Abstract
Enhanced Recovery After Surgery (ERAS) is a multidisciplinary approach that uses a combination of evidence-based methods to improve patient care. Different ERAS protocols are used in various surgical fields but for spine surgery there is no widely used standard ERAS protocol. We compiled and examined the multiple available publications on ERAS protocols for spine surgery. Some general commonalities exist between ERAS protocols; however, a great deal of variety is observed in the granularity of important details such as differing drug choices or specific dosing. To assess and relate the different available ERAS protocols, we conducted a comprehensive narrative literature review focused on comparing commonalities and differences among the following aspects of ERAS protocols: mechanisms of action, post-surgery pain levels, opioid consumption, utilization of muscle relaxants, use of anti-inflammation drugs, and ambulation after surgery. Our goal in this project was to simplify the search process for institutions who review the literature. In this review, certain ERAS elements such as early ambulation, blood loss, pain management, and patient positioning are further explored in more depth.
Keywords: anesthesia, postoperative, pain, protocol, enhanced recovery after surgery, spine surgery, opioid
Enhanced Recovery After Surgery (eRAS) is a multidisciplinary approach to improve patient care using a combination of evidence-based methods. the interventions found in eRAS protocols are based on the rationale that patient outcomes can be improved by controlling pain and optimizing fluid administration, early ambulation, and nutrition to prevent catabolism and immune dysfunction. there are different eRAS protocols available for different surgical fields with the shared goal of minimizing physiological patient stress [1]. eRAS protocols can be partitioned into their pre-operative, intra-operative, and post-operative management. the preoperative component of eRAS protocols includes pre-emptive analgesia (e.g. gabapentin/ pregabalin, acetaminophen), nutrition and fasting optimization, and patient education. the intra-operative component of eRAS protocols focuses on the choice of anaesthetic agents for induction and maintenance (e.g. propofol, ketamine, use of total intravenous anaesthesia [tIvA] vs. inhaled anaesthetics), and utilization of non-opioid analgesics (e.g. ketorolac, lidocaine). the post-operative component of eRAS protocols addresses early ambulation and physical therapy, early nutrition intake, wound care, and pain management [2, 3]. Different spine surgery eRAS protocols are used by various anaesthesia and surgical teams; however, there is no widely used standard eRAS for spine surgery [4].
To assess and relate the available different eRAS protocols, we conducted a comprehensive review focused on comparing the commonalities and differences among the following aspects: mechanisms of action, post-surgery pain levels, opioid consumption, utilization of anti-inflammation drugs use, use of muscle relaxants, and ambulation after surgery (tables 1 and 2).
TABLE 1.
Summary of perioperative stages of ERAS protocols from different institutions, highlighting drug use and early ambulation
| Protocol | Preoperative medications | Intraoperative medications | Postoperative medications |
|---|---|---|---|
| Rush University Medical Center [3] | 1,000 mg IV acetaminophen 600 mg dose of gabapentin OR 150 mg dose of pregabalin 10 mg cyclobenzaprine 10 mg oxycodone |
Standard propofol induction and maintenance with inhaled anaesthesia Ketamine use at induction Dexamethasone Fentanyl Methadone Lidocaine Acetaminophen |
NSAID Gabapentin and pregabalin Tramadol (as needed) |
| Weill Cornell [4, 5] | Acetaminophen Gabapentin Antiemetic prophylaxis | Total intravenous anaesthesia (TIVA) Ketorolac Lidocaine Dual antiemetic prophylactic therapy |
Acetaminophen Nonsteroidal anti-inflammatory drugs Two 50 mg tramadol doses or 5 mg oxycodone based on NRS pain score |
| University of Western Ontario [9] | Pregabalin Gabapentin 0.2 mg kg-1 methadone |
Selective COX-2 inhibitors Ketamine administration Either bolus (0.2-1 mg kg-1) or infusion (1–4 µg kg-1 min-1) Tramadol Analgesics mixture |
N/A |
| Perelman School of Medicine, University of Pennsylvania [2, 12] | 600 mg gabapentin | NSAIDs Opioids Anti-convulsants Other analgesia |
975 mg, q 6 h of acetaminophen Diazepam PO Cyclobenzaprine PO Ketorolac PO Inpatient physical therapy, wound care, and gum chewing (1 piece for 3 minutes daily) |
| The University of Texas [10] | 1000 mg acetaminophen 300 mg tramadol 3.75-150 mg pregabalin OR 100-300 mg gabapentin |
TIVA Lidocaine Ketamine IV dexmedetomidine infusion IV dexamethasone 10 mg q 6 h Lidocaine or other local anaesthetics TXA use (1 g bolus over 30 min followed by 0.5 g h-1 infusion) |
Early ambulation, early oral intake (POD 1), DVT prophylaxis, and physical therapy |
NSAID – nonsteroidal anti-inflammatory drug, TIVA – total intravenous anaesthesia, IV – intravenous, TXA – tranexamic acid, DVT – deep vein thrombosis
TABLE 2.
Summary of opioid, ketamine, anti-inflammation drugs, muscle relaxant medication, and early ambulation in ERAS protocols from different institutions
| Protocol | Opioids | Ketamine | Anti-inflammation | Muscle relaxant medication | Early ambulation |
|---|---|---|---|---|---|
| Rush University Medical Center [3] | Oxycodone (10 mg) Ketamine Fentanyl | Used intraoperatively | Dexamethasone | Cyclobenzaprine | N/A |
| Weill Cornell [4, 5] | N/A | N/A | Ketorolac | N/A | Early physical therapy within 2 hours of arriving to PACU |
| University of Western Ontario [9] | Methadone Tramadol | Ketamine: either bolus (0.2-1 mg kg-1) or infusion (1–4 µg kg-1 min-1) |
Selective COX-2 inhibitors | ||
| Perelman School of Medicine, University of Pennsylvania [2, 12] |
N/A | N/A | Ketorolac | Diazepam Cyclobenzaprine | 3–5 times daily starting 6 hours after surgery |
| University of Texas [10] | Tramadol | Used intraoperatively | IV dexamethasone 10 mg q 6 h | N/A | Yes, unspecified |
PACU – post-anaesthesia care unit
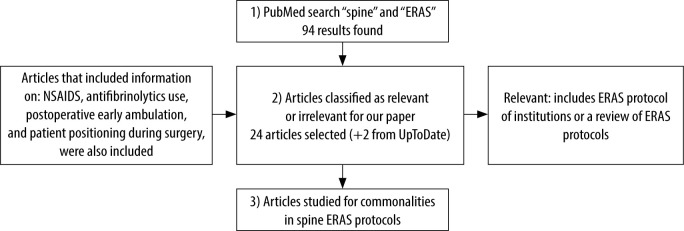
We conducted a PubMed search for relevant articles, focusing on the queries ‘eRAS’ and ‘spine’. Included relevant articles were those that described an institution’s eRAS protocol or reviews of eRAS protocols. After studying these relevant articles, further details on specific elements were compiled. these include NSAIDS, antifibrinolytics use, postoperative early ambulation, and patient positioning during surgery. Articles that focused on these topics (eRAS protocols not specific to spine surgery) were selected and reviewed as well (Figures 1 and 2).
FIGURE 1.
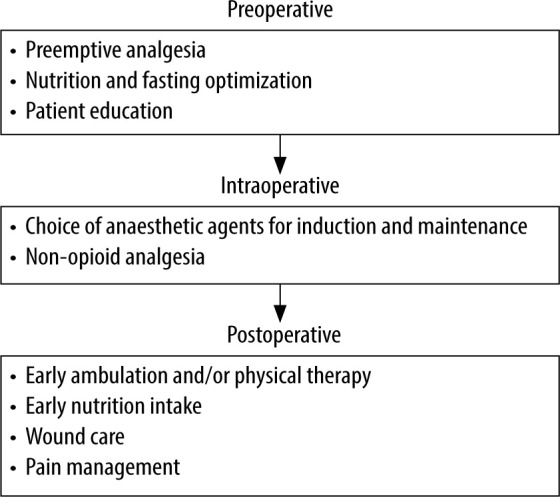
Overview of the three perioperative aspects of ERAS spine protocols
FIGURE 2.
Overview of the search process and selection of relevant articles for this review
At weill cornell Medical college (NYc, NY, USA), Soffin et al. [5] performed a retrospective matched cohort study of opioid-free anaesthesia in minimally invasive lumbar decompression spine surgery before developing an eRAS protocol for spine surgeries. In this study they compared patient outcomes in surgical patients who were treated with traditional opioid-containing anaesthesia and those who were treated with opioid-free anaesthesia. this study found that patients who were treated with opioid-containing intraoperative anaesthesia had significantly greater perioperative opioid consumption, with no significant difference in PAcU pain scores. Following this study, Soffin et al. [5] developed an eRAS protocol for minimally invasive lumbar decompression spine surgery. the protocol included 15 standard eRAS elements and focused on minimizing opioid usage [5].
At the University of Miami, wang et al. [6] reported their eRAS protocol for lumbar spine surgery, which focused on technical surgical means for improving patient outcomes. Before developing their eRAS protocol, they reviewed 42 cases of endoscopic decompression, expandable cage deployment, and percutaneous screw placement surgeries that were done without general anaesthesia. the University of Miami’s eRAS protocol strategy concentrates on minimizing aesthetic agents, in large, through use of short-acting sedatives and hence not limited to avoiding opioids [6].
Ali et al. [3, 4], at the Perelman School of Medicine in the University of Pennsylvania, conducted a prospective cohort analysis testing the elements of their spine surgery’s eRAS protocol in an intervention group compared to a historical control cohort. the eRAS intervention group was treated with the preoperative care elements found in their eRAS protocol. the historical control group was treated with standard care. Results of the study show that, in the eRAS group, intravenous opioids administration via patient-controlled analgesia (PcA) pumps were almost eliminated. In their study, 0.5% of patients in the eRAS group utilized a PcA pump to administer Iv opioids, compared to 54% of patients in the control group. Patients in the eRAS group had a much lower 1-month post-surgery opioid use (38.8% of patients in the eRAS group compared to 52.7% of patients in the control group). Administration of gabapentin (80.6% of eRAS patients vs. 21.6% of control patients) and ketorolac (23.9% of eRAS patients vs. 10.8% of control patients) were more frequent in the eRAS group than in the control group. Acetaminophen and cyclobenzaprine were used more frequently in the eRAS group. On post-operative days (POD) 0-3, pain scores were similar between the 2 groups. the authors suggested that these results show that postoperative opioid use can be reduced by using multiple nonopioid pain medications, and with that, PcA use may be unnecessary. early ambulation and mobilization were higher on POD 0–1 in the eRAS group [3, 4].
Grasu et al. [7] (the University of texas MD Anderson cancer center) conducted a comparative study for patient outcomes before and after the implementation of their eRAS protocol. the results of their comparative study show that on average the eRAS group received more non-opioid analgesics, and had higher intraoperative rates of lido-caine and ketamine infusions. the eRAS group also had lower average pain scores on POD 2 (2.0 ± 1.8 for eRAS group vs. 2.8 ± 2.2 for control) and POD 3 (1.6 ± 1.7 for eRAS group vs. 2.6 ± 2.3 for control) as well as on POD 1, but was not statistically significant on that day. eRAS patients also received a lower morphine equivalent daily dose (MeDD) (the eRAS group’s MeDD was 372.2 mg compared to the noneRAS group’s MeDD of 521.5 mg) and had a slightly shorter length of stay (lOS) (6.3 ± 2.2 days for the eRAS group vs. 6.8 ± 1.9 days for the control) in the hospital post-surgery. Of note, the eRAS group had a slightly higher 30-day hospital readmission rate (14.6% for the eRAS group compared to 8.9% for control group) and a higher 30-day complication rate (31.7% in the eRAS group and 17.9% in the control group) [7].
PREOPERATIVE COMPONENT
Bhatia et al. [8] describes the eRAS protocol of Rush University Medical centre’s Department of Anaesthesiology (chicago, Illinois, USA) with their multimodal synergistic anaesthesia strategy, which is focused on avoiding the negative side effects of opioids. their reported preoperative care included 1 g intravenous (Iv) acetaminophen, either a 600 mg dose of gabapentin or a 150 mg dose of pregabalin PO, 10 mg of the muscle relaxant cyclobenzaprine PO, and 10 mg of oxycodone PO. their reasoning for using a small dose of oxycodone preoperatively is to reduce the amount of opioids administered postoperatively. they also suggest administering cyclooxygenase (cOX) enzyme inhibitor (i.e. an NSAID) 3 days prior to the surgery, but at the surgeon’s discretion [8].
At weill cornell Medical college (NYc, NY, USA) the preoperative care includes oral pre-emptive analgesia containing acetaminophen and gabapentin plus antiemetic prophylaxis prior to surgery [2].
The eRAS protocol for lumbar spine surgeries at the University of Miami focuses on the minimization of anaesthetic agents using short-acting sedatives. the focus of their pre-operative care is on improving nutrition and limiting food fasting to 12 hours and liquid fasting to 8 hours before the surgery [6].
The eRAS protocol for spine surgery at the Perelman School of Medicine in the University of Pennsylvania defines the pre-operative component as the period up to a week before surgery. their pre-operative component is composed of patient education, nutrition optimizations, smoking cessation counselling, pre-habilitation with ambulation, drug and alcohol abuse interventions, sleep medicine for patients with sleep-apnoea, and discharge planning that is initiated prior to surgery. the nutrition component of the perioperative care includes clear carbohydrate fluids starting one day, and up to two hours, prior to surgery, with return to regular diet postoperatively on the day of the surgery if possible. the medication regimen used perioperatively to control pain levels follows an opioid-sparing multimodality. to do so, their eRAS protocol utilizes administration of 600 mg gabapentin PO preoperatively [3, 4].
At the University of texas MD Anderson cancer center, Grasu et al. [7] formed a multidisciplinary committee to develop their eRAS protocol. the preoperative component of their eRAS protocol includes patient education, preconditioning exercise 2 months prior to surgery, and optimization of comorbidities. Up until 2 hours prior to surgery patients are permitted to drink clear liquids, with no oral intake up until 8 hours prior to surgery. During the immediate preoperative period patients receive the following: 1000 mg acetaminophen, 300 mg tramadol, and either 75–150 mg pregabalin or 100-300 mg gabapentin [7].
INTRAOPERATIVE COMPONENT
At Rush University Medical center, practitioners opted for standard propofol induction and maintenance with an inhaled anaesthetic. In their reporting of this protocol, Bahatia et al. [8] supported ketamine use, as an adjuvant on induction, because it has been shown to reduce postoperative opioid consumption when administered intraoperatively. they recommend intraoperative administration of dexamethasone Iv to decrease postoperative pain scores and opioid consumption. Fentanyl, methadone, lidocaine, and acetaminophen have also shown favourable postoperative effects, and they have been utilized intraoperatively [8].
The intraoperative eRAS protocol at weill cornell Medical college (NYc, NY, USA) included tIvA technique, non-opioid analgesia with Iv ketorolac and lidocaine, and dual antiemetic prophylactic therapy. All surgeries were minimally invasive and with local anaesthetic infiltration at the end of the procedure [2].
The eRAS protocol for lumbar spine surgery at the University of Miami focuses on the minimization of anaesthetic agents through the use of short-acting sedatives. their intraoperative care emphasized local anaesthetics, and avoidance of urinary catheters [6].
The eRAS protocol at the Perelman School of Medicine defines peri-operative care as the period of one week before the surgery, during surgery, and immediately post-operation. their eRAS protocol recommends intraoperative administration of NSAIDs, opioids, anti-convulsants, and other analgesia decided at the discretion of the anaesthesiologist. local infiltration of bupivacaine during closure is also used, as well as 975 mg of oral acetaminophen every 6 hours postoperatively. the protocol also recommends diazepam, cyclobenzaprine, and ketorolac as needed. they avoided using Foley cathe ters in surgeries shorter than 2 hours in order to decrease complications (e.g. urinary tract infections, urethral trauma, kidney and bladder damage, bladder stones, and pseudo polyps) and enable easer patient ambulation and mobilization [3, 4].
The spine surgery eRAS protocol at the University of texas MD Anderson cancer center calls for intraoperative infusions of lidocaine, ketamine, and Iv dexmedetomidine infusions with a tIvA goal, 0.1–0.2 mg kg–1 single-dose Iv methadone, and also around-the-clock re-dosing of Iv acetaminophen and dexamethasone (10 mg). Surgical wound infiltration was done with intraoperative epidural analgesia or liposomal bupivacaine at the incision site based on provider preference. the protocol also calls for fluid therapy and haemodynamic optimization, restrictive blood transfusion (which they defined as haemoglobin ≤ 9–10 g dl–1 depending on comorbidities and haemodynamic stability) and tranexamic acid (tXA) use (1 g bolus over 30 min followed by a 0.5 g h–1 infusion), antiemetic prophylaxis, deep vein thrombosis (Dvt) prophylaxis, and maintenance of normal temperature [7].
POSTOPERATIVE COMPONENT
At Rush University Medical center postoperative management in the eRAS protocol continued preoperative medications such as NSAID and/or acetaminophen, gabapentin and pregabalin, and potentially also tramadol. they also utilized postoperative use of icepacks [8].
The postoperative care component of the eRAS protocol at weill cornell Medical college (NYc, NY, USA) included oral intake and mobilization using early physical therapy during the recovery period within 2 hours of arriving at the post-anaesthesia care unit (PAcU). Post-operative pain management began with acetaminophen and nonsteroidal anti-inflammatory drugs. For patients with a numeric rating scale pain (NRS) score of above 4, two 50 mg tramadol doses were prescribed, and 5 mg oxyco-done for those with NRS scores of above 8. Patients with post-operative nausea and vomiting (PONv) received treatment of metoclopramide (10 mg intravenously) or ondansetron (4 mg), and patients with refractory PONv received scopolamine (1.5 mg transdermally) [2].
At the University of Miami, the eRAS Protocol recommends that patients’ post-operative pain be managed with standard regimens of narcotic anal-gesics. their post-operative care also emphasizes early ambulation (0–1 days post-surgery) [6].
In the eRAS protocol at Perelman School of Medicine early postoperative ambulation is also stressed, in their case within 6 hours of surgery (when no activity restrictions exist). they recognize a cognitive behavioural phenomenon of post-operative fear of movement in spine surgery patients, which is associated with pain and disability. the postoperative period of the eRAS protocol consists of inpatient physical therapy, wound care, and gum chewing (1 piece for 3 minutes daily) to prevent ileus [3, 4].
At the University of texas MD Anderson cancer center the eRAS protocol’s postoperative care includes: early ambulation (POD 1 with movement 3 times per day from bed to chair) and physical therapy, early oral intake (clear liquids on POD 1 with diet advanced as tolerated), Dvt prophylaxis, pain management [gabapentin (300 mg q 8 h), celecoxib (200 mg q 12 h), tramadol (200 mg orally q 12 h), acetaminophen (1 g orally q 6 h), continuation of preop long-lasting opioids, and opioid administration via Iv hydromorphone or morphine PcA (if patient requirements were > 12 mg per day of hydromorphone or > 60 mg per day morphine, then cancer pain consultation would be obtained)] [7].
SYSTEMIC REVIEWS
The systematic reviews of eRAS protocols for spine surgery have focused on specific elements of existing eRAS protocols [9–11]. A review by corniola et al. [9] covered the MeDlINe search function, the cochrane library, eMBASe, ScienceDirect, and Google Scholar. corniola et al. [9] covered 7 eRAS publications that used different surgical procedures (1- or 2-level tlIF, 1-level fusion, 1-level microdiscectomy, non-instrumented lumbar and cervical surgery, instrumented and non-instrumented spine surgery). they found that 5 out of the 7 eRAS protocols had same-day discharge, 3 eRAS protocols used minimally invasive spine surgery that focuses on minimizing blood loss and muscular trauma, and one eRAS protocol included an awake surgery [12]. corniola et al. [9] reiterate that in the preoperative period, prolonged fasting should be avoided because it has been shown to have negative side effects on muscle catabolism. they emphasized that patients can eat a light meal up to 6 hours prior to surgery and can drink clear liquids up to 2 hours prior to surgery, and they also recommend carbohydrate supplementation. In the intraoperative period corniola et al. [9] recommend aggressive diet advancement so that patients eat and drink within hours after surgery [9].
A review by Dietz et al. [11] covered the PubMed and Ovid databases. they found that the use of eRAS was associated with a reduction in pain scores and reduced opioid consumption postoperatively, with no differences in complications or readmissions between the eRAS and control groups. they also found that 7 out of 19 eRAS studies had a reduction in length of stay. Several of the covered eRAS protocols noted a cost reduction. these cost reductions were demonstrated through nursing costs associated with decreased length of stay (46.8%), potential outpatient centre cost improvements, and a decrease in operation time [9, 11].
Feng et al. [13] showed a decrease in cost (U.S. $70,467 with eRAS vs. U.S. $71,426 without eRAS) when using 11 eRAS protocol elements for single-level MIS tlIF. this cost reduction is associated with reduced lOS, reduced operative time, reduced blood loss, and fewer complications [13]. Debono et al. [14] looked into lumbar microdiscectomies performed in ambulatory centres and with an eRAS philo sophy and, as expected, found it to be cheaper than lumbar microdiscectomies for in-patient settings (€224.08 ambulatory centre vs. €520.38 in-patient) [14]. wang and Grossman [12] reported a cost reduction of $3442 or 15.2% per procedure with application of eRAS protocol elements [12].
MINIMALLY INVASIVE SPINE SURGERY (MISS) APPROACH
Corniola et al. [9] noted that the minimally invasive spine surgery (MISS) approach complements the eRAS patient outcome improvement. the benefits of using a MISS include the following: 1) lower blood loss – one study found that using a minimally invasive procedure reduced blood loss to 185 ml compared to 255 ml in the open group. 2) Minimizing muscular trauma – in a study that compared open and MISS approaches for lumbar interbody fusion, patients whose operation used an open approach had significantly reduced multifidus muscle cross-sectional area (cSA) on post-op MRI. In another study, comparing open and MISS approaches in pedicle screw fixations showed similar trends in multifidus muscle cSA. this study also showed that the MISS group had lower serum creatine kinase levels than the open approach group. 3) Faster postoperative ambulation – a study showed that patients operated on using MISS approach had a faster postoperative ambulation by a factor of two (1.5 days vs. 3 days) [9, 11]. 4) Fewer postoperative infections associated with smaller incisions (for posterior [transforaminal] lateral interbody fusion [P/tlIF] 2 level infusion 4.6% of patients in MISS group experience postoperative infections vs. 7.0% in the non-MISS group) [15]. 5) Postoperative opioid use reduction – one study showed that using MISS can reduce postoperative opioid consumption from 4 weeks post operation in the open approach to 2 weeks with the MISS approach. Another study showed that patients whose operation used MISS approach had a total mean MeDD use of 17.4 mg compared to 35.7 mg in patients whose operation used an open approach. 6) Shorter hospitalization – 3.6 vs. 5.9 days with MISS compared to an open approach [9, 11].
BLOOD LOSS, PAIN MANAGEMENT, AND POSITION-RELATED COMPLICATIONS
Alboog et al. [16] at the University of western Ontario, canada conducted a systematic review examining 3 major patient outcomes after spine surgery: blood loss, pain management, and position-related complications. their team explored 3 antifibrinolytic agents: tXA, epsilon aminocaproic acid (eAcA), and aprotinin administered to lower blood loss and subsequent complications. they found that tXA reduced intraoperative, postoperative, and total blood loss. Reduction in blood loss attributed to tXA use did not appear to be affected by tXA dosage; no significant difference was observed between reduction in blood loss with the use of low dosage (10 mg kg–1 followed by up to 10 mg kg–1 h–1) and high dosage (bolus of 10-100 mg kg–1 followed by infusion greater than 10 mg kg–1 h–1). eAcA use showed no significant decrease in blood loss, and aprotinin showed decreased blood loss intraoperatively but with no decrease in total blood loss [16]. Qureshi et al. [17] reference tXA use in their study of perioperative management of blood loss in spine surgeries. It has been shown that patients treated with tXA during spine surgeries lost less blood, needed fewer blood-transfusions, and had less complications in orthopaedic surgeries. tXA appears to be a cost-efficient antifibrinolytic with a low side effect profile; a 2 g loading dose followed by 100 mg h–1 infusion of tXA has been shown to significantly reduce blood loss in spine surgeries. As Alboog et al. [16] mention, the tXA dosage has not been standardized and seems to vary based on the clinicians’ preference [17].
In their systematic review, Alboog et al. [16] also explored pain management options. they looked into several commonly used medications. First their study showed that commonly used acetaminophen is not more effective than placebo in reducing pain scores and opioid consumption. they recommended that NSAIDs be used with caution because there are concerns about nonunion of bone and bleeding. However, they note that the literature is conflicted in regard to NSAID-associated nonunion and increased bleeding [16]. there is a relationship between nonunion of bones in patients with fractures; however, a causal relationship with NSAIDs has not been proven, and the effect of these drugs on fracture healing in humans is uncertain. More caution from bleeding exacerbation by NSAIDs is warranted in elderly patients [24]. Alboog et al. [16] found non-selective NSAIDs to be less effective than selective cOX-2 inhibitors. Pregabalin or gabapentin were found to reduce post-operative opioid consumption and reduce opioid-related side effects [16]. Adjunctive ketamine administration – either bolus (0.2–1 mg kg–1)or continuous infusion (1–4 µg kg–1 min–1) – was found to significantly reduce postoperative pain scores at 6, 12, and 24 hours post-surgery. Adjunctive ketamine use was also found to reduce cumulative morphine consumption on POD 1. However, these effects were not significant after 24 hours [16, 18]. the opioids they examined were tramadol and methadone, which both have “atypical” mechanisms of action in that they are not limited to the mu opioid receptor. Mu receptor agonists, used for pain control, have several side effects, which limits their use [19, 20]. tramadol was found to reduce pain scores up to 6 hours post operation. 0.2 mg kg-1 of methadone at the start of the surgery had a better effect on pain scores than 2 mg of hydromorphone given at the end of surgery (median difference: –1 [–3, 0]). Patients treated with methadone at the start of surgery also had lower Iv and oral opioid use post-surgery. It is possible that methadone’s effectiveness in treating postoperative pain comes from its ability to antagonize the NMDA receptor. Also, it was demonstrated that methadone reduces serotonin and norepinephrine reuptake, which may also contribute to its beneficial effect on pain scores [16, 21]. Alboog et al. [16] also examined intramuscular injection of local anaesthetic and subcutaneous infiltration of liposomal bupivacaine. they found that each analgesic intervention only produced a mild reduction of pain scores and reduction of postoperative opioid consumption. they therefore advocate a multi-modal approach with a mixture of several analgesics that, together, are likely to be more beneficial. the synergistic effect of different analgesic combinations in spine surgery pain can be examined further.
Neuraxial regional anaesthesia was also explored. epidural anaesthesia was shown to reduce postoperative pain scores on POD 1 (standardized mean difference: –0.94 [–1.56, –0.31]). epidural anaesthesia was shown to reduce postoperative pain scores on POD 1 (standardized mean difference: –0.94 [–1.56, –0.31]) compared to intravenous patient-controlled analgesia. Intrathecal morphine (0.1–1 mg) reduced POD 1 pain scores (standardized mean difference: –0.47 [–0.69, –0.25]), but placed patients at a higher risk for pruritus (OR: 4.09 [1.84, 9.11]) and respiratory depression (OR: 3.48 [0.41, 29.32]). epidural steroid administered at closure reduced POD 1 pain scores (median difference: –0.97 [–0.14, –1.79]), but its risk assessment was not performed [16].
USE OF ANTIFIBRINOLYTICS
The systematic review of Alboog et al. [16] also explored the use of antifibrinolytics. In a review of the perioperative components of blood loss management by Graetz et al. [25], the use of antifibrinolytic medications was also explored. Graetz et al. [25] reported that antifibrinolytic medications are administered intravenously, with oral and topical administration of tXA reported in orthopaedic surgery. there is a reported association between use of tXA during surgery and postoperative seizures (albeit at high doses of 100 mg kg–1 Iv followed by 20–50 mg kg–1 h–1 with a total dose up to 259 mg kg–1). Aprotinin is an antifibrinolytic medication that is no longer used in the United States because of its association with increased mortality in cardiac surgery. Aprotinin was effective in reducing surgical bleeding and transfusion rates, but it was associated with increased mortality in cardiac surgery patients in a large trial, which led to its withdrawal and suspension [25]. tXA and eAcA have been used in cardiac as well as noncardiac major surgical procedures including spine surgeries. Dosing remains somewhat procedure and institution specific and not standardized. A review by Graetz et al. [25] specifies that data show no increased risk for thrombotic events with tXA or eAcA, even for cancer patients who are at increased risk. tXA has been associated with postoperative seizures, which may be a dose-related effect. eAcA should be administered slowly and with caution because rapid administration may induce hypotension, as well as bradycardia or other arrhythmias [22, 25].
Brown et al. [26] also use antifibrinolytic agents such as tXA and eAcA for spine surgeries. they suggest that antifibrinolytic agents reduce blood loss and have not been shown to cause morbidity or increased risk for thromboembolic events. Based on available meta-analysis and pharmacokinetic properties of antifibrinolytics, they recommend the following dosages for each medication: Iv tXA administration of 10 mg kg–1 bolus, followed by an infusion of 2 mg kg–1 h–1 until the end of the procedure. Iv eAcA administration of 100 mg kg–1 bolus followed by an infusion of 10 to 15 mg kg–1 h–1 until the end of the procedure. Brown et al. [26] specify that they do not use antifibrinolytic medications in patients who have conditions that put them at risk (e.g. patients undergoing vascular anastomosis or free fibula grafting, or those with a hypercoagulable state) [26].
EARLY AMBULATION
Early ambulation was mentioned in several of the reviewed eRAS protocols. early ambulation has positive effects on perioperative patient comorbidi-ties and length of stay in other surgical subspecial-ties [26]. to explore the benefits of early mobilization post-surgery, a published review of multiple spine surgery studies (and other surgical procedures) with an early mobilization and ambulation component was reviewed. It examined comorbidities such as hypertension, diabetes, obesity/elevated body mass index (BMI), hypothyroidism, osteoporosis, chronic obstructive pulmonary disease (cOPD), and coronary artery disease (cAD). Also, this review investigated pre-habilitation accomplished months prior to surgery. Pre-habilitation included a strict exercise regimen initiated 2 months before the surgery, pain-control medications, and protein drinks on the day prior to the operation. It concluded that patients treated with pre-habilitation and early ambulation had better outcomes in several postoperative measures. this included a shorter length of stay (5 average days instead of 7) and greater postoperative patient satisfaction [23].
PATIENT POSITIONING
Patient positioning was another topic of interest common in eRAS protocols for spine surgeries. In their systematic review of eRAS protocols for spine surgery, Alboog et al. [16] found that when the patient is positioned to have less abdominal pressure, blood loss is reduced [16]. when a patient is inadequately positioned during surgery, elevated abdominal pressures translate to increased pressure in the vena cava and epidural venous system, which thereby causes more bleeding. In his study of “the effect of patient positioning on intraabdominal pressure and blood loss in spinal surgery”, Dr. Park indirectly measured inferior vena cava pressure (IvcP) by measuring intraabdominal pressure (IAP) in patients undergoing posterior lumbar spinal fusion. Dr. Park found that with less pad support on a wilson frame, patients had an increase in IAP and an increase in blood loss [24].
CONCLUSIONS
This literature review was done as part of our process to put together an eRAS protocol for the elective spine procedures at our institution. we were particularly interested in mapping the differences in protocol-specific details among the various eRAS protocols available in the literature. General commonalities are evident across the eRAS protocols for spine surgeries on the broad strokes level. However, a great deal of variety is observed in the granularity of finer but important details such as different drug choices, doses, or routes of administration. Among the 5 eRAS protocols mentioned in this review, commonalities included the following: aiming to limit perioperative opioid usage, utilizing multimodal pain management, promoting early ambulation, and encouraging aggressive advancement of diet.
ACKNOWLEDGEMENTS
Financial support and sponsorship
Rutgers New Jersey Medical School Department of Anesthesiology.
conflicts of interest
none.
References
- 1.Elsarrag M, Soldozy S, Patel P, et al. Enhanced recovery after spine surgery: a systematic review. Neurosurg Focus 2019; 46: E3. doi: 10.3171/2019.1.Focus18700. [DOI] [PubMed] [Google Scholar]
- 2.Soffin EM, Vaishnav AS, Wetmore DS, et al. Design and implementation of an Enhanced Recovery After Surgery (ERAS) program for minimally invasive lumbar decompression spine surgery: initial experience. Spine (Phila Pa 1976) 2019; 44: E561-e570. doi: 10.1097/brs.0000000000002905. [DOI] [PubMed] [Google Scholar]
- 3.Ali ZS, Ma TS, Ozturk AK, et al. Pre-optimization of spinal surgery patients: development of a neurosurgical enhanced recovery after surgery (ERAS) protocol. Clin Neurol Neurosurg 2018; 164: 142-153. doi: 10.1016/j.clineuro.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Ali ZS, Flanders TM, Ozturk AK, et al. Enhanced recovery after elective spinal and peripheral nerve surgery: pilot study from a single institution. J Neurosurg Spine 2019. doi: 10.3171/2018.9.Spine18681. [DOI] [PubMed] [Google Scholar]
- 5.Soffin EM, Wetmore DS, Beckman JD, et al. Opioid-free anesthesia within an enhanced recovery after surgery pathway for minimally invasive lumbar spine surgery: a retrospective matched cohort study. Neurosurg Focus 2019; 46: E8. doi: 10.3171/2019.1.Focus18645. [DOI] [PubMed] [Google Scholar]
- 6.Development of an Enhanced Recovery After Surgery (ERAS) approach for lumbar spinal fusion. J Neurosurg Spine 2017; 26: 411-418. doi: 10.3171/2016.9.Spine16375. [DOI] [PubMed] [Google Scholar]
- 7.Grasu RM, Cata JP, Dang AQ, et al. Implementation of an Enhanced Recovery After Spine Surgery program at a large cancer center: a preliminary analysis. J Neurosurg Spine 2018; 29: 588-598. doi: 10.3171/2018.4.Spine171317. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia A, Buvanendran A. Anesthesia and postoperative pain control-multimodal anesthesia protocol. J Spine Surg (Hong Kong) 2019; 5 (Suppl 2): S160-S165. doi: 10.21037/jss.2019.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corniola MV, Debono B, Joswig H, Lemee JM, Tessitore E. Enhanced recovery after spine surgery: review of the literature. Neurosurg Focus 2019; 46: E2. doi: 10.3171/2019.1.Focus18657. [DOI] [PubMed] [Google Scholar]
- 10.Dietz N, Sharma M, Adams S, et al. Enhanced Recovery After Surgery (ERAS) for spine surgery: a systematic review. World Neuro-surgery 2019; 130: 415-426. doi: 10.1016/j.wneu.2019.06.181. [DOI] [PubMed] [Google Scholar]
- 11.Dietz N, Sharma M, Adams S, et al. Enhanced Recovery After Surgery (ERAS) for spine surgery: a systematic review. World Neurosurg 2019; 130: 415-426. doi: 10.1016/j.wneu.2019.06.181. [DOI] [PubMed] [Google Scholar]
- 12.Wang MY, Grossman J. Endoscopic minimally invasive transforaminal interbody fusion without general anesthesia: initial clinical experience with 1-year follow-up. Neurosurg Focus 2016; 40: E13. doi: 10.3171/2015.11.Focus15435. [DOI] [PubMed] [Google Scholar]
- 13.Feng C, Zhang Y, Chong F, et al. Establishment and implementation of an Enhanced Recovery After Surgery (ERAS) pathway tailored for minimally invasive transforaminal lumbar interbody fusion surgery. World Neurosurg 2019; 129: e317-e323. doi: 10.1016/j.wneu.2019.05.139. [DOI] [PubMed] [Google Scholar]
- 14.Debono B, Sabatier P, Garnault V, et al. Outpatient lumbar micro-discectomy in France: from an economic imperative to a clinical standard-an observational study of 201 cases. World Neurosurg 2017; 106: 891-897. doi: 10.1016/j.wneu.2017.07.065. [DOI] [PubMed] [Google Scholar]
- 15.McGirt MJ, Parker SL, Lerner J, Engelhart L, Knight T, Wang MY. Comparative analysis of perioperative surgical site infection after minimally invasive versus open posterior/transforaminal lumbar interbody fusion: analysis of hospital billing and discharge data from 5170 patients. J Neurosurg Spine 2011; 14: 771-778. doi: 10.3171/2011.1.Spine10571. [DOI] [PubMed] [Google Scholar]
- 16.Alboog A, Bae S, Chui J. Anesthetic management of complex spine surgery in adult patients: a review based on outcome evidence. Curr Opin Anaesthesiol 2019; 32: 600-608. doi: 10.1097/aco.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi R, Puvanesarajah V, Jain A, Hassanzadeh H. Perioperative Management of Blood Loss in Spine Surgery. Clin Spine Surg 2017; 30: 383-388. doi: 10.1097/bsd.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 18.Pendi A, Field R, Farhan SD, Eichler M, Bederman SS. Perioperative ketamine for analgesia in spine surgery: a meta-analysis of randomized controlled trials. Spine (Phila Pa 1976) 2018; 43: E299-e307. doi: 10.1097/brs.0000000000002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doi S, Mori T, Uzawa N, et al. Characterization of methadone as a β-arrestin-biased μ-opioid receptor agonist. Mol Pain 2016; 12. doi: 10.1177/1744806916654146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dayer P, Desmeules J, Collart L. Pharmacology of tramadol. Drugs 1997; 53 Suppl 2: 18-24. doi: 10.2165/00003495-199700532-00006. [DOI] [PubMed] [Google Scholar]
- 21.Murphy GS, Szokol JW, Avram MJ, et al. Clinical effectiveness and safety of intraoperative methadone in patients undergoing posterior spinal fusion surgery: a randomized, double-blinded, controlled trial. Anesthesiology 2017; 126: 822-833. doi: 10.1097/aln.0000000000001609. [DOI] [PubMed] [Google Scholar]
- 22.Levy JH, Koster A, Quinones QJ, Milling TJ, Key NS. Antifibrinolytic therapy and perioperative considerations. Anesthesiology 2018; 128: 657-670. doi: 10.1097/aln.0000000000001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein NE. A review article on the benefits of early mobilization following spinal surgery and other medical/surgical procedures. Surg Neurol Int 2014; 5 (Suppl 3): S66-73. doi: 10.4103/2152-7806.130674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park CK. The effect of patient positioning on intraabdominal pressure and blood loss in spinal surgery. Anesth Analg 2000; 91: 552-557. doi: 10.1097/00000539-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Graetz TJ, Nuttall G, Shander A, et al. Perioperative blood management: strategies to minimize transfusions [Internet]. UpToDate 2019. Avaliable from: https://www-uptodate-com.proxy.libraries.rutgers.edu/contents/perioperative-blood-management-strategies-to-minimize-transfusions?search=risks%20of%20antifibrinolytics%20spine%20surgery%20use&source=search_result&selectedTitle=3~150&usage_type=default&display_rank=3#H10487453 (Accesed: 18.05.2021).
- 26.Brown MJ, Pasternak JJ, Crowley M. Anesthesia for elective spine surgery in adults. UpToDate 2020. Avaliable from: https://wwwuptodate-com.proxy.libraries.rutgers.edu/contents/anesthesia-for-elective-spine-surgery-in-adults?search=Anesthesia%20for%20elective%20spine%20surgery%20in%20adults.%20J.J.%20Pasternak,%20M.%20Crowley&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (Accessed: 18.05.2021).