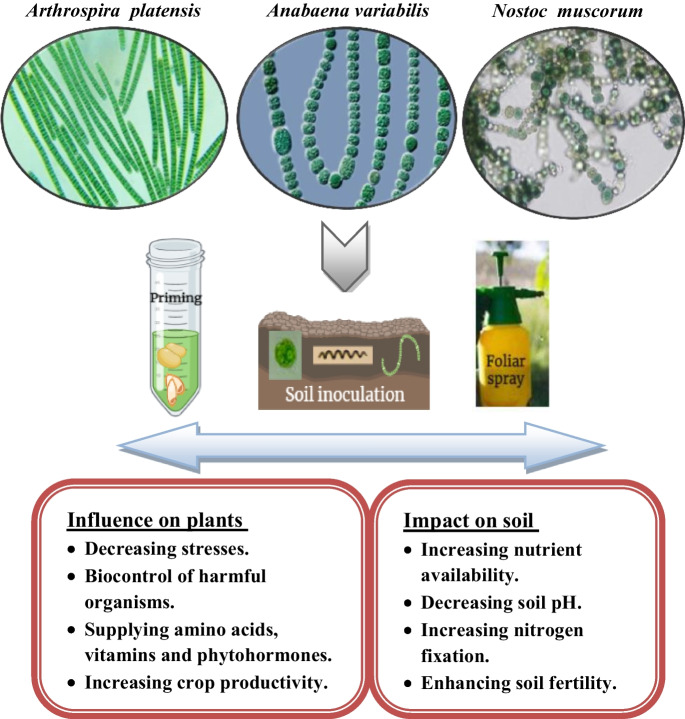
Abstract
Taking into consideration, the challenges faced by the environment and agro-ecosystem make increased for suggestions more reliable methods to help increase food security and deal with difficult environmental problems. Environmental factors play a critical role in the growth, development, and productivity of crop plants. Unfavorable changes in these factors, such as abiotic stresses, can result in plant growth deficiencies, yield reductions, long-lasting damage, and even death of the plants. In reflection of this, cyanobacteria are now considered important microorganisms that can improve the fertility of soils and the productivity of crop plants due to their different features like photosynthesis, great biomass yield, ability to fix the atmospheric N2, capability to grow on non-arable lands, and varied water sources. Furthermore, numerous cyanobacteria consist of biologically active substances like pigments, amino acids, polysaccharides, phytohormones, and vitamins that support plant growth enhancement. Many studies have exposed the probable role of these compounds in the alleviation of abiotic stress in crop plants and have concluded with evidence of physiological, biochemical, and molecular mechanisms that confirm that cyanobacteria can decrease the stress and induce plant growth. This review discussed the promising effects of cyanobacteria and their possible mode of action to control the growth and development of crop plants as an effective method to overcome different stresses.
Graphical Abstract
Keywords: Abiotic stress, Molecular mechanisms, Plants, Phytohormones, Soils
Introduction
The world exerts continuous efforts to end hunger, food instability, and undernourishment in all its forms in 2030, as reported by the latest 2022 State of Food Security and Nutrition in the World (SOFI) report (FAO et al. 2022(. About 828 million people suffered from hunger globally in 2021 (around 10.5% of the world population) which increased by 46 million in 2020 and 150 million since the COVID-19 pandemic was initiated. Africa has the heftiest local encumbrance. One in five people, or 20.2% of the population in Africa, starved, as compared to 9.1% in Asia, 8.6% in Latin America and the Caribbean, 5.8% in Oceania, and 2.5% in Northern America and Europe together (FAO et al. 2022(. Also, the continuous increase in the worldwide population caused an augmented request for different food supplies (El-shenody et al. 2023; Pathak et al. 2018; Ronga et al. 2019), besides, the reduction in available area for food crop production, infrequent water resources, accretion of xenobiotic compounds in the soils; and deficient soil quality. So, the main issues facing hunger are agricultural maintenance challenges, environmental subsidies, and climate change.
The present agricultural applications are deeply reliant on the application of synthetic chemicals such as fertilizers and pesticides, both as plant growth stimulators and as an agent for protecting plants from different stress conditions, and that facilitated many developing countries to meet the food necessity of their people (Ashour et al. 2021, 2023; Dmytryk and Chojnacka, 2018; Hassan et al. 2021).
Unfortunately, The repetition of using chemicals in agriculture may accumulate in the plants and soil, releasing and forming environmentally harmful products that can be a danger to humans (Pan et al. 2019). For example, about 50% of the useful nitrogen fertilizer is essentially used by plants, and the remaining 50% causes damage in surface waters (Collos and Harrison, 2014). So, it is a big challenge to available the food requirements of the population with limited resources and without worsening the environmental quality (Singh and Strong, 2016).
Cyanobacteria are an ideal solution for this problem due to their environment-friendly and low-cost farming. Since thousands of years ago, cyanobacterial biomass has been widely used in agriculture, but in the twentieth century, increased beneficial products obtained from cyanobacterial extracts have attracted the attention of farmers worldwide (Kumar et al. 2022; Pathak et al. 2018). As a result, researchers have focused their efforts on biologically-based products such as cyanobacteria, which have been evaluated as crop protection agents as well as for their bio-stimulating potential (Górka et al. 2018).
Cyanobacteria are prokaryotic microorganisms, and some are heterotrophs, capable of using various sources of carbon and organic nitrogen (Pham et al. 2017). It can be tailored to many environmental changes due to its diversity in morphological characteristics (Singh et al. 2014). An additional feature that makes algae and cyanobacteria more appropriate is that they do not need arable land for their growth. Cyanobacteria can be alive in the desert, in contaminated and stressed soils, and in other excessive environmental conditions (Rossi et al. 2017). Furthermore, they could grow with high productivity on remaining nutrients while supplementing yields of lipids (20–65% of dry weight), proteins, total fibers (33–50% higher than plants), and carbohydrates (El Shafay et al. 2021; Guihéneuf et al. 2016).
Furthermore, it can produce several bioactive compounds that can stimulate crop growth/protect and improve the soil nutrient status. Cyanobacteria are also beneficial for wastewater management and have the capability to break down numerous toxic compounds even pesticides (Cohen, 2006).
This review focused on providing an overview of cyanobacterial roles in the improvement of plant growth and yield depending on their biostimulants. In addition, it explains their roles in the alleviation of abiotic stress conditions on crop plants.
Cyanobacteria Growth and Extraction
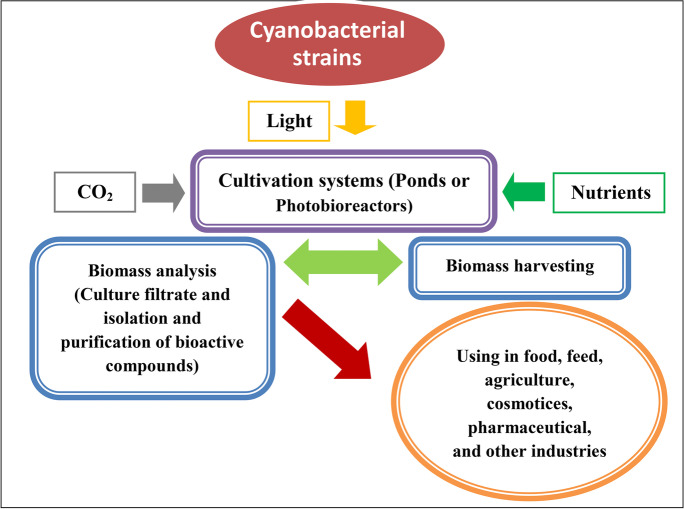
Cyanobacteria grow earlier than plants and are characterized by a simple genetic system and a high yield of biomass and metabolites (Wijffels et al. 2013). Good results for their products are dependent on the strain chosen and the effectiveness of the cultivation system (Balasubramaniam et al. 2021). Several basic parameters adjust the growth of the strains, including (1) light intensity, (2) pH, (3) gas exchange, (4) nutrient source, and (5) light/dark cycling (Balasubramaniam et al. 2021).
Cyanobacteria mass production might be achieved by two basic systems: open reservoirs (ponds or tanks), or closed containers (photobioreactors), which are supported by natural or artificial illumination (Fig. 1).
Fig. 1.
Cyanobacterial strains’ culture, harvesting, and downstream use
Biostimulants (Biomolecules) from Cyanobacteria
The possible applications of cyanobacteria embrace agriculture, wastewater management, bioenergy, and bioactive value-added compounds (Odjadjare et al. 2017). According to the literature, cyanobacterial metabolites can play an important role in soil decontamination and fertilization, plant protection against biotic and abiotic stress factors, and plant development (Górka et al. 2018; Ronga et al. 2019). Besides their role in adding organic nitrogen and complementary mineral nutrition to the soil, they are known as sources of numerous biologically active compounds and secondary metabolites including phytohormones (auxins, gibberellins, and cytokinins) that can be used in biotechnological and industrial fields (Guihéneuf et al. 2016). These bioactive substances can affect plant gene expression and encourage the accumulation of a wide range of compounds that help in the stimulation of plant growth and protection against biotic and abiotic stress (Han et al. 2018; Pan et al. 2019) (Table 1). Cyanobacteria are generally gaining attention as plant-growth-promoting and biocontrol agents in diverse crops, including rice, wheat, cotton, and legumes (Prasanna et al. 2015). The inoculation of these organisms influences various metabolic processes in plants since they activate the production of defense proteins and enzymes that lead to a greater immunity of plants against pathogens (Gonçalves, 2021). It is known that the inoculation of cyanobacteria directly in the soil or by seed engagement or priming causes an increase in the germination rate, better development of plants, and a higher production yield in a wide variety of cereal, horticultural, and vegetable crops (Singh et al. 2017; Toribio et al. 2021). From what was mentioned above, cyanobacteria are considered platforms for the potential development of products for soil improvement and crop production and protection, such as biofertilizers, organic fertilizers, biostimulants, and biocontrol agents as summarized in Table 2.
Table 1.
Cyanobacterial strains metabolites (Kollmen and Strieth, 2022)
| Class | Metabolites | Cyanobacterial strains |
|---|---|---|
| Phytohormones | Auxins, abscisic acid, cytokinins, gibberellins, ethylene |
Anabaena sp., Nostoc sp., Oscillatoria sp., Phormidium sp., Rhodospirillum sp., Scytonema sp., Synechocystis sp., and Westiellopsis prolifica |
| Phenolic compounds |
Flavonoids, phenolic acids, cell wall phenolics |
Anabaena sp., Arthrospira sp., Calothrix, Chroococcidiopsis, Leptolyngbya, Nostoc sp., Oscillatoria, Phormidium |
| Terpenoids |
Isoprene, limonene, β-phellandrene, linalool, farnesene, bisabole |
Anabaena ap., Synechocystis sp., Synechococcus sp. |
| Carotenoids |
β-carotene, astaxanthin, canthaxanthin, zeaxanthin, lutein, lycopene, phytoene, echinenone |
Anabaena sp., Cylindrospermum sp., Microcystis sp., Nostoc sp., Oscillatoria sp., Phormidium sp., Synechococcus sp., Spirulina sp., Tolypothrix sp. |
| Peptides |
Peptides, a free amino acids, proteins |
Aphanizomenon flos-aquae, Calothrix ghosei, Cylindrospermum musciola, Hapalosiphon intricatus, Microcystis aeruginosa, Nostoc muscorum, Nostoc sp., |
| Polysaccharides |
β-glucans, chitin, lipopolysaccharides, carrageenan |
Arthrospira platensis, Nostoc muscorum, Cylindrospermum musciola |
| Vitamins |
Riboflavin, ascorbic acid, thiamine, cobalamine, pyridoxine, nicotinic acid, folic acid, phenothene |
Anabaena sp., Chroococcus mimulus, Microcystis pulverana, Nostoc sp., Nostoc muscorum, Oscillatoria jasorvensis, Phormidium bijugatum, Arthrospira |
Table 2.
Cyanobacterial biomass and/or extracts’ main known activities in crop plants (Gonçalves, 2021)
| Type | Mode of action | Influence on crops |
|---|---|---|
| Biostimulants | Plants | Protection and production |
| Biofertilizers | Soils | Nutrition |
| Biopesticides | Pathogenic organisms | Protection |
Taking into account their potential benefits for the development of workable agriculture, both biomass, and extracts from cyanobacteria are commercially available on the market (Górka et al. 2018), as abridged in Table 3.
Table 3.
Some common cyanobacterial biostimulants are currently on the market (Gonçalves, 2021)
| Commercial name | Species | Mode of application |
|---|---|---|
| Spiragro Spiragrow | Arthrospira platensis | Foliar and radical |
| Floralgal Algafert | Arthrospira sp. | Foliar and radical |
| Shwe Awzar | Arthrospira sp. | Radical-soil conditioner |
| Microp | Unspecified cyanobacteria | Radical-soil conditioner |
| Agrialgae Phycoterra | Unspecified cyanobacteria | Foliar and radical |
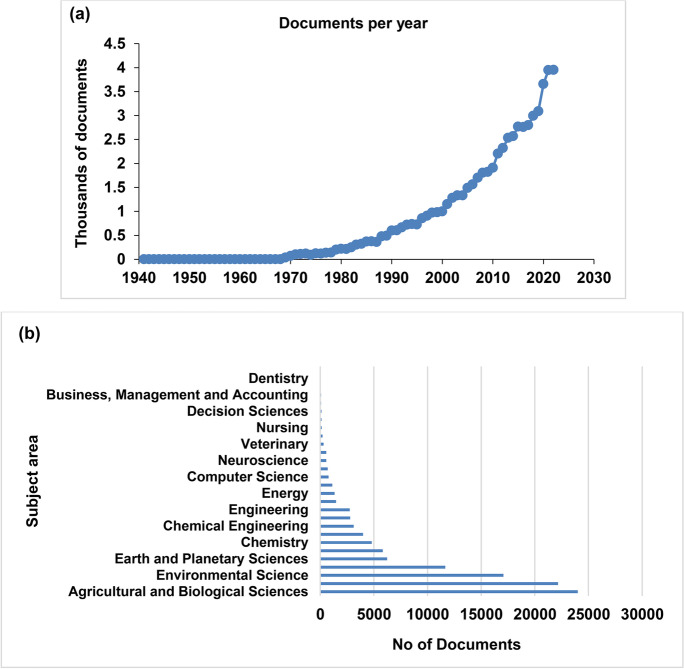
The attention to cyanobacteria is growing, as revealed by several studies in many fields (Fig. 2a) like Biological Sciences, Agricultural, Biochemistry, Environmental Science, Genetics and Molecular Biology, Immunology, and Microbiology (Fig. 2b).
Fig. 2.
Cyanobacteria by year (a) and by subject area (b) appraised in the Scopus® database until 2022 (updated to February 2023)
Role of Cyanobacteria in Agriculture
Plant Growth and Yield Improvement
Cyanobacteria can be applied to monocot and dicot crops as biofertilizers to raise plant growth and crop yield. Cyanobacteria as biofertilizers are inexpensive, they cost one-third of the price of chemical fertilizers (Prasanna et al. 2013). Nostoc and Anabaena are familiar genera for plant growth promotion (Prasanna et al. 2016). They can supply nitrogen to the plant by using nitrogenases enzyme to fix atmospheric nitrogen (Meeks and Elhai, 2002).
This helps plants to grow without nitrogen-deficient soils and eliminates the need to use expensive nitrogen fertilizers, which are frequently a source of pollution (Li et al. 2019). In the same context, Osman et al. (2020) reported that treatment of broad bean seeds with biomass of Nostoc muscorum induced growth parameters of root length (30%), shoot length (44%), root fresh weight (2-fold), shoot fresh weight (1.5-fold), root dry weight (67%), and shoot dry weight (1.6-fold). Photosynthetic pigment, carbohydrate, and protein contents were also increased by 52%, 20%, and 1.7-fold, respectively. Moreover, algal treatments improved the activity of antioxidant enzymes (peroxidase and catalase) and were accompanied by a decline in lipid peroxidation (Osman et al. 2020). In another study, Spirulina platensis was applied to radish (Raphanus sativus) with its filtrate as seed soaking and foliar spray in addition to its homogenate as a seed coating treatment. Spirulina increased growth, chlorophyll, and element content as compared to the control of radish seedlings (Godlewska et al. 2019). Crude bio-extracts obtained from 18 cyanobacteria and microalgae species were recommended as biostimulants for plant growth, nutrient uptake, chlorophyll content, and metabolite profiles of the tomato plant (Mutale-Joan et al. 2020). The authors detected that root and shoot lengths of the plant were significantly developed by 112.65% and 53.70% in response to Aphanothece sp treatment, respectively. In the meantime, the uptake of nitrogen, phosphorus, and potassium was amplified by 185.17%, 119.36%, and 78.04%, respectively, compared with the control plants. In the same manner, Osman et al. (2021) reported that priming wheat grains in Arthrospira platensis and Nostoc muscorum aqueous extract caused wheat growth stimulation and improvement in various morphological growth parameters. In addition, the biochemical content of pigments, carbohydrates, and proteins was boosted in the wheat seedlings. Moreover, using Anabaena laxa and Anabaena rhizobium caused an increase in yield by 104% and the nitrogen content by 50% in chickpea (Cicer arietinum L.) plants as compared to control plants (Bidyarani et al. 2016). In another experiment, Anabaena laxa amplified the yield by up to 39% and the protein content by 11% for pea plants (Prasanna et al. 2017).
In recent years, cyanobacterial polysaccharides have been applied to plants and stimulants for their properties and signaling capabilities (Elarroussi et al. 2016; Farid et al. 2019). A. platensis crude polysaccharides extract was applied to tomatoes and peppers by foliar spraying. The treatments amplified shoot dry weight by 1.4-fold in both species of plants, while the positive effects on root weight were much more noticeable in tomatoes (2.30-fold) than in peppers (67%) (Elarroussi et al. 2016). Crude polysaccharide extracts can hold other bioactive metabolites that may contribute to the experimental effects. For example, crude polysaccharides extracted from Phormidium tenue, consisting of 58% carbohydrates and 15% proteins have been shown to induce growth and superoxide dismutase activity in seedlings of the shrub Caragana korshinskii (Xu et al. 2013). Besides, it has been revealed that crude polysaccharides extracted from A. platensis contain phenolic compounds (about 45 mg gallic acid equivalent to g−1 of biomass), which show antioxidant activities in plants (Chaiklahan et al. 2013). Together, these findings support the hypothesis that cyanobacterial polysaccharides might be an active source of plant biostimulants for crop enhancement and protection against abiotic stresses (Elarroussi et al. 2016). Also, several metabolic pathways, such as photosynthesis and nitrate assimilation, seem to be affected by treatments with cyanobacterial polysaccharides. A gas chromatography–mass spectrometry (GC-MS) metabolomic examination also showed an increase in phytosterols (Farid et al. 2019). The rise in plant sterols might lead to the production of brassinosteroids, a group of oxidized steroids with hormonal activities accountable for increasing the effectiveness of photosynthetic carbon fixation and avoiding damage to photosynthetic pigments through stresses (Siddiqui et al. 2018). Table 4 listed some studies that exposed the beneficial roles of using cyanobacterial species in the growing of economic crops.
Table 4.
The beneficial roles of different cyanobacterial strains on plants
| Cyanobacterial strains | Plant | Beneficial role | Reference |
|---|---|---|---|
| Aphanothece sp | Tomato | Amplified uptake of nitrogen, phosphorus, and potassium by 185.17%, 119.36%, and 78.04%, respectively | (Mutale-Joan et al. 2020) |
| Anabaena laxa | Chickpea | 50 % higher grains yield | (Bidyarani et al. 2015) |
| Anabaena sp., Anabaena doliolum, Nostoc carneum and Nostoc piscinale | Maize | Yields were increased with 20–30% increases in all inoculated treatments | (Prasanna et al. 2016) |
| Nostoc muscorum | Barley | Increased in proteins (45.95%), amino acids (39.13), and nutrient content [N (28.4%), K (24.3%), Ca (12.9%), Mg (29.06%), and Fe (13.8%) | (Abo-Shady et al. 2018) |
| Nostoc muscorum | Broad bean | Induced growth parameters of root length (30%), shoot length (44%), root fresh weight (2-fold), shoot fresh weight (1.5-fold), root dry weight (67%), and shoot dry weight (1.6-fold) | (Osman et al. 2020) |
| Aphanothece sp | Tomato | Root and shoot lengths of the plant were significantly developed by 112.65% and 53.70%, respectively | (Mutale-Joan et al. 2020) |
| Arthrospira Platensis | Tomatoes and peppers | Induced shoot dry weight by 1.4-fold in both species of plants, while the positive effects on root weight were much more in tomatoes (2.30-fold) than in peppers (67%) | (Elarroussi et al. 2016) |
| Anabaena laxa | Pea | Increased the yield by up to 39% and the protein content by 11% | (Prasanna et al. 2017) |
Cyanobacteria as Phytoremediation Tool
Agricultural practices have influenced the environment's air, water, and soil. These environmental factors depend on factors like location and management practices used, such as fertilizers application. Also, adding nutrients and pesticides can cause runoff from agricultural fields into surface water and groundwater (Abdelsalam et al. 2019; Rohila et al. 2017). Biological treatment with cyanobacteria to remove pollutants is called (Bioremediation). Cyanobacteria could be used for the bioremediation of numerous organic pollutants like heavy metals, pesticides, surfactants, phenol, and catechol (Kumar et al. 2016; Singh et al. 2016) using several ways. Their capability to remove pollutants results from their high photosynthetic activity, which offers huge biomasses. These biomasses consist of numerous bioactive substances that could be used in many useful applications (Gonçalves, 2021). Furthermore, many species of cyanobacteria can grow best in contaminated environments, such as Oscillatoria limosa, Oscillatoria tenuis, Oscillatoria princeps, Anabaena torulosa, Nostoc sp., and Phormidium uncinatum (Gonçalves, 2021). Likewise, Anabaena flosaquae had the highest absorption ability for pollutants as compared to Microcystis aeruginosa (Corpuz et al. 2021).
Methods of Bioremediation
Cyanobacteria can accumulate organic or inorganic toxic substances and produce several detoxifying mechanisms, like bioaccumulation, biosorption, biotransformation, and biodegradation (Mondal et al. 2019). Biosorption is a physicochemical method that depends on different factors such as absorption, adsorption, surface area, ion exchange, and precipitation. Biosorption is a perfect method for the removal of many pollutants (phenolic compounds, heavy metals, herbicides, and pesticides). Moreover, it is friendly to the environment and a cheaper method for removing pollutants (Gadd, 2014). Cyanobacterial biodegradation happens extracellularly, intracellularly, or both, where the primary degradation arises extracellularly (Ventura et al. 2017). The amount of removal and biodegradation of pollutants are influenced by their concentration, cyanobacterial biomass, species, growth phase, and environmental conditions (Abou-El-Souod and El-Sheekh, 2016).
Heavy Metals and Organic Pollutant Removal
Heavy metals are certainly found in the environment at actual low levels, but due to human activities and industrial waste their concentration in the environment has extremely amplified. Heavy metals are very dangerous toxicants for living organisms thus they can bind to some cellular components, counting enzymes, proteins, and nucleic acids, and upset their normal functions (Abdelsalam et al. 2019; Das et al. 2008). Cyanobacteria can remove numerous heavy metals such as cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), and zinc (Zn) (Das et al. 2008) (Table 5).
Table 5.
Heavy metal, organic pollutant, and pesticides removal via some species of Cyanobacteria
| Heavy metals | Cyanobacteria | Removal percent | References |
|---|---|---|---|
| Cd | Nostoc linckia and Nostoc rivularis | 2–10-fold | (El-Enany and Issa, 2000) |
| Zn | Nostoc linckia and Nostoc rivularis | 10–30-fold | (El-Enany and Issa, 2000) |
| Cu, Co, Pb, and Mn | Nostoc muscorum and Anabaena subcylindrica | 12.5–81.8, 11.8–33.7, 26.4–100, and 32.7–100%, respectively | (El-Sheekh et al. 2005) |
| Zn | Spirulina platensis | 87%, 80%, and 70.5% when its initial concentration was 0.5, 1, and 2 mg/L, respectively | (Meng et al. 2012) |
| Cr | Nostoc PCC7936 | 50% | (Colica et al. 2010) |
| Sr | Gloeomargarita lithophora and Cyanothece sp | – | (Cam et al. 2016) |
| Organic pollutants | Cyanobacteria | References | |
| Phenol | Ochromonas danica | 65% | (Semple and Cain, 1996) |
| Diesel 99.5% (0.6% v/v) |
Phormidium sp., Oscillatoria sp., and Chroococcus sp. |
94% | (Chavan and Mukherji, 2008) |
|
Total petroleum hydrocarbon 99% (diesel 0.6% v/v) |
Phormidium sp., Oscillatoria sp., and Chroococcus sp. |
99% | (Chavan and Mukherji, 2010) |
| Phenanthrene |
Selenastrum capricornutum and Microcystis aeruginosa |
96–100% |
(Chan et al. 2006 and Bai et al. 2016) |
| Dimethyl phthalate |
Synechocystis sp. PCC6803 Synechococcus sp. PCC7942, and Cyanothece sp. PCC7822 |
11.8% | (Zhang et al. 2016) |
| Pesticides | Cyanobacteria | References | |
| Glyphosate (H) | Anabaena sp., and Nostoc sp | – | (Forlani et al. 2008) |
| Chlorpyrifos (I) | Spirulina platensis and Spirulina maxima | 60% | |
| Carbofuran (I) | Nostoc hatei | 12% | (Jha and Mishra, 2005) |
| Fluroxypyr (H) | Chlamydomonas reinhardtii | 57% | (Zhang et al. 2011) |
| Mancozeb (F) | Nostoc ellipsosporum, and Tolypothrix tenuis | – | (Barton et al. 2004) |
Heavy metals like Cd in soils can be prevented from translocation from roots to shoots as a response to seed priming in Spirulina platensis and can also stimulate seed germination and improve maize plant growth (Seifikalhor et al. 2020). Additionally, Limnococcus sp., Nostoc muscorum, and Synechococcus sp. can remove a wide variety of heavy metal ions like Cu, Ni, Pb, Cd, Zn, and Co (Al-Amin et al. 2021). In the same manner, Cyanobacterial can remove different organic compounds from different systems (Table 5). Likewise, Lyngby lagerlerimi, Nostoc linkia, and Oleria rubescens have been widely used to remove phenolic pollutants (El-Sheekh et al. 2012). Also, Spirulina maxima can degrade phenolic compounds, which are reflected as very toxic pollutants in the USA (Ebele et al. 2017).
Pesticides and Herbicides Removal
Pesticides contaminated soils and waters due to their bio-accumulative and persistent nature (Mastovska and Wylie, 2012). Their presence is harmful to plants, ecosystems, drinking water, and human health (Mastovska and Wylie, 2012). Pesticides are one of the most vital agricultural involvements that affect the quality and production of crop plants. It can be classified into herbicides (weed killers), fungicides (fungal destroyers), nematicides (nematode killers), insecticides (insect killers), and rodenticides (vertebrate poisons) (de Souza et al. 2020). In contrast, their excessive use and toxic properties make them a major issue for public health and the environment (Nicolopoulou-Stamati et al. 2016). Moreover, pesticides, accidental or highly occupational, result in many side effects such as dermatological, neurological, carcinogenic, respiratory, and reproductive, which lead finally to hospitalization and death (Thakur et al. 2014). An earlier study that reflects the beneficial role of cyanobacteria as Anabaena sp., Nostoc sp., and Arthrospira sp. can use glyphosate herbicides as a source of phosphorus, which helps in the removal of this herbicide from polluted soil (Forlani et al. 2008). Several cyanobacterial species were used to bioremediate toxic pesticides Table 5. Also, it has been found that Synechocystis sp. and Phormidium sp. can bioabsorb and remove the insecticide imidacloprid from the soil (Aminfarzaneh and Duygu, 2010). In the same way, Scytonema hofmanni and Fischerella sp. can remove the insecticide methyl parathion (Tiwari et al. 2017). In herbicide situations, priming faba bean seeds in Spirulina platensis stimulates the production of some amino acids that can protect them from the adverse effects of the fusillade herbicide on the plants (Osman et al. 2016). Besides, Microcystis aeruginosa was found to break down phenyl urea herbicides (Bayazıt et al. 2020). Moreover, Spirulina sp., Westiellopsis sp., and Oscillatoria sp. are recorded as the most generally used cyanobacteria for wastewater treatment (Das et al. 2017).
I insecticide, F fungicide, H herbicide
Cyanobacteria Protection Against Plant Abiotic Stresses
Effect of Different Abiotic Stresses on Crop Plants
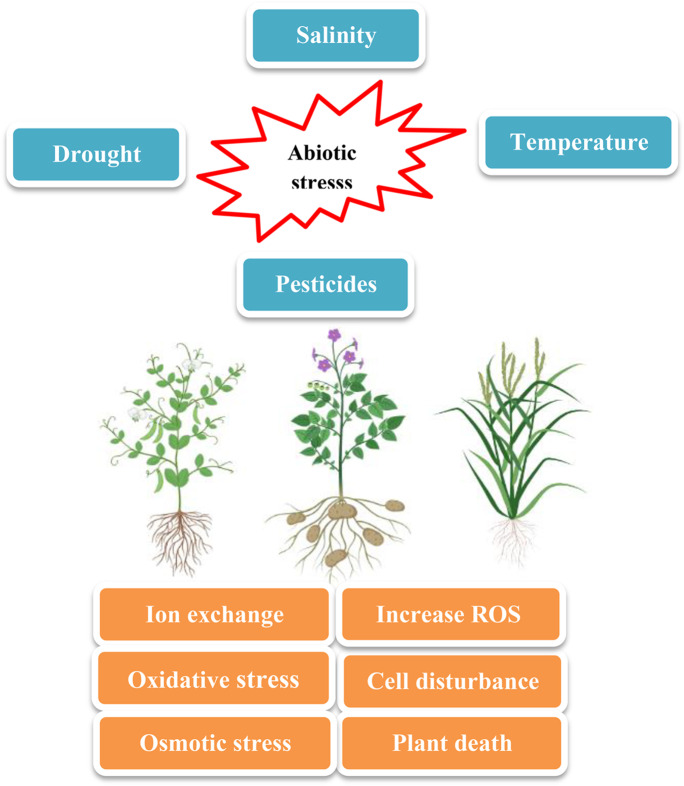
Plant stress can be classified into several types by numerous factors, including the type of stress (biotic and abiotic), the effect of the stress, and the persistence of the stress (short-term and long-term stresses) (Kranner et al. 2010). Alternatively, plant stresses can be classified into internal stresses (that come from within the plant) and external stresses (that exist outside the plant). When one or more stresses change the optimal conditions of the plant, it uses a special mechanism called “stress sensing” to detect this variation. When one or more stresses change the optimal conditions of the plant, it uses a special mechanism called “stress sensing” to detect this variation. There are four main phases: (1) alarm, (2) resistance, (3) exhaustion, and (4) regeneration phases of plant stress sensing and response based on the duration and intensity of the stress (Kranner et al. 2010). Stress resistance depends on species, genotype, age of the plant, tissue identity, duration, severity, and rate of stress. Many abiotic stresses such as salinity, temperature, drought, and pesticides (ex: herbicides) are established in plants as osmotic stresses, leading to the accumulation of reactive oxygen species (ROS) that harm carbohydrates, proteins, lipids, DNA, and also cause abnormal cell signaling (Mala et al. 2017) (Fig. 3). Abiotic stresses result in about half of all yield losses. For example, high temperatures (20%), low temperatures (7%), salinity (10%), drought (9%), and all other abiotic stresses account for about 4% (Ningombam et al. 2021).
Fig. 3.
Effect of abiotic stress on crop plants
Cold and Heat Stress
Cold and heat stress are preventive factors for stimulating crops (Ruelland et al. 2009). Low-temperature stress disturbs plant reproductive stages, causing late flowering and pollen production which causes crop yield reduction (Yadav, 2010) (Table 6). Besides that, cold stress-stimulated membrane dehydration (Saito and Matsuda, 2010). Likewise, Jatropha curcas is a key bioenergy crop, but it cannot yield biofuel under cold stress (Wang et al. 2018). Low-temperature stress not only declines grain yield but also upsets crop grain value. Also, Atayee and Noori (2020) observed that cold stress induced the ability to injury and death of many plants through a variety of physiological disruptions. As reported by Joshi et al. (2016), not only low temperatures but also high temperatures caused losses in grain weight and injury during grain developmental stages. In the same manner, the biosynthesis of phenolics, proline, secondary metabolites, and grain growth was inhabited as a result of heat stress (Kamal et al. 2017).
Table 6.
Effect of different abiotic stresses on crop plants
| Crop plants and stress | Effects | Reduction percent | References |
|---|---|---|---|
|
Low temperatures Wheat Barley Canola Chickpea Field pea |
Decrease grains yield and quality | – | |
|
High temperatures Wheat |
losses in grain weight and injury during grain developmental stages | Tiller number (70%), grain yield (67.3%), 1000-grain weight (47%), and vigour Index II (52.7%) | (Joshi et al. 2016) |
|
High temperatures Cotton |
Biosynthesis of phenolics, proline, secondary metabolites, and grain growth was inhabited | – | (Kamal et al. 2017) |
|
Salinity Maize |
Decreased in germination rate, root length, and shoot length | 32%, 80%, and 78% in germination rate, root length, and shoot length, respectively, | Khodarahmpour et al. (2011) |
|
Drought Isatis indigoticaFort |
Decreased chlorophyll content | 31% | (Hao et al. 2013) |
|
Drought Legumes grain |
Destroys many stages of development, especially the generation and function of reproductive organs | 27 to 87% | (Farooq et al. 2017) |
|
Drought Ornamental shrubs |
Reduction in plant physiological, metabolic, enzymatic, and grain yield processes | 50% | (Toscano et al. 2016) |
|
Glyphosate herbicide Dimorphandra wilsonii |
Simulated ROS that leads to a reduction in the germination of seeds by dropping the rates of seed respiration | – | (Gomes et al. 2017) |
|
Roundup herbicide Maize |
Setback the seed germination process | – | (Gomes et al. 2019) |
Salinity and Drought Stress
Salinity is one of the most abiotic stresses that affect plant growth and productivity (Table 6). Khodarahmpour et al. (2011) reported that in maize crops there was a reduction of 32%, 80%, and 78% in germination rate, root length, and shoot length, respectively, as a response to salinity stress. Panuccio et al. (2014) noted that shoot length and root length were significantly reduced in the response to the salinity stress. In the same manner, increased salinity had side effects on sorghum production and yield components (Shakeri et al. 2017). Drought stress can happen at any stage of plant production. Normal plant growth phases are very sensitive to soil moisture, which significantly caused a decline in crop yield (Krishnamurthy et al. 2010). Hao et al. (2013) noted that drought-stressed plants decreased chlorophyll content by 31% as compared to control plants. Also, Farooq et al. (2017) observed that drought destroys many stages of development, especially the generation and function of reproductive organs, by 27 to 87%. Moreover, drought stress caused a 50% reduction in plant physiological, metabolic, enzymatic, and grain yield processes (Toscano et al. 2016).
Herbicides Stress
Although herbicides can help in increasing crops' yield, not all herbicides can get to their targets, and an extensive part of them can remain in soils or be absorbed by other non-target plants (Parween et al. 2016). Repetitive use of one or more herbicides with the same mode of action can result in the generation of resistance in the weed population (Sharma et al. 2019). For example, Gomes et al. )2017) reported that Glyphosate simulated ROS in Dimorphandra wilsonii leads to a reduction in the germination of seeds by dropping the rates of seed respiration. Another formulation of glyphosate herbicides (Roundup) may setback the seed germination process of maize (Gomes et al. 2019). Similarly, Subedi et al. (2017) experimented with glyphosate herbicide on Lens culinaris L. and they established that seed germination declined as compared to the control. In the same context, Wang et al. (2018) reported that the application of herbicides can affect plant growth and progress and result in yield reduction. Herbicides stimulate reactive oxygen species (ROS) as a secondary effect when applied to plant cells (Caverzan et al. 2019), which react to different cell consistent (like proteins, lipids, pigments, and nucleic acids) and finally trigger lipid peroxidation, membrane damage, and enzyme activity disturbance (Singh et al. 2017) (Table 6).
Plants Detoxification Defense System
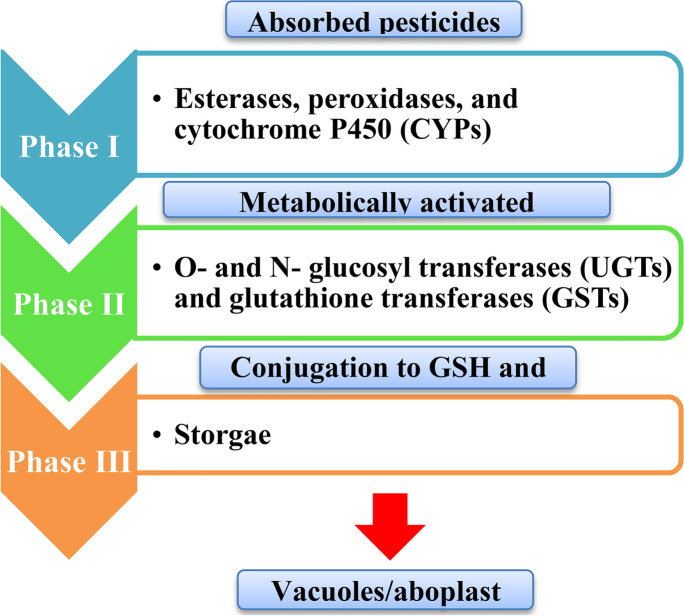
Plants have adopted a biotransformation process or detoxification system of xenobiotics (a chemical substance found within an organism that is not naturally produced or expected to be present within the organism) to protect themselves from the destructive attack of such compounds. These detoxification systems can be subdivided into four phases. The 1st phase of xenobiotics metabolism is manipulated by esterases, peroxidases, and cytochrome P450-dependent monooxygenases (CYPs), which are present in many isoforms (Coleman et al. 1997). The most common CYPs reactions are hydroxylation (Kreuz et al. 1996). Products from phase I do not always result in decreased phytotoxicity. Some xenobiotics already involve functional groups (OH, NH2, and COOH) and have exceeded the second phase completely. The 2nd phase of metabolism is stimulated by conjugating hydrophilic enzymes like transferases (O– and N–glucosyl transferases (UGTs) and glutathione transferases (GSTs) to the xenobiotic metabolite to form a water-soluble conjugate (Gaillard et al. 1994). The 3rd phase of metabolism comprises xenobiotic conjugates being placed in the large central vacuole of the cell, remarkably with the aid of adenosine triphosphate binding cassette transporter proteins (ABC) (Edwards et al. 2005) (Fig. 4). In the 4th phase of xenobiotic metabolism, products formed were introduced into the vacuole and could be transferred out into the cytoplasm and combined into cell wall components or other macromolecules (Gaillard et al. 1994; Edwards et al. 2005). Of such systems (phases), the one on which many recent studies have focused is the detoxification of herbicides through conjugation to the glutathione transferases (GSTs) (Baek et al. 2019; Sun et al. 2017; Taylor et al. 2013). The inhibitory outcome of abiotic stresses on plant growth is shown at many levels and includes a varied range of cellular processes that are controlled by hormones, amino acids, polyamines, polysaccharides, and enzymes that might be changed during stress (Wally et al. 2013).
Fig. 4.
Flow chart of the three-step pesticide detoxification system
Cyanobacteria’s Role Against Plant Abiotic Stresses
The decrease of the harmful effect of abiotic stresses observed in plants due to the interaction with cyanobacteria, which has been proved directly, by its action in the soil, or indirectly, due to the activation of specific responses in plants (Singh, 2014)
In the same context, A. platensis aqueous extract has been shown to contain a high amount of phytohormones like jasmonic acid, abscisic acid, and cytokinins, which participate in plant response to abiotic stresses (Tejada-Ruiz et al. 2020). Interestingly, the presence of salicylic acid, a vital signaling molecule responsible for the stimulation of defense responses in plants, was identified in the extracts of 28 cyanobacterial strains (Toribio et al. 2020). l-amino acids can work as signaling molecules to alleviate the damage caused by abiotic stresses (Oosten et al. 2017). New reports show that melatonin, a derivative of L-tryptophan, can help major seeds tolerate opposing environmental conditions at the germination stage (Kołodziejczyk et al. 2016). A. platensis biomass is known to be rich in l-amino acids and has been reported to motivate carbon metabolism, chlorophyll synthesis, and sugar content (Mógor et al. 2018), likewise providing useful properties through stress. Other cyanobacteria (Synechocystis sp. and Anabaena sp.) have been revealed to accumulate polyamines under stressful conditions, which synthesis comes from the decarboxylation of l-amino acids like l-arginine and l-ornithine (Mógor et al. 2017).
Application of cyanobacteria in crop plant fields alleviates adverse effects caused by salinity stress. Shariatmadari et al. (2015) observed that the application of cyanobacteria (Anabaena vaginicola ISB42, Anabaena oscillarioides ISB46, Anabaena torulosa, Anabaena sphaerica ISB23, Trichormus ellipsosporus, and Nostoc calcicola) in Mentha piperita fields exposed to salinity stress stimulated plant growth and oil content. Likewise, Rady et al. (2018) found that cyanobacteria alone or in combination with glutathione and ascorbic acid inoculated in salt-stressed soil cultivated with bean plants improved growth parameters like plant length, number of leaves, and fresh and dry weights of plants, as well as yield parameters. Moreover, photosynthetic pigments, relative water, stability of membrane, carbohydrates, proline, ascorbic acid, glutathione, N, P, and K+ion content, superoxide dismutase, and catalase activities were also stimulated compared to control plants. Furthermore, Brito et al. (2022) found that the cyanobacterial species Oculatella lusitanica LEGE stimulated salinity stress resistance in lettuce plants by increasing the non-enzymatic antioxidant system (H2O2, proline, and reduced glutathione). Regarding drought cases, soils inoculated by Spirulina meneghiniana and Anabaena oryzae improved lettuce plants’ growth as compared with non-inoculated soil plants (Ibraheem, 2007). Similarly, priming seeds of Senna notabilis and Acacia hilliana before cultivation in Microcoleus sp. and Nostoc sp. can increase germination and seedling growth (Muñoz-Rojas et al. 2018).
In the same context, the use of cyanobacteria helps in crop protection against the adverse effects of herbicides. Arthrospira platensis suspension applied to faba bean seeds was found to improve the harmful effects of Fusillade herbicide and caused an increase in root and shoot protein and amino acid content. Moreover, the same treatment increased the antioxidant enzymes and reduced the lipid peroxidation and proline content of the faba bean plants (Osman et al. 2016).
Furthermore, Abo-Shady et al. (2018) found that priming barley grains in the cyanobacterial suspension of Nostoc muscorum before cultivation removes the toxic effect of granstar herbicide on yield parameters (number of spikes/plant, spike length, the weight of spike, number of grains/spike and weight of 100 grains) of barley plants. Besides, it stimulated the production of some amino acids which were not present in the control plant and increased the concentration of the amino acids that were previously present.
The Mechanistic Approach Behind the Improvement and Protection of Plants by Using Cyanobacteria
Once cyanobacteria are exposed to oxidative stress, various compounds, such as antioxidants and secondary metabolites, can accumulate in cyanobacterial cells as an adjusted response to stress conditions (Kosar et al. 2015). Some enzymatic antioxidants such as superoxide dismutase (SOD), catalase, and glutathione peroxidase are created in microalgal cells due to abiotic stresses to defend against ROS-produced oxidative agents (Pikula et al. 2019). Likewise, cyanobacteria biostimulants inoculation in soil or foliar application has been shown to support the antioxidant activity of treated plants, hence alleviating the effects of stress-induced free radicals by direct scavenging and avoiding ROS formation (Ertani et al. 2019).
Cyanobacteria are a vital source of many biologically active compounds that can develop agricultural productivity. Cyanobacteria, use as crude or pure extracts. Furthermore, this range of metabolites influences crops’ production and improvement in many ways: (a) some extracts prompt higher crops’ productivity via an improvement in the soil quality (Table 4); (b) some metabolites perform straight on plant growth motivation (Table 4); and (c) others enrich crops’ progress through induce the protection against biotic and abiotic stress (Table 7). Additionally, cyanobacteria play an important role in the biogeochemical cycles of nitrogen, carbon, and oxygen, which is a feature of great significance in agricultural systems (Gonçalves, 2021).
Table 7.
Cyanobacteria-induced plants protection against different abiotic stresses
| Crop plants and stress | Cyanobacteria | Improvement effect | References |
|---|---|---|---|
|
Salinity Mentha piperita fields |
Anabaena vaginicola ISB42, Anabaena oscillarioides ISB46, Anabaena torulosa, Anabaena sphaerica ISB23, Trichormus ellipsosporus, and Nostoc calcicola | Stimulated germination percentage by 88, 87, 89, 86, 87, and 87%, respectively | (Shariatmadari et al. 2015) |
|
Salinity Lettuce plants |
Oculatella lusitanica LEGE | Increasing the non-enzymatic antioxidant reduced glutathione (66.67%) and decrease oxidative stress markers (H2O2, MDA, and proline by 32.5%, 21.43%, and 12.5%, respectively) | (Brito et al. 2022) |
|
High or low temperatures, salinity, and drought Poplar plants |
Aphanothece halophytica | Induced heat shock protein-like 70 (HSP70) and Photosynthetic activity (1.4-fold) | (Takabe et al. 2008) |
|
Irradiation, extreme temperatures, herbicides, oxidative, drought, and heat stress Tobacco plants |
Anabaena sp. | Induction of protein pattern by 1.5-fold | (Gharechahi et al. 2015) |
| Fusillade herbicide Faba bean | Arthrospira platensis | Decreased root and shoot proline content by 42.9% and 33.3%, respectively. Also, reduced the lipid peroxidation (MDA content by 98.7%) | (Osman et al. 2016) |
|
Granstar herbicide Barley plants |
Nostoc muscorum | Increased yield parameters [number of spikes/plant (90%), spike length (23.5%), the weight of spike (1.13-fold), number of grains/spike (82.4%), and weight of 100 grains (92.3%)] | (Abo-Shady et al. 2018) |
|
Brominal herbicide Wheat plants |
Arthrospira platensis | Induced by 1.13-fold for chl a, 1.05-fold for chl b, 88.89% for carotenoids, and 90.63% for total pigments, and also by 41.49 and 58.62% for carbohydrates and protein content, respectively | (Osman et al. 2022) |
In the same context, Gaafar et al. (2022) reported biochemical and molecular evidence explaining the protective effects of cyanobacteria, Arthrospira platensis, and Nostoc muscorum, for wheat crop plants against practice-specific herbicides. Priming wheat grains in the aqueous extract of these cyanobacteria stimulated the growth of the plants, especially after Broxyminyl herbicide spraying. Induction of antioxidant-defense enzymes, such as SOD, CAT, GPX, GST, and the non-enzymatic GSH molecules, was enhanced with a special performance for Arthrospira platensis treatment. Furthermore, detoxification genes, including GST (GSTZ, GSTU, and GSTL), TaGS, and TaGPX, were upregulated as a response to Arthrospira platensis and Nostoc muscorum application in combination with Brominal herbicide, which led to alleviating the toxic effect of Broxyminyl on wheat plants.
The transformation of plants with genes consequent from cyanobacteria has been significantly established since the 1990s, with genes complicated in carbon metabolism, fatty acid biosynthesis, and pigment biosynthesis (Ahmad et al. 2016). Transformation with the gene like heat shock protein 70 (HSP70) Aphanothece halophytica rises its tolerance against high or low temperatures, drought, and salinity (Takabe et al. 2008). Likewise, the overexpression of genes of flavodoxins from Anabaena sp. upsurges tobacco plant tolerance against numerous stresses (drought, high irradiation, risky temperatures, herbicides, and water shortage (Gharechahi et al. 2015; Li et al. 2017). Also, oxidative stress decrease has been shown with the transformation of tobacco plants with the gene that codes for a ferredoxin-NADP+ reductase (Gir´o et al. 2011). Finally, Fig. 5 represented a suggested mechanistic flow diagram approach behind the improvement and protection of plants by using Cyanobacteria.
Fig. 5.
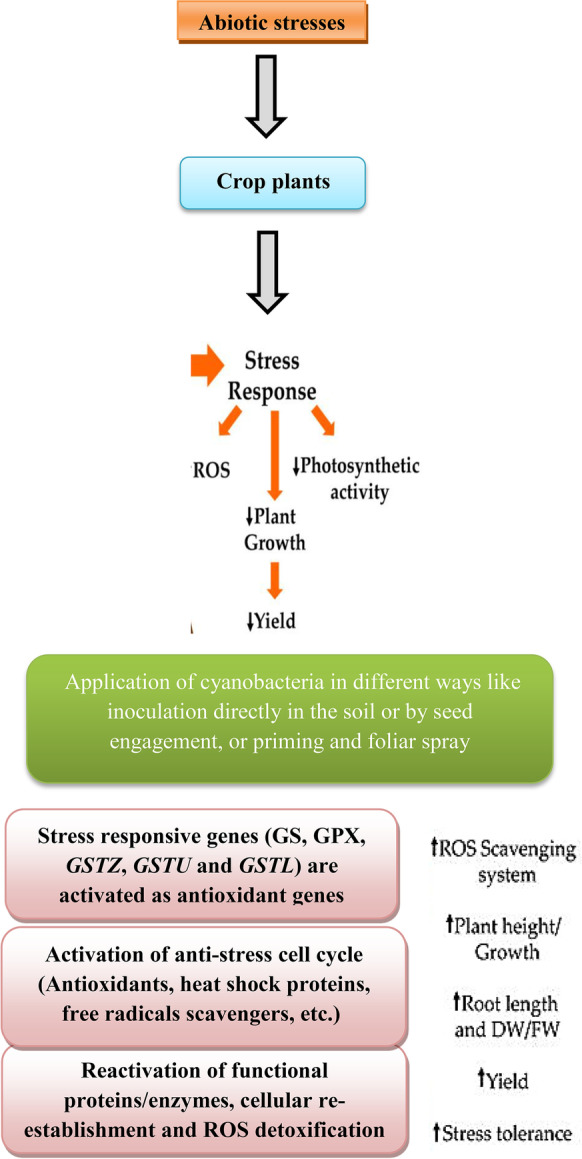
The mechanistic approach behind the improvement and protection of plants by using Cyanobacteria
Conclusion and future prospective
Plants exposed to risky environmental conditions cause abiotic stress, which can negatively impact crop production and development. To resolve this issue, the use of cyanobacteria can be a very hopeful option, as these compounds can (a) encourage the accumulation of antioxidant compounds, thus increasing the plant's tolerance to oxidative stress conditions; (b) improve the complete performance of higher plants, therefore stimulating their growth; (c) produce a wide variety of secondary metabolites with the ability to induce several plant protective mechanisms; and (d) chelate oxidizing numerous toxicants, xenobiotics, and complex organic compounds in the soils so that their bioremediation. Thus, they provide a lot of money spent on agriculture leading to nutrient-wealthy food that is healthy for the growth of the world population. Because of its safety and benefits, the mitigation strategies contesting unfavorable environmental conditions using cyanobacteria are important for stimulating the defensive mechanism system of plants against chemically hazardous pollutants and different abiotic stresses.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Authors’ Contributions
Project conceptualization: Osman MEH, El-Nagar MMF; writing first manuscript draft and editing: Ismail GA, El-Nagar MMF; writing final manuscript and approval: Osman MEH, Abo-shady AM, Gafaar RM, Ismail GA, El Nagar MMF. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Atef M. Abo-Shady, Email: atef.aboshady@science.tanta.edu.eg
Mohamed El-Anwar H. Osman, Email: mohamed.osman@science.tanta.edu.eg
Reda M. Gaafar, Email: redagaafar@science.tanta.edu.eg
Gehan A. Ismail, Email: gehan.ismail@science.tanta.edu.eg
Maysa M. F. El-Nagar, Email: pg_14055@science.tanta.edu.eg
References
- Abo-Shady AM, Osman MEH, El-Nagar MMF. Amelioration of the toxic effect induced by herbicide granstar on barley yield by the cyanobacterium Nostoc muscorum. Middle East Journal of Agriculture Research. 2018;7:1465–1472. [Google Scholar]
- Abdelsalam I, Elshobary M, Eladawy M, M., & Nagah, M. Utilization of multi-tasking non-edible plants for phytoremediation and bioenergy source-a review. Phyton. 2019;88(2):69–90. doi: 10.32604/phyton.2019.06831. [DOI] [Google Scholar]
- Abou-El-Souod GW, El-Sheekh MM. Biodegradation of basic fuchsin and methyl red by the blue-green algae Hydrocoleum oligotrichum and Oscillatoria limnetica. Environmental Engineering and Management Journal. 2016;15(2):279–286. doi: 10.30638/eemj.2016.028. [DOI] [Google Scholar]
- Ahmad R, Bilal M, Jeon JH, Kim HS, Park YI, Shah MM, Kwon SY. Improvement of biomass accumulation of potato plants by the transformation of cyanobacterial photorespiratory glycolate catabolism pathway genes. Plant Biotechnology. 2016;10:269–276. doi: 10.1007/s11816-016-0403-x. [DOI] [Google Scholar]
- Al-Amin A, Parvin F, Chakraborty J, Kim YI. Cyanobacteria mediated heavy metal removal: a review on the mechanism, biosynthesis, and removal capability. Environmental Technology Reviews. 2021;10(1):44–57. doi: 10.1080/21622515.2020.1869323. [DOI] [Google Scholar]
- Aminfarzaneh H, Duygu E. The effect of salicylic acid and triacontanol on biomass production and imidaclopirid removal capacity by cyanobacteria. Communications Faculty of Sciences University of Ankara Series C Biology. 2010;22(1):15–31. [Google Scholar]
- Ashour M, Al-Souti AS, Hassan SM, Ammar GAG, Goda AMA-S, El-Shenody R, et al. Commercial seaweed liquid extract as strawberry biostimulants and bioethanol production. Life. 2023;13(85):1–15. doi: 10.3390/life13010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashour M, Hassan SM, Elshobary ME, Ammar GAG, Gaber A, Alsanie WF, et al. Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum) Plants. 2021;10(6):1045. doi: 10.3390/plants10061045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atayee AR, Noori MS. Alleviation of cold stress in vegetable crops. Journal of Scientific Agriculture. 2020;4:38–44. doi: 10.25081/jsa.2020.v4.6110. [DOI] [Google Scholar]
- Bai L, Xu H, Wang C, Deng J, Jiang H. Extracellular polymeric substances facilitate the biosorption of phenanthrene on cyanobacteria Microcystis aeruginosa. Chemosphere. 2016;162:172e180. doi: 10.1016/j.chemosphere.2016.07.063. [DOI] [PubMed] [Google Scholar]
- Baek, Y. S., Goodrich, L. V., Brown, P. J., James, B. T., Moose, S. P., Lambert, K. N., & Riechers, D. E. (2019). Transcriptome profiling and genome-wide association studies reveal Gsts and other defense genes involved in multiple signaling pathways induced by herbicide safener in grain sorghum. Frontiers in Plant Science, 10. 10.3389/fpls.2019.00192 [DOI] [PMC free article] [PubMed]
- Balasubramaniam V, Gunasegavan RDN, Mustar S, Lee JC, Noh MFM. Isolation of industrial important bioactive compounds from microalgae. Molecules. 2021;26(4):1–45. doi: 10.3390/molecules26040943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton JW, Kuritz T, O’Connor LE, et al. Reductive transformation of methyl parathion by the cyanobacterium Anabaena sp. strain PCC7120. Applied Microbiology and Biotechnology. 2004;65:330–335. doi: 10.1007/s00253-004-1557-y. [DOI] [PubMed] [Google Scholar]
- Bayazıt G, Tastan BE, Gül D. Biosorption, isotherm, and kinetic properties of common textile dye by phormidium animale. Global NEST Journal. 2020;22(1):1–7. doi: 10.30955/gnj.002984. [DOI] [Google Scholar]
- Bidyarani N, Prasanna R, Babu S, Hossain F, Saxena AK. Enhancement of plant growth and yields in Chickpea (Cicer arietinum L.) through novel cyanobacterial and biofilmed inoculants. Microbiological Research. 2016;188:97–105. doi: 10.1016/j.micres.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Bidyarani N, Prasanna R, Chawla G, Babu S, Singh R. Deciphering the factors associated with the colonization of rice plants by cyanobacteria. Journal of Basic Microbiology. 2015;55(4):407–419. doi: 10.1002/jobm.201400591. [DOI] [PubMed] [Google Scholar]
- Brito Â, Rocha M, Kaštovský J, Vieira J, Vieira CP, Ramos V, et al. A new cyanobacterial species with a protective effect on lettuce grown under salinity stress: Envisaging sustainable agriculture practices. Journal of Applied Phycology. 2022;34(2):915–928. doi: 10.1007/s10811-022-02692-4. [DOI] [Google Scholar]
- Cam N, Benzerara K, Georgelin T, Jaber M, Lambert JF, Poinsot M, Skouri-Panet F, Cordier L. Selective uptake of alkaline earth metals by cyanobacteria forming intracellular carbonates. Environmental Science and Technology. 2016;50(21):11654e11662. doi: 10.1021/acs.est.6b02872. [DOI] [PubMed] [Google Scholar]
- Caverzan, A., Piasecki, C., Chavarria, G., Stewart, C. N., & Vargas, L. (2019). Defenses against ROS in crops and weeds: The effects of interference and herbicides. International Journal of Molecular Sciences, 20(5). 10.3390/ijms20051086 [DOI] [PMC free article] [PubMed]
- Chaiklahan R, Chirasuwan N, Triratana P, Loha V, Tia S, Bunnag B. Polysaccharide extraction from Spirulina sp. and its antioxidant capacity. International Journal of Biological Macromolecules. 2013;58:73–78. doi: 10.1016/j.ijbiomac.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Chan SM, Luan T, Wong MH, Tam NF. Removal and biodegradation of polycyclic aromatic hydrocarbons by Selenastrum capricornutum. Environmental Toxicology and Chemistry. 2006;25(7):1772e1779. doi: 10.1897/05-354R.1. [DOI] [PubMed] [Google Scholar]
- Chavan, A., Mukherji, S., June 15, 2008. Treatment of hydrocarbon-rich wastewater using oil-degrading bacteria and phototrophic microorganisms in rotating biological contactor: effect of N: P ratio. Journal of Hazardous Materials, 154 (1e3), 63e72. [DOI] [PubMed]
- Chavan A, Mukherji S. Effect of co-contaminant phenol on the performance of a laboratory-scale RBC with algal-bacterial biofilm treating petroleum hydrocarbon-rich wastewater. Journal of Chemical Technology and Biotechnology. 2010;85(6):851e859. [Google Scholar]
- Chittapun S, Limbipichai S, Amnuaysin N, Boonkerd R, Charoensook M. Effects of using cyanobacteria and fertilizer on growth and yield of rice, Pathum Thani I: a pot experiment. Journal of Applied Phycology. 2018;30(1):79–85. doi: 10.1007/s10811-017-1138-y. [DOI] [Google Scholar]
- Cohen RRH. Use of microbes for cost reduction of metal removal from metals and mining industry waste streams. Journal of Cleaner Production. 2006;14(12):1146–1157. doi: 10.1016/j.jclepro.2004.10.009. [DOI] [Google Scholar]
- Coleman JOD, Blake-Kalff MMA, Davies TGE. Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trends in Plant Science. 1997;2(4):144–151. doi: 10.1016/S1360-1385(97)01019-4. [DOI] [Google Scholar]
- Colica G, Mecarozzi PC, De Philippis R. Treatment of Cr (VI)-containing wastewaters with exopolysaccharide-producing cyanobacteria in pilot flow through and batch systems. Applied Microbiology and Biotechnology. 2010;87(5):1953–1961. doi: 10.1007/s00253-010-2665-5. [DOI] [PubMed] [Google Scholar]
- Collos Y, Harrison PJ. Acclimation and toxicity of high ammonium concentrations to unicellular algae. Marine Pollution Bulletin. 2014;80(1–2):8–23. doi: 10.1016/j.marpolbul.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Corpuz MVA, Borea L, Senatore V, Castrogiovanni F, Buonerba A, Oliva G, et al. Wastewater treatment and fouling control in an electro algae-activated sludge membrane bioreactor. Science of the Total Environment. 2021;786:147475. doi: 10.1016/j.scitotenv.2021.147475. [DOI] [PubMed] [Google Scholar]
- Das C, Naseera K, Ram A, Meena RM, Ramaiah N. Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris. Journal of Applied Phycology. 2017;29(1):235–243. doi: 10.1007/s10811-016-0910-8. [DOI] [Google Scholar]
- Das N, Vimala R, Karthika P. Biosorption of heavy metals–an overview. Indian Journal of Biotechnology. 2008;7:159–169. [Google Scholar]
- De Souza RM, Seibert D, Quesada HB, de Jesus Bassetti F, Fagundes-Klen MR, Bergamasco R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Safety and Environmental Protection. 2020;135:22–37. doi: 10.1016/j.psep.2019.12.035. [DOI] [Google Scholar]
- Dmytryk, A., & Chojnacka, K. A. (2018). Algae as fertilizers, biostimulants, and regulators of plant growth. Algae biomass: characteristics and applications: towards algae-based products, 115–122. 10.1007/978-3-319-74703-3_10
- Ebele AJ, Abou-Elwafa Abdallah M, Harrad S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerging Contaminants. 2017;3(1):1–16. doi: 10.1016/j.emcon.2016.12.004. [DOI] [Google Scholar]
- Edwards R, Brazier-hicks M, Dixon DP, Cummins IAN. Chemical manipulation of antioxidant defences in plants School of Biological and Biomedical Sciences. University of Durham. 2005;2296(04):1–32. doi: 10.1016/S0065-2296(04)42001-1. [DOI] [Google Scholar]
- Elarroussi H, Elmernissi N, Benhima R, Meftah El Kadmiri I, Bendaou N, Smouni A. Microalgae polysaccharides are a promising plant growth biostimulant. Journal of Algal Biomass Utilization. 2016;7(4):55–63. [Google Scholar]
- El-Enany AE, Issa AA. Cyanobacteria as a biosorbent of heavy metals in sewage water. Environmental Toxicology and Pharmacology. 2000;8(2):95e101. doi: 10.1016/S1382-6689(99)00037-X. [DOI] [PubMed] [Google Scholar]
- El-Sheekh MM, El-Shouny WA, Osman MEH, El-Gammal EWE. Growth and heavy metals removal efficiency of Nostoc muscorum and Anabaena subcylindrica in sewage and industrial wastewater effluents. Environmental Toxicology and Pharmacology. 2005;19:357–365. doi: 10.1016/j.etap.2004.09.005. [DOI] [PubMed] [Google Scholar]
- El-Sheekh MM, Ghareib M, EL-Souod, G. A. Biodegradation of phenolic and polycyclic aromatic compounds by some algae and cyanobacteria. Journal of Bioremediation & Biodegradation. 2012;3(1):26–29. doi: 10.4172/2155-6199.1000133. [DOI] [Google Scholar]
- El-shenody RA, Elshobary ME, Ragab GA, Huo S, Essa D. Towards biorefinery : Exploring the potential of seaweed-derived biodiesel and its residual biomass in improving the traits of Eruca vesicaria (L.) cav. South African Journal of Botany. 2023;155:361–371. doi: 10.1016/j.sajb.2023.02.029. [DOI] [Google Scholar]
- El Shafay SM, Gaber A, Alsanie WF, Elshobary ME. Influence of nutrient manipulation on growth and biochemical constituent in Anabaena variabilis and Nostoc muscorum to enhance biodiesel production. Sustainability. 2021;13(16):9081. doi: 10.3390/su13169081. [DOI] [Google Scholar]
- Ertani A, Nardi S, Francioso O, Sanchez-Cortes S, Di Foggia M, Schiavon M. Effects of two protein hydrolysates obtained from Chickpea (Cicer arietinum L.) and Spirulina platensis on Zea mays (L.) plants. Frontiers in Plant Science. 2019;10:1–13. doi: 10.3389/fpls.2019.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, IFAD, UNICEF, WFP & WHO . Food Security and Nutrition in the World Repurposing Food and Healthy Diets More Affordable. 2022. [Google Scholar]
- Farid R, Mutale-joan C, Redouane B, Mernissi Najib E, Abderahime A, Laila S, Arroussi Hicham E. Effect of microalgae polysaccharides on biochemical and metabolomics pathways related to plant defense in Solanum lycopersicum. Applied Biochemistry and Biotechnology. 2019;188(1):225–240. doi: 10.1007/s12010-018-2916-y. [DOI] [PubMed] [Google Scholar]
- Farooq M, Gogoi N, Barthakur S, Baroowa B, Bharadwaj N, Alghamdi SS, Siddique KHM. Drought stress in grain legumes during reproduction and grain filling. Journal of Agronomy and Crop Science. 2017;203(2):81–102. doi: 10.1111/jac.12169. [DOI] [Google Scholar]
- Forlani G, Pavan M, Gramek M, Kafarski P, Lipok J. Biochemical bases for a widespread tolerance of cyanobacteria to the phosphonate herbicide glyphosate. Plant and Cell Physiology. 2008;49(3):443–456. doi: 10.1093/pcp/pcn021. [DOI] [PubMed] [Google Scholar]
- Gaafar RM, Osman MEH, Abo-shady AM, Almohisen IAA, Badawy GA, El-Nagar; M.M.F., Ismail, G.A. Role of antioxidant enzymes and glutathione S-transferase in bromoxynil herbicide stress tolerance in wheat. Plants. 2022;11:1–16. doi: 10.3390/plants11202679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd GM. Biosorption: Current perspectives on concept, definition, and application. Bioresource Technology. 2014;160:3–14. doi: 10.1016/j.biortech.2013.12.102. [DOI] [PubMed] [Google Scholar]
- Gaillard C, Dufaud A, Tommasini R, Kreuz K, Amrhein N, Martinoia E. A herbicide antidote (safener) induces the activity of both the herbicide-detoxifying enzyme and of a vacuolar transporter for the detoxified herbicide. FEBS Letters. 1994;352(2):219–221. doi: 10.1016/0014-5793(94)00961-9. [DOI] [PubMed] [Google Scholar]
- Gharechahi J, Hajirezaei MR, Salekdeh GH. Comparative proteomic analysis of tobacco expressing cyanobacterial flavodoxin and its wild type under drought stress. Journal of Plant Physiology. 2015;175:48–58. doi: 10.1016/j.jplph.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Giró, M., Ceccoli, R. D., Poli, H. O., Carrillo, N., & Lodeyro, A. F.(2011). An in vivo system involving the co-expression of cyanobacterial flavodoxin and ferredoxin–NADP+ reductase confers increased tolerance to oxidative stress in plants. FEBS Open Bio, 1, 7–13. 10.1016/j.fob.2011.10.004. [DOI] [PMC free article] [PubMed]
- Godlewska K, Michalak I, Pacyga P, Baśladyńska S, Chojnacka K. Potential applications of cyanobacteria: spirulina platensis filtrates and homogenates in agriculture. World Journal of Microbiology and Biotechnology. 2019;35(6):1–18. doi: 10.1007/s11274-019-2653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MP, Le Manac’h SG, Hénault-Ethier L, Labrecque M, Lucotte M, Juneau P. Glyphosate-dependent inhibition of photosynthesis in willow. Frontiers in Plant Science. 2017;8:1–13. doi: 10.3389/fpls.2017.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MP, Richardi VS, Bicalho EM, da Rocha DC, Navarro-Silva MA, Soffiatti P, et al. Effects of Ciprofloxacin and Roundup on seed germination and root development of maize. Science of the Total Environment. 2019;651:2671–2678. doi: 10.1016/j.scitotenv.2018.09.365. [DOI] [PubMed] [Google Scholar]
- Gonçalves AL. The use of microalgae and cyanobacteria in the improvement of agricultural practices: a review on their biofertilising, biostimulating, and biopesticide roles. Applied Sciences (Switzerland) 2021;11(2):1–21. doi: 10.3390/app11020871. [DOI] [Google Scholar]
- Górka, B., Korzeniowska, K., Lipok, J., & Wieczorek, P. P. (2018). The Biomass of algae and algal extracts in agricultural production. Algae Biomass: Characteristics and Applications: Towards Algae-based Products, 103–114. 10.1007/978-3-319-74703-3_9
- Guihéneuf F, Khan A, Tran LSP. Genetic engineering: a promising tool to engender physiological, biochemical, and molecular stress resilience in green microalgae. Frontiers in Plant Science. 2016;7:1–8. doi: 10.3389/fpls.2016.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V, Ratha SK, Sood A, Chaudhary V, Prasanna R. New insights into the biodiversity and applications of cyanobacteria (blue-green algae)-Prospects and challenges. Algal Research. 2013;2(2):79–97. doi: 10.1016/j.algal.2013.01.006. [DOI] [Google Scholar]
- Han X, Zeng H, Bartocci P, Fantozzi F, Yan Y. Phytohormones and effects on growth and metabolites of microalgae: a review. Fermentation. 2018;4(2):1–15. doi: 10.3390/fermentation4020025. [DOI] [Google Scholar]
- Hao, X., Li, P., Feng, Y., Han, X., Gao, J., Lin, E., & Han, Y. (2013). Effects of fully open-air [CO2] elevation on leaf photosynthesis and ultrastructure of Isatis indigotica Fort. PLoS One, 8(9). 10.1371/journal.pone.0074600 [DOI] [PMC free article] [PubMed]
- Hassan SM, Ashour M, Soliman AAF, Hassanien HA, Alsanie WF, Gaber A, Elshobary ME. The potential of a new commercial seaweed extract in stimulating morpho-agronomic and bioactive properties of Eruca vesicaria (L.) cav. Sustainability (Switzerland) 2021;13(8):4485. doi: 10.3390/su13084485. [DOI] [Google Scholar]
- Ibraheem I. Cyanobacteria as alternative biological conditioners for bioremediation of barren soil. Egyptian Journal of Phycology. 2007;8(1):99–117. doi: 10.21608/egyjs.2007.114548. [DOI] [Google Scholar]
- Jha NM, Mishra KS. Biological responses of cyanobacteria to insecticides and their insecticide degrading potential. Bulletin of Environmental Contamination and Toxicology. 2005;75:374–381. doi: 10.1007/s00128-005-0764-2. [DOI] [PubMed] [Google Scholar]
- Joshi MA, Faridullah S, Kumar A. Effect of heat stress on crop phenology, yield, and seed quality attributes of wheat (Triticum aestivum L.) Journal of Agrometeorology. 2016;18(2):206–215. doi: 10.54386/jam.v18i2.937. [DOI] [Google Scholar]
- Kamal MA, Saleem MF, Shahid M, Awais M, Khan HZ, Ahmed K. Ascorbic acid triggered physiochemical transformations at different phenological stages of heat-stressed Bt cotton. Journal of Agronomy and Crop Science. 2017;203(4):323–331. doi: 10.1111/jac.12211. [DOI] [Google Scholar]
- Khodarahmpour Z, Motamedi MIM. Effects of NaCl salinity on maize (Zea mays L.) at germination and early seedling stage. African Journal of Biotechnology. 2011;11(2):298–303. doi: 10.5897/ajb11.2624. [DOI] [Google Scholar]
- Kirkwood AE, Nalewajko C, Fulthorpe RR. The impacts of cyanobacteria on pulp-and-paper wastewater toxicity and biodegradation of wastewater contaminants. Canadian Journal of Microbiology. 2005;51(7):531e540. doi: 10.1139/w05-030. [DOI] [PubMed] [Google Scholar]
- Kollmen J, Strieth D. The Beneficial Effects of Cyanobacterial Co-Culture on Plant Growth. Life (Basel) 2022;12(2):223. doi: 10.3390/life12020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołodziejczyk I, Dzitko K, Szewczyk R, Posmyk MM. Exogenous melatonin improves corn (Zea mays L.) embryo proteome in seeds subjected to chilling stress. Journal of Plant Physiology. 2016;193:47–56. doi: 10.1016/j.jplph.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Kosar F, Akram NA, Ashraf M. Exogenously applied 5- aminolevulinic acid modulates some key physiological characteristics and antioxidative defense systems in spring wheat (Triticum aestivum L.) seedlings under water stress. South African Journal of Botany. 2015;96:71–77. doi: 10.1016/j.sajb.2014.10.015. [DOI] [Google Scholar]
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. What is stress? Concepts, definitions, and applications in seed science. New Phytologist. 2010;188(3):655–673. doi: 10.1111/j.1469-8137.2010.03461.x. [DOI] [PubMed] [Google Scholar]
- Kreuz K, Tommasini R, Martinoia E. Old Enzymes for a New Job (Herbicide Detoxification in Plants) Plant Physiology. 1996;111(2):349–353. doi: 10.1104/pp.111.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy L, Kashiwagi J, Gaur PM, Upadhyaya HD, Vadez V. Sources of tolerance to terminal drought in the chickpea (Cicer arietinum L.) minicore germplasm. Field Crops Research. 2010;119(2–3):322–330. doi: 10.1016/j.fcr.2010.08.002. [DOI] [Google Scholar]
- Kumar D, Pandey LK, Gaur JP. Metal sorption by algal biomass: From batch to continuous system. Algal Research. 2016;18:95–109. doi: 10.1016/j.algal.2016.05.026. [DOI] [Google Scholar]
- Kumar A, Chaurasia U, Elshobary ME, Kumari S, Hussain T, Bharti AP, et al. Utilization of algae in crop improvement and crop protection for a better agricultural system. In: El-Sheekh MM, Abdullah N, Ahmad I, et al., editors. Handbook of Research on Algae as a Sustainable Solution for Food, Energy, and the Environment (442–470) IGI Global; 2022. [Google Scholar]
- Li H, Zhao Q, Huang H. Current status and challenges of salt-affected soil remediation by cyanobacteria. Science of the Total Environment. 2019;669:258–272. doi: 10.1016/j.scitotenv.2019.03.104. [DOI] [PubMed] [Google Scholar]
- Li Z, Yuan S, Jia H, Gao F, Zhou M, Yuan N, et al. Ectopic expression of a cyanobacterial flavodoxin in creeping bentgrass impacts plant development and confers broad abiotic stress tolerance. Plant Biotechnology Journal. 2017;15:433–446. doi: 10.1111/pbi.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mala R, Celsia ASR, Mahalakshmi R, Rajeswari S. IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017. Agronomic biofortification of Amaranthus dubius with macro nutrients and vitamin A; p. 12214. [Google Scholar]
- Mastovska K, Wylie PL. Evaluation of a new column backflushing set-up in the gas chromatographic-tandem mass spectrometric analysis of pesticide residues in dietary supplements. Journal of Chromatography A. 2012;1265:155–164. doi: 10.1016/j.chroma.2012.09.094. [DOI] [PubMed] [Google Scholar]
- Meeks JC, Elhai J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiology and Molecular Biology Reviews. 2002;66(1):94–121. doi: 10.1128/mmbr.66.1.94-121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Xia Y, Chen H. Bioremediation of surface water co-contaminated with zinc (II) and linear alkyl benzene sulfonates by Spirulina platensis. Physics and Chemistry of the Earth. 2012;47:152–155. doi: 10.1016/J.PCE.2011.06.003. [DOI] [Google Scholar]
- Mógor ÁF, Ördög V, Lima GPP, Molnár Z, Mógor G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. Journal of Applied Phycology. 2017;30(1):453–460. doi: 10.1007/s10811-017-1242-z. [DOI] [Google Scholar]
- Mógor ÁF, de Oliveira Amatussi J, Mógor G, Bocchetti de Lara G. Bioactivity of cyanobacterial biomass related to amino acids induces growth and metabolic changes on seedlings and yield gains of organic red beet. American Journal of Plant Sciences. 2018;09(05):966–978. doi: 10.4236/ajps.2018.95074. [DOI] [Google Scholar]
- Mondal M, Halder G, Oinam G, Indrama T, Tiwari ON. New and future developments in microbial biotechnology and bioengineering. Elsevier; 2019. Bioremediation of organic and inorganic pollutants using microalgae; pp. 223–235. [Google Scholar]
- Muñoz-Rojas M, Chilton A, Liyanage GS, Erickson TE, Merritt DJ, Neilan BA, Ooi MKJ. Effects of indigenous soil cyanobacteria on seed germination and seedling growth of arid species used in restoration. Plant and Soil. 2018;429(1–2):91–100. doi: 10.1007/s11104-018-3607-8. [DOI] [Google Scholar]
- Mutale-joan C, Redouane B, Najib E, Yassine K, Lyamlouli K, Laila S, et al. Screening of microalgae liquid extracts for their biostimulant properties on plant growth, nutrient uptake and metabolite profile of Solanum lycopersicum L. Scientific Reports. 2020;10(1):1–12. doi: 10.1038/s41598-020-59840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Frontiers in Public Health. 2016;4:1–8. doi: 10.3389/fpubh.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ningombam B, Devi AS, Darvhankar M. Effects of abiotic stresses on crop yield: A review. Pharmaceutical Innovations. 2021;10(5):418–422. [Google Scholar]
- Odjadjare EC, Mutanda T, Olaniran AO. Potential biotechnological application of microalgae: a critical review. Critical Reviews in Biotechnology. 2017;37(1):37–52. doi: 10.3109/07388551.2015.1108956. [DOI] [PubMed] [Google Scholar]
- Osman MEH, Abo-Shady AM, El-Nagar MMF. Cyanobacterial Arthrospira (Spirulina platensis) as safener against harmful effects of fusillade herbicide on faba bean plant. Rendiconti Lincei. 2016;27(3):455–462. doi: 10.1007/s12210-015-0498-y. [DOI] [Google Scholar]
- Osman MEH, Abo-Shady AM, El-Nagar MMF. Treatment of broad bean seeds with algal suspensions to study their effects on certain growth and yield parameters. Journal of Environmental Sciences. 2020;9(1):1–7. doi: 10.21608/JOESE.2020.147760. [DOI] [Google Scholar]
- Osman MEH, Abo-Shady AM, Gaafar RM, El-Nagar MMF, Ismail GA. Promoting wheat growth by priming grains with water extracts of Nostoc muscorum and Arthrospira platensis. Egyptian Journal of Botany. 2021;61(3):809–821. doi: 10.21608/ejbo.2021.78079.1697. [DOI] [Google Scholar]
- Osman, M. E. H., Abo-Shady, A. M., Gaafar, R. M., Ismail, G. A., & El-Nagar, M. M. F. (2022). Assessment of cyanobacteria and tryptophan role in the alleviation of the toxic action of brominal herbicide on wheat plants. Gesunde Pflanzen, 1-15. 10.1007/s10343-022-00785-1
- Pan S, Jeevanandam J, Danquah M. Grand Challenges in Marine Biotechnology. Springer Science and Business; 2019. Benefits of Algal Extracts in Sustainable Agriculture; pp. 501–553. [Google Scholar]
- Panuccio MR, Jacobsen SE, Akhtar SS, Muscolo A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB PLANTS. 2014;6:1–18. doi: 10.1093/aobpla/plu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parween T, Jan S, Mahmooduzzafar S, Fatma T, Siddiqui ZH. Selective Effect of Pesticides on Plant—A Review. Critical Reviews in Food Science and Nutrition. 2016;56(1):160–179. doi: 10.1080/10408398.2013.787969. [DOI] [PubMed] [Google Scholar]
- Pathak, J., Maurya, P. K., Singh, S. P., Häder, D. P., Sinha, R. P., Häder, D. P., & Sinha, R. P. (2018). Cyanobacterial farming for environment-friendly sustainable agriculture practices: Innovations and perspectives. Frontiers in Environmental Science, 6. 10.3389/fenvs.2018.00007
- Pham HTL, Nguyen LTT, Duong TA, Bui DTT, Doan QT, Nguyen HTT, Mundt S. Diversity and bioactivities of nostocacean cyanobacteria isolated from paddy soil in Vietnam. Systematic and Applied Microbiology. 2017;40(8):470–481. doi: 10.1016/j.syapm.2017.08.001. [DOI] [PubMed] [Google Scholar]
- Pikula KS, Zakharenko AM, Aruoja V, Golokhvast KS, Tsatsakis AM. Oxidative stress and its biomarkers in microalgal ecotoxicology. Current Opinion in Toxicology. 2019;13:8–15. doi: 10.1016/j.cotox.2018.12.006. [DOI] [Google Scholar]
- Prasanna R, Babu S, Rana A, Kabi SR, Chaudhary V, Gupta V, et al. Evaluating the establishment and agronomic proficiency of cyanobacterial consortia as organic options in wheat-rice cropping sequence. Experimental Agriculture. 2013;49(3):416–434. doi: 10.1017/S001447971200107X. [DOI] [Google Scholar]
- Prasanna R, Sharma E, Sharma P, Kumar A, Kumar R, Gupta V, et al. Soil fertility and establishment potential of inoculated cyanobacteria in rice crop grown under non-flooded conditions. Paddy and Water Environment. 2013;11(1–4):175–183. doi: 10.1007/s10333-011-0302-2. [DOI] [Google Scholar]
- Prasanna R, Bidyarani N, Babu S, Hossain F, Shivay YS, Nain L. Cyanobacterial inoculation elicits plant defense response and enhanced Zn mobilization in maize hybrids. Cogent Food & Agriculture. 2015;1(1):998507. doi: 10.1080/23311932.2014.998507. [DOI] [Google Scholar]
- Prasanna R, Kanchan A, Ramakrishnan B, Ranjan K, Venkatachalam S, Hossain F, et al. Cyanobacteria-based bioinoculants influence growth and yields by modulating the microbial communities favorably in the rhizospheres of maize hybrids. European Journal of Soil Biology. 2016;75:15–23. doi: 10.1016/j.ejsobi.2016.04.001. [DOI] [Google Scholar]
- Prasanna R, Ramakrishnan B, Simranjit K, Ranjan K, Kanchan A, Hossain F, Nain L. Cyanobacterial and rhizobial inoculation modulates the plant physiological attributes and nodules microbial communities of Chickpeas. Archives of Microbiology. 2017;199(9):1311–1323. doi: 10.1007/s00203-017-1405-y. [DOI] [PubMed] [Google Scholar]
- Rady MM, Taha SS, Kusvuran S. Integrative application of cyanobacteria and antioxidants improves common bean performance under saline conditions. Scientia Horticulturae. 2018;233:61–69. doi: 10.1016/j.scienta.2018.01.047. [DOI] [Google Scholar]
- Rohila AK, Ansul M, D., Kumar, A., & Kumar, K. Impact of agricultural practices on the environment. Asian Journal of Microbiology, Biotechnology & Environmental Sciences. 2017;19(2):381–384. [Google Scholar]
- Ronga D, Biazzi E, Parati K, Carminati D, Carminati E, Tava A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy. 2019;9(4):1–22. doi: 10.3390/agronomy9040192. [DOI] [Google Scholar]
- Rossi F, Li H, Liu Y, De Philippis R. Cyanobacterial inoculation (cyanobacterisation): Perspectives for the development of a standardized multifunctional technology for soil fertilization and desertification reversal. Earth-Science Reviews. 2017;171:28–43. doi: 10.1016/j.earscirev.2017.05.006. [DOI] [Google Scholar]
- Ruelland E, Vaultier MN, Zachowski A, Hurry V. Advances in Botanical Research. 1. Elsevier Ltd.; 2009. Chapter 2 Cold Signalling and Cold Acclimation in Plants. [Google Scholar]
- Saito K, Matsuda F. Metabolomics for functional genomics, systems biology, and biotechnology. Annual Review of Plant Biology. 2010;61:463–489. doi: 10.1146/annurev.arplant.043008.092035. [DOI] [PubMed] [Google Scholar]
- Seifikalhor M, Hassani SB, Aliniaeifard S. Seed priming by cyanobacteria (Spirulina platensis) and salep gum enhances tolerance of maize plant against cadmium toxicity. Journal of Plant Growth Regulation. 2020;39(3):1009–1021. doi: 10.1007/s00344-019-10038-7. [DOI] [Google Scholar]
- Semple KT, Cain RB. Biodegradation of phenols by the alga Ochromonas danica. Applied and Environmental Microbiology. 1996;62(4):1265–1273. doi: 10.1128/aem.62.4.1265-1273.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakeri E, Emam Y, Tabatabaei SA, Sepaskhah AR. Evaluation of grain sorghum (Sorghum bicolor L.) lines/cultivars under salinity stress using tolerance indicest. International Journal of Plan Production. 2017;11(1):101–116. [Google Scholar]
- Shariatmadari Z, Riahi H, Abdi M, Hashtroudi MS, Ghassempour AR. Impact of cyanobacterial extracts on the growth and oil content of the medicinal plant Mentha piperita L. Journal of Applied Phycology. 2015;27(6):2279–2287. doi: 10.1007/s10811-014-0512-2. [DOI] [Google Scholar]
- Sharma, K. K., Tripathy, V., Gopal, M., & Walia, S. (2019). Good agricultural practices and monitoring of herbicide residues in India. Herbicide Residue Research in India, 443–465. 10.1007/978-981-13-1038-6_16
- Siddiqui H, Hayat S, Bajguz A. Regulation of photosynthesis by brassinosteroids in plants. Acta Physiologiae Plantarum. 2018;40(3):1–15. doi: 10.1007/s11738-018-2639-2. [DOI] [Google Scholar]
- Singh SS, Kunui K, Minj RA, Singh P. Diversity and distribution pattern analysis of cyanobacteria isolated from paddy fields of Chhattisgarh. India. Journal of Asia-Pacific Biodiversity. 2014;7(4):462–470. doi: 10.1016/j.japb.2014.10.009. [DOI] [Google Scholar]
- Singh JS, Kumar A, Rai AN, Singh DP. Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Frontiers in Microbiology. 2016;7:1–19. doi: 10.3389/fmicb.2016.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JS, Strong PJ. Biologically derived fertilizer: a multifaceted bio-tool in methane mitigation. Ecotoxicology and Environmental Safety. 2016;124:267–276. doi: 10.1016/j.ecoenv.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Singh H, Pal S, Bhattacharya A. Oxidative stress caused by use of pre-emergent herbicides in wheat seedlings. International Journal of Current Microbiology and Applied Sciences. 2017;6(12):2580–2586. doi: 10.20546/ijcmas.2017.612.299. [DOI] [Google Scholar]
- Singh R, Parihar P, Singh M, Bajguz A, Kumar J, Singh S, et al. Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture, and medicine: Current status and future prospects. Frontiers in Microbiology. 2017;8:1–37. doi: 10.3389/fmicb.2017.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subedi, M., Willenborg, C. J., & Vandenberg, A. (2017). Influence of harvest aid herbicides on seed germination, seedling vigor, and milling quality traits of red lentils (Lens culinaris L.). Frontiers in Plant Science, 8. 10.3389/fpls.2017.00311 [DOI] [PMC free article] [PubMed]
- Sun L, Wu R, Su W, Gao Z, Lu C. Physiological basis for isoxadifen-ethyl induction of nicosulfuron detoxification in maize hybrids. PLoS One. 2017;12(3):1–16. doi: 10.1371/journal.pone.0173502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe T, Uchida A, Shinagawa F, Terada Y, Kajita H, Tanaka Y, et al. Overexpression of DnaK from a halotolerant cyanobacterium Aphanothece halophytica enhances the growth rate as well as abiotic stress tolerance of poplar plants. Plant Growth Regulation. 2008;56(3):265–273. doi: 10.1007/s10725-008-9306-3. [DOI] [Google Scholar]
- Taylor VL, Cummins I, Brazier-Hicks M, Edwards R. Protective responses induced by herbicide safeners in wheat. Environmental and Experimental Botany. 2013;88:93–99. doi: 10.1016/j.envexpbot.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejada-Ruiz S, Gonzalez-Lopez C, Rojas E, Jiménez-Becker S. Effect of the foliar application of microalgae hydrolysate (Arthrospira platensis) and silicon on the growth of pelargonium hortorum L.H Bailey under salinity conditions. Agronomy. 2020;10(11):4003–4011. doi: 10.3390/agronomy10111713. [DOI] [Google Scholar]
- Thakur DS, Khot R, Joshi PP, Pandharipande M, Nagpure K. Glyphosate poisoning with acute pulmonary edema. Toxicology International. 2014;21(3):328–330. doi: 10.4103/0971-6580.155389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari B, Singh S, Chakraborty S, Verma E, Mishra AK. Sequential role of biosorption and biodegradation in rapid removal degradation and utilization of methyl parathion as a phosphate source by a new cyanobacterial isolate Scytonema sp. BHUS-5. International Journal of Phytoremediation. 2017;19:884. doi: 10.1080/15226514.2017.1303807. [DOI] [PubMed] [Google Scholar]
- Toribio AJ, Suárez-Estrella F, Jurado MM, López MJ, López-González JA, Moreno J. Prospection of cyanobacteria producing bioactive substances and their application as potential photostimulation agents. Biotechnology Reports. 2020;6(26):e00449. doi: 10.1016/j.btre.2020.e00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribio AJ, Jurado MM, Suárez-Estrella F, López MJ, López-González JA, Moreno J. Seed biopriming with cyanobacterial extracts as an eco-friendly strategy to control damping-off caused by Pythium ultimum in seedbeds. Microbiological Research. 2021;248:126766. doi: 10.1016/j.micres.2021.126766. [DOI] [PubMed] [Google Scholar]
- Toscano S, Farieri E, Ferrante A, Romano D. Physiological and biochemical responses in two ornamental shrubs to drought stress. Frontiers in Plant Science. 2016;2(7):645. doi: 10.3389/fpls.2016.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oosten MJ, Pepe O, De Pascale S, Silletti S, Maggio A. The role of biostimulants and effectors as alleviators of abiotic stress in crop plants. Chemical and Biological Technologies in Agriculture. 2017;4(1):1–12. doi: 10.1186/s40538-017-0089-5. [DOI] [Google Scholar]
- Ventura SPM, Nobre BP, Ertekin F, Hayes M, Garciá-Vaquero M, Vieira F, et al. Microalgae-based biofuels and bioproducts. Woodhead Publishing; 2017. Extraction of value-added compounds from microalgae; pp. 461–483. [Google Scholar]
- Wally OSD, Critchley AT, Hiltz D, Craigie JS, Han X, Zaharia LI, et al. Regulation of phytohormone biosynthesis and accumulation in arabidopsis following treatment with commercial extract from the marine macroalga Ascophyllum nodosum. Journal of Plant Growth Regulation. 2013;32(2):324–339. doi: 10.1007/s00344-012-9301-9. [DOI] [Google Scholar]
- Wang X, Wu L, Xie J, Li T, Cai J, Zhou Q, et al. Herbicide isoproturon aggravates the damage of low-temperature stress and exogenous ascorbic acid alleviates the combined stress in wheat seedlings. Plant Growth Regulation. 2018;84(2):293–301. doi: 10.1007/s10725-017-0340-x. [DOI] [Google Scholar]
- Wijffels RH, Kruse O, Hellingwerf KJ. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Current Opinion in Biotechnology. 2013;24(3):405–413. doi: 10.1016/j.copbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Xu Y, Rossi F, Colica G, Deng S, De Philippis R, Chen L. Use of cyanobacterial polysaccharides to promote shrub performances in desert soils: a potential approach for the restoration of desertified areas. Biology and Fertility of Soils. 2013;49(2):143–152. doi: 10.1007/s00374-012-0707-0. [DOI] [Google Scholar]
- Yadav SK. Cold stress tolerance mechanisms in plants. A review. Agronomy for Sustainable Development. 2010;30(3):515–527. doi: 10.1051/agro/2009050. [DOI] [Google Scholar]
- Yadav P, Gupta RK, Singh RP, Yadav PK, Patel AK, Pandey KD. Sustainable Environmental Clean-up. Elsevier; 2021. Role of cyanobacteria in green remediation; pp. 187–210. [Google Scholar]
- Zhang S, Qiu CB, Zhou Y, et al. Bioaccumulation and degradation of pesticide fluroxypyr are associated with toxic tolerance in green alga Chlamydomonas reinhardtii. Ecotoxicology. 2011;20:337–347. doi: 10.1007/s10646-010-0583-z. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu L, Zhang S, Pan Y, Li J, Pan H, Xu S, Luo F. Biodegradation of dimethyl phthalate by freshwater unicellular cyanobacteria. BioMed Research International. 2016;2016:5178697. doi: 10.1155/2016/5178697. [DOI] [PMC free article] [PubMed] [Google Scholar]