Abstract
Use of the nonpathogenic yeast Saccharomyces boulardii in the treatment of infectious diarrhea has attracted growing interest. The present study designed to investigate the effect of this yeast on enteropathogenic Escherichia coli (EPEC)-associated disease demonstrates that S. boulardii abrogated the alterations induced by an EPEC strain on transepithelial resistance, [3H]inulin flux, and ZO-1 distribution in T84 cells. Moreover, EPEC-mediated apoptosis of epithelial cells was delayed in the presence of S. boulardii. The yeast did not modify the number of adherent bacteria but lowered by 50% the number of intracellular bacteria. Infection by EPEC induced tyrosine phosphorylation of several proteins in T84 cells, including p46 and p52 SHC isoforms, that was attenuated in the presence of S. boulardii. Similarly, EPEC-induced activation of the ERK1/2 mitogen-activated protein (MAP) kinase pathway was diminished in the presence of the yeast. Interestingly, inhibition of the ERK1/2 pathway with the specific inhibitor PD 98059 decreased EPEC internalization, suggesting that modulation of the ERK1/2 MAP pathway might account for the lowering of the number of intracellular bacteria observed in the presence of S. boulardii. Altogether, this study demonstrated that S. boulardii exerts a protective effect on epithelial cells after EPEC adhesion by modulating the signaling pathway induced by bacterial infection.
Saccharomyces boulardii is a thermophilic, nonpathogenic yeast administered in Western Europe for the prevention and treatment of a variety of diarrheal diseases (17, 29). However, the mechanisms by which S. boulardii controls diarrhea remain elusive. The efficacy of this yeast has been attributed to several of its properties, such as its effect on the mucosa leading to an increase in dissaccharidase activity (8) or stimulation of the immune response (7). In animals, administration of S. boulardii provides protection against intestinal lesions caused by several diarrheal pathogens (10, 33). In vitro studies have demonstrated that S. boulardii exerts antagonistic activity against various bacterial pathogens (6). Recent studies have reported the adhesion of the Salmonella enterica serovars Typhimurium and Enteritis and of enteropathogenic Escherichia coli (EPEC) and enterohemorrhagic E. coli to S. boulardii (24, 25).
EPEC is a major cause of diarrhea in the developing world (31, 34). The pathogenesis of EPEC infections involves a three-stage process. (i) EPEC adheres initially to intestinal epithelial cells in a pattern described as localized adherence (36). This pattern of adherence, characterized by microcolonies of bacteria associated with the epithelial cells, is dependent on the expression of the bacterial type IV bundle-forming pilus (BFP) (3). (ii) Next, the bacteria induce signal transduction pathways in host cells, leading to an elevation in the intracellular levels of Ca2+ and inositol triphosphate (16, 23) and the phosphorylation of cellular proteins (4, 35, 41). (iii) These signaling events culminate in the formation of attaching-and-effacing lesions which are characterized by localized degeneration of the microvilli, intimate contact between the bacteria and the infected cell, and the assembly of highly organized cytoskeletal structures in the epithelial cells just beneath the attached bacteria, forming cuplike pedestals (22, 27, 30). EPEC is also able to induce its internalization by nonphagocytic epithelial cells (2, 15).
The aim of our study was to investigate in vitro the effect of S. boulardii against EPEC infection using the T84 cell line derived from a colon carcinoma. This cell line has been extensively used to elucidate the mechanism of EPEC-induced diarrhea. EPEC infection results in a modification of the T84 barrier function, characterized by a drop in transepithelial resistance, an increase in permeability, and modification of the distribution of the tight junction-associated protein ZO-1 (32, 37). Our study reveals that S. boulardii maintains the barrier function and the viability of EPEC-infected T84 cells. Although the yeast does not modify the number of cell-associated bacteria, it reduces the number of intracellular bacteria. The phosphorylation of several proteins induced by EPEC in T84 cells is diminished in the presence of S. boulardii. Finally, the yeast interferes with the ERK1/2 mitogen-activated protein (MAP) kinase pathway that, as demonstrated in this study, is implicated in the invasive process of EPEC.
MATERIALS AND METHODS
Cell line, media, and bacterial and yeast strains.
The human colon T84 cell line was obtained from the European Collection of Animal Cell Cultures (Salisbury, England). The T84 culture medium contained a 1:1 mixture of Dulbecco-Vogt modified Eagle medium and Ham's F-12 medium (DMEM–F-12) supplemented with 50 μg of penicillin and 50 μg of streptomycin (Sigma) per ml and 5% fetal bovine serum (DAP). The bacterial wild-type (WT) strain E2348/69 (kindly provided by J. Kaper, Center for Vaccine Development, University of Maryland, Baltimore) was grown overnight in Luria-Bertani medium at 37°C, without shaking. The yeast S. boulardii (Laboratories Biocodex, Paris, France) was grown at 37°C, with shaking, in Halvorston minimal medium with 2% glucose.
Inhibitor.
The MEK1 inhibitor PD 98059 (1) (Calbiochem) was stored in dimethyl sulfoxide (DMSO) at −20°C.
Electrical resistance measurements.
T84 cells were grown on 4.6-cm2 porous filter membranes (0.4-μm pores; Nunc). Transmonolayer electrical resistance (TER) was measured with the Millicell-ERS apparatus (Millipore, Molsheim, France) as described previously (21). Under these conditions, high TER values (>1,000 Ω·cm2) were consistently obtained in 14-day postseeding monolayers.
Infection of filter-grown T84 monolayers with EPEC.
Prior to infection, the T84 medium was changed to medium without serum and antibiotics (DMEM–F-12). As previously reported (41), approximately 108 EPEC (or 100 bacteria/cell) were added to the apical surface of T84 monolayers and incubated at 37°C in a 5% CO2, water-jacketed incubator. When infection was performed in the presence of yeast, 10 yeasts/cell were added. This ratio did not modify intestinal cell viability (13). At the indicated times, transepithelial resistance, bacterial adhesion and invasion, inulin passage, and ZO-1 distribution were measured as described hereafter.
Adhesion and invasion assays.
Bacterial adhesion to T84 cells was quantified using the plate dilution method (35). Briefly, at the times of infection indicated in the legend to Table 1, bacteria present in the culture medium were eliminated by extensive washes with sterile phosphate-buffered saline (PBS). Cells were then trypsinized and lysed in water containing 0.1% bovine serum albumin (BSA). The cell lysates contained “cell-associated bacteria” corresponding to adherent as well as intracellular bacteria. For the determination of invasion, after PBS washes, monolayers were incubated for an additional hour with DMEM–F-12 containing 100 μg of gentamicin per ml. Since gentamicin was not concentrated in epithelial cells, intracellular bacteria survived to the incubation, while adherent and extracellular bacteria were killed (19). The monolayers were then washed with sterile PBS, and epithelial cells with intracellular bacteria were detached by trypsin and lysed as described elsewhere (35). The percentage of invasion was calculated as follows: percent invasion = number of intracellular bacteria/number of cell-associated (adherent and intracellular) bacteria.
TABLE 1.
Effect of S. boulardii on [3H]inulin flux across filter-grown T84 cells after EPEC infectiona
| Cell treatment | Mean [3H]inulin penetration (cpm) ± SEM after:
|
||
|---|---|---|---|
| 6 h | 12 h | 24 h | |
| None | 331 ± 64 | 740 ± 84 | 1,244 ± 156 |
| EPEC 2348/69 | 494 ± 24 | 2,324 ± 254∗ | 4,310 ± 223∗ |
| EPEC 2348/69 + S. boulardii | 488 ± 73 | 1,972 ± 245 | 2,489 ± 180 |
Bacteria (100/cell), yeasts (10/cell), and [3H]inulin were added apically. At 6, 12, and 24 h after infection, counts-per-minute values were determined in 100-μl aliquots removed from the basolateral compartment. The [3H]inulin flux was significantly increased in EPEC-infected cells (∗, significantly different versus controls) but remained comparable to and not significantly different from control monolayers in cells infected in the presence of S. boulardii. Each point corresponds to the mean value of at least four individual T84 monolayers.
Distribution of ZO-1.
ZO-1 distribution was analyzed after infection using immunochemistry and confocal microscopy. At the times indicated, the monolayers were washed extensively with PBS and fixed with 2% paraformaldehyde for 30 min. The cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min and then washed with PBS. Polyclonal rabbit anti-ZO-1 antibody (Zymed 61-7300) was incubated with the permeabilized cells for 45 min at 37°C. The monolayers were washed and then treated with fluorescein-conjugated anti-rabbit immunoglobulin G (Dakopatts F205) for 45 min at 37°C. After washes with PBS, the filters were excised from the supports, mounted, and observed under a Zeiss confocal laser scanning microscope.
Measurement of [3H]inulin passage.
Polarized monolayers of filter-grown T84 cells were infected with EPEC for 3 h as described above. After measurement of transepithelial resistance, 220,000 cpm of [3H]inulin (Mr, 5,200; hydrodynamic diameter, 11.5 Å; Amersham) was added to the apical surface. Unlabeled inulin (0.5 mM) was present in both apical and basolateral incubation medium. After a 2-h equilibration period, 100 μl (5%) of the basolateral fluid was removed at 1-h intervals and counted.
Preparation of cell lysates for Western blotting and immunoprecipitation.
T84 cells were seeded in 100-mm petri tissue culture dishes. At 70 to 90% confluence, the monolayers were washed twice with serum-free DMEM–F-12 and then grown in fresh culture medium supplemented with 0.1% BSA (Sigma A7030) for 12 h. Infection was carried out by the addition of 500 μl of a late-logarithmic bacterial culture (100 bacteria/cell) alone or in the presence of S. boulardii (10 yeasts/cell). At the times indicated, the infected cells were washed with PBS and solubilized for 30 min at 4°C in lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1% Nonidet P-40 [NP-40]; 2 mM Na3VO4; 1 mM EDTA; 1 μM aprotinin; 25 μM leupeptin; 1 μM pepstatin; 1 mM AEBSF; 10 mM NaF; 5 mM sodium PPi; 10 mM β-glycerophosphate). The lysate was sonicated and centrifuged at 15,000 rpm for 15 min at 4°C. The protein content of the supernatant was determined using Bio-Rad DC reagents. Immunoprecipitation and Western blotting were carried out as previously described (5).
Statistical analysis.
Results are presented as the mean ± the standard error of the mean (SEM). Tests of statistical significance were done by analysis of variance with the StatView program for Macintosh followed by the post hoc comparison with the Bonferroni-Dunn tests.
RESULTS
S. boulardii prevents the EPEC-induced decrease of T84 monolayer resistance.
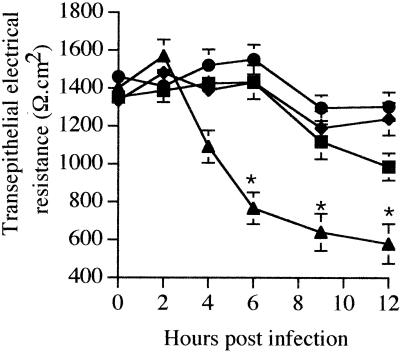
To determine the effect of S. boulardii on the EPEC-induced decrease of transepithelial resistance, T84 monolayers were apically infected with the E2348/69 strain alone or in the presence of S. boulardii, and the transepithelial resistance was monitored over 12 h. As shown in Fig. 1, incubation of T84 monolayers with S. boulardii alone had no effect on transepithelial resistance. In EPEC-infected cells, monolayer resistance was unchanged up until 4 h; at 6 h it had dropped significantly to 767 ± 84 Ω·cm2 (P < 0.02 versus control monolayers) and reached a plateau by 12 h of infection. In contrast, when infection was performed in the presence of S. boulardii, the transepithelial resistance remained at the level of uninfected monolayers up until 9 h of infection. At 12 h of infection, the resistance of these monolayers dropped slightly but did not differ significantly from control monolayers (P > 0.05). The barrier function and the viability of EPEC-infected T84 cells were thus preserved in the presence of S. boulardii.
FIG. 1.
S. boulardii prevents EPEC-induced decrease of transepithelial resistance in T84 monolayers. Bacteria (100 bacteria/cell) and yeast (10 yeasts/cell) were added to the apical surface of T84 cells. Resistance decreased in cells infected with EPEC alone (▴) but remained comparable to control monolayers (●) in cells infected in the presence of S. boulardii (■). S. boulardii alone did not affect the transepithelial resistance of T84 monolayers during the time of these experiments (⧫). Each point represents the mean value obtained from at least six individual T84 monolayers. Error bars show the standard deviation. The asterisk denotes a significant difference versus the control monolayers (P < 0.02) when compared by the Bonferroni-Dunn tests.
S. boulardii maintains the tight-junction structure of EPEC-infected T84 cells.
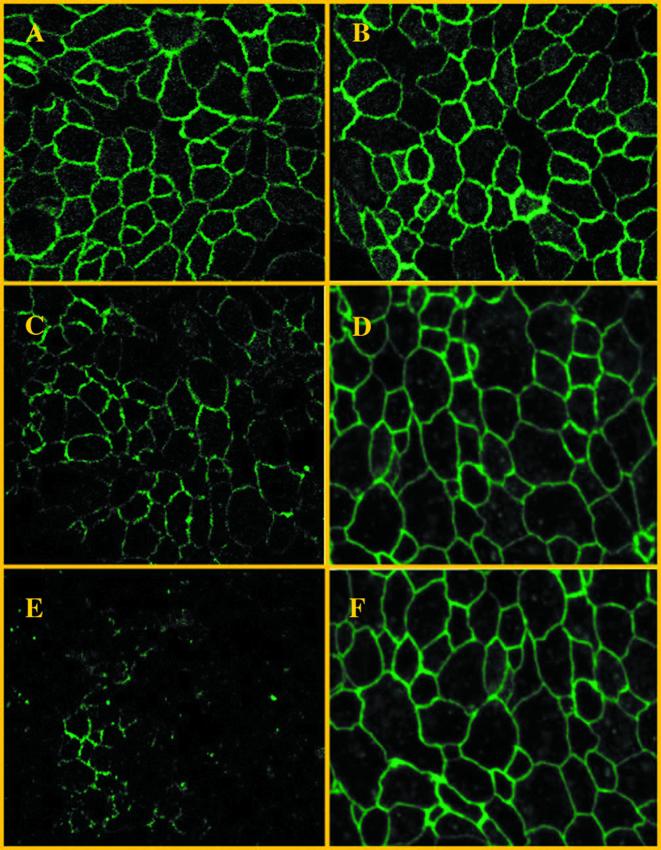
The decrease in transepithelial resistance is associated with the disruption of actin filaments and thereby with morphological modification of the structure of the tight junctions. To investigate the effect of S. boulardii on EPEC-induced changes in tight-junction structure, confocal microscopy was used to analyze the distribution of Zonula Occludens (ZO-1), a tight-junction-associated protein, in cells infected by E2348/69 alone or in the presence of yeast. Uninfected T84 monolayers exhibited (Fig. 2A) a well-defined ZO-1 staining pattern in the perijunctional region that remained unchanged in cells maintained in the presence of S. boulardii (Fig. 2B). In contrast, T84 monolayers infected for 6 h with E2348/69 showed diffuse ZO-1 staining (Fig. 2C) that disappeared after 12 h of infection (Fig. 2E). In contrast, ZO-1 protein distribution was preserved in cells infected by EPEC for 6 h (Fig. 2D) and 12 h (Fig. 2F) in the presence of S. boulardii, strongly supporting a protective role of S. boulardii on the tight junction structure in EPEC-infected T84 monolayers.
FIG. 2.
Representative confocal laser scanning micrographs (z-series, overlay) of T84 cell monolayers immunostained for ZO-1 with antibodies for this protein, followed by secondary antibodies conjugated to fluorescein isothiocyanate. (A and B) Normal distribution of ZO-1 in uninfected cells (A) and in cells exposed to S. boulardii (B). (C and E) Monolayers infected with E2348/69 alone for 6 h (C) and 12 h (E). (D and F) Monolayers infected for 6 h (D) or 12 h (F) in the presence of S. boulardii. Original magnification, ×2,400. The same results were obtained at least three times.
S. boulardii reduces the [3H]inulin flux in EPEC-infected T84 cells.
In intestinal epithelium, paracellular transport of molecules was under the control of tight junctions. Inulin (an inert compound with a molecular weight of 5,200 and an 11.5-Å radius) has been used as a marker of the gate function of tight junctions. To further examine the effect of S. boulardii on EPEC-induced changes in paracellular transport, [3H]inulin was added to the apical surface of T84 monolayers infected for 6, 12, and 24 h with E2348/69 alone or in the presence of S. boulardii. Inulin flux was measured by assaying the radioactivity that crossed the monolayers to the basolateral medium. As expected, [3H]inulin did not penetrate uninfected T84 monolayers with a high electrical resistance (Table 1). In monolayers infected for 12 and 24 h, [3H]inulin passage was significantly increased compared to control monolayers. In cells infected by EPEC in the presence of S. boulardii, [3H]inulin passage through T84 monolayers was significantly lower. This result indicates that the gate function of tight junctions was maintained in cells infected in the presence of yeast. Since increasing inulin flux was associated with cell death (28), this prompted us to investigate the effect of S. boulardii on EPEC-induced apoptosis.
S. boulardii prevents the caspase-3 activation induced by EPEC in T84 cells.
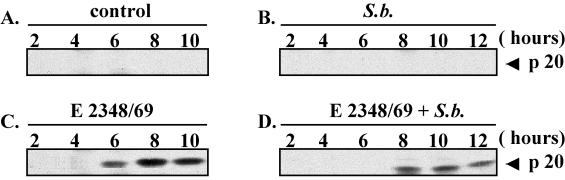
Programmed cell death, or apoptosis, can be initiated by a wide variety of stresses, including bacterial infection (42). The caspases are proteases that play a key role in the initiation and the execution of apoptosis. Among them, cpp32 (caspase-3) is the most extensively studied apoptotic caspase. It is synthesized as an inactive proenzyme of 32 kDa that is cleaved in cells undergoing apoptosis into two active forms of 12 and 20 kDa. We therefore investigated the generation of the active form of cpp32 (p20) in T84 cells infected by EPEC alone or in the presence of S. boulardii. As shown in Fig. 3B, the yeast alone did not induce the cleavage of cpp32 in T84 cells, indicating that S. boulardii did not affect cell viability. By contrast, accumulation of the activated p20cpp32 form was visualized after 6 h in cells infected with EPEC alone (Fig. 3C) but was absent in cells infected in the presence of S. boulardii (Fig. 3D). When yeast was present, the p20cpp32 form was detectable only after 8 h of infection. These results indicate that the apoptotic program induced by EPEC infection in T84 cells is delayed in the presence of yeast.
FIG. 3.
S. boulardii delays the activation of caspase-3 in EPEC-infected cells. T84 cells were infected with E2348/69 alone (C) or in the presence of S. boulardii (D). After various periods of infection, the cells were lysed and samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 9% polyacrylamide gel and analyzed by immunoblotting using anti-cpp32 antibody. The anti-caspase-3 recognizes the 32-kDa proform and the 20-kDa intermediate active form. (A) Control cells. (B) Cells incubated for various times with S. boulardii alone. The same results were obtained at least three times.
S. boulardii does modify the number of intracellular bacteria.
Since adhesion to intestinal cells is the first step in EPEC pathogenicity, we investigated the effect of S. boulardii on the number of bacteria adherent to T84 cells. The data in Table 2 reveal that S. boulardii did not modify the number of cell-associated (adherent and intracellular) bacteria. EPEC has been shown to be invasive in some culture systems (2, 15, 35). The invasion assay was therefore performed using gentamicin on cells infected in the absence or presence of S. boulardii. Use of this standard assay revealed that the number of intracellular bacteria recovered in T84 cells infected with EPEC alone ([4.38 ± 0.72] × 104 CFU/well) was significantly decreased, by 50% ([2.2 ± 0.41] × 104 CFU/well), in cells infected in the presence of S. boulardii.
TABLE 2.
Effect of S. boulardii on the ability of EPEC 2348/69 to adhere to and invade T84 cellsa
| Cell treatment | Mean no. ± SEM of:
|
% Invasion | |
|---|---|---|---|
| Cell-associated bacteria (106 CFU/well) | Intracellular bacteria (104 CFU/well) | ||
| EPEC | 2.84 ± 0.40 | 4.38 ± 0.72 | 1.54 |
| EPEC + S. boulardii | 2.49 ± 0.32 | 2.21 ± 0.41* | 0.88* |
Filter-grown T84 cells were infected for 6 h with bacteria (100/cell) alone or in the presence of S. boulardii (10/cell). Invasion was assessed by the gentamicin protection method. ∗, P < 0.04. Each point represents the mean value obtained from 10 to 12 individual T84 monolayers.
S. boulardii alters EPEC-induced tyrosine phosphorylation of T84 proteins.
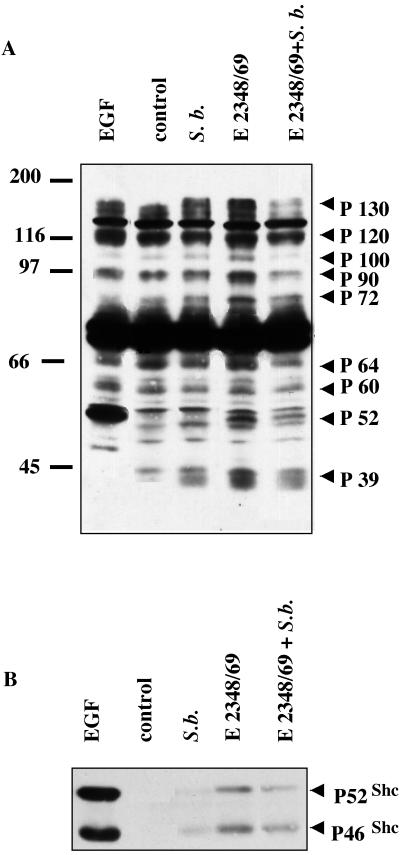
Bacteria trigger signals into the host cells that lead to their internalization (4). The decrease in the number of intracellular bacteria in the presence of S. boulardii prompted us to investigate the effect of the yeast on EPEC-induced transduction signals. The time course of infection showed that 1 h of infection is sufficient to induce protein tyrosine phosphorylation in T84 cells (data not shown). Therefore, T84 cells were infected for 1 h with EPEC alone or in the presence of yeast, and the whole-cell lysates were analyzed by anti-phosphotyrosine (αP-Tyr) Western blotting (Fig. 4A). In agreement with results obtained in EPEC-infected HeLa cells (35), EPEC infection of T84 cells induced the phosphorylation of several proteins (indicated by arrows in Fig. 4A). These proteins presented various molecular sizes: 130, 120, 100, 90, 72, 64, 60, 52, and 39 kDa. Coinfection in the presence of the yeast significantly decreased the degree of phosphorylation of most of these proteins, supporting the idea that S. boulardii might modify cellular response(s) to EPEC infection.
FIG. 4.
S. boulardii significantly affects EPEC-induced signaling in T84 cells. (A) T84 cells were infected with E2348/69 alone or in the presence of yeast cells. After a 1-h infection, the cells were lysed and samples were resolved by SDS-PAGE using a 9% polyacrylamide gel and analyzed by immunoblotting with αP-Tyr antibody. The control lane corresponds to uninfected cells. A positive control lane was obtained using cells treated for 15 min with 10 nM EGF. The positions of the molecular mass standards are shown on the left of the blot in kilodaltons. The proteins phosphorylated in infected T84 cells are indicated by arrows. (B) Lysates from uninfected T84 cells (control) and cells infected for 1 h with the WT strain (E2348/69) alone or in the presence of S. boulardii were immunoprecipitated with anti-SHC antibody. The immunocomplex was subjected to SDS-PAGE using a 9% polyacrylamide gel and then analyzed by immunoblotting using αP-Tyr antibody. A positive control was obtained using cells treated for 15 min with 10 nM EGF. The same results were obtained at least three times.
S. boulardii modifies tyrosine phosphorylation of SHC in T84 cells infected by the WT EPEC strain.
The anti-phosphotyrosine antibody reacted strongly with protein localized at a molecular mass of 52 kDa (Fig. 4A). This protein presented the same electrophoretic mobility as the major phosphorylated protein in EGF-treated T84 cells. Phosphorylation of this protein was significantly decreased in T84 cells infected in the presence of S. boulardii. In order to verify that this phosphoprotein corresponded to p52 SHC, immunoprecipitation was performed using an anti-SHC antibody, followed by αP-Tyr Western blotting. The results presented in Fig. 4B showed tyrosine phosphorylation of the p46 and p52 SHC isoforms in cells infected for 1 h with EPEC and in epidermal growth factor (EGF)-treated cells used as a positive control. Since phosphorylation of the p46 and p52 SHC isoforms was significantly decreased in cells infected in the presence of yeast, S. boulardii can thus downregulate the SHC downstream cellular response induced in T84 cells by EPEC infection.
S. boulardii decreases the activation of ERK1/2 MAP kinases by the WT EPEC strain.
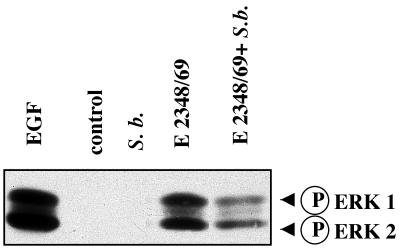
Since SHC is an upstream regulatory protein of the ERK1/2 MAP kinase pathway, the effect of the yeast on activation of the ERK1/2 pathway was thus investigated by Western blotting using an antiphosphorylated ERK1/2 antibody (Fig. 5). The activated forms of ERK1/2 were not detected in cell lysates obtained after a 1-h incubation with S. boulardii alone. Consistent with the SHC phosphorylation, active forms of ERK1/2 (p42 and p44) were present in cells infected for 1 h by EPEC. When infection was performed in the presence of S. boulardii, phosphorylation of both ERK1 and ERK2 was significantly decreased. Western blotting using a nonphosphorylated form of ERK1/2 revealed the presence of the same amount of these proteins (p42 and p44) (data not shown). S. boulardii thus decreased the activation of ERK1/2 induced by EPEC infection of T84 cells.
FIG. 5.
S. boulardii prevents the activation of ERK1/2 kinases in E2348/69-infected cells. T84 cells were infected with the WT strain E2348/69 alone or in the presence of S. boulardii. After 1 h of infection, cells were lysed and samples were resolved by SDS-PAGE using a 9% polyacrylamide gel and then analyzed by immunoblotting with anti-phosphorylated ERK1/2 antibodies. The control line corresponds to uninfected T84 cells. A positive control was obtained using cells treated for 15 min with 10 nM EGF. The same results were obtained at least three times.
Inhibition of invasion by PD 98059 treatment: correlation of invasion with the activation of ERK1/2 pathway.
Since we have shown that S. boulardii decreased both EPEC invasion and EPEC-induced MAP activation and since activation of ERK1/2 kinases has been implicated in the invasion of Listeria monocytogenes (38), we therefore addressed whether the activation of ERK1/2 MAP kinases could be involved in the invasion process of EPEC. To this end, T84 cells were preincubated for 90 min with 50 μM PD98059, an MEK1–ERK1/2 pathway inhibitor, prior to being exposed to bacterial infection. Even though PD98059 did not modify the number of cell-associated bacteria, EPEC invasion was reduced by 60% in cells incubated with PD98059 (Table 3), indicating that ERK1/2 MAP kinase activation was involved in the invasion process of EPEC into T84 cells.
TABLE 3.
Effect of PD 98059 on EPEC adhesion and invasiona
| Cell treatment | Mean no. ± SEM of:
|
% Invasion | |
|---|---|---|---|
| Cell-associated bacteria (105 CFU/well) | Intracellular bacteria (104 CFU/well) | ||
| EPEC | 1.95 ± 0.55 | 2.12 ± 0.22 | 10.0 |
| EPEC + DMSO | 2.76 ± 0.21 | 2.28 ± 0.3 | 8.4 |
| EPEC + PD 98059 | 2.0 ± 0.2 | 0.86 ± 0.164* | 4.3* |
Filter-grown T84 cells were pretreated for 90 min with PD 98059 prior to infection. To avoid any toxic effect of this inhibitor, cells were infected for 3 h with EPEC strain E2348/69 (100 bacteria/cell) alone or in the presence of DMSO (1:5,000) or PD 98059 (50 μM). Invasion was assessed by the gentamicin protection method. DMSO or PD 98059 was present during gentamicin incubation. Values were compared by Bonferroni-Dunn test, and asterisks denote when the difference was significant (P < 0.04) versus EPEC alone. Each point represents the mean value obtained from at least three individual T84 monolayers.
DISCUSSION
Monolayers of cultured polarized epithelial cells grown on filters were used to demonstrate that EPEC interaction with the apical cell surface induced a marked decrease in transepithelial resistance. Interestingly, S. boulardii prevented the decrease of transepithelial electrical resistance in EPEC-infected T84 cells. Since transepithelial resistance reflects the barrier function and the viability of monolayers, we investigated whether S. boulardii preserves these two parameters in infected T84 cells. In EPEC-infected cells, alteration of the tight junction structure modifies epithelial permeability to solutes transported through the paracellular pathway (32). Since tight junctions are size selective, the flux of inulin (a molecule with an 11.5-Å radius versus 6.7 Å for mannitol) increases only after a long period of infection (12 and 24 h), when the transepithelial resistance has been considerably lowered. Inulin flux was significantly attenuated in cells infected in the presence of yeasts. Since paracellular permeability to inert probes reflects the gate function of tight junctions (11, 28), the decrease in inulin flux suggests that damage to the intercellular tight junction caused by EPEC infection was reduced in the presence of yeasts. This observation was supported by the immunolocalization of ZO-1. This tight-junction-associated protein disappeared in cells infected with EPEC alone but remained present in cells when infection was performed in the presence of yeast.
Since inulin flux also reflects the killing effect of EPEC on T84 cells (28), the fact that the yeast lowered this flux suggests that cell viability is preserved when S. boulardii is present during the infection period. Increasing numbers of bacterial pathogens have been identified as mediators of apoptosis in vitro (42). Recently, Crane et al. (12) demonstrated that EPEC-infected cells died by apoptosis. The data presented in this study, based on detection of the active form of caspase-3, confirms this observation. EPEC triggers activation of this proapoptotic protease in T84 cells. When infection was performed in the presence of yeast cells, caspase activation by EPEC was delayed, indicating that S. boulardii protects T84 cells. Moreover, the active form of caspase was not detected in cells incubated with yeast cells alone. This observation, together with the fact that the yeast alone did not modify transepithelial resistance, demonstrates that T84-cell viability is not affected by incubation with S. boulardii. The protective effect of S. boulardii in EPEC infection of T84 cells prompted us to investigate the mechanism of action of this yeast.
Adhesion of EPEC to S. boulardii was recently reported by Gedek et al. (24, 25). Because S. boulardii does not permanently colonize animals or humans with normal flora (29), these authors proposed that the bacteria that bind the yeast are eliminated. Aggregation of the yeasts and bacteria is mediated by the bacterial type I pili and the mannose residues on the S. boulardii cell wall (24). EPEC adhesion to epithelial cells is mediated by type IV BFP, which is not inhibited by mannose; mannose-resistant adhesiveness to epithelial cells is considered a strong indication of enteropathogenicity for E. coli (34). Thus, the cell wall of S. boulardii rich in mannose is not expected to compete for EPEC adhesion site to enterocytes. Consistently, as shown in this study, S. boulardii did not significantly modify the number of adherent bacteria. However, S. boulardii significantly decreased, by 50%, the number of intracellular bacteria. Invasion of eucaryotic cells is an important virulence mechanism of many enteric pathogens such as Salmonella, Shigella, and Listeria species (20). EPEC strains have traditionally been considered noninvasive, but accumulating evidence raises doubt about this assumption. Intracellular EPEC have been observed in animal models (30, 39) and in biopsies from infected humans (18, 40). In vitro studies have revealed that EPEC are also capable of invading Hep-2, HeLa, and CaCo2 cells (2, 15, 35), supporting our results with T84 cells. As previously demonstrated for Salmonella enterica serovar Typhimurium and Yersinia pseudotuberculosis, EPEC strains take advantage of host signaling mechanisms to gain entry into the cell (reviewed in reference 4). We thus investigated the effect of S. boulardii on a cellular event(s) occurring after EPEC adhesion. As shown here, EPEC induced tyrosine phosphorylations of several proteins in T84 cells. Tyrosine phosphorylations of proteins during EPEC infection have been already reported in HeLa cells (35). These authors identified three tyrosine-phosphorylated proteins: a 90-kDa protein (Hp90), representing the major phosphorylation substrate, and two minor phosphorylated proteins of 39 kDa (Hp39) and 72 kDa (Hp72). In contrast to HeLa cells, Hp90 was not the major phosphorylated substrate in T84 cells. Tyrosine-phosphorylated proteins with molecular masses of 32 and 72 kDa probably corresponding to Hp32 and Hp72 were also detected in EPEC-infected T84 cells. As reported in this study EPEC triggered tyrosine phosphorylation of several other proteins with apparent masses of 130, 120, 100, 60, and 52 kDa that had not yet been reported. These proteins were barely phosphorylated when the infection was performed in the presence of yeast cells. Of these substrates we identified SHC, the upstream regulatory protein of the ERK1/2 MAP kinases, as a new phosphorylated substrate in EPEC-infected cells. MAP kinases are central in many host responses, including the mitogenic response to growth factors, the regulation of cytokine responses, stress responses, and cytoskeletal reorganization (14). Recently, MAP kinases have been implicated in the host response to bacterial infection (26, 38). As reported in this study, EPEC induces the activation of ERK1/2 MAP kinases in T84 cells. SHC isoforms were less phosphorylated in cells infected in the presence of S. boulardii: consequently, the activation of ERK1/2 MAP kinases was decreased in the presence of yeast. Activation of ERK1/2 MAP kinases has been implicated in the invasion process of L. monocytogenes, since a specific inhibitor of this pathway, namely, PD 98059, blocks invasion of HeLa cells by these bacteria (38). That study demonstrated that PD 98059 had no effect on the adherence of EPEC to host cells but significantly lowered EPEC invasion of T84 cells, underscoring the implication, at least in part, of ERK1/2 MAP kinases in this process. Thus, a decrease in the phosphorylation of ERK1/2 proteins may account for the lowering of the number of intracellular bacteria observed in cells infected in the presence of yeasts.
Since intracellular bacteria do not play an important role in the reduction of transepithelial resistance (9), the decrease in the number of intracellular bacteria cannot explain the presence of transepithelial resistance in cells infected in the presence of yeasts. Tyrosine phosphorylation of proteins is implicated in intercellular junction organization and modulation of the paracellular barrier (11). Other EPEC-induced tyrosine-phosphorylated proteins, dephosphorylated in the presence of yeasts, might therefore be implicated in the maintenance of transepithelial resistance. Future studies will be aimed at the identification of these proteins and the underlying mechanism of yeast-induced dephosphorylation.
ACKNOWLEDGMENTS
This study was supported by Laboratories BIOCODEX, the Région Provence-Alpes Côte d'Azur, the Conseil Général des Alpes Maritimes, the Faculté de Médecine de l'Université de Nice-Sophia Antipolis, and the Centre Hospitalier Régional de Nice.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Andrade J R C, Da Viega V D, De Santa Rosa M R, Suassuna I. An endocytic process in Hep-2 cells induced by enteropathogenic Escherichia coli. J Med Microbiol. 1989;28:49–57. doi: 10.1099/00222615-28-1-49. [DOI] [PubMed] [Google Scholar]
- 3.Bieber D, Sandra S W, Hu C-Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 4.Bliska J B, Falkow S. The role of host tyrosine phosphorylation in bacterial pathogenesis. Trends Genet. 1993;9:85–89. doi: 10.1016/0168-9525(93)90229-b. [DOI] [PubMed] [Google Scholar]
- 5.Bocciardi R, Mograbi B, Pasini B, Borrello M G, Pierotti M A, Bourget I, Fisher S, Romeo G, Rossi B. The multiple endocrine neoplasia type 2B point mutation switches the specificity of the Ret tyrosine kinase towards cellular substrates that are susceptible to interact with Crk and Nck. Oncogene. 1997;15:2257–2265. doi: 10.1038/sj.onc.1201413. [DOI] [PubMed] [Google Scholar]
- 6.Brugier S, Patte F. Antagonisme in vitro entre l'ultra-levure et différents germes bactériens. Med Paris. 1975;45:61–66. [Google Scholar]
- 7.Buts J P, Bernasconi P, Vaerman J P, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulin in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35:251–256. doi: 10.1007/BF01536771. [DOI] [PubMed] [Google Scholar]
- 8.Buts J P, Bernasconi P, Van Craynest M P, Maldague P, deMeyer R. Response of human and rat small intestinal mucosa to oral administration of Saccharomyces boulardii. Pediatr Res. 1986;20:192–196. doi: 10.1203/00006450-198602000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Canil C, Rosenshine I, Rushkowski S, Donnenberg M S, Kaper J B, Finlay B. Enteropathogenic Escherichia coli decreases transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castex F, Corthier G, Jouvert S, Elmer G W, Guibal J, Lucas F, Bastidel M. Prevention of experimental pseudomembranous cecitis by Saccharomyces boulardii: topographical histology of the mucosa, bacterial counts, and analysis of toxin production. Microecol Ther. 1989;18:113–116. [Google Scholar]
- 11.Collares-Buzato C B, Jepson M A, Simmons N L, Hirst B H. Increased tyrosine phosphorylation causes redistribution of adherence junction and tight junction proteins and perturbs paracellular barrier function in MDCK epithelia. Eur J Cell Biol. 1998;76:85–92. doi: 10.1016/S0171-9335(98)80020-4. [DOI] [PubMed] [Google Scholar]
- 12.Crane J K, Majumdar S, Pickhardt D F., III Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;67:2575–2584. doi: 10.1128/iai.67.5.2575-2584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czerucka D, Nano J L, Bernasconi P, Rampal P. Réponse à la toxine cholérique de deux lignées de cellules épithéliales intestinales. Effet de Saccharomyces boulardii. Gastroenterol Clin Biol. 1989;13:383–387. [PubMed] [Google Scholar]
- 14.Davis R J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993;268:14553–14556. [PubMed] [Google Scholar]
- 15.Donnenberg M S, Donohue-Rolfe A, Keusch G T. Epithelial cell invasion: overlooked property of enteropathogenic Escherichia coli (EPEC) associated with the EPEC adherence factor. J Infect Dis. 1989;160:452–459. doi: 10.1093/infdis/160.3.452. [DOI] [PubMed] [Google Scholar]
- 16.Dytoc M T, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 17.Elmer G W, Surawicz C M, McFarland L V. Biotherapeutic agents: a neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA. 1996;275:870–876. doi: 10.1001/jama.275.11.870. [DOI] [PubMed] [Google Scholar]
- 18.Fagundes-Neto U, Freymuller E, Gatti M S V, Schmitz L G, Scaletsky I. Enteropathogenic Escherichia coli O111ab/H2 penetrates the small bowel epithelium in an infant with acute diarrhoea. Acta Pediatr. 1995;84:453–455. doi: 10.1111/j.1651-2227.1995.tb13670.x. [DOI] [PubMed] [Google Scholar]
- 19.Falkow S, Small P, Isberg R, Hayes S F, Corwin D A. A molecular strategy for the study of bacterial invasion. Rev Infect Dis. 1987;9(Suppl.):S450–S455. doi: 10.1093/clinids/9.supplement_5.s450. [DOI] [PubMed] [Google Scholar]
- 20.Finlay B B, Falkow S. Comparison of the invasion strategies used by Salmonella choleraesuis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;70:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 21.Finlay B B, Falkow S. Salmonella interaction with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 22.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC) triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gedek B R. Adherence of Escherichia coli serogroup O157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses. 1999;42:261–264. doi: 10.1046/j.1439-0507.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- 25.Gedek B R, Amselgruber W. Mikrobieller antagonismus: zur eliminierung von enteropathogenen E. coli-keimen and Salmonella aus dem darm durch Saccharomyces boulardii. In: Ottenjann R, Muller J, Seifert J, editors. Okosystem Darm II-Mykrobiologie, Immunologie, Morphologie. Berlin, Germany: Springer-Verlag; 1990. pp. 184–186. [Google Scholar]
- 26.Hobbie S, Chen L M, Davis R J, Galan J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 27.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandel L J, Bacallao R, Zampighi G. Uncoupling of the molecular “fence” and paracellular “gate” functions in epithelial tight junctions. Nature. 1993;361:552–555. doi: 10.1038/361552a0. [DOI] [PubMed] [Google Scholar]
- 29.McFarland L V, Bernasconi P. Saccharomyces boulardii: a review of an innovative biotherapeutic agent. Microb Ecol Health Dis. 1993;6:157–171. [Google Scholar]
- 30.Moon H W, Whipp S C, Argenzio R A, Levine M M, Gianella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nataro J, Kaper J B. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;33:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues A C P, Nardi R M, Bambirra E A, Viera E C, Nicoli J R. Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol. 1996;91:251–256. doi: 10.1111/j.1365-2672.1996.tb04325.x. [DOI] [PubMed] [Google Scholar]
- 34.Rosa A C, Mariano A T, Tibana A, Gomes T A, Andrade J R. Enteropathogenicity markers in Escherichia coli isolated from infants with acute diarrhoea and healthy controls in Rio de Janeiro, Brazil. J Med Microbiol. 1998;47:781–790. doi: 10.1099/00222615-47-9-781. [DOI] [PubMed] [Google Scholar]
- 35.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scaletsky I, Silva M L, Trabulski L R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal monolayers diminishes barrier function. Am J Physiol. 1995;31:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 38.Tang P, Sutherland C L, Gold M R, Finlay B B. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect Immun. 1998;66:1106–1112. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzipori S, Robins-Browne R M, Gonis G, Hayes J, Withers M, McCartney E. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut. 1985;26:570–578. doi: 10.1136/gut.26.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulshen M H, Rollo J L. Pathogenesis of Escherichia coli gastroenteritis in man—another mechanism. N Engl J Med. 1980;302:99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- 41.Yuhan R, Koutsouris A, Savkovic S D, Heicht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;113:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]
- 42.Zychlinsky A, Sansonetti P. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]