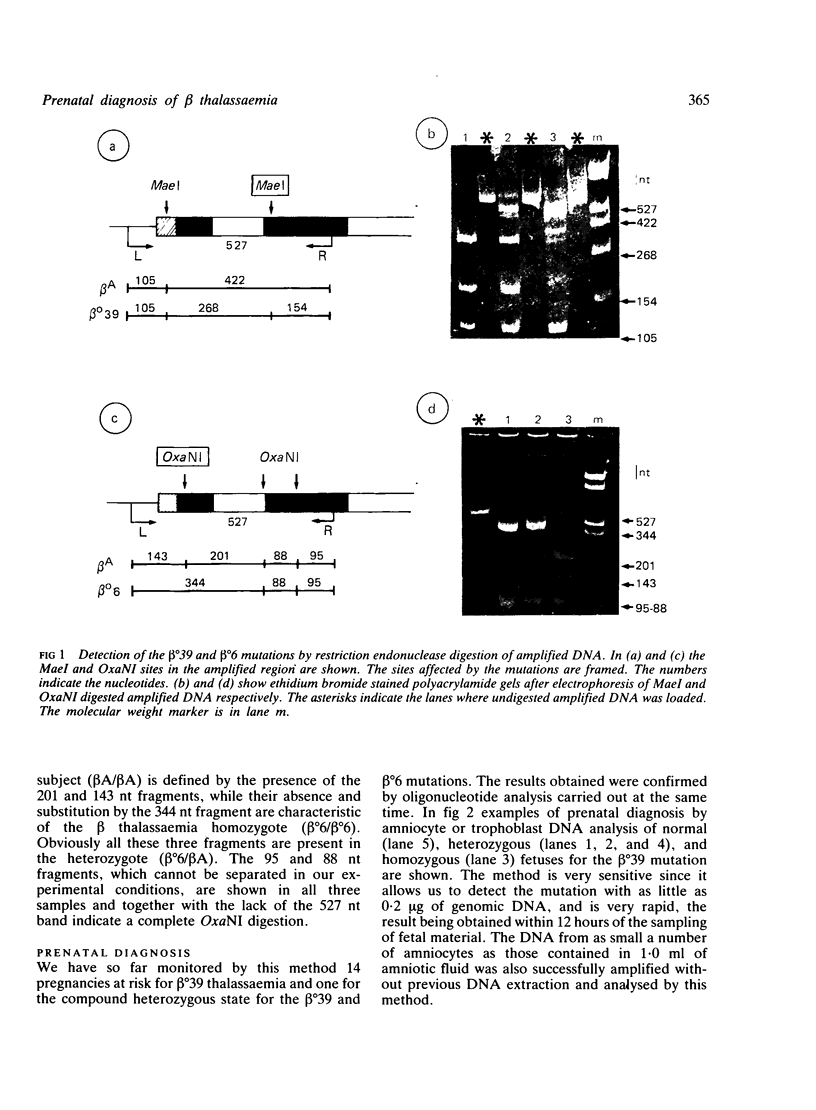
Abstract
In the Mediterranean area, 50% of the beta thalassaemia mutations abolish or create a restriction endonuclease site in the beta globin gene. This study describes a new procedure for prenatal detection of these beta thalassaemia defects based on the direct visualisation, on an ethidium bromide stained polyacrylamide gel, of the discrete DNA fragments produced by restriction endonuclease digestion of fetal DNA, enzymatically amplified using the DNA polymerase from the thermophilus bacterium Thermus aquaticus. We applied this procedure to the Sardinian population to detect the nonsense mutation at codon 39 and the frameshift at codon 6 of the beta globin gene; these are the most frequent beta thalassaemia mutations in this population, accounting for 95% and 2.2% of the beta thalassaemia chromosomes. The main advantages of this procedure are simplicity (no radioactivity), sensitivity (0.2 microgram of DNA), and rapidity (12 hours). The very small amount of fetal material required makes amniotic fluid cell culture unnecessary and may decrease the fetal loss rate associated with trophoblast sampling. By circumventing the use of radioactive and non-radioactive probes, the spread of this technology to the high risk areas will be facilitated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arous N., Galacteros F., Fessas P., Loukopoulos D., Blouquit Y., Komis G., Sellaye M., Boussiou M., Rosa J. Structural study of hemoglobin Knossos, beta 27 (B9) Ala leads to Ser. A new abnormal hemoglobin present as a silent beta-thalassemia. FEBS Lett. 1982 Oct 18;147(2):247–250. doi: 10.1016/0014-5793(82)81052-1. [DOI] [PubMed] [Google Scholar]
- Baird M., Driscoll C., Schreiner H., Sciarratta G. V., Sansone G., Niazi G., Ramirez F., Bank A. A nucleotide change at a splice junction in the human beta-globin gene is associated with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4218–4221. doi: 10.1073/pnas.78.7.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao A., Pirastu M., Rosatelli C. The prenatal diagnosis of thalassaemia. Br J Haematol. 1986 Jun;63(2):215–220. doi: 10.1111/j.1365-2141.1986.tb05543.x. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Alberti A., Kan Y. W. A beta-thalassemia lesion abolishes the same Mst II site as the sickle mutation. Nucleic Acids Res. 1983 Nov 25;11(22):7789–7794. doi: 10.1093/nar/11.22.7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chehab F. F., Doherty M., Cai S. P., Kan Y. W., Cooper S., Rubin E. M. Detection of sickle cell anaemia and thalassaemias. Nature. 1987 Sep 24;329(6137):293–294. doi: 10.1038/329293b0. [DOI] [PubMed] [Google Scholar]
- Chibani J., Vidaud M., Duquesnoy P., Bergé-Lefranc J. L., Pirastu M., Ellouze F., Rosa J., Goossens M. The peculiar spectrum of beta-thalassemia genes in Tunisia. Hum Genet. 1988 Feb;78(2):190–192. doi: 10.1007/BF00278196. [DOI] [PubMed] [Google Scholar]
- Chien A., Edgar D. B., Trela J. M. Deoxyribonucleic acid polymerase from the extreme thermophile Thermus aquaticus. J Bacteriol. 1976 Sep;127(3):1550–1557. doi: 10.1128/jb.127.3.1550-1557.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman J. G., Huisman T. H., Stojanovski N., Efremov G. D. Characterization of the beta+-thalassemia mutation in a homozygous Yugoslavian patient. Hemoglobin. 1984;8(5):529–534. doi: 10.3109/03630268408991739. [DOI] [PubMed] [Google Scholar]
- Gomes M. P., da Costa M. G., Braga L. B., Cordeiro-Ferreira N. T., Loi A., Pirastu M., Cao A. Beta-thalassemia mutations in the Portuguese population. Hum Genet. 1988 Jan;78(1):13–15. doi: 10.1007/BF00291226. [DOI] [PubMed] [Google Scholar]
- Goossens M., Dumez Y., Kaplan L., Lupker M., Chabret C., Henrion R., Rosa J. Prenatal diagnosis of sickle-cell anemia in the first trimester of pregnancy. N Engl J Med. 1983 Oct 6;309(14):831–833. doi: 10.1056/NEJM198310063091405. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Boehm C. D., Sexton J. P., Antonarakis S. E. beta-Thalassemia due to a deletion of the nucleotide which is substituted in the beta S-globin gene. Am J Hum Genet. 1983 Sep;35(5):1028–1033. [PMC free article] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Loi A., Pirastu M., Cao A., Ulbridh R., Hansmann I. Prenatal diagnosis of most common Mediterranean beta-thalassaemia mutants. Lancet. 1986 Feb 1;1(8475):274–274. doi: 10.1016/s0140-6736(86)90808-1. [DOI] [PubMed] [Google Scholar]
- Modell B. Chorionic villus sampling. Evaluating safety and efficacy. Lancet. 1985 Mar 30;1(8431):737–740. doi: 10.1016/s0140-6736(85)91272-3. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Markham A. F., Kazazian H. H., Jr Direct detection of the common Mediterranean beta-thalassemia gene with synthetic DNA probes. An alternative approach for prenatal diagnosis. J Clin Invest. 1983 Mar;71(3):775–779. doi: 10.1172/JCI110826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirastu M., Kan Y. W., Cao A., Conner B. J., Teplitz R. L., Wallace R. B. Prenatal diagnosis of beta-thalassemia. Detection of a single nucleotide mutation in DNA. N Engl J Med. 1983 Aug 4;309(5):284–287. doi: 10.1056/NEJM198308043090506. [DOI] [PubMed] [Google Scholar]
- Rosatelli C., Falchi A. M., Tuveri T., Scalas M. T., Di Tucci A., Monni G., Cao A. Prenatal diagnosis of beta-thalassaemia with the synthetic-oligomer technique. Lancet. 1985 Feb 2;1(8423):241–243. doi: 10.1016/s0140-6736(85)91026-8. [DOI] [PubMed] [Google Scholar]
- Rosatelli C., Leoni G. B., Tuveri T., Scalas M. T., Di Tucci A., Cao A. Beta thalassaemia mutations in Sardinians: implications for prenatal diagnosis. J Med Genet. 1987 Feb;24(2):97–100. doi: 10.1136/jmg.24.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Thein S. L., Wainscoat J. S., Lynch J. R., Weatherall D. J., Sampietro M., Fiorelli G. Direct detection of beta zero 39 thalassaemic mutation with Mae 1. Lancet. 1985 May 11;1(8437):1095–1095. doi: 10.1016/s0140-6736(85)92390-6. [DOI] [PubMed] [Google Scholar]
- Thein S. L., Wainscoat J. S., Old J. M., Sampietro M., Fiorelli G., Wallace R. B., Weatherall D. J. Feasibility of prenatal diagnosis of beta-thalassaemia with synthetic DNA probes in two Mediterranean populations. Lancet. 1985 Aug 17;2(8451):345–347. doi: 10.1016/s0140-6736(85)92493-6. [DOI] [PubMed] [Google Scholar]
- Trecartin R. F., Liebhaber S. A., Chang J. C., Lee K. Y., Kan Y. W., Furbetta M., Angius A., Cao A. beta zero thalassemia in Sardinia is caused by a nonsense mutation. J Clin Invest. 1981 Oct;68(4):1012–1017. doi: 10.1172/JCI110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R., Eskdale J., Coleman D. V., Niazi M., Loeffler F. E., Modell B. M. Direct gene analysis of chorionic villi: A possible technique for first-trimester antenatal diagnosis of haemoglobinopathies. Lancet. 1981 Nov 21;2(8256):1125–1127. doi: 10.1016/s0140-6736(81)90583-3. [DOI] [PubMed] [Google Scholar]