Abstract
Background
Difficulty in weaning from mechanical ventilation is encountered in appro-ximately 20% of patients in the intensive care unit. We assessed the utility of a combined lung, diaphragmatic, and cardiac ultrasound protocol to predict extubation failure.
Methods
All patients extubated following a successful spontaneous breathing trial (SBT) were included in the study. Lung ultrasonography score (LUS), diaphragmatic thickness fraction (DTF), changes in velocity time integral (VTI) to passive leg raise at the beginning of SBT, and change in LUS following SBT were recorded.
Results
A total of 60 patients who underwent successful SBT were included in the study. Twenty-seven patients required either non-invasive or invasive mechanical ventilation during the next 48 hours and were classified as weaning failure (Group F). The remaining 33 patients were designated as weaning success (Group S). Compared to group S, patients in Group F had significantly longer ICU length of stay (6.96 ± 4.30 days vs. 11.66 ± 3.85 days, P < 0.001), higher LUS change during SBT (1 [0–2] vs. 2 [1–4], P < 0.001), lower DTF (30.87 ± 5.32 vs. 27.88 ± 6.24, P = 0.04), and showed lower VTI increment to PLR (13.63 ± 3.44 vs. 9.11 ± 4.59, P < 0.001). Using a binary logistic regression model, DTF < 26% (odds ratio 6.20, 95% CI: 1.06–36.04) and VTI change to PLR < 10.2% (odds ratio 6.16, 95% CI: 1.14–33.13) were found to be significant predictors of weaning failure (P < 0.05). The AUROC for VTI and DTF for predicting weaning failure were 0.79 and 0.64, respectively.
Conclusions
An integrated ultrasound protocol using a combination of lung, diaphragm, and cardiac sonography was a reliable predictor of weaning failure.
Keywords: mechanical ventilation, weaning, ultrasound
The aim of providing mechanical ventilation in the intensive care unit (ICU) is to support the patient’s respiration until the patient can maintain adequate ventilatory homeostasis. Once the airway and cardiopulmonary functions are stabilized and the primary disease process is resolved, patients are subjected to a spontaneous breathing trial (SBT), typically 30 to 120 minutes in duration, to gauge readiness for ventilator weaning [1]. Weaning from ventilation may account for almost 40% of the patient time spent in the ICU, and in as many as 20% of cases significant difficulties may be encountered [2]. Weaning failure occurs in patients who fail the initial SBT and in patients who develop respiratory distress within 48 hours of extubation. These patients have significantly higher morbidity and mortality rates, including a longer length of stay in the ICU and in the hospital and a greater chance of requiring transition to a long-term acute care facility or nursing home, thereby incurring greater healthcare costs [2]. Therefore, an objective assessment of patients before and during an SBT is needed to identify predictors of weaning failures. The assessment should aim to diagnose reversible conditions (pulmonary oedema, left ventricular failure, and ischaemia) that might contribute to failure to wean a patient from mechanical ventilation and may indicate that the patient would benefit from directed therapy [3]. Bedside ultrasonography, which is increasingly available to intensive care physicians, provides immediate information about changes in pulmonary and cardiac function [2]. Recent investigations focusing on lung, diaphragm, or cardiac ultrasounds have highlighted the potential of ultra-sonography to enhance the prediction of extubation outcomes [4–14].
Soummer et al. [12] used a well-established lung ultrasound score (LUS) to characterize the lung aeration patterns in bilateral lung fields. Loss of lung aeration during SBT and higher baseline scores were associated with post-extubation distress and higher occurrences of reintubation. Another contributing factor is atrophy of diaphragmatic fibres, which has been well described in patients on prolonged mechanical ventilation [15]. While a patient is on mechanical ventilation, the thickness of the diaphragm in the zone of apposition and diaphragmatic excursion can be used to assess their diaphragmatic function [9]. Additionally, cardiovascular dysfunction during an SBT is increasingly recognized as a potential cause of weaning failure [6–8, 14]. It occurs when the heart is unable to tole-rate the SBT-associated increase in preload and afterload. Dres et al. [16] used the passive leg raise (PLR) test to assess the preload independence of the heart and found that failure to increase cardiac output by 10% in response to a PLR correlated with a higher likelihood of a failure in SBT.
The primary aim of this study was to assess the ability of an integrated lung, diaphragmatic, and cardiac ultrasound protocol to predict weaning success and extubation failure. The secondary objectives were to assess the potential contribution of lung, diaphragmatic, and cardiovascular dysfunction to failed weaning and to determine any other variables during an ICU stay that may be associated with weaning success/failure.
METHODS
The study was registered with the Clinical Trials Registry of India (reference number CTRI/2018/08/015368) and was performed across 2 intensive care units of a tertiary care university teaching hospital. Following institutional ethics committee approval (ref. no. IECPG-435/27.07.2016, RT 12/21.09.2016, dated 22.09.2016) and with informed written consent, patients were recruited for the study. Consent was obtained from the next of the kin for patients on mechanical ventilation who were considered for study recruitment. The period of recruitment was from October 2016 through June 2018.
Patients who underwent extubation following a successful SBT and who had been on mechanical ventilation for more than 48 hours were included in the study. To be considered for SBT, patients must have had a stable clinical status as defined by stable oxygenation (FiO2 < 0.5, PEEP ≤ 5 cm H2O), a respiratory rate of < 30 breaths per minute, an absence of fever, alertness and cooperation, haemodynamic stability, and the absence of high doses of vasopressor therapy (requirement of norepinephrine > 0.1 μg kg–1 min–1 or equivalent vasopressor doses).
Exclusionary criteria included short-term mecha-nical ventilation (< 48 hours) in the immediate postoperative period, age < 18 years, patients with spinal cord injuries above the T8 level, presence of significant cardiac arrhythmia, diaphragmatic paralysis, ICU-acquired neuromyopathy, planned non-invasive ventilation (NIV) following extubation, and poor ultrasound windows.
The SBT protocol was standardized across our study sites. The patients underwent a 120-minute SBT with standardized ventilator settings (pressure support ventilation, PS 5, PEEP 5, and FiO2 40%). SBT failure was recognized based on the following parameters: change in mental status, the onset of discomfort or diaphoresis, respiratory rate > 35 breaths per minute, haemodynamic instability (heart rate > 140 min-1, systolic blood pressure > 180 or < 90 mmHg), or laboured breathing.
All patients included in the study were examined by bedside ultrasonography before and after SBT. An ultrasound examination was performed on all patients by a single intensive care physician with more than 5 years of experience in performing critical care ultrasonography, who was blinded to the study protocols and results. Twenty patients were initially examined to assess the feasibility of the study protocols. These patients were not included in the final analysis. The physician scanned the thorax and sequentially measured the LUS and diaphragm thickening fraction (DTF) with the patient in a semi-recumbent position [15, 16]. After LUS and DTF were obtained, echocardiography was performed to measure the baseline velocity time integral (VTI) with the patient in a semi-recumbent position. Once the requisite readings were obtained, a PLR manoeuvre was performed, and the change in VTI to PLR was then recorded. Subsequently, all patients underwent SBT. A lung ultrasound was performed at the end of SBT by the same investigator by rescanning the requisite lung fields. All ultra-sonographic measurements were an average of 3 measurements to reduce intra-observer variability. The patient’s vitals (heart rate, respiratory rate, mean arterial pressure, and central venous pressure) and arterial blood gas values were recorded by the investigator before the start and at the end of SBT. The attending physician and the nurse were blinded to the ultrasound results. Patients who were extubated after a successful SBT trial were followed up until their discharge from the ICU. Weaning/extubation failure was defined as the requirement of non-invasive or invasive mechanical ventilation over the ensuing 48 hours (Group F). The patients who did not require any form of non-invasive or invasive mechanical ventilator support during the 48 hours following extubation were classified as a successful weaning (Group S).
The baseline patient data, including admission APACHE and SOFA scores, reason for ICU admission and mechanical ventilation, use of corticosteroids, neuromuscular blockers, sedative/analgesic use, the predominant mode of ventilation, and the time to SBT and ICU length of stay were recorded.
Sample size calculations and statistical analysis
This study aimed to determine whether the addition of an integrated ultrasound protocol using the measurements of LUS, DTF, and cardiac VTI response to PLR can be used to better predict weaning success or failure. We planned to perform an observational pilot study recruiting all patients who satisfy the inclusion criteria for 18 months following institutional Ethics Committee approval. We aimed to devise a scoring system using the 3 ultrasonographically measured variables of LUS, DTF, and VTI change to PLR. To develop an integrated scoring system of 3 variables, at least 30 events (10 per variable) were required, with an estimated incidence of weaning failure of 50%, n = 60 patients were needed. The data obtained were analysed using IBM SPSS Statistics (IBM Inc., Chicago, IL, USA) for Windows, version 20.0, and the online calculator https://www.openepi.com/. Parametric data are presented as the mean ± SD, while nonparametric data are presented as the median (interquartile range). Normally distributed data were compared using Student’s t-test. Non-normally distributed data were compared using the Mann-Whitney test. The χ2 test was used to compare categorical variables. Receiver operating characteristic (ROC) curves were constructed for each predictor of weaning failure, and the best cut-off values were obtained from Youden’s index. Subsequently, a binary logistic regression model was constructed from the variables that were significantly different in the weaning success and failure groups to identify predictors of weaning failure. Model fitness was tested by the goodness of fit test.
RESULTS
Figure 1 represents the flow of patients throughout the study. Sixty patients who underwent a successful SBT during the ICU stay followed by removal from mechanical ventilation were included in the final analysis. The baseline demographic parameters of the entire cohort of patients (n = 60) are represented in Table 1. Thirty-three patients (55%) did not require any non-invasive or invasive mechanical ventilator support during the next 48 hours and were considered to be successfully weaned; these patients were designated as Group S. The remaining 27 patients were classified as weaning failure (Group F).
FIGURE 1.
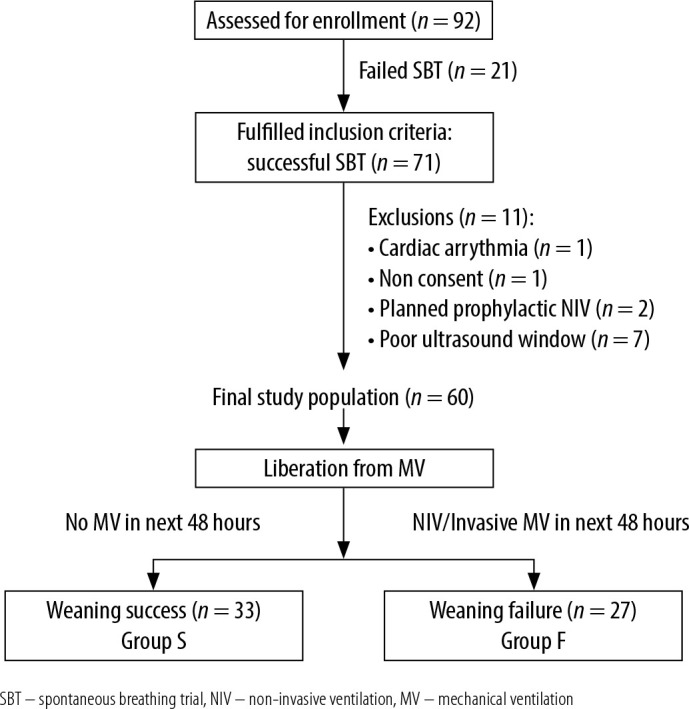
Flowchart depicting the flow of patients through the study
TABLE 1.
Baseline demographics of patients (n = 60)
| Parameters | Mean ± SD/median (IQR) |
|---|---|
| Age (years) | 42.7 ± 16.98 |
| Weight (kg) | 65.8 ± 11.35 |
| Sex, n (%) | |
| Male | 30 (50) |
| Female | 30 (50) |
| BMI (kg m–2) | 23.02 ± 3.47 |
| APACHE II | 17.13 ± 6.03 |
| Admission SOFA score | 9.43 ± 3.48 |
| SOFA score (at SBT) | 3.4 ± 1.68 |
| Time to 1st SBT (days) | 4.81 ± 2.19 |
| LOS in ICU (days) | 9.08 ± 4.70 |
| Cumulative fluid balance (at SBT) (mL) | 564.7 (315.4–937.5) |
| Haemoglobin | 10.03 ± 1.53 |
The data is presented as mean ± SD/median (IQR) as applicable. (*) Gender data are presented as absolute numbers, n = 30 for male participants, n = 30 for female participants.
BMI – body mass index, APACHE – Acute Physiology and Chronic Health Evaluation,
SOFA – Sequential Organ Failure Assessment, SBT – spontaneous breathing tial, LOS – length of stay, ICU – intensive care unit
The groups were comparable in terms of baseline variables, as depicted in Table 2. However, patients in Group F had a longer total ICU stay (LOS ICU) than those in Group S (P < 0.001).
TABLE 2.
Comparison of baseline variables in weaning success (Group S) and failure groups (Group F)
| Factor | group S (n = 33) | group F (n = 27) | P-value | |
|---|---|---|---|---|
| Age (years) | 42.33 ± 17.05 | 43.14 ±16.83 | 0.85 | |
| Weight (kg) | 66.23 ± 13.05 | 65.44 ± 9.06 | 0.78 | |
| BMI (kg m–2) | 23.12 ± 3.63 | 22.90 ± 3.32 | 0.81 | |
| APACHE II | 15.87 ± 5.20 | 18.66 ± 6.70 | 0.07 | |
| Admission SOFA score | 8.63 ± 3.38 | 9.40 ± 3.42 | 0.38 | |
| Comorbid illness, n (%) | ||||
| Endocrine | 10 (30) | 3 (11) | 0.06 | |
| Hypertension | 8 (24) | 7 (25) | 0.55 | |
| CAD | 5 (15) | 2 (7) | 0.30 | |
| CVA | 1 (3) | 2 (7) | 0.42 | |
| Respiratory | 6 (18) | 5 (18) | 0.61 | |
| Heart failure | 5 (15) | 2 (7) | 0.30 | |
| Valve disease | 1 (3) | 0 (0) | 0.55 | |
| Renal | 5 (15) | 8 (29) | 0.14 | |
| Category of admission, n (%) | ||||
| Medical | 16 (48) | 16 (59) | 0.284 | |
| Surgical | 17 (51) | 11 (40) | ||
| Source of admission, n | ||||
| ED | 11 | 8 | 0.25 | |
| Ward | 21 | 14 | ||
| ICU | 1 | 4 | ||
| Interhospital | 0 | 1 | ||
| Indication for MV, n | ||||
| Resp. | 12 | 16 | 0.27 | |
| CNS | 4 | 1 | ||
| Combination | 5 | 4 | ||
| Other | 12 | 6 | ||
| No of organ dysfunction, n | ||||
| 1 | 10 | 5 | 0.20 | |
| 2 | 19 | 14 | ||
| ≥ 3 | 4 | 8 | ||
| Corticosteroid use, n | 7 | 12 | 0.05 | |
| NMBA use, n | 3 | 7 | 0.08 | |
| Sedative use, n | ||||
| Fentanyl | 22 | 22 | 0.67 | |
| Morphine | 3 | 2 | ||
| Dexmedetomidine | 3 | 0 | ||
| Combination | 5 | 3 | ||
| SOFA score (at SBT) | 3.09 ± 1.40 | 3.77 ± 1.52 | 0.07 | |
| Mode of SBT, n | ||||
| T piece | 24 | 20 | 0.57 | |
| PS 5 | 9 | 7 | ||
| Time to 1st SBT (days) | 4.45 ± 2.01 | 5.25 ± 2.34 | 0.15 | |
| LOS in ICU (days) | 6.96 ± 4.30 | 11.66 ± 3.85 | < 0.001 | |
| Cumulative fluid balance (at SBT) (mL) | 2738.03 ± 725.50 | 3919.81 ± 2521.48 | 0.20 | |
| 24 hours fluid balance (preceding SBT) (mL) | 260.72 ± 682.90 | 419.50± 847.19 | 0.42 | |
CAD – coronary artery disease, CVA – cerebrovascular accidents, ED – emergency department, ICU – intensive care unit, MV – mechanical ventilation, Resp. – respiratory system, CNS – central nervous system, NMBA – neuromuscular blocking agent, SOFA – Sequential Organ Failure Assessment, SBT – Spontaneous Breathing Trial, LOS – length of stay
The comparison between Group S and Group F in terms of weaning variables is depicted in Table 3. The patients in Group S had a significantly lower LUS score both before and after SBT compared to Group F. The patients in Group F had a significantly higher change in LUS following SBT compared to Group S (P = 0.005). The patients in Group F had a lower DTF than those in Group S (P = 0.04). The patients in Group S showed a higher incremental VTI response to a passive leg raise compared to Group F (P < 0.001).
TABLE 3.
Comparison of weaning variables in the weaning success (Group S) and failure groups (Group F)
| Weaning parameters | group S (n = 33) | group F (n = 27) | P-value |
|---|---|---|---|
| LUS (before SBT) | 15.06 ± 2.16 | 17.44 ± 2.59 | < 0.001 |
| LUS (after SBT) | 16.12 ± 2.17 | 19.85 ± 2.56 | < 0.001 |
| DTF (%) | 30.87 ± 5.32 | 27.88 ± 6.24 | 0.04 |
| VTI change (%) | 13.63 ± 3.44 | 9.11 ± 4.59 | < 0.001 |
| RSBI | 80.90 ± 22.62 | 84.07 ± 16.19 | 0.54 |
| Vt (expired) (mL) | 377.01 ± 71.08 | 355.29 ± 46.13 | 0.17 |
| LUS change | 1 (0–2) | 2 (1–4) | 0.005 |
| Delta pO2 (mmHg) | 15.2 (10–21) | 13.3 (5–25) | 0.67 |
The data are presented as mean + SD/median (IQR) as applicable. LUS change and delta pO2 are presented as median (IQR).
LUS – lung ultrasound score, DTF – diaphragmatic thickness fraction, SBT – spontaneous breathing trial, VTI – velocity time integral, Vt – tidal volume, RSBI – rapid shallow breathing index
Comparing the cardiorespiratory variables before and after SBT, Group F patients had significantly higher CVP values before the commencement of SBT (CVP in cm of H2O: Group S 9.39 ± 3.18, Group F 10.92 ± 2.58, P = 0.04) and respiratory rate than Group S patients (after the conclusion of SBT) (RR/minute: Group S 20.24 ± 4.02, Group F 22.37 ± 3.45, P = 0.03). The pO2 values after SBT were found to be significantly higher in Group S (111.96 ± 33.30 mmHg) than in Group F (95.70 ± 14.45 mmHg) (P = 0.02).
The ROC curves for DTF (AUROC 0.64) and VTI to PLR (AUROC 0.79) are presented in Figure 2 and Figure 3. For DTF, the optimal cut-off point for predicting weaning success was ≥ 26% (sensitivity 90.91%, specificity 37.04%, +veLR 1.4439, –veLR 0.2455). The optimal cut-off value for a VTI change to PLR was ≥ 10.2% (sensitivity 84.85%, specificity 66.67%, 2.5455, +veLR 0.2273, –veLR 0.51).
FIGURE 2.
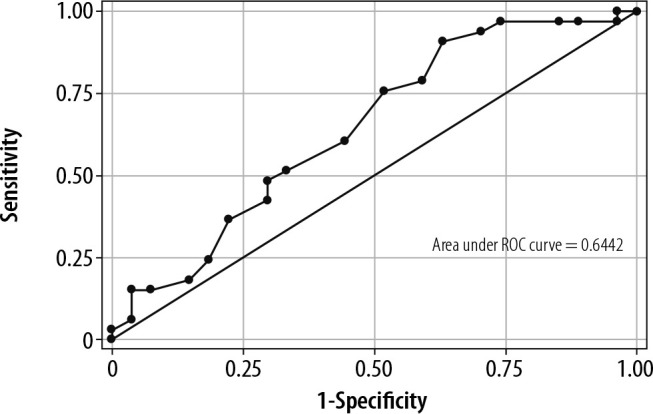
ROC curve for diaphragmatic thickness fraction (DTF)
FIGURE 3.
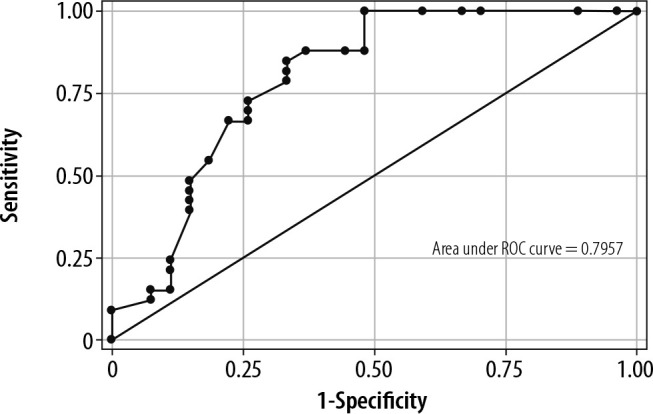
ROC curve for velocity time integral (VTI) change to passive leg raising (PLR)
Based on these observations, a binary logistic regression model was constructed using weaning failure or success as the dependent outcome (Table 4). In this model, DTF < 26% was a significant predictor of weaning failure in our study population with an odds ratio of 6.20 (95% CI: 1.06–36.04, P = 0.04). The percentage change in VTI to a PLR of less than 10.2% predicted weaning failure with an odds ratio of 6.16 (95% CI: 1.14–33.13, P = 0.03).
TABLE 4.
Binary logistic regression model showing predictors of extubation failure
| Factor | Odds ratio | 95% confidence interval | P-value | |
|---|---|---|---|---|
| Age | 1.023 | 0.97–1.07 | 0.34 | |
| DTF < 26% | 6.20 | 1.06–36.04 | 0.04* | |
| VTI change < 10.2 | 6.16 | 1.14–33.13 | 0.03* | |
| LUS change | ||||
| ≥ 1 | 1.62 | 0.20–13.12 | 0.64 | |
| ≥ 2 | 0.53 | 0.07–3.76 | 0.53 | |
| ≥ 3 | 0.67 | 0.044–10.07 | 0.77 | |
| CVP > 11 cm H20 | 0.46 | 0.10–25.01 | 0.30 | |
| Respiratory Rate > 20 | 0.65 | 0.15–2.84 | 0.57 | |
LUS – lung ultrasound score, DTF – diaphragmatic thickness fraction, SBT – spontaneous breathing trial, VTI – velocity time integral, Vt – tidal volume, CVP – central venous pressure
DISCUSSION
Bedside ultrasound can be used to assess cardiac function, diaphragmatic function, and lung aeration before discontinuation from mechanical ventilation [17]. Dysfunction of any of these structures could potentially lead to failed weaning attempts. Therefore, we based our study on the premise that using bedside ultrasound to assess functions of those organs could be a better guide to weaning than looking at them in isolation.
The LUS was first demonstrated as a measure to assess global and regional lung recruitment at the bedside, with higher LUS being more likely to lead to post-extubation respiratory distress [12]. In our study, patients who had successful weaning had lower values of LUS both before and after SBT compared to the group who had a failure of weaning. In addition, SBT was associated with loss of lung aeration, as suggested by the higher LUS after SBT compared to pre-SBT values in both groups.
Diaphragmatic dysfunction in critically ill patients can be secondary to ventilator-induced diaphragmatic injury, ICU-acquired critical illness neuromyopathy, or neuromuscular disorders or drugs. The measures of diaphragmatic thickening and diaphragmatic excursion have been found to correlate with trans-diaphragmatic pressure measurements [18]. Diaphragmatic excursion can be influenced by the amount of mechanical ventilatory support. In contrast, DTF reflects diaphragmatic contractile activity and is independent of the amount of ventilatory support [19]. The threshold value of DTF as an indicator of diaphragmatic dysfunction has been reported to vary from 30% to 36% in the literature [20]. In our study, patients in Group F had a lower DTF than those in Group S (P < 0.05), and the optimal cut-off for DTF was 26% for predicting weaning success in our study, which is similar to the study by Samanta et al. [21]. Perhaps in the Indian population a lower DTF cut-off may be a better predictor of weaning success than in the Western population.
The process of weaning from mechanical ventilation increases the patient’s cardiac workload. The greater venous return along with changes in intrathoracic pressures in spontaneously breathing patients can potentially lead to weaning-induced cardiovascular dysfunction in a heart operating on the flat portion of the Frank Sterling curve. How-ever, patients who can increase cardiac output in response to an increase in preload (as measured by an increase in VTI in response to PLR) may have a better tolerance to increased cardiac workload imposed by spontaneous breathing. While both systolic and diastolic dysfunction can contribute to weaning failure, diastolic dysfunction is the commoner of the two [22, 23]. Doppler echocardiography using tissue Doppler imaging (TDI) and pulsed-wave Doppler (PWD) can be used to elicit several indices reflecting diastolic dysfunction at the bedside, which has been found to correlate with weaning failure in various studies [6–8, 14]. Evaluation of diastolic dysfunction requires proficiency in advanced echocardiography. While an E/E' ratio greater than 15 measured by tissue Doppler can indicate elevated left ventricular end diastolic pressure, values between 8 and 15 are less reliable [24]. On the other hand, measurement of a VTI change to PLR acts as a stress test for the heart and could be used bedside to reflect both systolic and diastolic cardiovascular dysfunction [16]. Therefore, we used this manoeuvre and subsequent VTI changes to look for weaning-induced cardiovascular dysfunction in our patient cohort.
After completion of SBT, patients in Group F had significantly higher respiratory rates than those in Group S. The findings are similar to research by Lima et al. [25], who suggested that a respiratory rate with a cut-off value of 24 breaths per minute was a predictor of weaning failure.
Before the commencement of SBT, patients in Group F had higher CVP values than those in Group S. This could be an incidental finding or may be related to a more volume-replete state in patients more likely to be associated with weaning failure [26]. However, there was no difference in cumulative fluid balance or last 24-hour fluid balance before extubation in our study between the 2 groups.
Twenty-seven patients (45%) required some form of invasive or non-invasive mechanical ventilator support during the following 48 hours and were classified as weaning failure. This finding highlights the fact that good performance during SBT does not guarantee that the patient will have successful extubation [27]. Thirteen patients could be managed with non-invasive ventilator support, while 14 patients required reintubation and invasive mechanical ventilation. NIV can benefit patients following planned extubation by avoiding reintubation, while its role in patients with established respiratory failure is less clear [28]. In our mixed medical-surgical ICU population, NIV appreciably decreased reintubation rates. The reintubation rate in our study was 23%, which is consistent with previous studies [29].
The longer ICU LOS in weaning failure patients is in accordance with previous studies [30]. This has been attributed to a higher incidence of nosocomial infections and airway complications in these patients.
In the binary logistic regression model, we found that the changes in DTF and VTI to PLR were the only significant predictors of weaning outcomes. In the initial univariate analysis, a lower LUS score before and after SBT and a lower change in LUS score during SBT (delta LUS) were found to be significant factors associated with weaning success. However, this was not replicated in the multiple regression model. Statistically, a univariate analysis might detect factors that may be a chance association. Secondly, lung ultrasonography is predominantly effective in detecting surface parenchymal changes but insensitive in detecting deeper parenchymal changes when surrounded by an area of normal parenchyma. Thus, early changes in interstitial oedema might not be picked up by lung ultrasonography, which might be better predicted by the inability of the heart to increase VTI in response to a PLR. Interestingly, in the study by Bouhemad et al. [11], static LV filling pressure was not a good predictor of weaning failure. Using the dynamic measure of a VTI change to PLR may be a better reflection of the stress imposed on the heart by increased work of spontaneous breathing than any other static parameter.
The cause of weaning failure is essentially multi-factorial. Well-performed bedside ultrasonography will identify the interplay of several factors that could lead to difficulty in liberating a patient from mechanical ventilation. A patient on long-standing mechanical ventilation with coexistent diaphragmatic weakness and cardiac dysfunction may fail a trial of extubation due to varying combinations of both processes.
Based on the findings of our study, we suggest that all patients should be routinely screened for a combination of DTF and VTI changes to PLR before removing them from mechanical ventilation. An abnormality in any of these parameters will alert the intensivist to the possibility of encountering a difficult weaning. Such patients could benefit from a change in therapy prior to extubation. Patients with poor response to VTI or response to PLR would probably benefit from a deresuscitation approach to fluid management or vasodilators [31]. Patients with diaphragmatic dysfunction could benefit from inspiratory muscle training [32, 33]. However, bedside clinicians should not look at ultrasound data in isolation and rather correlate them with the clinical status of the patient to guide decision-making.
Our study has several limitations. Measuring a VTI change to PLR requires a good-quality apical 5-chamber view, which may be difficult to obtain in patients with poor echocardiographic windows. The major reason for patient exclusion in the current study was suboptimal echocardiographic visualisation (i.e. poor acoustic window). Secondly, we did not assess RV and LV diastolic function independently. However, the usefulness of the PLR test lies in its ability to account for both RV and LV preload independence. An isolated assessment of LV systolic function is likely to miss patients with weaning-induced cardiovascular dysfunction due to RV failure or LV diastolic dysfunction.
The rate of extubation failure was relatively high (47%), and patients who needed NIV post extubation were included in Group F. However, the rate of reintubation following extubation (24%) was comparable to that in previous studies. Recently, it has been suggested that patients requiring NIV after extubation be classified in an intermediary category called “weaning in progress” [34]. Another limitation of ultrasonography is the considerable intra- and interobserver variability. Therefore, we allowed only one dedicated physician to perform all ultrasonographic examinations, to eliminate interobserver variability, and all measurements were averaged to reduce intra-observer variability. Finally, there might be other factors associated with weaning failure in either group, which may not have been accounted for in the current study.
Conclusions
The use of an integrated ultrasound protocol can be useful in identifying patients who are likely to fail extubation. It is important that clinicians image the heart, lungs, and diaphragm in combination and integrate the information obtained to modify therapy and identify patients at high risk of extubation failure.
ACKNOWLEDGEMENTS
Presentation
The above study was presented at the ESICM ANNUAL Conference held in Paris on 22 October 2018.
Financial support and sponsorship
none.
Conflict of interest
none.
References
- 1.McConville JF, Kress JP. Weaning patients from the ventilator. N Engl J Med 2012; 367: 2233-2239. doi: 10.1056/NEJMra1203367. [DOI] [PubMed] [Google Scholar]
- 2.Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med 1995; 332: 345-350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 3.Oropello J, Rahmanian M. Can chest sonography predict and facilitate successful ventilator weaning? Crit Care Med 2013; 41: 2065-2067. doi: 10.1097/CCM.0b013e3182963e91. [DOI] [PubMed] [Google Scholar]
- 4.Mongodi S, Via G, Bouhemad B, Storti E, Mojoli F, Braschi A. Usefulness of combined bedside lung ultrasound and echocardiography to assess weaning failure from mechanical ventilation: a suggestive case. Crit Care Med 2013; 41: e182-185. doi: 10.1097/CCM.0b013e31828e928d. [DOI] [PubMed] [Google Scholar]
- 5.Papanikolaou J, Makris D, Saranteas T, et al. New insights into weaning from mechanical ventilation: Left ventricular diastolic dysfunction is a key player. Intensive Care Med 2011; 37: 1976-1985. doi: 10.1007/s00134-011-2368-0. [DOI] [PubMed] [Google Scholar]
- 6.Caille V, Amiel JB, Charron C, Belliard G, Vieillard-Baron A, Vignon P. Echocardiography: a help in the weaning process. Crit Care 2010; 14: R120. doi: 10.1186/cc9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schifelbain LM, Vieira SRR, Brauner JS, Pacheco DM, Naujorks AA. Echocardiographic evaluation during weaning from mechanical ventilation. Clinics (Sao Paulo) 2011; 66: 107-111. doi: 10.1590/s1807-59322011000100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamia B, Maizel J, Ochagavia A, et al. Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med 2009; 37: 1696-1701. doi: 10.1097/CCM.0b013e31819f13d0. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari G, De Filippi G, Elia F, Panero F, Volpicelli G, Aprà F. Diaphragm ultrasound as a new index of discontinuation from mechanical ventilation. Crit Ultrasound J 2014; 6: 8. doi: 10.1186/2036-7902-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang JR, Tsai TH, Jerng JS, Yu CJ, Wu HD, Yang PC. Ultrasonogra-phic evaluation of liver/spleen movements and extubation outcome. Chest 2004; 126: 179-185. doi: 10.1378/chest.126.1.179. [DOI] [PubMed] [Google Scholar]
- 11.Bouhemad B, Liu ZH, Arbelot C, et al. Ultrasound assessment of antibiotic-induced pulmonary reaeration in ventilator-associated pneumonia. Crit Care Med 2010; 38: 84-92. doi: 10.1097/CCM.0b013e3181b08cdb. [DOI] [PubMed] [Google Scholar]
- 12.Soummer A, Perbet S, Brisson H, et al. Ultrasound assessment of lung aeration loss during a successful weaning trial predicts postextubation distress. Crit Care Med 2012; 40: 2064-2072. doi: 10.1097/CCM.0b013e31824e68ae. [DOI] [PubMed] [Google Scholar]
- 13.Mayo PH, Beaulieu Y, Doelken P, et al. American college of chest physicians/ la societédé réanimation de langue française statement on competence in critical care ultrasonography. Chest 2009; 135: 1050-1060. doi: 10.1378/chest.08-2305. [DOI] [PubMed] [Google Scholar]
- 14.Moschietto S, Doyen D, Grech L, Dellamonica J, Hyvernat H, Bernardin G. Transthoracic Echocardiography with Doppler Tissue Imaging predicts weaning failure from mechanical ventilation: Evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care 2012; 16: R81. doi: 10.1186/cc11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goligher EC, Fan E, Herridge MS, et al. Evolution of diaphragm thickness during mechanical ventilation: Impact of inspiratory effort. Am J Respir Crit Care Med 2015; 192: 1080-1088. doi: 10.1164/rccm.201503-0620OC. [DOI] [PubMed] [Google Scholar]
- 16.Dres M, Teboul JL, Anguel N, Guerin L, Richard C, Monnet X. Passive leg raising performed before a spontaneous breathing trial predicts weaning-induced cardiac dysfunction. Intensive Care Med 2015; 41: 487-494. doi: 10.1007/s00134-015-3653-0. [DOI] [PubMed] [Google Scholar]
- 17.Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, Vieillard-Baron A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med 2016; 42: 1107-1117. doi: 10.1007/s00134-016-4245-3. [DOI] [PubMed] [Google Scholar]
- 18.Lerolle N, Guérot E, Dimassi S, et al. Ultrasonographic diagnostic criterion for severe diaphragmatic dysfunction after cardiac surgery. Chest 2009; 135: 401-407. doi: 10.1378/chest.08-1531. [DOI] [PubMed] [Google Scholar]
- 19.Turton P, ALAidarous S, Welters I. A narrative review of diaphragm ultrasound to predict weaning from mechanical ventilation: where are we and where are we heading? Ultrasound J 2019; 11: 2. doi: 10.1186/s13089-019-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinino E, Gartman EJ, Sethi JM, McCool FD. Diaphragm ultrasound as a predictor of successful extubation from mechanical ventilation. Thorax 2014; 69: 423-427. doi: 10.1136/thoraxjnl-2013-204111. [DOI] [PubMed] [Google Scholar]
- 21.Samanta S, Singh RK, Baronia AK, Poddar B, Azim A, Gurjar M. Diaphragm thickening fraction to predict weaning-a prospective exploratory study. J Intensive Care 2017; 5: 62. doi: 10.1186/s40560-017-0258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleh M, Vieillard-Baron A. On the role of left ventricular diastolic function in the critically ill patient. Intensive Care Med 2012; 38: 189-191. doi: 10.1007/s00134-011-2448-1. [DOI] [PubMed] [Google Scholar]
- 23.Sanfilippo F, Di Falco D, Noto A, et al. Association of weaning failure from mechanical ventilation with transthoracic echocardiography parameters: a systematic review and meta-analysis. Br J Anaesth 2021; 126: 319-330. doi: 10.1016/j.bja.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 24.Vetrugno L, Guadagnin GM, Brussa A, et al. Mechanical ventilation weaning issues can be counted on the fingers of just one hand: part 1. Ultrasound J 2020; 12: 9. doi: 10.1186/s13089-020-00161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima EJS. Respiratory rate as a predictor of weaning failure from mechanical ventilation. Braz J Anesthesiol 2013; 63: 1-6. doi: 10.1016/j.bjane.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Upadya A, Tilluckdharry L, Muralidharan V, Amoateng-Adjepong Y, Manthous CA. Fluid balance and weaning outcomes. Intensive Care Med 2005; 31: 1643-1647. doi: 10.1007/s00134-005-2801-3. [DOI] [PubMed] [Google Scholar]
- 27.Deab SAEAES, Bellani G. Extubation failure after successful spontaneous breathing trial: prediction is still a challenge! Respir Care 2014; 59: 301-302. doi: 10.4187/respcare.03037. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Lin C, Fan H, Li Z. The efficacy of noninvasive ventilation in managing postextubation respiratory failure: a meta-analysis. Heart Lung 2014; 43: 99-104. [DOI] [PubMed] [Google Scholar]
- 29.Whitmore D, Mahambray T. Reintubation following planned extubation: incidence, mortality and risk factors. Intensive Care Med Exp 2015; 3 (Suppl 1): A684. doi: 10.1186/2197-425X-3-S1-A684. [DOI] [Google Scholar]
- 30.Epstein SK. Decision to extubate. Intensive Care Med 2002; 28: 535-546. doi: 10.1007/s00134-002-1268-8. [DOI] [PubMed] [Google Scholar]
- 31.Dres M, Teboul JL, Monnet X. Weaning the cardiac patient from mechanical ventilation. Curr Opin Crit Care 2014; 20: 493-498. doi: 10.1097/MCC.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 32.Tenório LHS, Santos AC, Câmara Neto JB, et al. The influence of inspiratory muscle training on diaphragmatic mobility, pulmonary function and maximum respiratory pressures in morbidly obese individuals: a pilot study. Disabil Rehabil 2013; 35: 1915-1920. doi: 10.3109/09638288.2013.769635. [DOI] [PubMed] [Google Scholar]
- 33.Kodric M, Trevisan R, Torregiani C, et al. Inspiratory muscle training for diaphragm dysfunction after cardiac surgery. J Thorac Cardiovasc Surg 2013; 145: 819-823. doi: 10.1016/j.jtcvs.2012.07.087. [DOI] [PubMed] [Google Scholar]
- 34.MacIntyre NR, Cook DJ, Ely EW Jr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001; 120 (6 Suppl): 375S-395S. doi: 10.1378/chest.120.6_suppl.375s. [DOI] [PubMed] [Google Scholar]


