP1-SKP2: 5.3, SKP1-FBXW7: 5.92, CUL1-RBX1-SKP1-FBXW7: 6.52, CAND1-CUL1-RBX1-SKP1-FBXW7: 6.04
Cryo-EM sample preparation
Samples for CAND1-SCFSKP2, CAND1-SCFFBXO6, CAND1-SCFFBXW7 (with phosphorylated CyclinE peptide required for sample homogeneity) and their mutant versions were generated by incubating 10 μM CUL1-RBX1, 11 μM CAND1, 11 μM SKP1-Fbp, (and 50 μM CyclinE phosphopeptide in the case of FBXW7). It was necessary to include CyclinE phosphopeptide in samples with FBXW7 to obtain interpretable cryo-EM data. To generate the CAND1-SCFSKP2 sample with substrate, 11 μM CKSHS1, 11 μM CyclinA-CDK2, 20 μM p27 C-terminal phosphopeptide and p27 N-terminal kinase inhibitory domain were also included. Samples were mixed on ice for 30 min in 25 mM HEPES, 150 mM NaCl, 1 mM DTT, pH 7.5. Samples were plunged with various dilutions for optimal particle density. CAND1-CUL1-RBX1 was generated by incubating 10 μM CAND1 and 10 μM CUL1-RBX1 in the same condition above. 3.5 μL of these samples were applied to Quantifoil R1.2/1.3 holey carbon grids and plunge frozen with a Vitrobot Mark IV in liquid ethane.
Cryo-electron microscopy
All datasets were prescreened through data collection on a Glacios at 200kV equipped with a K2 Summit direct detector in counting mode. Overnight collections of ∼2000 movies per dataset were recorded at 1.885Å/pixel with a nominal magnification of 22,000x. Total dose of ∼60 e-/Å2 were fractionated over 40 frames, with a defocus range of -1.5 μm ∼ -3.0 μm.
High resolution datasets were collected on a Titan Krios at 300kV equipped with a Quantum-LS energy filter and a Gatan K3 direct detector in counting mode and in CDS mode. Movies were collected at 0.8512 Å/pixel with a nominal magnification of 105,000x. Total dose of ∼68e-/Å2 were fractionated over 30 frames, with a defocus range of -0.5 μm ∼ -2.0 μm.
Data processing
For datasets collected on the Glacios, frames were motion-corrected using RELION-3.1.69 CTF was estimated by CTFFIND-4.1 and particles were picked using Gautomatch-0.56 (K. Zhang, MRC Laboratory of Molecular Biology, Cambridge).70 All downstream processing including 2D classification, initial model generation, 3D classification, and 3D refinements were performed using RELION.
For datasets collected on the Titan Krios, Focus74 was used for on-the-fly motion correction (MotionCorr2-1.1), CTF estimation (Gctf-1.06), and particle picking (Gautomatch-0.56).71,74 All data were imported to RELION-3.1, and all processed in RELION. DeepEMhancer was used for final post-processing.73 Detailed processing strategies are described in Methods S1 with schematics of classifications shown. In brief, classification was performed initially to find conformations that drastically differ from one another. Once particles were sorted into major conformations, all classes were again subject to iterative classifications using the angles obtained from 3D refinement to further search for classes with different densities of domains. Each meaningful class was subject to 3D refinement and subsequent masked 3D refinement, and only the classes that provide subnanometer resolution were used for interpretation as they allow unambiguous placing of secondary structure domains. For example, for the sample with CAND1 and SCFFBXW7 shown in Figure 2, while the two conformations with no CAND1 look similar overall, they show different conformations of RBX1 and FBXW7, and thus classes were separated. Classification was performed until the set of particles no longer provided additional classes, or not enough particles to further classify. In the case of the dataset from the sample including components of the SKP2 substrate complex, all classes showed CKSHS1, which acts as a protein glue between SKP2 and the p27 phosphopeptide. The majority of particles also showed CyclinA-CDK2-p27. Nonetheless, the addition of these proteins binding the Fbp shifted the proportion of particles observed in CAND1 engaged, CAND1 rolling, and no CAND1 conformations compared to CAND1-SCFSKP2 alone.
Model building and refinement
Final models were generated for parts that allow unambiguous docking of all individual components. Previously determined structures of CUL1, RBX1, CAND1, SKP1, and SKP2 (PDB IDs: 6TTU, 1U6G, 1LDK, 7B5L40,45,48,50) were manually placed and fitted to the density using UCSF Chimera.75,76 Portions of proteins for which there was little to no density were excluded from modeling; as such, parts of CAND1’s anti-SKP1 domain and portions of the central arch, and RBX1’s RING domain were not included in coordinates for the CAND1 rolling conformations. For SKP2, the N-terminal domain was not visible and thus excluded from all structures; the F-box domain was modeled with side chains, and the leucine-rich repeat substrate binding domain was modeled by wholesale docking of the previously published SKP2 structure into each map. The initial models were fitted through rigid body refinements for bulk movements and were subject to iterative manual model rebuilding using COOT by inspecting maps generated by either DeepEMhancer or RELION postprocessing. Coordinates were subjected to real space refinements using COOT, ultimately refined with Phenix.refine.77,78
IκBα (NFKBIA) degradation assay
The assay was performed as previously described.10 In summary, ∼0.7 million cells were seeded in 6-well plates and cultured overnight before exchanging the media to FBS free DMEM for 3h. Cells were then treated with 100 μg/ml cycloheximide for 10 min before addition of 25 ng/ml of TNF-α. At indicated times after TNF-α treatment cells were washed with PBS and lysed in 3x SDS sample buffer followed by boiling for 5 min at 98˚C and brief sonication. Samples were fractionated by SDS-PAGE and analyzed by immunoblot. Peroxidase-conjugated secondary antibodies were detected using SuperSignal West Pico PLUS Chemiluminescent Substrate (ThermoFisher Scientific) and imaged on an Amersham ImageQuant 800 (Cytiva) using the Chemiluminescent function. When showing immunoblots brightness and contrast for the chemiluminescent signal were adjusted in Fiji. For plotting relative phospho-IκBα levels, the phospho-IκBα band intensities were determined in Fiji and normalized to the Vinculin loading control. To gain relative phospho-IκBα levels the maximal normalized phospho-IκBα band intensity per replicate was set as 1 and the minimal normalized phospho-IκBα band intensity as 0. Data are shown as mean±SEM of three biological replicates (n=3).
Affinity-purification mass spectrometry
Around 50 μL of compacted cells were lysed in 500 μl lysis buffer (25 mM HEPES pH 7.4, 100 mM NaCl, 1% IGEPAL CA-63, 5% glycerol, 1x protease inhibitor, 1 μM MLN4924, 1 μM CSN5i-3 and 1.5 μM GST-RBX1-CUL1). Lysates were briefly sonicated (10s, 1s on/off, 10% amplitude, Bandelin Sonopuls HD 4200, TS 103) and cleared by centrifugation at ∼20,000xg for 3 min at 4˚C followed by filtration using 0.22 μm spin filters. Afterwards, 9 μL of magnetic anti-FLAG beads were added to the soluble fraction and incubated for 45 min at 4˚C while rotating. The flow-through was diluted 1:1 in lysis buffer without GST-RBX1-CUL1, 40 μL of magnetic glutathione beads were added and incubated for 2h at 4˚C while rotating. Beads were washed 2x with lysis buffer, 2x with wash buffer (lysis buffer without IGEPAL CA-63 and GST-RBX1-CUL1) and 2 x with HBS (25 mM HEPES pH 7.4, 100 mM NaCl). After removing as much of the liquid as possible, beads were resuspended in 45 μL (FLAG) or 90 μL (glutathione) denaturing lysis buffer (100 mM Tris pH 8.5, 1% SDC) and boiled for 5 min at 98˚C. After addition of CAA and TCEP to final concentrations of 40 mM and 10 mM, respectively, samples were incubated at 45˚C for 5 min and then digested overnight at 37˚C using trypsin and Lys-C at 1:100 w/w. Samples were loaded onto SDB-RPS StageTips80 after being diluted in 1% trifluoroacetic acid (TFA) in isopropanol to a final volume of 200 μL. StageTips were washed 1x with 200 μL 1% TFA in isopropanol and 2x with 200 μL in 0.2% TFA/ 2% acetonitrile (ACN) before being eluted with 60 μL of 1.25% ammonium hydroxide in 80% ACN and dried using a SpeedVac centrifuge. Prior to LC-MS/MS analysis, samples were resuspended in buffer A∗ (0.2% TFA. 2% ACN) and peptide concentrations estimated by measuring absorbance at 280 nm and normalized to 0.1 mg/ml.
Affinity-purification immunoblots
Affinity purification was performed as described above. Afterwards beads were resuspended in 50 μl reducing sample buffer, fractionated by SDS-PAGE, and analyzed by immunoblot as described above.
LC-MS/MS measurements
Samples were loaded onto a 50 cm reverse-phase column (75 μm inner diameter, packed in house with ReproSil-Pur C18-AQ 1.9 μm resin). The column was maintained at 60˚C using a homemade column oven. For nano-flow liquid chromatography an EASY-nLC 1200 system directly coupled with the mass spectrometer (Orbitrap Exploris 480, Thermo Fisher Scientific) via a nano-electrospray source was used. Peptide separation was performed using a binary buffer system composed of buffer A (0.1% formic acid (FA)) and buffer B (0.1% FA, 80% ACN) at a flow rate of 300 nl/min. Per measurement 200ng of peptide material was loaded and separated using a gradient starting at 5% buffer B, followed by a stepwise increase to 30% in 45 min, 65% in 8 min, and 95% in 2 min, staying at 95% for 5min. Samples were acquired in data independent acquisition mode using a scan range of 300-1650 m/z at a resolution of 120,000. The automatic gain control (AGC) target was set to 3e6 at a maximum injection time of 20 ms and precursor ions were fragmented by higher-energy collisional dissociation at NCE of 28%. Fragment ions were analyzed in 32 DIA windows at a resolution of 30,000 with AGC adjusted to 10e6 (Table S4).
MS data analysis
DIA raw files were processed using Spectronaut81 using default settings for directDIA. For data analysis the Perseus software package (version 1.6.7.0) was used. Protein group intensities were log2-transformed and data sets filtered to contain more than 50% valid values in at least one experimental condition with missing values being imputed based on normal distribution with a width of 0.3 and a downshift of 1.8. Intensities for CUL1, SKP1, CAND1 and F-box proteins were extracted and filtered to have an average coefficient of variation within experimental conditions of <15%. Protein group intensities for FLAG-immunopurifications were normalized to CUL1 levels, log2-fold-changes calculated and plotted in GraphPad Prism. For plotting of the percentage of CUL1-associated versus free Fbps CUL1 normalized protein group intensities for GST-pulldowns were corrected based on total eluate volume compared to FLAG-immunopurifications. The sum of corrected GST-pulldown and FLAG-immunopurification protein group intensities was assumed as the total Fbp level and the FLAG-immunopurification intensity as the CUL1 bound Fbp level9 (Table S4).
Quantification and statistical analysis
Statistical analyses for all experiments are described in the figure legends and the method details. Cryo-EM density map resolution estimation (Tables S1–S3) were based on the 0.143 FSC criterion.
Acknowledgments
We gratefully acknowledge X. Liu and R. Deshaies for WT and CAND-null cells containing endogenously tagged 3xFLAG-CUL1; F. Adolf for SKP1-FBXO6; J. Kellermann, J.R. Prabu, S.v. Gronau, I. Paron, T. Heymann, J.W. Harper, R.V. Farese Jr., D. Bollschweiler, T. Schäfer, S. Pettera, S. Uebel, P. Rodrigues, F. Bassermann, I. Chen, G. Kleiger, and Schulman lab for assistance. Support: Max Planck Gesellschaft, the European Commission (ERC AdG Nedd8Activate), NIH R01CA247365, and Leibniz Prize from the Deutsche Forschungsgemeinschaft to B.A.S.; NIH P30CA021765 to St. Jude Cancer Center.
Author contributions
K.B. and B.A.S. conceived the project. K.B., D.C.S., and M.T.K. generated protein complexes. K.B., D.C.S., and L.T.H. developed assays. K.B. performed cryo-EM. D.C.S. conducted biochemistry. L.T.H. performed cellular assays, and proteomics supervised by M.M. K.B., D.C.S., L.T.H., and B.A.S. prepared manuscript with input from all authors. B.A.S. supervised.
Declaration of interests
B.A.S. is also Honorary Professor at Technical University of Munich and serves on Interline Therapeutics and BioTheryX SABs. B.A.S. and D.C.S. co-invented intellectual property licensed to CinSano. M.M. is also Professor at Novo Nordisk Foundation Center for Protein Research.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: April 6, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cell.2023.02.035.
Supplemental information
Data and code availability
-
•
Cryo-EM data are available from the Electron Microscopy Data Bank under accession codes: EMD-14561, EMD-14563, EMD-14594, EMD-16582, EMD-14595, EMD-14597, EMD-14598, EMD-16576, EMD-16575, EMD-16579, EMD-16580, EMD-16581, EMD-16583, EMD-16584, EMD-16585, EMD-16586, EMD-16587, EMD-16588, EMD-16589, EMD-14615, EMD-14616, EMD-16577, EMD-16578, EMD-14603, EMD-14604, EMD-14601, EMD-14602, EMD-14599, EMD-14600, EMD-14605, EMD-14606, EMD-14607, EMD-14608, EMD-14609, EMD-14610, EMD-14614, EMD-14613, EMD-14612, EMD-14611, EMD-16617, EMD-16621, EMD-16622, EMD-16623, EMD-16625, EMD-16764, EMD-16765, EMD-16766. PDB coordinates are available from the RCSB Protein Data Bank under accession codes: 7Z8R, 7Z8T, 7ZBW, 7Z8V, 7ZBZ, 8CDK, 8CDJ. Mass spectrometry data have been deposited on the ProteomeXchange Consortium via the PRIDE database under the accession code: PXD038661. Raw image data are deposited in Mendeley under: https://doi.org/10.17632/9c6zfpsxfh.1. Accession code numbers are listed in the key resources table.
-
•
This paper does not report original code. All computational approaches and software used are described in the STAR Methods and listed key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Murata S., Yashiroda H., Tanaka K. Molecular mechanisms of proteasome assembly. Nat. Rev. Mol. Cell Biol. 2009;10:104–115. doi: 10.1038/nrm2630. [DOI] [PubMed] [Google Scholar]
- 2.Tomko R.J., Taylor D.W., Chen Z.A., Wang H.W., Rappsilber J., Hochstrasser M. A Single alpha Helix Drives Extensive Remodeling of the proteasome Lid and Completion of Regulatory Particle Assembly. Cell. 2015;163:432–444. doi: 10.1016/j.cell.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis J.H., Tan Y.Z., Carragher B., Potter C.S., Lyumkis D., Williamson J.R. Modular assembly of the bacterial large ribosomal subunit. Cell. 2016;167:1610–1622.e15. doi: 10.1016/j.cell.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh S., Vanden Broeck A., Miller L., Chaker-Margot M., Klinge S. Nucleolar maturation of the human small subunit processome. Sci. N. Y. NY. 2021;373 doi: 10.1126/science.abj5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnell H.M., Walsh R.M., Rawson S., Hanna J. Chaperone-mediated assembly of the proteasome core particle - recent developments and structural insights. J. Cell Sci. 2022;135 doi: 10.1242/jcs.259622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce N.W., Lee J.E., Liu X., Sweredoski M.J., Graham R.L., Larimore E.A., Rome M., Zheng N., Clurman B.E., Hess S., et al. Cand1 promotes assembly of new SCF complexes through dynamic exchange of F box proteins. Cell. 2013;153:206–215. doi: 10.1016/j.cell.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zemla A., Thomas Y., Kedziora S., Knebel A., Wood N.T., Rabut G., Kurz T. CSN- and CAND1-dependent remodelling of the budding yeast SCF complex. Nat. Commun. 2013;4:1641. doi: 10.1038/ncomms2628. [DOI] [PubMed] [Google Scholar]
- 8.Wu S., Zhu W., Nhan T., Toth J.I., Petroski M.D., Wolf D.A. CAND1 controls in vivo dynamics of the cullin 1-RING ubiquitin ligase repertoire. Nat. Commun. 2013;4:1642. doi: 10.1038/ncomms2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reitsma J.M., Liu X., Reichermeier K.M., Moradian A., Sweredoski M.J., Hess S., Deshaies R.J. Composition and regulation of the cellular repertoire of SCF ubiquitin ligases. Cell. 2017;171:1326–1339.e14. doi: 10.1016/j.cell.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Reitsma J.M., Mamrosh J.L., Zhang Y., Straube R., Deshaies R.J. Cand1-mediated adaptive exchange mechanism enables variation in F-box protein expression. Mol. Cell. 2018;69:773–786.e6. doi: 10.1016/j.molcel.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichermeier K.M., Straube R., Reitsma J.M., Sweredoski M.J., Rose C.M., Moradian A., den Besten W., Hinkle T., Verschueren E., Petzold G., et al. PIKES analysis reveals response to degraders and key regulatory mechanisms of the CRL4 network. Mol. Cell. 2020;77:1092–1106.e9. doi: 10.1016/j.molcel.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Enchev R.I., Scott D.C., da Fonseca P.A., Schreiber A., Monda J.K., Schulman B.A., Peter M., Morris E.P. Structural basis for a reciprocal regulation between SCF and CSN. Cell Rep. 2012;2:616–627. doi: 10.1016/j.celrep.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emberley E.D., Mosadeghi R., Deshaies R.J. Deconjugation of Nedd8 from Cul1 is directly regulated by Skp1-F-box and substrate, and the COP9 signalosome inhibits deneddylated SCF by a noncatalytic mechanism. J. Biol. Chem. 2012;287:29679–29689. doi: 10.1074/jbc.M112.352484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer E.S., Scrima A., Böhm K., Matsumoto S., Lingaraju G.M., Faty M., Yasuda T., Cavadini S., Wakasugi M., Hanaoka F., et al. The molecular basis of CRL4DDB2/CSA ubiquitin ligase architecture, targeting, and activation. Cell. 2011;147:1024–1039. doi: 10.1016/j.cell.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y., Dai X., Zhao Y. AtCAND1, a HEAT-repeat protein that participates in auxin signaling in Arabidopsis. Plant Physiol. 2004;135:1020–1026. doi: 10.1104/pp.104.044495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang H.W., Zhang W., Gray W.M. Arabidopsis ETA2, an apparent ortholog of the human cullin-interacting protein CAND1, is required for auxin responses mediated by the SCF(TIR1) ubiquitin ligase. Plant Cell. 2004;16:1883–1897. doi: 10.1105/tpc.021923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng S.H., Shen Y.P., Sullivan J.A., Rubio V., Xiong Y., Sun T.P., Deng X.W. Arabidopsis CAND1, an unmodified CUL1-interacting protein, is involved in multiple developmental pathways controlled by ubiquitin/proteasome-mediated protein degradation. Plant Cell. 2004;16:1870–1882. doi: 10.1105/tpc.021949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo S.C., Hannink M. CAND1-mediated substrate adaptor recycling is required for efficient repression of Nrf2 by Keap1. Mol. Cell. Biol. 2006;26:1235–1244. doi: 10.1128/MCB.26.4.1235-1244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayor-Ruiz C., Jaeger M.G., Bauer S., Brand M., Sin C., Hanzl A., Mueller A.C., Menche J., Winter G.E. Plasticity of the cullin-RING ligase repertoire shapes sensitivity to ligand-induced protein degradation. Mol. Cell. 2019;75:849–858.e8. doi: 10.1016/j.molcel.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Willems A.R., Schwab M., Tyers M. A hitchhiker's guide to the cullin ubiquitin ligases: SCF and its kin. Biochim. Biophys. Acta. 2004;1695:133–170. doi: 10.1016/j.bbamcr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Sarikas A., Hartmann T., Pan Z.Q. The cullin protein family. Genome Biol. 2011;12:220. doi: 10.1186/gb-2011-12-4-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusnac D.V., Zheng N. Structural biology of CRL ubiquitin ligases. Adv. Exp. Med. Biol. 2020;1217:9–31. doi: 10.1007/978-981-15-1025-0_2. [DOI] [PubMed] [Google Scholar]
- 23.Skaar J.R., Pagan J.K., Pagano M. Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 2013;14:369–381. doi: 10.1038/nrm3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harper J.W., Schulman B.A. Cullin-RING ubiquitin ligase regulatory circuits: A quarter century beyond the F-box hypothesis. Annu. Rev. Biochem. 2021;90:403–429. doi: 10.1146/annurev-biochem-090120-013613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K., Deshaies R.J., Liu X. Assembly and regulation of CRL ubiquitin ligases. Adv. Exp. Med. Biol. 2020;1217:33–46. doi: 10.1007/978-981-15-1025-0_3. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen K.M., Busino L. The biology of F-box proteins: the SCF family of E3 ubiquitin ligases. Adv. Exp. Med. Biol. 2020;1217:111–122. doi: 10.1007/978-981-15-1025-0_8. [DOI] [PubMed] [Google Scholar]
- 27.Hao B., Zheng N., Schulman B.A., Wu G., Miller J.J., Pagano M., Pavletich N.P. Structural basis of the Cks1-dependent recognition of p27(Kip1) by the SCF(Skp2) ubiquitin ligase. Mol. Cell. 2005;20:9–19. doi: 10.1016/j.molcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Orlicky S., Tang X., Willems A., Tyers M., Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112:243–256. doi: 10.1016/s0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 29.Wu G., Xu G., Schulman B.A., Jeffrey P.D., Harper J.W., Pavletich N.P. Structure of a beta-TrCP1-Skp1-beta-catenin complex: destruction motif binding and lysine specificity of the SCF(beta-TrCP1) ubiquitin ligase. Mol. Cell. 2003;11:1445–1456. doi: 10.1016/s1097-2765(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 30.Hao B., Oehlmann S., Sowa M.E., Harper J.W., Pavletich N.P. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol. Cell. 2007;26:131–143. doi: 10.1016/j.molcel.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Pan Z.Q., Kentsis A., Dias D.C., Yamoah K., Wu K. Nedd8 on cullin: building an expressway to protein destruction. Oncogene. 2004;23:1985–1997. doi: 10.1038/sj.onc.1207414. [DOI] [PubMed] [Google Scholar]
- 32.Scott D.C., Sviderskiy V.O., Monda J.K., Lydeard J.R., Cho S.E., Harper J.W., Schulman B.A. Structure of a RING E3 trapped in action reveals ligation mechanism for the ubiquitin-like protein NEDD8. Cell. 2014;157:1671–1684. doi: 10.1016/j.cell.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyapina S., Cope G., Shevchenko A., Serino G., Tsuge T., Zhou C., Wolf D.A., Wei N., Shevchenko A., Deshaies R.J. Promotion of NEDD-CUL1 conjugate cleavage by COP9 signalosome. Science. 2001;292:1382–1385. doi: 10.1126/science.1059780. [DOI] [PubMed] [Google Scholar]
- 34.Bornstein G., Ganoth D., Hershko A. Regulation of Neddylation and deneddylation of cullin1 in SCFSkp2 ubiquitin ligase by F-box protein and substrate. Proc. Natl. Acad. Sci. USA. 2006;103:11515–11520. doi: 10.1073/pnas.0603921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lingaraju G.M., Bunker R.D., Cavadini S., Hess D., Hassiepen U., Renatus M., Fischer E.S., Thomä N.H. Crystal structure of the human COP9 signalosome. Nature. 2014;512:161–165. doi: 10.1038/nature13566. [DOI] [PubMed] [Google Scholar]
- 36.Mosadeghi R., Reichermeier K.M., Winkler M., Schreiber A., Reitsma J.M., Zhang Y., Stengel F., Cao J., Kim M., Sweredoski M.J., et al. Structural and kinetic analysis of the COP9-Signalosome activation and the cullin-RING ubiquitin ligase deneddylation cycle. eLife. 2016;5 doi: 10.7554/eLife.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavadini S., Fischer E.S., Bunker R.D., Potenza A., Lingaraju G.M., Goldie K.N., Mohamed W.I., Faty M., Petzold G., Beckwith R.E., et al. Cullin-RING ubiquitin E3 ligase regulation by the COP9 signalosome. Nature. 2016;531:598–603. doi: 10.1038/nature17416. [DOI] [PubMed] [Google Scholar]
- 38.Zheng J., Yang X., Harrell J.M., Ryzhikov S., Shim E.H., Lykke-Andersen K., Wei N., Sun H., Kobayashi R., Zhang H. CAND1 binds to unneddylated CUL1 and regulates the formation of SCF ubiquitin E3 ligase complex. Mol. Cell. 2002;10:1519–1526. doi: 10.1016/s1097-2765(02)00784-0. [DOI] [PubMed] [Google Scholar]
- 39.Liu J., Furukawa M., Matsumoto T., Xiong Y. NEDD8 modification of CUL1 dissociates p120(CAND1), an inhibitor of CUL1-SKP1 binding and SCF ligases. Mol. Cell. 2002;10:1511–1518. doi: 10.1016/s1097-2765(02)00783-9. [DOI] [PubMed] [Google Scholar]
- 40.Goldenberg S.J., Cascio T.C., Shumway S.D., Garbutt K.C., Liu J., Xiong Y., Zheng N. Structure of the Cand1-Cul1-Roc1 complex reveals regulatory mechanisms for the assembly of the multisubunit cullin-dependent ubiquitin ligases. Cell. 2004;119:517–528. doi: 10.1016/j.cell.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt M.W., McQuary P.R., Wee S., Hofmann K., Wolf D.A. F-box-directed CRL complex assembly and regulation by the CSN and CAND1. Mol. Cell. 2009;35:586–597. doi: 10.1016/j.molcel.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y., Jost M., Pak R.A., Lu D., Li J., Lomenick B., Garbis S.D., Li C.M., Weissman J.S., Lipford J.R., Deshaies R.J. Adaptive exchange sustains cullin-RING ubiquitin ligase networks and proper licensing of DNA replication. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2205608119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlierf A., Altmann E., Quancard J., Jefferson A.B., Assenberg R., Renatus M., Jones M., Hassiepen U., Schaefer M., Kiffe M., et al. Targeted inhibition of the COP9 signalosome for treatment of cancer. Nat. Commun. 2016;7 doi: 10.1038/ncomms13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiśniewski J.R., Hein M.Y., Cox J., Mann M. A "proteomic ruler" for protein copy number and concentration estimation without spike-in standards. Mol. Cell. Proteomics. 2014;13:3497–3506. doi: 10.1074/mcp.M113.037309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng N., Schulman B.A., Song L., Miller J.J., Jeffrey P.D., Wang P., Chu C., Koepp D.M., Elledge S.J., Pagano M., et al. Structure of the Cul1-Rbx1-Skp1-F boxSkp2 SCF ubiquitin ligase complex. Nature. 2002;416:703–709. doi: 10.1038/416703a. [DOI] [PubMed] [Google Scholar]
- 46.Schulman B.A., Carrano A.C., Jeffrey P.D., Bowen Z., Kinnucan E.R., Finnin M.S., Elledge S.J., Harper J.W., Pagano M., Pavletich N.P. Insights into SCF ubiquitin ligases from the structure of the Skp1-Skp2 complex. Nature. 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- 47.Mena E.L., Jevtić P., Greber B.J., Gee C.L., Lew B.G., Akopian D., Nogales E., Kuriyan J., Rape M. Structural basis for dimerization quality control. Nature. 2020;586:452–456. doi: 10.1038/s41586-020-2636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horn-Ghetko D., Krist D.T., Prabu J.R., Baek K., Mulder M.P.C., Klügel M., Scott D.C., Ovaa H., Kleiger G., Schulman B.A. Ubiquitin ligation to F-box protein targets by SCF-RBR E3-E3 super-assembly. Nature. 2021;590:671–676. doi: 10.1038/s41586-021-03197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ganoth D., Bornstein G., Ko T.K., Larsen B., Tyers M., Pagano M., Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat. Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- 50.Baek K., Krist D.T., Prabu J.R., Hill S., Klügel M., Neumaier L.M., von Gronau S., Kleiger G., Schulman B.A. NEDD8 nucleates a multivalent cullin-RING-UBE2D ubiquitin ligation assembly. Nature. 2020;578:461–466. doi: 10.1038/s41586-020-2000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winston J.T., Koepp D.M., Zhu C.H., Elledge S.J., Harper J.W. A family of mammalian F-box proteins. Curr. Biol. 1999;9:1180–11S3. doi: 10.1016/S0960-9822(00)80021-4. [DOI] [PubMed] [Google Scholar]
- 52.Welcker M., Wang B.Y., Rusnac D.V., Hussaini Y., Swanger J., Zheng N., Clurman B.E. Two diphosphorylated degrons control c-Myc degradation by the Fbw7 tumor suppressor. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abl7872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida Y., Chiba T., Tokunaga F., Kawasaki H., Iwai K., Suzuki T., Ito Y., Matsuoka K., Yoshida M., Tanaka K., Tai T. E3 ubiquitin ligase that recognizes sugar chains. Nature. 2002;418:438–442. doi: 10.1038/nature00890. [DOI] [PubMed] [Google Scholar]
- 54.Mizushima T., Hirao T., Yoshida Y., Lee S.J., Chiba T., Iwai K., Yamaguchi Y., Kato K., Tsukihara T., Tanaka K. Structural basis of sugar-recognizing ubiquitin ligase. Nat. Struct. Mol. Biol. 2004;11:365–370. doi: 10.1038/nsmb732. [DOI] [PubMed] [Google Scholar]
- 55.Angers S., Li T., Yi X., MacCoss M.J., Moon R.T., Zheng N. Molecular architecture and assembly of the DDB1-CUL4A ubiquitin ligase machinery. Nature. 2006;443:590–593. doi: 10.1038/nature05175. [DOI] [PubMed] [Google Scholar]
- 56.Cardote T.A.F., Gadd M.S., Ciulli A. Crystal structure of the Cul2-Rbx1-EloBC-VHL ubiquitin ligase complex. Structure. 2017;25:901–911.e3. doi: 10.1016/j.str.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duda D.M., Borg L.A., Scott D.C., Hunt H.W., Hammel M., Schulman B.A. Structural insights into NEDD8 activation of cullin-RING ligases: conformational control of conjugation. Cell. 2008;134:995–1006. doi: 10.1016/j.cell.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamoah K., Oashi T., Sarikas A., Gazdoiu S., Osman R., Pan Z.Q. Autoinhibitory regulation of SCF-mediated ubiquitination by human cullin 1's C-terminal tail. Proc. Natl. Acad. Sci. USA. 2008;105:12230–12235. doi: 10.1073/pnas.0806155105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saha A., Deshaies R.J. Multimodal activation of the ubiquitin ligase SCF by Nedd8 conjugation. Mol. Cell. 2008;32:21–31. doi: 10.1016/j.molcel.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fairweather S.J., Shah N., Brӧer S. Heteromeric Solute Carriers: Function, Structure, Pathology and Pharmacology. Adv. Exp. Med. Biol. 2021;21:13–127. doi: 10.1007/5584_2020_584. [DOI] [PubMed] [Google Scholar]
- 61.Evans R.M., Mangelsdorf D.J. Nuclear receptors, RXR, and the big bang. Cell. 2014;157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ji A.X., Privé G.G. Crystal structure of KLHL3 in complex with Cullin3. PLOS One. 2013;8 doi: 10.1371/journal.pone.0060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y.K., Kwak M.J., Ku B., Suh H.Y., Joo K., Lee J., Jung J.U., Oh B.H. Structural basis of intersubunit recognition in elongin BC-cullin 5-SOCS box ubiquitin-protein ligase complexes. Acta Crystallogr. D Biol. Crystallogr. 2013;69:1587–1597. doi: 10.1107/S0907444913011220. [DOI] [PubMed] [Google Scholar]
- 64.Duda D.M., Olszewski J.L., Schuermann J.P., Kurinov I., Miller D.J., Nourse A., Alpi A.F., Schulman B.A. Structure of HHARI, a RING-IBR-RING ubiquitin ligase: autoinhibition of an Ariadne-Family E3 and insights into ligation mechanism. Structure. 2013;21:1030–1041. doi: 10.1016/j.str.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott D.C., Rhee D.Y., Duda D.M., Kelsall I.R., Olszewski J.L., Paulo J.A., de Jong A., Ovaa H., Alpi A.F., Harper J.W., Schulman B.A. Two distinct types of E3 ligases work in unison to regulate substrate ubiquitylation. Cell. 2016;166:1198–1214.e24. doi: 10.1016/j.cell.2016.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weissmann F., Petzold G., VanderLinden R., Huis In 't Veld P.J., Brown N.G., Lampert F., Westermann S., Stark H., Schulman B.A., Peters J.M. biGBac enables rapid gene assembly for the expression of large multisubunit protein complexes. Proc. Natl. Acad. Sci. USA. 2016;113:E2564–E2569. doi: 10.1073/pnas.1604935113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L., Blackburn E.H. Human Rif1 protein binds aberrant telomeres and aligns along anaphase midzone microtubules. J. Cell Biol. 2004;167:819–830. doi: 10.1083/jcb.200408181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zivanov J., Nakane T., Forsberg B.O., Kimanius D., Hagen W.J., Lindahl E., Scheres S.H. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7 doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rohou A., Grigorieff N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015;192:216–221. doi: 10.1016/j.jsb.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 2016;193:1–12. doi: 10.1016/j.jsb.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng S.Q., Palovcak E., Armache J.P., Verba K.A., Cheng Y., Agard D.A. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods. 2017;14:331–332. doi: 10.1038/nmeth.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sanchez-Garcia R., Gomez-Blanco J., Cuervo A., Carazo J.M., Sorzano C.O.S., Vargas J. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 2021;4:874. doi: 10.1038/s42003-021-02399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Biyani N., Righetto R.D., McLeod R., Caujolle-Bert D., Castano-Diez D., Goldie K.N., Stahlberg H. Focus: the interface between data collection and data processing in cryo-EM. J. Struct. Biol. 2017;198:124–133. doi: 10.1016/j.jsb.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 76.Goddard T.D., Huang C.C., Meng E.C., Pettersen E.F., Couch G.S., Morris J.H., Ferrin T.E. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Afonine P.V., Klaholz B.P., Moriarty N.W., Poon B.K., Sobolev O.V., Terwilliger T.C., Adams P.D., Urzhumtsev A. New tools for the analysis and validation of cryo-EM maps and atomic models. Acta Crystallogr. D Struct. Biol. 2018;74:814–840. doi: 10.1107/S2059798318009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scott D.C., Schulman B.A. Dual-color pulse-chase ubiquitination assays to simultaneously monitor substrate priming and extension. Methods Enzymol. 2019;618:29–48. doi: 10.1016/bs.mie.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 80.Kulak N.A., Pichler G., Paron I., Nagaraj N., Mann M. Minimal, encapsulated proteomic-sample processing applied to copy-number estimation in eukaryotic cells. Nat. Methods. 2014;11:319–324. doi: 10.1038/Nmeth.2834. [DOI] [PubMed] [Google Scholar]
- 81.Bruderer R., Bernhardt O.M., Gandhi T., Miladinović S.M., Cheng L.Y., Messner S., Ehrenberger T., Zanotelli V., Butscheid Y., Escher C., et al. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Mol. Cell. Proteomics. 2015;14:1400–1410. doi: 10.1074/mcp.M114.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Cryo-EM data are available from the Electron Microscopy Data Bank under accession codes: EMD-14561, EMD-14563, EMD-14594, EMD-16582, EMD-14595, EMD-14597, EMD-14598, EMD-16576, EMD-16575, EMD-16579, EMD-16580, EMD-16581, EMD-16583, EMD-16584, EMD-16585, EMD-16586, EMD-16587, EMD-16588, EMD-16589, EMD-14615, EMD-14616, EMD-16577, EMD-16578, EMD-14603, EMD-14604, EMD-14601, EMD-14602, EMD-14599, EMD-14600, EMD-14605, EMD-14606, EMD-14607, EMD-14608, EMD-14609, EMD-14610, EMD-14614, EMD-14613, EMD-14612, EMD-14611, EMD-16617, EMD-16621, EMD-16622, EMD-16623, EMD-16625, EMD-16764, EMD-16765, EMD-16766. PDB coordinates are available from the RCSB Protein Data Bank under accession codes: 7Z8R, 7Z8T, 7ZBW, 7Z8V, 7ZBZ, 8CDK, 8CDJ. Mass spectrometry data have been deposited on the ProteomeXchange Consortium via the PRIDE database under the accession code: PXD038661. Raw image data are deposited in Mendeley under: https://doi.org/10.17632/9c6zfpsxfh.1. Accession code numbers are listed in the key resources table.
-
•
This paper does not report original code. All computational approaches and software used are described in the STAR Methods and listed key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.