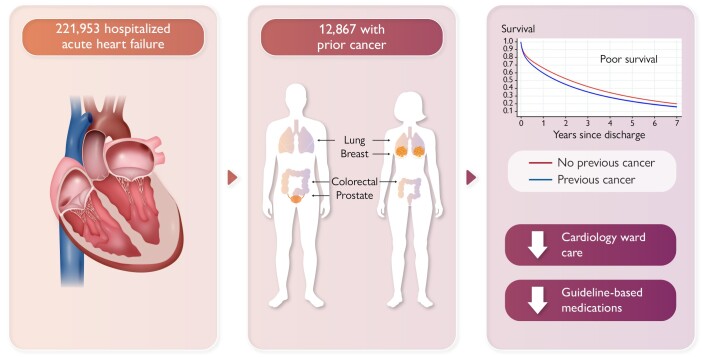
Abstract
Aims
Currently, little evidence exists on survival and quality of care in cancer patients presenting with acute heart failure (HF). The aim of the study is to investigate the presentation and outcomes of hospital admission with acute HF in a national cohort of patients with prior cancer.
Methods and results
This retrospective, population-based cohort study identified 221 953 patients admitted to a hospital in England for HF during 2012–2018 (12 867 with a breast, prostate, colorectal, or lung cancer diagnosis in the previous 10 years). We examined the impact of cancer on (i) HF presentation and in-hospital mortality, (ii) place of care, (iii) HF medication prescribing, and (iv) post-discharge survival, using propensity score weighting and model-based adjustment. Heart failure presentation was similar between cancer and non-cancer patients. A lower percentage of patients with prior cancer were cared for in a cardiology ward [−2.4% age point difference (ppd) (95% CI −3.3, −1.6)] or were prescribed angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists (ACEi/ARB) for heart failure with reduced ejection fraction [−2.1 ppd (−3.3, −0.9)] than non-cancer patients. Survival after HF discharge was poor with median survival of 1.6 years in prior cancer and 2.6 years in non-cancer patients. Mortality in prior cancer patients was driven primarily by non-cancer causes (68% of post-discharge deaths).
Conclusion
Survival in prior cancer patients presenting with acute HF was poor, with a significant proportion due to non-cancer causes of death. Despite this, cardiologists were less likely to manage cancer patients with HF. Cancer patients who develop HF were less likely to be prescribed guideline-based HF medications compared with non-cancer patients. This was particularly driven by patients with a poorer cancer prognosis.
Graphical Abstract
Graphical abstract.
Introduction
Survival in heart failure (HF) patients is often described as being akin to the prognosis of some cancers. However, with recent improvements in early detection and cancer treatment, patients with cancer are living longer.1 As a result, optimal management of co-morbidities and cardiovascular risk factors is becoming an increasingly important determinant of outcomes.2 Cancer survivors are at increased risk of developing cardiovascular diseases including heart failure (HF) which is associated with a poor prognosis.3 The association between cancer and HF is, in part, attributable to overlapping risk factors and pathophysiological pathways.4–6 Additionally, cancer treatments can contribute to cardiac dysfunction through chemotherapy-induced cardiotoxicity and/or chest radiotherapy.7–9 A multidisciplinary cardio-oncology approach is necessary to improve outcomes in cancer survivors with HF.10,11
Guideline-based management of HF alleviates patients’ symptoms, reduces hospital admissions, and improves outcomes.12 Effective treatments include angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists (ACEi/ARB), beta-blockers, mineralocorticoid receptor antagonists (MRA), and, more recently, the angiotensin receptor–neprilysin inhibitor and sodium–glucose co-transporter 2 inhibitors.12 In cancer patients, ACEi and beta-blockers have been shown to improve cardiac function in patients with chemotherapy-induced cardiotoxicity.9,13 These data are currently limited to anthracycline and anti-HER2–based treatments with little data on other classes of cancer therapies such as those frequently used to treat prostate, lung, and colon cancer. Although outcome data for other HF medications in a cancer-specific population are currently lacking, it is likely the benefits on HF outcomes are no different to the general HF population. However, it is less clear whether survival in cancer patients presenting with acute HF is primarily driven by HF or cancer outcomes. There is also little existing evidence on whether cancer patients with HF receive the same care as HF patients without cancer.
The objective of this study was to compare the presentation, treatments, and outcomes between hospitalized HF patients with or without a preceding diagnosis of one of the four most common cancers in the UK (breast, prostate, colon/rectum, lung) using the Virtual Cardio-Oncology Research Initiative (VICORI) research platform.14 The VICORI data sets link English national cancer registry and cardiovascular audit data with hospital coding and death certification data. This provides a unique opportunity to investigate the interplay between cardiovascular diseases and cancer.
Methods
Ethical approval and consent to participate
This study was reviewed and approved by the VICORI Consortium Project Review Panel. The VICORI research programme has received favourable ethical opinion from the North East—Newcastle and North Tyneside 2 Research Ethics Committee (REC reference 18/NE/0123). The study was performed in accordance with the Declaration of Helsinki.
Study design and databases
This is a retrospective, population-based cohort study using linked national cancer registry and HF audit databases. The VICORI study was approved by the UK Health Research Authority and the National Research Ethics Service (18/NE/0123). This study was reviewed and approved by the VICORI Consortium Project Review Panel and the National Disease Registration Service (NDRS) Project Review Panel.
The National Heart Failure Audit (NHFA) collects information on adults with an unscheduled (non-elective) admission to a hospital in England and Wales who have a death or discharge with a diagnosis of HF in the primary position (ICD-10 code I11.0, I25.5, I42.0, I42.9, I50.0, I50.1, or I50.9).15 The National Disease Registration Service compiles a comprehensive, quality-assured data set referred to as the National Cancer Registration Dataset (NCRD); this is collated using a wide range of data sources to register all tumours diagnosed for residents of England.16 Until recently, pseudonymized cardiovascular audit and cancer registry data for a single patient could not be linked. The VICORI is a research platform that links patient-level records from the NHFA, with the NDRS, and the Office of National Statistics death registration. Detailed information on the VICORI linkage process has been previously published.14 More details are given in the Supplementary Material.
Study population
All adults (≥18 years of age) with a first admission to the hospital for HF recorded in the NHFA from 1 January 2012 to 31 March 2018 (most recent NHFA data) were included; subsequent HF admissions were excluded. The NHFA data collection is nationally mandated and from 2012 contains high-quality data.17 We did not consider any first admission with HF recorded before 2012.
Cohorts
We defined our cancer cohort as linked patients from the NCRD,16 diagnosed within 10 years before the HF admission with the most common tumour sites identified by ICD-10 coding: breast (C50 females only), prostate (C61 males only), colorectal (C18–C20), and trachea, bronchus, and lung cancer (C33–C34). We analysed the cancer patients together and stratified by tumour site, but data on the stage of cancer at the time of cancer diagnosis and cardiovascular risk factors were limited. The comparator population consisted of HF patients without a diagnosis of malignant cancer (i.e. not identified in the NCRD) in the 10 years prior to HF admission. Comparator patients for breast cancer were restricted to females, for prostate cancer were males, and for colorectal and lung cancer were all patients without cancer.
Outcomes
Primary outcomes were (i) HF presentation (phenotype and in-hospital mortality), (ii) place of care (cardiology vs. non-cardiology vs. unknown ward care), (iii) HF medication prescribing, and (iv) post-discharge survival. Data completeness for left ventricular systolic dysfunction, as identified through echocardiography or other gold standard tests, is good and was used to identify heart failure with reduced ejection fraction (HFrEF). Heart failure severity was determined using the New York Heart Association (NYHA) Classification standard breathlessness score (1, least severe; 4, most severe including symptoms at rest/increase with physical activity).18
For patients with HFrEF discharged alive from hospital, HF management medications prescribed at discharge were obtained from the NHFA and included ACEi/ARBs, beta-blockers, loop diuretics, MRAs, and digoxin. Hospital discharge medications are only reported for patients with HFrEF because these medications are indicated for patients with HFrEF. Finally, for patients that did not die in the hospital, the date of death was obtained from the Office of National Statistics for post-discharge survival analyses. Patients were censored at the end of the study, 26 November 2018.
Statistical analyses
Stata/SE 15.1 was used for all analyses. Propensity weighting ensures that the distribution of known confounders is the same across exposure groups.19 In this study, we reweighted the distribution of confounders in patients without cancer to that of patients with cancer to provide estimates of the average effects in a cancer population.20 Potential confounding factors were selected a priori from the NHFA and consisted of age at HF admission, sex, ethnicity (categorized as White, Black, South Asian, other, unknown), year of HF admission, and the following pre-existing diseases: ischaemic heart disease, valve disease, diabetes, and chronic obstructive pulmonary disease. For cancer patients, ethnicity was obtained from the NDRS if unavailable in the NHFA.
Propensity scores were calculated for the comparison of the overall cancer cohort to patients without cancer and separately for each cancer site in comparison to patients without cancer. We used propensity score weighting to estimate the percentage of patients with and without cancer that experienced each outcome and the difference with 95% confidence interval. Standardized differences in baseline characteristics at HF admission were examined before and after propensity weighting for each exposure comparison.
Flexible parametric survival models were used to examine post-hospital discharge survival. All survival analyses excluded patients who died in the hospital or in whom hospital discharge date was after the end of survival follow-up. A restricted cubic spline was used to model the baseline log cumulative hazard of mortality, with four degrees of freedom. Cancer status was included as a binary variable with an interaction with follow-up time to allow for non-proportional hazards.21 Crude survival plots were created for cancer and non-cancer populations prior to any adjustment for confounders. For adjusted survival plots, age at admission, calendar year of admission, sex, ethnicity, and pre-existing diseases were included as covariates in the survival model, with restricted cubic splines used for age and calendar year of admission. Adjusted post-discharge survival curves were obtained for each exposure comparison, standardizing to the covariate distribution of cancer patients.
Analyses of post-discharge non-cancer–related mortality was investigated by censoring deaths from any cancer, ICD-10 C00-C97, using underlying cause of death information obtained from the Office of National Statistics (via the NDRS). For patients without cancer at the time of HF diagnosis, we searched for any future linkage with the NDRS and recorded cause of death from the Office of National Statistics if available. For non-cancer patients with no linkage to the NDRS, cause of death information was not available, and we made an assumption that these patients did not die of cancer. We obtained non-cancer net survival estimates, which describe survival free from non-cancer–related mortality in a population where cancer deaths cannot apply.
We investigated effect modification by grouping cancer into four distinct groups: lung cancer, non-lung cancers (breast, prostate, or colorectal) with recent diagnosis (≤1 year), non-lung cancers with diagnosis >1 and ≤3 years before HF, and non-lung cancers with diagnosis >3 years before HF. For each group, we estimated the difference in discharge medication prescription and all-cause mortality compared with controls.
Patient and public involvement
A group of patient representatives provided the study team with information on the experience of patients with cancer and heart disease and guided the key questions for the VICORI programme. The lead patient representatives attended the study management group meetings, provided guidance on study design and prioritization of research questions, and ensured study information and findings are disseminated, available, and accessible to patients and the public.
Results
The HF cohort comprised 221 953 patients admitted to the hospital for HF including 12 867 (5.8%) patients with a prior cancer diagnosis (of breast, prostate, colorectal, or lung cancer) and 209 086 without a cancer diagnosis (Table 1). Mean age was 78.1 years (SD 12.6) and 53.7% of the patients were male. Most patients were White (54.9%) or of unknown ethnicity (39.1%). There was a high prevalence of pre-existing diseases including ischaemic heart disease (38.8%), diabetes (29.9%), valve disease (21.7%), and chronic obstructive pulmonary disease (16.2%). Approximately half of patients presented with HFrEF, and most of these hospitalized patients (72.8%) had NYHA Classification 3 or 4 (Table 1).
Table 1.
Differences in baseline cohort characteristics before and after propensity weighting between patients with and without cancer who were admitted to the hospital for heart failure during 1 January 2012–31 March 2018
| Baseline | Total | Before propensity weighting | After propensity weighting | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cancer | Difference | Standardized difference | Cancer | Difference | Standardized difference | ||||
| Yes | No | Yes | No | ||||||
| Number of patients | 221 953 | 12 867 | 209 086 | — | — | 12 867 | 209 086 | — | — |
| Age (years), mean (SD) | 78.1 (12.6) | 80.8 (8.9) | 77.9 (12.7) | 2.8 | −0.259 | 80.8 | 80.8 | 0.0 | 0.001 |
| Sex, n (%) | |||||||||
| Male | 119 241 (53.7%) | 7923 (61.6%) | 111 318 (53.2%) | 8.4 | 0.169 | 61.6% | 61.4% | 0.2 | 0.003 |
| Female | 102 712 (46.3%) | 4944 (38.4%) | 97 768 (46.8%) | −8.4 | 38.4% | 38.6% | −0.2 | −0.003 | |
| Ethnicity, n (%) | |||||||||
| Unknown | 86 852 (39.1%) | 4769 (37.1%) | 82 083 (39.3%) | −2.2 | 0.045 | 37.1% | 37.1% | 0.0 | 0.000 |
| Black | 3245 (1.5%) | 176 (1.4%) | 3069 (1.5%) | −0.1 | 0.008 | 1.4% | 1.4% | 0.0 | 0.001 |
| Other | 7806 (3.5%) | 434 (3.4%) | 7372 (3.5%) | −0.1 | 0.008 | 3.4% | 3.4% | 0.0 | 0.000 |
| South Asian | 2279 (1.0%) | 58 (0.5%) | 2221 (1.1%) | −0.6 | 0.071 | 0.5% | 0.5% | 0.0 | 0.000 |
| White | 121 771 (54.9%) | 7430 (57.7%) | 114 341 (54.7%) | 3.0 | 0.062 | 57.7% | 57.7% | 0.0 | 0.000 |
| Pre-existing conditions | |||||||||
| Ischaemic heart disease, n (%) | |||||||||
| No | 125 801 (56.7%) | 7409 (57.6%) | 118 392 (56.6%) | 1.0 | 0.019 | 57.6% | 57.6% | 0.0 | 0.000 |
| Yes | 86 183 (38.8%) | 4917 (38.2%) | 81 266 (38.9%) | −0.7 | 0.013 | 38.2% | 38.2% | 0.0 | 0.000 |
| Unknown | 9969 (4.5%) | 541 (4.2%) | 9428 (4.5%) | −0.3 | 0.015 | 4.2% | 4.2% | 0.0 | 0.000 |
| Valve disease, n (%) | |||||||||
| No | 162 473 (73.2%) | 9536 (74.1%) | 152 937 (73.1%) | 1.0 | 0.022 | 74.1% | 74.1% | 0.0 | 0.000 |
| Yes | 48 270 (21.7%) | 2742 (21.3%) | 45 528 (21.8%) | −0.5 | 0.011 | 21.3% | 21.3% | 0.0 | 0.000 |
| Unknown | 11 210 (5.1%) | 589 (4.6%) | 10 621 (5.1%) | −0.5 | 0.023 | 4.6% | 4.6% | 0.0 | 0.000 |
| Diabetes, n (%) | |||||||||
| No | 148 740 (67.0%) | 8967 (69.7%) | 139 773 (66.8%) | 2.9 | 0.061 | 69.7% | 69.7% | 0.0 | 0.000 |
| Yes | 66 419 (29.9%) | 3531 (27.4%) | 62 888 (30.1%) | −2.7 | 0.058 | 27.4% | 27.5% | −0.1 | 0.000 |
| Unknown | 6794 (3.1%) | 369 (2.9%) | 6425 (3.1%) | −0.2 | 0.012 | 2.9% | 2.9% | 0.0 | 0.000 |
| Chronic obstructive pulmonary disease, n (%) | |||||||||
| No | 174 230 (78.5%) | 9954 (77.4%) | 164 276 (78.6%) | −1.2 | 0.029 | 77.4% | 77.3% | 0.1 | 0.000 |
| Yes | 36 013 (16.2%) | 2306 (17.9%) | 33 707 (16.1%) | 1.8 | 0.048 | 17.9% | 17.9% | 0.0 | 0.000 |
| Unknown | 11 710 (5.3%) | 607 (4.7%) | 11 103 (5.3%) | −0.6 | 0.027 | 4.7% | 4.7% | 0.0 | 0.000 |
| HF presentation | |||||||||
| HFrEF, n (%) | |||||||||
| No | 97 927 (44.1%) | 5838 (45.4%) | 92 089 (44.0%) | 1.4 | 0.027 | 45.4% | 44.7% | 0.7 | 0.013 |
| Yes | 119 446 (53.8%) | 6750 (52.5%) | 112 696 (53.9%) | −1.4 | 0.029 | 52.5% | 53.2% | −0.7 | −0.016 |
| Unknown | 4580 (2.1%) | 279 (2.2%) | 4301 (2.1%) | 0.1 | 0.008 | 2.2% | 2.0% | 0.2 | 0.010 |
| NYHA Classification, n (%) | |||||||||
| 1 | 13 904 (6.3%) | 763 (5.9%) | 13 141 (6.3%) | −0.4 | 0.015 | 5.9% | 6.2% | −0.3 | −0.012 |
| 2 | 31 697 (14.3%) | 1753 (13.6%) | 29 944 (14.3%) | −0.7 | 0.020 | 13.6% | 14.1% | −0.5 | −0.014 |
| 3 | 90 471 (40.8%) | 5263 (40.9%) | 85 208 (40.8%) | 0.1 | 0.003 | 40.9% | 41.0% | −0.1 | −0.002 |
| 4 | 71 043 (32.0%) | 4273 (33.2%) | 66 770 (31.9%) | 1.3 | 0.027 | 33.2% | 32.2% | 1.0 | 0.022 |
| Unknown | 14 838 (6.7%) | 815 (6.3%) | 14 023 (6.7%) | −0.4 | 0.015 | 6.3% | 6.5% | −0.2 | −0.007 |
HFrEF, failure with reduced ejection fraction; NYHA, New York Heart Association. Age is listed in years as mean (SD). All other information is number (%).
Propensity score includes the variables: age at HF admission, sex, ethnicity year of HF admission, ischaemic heart disease, valve disease, diabetes, and chronic obstructive pulmonary disease.
Differences in baseline characteristics between patients with and without cancer were eliminated after propensity score weighting (all standardized differences <0.005, Table 1), with propensity score distributions exhibiting satisfactory overlap between HF patients with and without cancer. Similarly, baseline differences between each tumour site and the corresponding patients without cancer were eliminated after propensity score weighting (all standardized differences <0.001, Supplementary material online, Table S1).
Amongst cancer patients, there were 3216 (25.0%) breast, 5118 (39.8%) prostate, 3199 (24.9%) colorectal, and 1334 (10.4%) lung cancer patients. Nearly half (47.1%) were missing cancer stages with lung cancer patients having a higher proportion of advanced disease than other tumour sites (see Supplementary material online, Table S2). The cancer stage distribution varied greatly between the different cancer sites.
Heart failure presentation and in-hospital outcomes
There were minimal differences in HF phenotype and severity (NYHA Classification), between patients with and without prior cancer, except in lung cancer patients where a lower percentage presented with HFrEF compared with patients without cancer [−4.3% age point difference (ppd) (95% CI −7.0, −1.6) after adjustment] (Table 2). A lower percentage of cancer patients were cared for in a cardiology ward compared with patients without cancer [−2.4 ppd (95% CI −3.3, −1.6) after adjustment; Table 2]. This was most pronounced in lung cancer patients where only 33.7% received care in a cardiology ward compared with 43.7% of patients without cancer [−10.1 ppd (95% CI −12.6, −7.5) after adjustment]. In-hospital mortality was 5.9% in cancer patients compared with 5.0% in patients without cancer [0.7 ppd (95% CI 0.3, 1.1) after adjustment]. The difference was highest between patients with lung cancer (7.0%) vs. without [4.6%, 2.4 ppd (95% CI 1.0, 3.8) after adjustment] (Table 2).
Table 2.
Propensity-weighted difference (95% confidence interval) in primary outcomes and discharge medication for patients with heart failure admission by cancer diagnosis and tumour site
| Outcome | Propensity-weighted | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Cancer | Tumour site | ||||||||||||||
| Yes | No | Diff (95% CI) | Breast cancer | Prostate cancer | Colorectal cancer | Lung cancer | ||||||||||
| Yes | No | Diff (95% CI) | Yes | No | Diff (95% CI) | Yes | No | Diff (95% CI) | Yes | No | Diff (95% CI) | |||||
| Number of patients | 221 953 | 12 867 | 209 086 | 3216 | 97 768 | 5118 | 111 318 | 3199 | 209 086 | 1334 | 209 086 | |||||
| HF hospital admission | ||||||||||||||||
| Main place of care | ||||||||||||||||
| Other | 123 521 (55.7%) | 59.7% | 57.2% | 2.5 (1.6, 3.3) | 62.4% | 61.5% | 0.9 (−0.8, 2.6) | 56.5% | 54.2% | 2.3 (0.9, 3.7) | 59.4% | 58.1% | 1.4 (−0.4, 3.1) | 66.0% | 55.9% | 10.1 (7.5, 12.6) |
| Cardiology | 97 592 (44.0%) | 40.0% | 42.4% | −2.4 (−3.3, −1.6) | 37.2% | 38.1% | −0.9 (−2.6, 0.8) | 43.2% | 45.4% | −2.2 (−3.6, −0.8) | 40.2% | 41.6% | −1.3 (−3.0, 0.4) | 33.7% | 43.7% | −10.1 (−12.6, −7.5) |
| Unknown | 840 (0.4%) | 0.3% | 0.4% | 0.0 (−0.1, 0.1) | 0.4% | 0.4% | 0.0 (−0.2, 0.2) | 0.3% | 0.4% | −0.1 (−0.2, 0.1) | 0.3% | 0.4% | 0.0 (−0.2, 0.2) | 0.4% | 0.4% | 0.0 (−0.3, 0.3) |
| Died in the hospital | ||||||||||||||||
| No | 211 249 (95.2%) | 94.2% | 94.9% | −0.7 (−1.1, −0.3) | 94.7% | 95.2% | −0.5 (−1.3, 0.3) | 94.1% | 94.6% | −0.5 (−1.2, 0.2) | 94.2% | 94.6% | −0.4 (−1.2, 0.4) | 93.0% | 95.4% | −2.4 (−3.8, −1.0) |
| Yes | 10 704 (4.8%) | 5.9% | 5.0% | 0.7 (0.3, 1.1) | 5.3% | 4.8% | 0.5 (−0.3, 1.3) | 5.9% | 5.4% | 0.5 (−0.2, 1.2) | 5.8% | 5.4% | 0.4 (−0.4, 1.2) | 7.0% | 4.6% | 2.4 (1.0, 3.8) |
| HF presentation | ||||||||||||||||
| HFrEF, n (%) | ||||||||||||||||
| No | 97 927 (44.1%) | 45.4% | 44.7% | 0.6 (−0.3, 1.5) | 53.3% | 53.9% | −0.5 (−2.3, 1.2) | 39.9% | 38.8% | 1.1 (−0.3, 2.5) | 45.2% | 45.2% | 0.0 (−1.8, 1.7) | 47.6% | 43.7% | 3.9 (1.2, 6.6) |
| Yes | 119 446 (53.8%) | 52.5% | 53.2% | −0.8 (−1.7, 0.1) | 44.1% | 43.9% | 0.1 (−1.6, 1.9) | 58.0% | 59.2% | −1.2 (−2.6, 0.1) | 53.0% | 52.8% | 0.3 (−1.5, 2.0) | 50.0% | 54.3% | −4.3 (−7.0, −1.6) |
| Unknown | 4580 (2.1%) | 2.2% | 2.0% | 0.1 (−0.1, 0.4) | 2.6% | 2.2% | 0.4 (−0.2, 0.9) | 2.1% | 2.0% | 0.1 (−0.3, 0.5) | 1.8% | 2.0% | −0.3 (−0.7, 0.2) | 2.4% | 1.9% | 0.5 (−0.4, 1.3) |
| NYHA Classification, n (%) | ||||||||||||||||
| 1 | 13 904 (6.3%) | 5.9% | 6.2% | −0.3 (−0.7, 0.1) | 5.5% | 6.2% | −0.7 (−1.5, 0.1) | 6.5% | 6.4% | 0.2 (−0.5, 0.9) | 5.9% | 6.2% | −0.3 (−1.1, 0.5) | 4.7% | 5.6% | −0.9 (−2.1, 0.2) |
| 2 | 31 697 (14.3%) | 13.6% | 14.1% | −0.5 (−1.1, 0.1) | 13.4% | 14.3% | −0.9 (−2.1, 0.3) | 14.2% | 14.0% | 0.2 (−0.8, 1.2) | 13.7% | 14.0% | −0.4 (−1.6, 0.8) | 11.8% | 13.7% | −1.9 (−3.6, −0.1) |
| 3 | 90 471 (40.8%) | 40.9% | 41.0% | −0.1 (−1.0, 0.8) | 40.9% | 40.6% | 0.3 (−1.4, 2.0) | 41.3% | 41.3% | 0.0 (−1.4, 1.4) | 39.5% | 41.0% | −1.4 (−3.1, 0.3) | 42.7% | 41.6% | 1.1 (−1.5, 3.8) |
| 4 | 71 043 (32.0%) | 33.2% | 32.2% | 1.0 (0.2, 1.9) | 33.6% | 32.3% | 1.3 (−0.3, 3.0) | 31.9% | 31.9% | 0.0 (−1.3, 1.3) | 34.4% | 32.3% | 2.0 (0.4, 3.7) | 34.5% | 33.0% | 1.5 (−1.1, 4.0) |
| Unknown | 14 838 (6.7%) | 6.3% | 6.5% | −0.2 (−0.6, 0.3) | 6.6% | 6.7% | −0.1 (−1.0, 0.8) | 6.1% | 6.4% | −0.4 (−1.0, 0.3) | 6.5% | 6.5% | 0.0 (−0.8, 0.9) | 6.2% | 6.0% | 0.2 (−1.1, 1.5) |
| Discharge medication a | ||||||||||||||||
| Number of patients with HFrEF and did not die in the hospital | 114 001 | 6385 | 107 616 | — | 1355 | 40 913 | — | 2794 | 66 703 | — | 1604 | 107 616 | — | 632 | 107 616 | — |
| ACEi/ARB | ||||||||||||||||
| No | 28 341 (24.9%) | 28.4% | 26.7% | 1.7 (0.6, 2.9) | 25.5% | 26.3% | −0.8 (−3.2, 1.6) | 28.8% | 27.3% | 1.6 (−0.1, 3.3) | 30.0% | 27.7% | 2.3 (0.1, 4.6) | 29.1% | 25.1% | 4.1 (0.5, 7.6) |
| Yes | 78 037 (68.5%) | 64.1% | 66.2% | −2.1 (−3.3, −0.9) | 67.6% | 66.9% | 0.7 (−1.8, 3.2) | 63.8% | 65.4% | −1.7 (−3.5, 0.2) | 62.3% | 65.0% | −2.7 (−5.1, −0.3) | 62.5% | 68.4% | −5.9 (−9.7, −2.1) |
| Unknown/NA | 7623 (6.7%) | 7.5% | 7.1% | 0.4 (−0.3, 1.1) | 6.9% | 6.8% | 0.1 (−1.3, 1.5) | 7.4% | 7.3% | 0.1 (−0.9, 1.1) | 7.7% | 7.3% | 0.4 (−0.9, 1.7) | 8.4% | 6.6% | 1.8 (−0.3, 4.0) |
| Beta-blocker | ||||||||||||||||
| No | 22 044 (19.3%) | 21.0% | 20.6% | 0.4 (−0.7, 1.4) | 19.9% | 20.3% | −0.4 (−2.5, 1.8) | 20.8% | 21.1% | −0.2 (−1.8, 1.3) | 20.9% | 21.0% | −0.2 (−2.2, 1.9) | 24.1% | 20.9% | 3.1 (−0.2, 6.5) |
| Yes | 84 052 (73.7%) | 71.5% | 72.0% | −0.6 (−1.7, 0.6) | 73.3% | 72.6% | 0.7 (−1.7, 3.1) | 71.7% | 71.3% | 0.4 (−1.3, 2.1) | 71.1% | 71.4% | −0.3 (−2.5, 2.0) | 67.4% | 72.1% | −4.7 (−8.4, −1.1) |
| Unknown/NA | 7905 (6.9%) | 7.6% | 7.4% | 0.2 (−0.5, 0.9) | 6.8% | 7.1% | −0.3 (−1.7, 1.1) | 7.5% | 7.6% | −0.2 (−1.2, 0.8) | 8.0% | 7.6% | 0.4 (−0.9, 1.8) | 8.5% | 6.9% | 1.6 (−0.5, 3.8) |
| Loop diuretic | ||||||||||||||||
| No | 12 507 (11.0%) | 10.9% | 10.2% | 0.6 (−0.2, 1.4) | 10.7% | 10.8% | −0.1 (−1.8, 1.5) | 10.4% | 10.0% | 0.4 (−0.8, 1.6) | 10.8% | 10.0% | 0.8 (−0.8, 2.3) | 13.3% | 10.3% | 2.9 (0.3, 5.6) |
| Yes | 93 848 (82.3%) | 82.4% | 83.0% | −0.6 (−1.6, 0.3) | 83.0% | 82.7% | 0.2 (−1.8, 2.3) | 82.9% | 83.0% | −0.2 (−1.6, 1.3) | 82.5% | 83.2% | −0.7 (−2.5, 1.2) | 79.0% | 83.2% | −4.3 (−7.4, −1.1) |
| Unknown/NA | 7646 (6.7%) | 6.8% | 6.7% | 0.0 (−0.6, 0.6) | 6.3% | 6.4% | −0.1 (−1.4, 1.2) | 6.7% | 7.0% | −0.2 (−1.2, 0.7) | 6.7% | 6.8% | −0.1 (−1.3, 1.1) | 7.8% | 6.4% | 1.3 (−0.8, 3.4) |
| MRA | ||||||||||||||||
| No | 51 823 (45.5%) | 48.1% | 46.9% | 1.2 (−0.1, 2.5) | 48.0% | 48.3% | −0.3 (−3.0, 2.4) | 47.0% | 46.6% | 0.4 (−1.5, 2.3) | 49.0% | 47.6% | 1.4 (−1.1, 3.8) | 50.6% | 45.4% | 5.2 (1.3, 9.2) |
| Yes | 45 363 (39.8%) | 36.1% | 37.8% | −1.7 (−2.9, −0.5) | 36.5% | 36.0% | 0.5 (−2.1, 3.1) | 37.6% | 38.1% | −0.5 (−2.4, 1.3) | 34.9% | 36.8% | −1.9 (−4.3, 0.4) | 32.3% | 39.8% | −7.6 (−11.2, −3.9) |
| Unknown/NA | 16 815 (14.7%) | 15.8% | 15.3% | 0.5 (−0.4, 1.4) | 15.5% | 15.7% | −0.2 (−2.2, 1.7) | 15.4% | 15.3% | 0.2 (−1.2, 1.5) | 16.1% | 15.6% | 0.6 (−1.3, 2.4) | 17.1% | 14.8% | 2.3 (−0.6, 5.3) |
| Digoxin | ||||||||||||||||
| No | 70 564 (61.9%) | 61.1% | 61.4% | −0.4 (−1.6, 0.9) | 60.6% | 60.8% | −0.2 (−2.8, 2.5) | 61.8% | 62.1% | −0.3 (−2.1, 1.6) | 61.0% | 61.6% | −0.6 (−3.0, 1.8) | 59.0% | 60.8% | −1.8 (−5.6, 2.1) |
| Yes | 17 998 (15.8%) | 16.3% | 16.1% | 0.2 (−0.8, 1.1) | 18.5% | 17.2% | 1.3 (−0.8, 3.4) | 15.1% | 15.1% | 0.0 (−1.3, 1.4) | 16.7% | 15.9% | 0.8 (−1.0, 2.6) | 16.0% | 16.9% | −0.9 (−3.8, 2.0) |
| Unknown/NA | 25 439 (22.3%) | 22.6% | 22.4% | 0.2 (−0.9, 1.3) | 21.0% | 22.0% | −1.1 (−3.3, 1.1) | 23.0% | 22.8% | 0.3 (−1.3, 1.9) | 22.3% | 22.5% | −0.2 (−2.3, 1.9) | 25.0% | 22.4% | 2.6 (−0.8, 6.0) |
HF, heart failure; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association; ACEi/ARB, angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists; MRA, mineralocorticoid receptor antagonists; Diff, difference. Lung cancer includes trachea, bronchus, and lung cancers. The no breast cancer group includes females only, whilst the no prostate cancer group includes males only.
Propensity score includes the variables: age at HF admission, sex, ethnicity year of HF admission, ischaemic heart disease, valve disease, diabetes, and chronic obstructive pulmonary disease.
Discharge medication was evaluated for patients who did not die in the hospital and had left ventricular systolic dysfunction.
Discharge medications
Of the 114 001 HF patients who had HFrEF and were discharged alive, 64.1% of the cancer patients received ACEi/ARB on hospital discharge compared with 66.2% of patients without cancer [−2.1 ppd (95% CI −3.3, −0.9) after adjustment; Table 2, Supplementary material online, Figure S1], driven primarily by lower prescribing in the lung cancer population [62.5%; −5.9 ppd (95% CI −9.7, −2.1) after adjustment]. The percentage of patients with and without cancer that were prescribed other HF management medications was comparable (≤2 ppd after adjustment) except for lung cancer patients where fewer patients received MRA [−7.6 ppd (95% CI −11.2, −3.9) after propensity score adjustment], beta-blockers [−4.7 ppd (95% CI −8.4, −1–1)], and loop diuretics [−4.3 ppd (95% CI −7.4, −1.1)] compared with patients without lung cancer. Amongst HF patients with non-lung cancer, there was no clear effect of time since cancer diagnosis on the likelihood of being prescribed HF discharge medication (see Supplementary material online, Figure S2).
Post-discharge survival
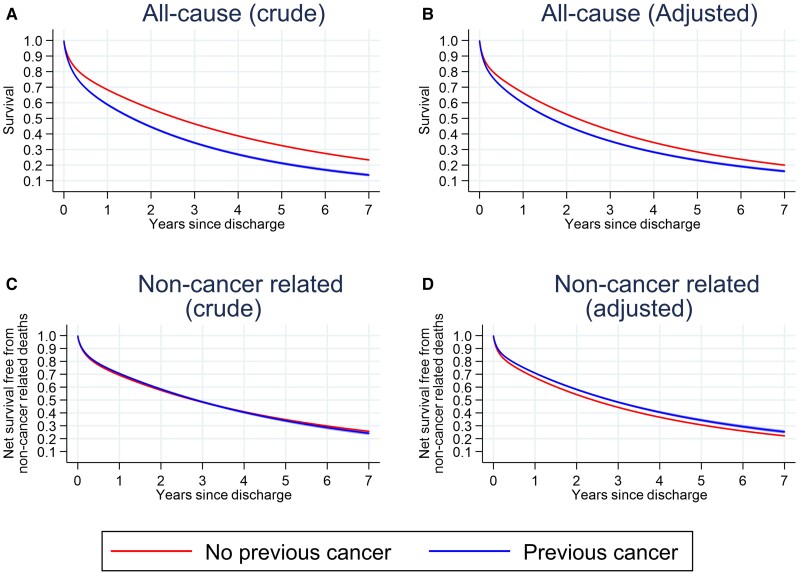
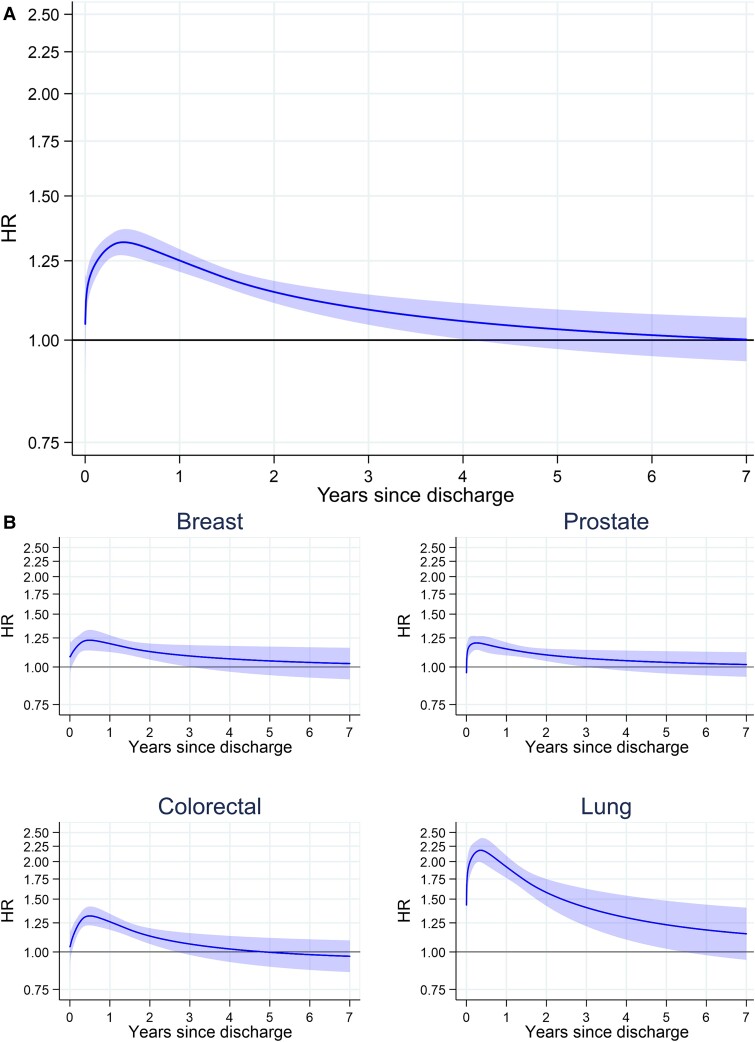
Survival post-acute HF discharge was estimated in 211 224 patients discharged alive with available follow-up. There were 117 533 post-discharge deaths during a mean follow-up time of 2.0 years (SD 1.7, range 0.0–6.9). Overall survival after an acute heart failure admission was low, regardless of prior cancer status. Median post-discharge survival was 2.5 years (95% CI 2.4, 2.5), 1.6 years (95% CI 1.5, 1.6) for patients with cancer, and 2.6 years (95% CI 2.6, 2.6) for patients without cancer (Figure 1A). This difference was attenuated but remained after adjusting for baseline characteristics (Figure 1B). A significantly increased rate of death for cancer patients was present in the first few years after hospital discharge and remained elevated in lung cancer patients up to 5 years after discharge (Figure 2). Despite this, most of the 7569 post-discharge deaths in cancer patients had non-cancer–related underlying causes with 3261 (41%) from diseases of the circulatory system and a further 920 (12%) from respiratory diseases. This compared with 2568 (32%) deaths with malignant neoplasm as the underlying cause (Table 3). Patients with and without cancer had similar non-cancer–related net survival, which was extremely poor for both groups of patients (Figures 1C and 1D).
Figure 1.
All-cause survival, post-hospital discharge, for heart failure by cancer diagnosis. Survival rates after hospital discharge for heart failure by cancer diagnosis with differing levels of adjustment. (A) Crude and (B) adjusted non-cancer–related net survival; (C) crude and (D) adjusted non-cancer related. Adjusted for age at admission, year of admission, sex, ethnicity (White, Black, South Asian, other, unknown), New York Heart Association class, and the following pre-existing diseases: valve disease, ischaemic heart disease, diabetes, and chronic obstructive pulmonary disease. n = 211 224 patients discharged alive and with available post-discharge follow-up.
Figure 2.
Hazard ratios for post-discharge mortality compared with no previous cancer. Marginal hazard ratios for post-discharge mortality comparing cancer to no previous cancer. (A) Marginal hazard ratio of mortality in all cancer patients relative to patients without cancer. (B) Marginal hazard ratio of mortality in breast cancer relative to women without cancer, prostate cancer relative to men without cancer, both colorectal and lung cancer are relative to patients without cancer. Adjusted for age at admission, year of admission, sex, ethnicity (White, Black, South Asian, other, unknown), New York Heart Association class, and the following pre-existing diseases: valve disease, ischaemic heart disease, diabetes, and chronic obstructive pulmonary disease. Lung cancer includes trachea, bronchus, and lung cancers. n = 211 224 patient discharged alive and with available post-discharge follow-up.
Table 3.
Distribution of underlying causes of death after hospital discharge for HF in patients with prior cancer
| ICD-10 | Description | n (%) | |
|---|---|---|---|
| A | A00-B99 | Certain infectious and parasitic diseases | 68 (0.9) |
| B | 5 (0.1) | ||
| C | C00-C97 | Malignant neoplasms | 2568 (32.3) |
| D | D00-D48 | Other neoplasms | 37 (0.5) |
| D50-D89 | Diseases of the blood and blood-forming organs and certain disorders involving the immune mechanism | 8 (0.1) | |
| E | E00-E89 | Endocrine, nutritional, and metabolic diseases | 128 (1.6) |
| F | F01-F99 | Mental, behavioural, and neurodevelopmental disorders | 206 (2.6) |
| G | G00-G99 | Diseases of the nervous system | 90 (1.1) |
| H | H00-H59 | Diseases of the eye and adnexa | 0 (0.0) |
| H60-H95 | Diseases of the ear and mastoid process | 0 (0.0) | |
| I | I00-I99 | Diseases of the circulatory system | 3261 (41.0) |
| I00-I02 | − of which acute rheumatic fever | 0 (0.0) | |
| I05-I09 | − of which chronic rheumatic heart diseases | 32 (0.4) | |
| I10-I16 | − of which hypertensive diseases | 138 (1.7) | |
| I20-I25 | − of which ischaemic heart diseases | 1681 (21.1) | |
| I26-I28 | − of which pulmonary heart disease and diseases of pulmonary circulation | 51 (0.6) | |
| I30-I49 | − of which other forms of heart disease | 635 (8.0) | |
| I50–15A | − of which heart failure | 447 (5.6) | |
| I60-I69 | − of which cerebrovascular diseases | 206 (2.6) | |
| I70-I79 | − of which diseases of arteries, arterioles, and capillaries | 63 (0.8) | |
| I80-I89 | − of which diseases of veins, lymphatic vessels, and lymph nodes, not elsewhere classified | 8 (0.1) | |
| I95-I99 | − of which other and unspecified disorders of the circulatory system | 0 (0.0) | |
| J | J00-J99 | Diseases of the respiratory system | 920 (11.6) |
| K | K00-K95 | Diseases of the digestive system | 206 (2.6) |
| L | L00-L99 | Diseases of the skin and subcutaneous tissue | 46 (0.6) |
| M | M00-M99 | Diseases of the musculoskeletal system and connective tissue | 46 (0.6) |
| N | N00-N99 | Diseases of the genitourinary system | 148 (1.9) |
| O | O00-O99 | Pregnancy, childbirth, and the puerperium | 0 (0.0) |
| P | P00-P96 | Certain conditions originating in the perinatal period | 0 (0.0) |
| Q | Q00-Q99 | Congenital malformations, deformations, and chromosomal abnormalities | 9 (0.1) |
| R | R00-R99 | Symptoms, signs, and abnormal clinical and laboratory findings, not elsewhere classified | 62 (0.8) |
| S | S00-T88 | Injury, poisoning, and certain other consequences of external causes | 0 (0.0) |
| T | 0 (0.0) | ||
| V | V00-Y99 | External causes of morbidity | 3 (0.0) |
| Y | 3 (0.0) | ||
| Z | Z00-Z99 | Factors influencing health status and contact with health services | 0 (0.0) |
| Multiple underlying causes of death recorded (usually indicating deaths due to accidents, poisonings, and violence) | 102 (1.3) | ||
| Missing | 40 (0.5) | ||
| Total deaths | 7948 (100.0) | ||
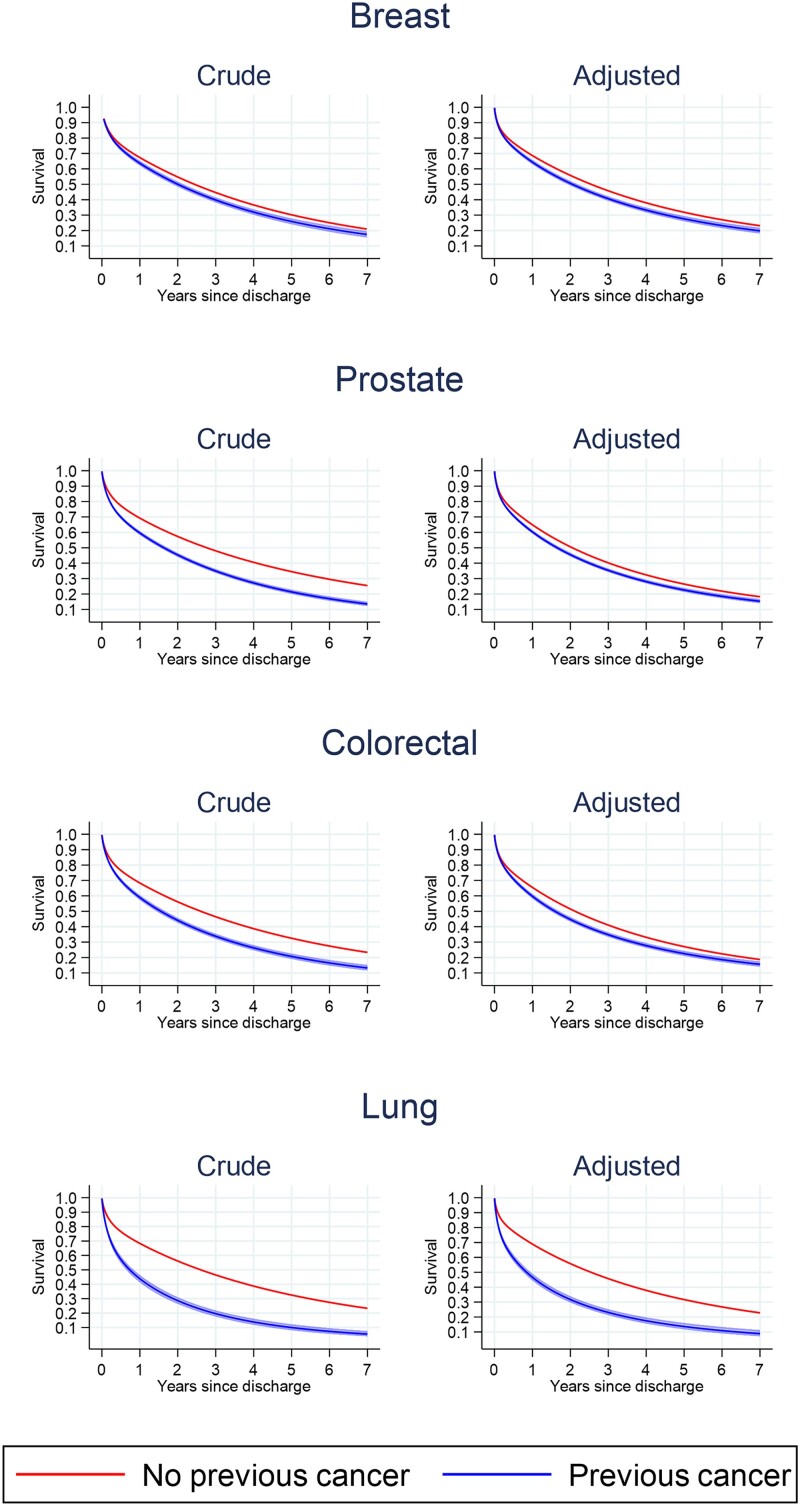
Lung cancer patients had the worst median survival post-acute HF discharge. Median survival was 2.0 years (95% CI 1.9, 2.1) for women with breast cancer, 1.6 years (95% CI 1.5, 1.7) for men with prostate cancer, 1.5 years (95% CI 1.4, 1.6) for patients with colorectal cancer, 0.8 years (95% CI 0.7, 0.9) for patients with lung cancer, and 2.6 years (95% CI 2.6, 2.6) for patients without cancer (2.4 years for women and 2.7 years for men) (Figure 3). After adjustment for confounders, survival differences remained between prior cancer and non-cancer patients, though to a lesser degree in comparisons with non-lung (breast, prostate, and colorectal) cancer cohorts. Amongst non-lung cancer patients admitted with acute HF, prognosis was slightly poorer in patients with a recent cancer diagnosis, whilst for patients diagnosed more than 3 years ago, prognosis was similar to patients without cancer (see Supplementary material online, Figure S3).
Figure 3.
All-cause survival post-hospital discharge for heart failure by tumour site. Crude and adjusted all-cause survival post-hospital discharge for each tumour site compared with patients without cancer. Each tumour site compared with patients without cancer. Crude and adjusted for age at admission, year of admission, sex, ethnicity (White, Black, South Asian, other, unknown), New York Class Association, and the following pre-existing diseases: valve disease, ischaemic heart disease, diabetes, and chronic obstructive pulmonary disease. The no breast cancer group includes females only, whilst the no prostate cancer group includes males only. Lung cancer includes trachea, bronchus, and lung cancers. n = 211 224 patients discharged alive and with available post-discharge follow-up.
Discussion
We identified a large, diverse, nationally representative cohort of 12 867 cancer survivors and 209 086 patients without cancer admitted to the hospital with HF. In this cohort, we found, firstly, survival following hospital discharge for HF was very poor for prior cancer patients with only 23% remaining alive at 5 years. Secondly, whilst survivors of prior cancer had worse survival following HF compared with patients without previous cancer, mortality in cancer patients with HF was driven primarily by non-cancer causes. As a result, and particularly in non-lung cancer patients, differences in adjusted survival between cancer and non-cancer patients following HF discharge were relatively small. Thirdly, fewer survivors of prior cancer admitted to the hospital with HF were managed by cardiology specialists compared with similar patients without cancer, and finally, survivors of prior cancer presenting with HFrEF were less likely to receive guideline-based therapies,12 particularly ACEi/ARB, compared with similar patients without cancer. This disparity was most evident for lung cancer survivors.
Cardiovascular co-morbidities in cancer patients may arise as a direct consequence of complications of cancer or cancer treatment, shared cancer–cardiovascular risk factors, or simply as coincidental diseases.2,4–6 As cancer treatment and outcomes improve, optimal management of cardiovascular co-morbidities is increasingly important in further improving survival.22 This study supports this new cardio-oncological paradigm. It is perhaps not surprising that the combination of previous cancer and an admission to the hospital with acute heart failure in this study carried a particularly dire prognosis.23 However, importantly, from the adjusted survival analysis presented, it appears that mortality in the cancer population is driven primarily by non-cancer causes of death. The partial exception is lung cancer patients and to a lesser extent those with a recent cancer diagnosis (where the cancer prognosis is worse). These findings suggest that in cancer patients presenting with a heart failure admission, improving heart failure care and management of co-morbidities in particular has the potential for improving survival.
Given this, our findings of potential deficits in specialist hospital care and evidence-based management suggest there may be opportunities to improve outcomes in cancer patients presenting with acute heart failure. Specifically, we have shown cancer patients with HFrEF were less likely than patients without cancer to be prescribed ACEi/ARB with the largest effect seen in lung cancer patients in whom a deficit of MRA and beta-blocker prescribing was also noted. This supports other research showing that HF management therapies are under-prescribed for cancer patients.24 For example, a study by Ohtani et al. found that only 51.9% of cancer patients who developed anthracycline-induced cardiotoxicity received HF management therapy including renin–angiotensin inhibitor and/or beta-blocker therapy.25 Another study found that only 48% of patients that experienced cardiotoxicity commenced on beta-blocker and/or ACEi therapy.26 In some cases, under-prescription may be appropriate due to a contraindication, for example, during the terminal phase of cancer care or where oral treatment is limited. However, it is well established that HF medications improve symptoms and reduce HF admissions as well as improve prognosis.27–29 There is therefore a strong rationale for optimizing treatments, even in non-curable cancers. Further research will be needed to determine to what extent the treatment differences demonstrated are a reflection of appropriate clinical management of patients with a poor cancer prognosis and whether improving treatment in this group has the potential to improve outcomes.
One of the primary reasons reported for underutilization of HF management therapy in cancer patients following hospital admission includes the absence of formal cardiology referral.30 Patients with HF looked after by an appropriate specialist multidisciplinary team receive significantly more guideline-recommended HF management therapy and have better outcomes.10 In a previous study, cardiology consultation in cancer patients has also been associated with a significantly higher frequency of HF management therapy prescription [100% vs. 52% for ACEi/ARB (P < 0.0001); 94% vs. 41% for beta-blocker (P < 0.0001)].31 Our study confirms that a lower percentage of cancer patients admitted with acute HF were seen in a cardiology ward. Again, the largest difference was seen in lung cancer patients. It is likely that patients whose main place of care is a non-cardiology ward do not receive a consultation with a cardiologist, which likely contributes to the suboptimal management of HF. These findings suggest that increased access to specialist cardiology or cardio-oncological care may improve treatment in cancer patients with HF.
Limitations
The study has a number of limitations. The heart failure audit is limited to patients with an acute heart failure admission. This analysis cannot be extended to heart failure patients managed in primary care or an ambulatory setting. Propensity matching was limited to potentially relevant confounders reliably recorded in the audit data. Prescription of HF management medication at the point discharge was used as a proxy for continuing treatment. We did not have information on maintenance of medication at admission or for the duration of follow-up. The data analysed are retrospective, and therefore, we are at present unable to investigate newer heart failure medications such as angiotensin receptor–neprilysin inhibitor and sodium–glucose transporter 2 inhibitor therapies. Likewise, we are not able to include analysis of the timing and nature of cancer treatments. This will be an important future analysis. The NHFA does not include absolute values of ejection fraction, and so the field for left ventricular systolic dysfunction was used as a surrogate for HFrEF. Natriuretic peptide data are poorly completed with only 8% coverage in the audit and were therefore not used in this analysis. Cause of death information was only available for cancer patients from linked mortality data; thus, for the cause-specific analysis, it was assumed that cancer deaths were negligible in the controls. This was felt to be a safe assumption as the whole registry was searched for cancer diagnoses after HF presentation and death certificate cancer deaths are included in the registry. However, this meant that we could only compare non-cancer–related mortality between cases and controls, and we were unable to compare HF-specific mortality. Primary cause of death information may also be inaccurate in co-morbid populations, which may partly explain the apparent higher non-cancer–related survival in cancer patients (Figure 1D). Further, whereas tumour stage is now well recorded overall in the NCRD, tumour stage was not well-recorded in these patients with HF (53% missing) due to many cancers being diagnosed before 2012 where staging completeness was markedly lower. Therefore, we decided not to include tumour stage within the analyses. Cardiology ward care, as recorded in the audit, is used here as a surrogate for specialist care. We did not have information on prior cardiovascular risk factors or post-discharge care provided by HF community nurse specialists. We also did not have information about post-discharge quality of life. Finally, as for all observational studies, there is a risk of residual confounding limiting causal inferences.
Conclusions
Survival of cancer patients presenting with acute HF is very poor and is driven primarily by non-cancer causes (i.e. HF). Cancer patients with acute HF are less likely to be managed by a cardiology specialist and are less likely to receive evidence-based treatments. This is particularly true for patients with a poorer cancer prognosis. More research will be needed to determine if these treatment differences are a reflection of appropriate prognosis-guided clinical management or if optimal HF management guided by a cardio-oncology specialist multidisciplinary team has any potential to improve outcomes in cancer patients.
Supplementary Material
Acknowledgements
The VICORI collaborative: Sarah Darby, Chris Gale, Mike Hawkins, Alexander Lyon, Jem Rashbass, Raoul Reulen, Alistair Ring Adam Timmis, Sally Vernon Jennifer Lai, and our lead lay representative, Paul Charlton, and the past and present VICORI patient panel.
We acknowledge support from National Institute for Cardiovascular Outcomes Research staff (James Chal, Akosua Donkor, Nadeem Fazal, Anil Gunesh, Andrew Harrison), audit leads (Abbas Khushnood, Andrew Goodwin, Peter Ludman, Theresa McDonagh, Francis Murgatroyd), and NHS Digital, particularly their staff (Brian Shand, Sally Vernon). Particular thanks to David Forman as external member for chairing the VICORI project review panel.
Contributor Information
Briana Coles, Biostatistics Research Group, Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK.
Catherine A Welch, Biostatistics Research Group, Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK; National Disease Registration Service, NHS Digital, Wellington Place, Leeds, LS1 4AP, UK.
Rishabh S Motiwale, Biostatistics Research Group, Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK; School of Medicine, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK.
Lucy Teece, Biostatistics Research Group, Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK; National Disease Registration Service, NHS Digital, Wellington Place, Leeds, LS1 4AP, UK.
Clare Oliver-Williams, Biostatistics Research Group, Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK.
Clive Weston, Department of Cardiology, Glangwili General Hospital, Dolgwili Road, Carmarthen, SA31 2AF, UK.
Mark A de Belder, National Institute for Cardiovascular Outcomes Research, Arden & GEM Commissioning Support Unit, St John's House, 30 East Street, Leicester, LE1 6NB, UK.
Paul C Lambert, Biostatistics Research Group, Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK; Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, 171 77, Stockholm, Sweden.
Mark J Rutherford, Biostatistics Research Group, Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK.
Lizz Paley, National Disease Registration Service, NHS Digital, Wellington Place, Leeds, LS1 4AP, UK.
Umesh T Kadam, Diabetes Research Centre, University of Leicester, Leicester General Hospital, Gwendolen Road, Leicester, LE5 4PW, UK; Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK.
Clare A Lawson, Diabetes Research Centre, University of Leicester, Leicester General Hospital, Gwendolen Road, Leicester, LE5 4PW, UK; Department of Cardiovascular Sciences, University of Leicester and NIHR Leicester Biomedical Research Centre, University of Leicester, Glenfield Hospital, Groby Road, Leicester, LE3 9QP, UK.
John Deanfield, National Institute for Cardiovascular Outcomes Research, Arden & GEM Commissioning Support Unit, St John's House, 30 East Street, Leicester, LE1 6NB, UK; Institute of Cardiovascular Science, University College London, 62 Huntley Street, London, WC1E 6DD, UK.
Michael D Peake, National Disease Registration Service, NHS Digital, Wellington Place, Leeds, LS1 4AP, UK; Department of Respiratory Medicine, University of Leicester, Glenfield Hospital, Groby Road, Leicester, LE3 9QP, UK.
Theresa McDonagh, King's College Hospital, Denmark Hill, London, SE5 9RS, UK.
Michael J Sweeting, Biostatistics Research Group, Department of Health Sciences, George Davies Centre, University of Leicester, University Road, Leicester, LE1 7RH, UK; Statistical Innovation, AstraZeneca, City House, Hills Road, Cambridge CB2 1RY, UK.
David Adlam, Department of Cardiovascular Sciences, University of Leicester and NIHR Leicester Biomedical Research Centre, University of Leicester, Glenfield Hospital, Groby Road, Leicester, LE3 9QP, UK.
VICORI collaborative:
Sarah Darby, Chris Gale, Mike Hawkins, Alexander Lyon, Jem Rashbass, Raoul Reulen, Alistair Ring Adam Timmis, Sally Vernon Jennifer Lai, Paul Charlton, Akosua Donkor, Nadeem Fazal, Anil Gunesh, Andrew Harrison, Abbas Khushnood, Andrew Goodwin, Peter Ludman, Theresa McDonagh, Francis Murgatroyd, Brian Shand, Sally Vernon, and David Forman
Author contributions
Briana Coles, Theresa McDonagh, Michael D Peake, John Deanfield, Clare A Lawson, Umesh T Kadam, Lizz Paley, Michael J Sweeting, Mark J Rutherford, Mark A de Belder, Clive Weston, Clare OLIVER-WILLIAMS, Lucy Teece, Rishabh S Motiwale, Catherine A Welch (Ph.D.), Paul C Lambert, and David Adlam.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Funding
This study was jointly funded by the Cancer Research UK (C53325/A21134) and the British Heart Foundation (SP/16/5/32415). This project was supported by funding from the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands (ARC EM). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care. The funders had no role in the study design, in the collection, analysis, or interpretation of the data, in the writing of the report, or in the decision to submit the article for publication.
Data availability
Data cannot be shared for ethical/privacy reasons.
Publication ethics and malpractice
In this article, we followed the guidance provided in the Committee on Publication Ethics (COPE) Core Practices and subscribed to the International Committee of Medical Journal Editors (ICMJE) Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly work in Medical Journals.
References
- 1. Arnold M, Rutherford MJ, Bardot A, Ferlay J, Andersson TM, Myklebust TA, . et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol 2019;20:1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adlam D, Peake MD. Cancer and heart disease: new bedfellows in the cardiovascular landscape. Eur Heart J Qual Care Clin Outcomes 2017;3:168–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR , et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019;394:1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Boer RA, Hulot JS, Tocchetti CG, Aboumsallem JP, Ameri P, Anker SD , et al. Common mechanistic pathways in cancer and heart failure. A scientific roadmap on behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020;22:2272–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meijers WC, de Boer RA. Common risk factors for heart failure and cancer. Cardiovasc Res 2019;115:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zamorano JL, Gottfridsson C, Asteggiano R, Atar D, Badimon L, Bax JJ, . et al. The cancer patient and cardiology. Eur J Heart Fail 2020;22:2290–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopez-Sendon J, Alvarez-Ortega C, Zamora Aunon P, Buno Soto A, Lyon AR, Farmakis D, . et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020;41:1720–1729. [DOI] [PubMed] [Google Scholar]
- 8. Tocchetti CG, Ameri P, de Boer RA, D'Alessandra Y, Russo M, Sorriento D, . et al. Cardiac dysfunction in cancer patients: beyond direct cardiomyocyte damage of anticancer drugs: novel cardio-oncology insights from the joint 2019 meeting of the ESC Working Groups of Myocardial Function and Cellular Biology of the Heart. Cardiovasc Res 2020;116:1820–1834. [DOI] [PubMed] [Google Scholar]
- 9. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, . et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 2016;37:2768–2801. [DOI] [PubMed] [Google Scholar]
- 10. Horne BD, Roberts CA, Rasmusson KD, Buckway J, Alharethi R, Cruz J, . et al. Risk score-guided multidisciplinary team-based care for heart failure inpatients is associated with lower 30-day readmission and lower 30-day mortality. Am Heart J 2020;219:78–88. [DOI] [PubMed] [Google Scholar]
- 11. Lancellotti P, Suter TM, Lopez-Fernandez T, Galderisi M, Lyon AR, Van der Meer P, . et al. Cardio-oncology services: rationale, organization, and implementation. Eur Heart J 2019;40:1756–1763. [DOI] [PubMed] [Google Scholar]
- 12. Authors/Task Force M, McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, . et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 13. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, . et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol 2020;31:171–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sweeting MJ, Oliver-Williams C, Teece L, Welch CA, de Belder MA, Coles B, . et al. Data resource profile: the Virtual Cardio-Oncology Research Initiative (VICORI) linking national English cancer registration and cardiovascular audits. Int J Epidemiol 2022;50(6):1768–1779. 10.1093/ije/dyab082. Epub 2021 Jun 5. PMID: 34999882; PMCID: PMC8743125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. [NICOR. National Heart Failure Audit 2019 summary report 2019:. Accessed online <]. https://www.nicor.org.uk/wp-content/uploads/2019/09/Heart-Failure-2019-Report-final.pdf > on 21 Aug 2021.
- 16. Henson KE, Elliss-Brookes L, Coupland VH, Payne E, Vernon S, Rous B, . et al. Data resource profile: national cancer registration dataset in England. Int J Epidemiol 2020;49, 16–16h. 10.1093/ije/dyz076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. https://www.nicor.org.uk/heart-failure-heart-failure-audit/.
- 18. [Commission TJ. Specifications manual for joint commission national quality measures (v2016A). Accessed online <]. https://manual.jointcommission.org/releases/TJC2016A/DataElem0439.html > on 21 Aug 2021.
- 19. Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology 2003;14:680–686. [DOI] [PubMed] [Google Scholar]
- 20. Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013;6:604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Royston P, Lambert PC. Flexible parametric survival analysis using stata: beyond the cox model. College Station, TX: Stata Press; 2011. [Google Scholar]
- 22. Pilleron S, Sarfati D, Janssen-Heijnen M, Vignat J, Ferlay J, Bray F, . et al. Global cancer incidence in older adults, 2012 and 2035: a population-based study. Int J Cancer 2019;144:49–58. [DOI] [PubMed] [Google Scholar]
- 23. Mamas MA, Sperrin M, Watson MC, Coutts A, Wilde K, Burton C, . et al. Do patients have worse outcomes in heart failure than in cancer? A primary care-based cohort study with 10-year follow-up in Scotland. Eur J Heart Fail 2017;19:1095–1104. [DOI] [PubMed] [Google Scholar]
- 24. [NICE. Chronic heart failure in adults: diagnosis and management. Accessed online <]. https://www.nice.org.uk/guidance/ng106 > on 21 Aug 2021.
- 25. Ohtani K, Fujino T, Ide T, Funakoshi K, Sakamoto I, Hiasa KI, . et al. Recovery from left ventricular dysfunction was associated with the early introduction of heart failure medical treatment in cancer patients with anthracycline-induced cardiotoxicity. Clin Res Cardiol 2019;108:600–611. [DOI] [PubMed] [Google Scholar]
- 26. Khan AA, Ashraf A, Singh R, Rahim A, Rostom W, Hussain M, . et al. Incidence, time of occurrence and response to heart failure therapy in patients with anthracycline cardiotoxicity. Intern Med J 2017;47:104–109. [DOI] [PubMed] [Google Scholar]
- 27. Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, . et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–220. [DOI] [PubMed] [Google Scholar]
- 28. Abid L, Charfeddine S, Kammoun I, Ben Halima M, Ben Slima H, Drissa M , et al. Epidemiology of heart failure and long-term follow-up outcomes in a north-African population: results from the NAtional Tunisian REgistry of heart failure (NATURE-HF). PLoS One 2021;16:e0251658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Slowik A, Jagielski P, Potocki P, Streb J, Ochenduszko S, Wysocki P, . et al. Anthracycline-induced cardiotoxicity prevention with angiotensin-converting enzyme inhibitor ramipril in women with low-risk breast cancer: results of a prospective randomized study. Kardiol Pol 2020;78:131–137. [DOI] [PubMed] [Google Scholar]
- 30. Emdin CA, Hsiao AJ, Kiran A, Conrad N, Salimi-Khorshidi G, Woodward M, . et al. Referral for specialist follow-up and its association with post-discharge mortality among patients with systolic heart failure (from the National Heart Failure Audit for England and Wales). Am J Cardiol 2017;119:440–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ammon M, Arenja N, Leibundgut G, Buechel RR, Kuster GM, Kaufmann BA, . et al. Cardiovascular management of cancer patients with chemotherapy-associated left ventricular systolic dysfunction in real-world clinical practice. J Card Fail 2013;19:629–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data cannot be shared for ethical/privacy reasons.