Abstract
Background
COVID-19 is associated with severe respiratory distress and high mortality. We investigated the evolution of the respiratory mechanics in COVID-19 acute respiratory distress syndrome (ARDS) and the occurrence of a restrictive respiratory pattern.
Methods
A retrospective, single-centre study including patients admitted to the ICU during the first wave of the pandemic (March-April 2020).
Results
A total of 141 consecutive patients were included. Many patients developed a restrictive pattern of respiratory mechanics during the course of the disease. Fifty-two patients died in the hospital (36.8%). In 29 cases (58% of the deceased) death was associated with a pattern of pulmonary mechanics, indicating a restrictive evolution of ARDS. Other diagnoses related to death were pulmonary embolism (n = 7, 14%), septic shock (n = 17, 33%), and other causes (n = 10, 20%), with some patients combining at least 2 of these diagnoses. In a multivariate analysis, age (OR = 1.06; 95% CI: 1.01–1.12; P = 0.029) and the administration of steroid pulses (OR = 2.7; 95% CI: 1.1–6.8; P = 0.03) were associated with the development of a restrictive pulmonary pattern and a higher level of plasmatic interleukin-6.
Conclusions
COVID-19 ARDS is associated with high mortality associated with a specific pattern of respiratory mechanics and sustained activation of innate immunological response. Age and administration of high-dose steroid pulses are associated with this clinical picture.
Keywords: COVID-19, adult, interleukins, restrictive lung disease, respiratory distress syndrome, respiration, artificial, late respiratory distress syndrome
Infection by the novel coronavirus SARS-CoV-2 produces a wide spectrum of clinical patterns, which are generating debate in the scientific community. Efforts to describe the clinical characteristics of this disease are made harder by the current pandemic situation in which most countries are either being heavily hit or are slowly recovering after enduring hard months of battle. In particular, many authors have tried to describe different phenotypes of COVID-19-associated acute respiratory distress syndrome (ARDS) (C-ARDS) to individualize treatment and respiratory management [1, 2], but mortality of mechanically ventilated COVID-19 patients remains very high and was estimated to be around 45%, although some studies have reported slightly lower figures [3–5].
The host immune response to infection by SARS-CoV-2 plays a key role in COVID-19 and has been the target of many therapies for the disease; moreover, it may influence the mechanical characteristics of the lungs during the course of the disease. This is due to the inability of the lung to repair itself following sustained injury, which results in persistent inflammatory stimulus and elevation of cytokines, which predicts poor outcome in ARDS [6]. Lung fibrosis is a known consequence of persistent ARDS after a long course of mechanical ventilation [7, 8], and it is associated with inflammatory dysregulation and extremely high mortality [7, 9]. However, few studies have presented data regarding the respiratory mechanics that patients present after a long course of mechanical ventilation for ARDS or for C-ARDS [10–13]. Recently the respiratory mechanics of patients with lung fibrosis were described with the paradigm of the “squishy ball” [14, 15]. This is a respiratory pattern that can often be seen in the later stages of persistent ARDS after a course of mechanical ventilation. In this study we reviewed the respiratory mechanics of patients with C-ARDS and evaluated the incidence of a pattern of respiratory mechanics compatible with restrictive or fibrotic disease – the “squishy ball” [14]. We investigated risk factors for the development of this respiratory pattern, and its association with cytokine production and death.
METHODS
Approval for this study was provided by the Ethical Committee CEI (Ethical Committee Nº 224/20), who waived the need for informed consent.
We conducted a retrospective review of the patient data originating from the electronic information system of the hospital. We included patients with positive SARS-CoV-2 PCR in nasal swab or bronchoalveolar aspirate, age > 18 years, and hospitalization in an ICU. Patients hospitalized between 1 March and 30 April 2020 were included in the study. No sample size calculations were performed.
All patients were treated following the hospital protocol for COVID-19 and its updated versions, which were published throughout the outbreak following newly acquired evidence from clinical trials. The protocol included the administration of hydroxychloroquine (loading dose 400 mg every 12 h for 1 day and 200 mg every 12 h for an additio-nal 4 days), lopinavir/ritonavir (400/100 mg every 12 h for 7 days), azithromycin (250 mg per day for 5 days), and low-dose methylprednisolone after admission to the ICU (1 mg kg-1 per day for 5 days tapering to 0.5 mg kg-1 per day for 5 days). High-dose steroid pulses (> 2 boluses of ≥ 250 mg methylprednisolone) were discretionarily used by some physicians in hospital wards prior to ICU admission. Tocilizumab was used (600 mg IV if > 75 kg, 400 mg if ≤ 75 kg) when plasma IL-6 was > 40 pg mL-1 and PaO2/FiO2 ratio < 200. Lungs were ventilated following a protective ventilation strategy aiming at a tidal volume ≤ 8 mL kg-1 (ideal body weight), plateau pressure < 30 cm H2O and driving pressure ≤ 15 cm H2O. Neuromuscular blockade, prone positioning (PP), and recruitment manoeuvres (RM) were also included in a protocolized approach to ventilatory treatment. Neuromuscular blockade was used only if PaO2/FiO2 ratio < 150 and patient/ventilator dyssynchrony was present. Prone positioning was indicated when PaO2/FiO2 ratio < 150 for more than 12 hours of controlled mechanical ventilation, and cycles of at least 16 hours of prone positioning were applied, which could be repeated after 4 hours of supine ventilation if criteria were met. Finally, the use of recruitment manoeuvres was restricted to cases of refractory hypoxaemia not responding to ordinary ventilatory treatment.
The data on respiratory mechanics was evaluated for each patient daily. We observed a pattern of very low compliance (< 25 mL cm H2O–1), no response to PEEP trial, and low optimal PEEP level of < 6 cm H2O (optimal PEEP being the PEEP level that achieved the maximal static compliance), and no response to prone positioning in patients who required a prolonged course of invasive mechanical ventilation. We referred to this pattern as “restrictive” [14] and evaluated factors correlated with the development of this respiratory pattern.
Four clinical diagnoses were defined to assess the causes of death: (1) refractory respiratory failure, (2) pulmonary embolism (diagnosed by CT scan or clinical signs of right ventricular failure by echocardiography), (3) septic shock, and (4) other causes (haemorrhage, neurological disorders).
Plasma levels of TNF-a and interleukins (IL) were monitored to assess changes in the immune response and help define the best therapeutic strategy. Serum cytokines were quantified using the BD Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit (BD Biosciences, Franklin Lakes, NJ, USA). Serum samples were mixed with beads coated with capture antibodies specific for TNF-a, IL-1b, IL-6, IL-8, IL-10, and IL-12p70 and then conducted according to the manufacturer’s instructions. Beads were analysed by flow cytometry using a FACSCanto II cytometer. Analysis was performed using FCAO Array Software v3.0 (BD Biosciences).
Statistical analysis
Data are presented as median (IQR) for continuous variables because a normal distribution could not be assumed as per Shapiro-Wilks normality tests. Categorical variables are represented as n (%). Univariable analyses for continuous variables were performed using Wilcoxon rank sum tests, and chi-squared tests were used for categorical variables. We analysed the association between the restrictive mechanical pattern and steroid pulses after adjusting for severity (APACHE II) and age using a multivariable logistic regression model. A multilevel linear mixed model was used to estimate the association between the restrictive pattern and (logarithm) IL-6 overtime. The model included fixed effects for the restrictive pattern, time, and the interaction term restrictive pattern x time and random intercepts for patients. Statistical significance was defined as a P-value ≤ 0.05. Statistical analysis was performed with Stata version 16 (StataCorp LP, College Station, TX, USA).
RESULTS
Between 2 March and 29 April we attended to 141 patients in our ICUs, of whom 52 (36.8%) died before leaving the ICU. The characteristics of the population are described in Table 1. At admission 84 patients (60%) had severe ARDS; all patients in the population were treated with invasive mechanical ventilation. Thirty-four patients (65%) died with refractory respiratory failure and persistent hypoxaemia (PaO2/FiO2 < 100). Thirty-one patients presented during mechanical ventilation with a restrictive pattern of respiratory mechanics. The appearance of the restrictive pattern of respiratory mechanics occurred after a median of 10 days of mechanical ventilation (IQR 6–14) and a median of 23 days after the onset of COVID-19 symptoms (IQR 14–26). This pattern of respiratory mechanics was associated with severely impaired gas exchange and a PaO2/FiO2 ratio < 150. Twenty-nine of these patients died (56% of the deceased). Data regarding the evolution of the respiratory mechanics shown in mechanically ventilated patients over time are displayed in the Supplementary Table.
TABLE 1.
Characteristics of the population
| Patients | N = 141 | |
|---|---|---|
| Age, years (IQR) | 61 (57–67) | |
| Sex – male, n (%) | 108 (76.6) | |
| BMI, median (IQR) | 28 (26–32) | |
| APACHE II (IQR) | 15 (10–19) | |
| Comorbidities, n (%) | ||
| Hypertension | 60 (42.5) | |
| Chronic ischaemic heart disease | 20 (14.2) | |
| Chronic kidney disease | 7 (4.9) | |
| Chronic kidney disease – patients in dialysis | 1 (0.7) | |
| Chronic obstructive pulmonary disease | 10 (7.1) | |
| Diabetes | 24 (17) | |
| Chronic liver disease (MELD > 10) | 1 (0.7) | |
| Immunosuppressive therapy, n (%) | 4 (2.8%) | |
| Time from hospital admission to ICU admission, median (IQR) | 4 (1-6) | |
| ICU length of hospitalization, median (IQR) | 14 (8–17) | |
| Pharmacologic treatment, n (%) | ||
| Lopinavir/Ritonavir | 130 (92) | |
| Hydroxychloroquine | 130 (92) | |
| Remdesivir | 13 (9) | |
| Interferon | 23 (16) | |
| Tocilizumab | 96 (68) | |
| Anakinra | 8 (5) | |
| Corticosteroids | 124 (88) | |
| Respiratory characteristics and treatment | ||
| PaO2/FiO2 ratio on first day of MV, median (IQR) | 124 (69–156) | |
| PEEP on first day of MV, median (IQR) | 12 (10–15) | |
| Respiratory compliance on first day of MV, median (IQR) | 40 (31–47) | |
| Tidal volume on first day of MV, median (IQR) | 470 (450–500) | |
| Prone position, n (%) | 125 (89.0) | |
| Patients with restrictive pattern, n (%) | 31 (21.9) | |
| Days of mechanical ventilation before appearance of restrictive pattern – median (IQR) | 10 (6–14) | |
BMI – body mass index, MELD – model for end-stage liver disease, MV – mechanical ventilation
SUPPLEMENTARY MATERIAL
Other diagnoses related to death were pulmonary embolism (n = 7, 13%), septic shock (n = 17, 33%), and other causes (n = 10, 19%), with some patients combining at least 2 of these diagnoses. Thus, patients who developed a restrictive respiratory pattern during the disease were very likely to die with severe hypoxaemia. Severe lung injury was observed in the first autopsy performed at our hospital, showing diffuse alveolar damage in the advanced organization/proliferation phase together with areas of well-developed fibrosis (Figure 1) [16].
FIGURE 1.
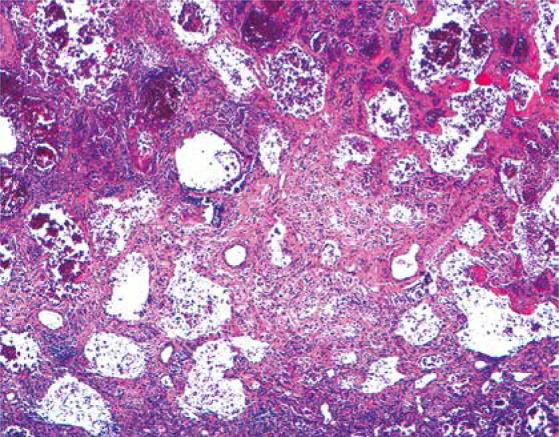
Histological section of the lung showing severe reduction of air spaces with septal thickening and fibrosis
We analysed several risk factors that are potentially associated with the development of this pattern. In a univariate model, age, severity of disease at ICU admission, and the use of steroid pulses showed a significant association with the development of this pattern of respiratory mechanics (Table 2).
TABLE 2.
Risk factors for developing a restrictive pattern. Obesity was defined as a body mass index > 30. Steroid pulses included the administration of more than 2 doses > 250 mg of methylprednisolone for more than one day. In brackets: percentage or range
| Factor | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| Total (N = 141) | Restrictive (n = 31) | Non-restrictive (n = 110) | P-value | OR | 95% CI | P-value | |
| Age, years | 62 (57–67) | 65 (57–71) | 61 (56–66) | 0.042 | 1.06 | 1.01–1.12 | 0.029 |
| Sex – female | 33 (23.4) | 9 (29.0) | 24 (21.8) | 0.40 | – | – | – |
| Obesity | 42 (29.8) | 8 (25.8) | 34 (30.9) | 0.58 | – | – | – |
| APACHE II | 15 (12-16) | 15 (15-18) | 15 (10-15) | 0.006 | – | – | NS |
| Infection | 60 (42.6) | 16 (51.6) | 44 (40.0) | 0.25 | – | – | – |
| Tocilizumab | 96 (68.0) | 25 (80.6) | 71 (64.5) | 0.079 | – | – | – |
| Steroid pulses | 30 (21.3) | 11 (35.5) | 19 (17.3) | 0.029 | 2.7 | 1.1–6.8 | 0.03 |
In a multivariate model we analysed the association of high-dose steroid pulses and the pulmonary restrictive pattern adjusted by age and APACHE II. After adjusting, age (OR = 1.06; 95% CI: 1.01–1.12; P = 0.029) and the administration of steroid pulses were associated with higher risk (OR = 2.7; 95% CI: 1.1–6.8; P = 0.03) (Table 2).
Cytokines (IL-1b, IL-6, IL-8, IL-10, IL-12, and TNF-a) were measured routinely in the plasma of patients hospitalized in the ICU. Logarithmic values of IL-6 were significantly higher and increased significantly over time in patients who developed a restrictive pattern of respiratory mechanics (P < 0.001), but they appeared to slightly decrease in patients who did not (P = 0.084) (Figure 2).
FIGURE 2.
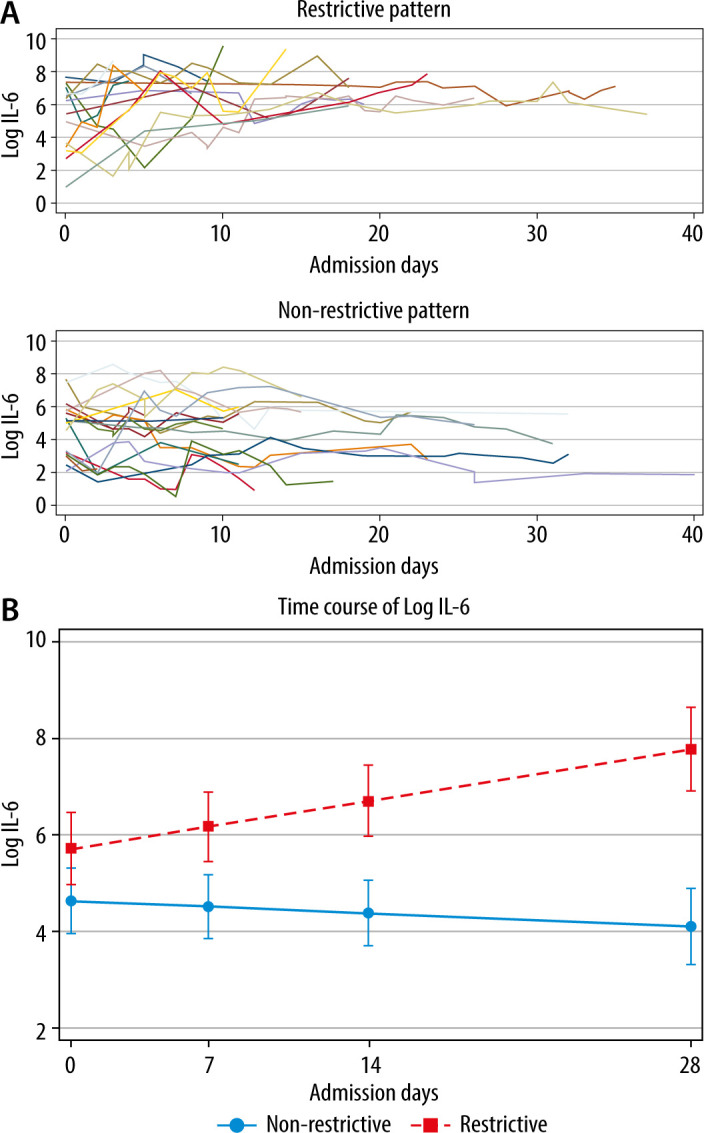
a) Log IL-6 measurements over time in both restrictive (upper panel) and non-restrictive groups (lower panel). b) Log IL-6 significantly increased over time in the restrictive group (P < 0.001) while appearing to slightly decrease in the nonrestrictive group (P = 0.084)
DISCUSSION
This study evaluated the incidence and impact on clinical outcome of a ventilatory pattern compatible with acquired restrictive pulmonary disease in COVID-19 mechanically ventilated patients. We showed that patients who present this ventilatory pattern have a high incidence of mortality, as well as a strong activation of the innate immune system.
To our knowledge, this is the first study to describe this type of ventilatory pattern in late COVID-19- associated ARDS. We observed the emergence of a restrictive pattern in the mechanical properties of the lungs of 31 patients, of whom 29 (93%) subsequently died, constituting 56% of the overall mortality registered in our study. Early reports in ARDS have shown a similar pattern of restrictive disease to be present in the late stages of the syndrome [17]. However, later studies during the era of lung protective ventilation did not show the same results [18]. The high incidence of this type of respiratory pattern is concerning, and it could be related to ventilator-induced lung injury, as was probably the case in the earlier reports of late ARDS lung mechanics before the era of lung protective ventilation [18]. However, it must be underlined that the patients included in this study were all treated following strict lung protective strategies in a tertiary hospital that cares for a high volume of ARDS patients every year. The mechanical properties of the lungs of patients who developed a restrictive pattern as per our criteria were similar to the ones shown by ARDS patients with fibrotic lungs (due to idiopathic pulmonary fibrosis) published in the literature [14, 15]. In particular, the application of incremental PEEP produced an increase in elastance and therefore an increase in parenchymal stress [14, 15]. In our experience, in the initial phase after intubation, COVID-19 patients are usually characterized by a positive response to RM and PP. However, despite lung protection, a remarkable reduction in lung compliance gradually appears in some ICU patients, usually after 10 days of disease. Several studies in C-ARDS and ARDS by other aetiologies have described lung mechanics to be highly variable and not related with the impairment of gas exchange [10–13, 19–21]. In some cases, lung compliance on the first day of mechanical ventilation was associated with mortality [20, 21]; however, the association was not as clear in other reports [19]. In particular, lung recruitability was found to be high in the majority of patients in the first hours of mechanical ventilation, and to follow a variable pattern in the following days of treatment [22]. The parameters of respiratory mechanics over time show a progressive worsening with reduced compliance associated with stable PaO2/FiO2 ratio and worsening ventilatory ratio; however, none of these studies provides respiratory mechanics parameters beyond day 7 of mechanical ventilation, and there is no description of the emergence of specific patterns in the study population [12]. The pattern of respiratory mechanics analysed in this study appeared after 10 days (IQR 6–14) of protective mechanical ventilation, and it is compatible with clinically relevant lung fibrosis. None of the patients included in this study underwent lung biopsy; therefore, the clinical suspicion of lung fibrosis given by the restrictive pattern of respiratory mechanics cannot be confirmed. However, in all the autopsies performed on COVID-19 ICU patients in our centre, areas of well-developed pulmonary fibrosis were described [16]. Several reports of pulmonary fibrosis following severe COVID-19 have been published recently and confirm the high prevalence of this finding [23, 24], which is associated with the severity of the viral pneumonia [25]. McGroder et al. [23] found significant alteration in the post-discharge CT scans of 72% of C-ARDS survivors who had been mechanically ventilated, whereas Zou et al. [24] found them in 100% of ICU survivors. Moreover, in the study by Zou et al. [24] there was a clear association between fibrotic changes and the level of inflammatory markers and notably IL-6 during the acute phase of the disease. These findings coincide with previous observations showing that the development of pulmonary fibrosis is common after a SARS coronavirus infection [26]. Fibrosis in acute lung injury is related to complex biological reactions where inflammatory cytokines and neutrophils act as primers [27]. Sequential exudative (early) and fibroproliferative (late) phases defined although proliferation have been observed early in ARDS [28]. This pathogenic mechanism has been thoroughly studied in the last decades, but no successful treatment has been found. Ventilator-induced lung injury (VILI) is associated with fibrosis, and the implementation of protective ventilation leads to a reduction in mortality [29].
We have analysed several factors that might have an influence on the restrictive progression. Both sepsis [30] and obesity [31] involve a degree of inflammatory dysregulation despite being 2 different processes causing distinct alteration in immune physiology. In our analysis neither was associated with the development of clinical pulmonary restrictive features. A predominant role of the innate over the adaptive immunity is probably related to the persistent release of mediators that finally favour the fibroproliferative phase in lung injury. The blockade of the adaptive response may be secondary to immunosenescence (T-cell exhaustion), lymphopaenia secondary to viral infection, or immunosuppression [32]. Thus, immunosenescence in older patients may explain the greater risk of developing a restrictive pulmonary pattern associated with age [23]. High-dose steroid pulses (on top of the normal treatment with steroids given to ARDS patients) were also related to the development of this pattern. A possible mechanism to explain this finding is an excess of generalized immunosuppression affecting the adaptive response, which induces a rebound of the innate immune response [32]. However, it is difficult to interpret these data because the criteria for the use of steroid pulses were not clearly defined. On the other hand, it must be underlined that steroid pulses were in all cases administered in hospital wards and before ICU admission was needed. Moreover, a recent study has demonstrated different patterns of response in C-ARDS patients to therapy with corticosteroids, indicating possible harm from these drugs in specific subpopulations [33].
Patients who developed a clinical pulmonary restrictive pattern presented a different immune response compared to other COVID-19 ARDS patients, as described by cytokine production. In fact, they showed a dramatic increase in plasma levels of IL-6 but not in the other cytokines measured (IL-1b, IL-8, IL-10, IL-12 and TNF-a). This suggests that IL-6 is the main inflammatory cytokine in SARS-CoV-2 associated ARDS and its late fibroproliferative stages. Persistent activation of the innate immune system, suggested by high IL-6 values, can contribute to the development of pulmonary fibrosis and highlights the importance of an early control of innate cell inflammation because it can derive in the production of mediators (e.g. oxygen reactive species or proteases), which may induce lung damage resulting in fibrosis. The reason for the persistent inflammation is unknown.
ARDS and idiopathic pulmonary fibrosis acute exacerbations (IPF-AE) share many clinical and histological features. IPF-AE may be related to intrinsic biological dysfunction of the lung, which makes some individuals more susceptible to external insults such as VILI [9, 14], hyperoxia, and viral infections [34], all of which are present in COVID-19 patients. The restrictive pattern observed in COVID-19 probably shares biological processes with both late ARDS and IPF-AE; thus, we may speculate that previous experience in these scenarios may be useful in the case of COVID-19. Many drugs have been used to prevent or treat fibrosis in ARDS. The use of early low-dose steroids (methylprednisolone 1 mg kg–1 per day or equivalent doses of dexamethasone) has been advocated in ARDS [35, 36] and has shown positive effects in COVID-19-associated ARDS [37]. However, high-dose steroid pulses or late use of low-dose steroids raise concerns because they might increase mortality [38, 39].
There are several limitations to this study. The fact that it is single-centre, retrospective, and includes a relatively low number of cases precludes the generalizability of our findings. Second, we evaluated the respiratory mechanics of patients but did not perform further tests such as lung biopsies or HRCT to confirm the clinical suspicion of lung fibrosis. Moreover, we did not evaluate the smoking status, which has been correlated with severe C-ARDS. In respect to IL-6, the persistence of high levels of this mediator might be influenced by the administration of tocilizumab because the IL-6 receptor blockade can increase or decrease the plasmatic levels of IL-6. However, because the protocol for the use of tocilizumab was common for all patients, this should have been seen in non-restrictive patients as well, because tocilizumab was administered whenever IL-6 levels were raised (as was the case in all the patients analysed). Because IL-6 levels were measured routinely and all our patients were treated according to the same protocol, it is not possible to discern the role of tocilizumab in this regard. Regarding the role of steroid boluses, we must acknowledge the possibility of a survival bias because patients who were given early steroid boluses may have survived the initial stage of the disease to then develop a restrictive pattern later on. The strength of the study is that we have included a homogeneous cohort of patients with ARDS caused by the same pathology and were treated with the same pharmacological and ventilatory protocol during their ICU stay, thus limiting confounding factors based on different therapeutic approaches.
We conclude that COVID-19 patients requiring a prolonged course of mechanical ventilation often develop a restrictive pattern of respiratory mechanics despite the use of protective ventilation strategies. This ventilatory pattern is associated with sustained activation of the innate immunological response, and it has a very high mortality rate. Age and the administration of high-dose steroid pulses prior to ICU admission are the main risk factors associated with the development of this respiratory pattern.
ACKNOWLEDGMENTS
Assistance with the article
The authors would like to thank Javier Zamora for statistical analysis and the Pathology Department of Hospital Ramón y Cajal for providing the image of the first autopsy performed in the hospital.
Financial support and sponsorship
none.
Conflicts of interest
none.
Presentation
Poster presentation at eCOVID-19 Spanish national congress, May 2020.
References
- 1.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46: 1099-1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan E, Beitler JR, Brochard L, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med 2020; 8: 816-821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim ZJ, Subramaniam A, Ponnapa Reddy M, et al. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation: a meta-analysis. Am J Respir Crit Care Med 2021; 203: 54-66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al.; COVID-19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323: 1574-1581. doi: 10.1001/jama.2020.5394. Erratum In: JAMA 2021; 325: 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al.; COVID-19 Spanish ICU Network . Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med 2020; 46: 2200-2211. doi: 10.1007/s00134-020-06192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995; 107: 1062-1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 7.Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest 1995; 107: 196-200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- 8.Papazian L, Doddoli C, Chetaille B, et al. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med 2007; 35: 755-762. doi: 10.1097/01.CCM.0000257325.88144.30. [DOI] [PubMed] [Google Scholar]
- 9.Cabrera-Benitez NE, Laffey JG, Parotto M, et al. Mechanical ventilation-associated lung fibrosis in acute respiratory distress syndrome: a significant contributor to poor outcome. Anesthesiology 2014; 121: 189-198. doi: 10.1097/ALN.0000000000000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haudebourg AF, Perier F, Tuffet S, et al. Respiratory mechanics of COVID-19-versus non-COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 202: 287-290. doi: 10.1164/rccm.202004-1226LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbeta E, Motos A, Torres A, et al.; Covid Clinic Critical Care Group :. SARS-CoV-2-induced acute respiratory distress syndrome: pulmonary mechanics and gas-exchange abnormalities. Ann Am Thorac Soc 2020; 17: 1164-1168. doi: 10.1513/AnnalsATS.202005-462RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schenck EJ, Hoffman K, Goyal P, et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc 2020; 17: 1158-1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziehr DR, Alladina J, Petri CR, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med 2020; 201: 1560-1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchioni A, Tonelli R, Rossi G, et al. Ventilatory support and mechanical properties of the fibrotic lung acting as a “squishy ball”. Ann Intensive Care 2020; 10: 13. doi: 10.1186/s13613-020-0632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin MJ, Moua T. Mechanical ventilation and predictors of in-hospital mortality in fibrotic interstitial lung disease with acute respiratory failure: a cohort analysis through the paradigm of acute respiratory distress syndrome. Crit Care Med 2020; 48: 993-1000. doi: 10.1097/CCM.0000000000004366. [DOI] [PubMed] [Google Scholar]
- 16.COVID-19 Autopsy . Electronic address: anapat.hrc@salud.madrid.org. The first COVID-19 autopsy in Spain performed during the early stages of the pandemic. Rev Esp Patol 2020; 53: 182-187. doi: 10.1016/j.patol.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matamis D, Lemaire F, Harf A, Brun-Buisson C, Ansquer JC, Atlan G. Total respiratory pressure-volume curves in the adult respiratory distress syndrome. Chest 1984; 86: 58-66. doi: 10.1378/chest.86.1.58. [DOI] [PubMed] [Google Scholar]
- 18.Nunes S, Valta P, Takala J. Changes in respiratory mechanics and gas exchange during the acute respiratory distress syndrome. Acta Anaesthesiol Scand 2006; 50: 80-91. doi: 10.1111/j.1399-6576.2005.00767.x. [DOI] [PubMed] [Google Scholar]
- 19.Li Bassi G, Suen JY, Dalton HJ, et al.; COVID-19 Critical Care Consortium . An appraisal of respiratory system compliance in mechanically ventilated covid-19 patients. Crit Care 2021; 25: 199. doi: 10.1186/s13054-021-03518-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandenbunder B, Ehrmann S, Piagnerelli M, et al.; COVADIS study group . Static compliance of the respiratory system in COVID-19 related ARDS: an international multicenter study. Crit Care 2021; 25: 52. doi: 10.1186/s13054-020-03433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panwar R, Madotto F, Laffey JG, van Haren FMP. Compliance phenotypes in early acute respiratory distress syndrome before the COVID-19 pandemic. Am J Respir Crit Care Med 2020; 202: 1244-1252. doi: 10.1164/rccm.202005-2046OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beloncle FM, Pavlovsky B, Desprez C, et al. Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care 2020; 10: 55. doi: 10.1186/s13613-020-00675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGroder CF, Zhang D, Choudhury MA, et al. Pulmonary fibrosis 4 months after COVID-19 is associated with severity of illness and blood leucocyte telomere length. Thorax 2021: thoraxjnl-2021-217031. doi: 10.1136/thoraxjnl-2021-217031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zou JN, Sun L, Wang BR, et al. The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT. PLoS One 2021; 16: e0248957. doi: 10.1371/journal.pone.0248957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J 2020; 55: 2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus-induced pulmonary fibrosis. Antiviral Res 2017; 143: 142-150. doi: 10.1016/j.antiviral.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome. Association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med 1995; 122: 17-23. doi: 10.7326/0003-4819-122-1-199501010-00003. [DOI] [PubMed] [Google Scholar]
- 28.Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med 2000; 162: 1783-1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 29.Pierrakos C, Vincent JL. The changing pattern of acute respiratory distress syndrome over time: a comparison of two periods. Eur Respir J 2012; 40: 589-595. doi: 10.1183/09031936.00130511. [DOI] [PubMed] [Google Scholar]
- 30.Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med 2017; 45: 253-262. doi: 10.1097/CCM.0000000000002074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr 2006; 83: 461S-465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 32.Vardhana SA, Wolchok JD. The many faces of the anti-COVID immune response. J Exp Med 2020; 217: e20200678. doi: 10.1084/jem.20200678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha P, Furfaro D, Cummings MJ, et al. Latent class analysis reveals COVID-19-related ARDS subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med 2021. doi: 10.1164/rccm.202105-1302OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idio-pathic pulmonary fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016; 194: 265-275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 35.Meduri GU, Golden E, Freire AX, et al. Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 2007; 131: 954-963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 36.Villar J, Ferrando C, Martínez D, et al.; dexamethasone in ARDS network . Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med 2020; 8: 267-276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 37.RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384: 693-704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg KP, Hudson LD, Goodman RB, et al.; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network . Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006; 354: 1671-1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 39.Takaki M, Ichikado K, Kawamura K, Gushima Y, Suga M. The negative effect of initial high-dose methylprednisolone and tapering regimen for acute respiratory distress syndrome: a retrospective propensity matched cohort study. Crit Care 2017; 21: 135. doi: 10.1186/s13054-017-1723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY MATERIAL


