Abstract
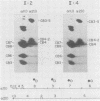
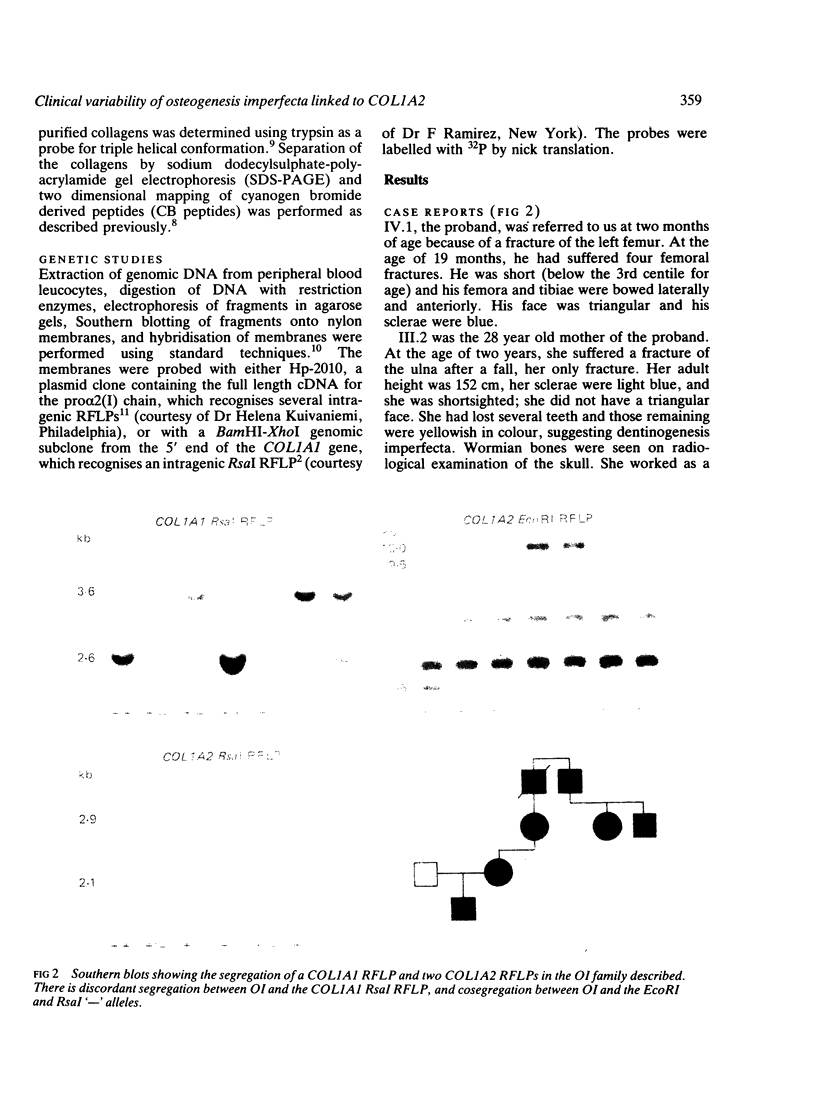
We report a family in which dominant osteogenesis imperfecta segregates with a COL1A2 haplotype and is associated with a structural defect in the helical region of the type I procollagen molecule. All affected subjects had short stature, dentinogenesis imperfecta, and myopia; however, great differences were observed in the number of fractures and in the degree of bone deformity. Identical biochemical changes were found in the type I collagen molecules synthesised by fibroblasts of subjects with severe or minimal bone fragility. These results confirm that mutations in the triple helical region of alpha 2(I) chains produce a milder phenotype than analogous mutations in the alpha 1(I) chains, but indicate that, in addition to defects in the type I collagen molecule, other factors may modulate the degree of bone involvement in osteogenesis imperfecta.
Full text
PDF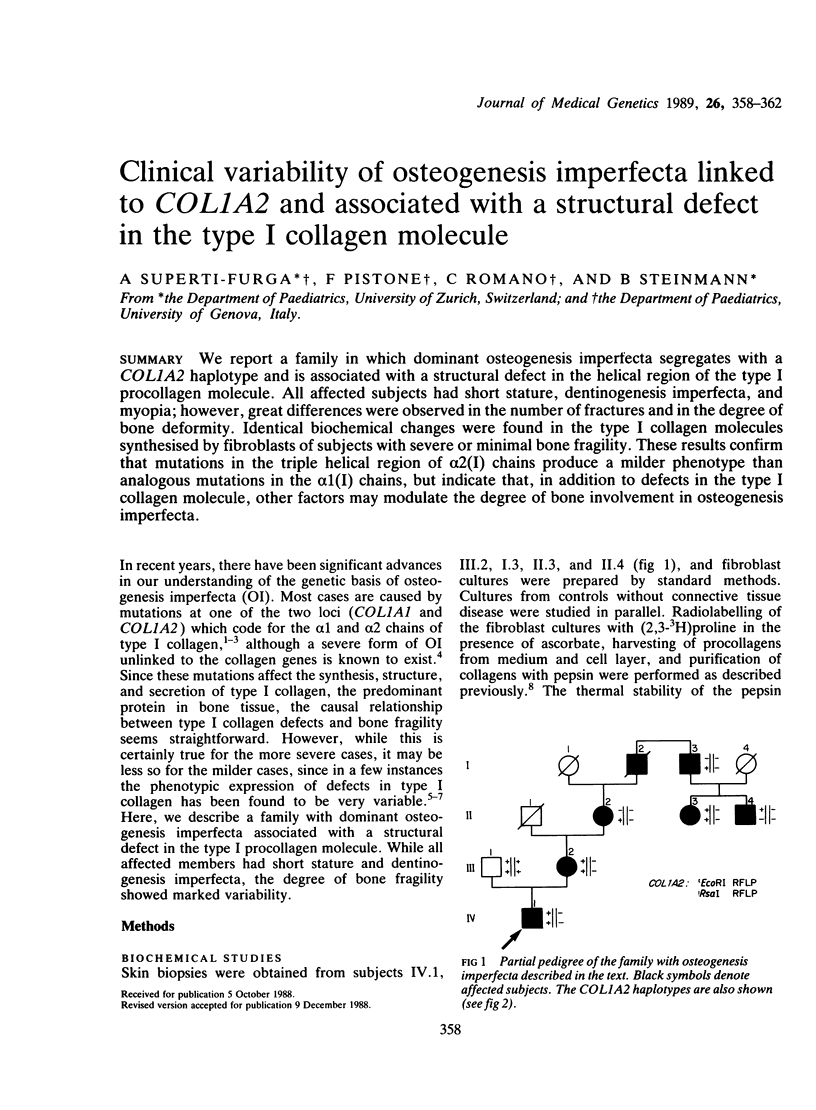



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitchison K., Ogilvie D., Honeyman M., Thompson E., Sykes B. Homozygous osteogenesis imperfecta unlinked to collagen I genes. Hum Genet. 1988 Mar;78(3):233–236. doi: 10.1007/BF00291667. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Tsipouras P., Bonadio J. F., Starman B. J., Schwartz R. C. Perinatal lethal osteogenesis imperfecta (OI type II): a biochemically heterogeneous disorder usually due to new mutations in the genes for type I collagen. Am J Hum Genet. 1988 Feb;42(2):237–248. [PMC free article] [PubMed] [Google Scholar]
- Cohn D. H., Byers P. H. Osteogenesis imperfecta and other inherited disorders of the structure and synthesis of type I collagen: models for the analysis of mutations that result in inherited chondrodysplasias. Pathol Immunopathol Res. 1988;7(1-2):132–138. doi: 10.1159/000157108. [DOI] [PubMed] [Google Scholar]
- Falk C. T., Schwartz R. C., Ramirez F., Tsipouras P. Use of molecular haplotypes specific for the human pro alpha 2(I) collagen gene in linkage analysis of the mild autosomal dominant forms of osteogenesis imperfecta. Am J Hum Genet. 1986 Mar;38(3):269–279. [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Sabol C., Tromp G., Sippola-Thiele M., Prockop D. J. A 19-base pair deletion in the pro-alpha 2(I) gene of type I procollagen that causes in-frame RNA splicing from exon 10 to exon 12 in a proband with atypical osteogenesis imperfecta and in his asymptomatic mother. J Biol Chem. 1988 Aug 15;263(23):11407–11413. [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Chu M. L., Prockop D. J. Structure of a full-length cDNA clone for the prepro alpha 2(I) chain of human type I procollagen. Comparison with the chicken gene confirms unusual patterns of gene conservation. Biochem J. 1988 Jun 15;252(3):633–640. doi: 10.1042/bj2520633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillence D. O., Senn A., Danks D. M. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979 Apr;16(2):101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippola M., Kaffe S., Prockop D. J. A heterozygous defect for structurally altered pro-alpha 2 chain of type I procollagen in a mild variant of osteogenesis imperfecta. The altered structure decreases the thermal stability of procollagen and makes it resistant to procollagen N-proteinase. J Biol Chem. 1984 Nov 25;259(22):14094–14100. [PubMed] [Google Scholar]
- Steinmann B., Superti-Furga A., Royce P. M. Imperfect collagenesis in osteogenesis imperfecta. The consequences of cysteine-glycine substitutions upon collagen structure and metabolism. Ann N Y Acad Sci. 1988;543:47–61. doi: 10.1111/j.1749-6632.1988.tb55315.x. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Gugler E., Gitzelmann R., Steinmann B. Ehlers-Danlos syndrome type IV: a multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J Biol Chem. 1988 May 5;263(13):6226–6232. [PubMed] [Google Scholar]
- Superti-Furga A., Royce P. M., Pistone F. M., Romano C., Steinmann B. Delayed triple-helix formation of abnormal type I collagen is corrected by reduced temperature. Studies of a family with variable expression of osteogenesis imperfecta. Ann N Y Acad Sci. 1988;543:85–92. doi: 10.1111/j.1749-6632.1988.tb55319.x. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A., Steinmann B. Impaired secretion of type III procollagen in Ehlers-Danlos syndrome type IV fibroblasts: correction of the defect by incubation at reduced temperature and demonstration of subtle alterations in the triple-helical region of the molecule. Biochem Biophys Res Commun. 1988 Jan 15;150(1):140–147. doi: 10.1016/0006-291x(88)90497-4. [DOI] [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Anderson, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986 Jul 12;2(8498):69–72. doi: 10.1016/s0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- Wenstrup R. J., Cohn D. H., Cohen T., Byers P. H. Arginine for glycine substitution in the triple-helical domain of the products of one alpha 2(I) collagen allele (COL1A2) produces the osteogenesis imperfecta type IV phenotype. J Biol Chem. 1988 Jun 5;263(16):7734–7740. [PubMed] [Google Scholar]
- Wenstrup R. J., Tsipouras P., Byers P. H. Osteogenesis imperfecta type IV. Biochemical confirmation of genetic linkage to the pro alpha 2(I) gene of type I collagen. J Clin Invest. 1986 Dec;78(6):1449–1455. doi: 10.1172/JCI112735. [DOI] [PMC free article] [PubMed] [Google Scholar]