Abstract
BACKGROUND:
This study aimed to explore the changes of programmed death-ligand 1 (PD-L1) and programmed death-1 (PD-1) expression on antigen-presenting cells (APCs) and evaluate their association with organ failure and mortality during early sepsis.
METHODS:
In total, 40 healthy controls and 198 patients with sepsis were included in this study. Peripheral blood was collected within the first 24 h after the diagnosis of sepsis. The expression of PD-L1 and PD-1 was determined on APCs, such as B cells, monocytes, and dendritic cells (DCs), by flow cytometry. Cytokines in plasma, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), IL-6, IL-10, and IL-17A were determined by Luminex assay.
RESULTS:
PD-1 expression decreased significantly on B cells, monocytes, myeloid DCs (mDCs), and plasmacytoid DCs (pDCs) as the severity of sepsis increased. PD-1 expression was also markedly decreased in non-survivors compared with survivors. In contrast, PD-L1 expression was markedly higher on mDCs, pDCs, and monocytes in patients with sepsis than in healthy controls and in non-survivors than in survivors. The PD-L1 expression on APCs (monocytes and DCs) was weakly related to organ dysfunction and inflammation. The area under the receiver operating characteristic curve (AUC) of the PD-1 percentage of monocytes (monocyte PD-1%)+APACHE II model (0.823) and monocyte PD-1%+SOFA model (0.816) had higher prognostic value than other parameters alone. Monocyte PD-1% was an independent risk factor for 28-day mortality.
CONCLUSION:
The severity of sepsis was correlated with PD-L1 or PD-1 over-expression on APCs. PD-L1 in monocytes and DCs was weakly correlated with inflammation and organ dysfunction during early sepsis. The combination of SOFA or APACHE II scores with monocyte PD-1% could improve the prediction ability for mortality.
Keywords: Inflammation, Programmed death-ligand 1, Programmed death-1, Antigen-presenting cells
INTRODUCTION
Sepsis is the primary cause of death due to infection and is associated with a high hospital mortality rate worldwide, causing 5.3 million deaths annually.[1,2] The mortality in the hospital was estimated to be 17% for sepsis and 26% for severe sepsis worldwide.[3] The latest version of Sepsis-3 defines sepsis as characterized by life-threatening organ failure and dysfunction due to infection in the host.[4] In the latest version, the definition is established based on the balance between host immune status and pathogens.
The development of sepsis is initiated through a hyper-inflammatory phase, followed by a prolonged immune suppression phase. Severe cytokine storms and low volume perfusion caused by overwhelming inflammation during septic shock could lead to acute organ dysfunction, which contributes to death from sepsis.[5] These two extreme phases are compatible, and a mixture of these phenotypes has been detected in most patients.[6]
Checkpoint regulators are membrane-bound proteins widely expressed on immune cells. They serve as a second signal to direct the immune response of T cells.[7] Programmed death-1 (PD-1) (CD279) and its ligand programmed death-ligand 1 (PD-L1) (CD274) are important checkpoint inhibitors that induce apoptosis, disability and depletion of T cells, causing them to be irresponsible to antigen stimulation.[8] PD-1 or PD-L1 has been proven to be overexpressed among critically ill patients with sepsis. The expression of inhibitory receptors, such as B- and T-lymphocyte attenuator (BTLA), PD-1, cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), and inhibitory ligands (PD-L1, PD-L2, herpesvirus entry mediator [HVEM]), was increased in ICU patients who died of sepsis.[9] PD-1 and PD-L1 on neutrophils could be early predictors for subsequent sepsis.[10] Studies have also revealed that PD-L1 expression on monocytes and natural killer (NK) cells is a promising independent prognostic marker for septic shock or mortality.[7,11]
Antigen-presenting cells (APCs) present antigens and secrete inflammatory cytokines. Increased expression of PD-1 or PD-L1 on the innate myoid cell axis is involved in inhibitory signaling in the APC process and is responsible for immunosuppression in sepsis.[7] However, little is known about the crosstalk between PD-1 and PD-L1 on APCs and inflammation during sepsis. Accordingly, in this study, we aimed to evaluate PD-L1 expression on different APCs and inflammatory cytokines during early sepsis, explore their possible relationships, and test their ability to predict organ failure and prognosis in early sepsis.
METHODS
Study population and data collection
This study was an observational, single-center cohort study conducted in the emergency department (ED) of Beijing Chaoyang Hospital, a tertiary teaching hospital in China. From October 2018 to August 2019, consecutive patients meeting the Sepsis-3 criteria[4] were enrolled in this study from the emergency intensive care unit. Sepsis is defined as an infection-caused change in baseline with a total Sequential Organ Failure Assessment (SOFA) score ≥2 points. Septic shock is identified as a subtype of sepsis with a serum lactate level >2 mmol/L and persisting hypotension, which requires the administration of vasopressors to maintain mean arterial pressure (MAP) ≥65 mmHg (1 mmHg=0.133 kPa) despite adequate volume resuscitation. The inclusion criteria were adult patients who met the Sepsis-3 criteria. Exclusion criteria included patients with any of the following conditions: (1) less than 18 years old; (2) received long-term hormone or immunosuppressive therapy; (3) experienced major surgery or trauma during the past three months; (4) suffered from blood systemic diseases, hepatitis, liver cirrhosis, or end-stage renal disease; (5) suffered from autoimmune disease or immunodeficiency disease; (6) diagnosed with cancer and received radiotherapy or chemotherapy; (7) during the pregnancy period; (8) patients or representatives refused to participate in any stage of the study. All patients received standardized treatment based on the International Guidelines for the Management of Sepsis and Septic Shock (2016).[12] Patients were treated with antibiotics on or before the date of sepsis diagnosis when infection parameters were elevated or radiological imaging indicated infection sites. Patients received mechanical ventilation when they (1) were conscious or had irregular breathing; (2) had airway obstruction; (3) were prone to vomiting and aspiration; and (4) had severe hypoxia or/and carbon dioxide retention. Volunteers who had never experienced the above-mentioned diseases, hypertension, diabetes, or major surgery were regarded as the healthy control group. The control group was matched by age and sex with the sepsis group. There was no significant difference in sex or age. Demographic and clinical data were extracted. Acute Physiology and Chronic Health Evaluation (APACHE) II scores[13] and SOFA scores[14] at day 1 of enrollment were calculated. The 28-day mortality was recorded. The study was approved by the Beijing Chao-yang Hospital Ethics Committee. Written informed consent was obtained from all the subjects or their legally authorized representatives.
Laboratory tests for immune-related antigens in leukocyte subsets
Ethylenediamine tetraacetic acid (EDTA) anticoagulant venous blood was collected within 24 h after sepsis was diagnosed in patients who met the inclusion criteria. Flow cytometry was performed within 3 h of sample collection using 100 μL residual venous blood for each leukocyte subtype. Cytometry was carried out using a Gallios flow cytometer (Beckman Coulter, USA). The data were analyzed using Gallios software version 1.0 (Beckman Coulter, USA). Monoclonal antibodies and their isotype controls were all purchased from BD Biosciences (San Jose, USA). The expression of PD-1 (allophycocyanin, MIH4) and PD-L1 (phycoerythrin, MIH4) was measured on B cells, monocytes, and dendritic cells (DCs). B cells were gated by CD3- CD19+ (anti-CD3: Pacific Blue™, UCHT1; anti-CD19: PE-Cy7, SJ25C1); monocytes were gated by CD14+ (anti-CD14: Pacific Blue™, M5E2); DCs were gated by HLADR+ CD11c+ (anti-CD11c: BV421, B-LY6) for myeloid DCs and HLA-DR+ CD123+ (anti-CD123: PE-Cy7, 7G3) for plasmacytoid DCs. At least 10,000 events were collected in the lymphocyte or mononuclear cell gate for each sample. Fluorescence was compensated using anti-mouse COMP antibody (BD Biosciences, USA). The results are expressed as percentages of positively gated cells or mean fluorescence intensities. Representative plots are shown in supplementary Figure 1.
Evaluation of plasma biomarkers
Residual venous blood (4 mL) taken for routine tests on day 1 of enrollment was used for subsequent determination. The blood was centrifuged, and plasma was collected and stored at –80 °C for plasma biomarker determination.
Cytokine concentrations of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-4 (IL-4), IL-6, IL-10, and IL-17A were measured with a ProcartaPlex Multiplex Luminex assay customized kit (Invitrogen, USA) according to the manufacturer’s protocol.
Statistical analysis
The variables were described as medians (interquartile ranges) for continuous variables that did not conform to the normal distribution or homogeneity of variance test. Discontinuous variables were described as counts or percentages. Categorical data were compared using the Pearson Chi-square test or Fisher’s exact test as appropriate. For comparison of continuous variables, Kruskal-Wallis H-tests or Mann-Whitney U-tests were applied where appropriate. Correlations between two independent parameters were assessed using the Spearman rank correlation test. The results with two-tailed P-values of less than 0.05 were considered significant. All data were analyzed using SPSS Statistics 25.0 software (IBM, USA). The figures were prepared using GraphPad Prism 8.0 software (GraphPad Software, USA).
RESULTS
Baseline characteristics of the enrolled participants
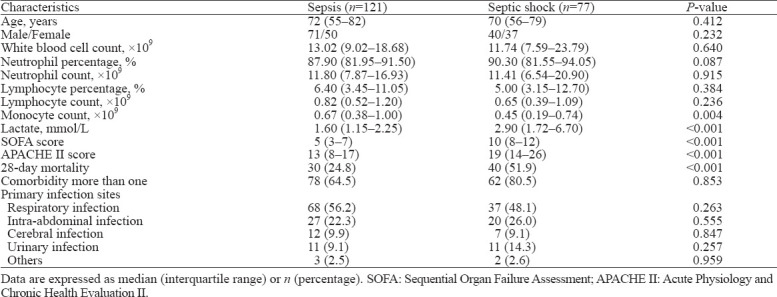
The flow diagram of the present study is shown in supplementary Figure 2. In total, 40 heathy controls and 198 adult patients diagnosed with sepsis according to Sepsis-3, including 111 males and 87 females, were enrolled in the study. Patients were divided into sepsis and septic shock subgroups according to severity. Baseline characteristics are presented in Table 1, Table 2, and supplementary Table 1. There were 121 sepsis patients and 77 septic shock patients based on severity. Alternatively, patients were grouped as 128 survivors and 70 non-survivors according to the outcomes observed at day 28. The cohort was composed of 105 (53.0%) patients with respiratory infection, 47 (23.7%) patients with intra-abdominal infection, 19 (9.6%) patients with cerebral infection, 22 (11.1%) patients with urinary infection, and 5 (2.5%) with other infections. APACHE II scores, SOFA scores and lactate levels were significantly higher in non-survivors than in survivors and in septic shock than in sepsis. No statistically significant differences were detected between the survivor and non-survivor groups or the sepsis and septic shock groups for age, sex, or primary infection sites.
Table 1.
Baseline characteristics of the participants
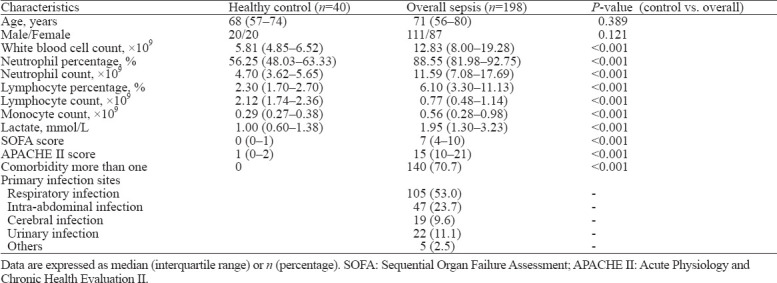
Table 2.
Baseline characteristics of subgroups of sepsis patients on admission
Comparisons of PD-1 and PD-L1 on different APCs according to disease severity and outcomes
As shown in Figure 1 and supplementary Figure 3, the expression of PD-1 decreased significantly on B cells, monocytes, myeloid DCs (mDCs), and plasmacytoid DCs (pDCs) as the severity of sepsis increased. The expression of PD-1 was also markedly decreased in non-survivors compared with survivors. In contrast, the expression of PD-L1 was markedly higher on mDCs, pDCs, and monocytes in patients with sepsis than in healthy controls and in non-survivors than in survivors.
Figure 1.
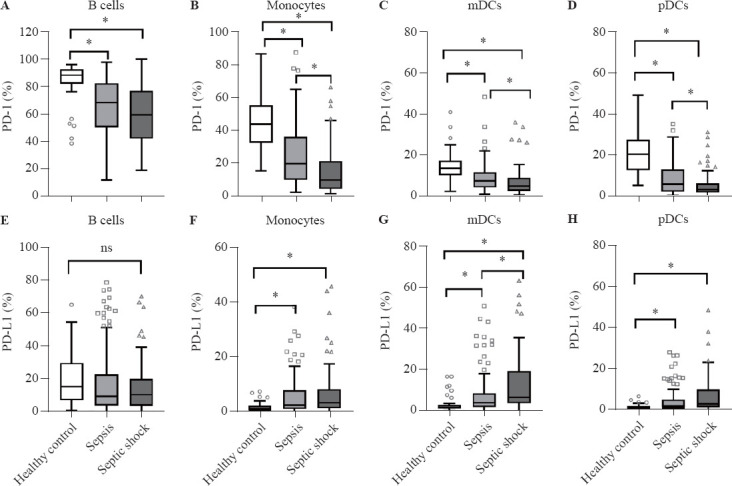
Surface biomarker expression on leukocyte subsets in controls (n=40) and patients with sepsis (n=121) or septic shock (n=77). PD-1 and PD-L1 expression was evaluated on B cells (A, E), monocytes (B, F), myeloid dendritic cells (mDCs) (C, G), and plasmacytoid dendritic cells (pDCs) (D, H). *P<0.05. ns: no significance. PD-L1: programmed death-ligand 1; PD-1: programmed death-1.
Comparisons of cytokines according to disease severity and outcomes
As shown in supplementary Figures 4 and 5, the levels of IL-6 and IL-10 were significantly higher in patients with sepsis than in healthy controls and increased in parallel with the severity of sepsis. The supplementary Figure 5 shows that non-survivors had significantly higher levels of IL-6 and IL-10 than survivors and healthy controls. IL-17A and IL-4 were significantly higher in sepsis patients than in healthy controls, while no significant difference was found between survivors and non-survivors.
Association between cytokines, PD-1 or PD-L1 expression and scoring systems
As shown in supplementary Table 2, associations between cytokines, PD-1 or PD-L1 expression and scoring systems are displayed. PD-1 expression on monocytes showed a weak negative correlation with SOFA score (Spearman’s ρ= –0.310, P<0.001). IL-6 was positively correlated with SOFA score (Spearman’s ρ=0.235, P=0.001) and APACHE II score (Spearman’s ρ=0.209, P=0.003). IL-10 was also positively correlated with SOFA score (Spearman’s ρ=0.334, P<0.001) and APACHE II score (Spearman’s ρ=0.253, P<0.001). The PD-1 percentage of monocytes (monocyte PD-1%) showed a weak negative correlation with IL-6 (Spearman’s ρ= –0.251, P<0.001) and IL-10 (Spearman’s ρ= –0.266, P<0.001). The PD-L1 percentage (PD-L1%) of B cells showed a weak negative correlation with IL-4 (Spearman’s ρ= –0.214, P=0.002). PD-L1 in mDCs showed a weak positive correlation with IFN-γ (Spearman’s ρ=0.271, P<0.001), IL-4 (Spearman’s ρ=0.243, P=0.001), IL-6 (Spearman’s ρ=0.218, P=0.002), TNF-α (Spearman’s ρ=0.280, P<0.001), and IL-17A (Spearman’s ρ=0.239, P=0.001).
Independent predictors for 28-day mortality during sepsis
Univariate analysis showed that parameters, including IL-6, IL-10, monocyte PD-1%, and PD-L1% of mDCs (mDC PD-L1%), were significantly different between survivors and non-survivors. The above parameters were included in the binary logistic regression model. The results are shown in supplementary Table 3. Monocyte PD-1% was an independent risk factor for 28-day mortality (risk ratio 0.971, 95% confidence interval [95% CI] 0.951–0.991; P=0.005).
Predictive performance of PD-1 and PD-L1 on APCs, clinical severity, and inflammatory cytokines alone and in combination
The area under the receiver operating characteristic curve (AUC) of the biomarkers in predicting 28-day mortality of sepsis was 0.811 (SOFA score), followed by 0.809 (APACHE II score), 0.675 (IL-10), 0.607 (IL-6), 0.591 (mDC PD-L1%), and 0.575 (pDC PD-L1%), as presented in Figure 2 and supplementary Table 4. We also compared the AUC of the parameters in combination. The AUC of the monocyte PD-1%+ APACHE II model (0.823) was higher than that of the monocyte PD-1%+SOFA model (0.816), followed by that of the mDC PD-L1%+SOFA model (0.813). No statistically significant difference was found between the combined model and the other isolated indicators for mortality prediction.
Figure 2.
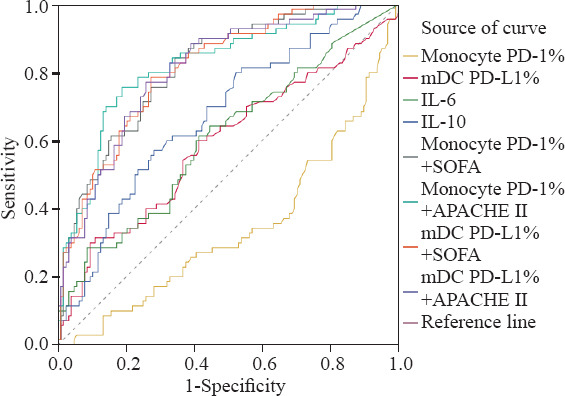
The receiver operating characteristic curve of PD-1 or PD-L1 expression of antigen-presenting cells, scores, cytokines alone or in combination in predicting 28-day outcome.PD-1: programmed death-1; PD-L1: programmed death-ligand 1; mDC: myeloid dendritic cell; IL-6: interleukin-6; IL-10: interleukin-10; SOFA: Sequential Organ Failure Assessment; APACHE II: Acute Physiology and Chronic Health Evaluation II.
DISCUSSION
In the present study, PD-L1 was up-regulated on APCs and correlated with SOFA and APACHE II scores during early sepsis. The PD-1 or PD-L1 axis plays an important role in immunosuppressive mechanisms during sepsis.[15] It was reported that the induction of an adaptive response was impaired in patients with severe endotoxin tolerance status, which was dependent on the PD-L1 or PD-1 pathway;[16] PD-1-deficient mice showed increased survival and maintained macrophage function, demonstrating improved bacterial clearance and reduced inflammation.[17,18] In line with these reports, we found that monocyte PD-1% and lactate were independent risk factors for 28-day mortality. Monocyte PD-1% combined with the APACHE II or SOFA model had higher prognostic value than other parameters. It was observed that in septic newborns with complications, there was a higher percentage of intermediate monocytes with PD-1 expression. PD-1 might indicate the immunosuppressive phase of sepsis in prematurely born children with sepsis.[19] PD-1+ monocytes presented a preference toward M2 polarization and had a deficiency in supporting CD8 T cells in hepatocellular carcinoma.[20] However, as this was an observational, single-center clinical study, this result will eventually need to be validated by studies with larger scales. Further basic research is needed to interpret the potential function of this subpopulation.
Prior observational clinical studies have shown that critically ill patients who developed severe septic shock expressed markedly elevated levels of PD-1 or PD-L1 on various leukocyte subsets, commonly T cells.[6,7,21] The novelty of the present study was that PD-L1 on APCs significantly increased as the severity of sepsis increased. In line with previous studies, PD-L1 on APCs has been demonstrated to be an important indicator reflecting prognosis during sepsis. Our previous research showed that the increased expression of PD-L1 on the monocyte surface was an independent risk factor for risk stratification and prognosis at 3–4 d of sepsis.[11] The combination of monocyte PD-L1% and plasma infection biomarker presepsin or procalcitonin (PCT) can also help to improve the prognostic value of sepsis.[22] An increased percentage of PD-L1+ natural killer (NK) cells has also been suggested as a novel prognostic biomarker in predicting sepsis.[23]
Classical studies have suggested that PD-L1 on APCs binds to PD-1 on effector T cells, leading to T-cell apoptosis, anergy, and exhaustion, and plays a critical role in T-cell tolerance.[15,24,25] In contrast, PD-L1 binds to PD-1 on inhibitory Tregs and promotes proliferation.[26] Few studies have focused on the effects of PD-L1 activation on APCs themselves. It was reported that reduced monocyte phagocytic function correlated with higher expression of PD-L1 on total monocytes in sepsis. In addition, ex vivo incubation of whole blood with anti-PD-L1 and anti-PD-1 mAbs was able to increase the phagocytosis function of monocytes.[6] Recent tumor studies have shown that incubation with soluble CD80 or PD-1 increased the proliferation, survival and activation of tumor-associated macrophages (TAMs). Anti-PD-L1 antibody treatment induced the transformation of TAMs to a pro-inflammatory phenotype (M1-like macrophages) and increased the secretion of pro-inflammatory cytokines.[27, 28] The role of APC-expressed PD-L1 in the pathogenesis of sepsis remains to be elucidated. Therefore, we focused on PD-L1 expression on APCs, the first barrier in fighting against infection, and found their association with inflammation, organ failure, and mortality during early sepsis.
The regulation of nuclear factor-kappa B (NF-κB), the key factor involved in inflammation, might account for the correlation observed in our study. Peripheral blood mononuclear cells (PBMCs) from non-survivors of sepsis had increased levels of NF-κB activation. Increased NF-κB activation was also strongly correlated with the severity of illness (APACHE II score) and associated with higher mortality.[29,30] The association between PD-L1 and NF-κB, however, has not been thoroughly studied in sepsis. A study showed that TNF-α induced the NF-κB pathway and promoted demethylated PD-L1 promoter expression in non-small cell lung carcinoma. Inhibition of NF-κB resulted in the abolition of PD-L1 expression.[31] Other studies confirmed that NF-κB occupied the CD274 promoter and acted as a regulator of PD-L1 mRNA expression in various cancer types.[32,33] However, the underlying regulatory mechanism during sepsis still requires future research.
There are some limitations to our study. First, this was a single-center, observational study, and the sample size was relatively small. Further large-scale studies should be conducted to validate our findings. Second, the observation period was relatively short. We only traced the patients for 28 d and did not evaluate long-term changes in the immune system. Third, we only picked a single time point to observe the immune condition, which could not reflect the dynamic changes during the sepsis course. Patients should be stratified and discussed to avoid bias. Fourth, only a correlation between PD-L1 on APCs and cytokines was observed. The possible role of inflammation master molecules, such as NF-κB, should be elucidated to explain the mechanism of our findings. Finally, the flow cytometry results may have differed due to different protocols and processing software. Thus, the results of our study could only reflect trends in relative expression levels during sepsis.
CONCLUSIONS
PD-L1 was over-expressed on APCs during early sepsis and correlated with the severity of sepsis. PD-L1 on APCs (monocytes and DCs) was weakly correlated with inflammation and organ dysfunction during early sepsis. The combination of SOFA or APACHE II scores with monocyte PD-1% could improve the ability for mortality prediction.
Footnotes
Funding: None.
Ethical approval: All operations were in compliance with the ethics standards of Beijing Chaoyang Hospital. Informed consents for participation were obtained from patients or their legally authorized representatives. Ethical approval was acquired from Beijing Chaoyang Hospital Medical Ethics Committee (Ethical number: 2013-KE-1). The study conformed to the Declaration of Helsinki.
Conflicts of interest: The authors do not have a financial interest or relationship to disclose regarding this research project.
Contributors: CSL contributed to the study conception and design, revised the manuscript, and provided research funding. JBL, MLD, YNY, CCH, ZRT, and MRX were involved in sample collection and material preparation, data collection and analysis during the experimental process. JBL collected data, drafted the manuscript, performed statistical analysis, and critically revised the manuscript. All authors commented on previous versions of the manuscript. All authors consented to the publication of the manuscript and agreed to be responsible for the manuscript.
All the supplementary files in this paper are available at http://wjem.com.cn.
REFERENCES
- 1.Duan LW, Qu JL, Wan J, Xu YH, Shan Y, Wu LX, et al. Effects of viral infection and microbial diversity on patients with sepsis:A retrospective study based on metagenomic next-generation sequencing. World J Emerg Med. 2021;12((1)):29–35. doi: 10.5847/wjem.j.1920-8642.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin J, Chen Y, Huang JL, Yan L, Kuang ZS, Xue MM, et al. Prognosis-related classification and dynamic monitoring of immune status in patients with sepsis:A prospective observational study. World J Emerg Med. 2021;12((3)):185–91. doi: 10.5847/wjem.j.1920-8642.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of hospital-treated Sepsis current estimates and limitations. Am J Respir Crit Care Med. 2016;193((3)):259–72. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 4.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression:from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patera AC, Drewry AM, Chang K, Beiter ER, Osborne D, Hotchkiss RS. Frontline science:defects in immune function in patients with sepsis are associated with PD-1 or PD-L1 expression and can be restored by antibodies targeting PD-1 or PD-L1. J Leukoc Biol. 2016;100(6):1239–54. doi: 10.1189/jlb.4HI0616-255R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakeley ME, Gray CC, Monaghan SF, Heffernan DS, Ayala A. Check point inhibitors and their role in immunosuppression in sepsis. Crit Care Clin. 2020;36(1):69–88. doi: 10.1016/j.ccc.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Yan J, Ma LQ, Bi W, Wu CJ. Effects of Maxingloushi decoction on immune inflammation and programmed death markers in mice with chronic obstructive pulmonary disease. World J Emerg Med. 2022;13((1)):32–7. doi: 10.5847/wjem.j.1920-8642.2022.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA. 2011;306(23):2594–605. doi: 10.1001/jama.2011.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar-Hari M, Datta D, Wilson J, Assi V, Stephen J, Weir CJ, et al. Early PREdiction of sepsis using leukocyte surface biomarkers:the ExPRES-sepsis cohort study. Intensive Care Med. 2018;44((11)):1836–48. doi: 10.1007/s00134-018-5389-0. [DOI] [PubMed] [Google Scholar]
- 11.Shao R, Fang YY, Yu H, Zhao LX, Jiang ZF, Li CS. Monocyte programmed death ligand-1 expression after 3-4 days of sepsis is associated with risk stratification and mortality in septic patients:a prospective cohort study. Crit Care. 2016;20(1):124. doi: 10.1186/s13054-016-1301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign:international guidelines for management of sepsis and septic shock:2016. Intensive Care Med. 2017;43((3)):304–77. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 13.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–36. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, Willatts S, de Mendonça A, Bruining H, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22((7)):707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 15.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192((7)):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avendaño-Ortiz J, Maroun-Eid C, Martín-Quirós A, Toledano V, Cubillos-Zapata C, Gómez-Campelo P, et al. PD-L1 overexpression during endotoxin tolerance impairs the adaptive immune response in septic patients via HIF1α. J Infect Dis. 2018;217(3):393–404. doi: 10.1093/infdis/jix279. [DOI] [PubMed] [Google Scholar]
- 17.Young WA, Fallon EA, Heffernan DS, Efron PA, Cioffi WG, Ayala A. Improved survival after induction of sepsis by cecal slurry in PD-1 knockout murine neonates. Surgery. 2017;161(5):1387–93. doi: 10.1016/j.surg.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X, Venet F, Wang YL, Lepape A, Yuan ZL, Chen YP, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106(15):6303–8. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zasada M, Lenart M, Rutkowska-Zapała M, Stec M, Durlak W, Grudzień A, et al. Analysis of PD-1 expression in the monocyte subsets from non-septic and septic preterm neonates. PLoS One. 2017;12(10):e0186819. doi: 10.1371/journal.pone.0186819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun J, Yu GH, Hu PP, Chao Y, Li XY, Chen XB, et al. PD-1 expression is elevated in monocytes from hepatocellular carcinoma patients and contributes to CD8 T cell suppression. Immunol Res. 2020;68((6)):436–44. doi: 10.1007/s12026-020-09155-3. [DOI] [PubMed] [Google Scholar]
- 21.Guignant C, Lepape A, Huang X, Kherouf H, Denis L, Poitevin F, et al. Programmed death-1 levels correlate with increased mortality, nosocomial infection and immune dysfunctions in septic shock patients. Crit Care. 2011;15(2):R99. doi: 10.1186/cc10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Tang Z, Xie M, Hang C, Yu Y, Li C. Association between elevation of plasma biomarkers and monocyte dysfunction and their combination in predicting sepsis:an observational single-centre cohort study. Innate Immun. 2020;26(6):514–27. doi: 10.1177/1753425920926602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang WQ, Li XS, Wen MY, Liu XY, Wang KR, Wang QS, et al. Increased percentage of PD-L1+natural killer cells predicts poor prognosis in sepsis patients:a prospective observational cohort study. Crit Care. 2020;24(1):617. doi: 10.1186/s13054-020-03329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo AD, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203((4)):883–95. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101(29):10691–6. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen LP, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13(4):227–42. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong HZ, Mittman S, Rodriguez R, Moskalenko M, Pacheco-Sanchez P, Yang YG, et al. Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019;79((7)):1493–506. doi: 10.1158/0008-5472.CAN-18-3208. [DOI] [PubMed] [Google Scholar]
- 28.Sun NY, Chen YL, Wu WY, Lin HW, Chiang YC, Chang CF, et al. Blockade of PD-L1 enhances cancer immunotherapy by regulating dendritic cell maturation and macrophage polarization. Cancers. 2019;11(9):1400. doi: 10.3390/cancers11091400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnalich F, Garcia-Palomero E, López J, Jiménez M, Madero R, Renart J, et al. Predictive value of nuclear factor kappa B activity and plasma cytokine levels in patients with sepsis. Infect Immun. 2000;68(4):1942–5. doi: 10.1128/iai.68.4.1942-1945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Böhrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Männel D, et al. Role of NFkappa B in the mortality of sepsis. J Clin Invest. 1997;100(5):972–85. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asgarova A, Asgarov K, Godet Y, Peixoto P, Nadaradjane A, Boyer-Guittaut M, et al. PD-L1 expression is regulated by both DNA methylation and NF-κB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology. 2018;7(5):e1423170. doi: 10.1080/2162402X.2017.1423170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouillez A, Rajabi H, Jin C, Samur M, Tagde A, Alam M, et al. MUC1-C integrates PD-L1 induction with repression of immune effectors in non-small-cell lung cancer. Oncogene. 2017;36(28):4037–46. doi: 10.1038/onc.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, et al. Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Cancer Res. 2015;75(23):5034–45. doi: 10.1158/0008-5472.CAN-14-3098. [DOI] [PubMed] [Google Scholar]


