Abstract
Knowledge of species’ functional traits is essential for understanding biodiversity patterns, predicting the impacts of global environmental changes, and assessing the efficiency of conservation measures. Bats are major components of mammalian diversity and occupy a variety of ecological niches and geographic distributions. However, an extensive compilation of their functional traits and ecological attributes is still missing. Here we present EuroBaTrait 1.0, the most comprehensive and up-to-date trait dataset covering 47 European bat species. The dataset includes data on 118 traits including genetic composition, physiology, morphology, acoustic signature, climatic associations, foraging habitat, roost type, diet, spatial behaviour, life history, pathogens, phenology, and distribution. We compiled the bat trait data obtained from three main sources: (i) a systematic literature and dataset search, (ii) unpublished data from European bat experts, and (iii) observations from large-scale monitoring programs. EuroBaTrait is designed to provide an important data source for comparative and trait-based analyses at the species or community level. The dataset also exposes knowledge gaps in species, geographic and trait coverage, highlighting priorities for future data collection.
Subject terms: Ecology, Zoology
Background & Summary
Functional traits are becoming increasingly important in large-scale ecological and evolutionary analyses, notably because they allow integrating individual-level information to species level1. Functional traits can be defined as any feature measurable at the individual level that can influence fitness2. Trait-based approaches functionally link individual organisms to community structure and dynamics through their physiological, morphological, or life-history attributes3 and facilitate generalisations across species and their assemblages4. Trait-based approaches have become increasingly popular in biogeography5, community ecology1, macroecology6, evolution7, conservation biology8 and ecosystem functioning9. They are now widely used to estimate biodiversity patterns and trends10,11, as well as to unveil the mechanisms underlying species assemblages12. By understanding how traits covary and are related to environmental variables we can infer general ecological principles that overcome taxonomic gaps. The use of a species-level trait dataset is particularly relevant when working at the community level and in the context of rapid environmental changes.
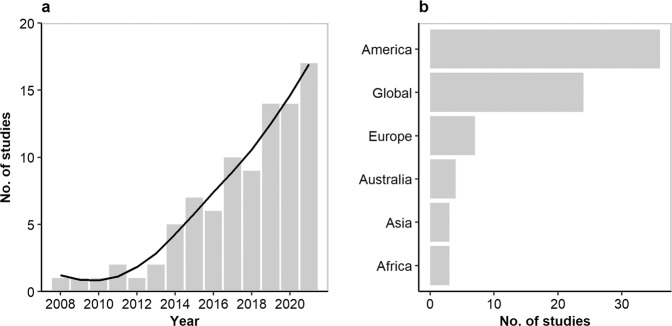
With 1,439 species distributed across the globe13, bats (order Chiroptera) account for ca. a fifth of global mammalian diversity. While bats are the second richest taxonomic order of mammals, they have been so far understudied compared to other groups14. In the absence of in-depth knowledge of how species respond to environmental gradients, we can make inferences on understudied species using traits of the most closely related taxa. Trait-based approaches are becoming more common in bat research15–91 (Fig. 1). For example, Conenna, et al.92 investigated the traits that favour bat persistence in arid environments across the globe. Similarly, Blakey, et al.93 investigated traits that explain bat community changes after wildfires. Jung and Threlfall55 studied traits relevant for tolerance of urban environments in bats. These studies help overcome the taxonomic knowledge gaps and derive general principles that can explain patterns of bat distribution and abundance, and their responses to the environment. This is especially important in the context of global change, where there is a pressing need for large-scale predictions of biodiversity responses to environmental change94.
Fig. 1.
Number of peer-reviewed studies per year (a) and per geographic area (b) that implemented a trait-based approach to study bats15–91. Data were extracted from a systematic literature search conducted in Web of Science and Google Scholar on the 15th of November 2021 using the following search string terms: (Bat* OR Chiroptera) AND (“trait-base*” OR “trait diversity” OR “functional diversity” OR trait*). The black solid line represents the LOESS (locally weighted scatterplot smoothing) fit to the observed relationship. See raw data and details in Supplementary Material 1.
The application of trait-based approaches in bat research and the inclusion of bats in wider trait-based studies in Europe and elsewhere (Fig. 1) have been limited to date due to lack of relevant and reliable trait data for most bat species. Many European bats have been studied extensively, but the most reliable information on their functional traits has been scattered across the scientific and grey literature and unpublished data held by researchers and special interest groups. There are several ongoing national95,96 and regional97 bat monitoring programs across Europe that are now old enough to provide highly reliable data on population trends and distributions. These programs also collect systematic and standardised data on morphology, echolocation, hibernation patterns and roost selection that are extremely useful to bat functional ecology.
Here, we present EuroBaTrait 1.0, a comprehensive open-access dataset of 118 traits for 47 European bat species built by compiling data from (i) a systematic literature and dataset search, (ii) unpublished data from pan-European bat experts and (iii) large-scale bat monitoring programs in France. EuroBaTrait 1.0 aims to fill the current knowledge gap to facilitate integrative trait-based evolutionary and ecological research on bat species, and mammals more generally, at the European scale and beyond. As our objective was to build a living dataset, EuroBaTrait 1.0 is intended to be updated annually and its geographic and taxonomic scope can be expanded over time.
Methods
Taxon and geographic coverage
Our dataset includes the complete bat fauna of Europe based on the Handbook of the Mammals of Europe98. This represents 47 bat species from 12 genera (Table 1). The taxonomic nomenclature follows the Handbook of the Mammals of Europe. The geographic coverage is mainland Europe and European islands, including the British Isles, Mediterranean islands and Macaronesia (Supplementary Material 2). The dataset is based mainly on recent publications, following recent taxonomic revisions (i.e. 2021)13
Table 1.
List of the 47 bat species included in EuroBaTrait 1.0.
| Family | Species: scientific name | Species: vernacular name | Taxon URI |
|---|---|---|---|
| Miniopteridae | Miniopterus schreibersii | Schreibers’ bent-winged bat | https://www.gbif.org/species/9796816 |
| Molossidae | Tadarida teniotis | European free-tailed bat | https://www.gbif.org/species/2433009 |
| Pteropodidae | Rousettus aegyptiacus | Egyptian fruit bat | https://www.gbif.org/species/2432953 |
| Rhinolophidae | Rhinolophus blasii | Blasius’ horseshoe bat | https://www.gbif.org/species/2432666 |
| Rhinolophidae | Rhinolophus euryale | Mediterranean horseshoe bat | https://www.gbif.org/species/2432621 |
| Rhinolophidae | Rhinolophus ferrumequinum | Greater horseshoe bat | https://www.gbif.org/species/2432655 |
| Rhinolophidae | Rhinolophus hipposideros | Lesser horseshoe bat | https://www.gbif.org/species/2432614 |
| Rhinolophidae | Rhinolophus mehelyi | Mehely’s horseshoe bat | https://www.gbif.org/species/2432667 |
| Vespertilionidae | Barbastella barbastellus | Barbastelle bat | https://www.gbif.org/species/2432582 |
| Vespertilionidae | Eptesicus anatolicus | Anatolian serotine bat | https://www.gbif.org/species/5787592 |
| Vespertilionidae | Eptesicus bottae | Botta’s serotine bat | https://www.gbif.org/species/2432346 |
| Vespertilionidae | Eptesicus isabellinus | Isabelline serotine bat | https://www.gbif.org/species/5787585 |
| Vespertilionidae | Eptesicus nilssonii | Northern bat | https://www.gbif.org/species/7261816 |
| Vespertilionidae | Eptesicus serotinus | Common serotine bat | https://www.gbif.org/species/2432359 |
| Vespertilionidae | Hypsugo savii | Savi’s pipistrelle | https://www.gbif.org/species/7261861 |
| Vespertilionidae | Myotis alcathoe | Alcathoe bat | https://www.gbif.org/species/4266346 |
| Vespertilionidae | Myotis bechsteinii | Bechstein’s bat | https://www.gbif.org/species/2432427 |
| Vespertilionidae | Myotis blythii | Lesser mouse-eared bat | https://www.gbif.org/species/2432414 |
| Vespertilionidae | Myotis brandtii | Brandt’s bat | https://www.gbif.org/species/7261875 |
| Vespertilionidae | Myotis capaccinii | Long-fingered bat | https://www.gbif.org/species/2432430 |
| Vespertilionidae | Myotis crypticus | Cryptic myotis | https://www.gbif.org/species/9918569 |
| Vespertilionidae | Myotis dasycneme | Pond bat | https://www.gbif.org/species/2432452 |
| Vespertilionidae | Myotis daubentonii | Daubenton’s bat | https://www.gbif.org/species/2432439 |
| Vespertilionidae | Myotis davidii | David’s myotis | https://www.gbif.org/species/4266349 |
| Vespertilionidae | Myotis emarginatus | Geoffroy’s bat | https://www.gbif.org/species/2432470 |
| Vespertilionidae | Myotis escalerai | Escalera’s bat | https://www.gbif.org/species/8181305 |
| Vespertilionidae | Myotis myotis | Greater mouse-eared bat | https://www.gbif.org/species/2432416 |
| Vespertilionidae | Myotis mystacinus | Common whiskered bat | https://www.gbif.org/species/9754263 |
| Vespertilionidae | Myotis nattereri | Natterer’s bat | https://www.gbif.org/species/2432389 |
| Vespertilionidae | Myotis punicus | Maghreb mouse-eared bat | https://www.gbif.org/species/4266337 |
| Vespertilionidae | Nyctalus azoreum | Azorean bat | https://www.gbif.org/species/5218523 |
| Vespertilionidae | Nyctalus lasiopterus | Greater noctule | https://www.gbif.org/species/5218525 |
| Vespertilionidae | Nyctalus leisleri | Leisler’s noctule | https://www.gbif.org/species/5218522 |
| Vespertilionidae | Nyctalus noctula | Common noctule | https://www.gbif.org/species/5218524 |
| Vespertilionidae | Pipistrellus kuhlii | Kuhl’s pipistrelle | https://www.gbif.org/species/5218464 |
| Vespertilionidae | Pipistrellus maderensis | Madeira pipistrelle | https://www.gbif.org/species/5218476 |
| Vespertilionidae | Pipistrellus nathusii | Nathusius’ pipistrelle | https://www.gbif.org/species/5218471 |
| Vespertilionidae | Pipistrellus pipistrellus | Common pipistrelle | https://www.gbif.org/species/5218465 |
| Vespertilionidae | Pipistrellus pygmaeus | Soprano pipistrelle | https://www.gbif.org/species/5707150 |
| Vespertilionidae | Plecotus auritus | Brown long-eared bat | https://www.gbif.org/species/5218507 |
| Vespertilionidae | Plecotus austriacus | Gray long-eared bat | https://www.gbif.org/species/5739437 |
| Vespertilionidae | Plecotus gaisleri | Gaisler’s long-eared bat | https://www.gbif.org/species/10893276 |
| Vespertilionidae | Plecotus kolombatovici | Balkan long-eared bat | https://www.gbif.org/species/5739445 |
| Vespertilionidae | Plecotus macrobullaris | Alpine long-eared bat | https://www.gbif.org/species/5787719 |
| Vespertilionidae | Plecotus sardus | Sardinian long-eared bat | https://www.gbif.org/species/5739436 |
| Vespertilionidae | Plecotus teneriffae | Tenerife long-eared bat | https://www.gbif.org/species/5218519 |
| Vespertilionidae | Vespertilio murinus | Parti-coloured bat | https://www.gbif.org/species/2432564 |
Trait categories
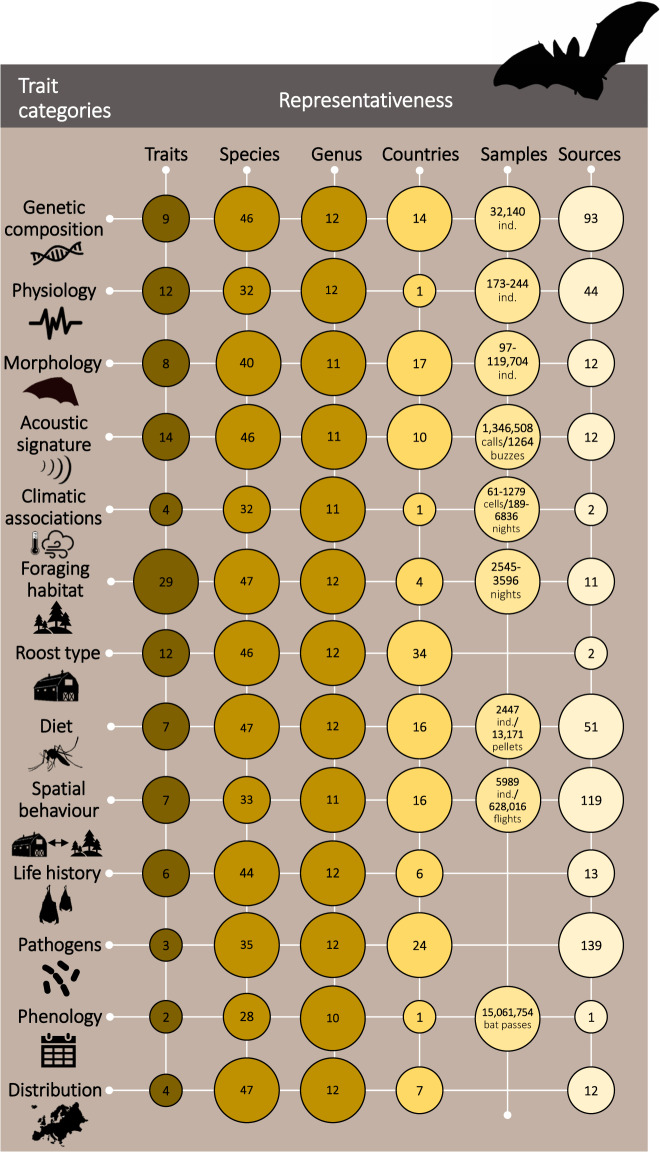
Based on the literature and expert-knowledge, we selected a comprehensive set of relevant traits, including both commonly used and bat-specific traits. These traits reflect a variety of ecological strategies, niches, and functional roles that are routinely collected for bats and other taxa to enable joint analyses with other datasets. Traits were divided into 13 categories: genetic composition (N = 9), physiology (N = 12), morphology (N = 8), acoustic signature (N = 14), climatic associations (N = 4), foraging habitat (N = 29), roost type (N = 12), diet (N = 7), spatial behaviour (N = 7), life history (N = 6), pathogens (N = 3), phenology (N = 2), and distribution (N = 5). While in theory a given trait can fall into different categories, we affiliated each trait to a single, most relevant category for clarity and to simplify analysis.
Dataset entry
All traits are given at the species and country/European level in the final dataset, but we also provide individual-level data (when available). Each trait value (i.e. dataset entry) is accompanied (whenever possible and applicable) by (i) a location (x,y coordinates and/or country), (ii) contextual characteristics, (iii) source, and (iv) estimates of precision. For aggregated values such as means and medians, we also provide the number of replicates and a measure of dispersion.
Data sources and acquisition
We followed three strategies to compile the EuroBaTrait 1.0 dataset. First, we conducted a systematic literature and dataset search and retrieved trait data from published books, taxonomic monographs, scientific articles, online resources, and existing datasets. Second, we put out a call to request unpublished data from European bat experts via the network of COST Action Network CA18107 “ClimBats” (https://climbats.eu/). Third, we used data from two large-scale French standardised bat monitoring programs (“Vigie-Chiro” and “CACCHI” programs; https://www.vigienature.fr/fr/chauves-souris & https://croemer3.wixsite.com/teamchiro/cacchi) to retrieve key traits and to derive traits that could not be collected following the first two strategies. The first version of the trait dataset was finalised after the three strategies had been completed for all taxa.
Literature and dataset search
We conducted the literature and dataset search using Web of Science and Google Scholar. We implemented a multi-step approach to target our search. In step 1, we based our search at the trait category level using the search string “(Bat* OR Chiroptera)” in addition to the name of a given trait category (see section 2.2 Trait categories) (e.g. “(Bat* OR Chiroptera)” and “acoustic*”). In step 2, we looked for each specific trait name (e.g. “(Bat* OR Chiroptera)” and “call duration”). In step 3, we focused our search on missing data. For this we used as a search string the name of the missing trait and the Latin name of the target bat species (e.g. “Plecotus auritus” and “call duration”). Altogether, we included trait data from six published books, nine taxonomic monographs, 378 scientific articles (published in indexed journals that were checked using clarivate https://mjl.clarivate.com/), 84 articles from the grey literature, 19 unpublished data sources, and three datasets (AnAge99, date of access: 02/02/2021, IUCN100, date of access: 20/01/2021; DBatVir101, date of access: 18/01/2021).
ClimBats COST Action network
Morphological measures taken from bats captured for research and monitoring are rarely published, so we asked European bat experts to provide data for traits that are usually not found in the literature. We contacted experts through the ClimBats network (representing 28 countries and ca. 100 experts), organised meetings to explain the project goals, and asked for raw or summarised unpublished trait data (e.g. morphological measurements). Data were also provided by the UK Bat Conservation Trust National Bat Monitoring Programme (https://www.bats.org.uk/our-work/national-bat-monitoring-programme). In total, we retrieved 279 trait values (i.e. dataset entry) for two morphological traits (mean forearm length and body mass) measured on 37 species in 12 countries (Bulgaria, Germany, Ireland, Italy, Luxembourg, Moldova, Norway, Poland, Portugal, Slovakia, Spain and the United Kingdom).
Large-scale bat monitoring programs
We used “CACCHI” (capture data) and the French national-scale citizen-science bat monitoring program “Vigie-Chiro” (acoustic data) which are coordinated by the French National Museum of Natural History (MNHN) to retrieve data on key traits and identify missing ones. CACCHI is a French bat monitoring program that aims to achieve national consistency regarding technical and ethical aspects of capture practices and data collection. French bat workers contribute to this program by providing data collected in a local context, thus giving the opportunity to build a dataset on a large spatio-temporal scale. We retrieved four traits from CACCHI, including mean forearm length and body mass (>60,000 and >6,000 dataset entries, respectively). Vigie-Chiro is one of the largest citizen-science bat monitoring programs in Europe with a standardized stationary point survey (among three) corresponding to a total of 16,349 sites acoustically monitored during 2015–2021 by >500 participants (see an overall description of Vigie-Chiro in Supplementary Material 3). We retrieved 33 species-level traits from the data collected by Vigie-Chiro, including acoustic signature, climatic associations, foraging habitat, and phenology (see full details in Supplementary Material 3). In brief, traits related to acoustic signature (i.e. buzz duration, buzz peak frequency, buzz rate, call duration, call maximum/minimum frequency, call frequency at half call duration, call peak frequency, call slope and interpulse interval) were directly extracted from the reference library of calls using TADARIDA software102. For traits related to climatic associations (i.e. responses to nightly temperature, precipitation, and wind speed) and foraging habitats (i.e. responses to deciduous forest, coniferous forest, dense urban area, freshwater, cropland and grassland at three spatial scales (50 m, 500 m and 5000 m radius buffer scale)), we used data from the stationary point protocol. We conducted a series of univariate generalized linear mixed models (‘glmmTMB’ R package103) with species-specific bat activity as response variables and foraging habitat and weather conditions as explanatory ones. The latter were standardized (mean = 0, SD = 1) and we extracted the slope of the relationship to inform the trait value. Finally, we derived the traits related to phenology (Kurtosis index and Skewness index of the seasonal activity pattern) with the ‘moments’ R package104, which required plotting bat activity obtained from the stationary point protocol as a function of Julian day.
Data Records
The EuroBaTrait 1.0 dataset is available at figshare105 and through the R Shiny App (https://jasja.shinyapps.io/ClimBats/) under the terms of a Creative Commons Attribution 4.0 International waiver. The CC-BY-4.0 waiver facilitates the discovery, re-use, and citation of the dataset. As this is a dynamic and living dataset, future updates of the dataset will be made directly on figshare and associated R Shiny App. We will follow the same technical validation as described hereafter.
Dataset structure
We followed the general recommendations proposed by Schneider, et al.106 to organise the dataset. The dataset consists of 16 tables linked by unique identifiers. The “Taxon” table includes the full scientific name and family of all taxa and a uniform resource identifier linked to GBIF (https://www.gbif.org/, Table 1). The “Trait description” table represents the metadata, in which we describe the different traits (name, category, unit, data type) and define each trait following (when available) current glossaries (e.g. we followed Blatteis, et al.107 for physiology). The “Trait reference” table lists the full references used to build the dataset. The dataset also includes one table per trait category – namely, genetic composition, physiology, morphology, acoustic signature, climatic associations, foraging habitat, roost type, diet, spatial behaviour, life history, pathogens, phenology, and distribution – with the core observation values (SD, CV and N referring to standard deviation, coefficient of variation and sample size, respectively) and associated information. We decided not to impute missing values to highlight research gaps and data needs.
Dataset completeness
Assessing dataset completeness is crucial for identifying knowledge gaps and highlighting future data collection priorities. The overall completeness of the dataset can be assessed based on species coverage, geographic coverage, and trait resolution. The EuroBaTrait 1.0 dataset has broad taxonomic coverage (Fig. 2). At the trait category level, the species coverage of trait information is (i) complete or nearly complete (i.e. at least one trait from these categories encompasses >95% of the species) for foraging habitat (100%), diet (100%), distribution (100%), acoustic signature (98%), roost type (98%), genetic composition (96%) and morphology (96%), (ii) moderately complete (between 75% and 95%) for life history (94%) and pathogens (72%), and (iii) at a low level of completeness (<75%) for spatial behaviour (66%), climatic associations (60%), phenology (60%) and physiology (55%). Note that the number of traits documented differ greatly amongst trait categories (see section 2.2 Trait categories and Fig. 2). At the trait level, species coverage ranges from 100% (e.g. geographic range in distribution) to 2% (e.g. heart rate during flight or rest in physiology). The physiology category has the lowest number of traits covered per species (Fig. 2) and it is evident that more research effort is needed to improve our knowledge on bat physiology. Regardless of trait type, we lack information on the four island endemic species (Nyctalus azoreum, Pipistrellus maderensis, Plecotus sardus, and Plecotus teneriffae) with >75% of traits missing for these species. Regarding geographic coverage, there is a clear longitudinal bias for many trait categories with most data originating from Western Europe (Fig. 3). Nevertheless, this bias is more evident for some traits (e.g. foraging habitat and spatial behaviour) than others (e.g. roost type and pathogens, Supplementary Material 4) because few traits and species have been comprehensively measured in many locations throughout Europe. Finally, when looking at the trait resolution, quantitative data (continuous and discrete) were available for 71% of the traits. This proportion, however, largely varies between trait categories (e.g. 0% for roost and 100% for morphology). Increasing species and geographic coverage as well as trait resolution (from categorical to quantitative data, whenever possible) remains a real challenge that needs to be overcome to implement a more robust trait-based approach108. To that end, enhancing collaboration among researchers/practitioners is the way forward, as witnessed here with the collection of the largest morphological dataset on bats in Europe if not in the world (measurements on body mass of ca. 55,000 individuals from 37 species and forearm length of ca. 120,000 individuals from 39 species).
Fig. 2.
Trait by species matrix illustrating the EuroBaTrait 1.0 dataset completeness.
Fig. 3.
Number of trait categories (genetic composition, physiology, morphology, acoustic signature, climatic associations, foraging habitat, roost type, diet, spatial behaviour, life history, pathogens, phenology, and distribution) provided at the country level across all species. For sake of clarity and for highlighting gaps in geographic coverage, we did not consider in this map traits provided across a given species’ range or at regional level. Details on each trait category are provided in Supplementary Material 4.
Current limitations
We acknowledge that many traits could not be compiled for the EuroBaTrait dataset 1.0 at their finest resolution. This is particularly the case for the diet trait category. There are many published and unpublished studies in different languages depicting European bat diets using traditional (e.g. microscopic faecal or stomach content analysis) or molecular (e.g. DNA metabarcoding) methods. However, gathering such a large amount of information at the highest resolution was not feasible at this stage, and we aim to provide a much more comprehensive treatment of this trait category in future releases of the EuroBaTrait dataset.
Trait quality, here defined as the degree of confidence in a trait value, is another important component to consider when assessing dataset completeness. However, evaluation of trait quality is subject to a certain degree of subjectivity. To allow future users of the dataset to evaluate trait information quality, we provide for each trait value the original sources and contextual characteristics that may include (when available) sample size (e.g. number of individuals) associated with the trait values. For quantitative continuous data, we also derived the coefficient of variation as a standardised measure of dispersion of the values around the mean.
Data visualization
We created a R Shiny app to help the users visualizing the trait data. It is freely accessible at the following URL: https://jasja.shinyapps.io/ClimBats/.
Technical Validation
We employed two main strategies to ensure the accuracy in the data included in the EuroBaTrait 1.0 dataset. First, we looked for erroneous data in the dataset using a series of plots (e.g. boxplots and frequency histograms to detect potential outliers across species in continuous data) alongside traditional statistical validation techniques (e.g. outlier test) in R v4.1.1109. When an outlier was detected, we looked at the original sources and cross-checked with other sources (e.g. Handbook of the Mammals of Europe) the plausibility and reliability of the observation. Second, we asked several experts to conduct data quality control and error detection in their field of expertise. Experts on a given bat species revised the trait associated with that species, while experts on a given trait category revised all traits from that category. This allowed us to cross validate the full dataset. Furthermore, as we provide the original references for each trait value collected during the literature and dataset search and describe in detail the methods used for the traits computed with data from the large-scale bat monitoring programs, users can assess the validity and accuracy of the original sources (note that >75% of our data sources are peer-reviewed articles published in indexed scientific journals) and method used. Finally, we strongly encourage users to report any errors and additional data sources directly to the corresponding authors. As it is a living dataset, the inclusion of new data will rigorously follow the same technical validation as described above.
Usage Notes
Overview
Here we present the first comprehensive dataset of functional traits in European bats (Fig. 4). The dataset has numerous applications in community ecology, macroecology, biogeography, conservation biology, ecophysiology, evolutionary biology, and virology/epidemiology for species and/or community approaches. For example, the dataset enables researchers to investigate functional redundancy and complementarity of coexisting bat species, to establish linkages among functional traits, as well as between functional traits and ecosystem functioning, and to assess trait-environment relationships and their impacts on bat distributional patterns92. This in turn is relevant for addressing relevant questions in conservation and global change biology. The paucity of trait data on bats has, until now, prevented the testing of large-scale correlations between the intrinsic characteristics of species and major drivers of decline in this taxon11,110,111. The EuroBaTrait dataset will contribute to assessing and quantifying the role of each trait in determining bats’ risk of extinction.
Fig. 4.
Representativeness of the EuroBaTrait 1.0 dataset. Summary of the 13 trait categories included in the trait dataset and their coverage in terms of number of traits included under the category, number of species and genera with data for at least one of the traits under the category, number of countries with data for at least one trait under the category (regional or global data were not included in these calculations), number of samples (individuals, calls or nights) used to generate values for the traits, and number of data sources on which the trait values are based.
EuroBaTrait is also designed to be used to investigate functional responses of bat assemblages to various environmental drivers of change and their impacts on ecosystem functions and services5. This could be done by relating environmental variables or processes to traits, i.e. either by calculating complementary and integrative functional diversity metrics such as functional richness, evenness or dispersion112 or by computing individual Community-Weighted Mean trait values113. Such trait-based approaches allow the inference of several key properties of bat communities including effective functional originality based on both rarity and distinctiveness of species in functional trait spaces to better support bat conservation programmes114,115. Three-table ordination methods can also be used to directly link bat trait syndromes to spatial or temporal environmental gradients (e.g.43–48). These trait-based analyses constitute a methodological corpus that is still seldom used by bat ecologists, while it can provide a relevant standard analysis approach to study the response of bat functional diversity and trait assemblages to bioclimatic variables, or environmental degradation and conservation measures (Fig. 1; see for example17,20,30,32,38,41,51,67,83,89,116). Finally, bats are increasingly acknowledged for performing key ecological functions in semi-natural habitats as well as in production forests and agroecosystems, that ultimately provide valuable ecosystem services117–120. As a result, the functional trait-based characterisation of bat communities to determine their role in ecological networks will greatly benefit from the EuroBaTrait dataset in future bat ecological research.
From a static to a dynamic trait dataset
The data descriptor was peer reviewed in 2023 based on the data available on the platform at the time105. It is our intention to keep improving and enriching the dataset over time, so while the paper presents version 1, new versions may be released in the future. For this reason, we invite anyone to (i) share their data in a standardized way via the R Shiny app at https://jasja.shinyapps.io/ClimBats/ and (ii) cite the dataset stored at figshare according to the specific version used as well as this publication, when using all or part of the dataset. We also encourage the wider bat research community to build on this first version and engage in updating it via meetings and workshops during dedicated bat congress (e.g. European Bat Research Symposiums).
Towards an open access individual-level trait dataset
We encourage researchers and practitioners to collect, store publicly and publish trait values at the individual level. As most functional traits tend to vary greatly among individuals according to age, sex, condition, state, gene pool and behavioural personality121, this individual-level variation may persist across time and space and upscale at the local community level (e.g. mean individual specialisation or mean home range size and composition)122,123. Natural populations of a given species are composed of sets of individuals that occupy subsets of the species’ niche. For instance, several studies of bats provide evidence of individual specialisation in diet124. Furthermore, trait-based approaches accounting for within-individual variation in species traits can inform us about the role of phenotypic plasticity in species’ responses to anthropogenic threats and drivers of global change (e.g.125–127). Within-individual data are particularly valuable in seasonal species like bats, which have to cope with reproduction, hibernation and/or migration. While we acknowledge that some traits can only be measured at the species level, the lack of individual-level trait information we observed during data collection likely arises from the fact that: (i) only summarised information (e.g. mean/median, accompanied with an estimate of precision at best) has traditionally been provided in the published literature, (ii) researchers/practitioners and/or institutions have limited time and resources, as well as almost no formal recognition (although the situation is now improving in many countries), for publishing such data128. We provide in Supplementary Material 5 trait information collected at the individual level, but we strongly encourage the bat expert community and associated institutions to share and open their data (FAIR Data principles) to enable the progression from a species-level trait dataset to an individual-level trait dataset.
Supplementary information
A species-level trait dataset of bats in Europe and beyond -- Supplementary Material
A species-level trait dataset of bats in Europe and beyond -- Supplementary Material
Acknowledgements
This article is based on work from COST Action CA18107 ‘Climate change and bats: from science to conservation – ClimBats’ (https://climbats.eu/), supported by COST (European Cooperation in Science and Technology). We deeply thank the Vigie-Chiro volunteers for data collection, and the CC-IN2P3 and PCIA-MNHN for providing computing and storage facilities to process and archive in the long-term all the acoustic recordings of Vigie-Chiro, and Didier Bas for his help in this process. We thank the Bat Conservation Trust National Bat Monitoring Programme (NBMP) for providing data, and especially Phillip Briggs for collating the data. We thank the Groupe Mammalogique Breton, Office National des Forêts, Groupe Chiroptères Corse, Coordination mammalogique du Nord de la France, Chauve-Souris Auvergne, Ligue pour la Protection des Oiseaux - Rhône-Alpes, Groupe Chiroptères Poitou-Charente, Groupe d'Étude et de Protection des Mammifères d’Alsace, Labex ECOFECT (Université de Lyon), Groupe Chiroptères de Provence, Commission de Protection des Eaux, du Patrimoine, de l’Environnement, du Sous-sol et des Chiroptères de Lorraine, Commission de Protection des Eaux, du Patrimoine, de l’Environnement, du Sous-sol et des Chiroptères de Franche-Comté, Benjamin Allegrini, Gildas Monnier, Raphael Colombo, Arnaud Le Houédec, James Jean-Baptiste, Rémy Grignon, Marie-Jo Dubourg-Savage, and all bat workers who have participated in the CACCHI programs. We thank Laurent Dacheux from the Institut Pasteur for the discussion around the dataset.
Author contributions
J.S.P.F. and N.T. contributed equally to the construction of the EuroBaTrait 1.0 dataset and the manuscript. J.S.P.F., N.T., L.S. and O.R. conceived the idea. J.S.P.F., O.R. and N.T. coordinated the collection of traits and J.S.P.F. and N.T. compiled most of the trait data. J.S.P.F. conducted the literature review and structured the EuroBaTrait dataset. J.S.P.F. led the writing of the manuscript with the support of L.B., A.B.L., C.K., I.L.V., M.P., L.S., C.S. and O.R. J.D. created the Shiny App. D.R. secured the funding and coordinated the COST Action. All authors provided and/or calculated trait data and provided comments to the manuscript and/or to the dataset.
Funding
We would like to acknowledge funding from the EU Framework Horizon 2020 through the COST Action CA18107 ‘Climate change and bats: from science to conservation – ClimBats’ (https://climbats.eu/). Jeremy Froidevaux was funded by the Région Bretagne (SAD grant number 19041) and the Leverhulme Trust (grant number: ECF-2020-571). Nia Toshkova was funded by the Bulgarian National Science Fund (CP-06-COST/15 from 16.12.2020) and а PhD Fellowship from Karoll Knowledge Foundation. Orly Razgour was supported through a Natural Environment Research Council Independent Research Fellowship (NE/M018660/1).
Code availability
The code to create the R Shiny App is available on GitHub (https://github.com/J4SJA/ClimBatsApp).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jérémy Froidevaux, Nia Toshkova.
Contributor Information
Jérémy S. P. Froidevaux, Email: jeremy.froidevaux@stir.ac.uk
Danilo Russo, Email: danrusso@unina.it.
Orly Razgour, Email: o.razgour@exeter.ac.uk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-023-02157-4.
References
- 1.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends in Ecology & Evolution. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Violle C, et al. Let the concept of trait be functional! Oikos. 2007;116:882–892. doi: 10.1111/j.0030-1299.2007.15559.x. [DOI] [Google Scholar]
- 3.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. doi: 10.1046/j.1365-2435.2002.00664.x. [DOI] [Google Scholar]
- 4.Zakharova L, Meyer K, Seifan M. Trait-based modelling in ecology: a review of two decades of research. Ecological Modelling. 2019;407:108703. doi: 10.1016/j.ecolmodel.2019.05.008. [DOI] [Google Scholar]
- 5.Violle C, Reich PB, Pacala SW, Enquist BJ, Kattge J. The emergence and promise of functional biogeography. Proceedings of the National Academy of Sciences. 2014;111:13690–13696. doi: 10.1073/pnas.1415442111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González‐Suárez M, Zanchetta Ferreira F, Grilo C. Spatial and species‐level predictions of road mortality risk using trait data. Global Ecology and Biogeography. 2018;27:1093–1105. doi: 10.1111/geb.12769. [DOI] [Google Scholar]
- 7.Groussin M, et al. Unraveling the processes shaping mammalian gut microbiomes over evolutionary time. Nature Communications. 2017;8:1–12. doi: 10.1038/ncomms14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santini, L. et al. The interface between macroecology and conservation: existing links and untapped opportunities. Frontiers in Biogeography13 (2021).
- 9.Gross N, et al. Functional trait diversity maximizes ecosystem multifunctionality. Nature Ecology & Evolution. 2017;1:1–9. doi: 10.1038/s41559-017-0132. [DOI] [PubMed] [Google Scholar]
- 10.Angert AL, et al. Do species’ traits predict recent shifts at expanding range edges? Ecology Letters. 2011;14:677–689. doi: 10.1111/j.1461-0248.2011.01620.x. [DOI] [PubMed] [Google Scholar]
- 11.Pacifici M, et al. Species’ traits influenced their response to recent climate change. Nature Climate Change. 2017;7:205–208. doi: 10.1038/nclimate3223. [DOI] [Google Scholar]
- 12.Potter AB, et al. Mechanisms of dietary resource partitioning in large‐herbivore assemblages: A plant‐trait‐based approach. Journal of Ecology. 2022;110:817–832. doi: 10.1111/1365-2745.13843. [DOI] [Google Scholar]
- 13.Simmons, N. B. & Cirranello, A. L. Bat Species of the World: A taxonomic and geographic database. Accessed on 11/31/2021 (2021).
- 14.dos Santos JW, et al. Drivers of taxonomic bias in conservation research: a global analysis of terrestrial mammals. Animal Conservation. 2020;23:679–688. doi: 10.1111/acv.12586. [DOI] [Google Scholar]
- 15.Alberdi A, et al. DNA metabarcoding and spatial modelling link diet diversification with distribution homogeneity in European bats. Nature Communications. 2020;11:1154. doi: 10.1038/s41467-020-14961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bader E, et al. Mobility explains the response of aerial insectivorous bats to anthropogenic habitat change in the Neotropics. Biological Conservation. 2015;186:97–106. doi: 10.1016/j.biocon.2015.02.028. [DOI] [Google Scholar]
- 17.Barbaro L, et al. Biotic predictors complement models of bat and bird responses to climate and tree diversity in European forests. Proceedings of the Royal Society B. 2019;286:20182193. doi: 10.1098/rspb.2018.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker DJ, et al. Ecological and evolutionary drivers of haemoplasma infection and bacterial genotype sharing in a Neotropical bat community. Molecular ecology. 2020;29:1534–1549. doi: 10.1111/mec.15422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belmaker J, Jetz W. Relative roles of ecological and energetic constraints, diversification rates and region history on global species richness gradients. Ecology Letters. 2015;18:563–571. doi: 10.1111/ele.12438. [DOI] [PubMed] [Google Scholar]
- 20.Blakey RV, Law BS, Kingsford RT, Stoklosa J. Terrestrial laser scanning reveals below-canopy bat trait relationships with forest structure. Remote Sensing of Environment. 2017;198:40–51. doi: 10.1016/j.rse.2017.05.038. [DOI] [Google Scholar]
- 21.Bogoni JA, Carvalho‐Rocha V, Ferraz KM, Peres CA. Interacting elevational and latitudinal gradients determine bat diversity and distribution across the Neotropics. Journal of Animal Ecology. 2021;90:2729–2743. doi: 10.1111/1365-2656.13594. [DOI] [PubMed] [Google Scholar]
- 22.Bowler DE, et al. A cross-taxon analysis of the impact of climate change on abundance trends in central Europe. Biological Conservation. 2015;187:41–50. doi: 10.1016/j.biocon.2015.03.034. [DOI] [Google Scholar]
- 23.Burns LE, Broders HG. Correlates of dispersal extent predict the degree of population genetic structuring in bats. Conservation genetics. 2014;15:1371–1379. doi: 10.1007/s10592-014-0623-y. [DOI] [Google Scholar]
- 24.Byamungu RM, et al. Abiotic and biotic drivers of functional diversity and functional composition of bird and bat assemblages along a tropical elevation gradient. Diversity and Distributions. 2021;27:2344–2356. doi: 10.1111/ddi.13403. [DOI] [Google Scholar]
- 25.Carrasco-Rueda F, Loiselle BA. Dimensions of phyllostomid bat diversity and assemblage composition in a tropical forest-agricultural landscape. Diversity. 2020;12:238. doi: 10.3390/d12060238. [DOI] [Google Scholar]
- 26.Carstens BC, Morales AE, Field K, Pelletier TA. A global analysis of bats using automated comparative phylogeography uncovers a surprising impact of Pleistocene glaciation. Journal of Biogeography. 2018;45:1795–1805. doi: 10.1111/jbi.13382. [DOI] [Google Scholar]
- 27.Carvalho RD, Cianciaruso MV, Trindade-Filho J, Sagnori MD, Loyola RD. Drafting a blueprint for functional and phylogenetic diversity conservation in the Brazilian Cerrado. Natureza & Conservação. 2010;8:171–176. doi: 10.4322/natcon.00802011. [DOI] [Google Scholar]
- 28.Carvalho WD, Lourenço EC, Costa LM, Bergallo HG, Esbérard CEL. Patterns and drivers determining phyllostomid bat diversity in land-bridge islands off the south-east coast of Brazil. Biological Journal of the Linnean Society. 2021;134:604–619. doi: 10.1093/biolinnean/blab112. [DOI] [Google Scholar]
- 29.Carvalho WD, et al. Taxonomic, functional and phylogenetic bat diversity decrease from more to less complex natural habitats in the Amazon. Oecologia. 2021;197:223–239. doi: 10.1007/s00442-021-05009-3. [DOI] [PubMed] [Google Scholar]
- 30.Charbonnier YM, et al. Bat and bird diversity along independent gradients of latitude and tree composition in European forests. Oecologia. 2016;182:529–537. doi: 10.1007/s00442-016-3671-9. [DOI] [PubMed] [Google Scholar]
- 31.Cisneros LM, et al. Multiple dimensions of bat biodiversity along an extensive tropical elevational gradient. Journal of Animal Ecology. 2014;83:1124–1136. doi: 10.1111/1365-2656.12201. [DOI] [PubMed] [Google Scholar]
- 32.Cisneros LM, Fagan ME, Willig MR. Effects of human‐modified landscapes on taxonomic, functional and phylogenetic dimensions of bat biodiversity. Diversity and Distributions. 2015;21:523–533. doi: 10.1111/ddi.12277. [DOI] [Google Scholar]
- 33.Cisneros LM, Fagan ME, Willig MR. Environmental and spatial drivers of taxonomic, functional, and phylogenetic characteristics of bat communities in human-modified landscapes. PeerJ. 2016;4:e2551. doi: 10.7717/peerj.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collen B, et al. Investing in evolutionary history: implementing a phylogenetic approach for mammal conservation. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:2611–2622. doi: 10.1098/rstb.2011.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooke RS, Eigenbrod F, Bates AE. Projected losses of global mammal and bird ecological strategies. Nature Communications. 2019;10:2279. doi: 10.1038/s41467-019-10284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cox D, Gardner A, Gaston K. Diel niche variation in mammals associated with expanded trait space. Nature Communications. 2021;12:1753. doi: 10.1038/s41467-021-22023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carvalho WD, Martins MA, Esbérard CE, Palmeirim JM. Traits that allow bats of tropical lowland origin to conquer mountains: bat assemblages along elevational gradients in the South American Atlantic Forest. Journal of Biogeography. 2019;46:316–331. doi: 10.1111/jbi.13506. [DOI] [Google Scholar]
- 38.Duchamp JE, Swihart RK. Shifts in bat community structure related to evolved traits and features of human-altered landscapes. Landscape Ecology. 2008;23:849–860. doi: 10.1007/s10980-008-9241-8. [DOI] [Google Scholar]
- 39.Farneda FZ, et al. Predicting biodiversity loss in island and countryside ecosystems through the lens of taxonomic and functional biogeography. Ecography. 2020;43:97–106. doi: 10.1111/ecog.04507. [DOI] [Google Scholar]
- 40.Farneda FZ, Meyer CF, Grelle CE. Effects of land‐use change on functional and taxonomic diversity of Neotropical bats. Biotropica. 2020;52:120–128. doi: 10.1111/btp.12736. [DOI] [Google Scholar]
- 41.Farneda FZ, et al. Trait‐related responses to habitat fragmentation in Amazonian bats. Journal of Applied Ecology. 2015;52:1381–1391. doi: 10.1111/1365-2664.12490. [DOI] [Google Scholar]
- 42.Farneda FZ, et al. Functional recovery of Amazonian bat assemblages following secondary forest succession. Biological Conservation. 2018;218:192–199. doi: 10.1016/j.biocon.2017.12.036. [DOI] [Google Scholar]
- 43.Frank HK, Frishkoff LO, Mendenhall CD, Daily GC, Hadly EA. Phylogeny, traits, and biodiversity of a Neotropical bat assemblage: close relatives show similar responses to local deforestation. The American Naturalist. 2017;190:200–212. doi: 10.1086/692534. [DOI] [PubMed] [Google Scholar]
- 44.Garcia-Herrera LV, et al. Functional traits of bats associated with the use of wetlands in Colombian tropical dry forests. Acta Chiropterologica. 2020;22:283–294. doi: 10.3161/15081109ACC2020.22.2.005. [DOI] [Google Scholar]
- 45.García-Morales R, et al. Deforestation impacts on bat functional diversity in tropical landscapes. PLoS One. 2016;11:e0166765. doi: 10.1371/journal.pone.0166765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González‐Maya JF, Víquez‐R LR, Arias‐Alzate A, Belant JL, Ceballos G. Spatial patterns of species richness and functional diversity in Costa Rican terrestrial mammals: implications for conservation. Diversity and Distributions. 2016;22:43–56. doi: 10.1111/ddi.12373. [DOI] [Google Scholar]
- 47.Grilo C, Koroleva E, Andrášik R, Bíl M, González‐Suárez M. Roadkill risk and population vulnerability in European birds and mammals. Frontiers in Ecology and the Environment. 2020;18:323–328. doi: 10.1002/fee.2216. [DOI] [Google Scholar]
- 48.Guy C, Ratcliffe JM, Mideo N. The influence of bat ecology on viral diversity and reservoir status. Ecology and Evolution. 2020;10:5748–5758. doi: 10.1002/ece3.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guy C, Thiagavel J, Mideo N, Ratcliffe JM. Phylogeny matters: revisiting ‘a comparison of bats and rodents as reservoirs of zoonotic viruses’. Royal Society Open Science. 2019;6:181182. doi: 10.1098/rsos.181182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han BA, et al. Undiscovered bat hosts of filoviruses. PLoS neglected tropical diseases. 2016;10:e0004815. doi: 10.1371/journal.pntd.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanspach J, Fischer J, Ikin K, Stott J, Law BS. Using trait‐based filtering as a predictive framework for conservation: a case study of bats on farms in southeastern Australia. Journal of Applied Ecology. 2012;49:842–850. doi: 10.1111/j.1365-2664.2012.02159.x. [DOI] [Google Scholar]
- 52.Herrera JP, Duncan N, Clare E, Fenton MB, Simmons N. Disassembly of fragmented bat communities in Orange Walk District, Belize. Acta Chiropterologica. 2018;20:147–159. doi: 10.3161/15081109ACC2018.20.1.011. [DOI] [Google Scholar]
- 53.Holt BG, et al. Environmental variation is a major predictor of global trait turnover in mammals. Journal of Biogeography. 2018;45:225–237. doi: 10.1111/jbi.13091. [DOI] [Google Scholar]
- 54.Jakobsson S, Wood H, Ekroos J, Lindborg R. Contrasting multi-taxa functional diversity patterns along vegetation structure gradients of woody pastures. Biodiversity and Conservation. 2020;29:3551–3572. doi: 10.1007/s10531-020-02037-y. [DOI] [Google Scholar]
- 55.Jung K, Threlfall CG. Trait-dependent tolerance of bats to urbanization: a global meta-analysis. Proceedings of the Royal Society B. 2018;285:20181222. doi: 10.1098/rspb.2018.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kamilar JM, Tecot SR. Connecting proximate mechanisms and evolutionary patterns: pituitary gland size and mammalian life history. Journal of Evolutionary Biology. 2015;28:1997–2008. doi: 10.1111/jeb.12715. [DOI] [PubMed] [Google Scholar]
- 57.Kellner KF, Duchamp JE, Swihart RK. Niche breadth and vertebrate sensitivity to habitat modification: signals from multiple taxa across replicated landscapes. Biodiversity and Conservation. 2019;28:2647–2667. doi: 10.1007/s10531-019-01785-w. [DOI] [Google Scholar]
- 58.Kosman E, Scheiner SM, Gregorius HR. Severe limitations of the FEve metric of functional evenness and some alternative metrics. Ecology and Evolution. 2021;11:123–132. doi: 10.1002/ece3.6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laurindo RdS, et al. Drivers of bat roles in Neotropical seed dispersal networks: abundance is more important than functional traits. Oecologia. 2020;193:189–198. doi: 10.1007/s00442-020-04662-4. [DOI] [PubMed] [Google Scholar]
- 60.Lentini PE, Bird TJ, Griffiths SR, Godinho LN, Wintle BA. A global synthesis of survival estimates for microbats. Biology Letters. 2015;11:20150371. doi: 10.1098/rsbl.2015.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luis AD, et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proceedings of the Royal Society B: Biological Sciences. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Magg N, Ballenthien E, Braunisch V. Faunal surrogates for forest species conservation: a systematic niche-based approach. Ecological Indicators. 2019;102:65–75. doi: 10.1016/j.ecolind.2019.01.084. [DOI] [Google Scholar]
- 63.Mancini MCS, de Souza Laurindo R, Hintze F, de Macêdo Mello R, Gregorin R. Different bat guilds have distinct functional responses to elevation. Acta Oecologica. 2019;96:35–42. doi: 10.1016/j.actao.2019.03.004. [DOI] [Google Scholar]
- 64.Martínez-Ferreira SR, et al. Taxonomic and functional diversity and composition of bats in a regenerating neotropical dry Forest. Diversity. 2020;12:332. doi: 10.3390/d12090332. [DOI] [Google Scholar]
- 65.Mazel F, et al. The geography of ecological niche evolution in mammals. Current Biology. 2017;27:1369–1374. doi: 10.1016/j.cub.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Melo AS, Cianciaruso MV, Almeida‐Neto M. tree NODF: nestedness to phylogenetic, functional and other tree‐based diversity metrics. Methods in Ecology and Evolution. 2014;5:563–572. doi: 10.1111/2041-210X.12185. [DOI] [Google Scholar]
- 67.Moir M, Richards LR, Rambau RV, Cherry MI. Functional diversity and trait filtering of insectivorous bats relate to forest biogeography and fragmentation in South Africa. Journal of Biogeography. 2021;48:1170–1182. doi: 10.1111/jbi.14069. [DOI] [Google Scholar]
- 68.Mollentze N, Streicker DG. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proceedings of the National Academy of Sciences. 2020;117:9423–9430. doi: 10.1073/pnas.1919176117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monadjem A, Conenna I, Taylor PJ, Schoeman MC. Species richness patterns and functional traits of the bat fauna of arid southern Africa. Hystrix, the Italian Journal of Mammalogy. 2018;29:19–24. [Google Scholar]
- 70.Núñez SF, et al. Echolocation and stratum preference: key trait correlates of vulnerability of insectivorous bats to tropical forest fragmentation. Frontiers in Ecology and Evolution. 2019;7:373. doi: 10.3389/fevo.2019.00373. [DOI] [Google Scholar]
- 71.Olival KJ, et al. Host and viral traits predict zoonotic spillover from mammals. Nature. 2017;546:646–650. doi: 10.1038/nature22975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peñaranda DA, Simonetti JA. Predicting and setting conservation priorities for Bolivian mammals based on biological correlates of the risk of decline. Conservation Biology. 2015;29:834–843. doi: 10.1111/cobi.12453. [DOI] [PubMed] [Google Scholar]
- 73.Penone C, et al. Global mammal beta diversity shows parallel assemblage structure in similar but isolated environments. Proceedings of the Royal Society B: Biological Sciences. 2016;283:20161028. doi: 10.1098/rspb.2016.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phelps K, Jose R, Labonite M, Kingston T. Assemblage and species threshold responses to environmental and disturbance gradients shape bat diversity in disturbed cave landscapes. Diversity. 2018;10:55. doi: 10.3390/d10030055. [DOI] [Google Scholar]
- 75.Pineda E, Moreno C, Escobar F, Halffter G. Frog, bat, and dung beetle diversity in the cloud forest and coffee agroecosystems of Veracruz, Mexico. Conservation Biology. 2005;19:400–410. doi: 10.1111/j.1523-1739.2005.00531.x. [DOI] [Google Scholar]
- 76.Presley SJ, et al. Phylogenetic and functional underdispersion in Neotropical phyllostomid bat communities. Biotropica. 2018;50:135–145. doi: 10.1111/btp.12501. [DOI] [Google Scholar]
- 77.Ramírez‐Mejía AF, Urbina‐Cardona JN, Sánchez F. Functional diversity of phyllostomid bats in an urban–rural landscape: A scale‐dependent analysis. Biotropica. 2020;52:1168–1182. doi: 10.1111/btp.12816. [DOI] [Google Scholar]
- 78.Rossoni DM, Costa BM, Giannini NP, Marroig G. A multiple peak adaptive landscape based on feeding strategies and roosting ecology shaped the evolution of cranial covariance structure and morphological differentiation in phyllostomid bats. Evolution. 2019;73:961–981. doi: 10.1111/evo.13715. [DOI] [PubMed] [Google Scholar]
- 79.Scheiner SM, Kosman E, Presley SJ, Willig MR. Decomposing functional diversity. Methods in Ecology and Evolution. 2017;8:809–820. doi: 10.1111/2041-210X.12696. [DOI] [Google Scholar]
- 80.Stevens RD, Grimshaw JR. Relative contributions of ecological drift and selection on bat community structure in interior Atlantic Forest of Paraguay. Oecologia. 2020;193:645–654. doi: 10.1007/s00442-020-04683-z. [DOI] [PubMed] [Google Scholar]
- 81.Stobo-Wilson AM, et al. Sharing meals: Predation on Australian mammals by the introduced European red fox compounds and complements predation by feral cats. Biological Conservation. 2021;261:109284. doi: 10.1016/j.biocon.2021.109284. [DOI] [Google Scholar]
- 82.Thaxter CB, et al. Bird and bat species’ global vulnerability to collision mortality at wind farms revealed through a trait-based assessment. Proceedings of the Royal Society B: Biological Sciences. 2017;284:20170829. doi: 10.1098/rspb.2017.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Threlfall C, Law B, Penman T, Banks PB. Ecological processes in urban landscapes: mechanisms influencing the distribution and activity of insectivorous bats. Ecography. 2011;34:814–826. doi: 10.1111/j.1600-0587.2010.06939.x. [DOI] [Google Scholar]
- 84.Turcios-Casco MA, et al. Ecological gradients explain variation of phyllostomid bat (Chiroptera: Phyllostomidae) diversity in Honduras. Mammalian Biology. 2021;101:949–961. doi: 10.1007/s42991-021-00152-z. [DOI] [Google Scholar]
- 85.Turmelle AS, Olival KJ. Correlates of viral richness in bats (order Chiroptera) EcoHealth. 2009;6:522–539. doi: 10.1007/s10393-009-0263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walsh MG, Mor SM, Maity H, Hossain S. A preliminary ecological profile of Kyasanur Forest disease virus hosts among the mammalian wildlife of the Western Ghats, India. Ticks and tick-borne diseases. 2020;11:101419. doi: 10.1016/j.ttbdis.2020.101419. [DOI] [PubMed] [Google Scholar]
- 87.Wells K, Morand S, Wardeh M, Baylis M. Distinct spread of DNA and RNA viruses among mammals amid prominent role of domestic species. Global Ecology and Biogeography. 2020;29:470–481. doi: 10.1111/geb.13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Willoughby AR, Phelps KL, Consortium P, Olival KJ. A comparative analysis of viral richness and viral sharing in cave-roosting bats. Diversity. 2017;9:35. doi: 10.3390/d9030035. [DOI] [Google Scholar]
- 89.Wordley CF, Sankaran M, Mudappa D, Altringham JD. Bats in the Ghats: Agricultural intensification reduces functional diversity and increases trait filtering in a biodiversity hotspot in India. Biological Conservation. 2017;210:48–55. doi: 10.1016/j.biocon.2017.03.026. [DOI] [Google Scholar]
- 90.Worsley-Tonks KE, et al. Using host traits to predict reservoir host species of rabies virus. PLoS neglected tropical diseases. 2020;14:e0008940. doi: 10.1371/journal.pntd.0008940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zamora‐Gutierrez V, Rivera‐Villanueva AN, Martinez Balvanera S. Castro‐Castro, A. & Aguirre‐Gutiérrez, J. Vulnerability of bat–plant pollination interactions due to environmental change. Global Change Biology. 2021;27:3367–3382. doi: 10.1111/gcb.15611. [DOI] [PubMed] [Google Scholar]
- 92.Conenna I, et al. Global patterns of functional trait variation along aridity gradients in bats. Global Ecology and Biogeography. 2021;30:1014–1029. doi: 10.1111/geb.13278. [DOI] [Google Scholar]
- 93.Blakey RV, et al. Bats in a changing landscape: Linking occupancy and traits of a diverse montane bat community to fire regime. Ecology and Evolution. 2019;9:5324–5337. doi: 10.1002/ece3.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thurman LL, et al. Persist in place or shift in space? Evaluating the adaptive capacity of species to climate change. Frontiers in Ecology and the Environment. 2020;18:520–528. doi: 10.1002/fee.2253. [DOI] [Google Scholar]
- 95.Barlow K, et al. Citizen science reveals trends in bat populations: the National Bat Monitoring Programme in Great Britain. Biological Conservation. 2015;182:14–26. doi: 10.1016/j.biocon.2014.11.022. [DOI] [Google Scholar]
- 96.Kerbiriou, C. et al. Vigie-Chiro: 9 ans de suivi des tendances des espèces communes. Symbioses, 1–4 (2015).
- 97.Van der Meij T, et al. Return of the bats? A prototype indicator of trends in European bat populations in underground hibernacula. Mammalian Biology. 2015;80:170–177. doi: 10.1016/j.mambio.2014.09.004. [DOI] [Google Scholar]
- 98.Russo, D. Chiroptera. (Springer Cham, 2023).
- 99.de Magalhaes JP, Costa J. A database of vertebrate longevity records and their relation to other life‐history traits. Journal of evolutionary biology. 2009;22:1770–1774. doi: 10.1111/j.1420-9101.2009.01783.x. [DOI] [PubMed] [Google Scholar]
- 100.IUCN. The IUCN Red List of Threatened Species. Version 2020-1. (2020).
- 101.Chen, L., Liu, B., Yang, J. & Jin, Q. DBatVir: the database of bat-associated viruses. Database2014 (2014). [DOI] [PMC free article] [PubMed]
- 102.Bas Y, Bas D, Julien J-F. Tadarida: A toolbox for animal detection on acoustic recordings. Journal of Open Research Software. 2017;5:6. doi: 10.5334/jors.154. [DOI] [Google Scholar]
- 103.Brooks ME, et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R journal. 2017;9:378–400. doi: 10.32614/RJ-2017-066. [DOI] [Google Scholar]
- 104.Komsta, L. & Novomestky, F. Moments, cumulants, skewness, kurtosis and related tests. R package version14 (2015).
- 105.Froidevaux JSP, 2023. figshare. [DOI]
- 106.Schneider FD, et al. Towards an ecological trait‐data standard. Methods in Ecology and Evolution. 2019;10:2006–2019. doi: 10.1111/2041-210X.13288. [DOI] [Google Scholar]
- 107.Blatteis C, et al. Glossary of terms for thermal physiology. Japanese Journal of Physiology. 2001;51:245–280. [Google Scholar]
- 108.Kohli BA, Jarzyna MA. Pitfalls of ignoring trait resolution when drawing conclusions about ecological processes. Global Ecology and Biogeography. 2021;30:1139–1152. doi: 10.1111/geb.13275. [DOI] [Google Scholar]
- 109.R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2021).
- 110.Cazalis V, et al. Bridging the research-implementation gap in IUCN Red List assessments. Trends in Ecology & Evolution. 2022;37:359–370. doi: 10.1016/j.tree.2021.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Davidson AD, Hamilton MJ, Boyer AG, Brown JH, Ceballos G. Multiple ecological pathways to extinction in mammals. Proceedings of the National Academy of Sciences. 2009;106:10702–10705. doi: 10.1073/pnas.0901956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mouillot D, Graham NA, Villéger S, Mason NW, Bellwood DR. A functional approach reveals community responses to disturbances. Trends in Ecology & Evolution. 2013;28:167–177. doi: 10.1016/j.tree.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 113.Ricotta C, Moretti M. CWM and Rao’s quadratic diversity: a unified framework for functional ecology. Oecologia. 2011;167:181–188. doi: 10.1007/s00442-011-1965-5. [DOI] [PubMed] [Google Scholar]
- 114.Pavoine S, Ricotta C. On the relationships between rarity, uniqueness, distinctiveness, originality and functional/phylogenetic diversity. Biological Conservation. 2021;263:109356. doi: 10.1016/j.biocon.2021.109356. [DOI] [Google Scholar]
- 115.Mouillot, D. et al. The dimensionality and structure of species trait spaces. Ecology Letters (2021). [DOI] [PubMed]
- 116.Laforge A, et al. Road density and forest fragmentation shape bat communities in temperate mosaic landscapes. Landscape and Urban Planning. 2022;221:104353. doi: 10.1016/j.landurbplan.2022.104353. [DOI] [Google Scholar]
- 117.Maas B, et al. Bird and bat predation services in tropical forests and agroforestry landscapes. Biological Reviews. 2016;91:1081–1101. doi: 10.1111/brv.12211. [DOI] [PubMed] [Google Scholar]
- 118.Kunz TH, Braun de Torrez E, Bauer D, Lobova T, Fleming TH. Ecosystem services provided by bats. Annals of the New York Academy of Sciences. 2011;1223:1–38. doi: 10.1111/j.1749-6632.2011.06004.x. [DOI] [PubMed] [Google Scholar]
- 119.Charbonnier Y, et al. Pest control services provided by bats in vineyard landscapes. Agriculture, Ecosystems & Environment. 2021;306:107207. doi: 10.1016/j.agee.2020.107207. [DOI] [Google Scholar]
- 120.Puig-Montserrat X, et al. Pest control service provided by bats in Mediterranean rice paddies: linking agroecosystems structure to ecological functions. Mammalian Biology. 2015;80:237–245. doi: 10.1016/j.mambio.2015.03.008. [DOI] [Google Scholar]
- 121.Carter AJ, Feeney WE, Marshall HH, Cowlishaw G, Heinsohn R. Animal personality: what are behavioural ecologists measuring? Biological Reviews. 2013;88:465–475. doi: 10.1111/brv.12007. [DOI] [PubMed] [Google Scholar]
- 122.Toscano BJ, Gownaris NJ, Heerhartz SM, Monaco CJ. Personality, foraging behavior and specialization: integrating behavioral and food web ecology at the individual level. Oecologia. 2016;182:55–69. doi: 10.1007/s00442-016-3648-8. [DOI] [PubMed] [Google Scholar]
- 123.Laforge A, et al. Landscape composition and life‐history traits influence bat movement and space use: Analysis of 30 years of published telemetry data. Global Ecology and Biogeography. 2021;30:2442–2454. doi: 10.1111/geb.13397. [DOI] [Google Scholar]
- 124.Kerches‐Rogeri P, Niebuhr BB, Muylaert RL, Mello MAR. Individual specialization in the use of space by frugivorous bats. Journal of Animal Ecology. 2020;89:2584–2595. doi: 10.1111/1365-2656.13339. [DOI] [PubMed] [Google Scholar]
- 125.Fontana S, Petchey OL, Pomati F. Individual‐level trait diversity concepts and indices to comprehensively describe community change in multidimensional trait space. Functional Ecology. 2016;30:808–818. doi: 10.1111/1365-2435.12551. [DOI] [Google Scholar]
- 126.Fontana S, Thomas MK, Moldoveanu M, Spaak P, Pomati F. Individual-level trait diversity predicts phytoplankton community properties better than species richness or evenness. The ISME journal. 2018;12:356–366. doi: 10.1038/ismej.2017.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Palacio FX, Fernández GJ, Ordano M. Does accounting for within-individual trait variation matter for measuring functional diversity? Ecological Indicators. 2019;102:43–50. doi: 10.1016/j.ecolind.2019.02.018. [DOI] [Google Scholar]
- 128.Gallagher RV, et al. Open Science principles for accelerating trait-based science across the Tree of Life. Nature Ecology & Evolution. 2020;4:294–303. doi: 10.1038/s41559-020-1109-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Froidevaux JSP, 2023. figshare. [DOI]
Supplementary Materials
A species-level trait dataset of bats in Europe and beyond -- Supplementary Material
A species-level trait dataset of bats in Europe and beyond -- Supplementary Material
Data Availability Statement
The code to create the R Shiny App is available on GitHub (https://github.com/J4SJA/ClimBatsApp).