Abstract
Cyclin-dependent kinase-like 5 (CDKL5) deficiency disorder (CDD) is a devastating rare neurodevelopmental disease without a cure, caused by mutations of the serine/threonine kinase CDKL5 highly expressed in the forebrain. CDD is characterized by early-onset seizures, severe intellectual disabilities, autistic-like traits, sensorimotor and cortical visual impairments (CVI). The lack of an effective therapeutic strategy for CDD urgently demands the identification of novel druggable targets potentially relevant for CDD pathophysiology. To this aim, we studied Class I metabotropic glutamate receptors 5 (mGluR5) because of their important role in the neuropathological signs produced by the lack of CDKL5 in-vivo, such as defective synaptogenesis, dendritic spines formation/maturation, synaptic transmission and plasticity. Importantly, mGluR5 function strictly depends on the correct expression of the postsynaptic protein Homer1bc that we previously found atypical in the cerebral cortex of Cdkl5−/y mice. In this study, we reveal that CDKL5 loss tampers with (i) the binding strength of Homer1bc-mGluR5 complexes, (ii) the synaptic localization of mGluR5 and (iii) the mGluR5-mediated enhancement of NMDA-induced neuronal responses. Importantly, we showed that the stimulation of mGluR5 activity by administering in mice specific positive-allosteric-modulators (PAMs), i.e., 3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) or RO6807794, corrected the synaptic, functional and behavioral defects shown by Cdkl5−/y mice. Notably, in the visual cortex of 2 CDD patients we found changes in synaptic organization that recapitulate those of mutant CDKL5 mice, including the reduced expression of mGluR5, suggesting that these receptors represent a promising therapeutic target for CDD.
Subject terms: Developmental disorders, Cellular neuroscience
Introduction
CDKL5 is a serine/threonine kinase highly expressed in the forebrain during the peak of synaptogenesis [1]. CDKL5 phosphorylates several substrates and is involved in a broad variety of cellular processes such as gene expression, neuronal migration, axon outgrowth, dendritic morphogenesis, synapses development and function [2–5]. In the nucleus CDKL5 has been shown to interact with epigenetic factors, such as methyl-CpG-binding protein 2 (MeCP2) and DNA Methyltransferase 1 (DNMT1) [6, 7], nevertheless the role of CDKL5 in regulating gene expression is still not fully understood. Recently, several cytoplasmic targets of CDKL5 phosphorylation, including MAP1S, EB2 and ARHGEF2, have been identified pointing to a major role of this kinase in the control of cytoskeletal function. Moreover, CDKL5 has been found to accumulate at synapses where it can interact with the palmitoylated form of postsynaptic density protein-95 (PSD-95) [8]. The interaction with PSD-95 facilitates the phosphorylation of the adhesion molecule netrin-G1 ligand (NGL-1) [9] promoting the maturation of dendritic spines, i.e., the vast majority of glutamatergic postsynaptic sites in the forebrain, as well as the formation and function of excitatory connections. In addition, Barbiero et al. (2017) [10] showed that IQ motif containing GTPase activating protein 1 (IQGAP1) can interact with CDKL5 and thus mediate the formation of complexes with post-synaptic proteins such as PSD-95 or both AMPA- and NMDA-glutamatergic receptors. Interestingly, shRNA-mediated knockdown of CDKL5 can influence the synaptic expression of the GluA2 subunit [11] further highlighting that the involvement of CDKL5 in glutamatergic neurotransmission is yet to be unfolded.
To study the consequences of the lack of CDKL5 in-vivo, different CDKL5-/y mouse lines have been recently generated [12–14]. These mutants display a broad spectrum of behavioral abnormalities, including hind-limb clasping, motor hyperactivity, abnormal eye tracking, learning and memory deficits, and autistic–like phenotypes [13] closely modeling human CDD [15]. Despite epileptic seizures have so far been reported exclusively in aging heterozygous female mice [16], CDKL5-/y mice exhibit multiple defects recapitulating the disease, such as sensorimotor, visual and auditory impairments [14, 17, 18]. For example, cortical visual impairment (CVI), that is correlated with developmental delay in CDD patients [15], is found in CDKL5 mutant mice starting from P27-P28 both in heterozygous and homozygous animals [14, 17]. Aberrant sensory processing in mice lacking CDKL5 is associated with severe abnormalities of the cerebral cortex, including altered dendritic arborization of pyramidal neurons, the downregulation of the postsynaptic scaffolding proteins PSD-95 and Homer, and the disruption of AKT-mTOR signaling [12, 14, 19–21]. Moreover, we previously reported that CDKL5 plays a key role in the dynamic of dendritic spines turn-over in the primary somatosensory (S1) cortex [19] by promoting their stabilization. In addition, S1 cortex of CDKL5-/y mice show impaired excitatory synaptic transmission and maintenance of long-term potentiation induced by theta-burst stimulation, emphasizing the role of CDKL5 in excitatory cortical connectivity [18, 19].
Given all the above, we reasoned that identifying druggable targets with relevant synaptic function will speed up the discovery of novel therapeutic options for CDD. Here we report that both the expression and function of a member of group I metabotropic glutamate receptors, mGluR5, are abnormal in Cdkl5−/y mice cerebral cortex and that the administration of selective mGluR5 positive allosteric modulators (PAMs) can rescue synaptic, cellular, functional and behavioural defects shown by mutant mice.
Materials and methods
All procedures were performed in accordance with the European Community Council Directive 2010/63/UE for care and use of experimental animals with protocols approved by the Italian Minister for Scientific Research (Authorization number 175/2015-PR) and the Bioethics Committee of the University of Torino, Italy. Complete methods, experimental procedures and statistics are reported in the Supplementary Materials section.
All data values are reported in Table S1. Statistical analysis and the n for each experimental group are reported in figure legends.
Results
Altered mGluR5/Homer1bc organization in the cerebral cortex of Cdkl5−/y mice
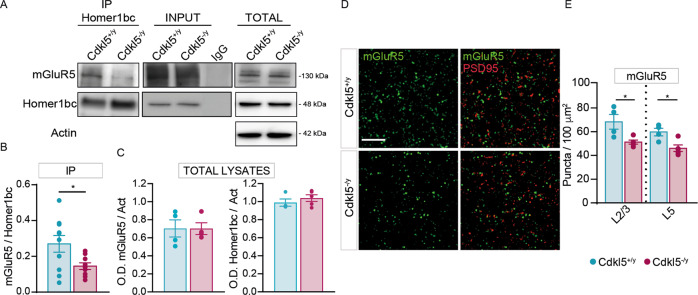
We focused on mGluR5 because of their role in mechanisms involved in CDD such as synaptogenesis, dendritic spines formation/maturation and synaptic plasticity [22–25]. Moreover, mGluR5 must interact with Homer1bc, that is downregulated in the cortex of Cdkl5−/y mice [17, 20], to exert signaling functions within the PSD [26–29]. First, we evaluated the strength of mGluR5-Homer1bc binding in mutant mice. Intriguingly, co-immunoprecipitation (co-IP) assays of cortical synaptosomal fraction (Fig. 1A) revealed that the amount of mGluR5 immunoprecipitated with Homer1bc was significantly reduced in Cdkl5−/y mice compared to Cdkl5+/y animals (O.D mGluR5/Homer1bc *p < 0.05; Fig. 1B), while the total amount of both Homer1bc and mGluR5 did not change between genotypes (O.D mGluR5/Act and O.D Homer1b/Act p > 0.05; Fig. 1C).
Fig. 1. CDKL5 loss is responsible for both the disruption of mGluR5-Homer1bc interaction and the reduction of mGluR5 localization in the cortical neuropil.
A Co-IP of cortical synaptosomal fraction (P2) from P56 mice by using anti-Homer1bc. IgG: control lane in the absence of antibodies. Immunoprecipitates, inputs (P2) and total cortical lysates were analyzed by immunoblotting for mGluR5 and Homer1bc. B, C Bar graphs showing Co-IP (B) and total cortical lysates (C) quantitation expressed as optical density (O.D.). D Confocal microscopy images showing mGluR5+ (green) and PSD-95+ (red) immunopuncta in layers II/III of S1 cortex (scale bar: 5 µm). E Bar graphs displaying the density of mGluR5+ puncta. Student T test *p < 0.05 (Co-IP: n = 8; WB: n = 4 IFL: n = 4).
We next assessed mGluR5 expression in the neuropil by performing immunofluorescence experiments on S1 cortices from Cdkl5−/y and Cdkl5+/y mice (Fig. 1D). By using a fixation/staining protocol improved for postsynaptic protein localization [21, 30], mGluR5 immunofluorescence (Fig. 1D) resulted in discrete puncta that were found closely localized, but only rarely overlapping, with PSD-95+ puncta in agreement with previously reported perisynaptic localization of mGluR5 [31]. Interestingly, the density of mGluR5-puncta was strongly reduced in layers II-III and V of S1cortex in Cdkl5−/y mice compared to controls (layers II-III and V *p < 0.05; Fig. 1D, E). These data indicate that Cdkl5 loss interferes with Homer1bc-dependent insertion/stabilization of mGluR5 in the postsynaptic membrane.
Excitatory neurotransmission and mGluR5-mediated signaling are severely disrupted in Cdkl5−/y cortical neurons
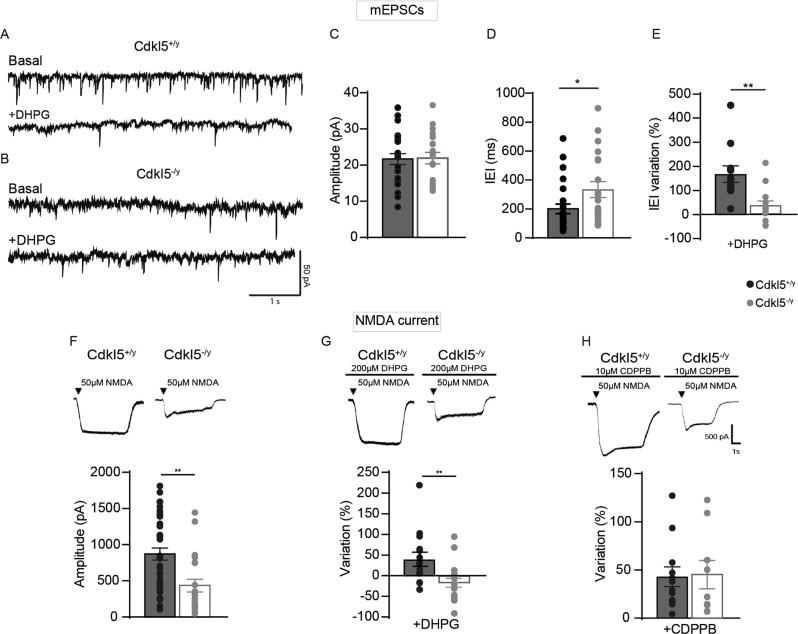
Our data suggest that mGluR5 function might be compromised in the absence of CDKL5 [32, 33]. To test this idea, we recorded spontaneous miniature excitatory postsynaptic currents (mEPSCs) in neuronal cultures of the S1 cortex from both Cdkl5+/y and Cdkl5−/y mice (Fig. 2A–D, upper part), before and after mGluR5 activation. As we reported previously in acute cortical slices [19], mEPSCs recorded from CDKL5-null neurons showed an increased inter-event interval (IEI) (Cdkl5+/y vs Cdkl5−/y *p < 0.05; Fig. 2D) while the mean peak amplitude was similar between genotypes (Cdkl5+/y vs Cdkl5−/y p > 0.05; Fig. 2C). Intriguingly, 2-minutes stimulation with the selective mGluR5 agonist DHPG (100 μM) produced a significant increase in the IEI of mEPSCs in Cdkl5+/y cultures [34, 35] but not in Cdkl5−/y neurons (Fig. 2E).
Fig. 2. CDKL5 loss tampers with both mEPSCs and NMDA current.
A Sample traces of miniature excitatory postsynaptic current (mEPSC) recorded from Cdkl5+/y neurons (A, upper part) and Cdkl5-/y neurons (B, upper part) and after the application of DHPG (A, B lower part). C, D Bar graphs showing the mean average amplitude (C) and the inter-event interval (IEI) of mEPSCs (D). E Bar graphs displaying the % of IEI variation IEI after the application of DHPG (100 µM). F Representative traces of currents obtained with patch-clamp recordings on S1 neurons cultures from Cdkl5+/y and Cdkl5-/y embryos after NMDA (50 µM) application (upper part), bar graphs showing differences of INMDA current between genotypes (lower part). G Representative traces of NMDA currents on S1 neurons after 2-min application of DHPG (100 µM-upper part); bar graphs showing the % change of INMDA after the application of DHPG (lower part). H Representative traces of NMDA after 2-min CDPPB + NMDA application (upper part), bar graphs showing the % change of INMDA current after the application of CDPPB (lower part). Student’s t-test, chi-square, two-way ANOVA followed by Fisher’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001 (mEPSC Cdkl5+/y n = 22 cells, Cdkl5-/y n = 28; minis+DHPG Cdkl5+/y: n = 12 cells; minis+DHPG Cdkl5-/y: n = 13 cells. NMDA: Cdkl5+/y n = 36 cells, Cdkl5-/y n = 23 cells; NMDA + DHPG Cdkl5+/y n = 15 cells and NMDA + DHPG Cdkl5-/y n = 14 cells; NMDA + CDPPB Cdkl5+/y n = 12 cells; NMDA + CDPPB Cdkl5-/y n = 9 cells).
Next, we tested NMDA-mediated responses because these receptors activity can be modulated by mGluR5 [36]. When NMDA currents were elicited by the application of NMDA (50 μM) [37], Cdkl5−/y cultures showed a significant reduction of INMDA compared to Cdkl5+/y neurons (Cdkl5+/y vs Cdkl5−/y **p < 0.01; Fig. 2F). Intriguingly, the application of NMDA together with DHPG (100 μm) increased INMDA in Cdkl5+/y cells, (Fig. 2G; see also [38]) while it produced a small decrease in Cdkl5−/y neurons, as illustrated by the sharp difference in the percentage of INMDA variation between genotypes (DHPG-Cdkl5+/y vs DHPG-Cdkl5-/y *p < 0.05; Fig. 2G). Strikingly, while 73% of Cdkl5+/y cortical neurons (11/15 cells) showed potentiated INMDA after the application of DHPG, most of Cdkl5−/y neurons did not respond to DHPG (10/14; 71%) as shown by plotted data (Fig. 2G). These results disclose that loss of CDKL5 disrupts excitatory neurotransmission at multiple sites and severely affects mGluR5 normal function.
CDPPB potentiates NMDAR current in cortical neurons lacking CDKL5
We and others have previously shown that in conditions where INMDA is not sensitive to DHPG, the application of selective mGluR5 PAMs can instead elicit the strengthening of this current [38, 39]. Among these, 3-Cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) offers several advantages compared to agonist drugs such as higher subtype selectivity, reduced desensitization, and more subtle modulatory effects on receptor function [40]. Thus, we examined the effect produced by CDPPB on cortical neurons by measuring NMDA current. Intriguingly, 2 min bath application of CDPPB (10 µM) preceding NMDA (50 µM) administration produced a comparable increase of INMDA (Fig. 2F, lower part) in both genotypes (CDPPB-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.05; Fig. 2H) when compared to the average amplitude of INMDA measured after administration of NMDA alone. Consistently, in the case of CDPPB application, the majority of both Cdkl5−/y and Cdkl5+/y neurons showed potentiated INMDA (Cdkl5+/y: 13/18, 78%; Cdkl5−/y 10/12, 83%) resulting in a significantly increase compared to DHPG-treated Cdkl5−/y neurons (chi-square DHPG-Cdkl5-/y: 29% vs CDPPB-Cdkl5-/y: 83% ****p < 0.0001). These results show that positive allosteric modulation can rescue mGluR5-dependent strengthening of NMDA-mediated activation in Cdkl5−/y neurons.
CDPPB treatment ameliorates visual, sensorimotor and memory functions in Cdkl5−/y mice
Encouraged by the positive effects we obtained on synaptic currents, we evaluated the therapeutic potential of CDPPB by treating mice with one intraperitoneal injection (i.p.) of CDPPB (3 mg/Kg), as in Vicidomini et al. (2017) [38], that were subsequently exposed to a battery of tests.
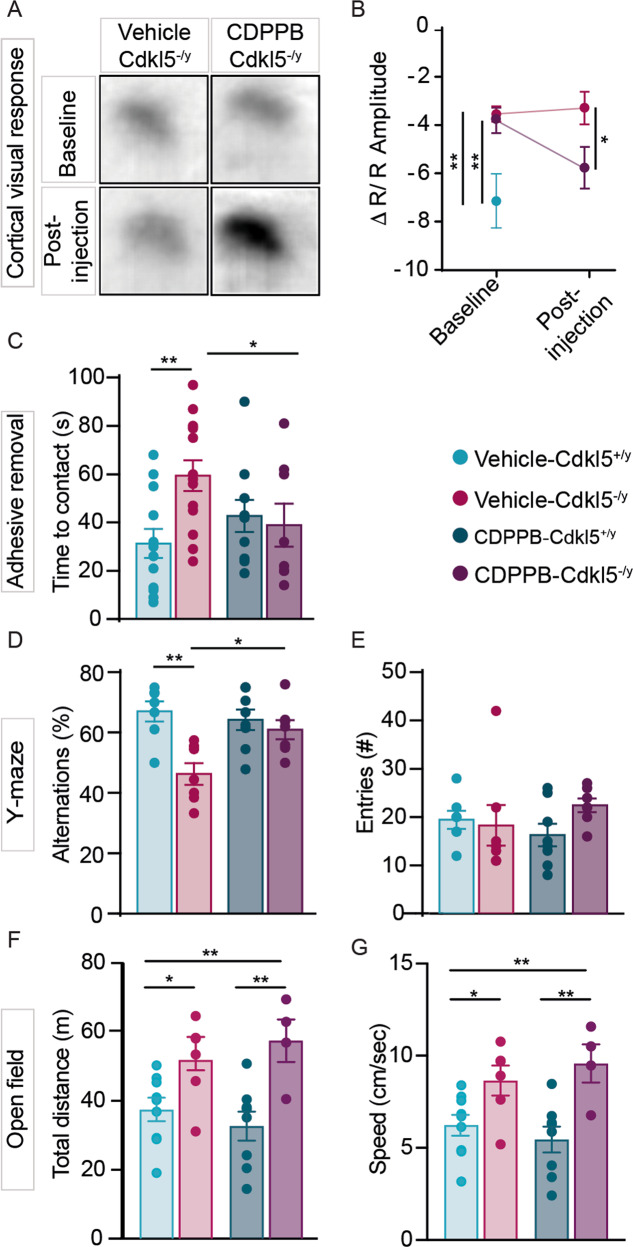
We investigated cortical visual responses by transcranial intrinsic optical signal (IOS) imaging before and after CDPPB administration in the same animals. As expected from our previous data [17, 20], baseline response amplitude of Cdkl5−/y mice was strongly decreased compared to Cdkl5+/y littermates (Cdkl5+/y vs vehicle-Cdkl5-/y / CDPPB-Cdkl5-/y **p < 0.01, Fig. 3A, B). After CDPPB treatment, visual responses approached Cdkl5+/y levels (Cdkl5+/y vs CDPPB-Cdkl5-/y post-injection p = 0.6; Cdkl5+/y vs vehicle-Cdkl5-/y post-injection *p < 0.05) significantly increasing from their baseline values (vehicle-Cdkl5−/y post-injection vs CDPPB-Cdkl5-/y post-injection *p < 0.05; CDPPB-Cdkl5-/y baseline vs CDPPB-Cdkl5-/y post-injection *p < 0.05; vehicle-Cdkl5-/y baseline vs vehicle-Cdkl5-/y post-injection p = 0.90). By contrast, visual response remained impaired in vehicle-treated mutants. These experiments indicate that cortical response to visual stimulation is ameliorated by CDPPB treatment in Cdkl5−/y mice.
Fig. 3. Acute CDPPB treatment rescues visual response, sensorimotor and memory deficits in Cdkl5-/y mice.
A Samples images showing differences of IOS evoked responses in vehicle- and CDPPB-treated Cdkl5-/y mice. B Trajectory of the IOS amplitude in vehicle-Cdkl5+/y, vehicle-Cdkl5+/y and CDPPB-Cdkl5-/y treated mice. C Bar graphs showing contact latency with the tape placed under mice’s forepaw. D, E Bar graphs showing the percentage of the correct alternations (D) and the number of entries (E) made by Cdkl5+/y and Cdkl5-/y mice, treated with either vehicle or CDPPB, in the Y-maze. F, G Bar graphs showing the total distance traveled (F) and the mean speed (G) in the open field arena of mice treated with either vehicle or CDPPB. One-way ANOVA followed by Tukey’s multiple comparison; two-way ANOVA followed by Sidak or Bonferroni’s multiple comparison test, *p < 0.05, **p < 0.01 (IOS: vehicle-Cdkl5+/y n = 3, vehicle-Cdkl5-/y n = 8, CDPPB-Cdkl5-/y n = 6; behavioural tests: vehicle-Cdkl5+/y n = 12, vehicle-Cdkl5-/y n = 13, CDPPB-Cdkl5+/y n = 8, CDPPB-Cdkl5-/y n = 7).
When assessed for sensorimotor responses in the adhesive tape-removal test [41, 42], Cdkl5−/y mice displayed a significant increase in time-to-contact the tape compared to Cdkl5+/y mice (vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y **p < 0.01; Fig. 3C). Importantly, a single CDPPB injection produced a reduction of the latency exclusively in mutant mice whose performance became similar to controls (vehicle-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.4; Fig. 3C). Moreover, the number of correct spontaneous alternations in the Y-maze paradigm for working memory was decreased in Cdkl5−/y mice compared to Cdkl5+/y animals (vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y **p < 0.01; Fig. 3D), confirming previous observations [43]. Intriguingly, working memory was rescued in Cdkl5−/y mice by CDPPB (vehicle-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.4; Fig. 3D), while it did not affect memory in Cdkl5+/y mice. Also, total number of arms entries did not change between genotypes under either treated or untreated conditions (Fig. 3E). To assess locomotor activity, we used the open-field test. As previously reported [44], Cdkl5−/y mice showed an increase in both total distance traveled (vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y *p < 0.05; Fig. 3F) and speed (vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y *p < 0.05; Fig. 3G) that was not changed by CDPPB treatment (vehicle-Cdkl5−/y vs CDPPB-Cdkl5-/y p > 0.4; Fig. 3F, G). These data indicate that the action of CDPPB can reverse atypical visual cortical response, sensorimotor and short-term memory impairments in Cdkl5−/y mice, but not locomotor activity.
mGluR5 PAMs rescue both synaptic and activity defects in Cdkl5−/y cerebral cortex
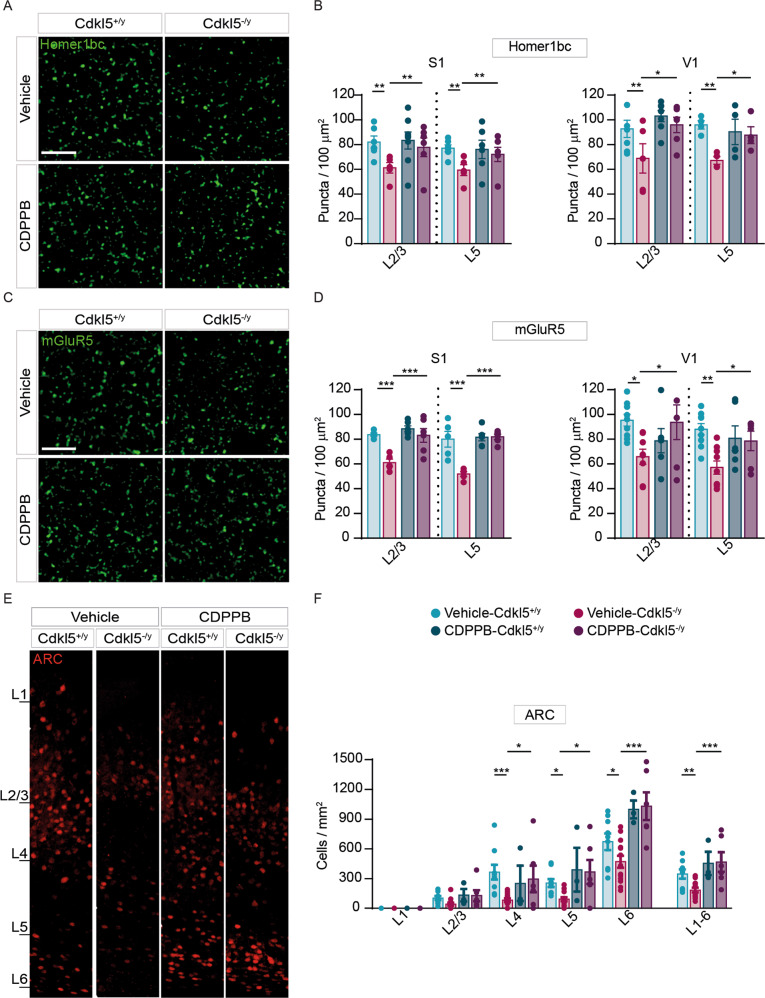
In parallel with the observed behavioral and functional rescues, acute CDPPB treatment normalized both number and organization of postsynaptic sites as well as neuronal activity in primary cortices of Cdkl5−/y mice. CDPPB increased the density of Homer1bc+ puncta in both S1 and V1 cortices of Cdkl5−/y mice (S1: layers II-III and V vehicle-Cdkl5-/y vs CDPPB-Cdkl5-/y **p < 0.01. V1: layers II-III and V vehicle-Cdkl5-/y vs CDPPB-Cdkl5-/y *p < 0.05; Fig. 4A, B), reproducing Cdkl5+/y mice conditions (S1 and V1: layers II-III and V: vehicle-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.3; Fig. 4A, B). Intriguingly, CDPPB treatment also normalized mGluR5+ puncta density in both S1 and V1 cortices of Cdkl5−/y mice (S1: layers II-III and V: vehicle-Cdkl5-/y vs CDPPB-Cdkl5-/y ***p < 0.001; vehicle-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.3. V1: layers II-III and V: vehicle-Cdkl5-/y vs CDPPB-Cdkl5-/y *p < 0.05. S1 and V1: vehicle-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.3; Fig. 4C, D). Finally, the density of cells expressing ARC, an immediate-early gene (IEG) induced by mGluR5 activation [45, 46], was restored in the V1 cortex of CDKL5-mutants after a single CDPPB administration (layers I-VI: vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y **p < 0.01; vehicle-Cdkl5-/y vs CDPPB-Cdkl5-/y ***p < 0.001; Fig. 4E, F).
Fig. 4. Structural defects exhibited by Cdkl5-/y mice cortices are rescued by an acute CDPPB injection.
A, C Representative confocal images showing Homer1bc+ and mGluR5+ puncta in layer II-III of S1 cortex from either vehicle- or CDPPB-treated mice (scale bar: 5 µm). B, D Bar graphs showing both Homer1bc+ (B) and mGluR5+ (D) immunopuncta density in layers II-III and V of both S1 and V1 cortices in either vehicle- or CDPPB-treated mice. E Confocal images of ARC immunostaining on coronal sections of the V1 cortex from mice treated with vehicle or CDPPB (scale bar: 25 µm), and relative ARC+ cells density quantitation (F) throughout the cortical layers. Two-way ANOVA followed by Fisher’s multiple comparison test, *p < 0.05, **p < 0.01, ***p < 0.001; (n = 6 animals for each genotype).
To increase the reproducibility of our study, we treated another group of Cdkl5−/y and Cdkl5+/y animals with a different mGluR5 PAM, the RO6807794 (RO68) compound [47]. Two hours after an i.p. injection with RO68 (0.3 mg/kg as in [45]), the density of Homer1bc+ puncta in S1 cortex of Cdkl5−/y mice was increased (S1: layers II-III and V vehicle-Cdkl5-/y vs CDPPB-Cdkl5-/y *p < 0.05. V1: layers II-III and V vehicle-Cdkl5-/y vs CDPPB-Cdkl5-/y *p < 0.05; Fig. S1A, B) reproducing Cdkl5+/y mice conditions (S1 layers II-III and V: vehicle-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.3; Fig. S1A, B). Intriguingly, RO68 was also able to restore neuronal activity in S1 cortex in Cdkl5−/y mice (Fig. S1C) throughout cortical layers (vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y ***p < 0.001; vehicle-Cdkl5-/y vs RO68-Cdkl5-/y ***p < 0.001), as indicated by c-Fos+ cell density (Fig. S1D; see also [21]), that reached the magnitude of Cdkl5+/y mice (vehicle-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.05). These results strongly support the idea that the atypical circuit organization, both structural and molecular, shown by the cerebral cortex of Cdkl5−/y mice can be rescued by activating mGluR5-mediated signaling.
A protracted treatment with CDPPB effectively restores Cdkl5−/y mice deficits
To assess the therapeutic potential of mGluR5 activation, we treated animals for five consecutive days with CDPPB that 24 h after the last injection were behaviourally tested and then sacrificed for brain analyses. The density of Homer1bc+ puncta was restored in both upper and deeper layers of the S1 cortex in treated mutant mice (layers II-III and V: vehicle-Cdkl5-/y vs CDPPB-Cdkl5-/y **p < 0.01; Fig. S2A, B), while protracted CDPPB had no effect on Homer1bc expression in Cdkl5+/y animals (layers II-III and V: vehicle-Cdkl5+/y vs CDPPB-Cdkl5+/y p = 0.9; Fig. S2B, C). Next, we analysed hind-limb clasping, a sign displayed by Cdkl5−/y mice [5, 12, 46]. In line with previous studies, vehicle-treated mutants showed increased hind-limb clasping compared to controls (vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y ***p < 0.001; Fig. S2C and [12]) whereas after CDPPB treatment Cdkl5−/y mice spent significantly less time clasping their hind paws (vehicle-Cdkl5−/y vs CDPPB-Cdkl5-/y **p < 0.01; Fig. S2C). Moreover, the differences shown by the two genotypes in the adhesive tape-removal test were abolished by 5-days CDPPB treatment (vehicle-Cdkl5+/y vs. CDPPB-Cdkl5-/y p > 0.7; Fig. S2D). Intriguingly, visual response was also significantly improved after the prolonged CDPPB treatment. While the baseline response amplitude was strongly reduced in Cdkl5−/y mice compared to Cdkl5+/y littermates (Cdkl5+/y vs vehicle-Cdkl5-/y **p < 0.01; Cdkl5+/y vs CDPPB-Cdkl5-/y *p < 0.05, fig S2F, G), no differences were shown by the two Cdkl5−/y groups (vehicle-Cdkl5−/y vs pre-CDPPB-Cdkl5-/y p > 0.3; Fig. S2F). After CDPPB treatment, mutants showed robust changes in response amplitude (pre-CDPPB-Cdkl5−/y vs post-CDPPB-Cdkl5-/y **p < 0.01) highlighting a substantial effect of treatment during time. Importantly, after CDPPB-treatment, Cdkl5−/y mice reached amplitude values significantly different from vehicle-treated mutants (CDPPB-Cdkl5−/y vs vehicle-Cdkl5-/y post-injection **p < 0.01).
Finally, we evaluated the effects of sub-chronic CDPPB treatment on cortical activation assessing both c-Fos and ARC expression. Intriguingly, the reduction of IEGs expression shown by mutant mice (c-FOS: vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y **p < 0.01 Fig. S2H, I; ARC: vehicle-Cdkl5+/y vs vehicle-Cdkl5-/y **p < 0.01; Fig. S2J, K) was abolished by the treatment (c-FOS and ARC vehicle-Cdkl5+/y vs CDPPB-Cdkl5-/y p > 0.05; Fig. S2I, K).
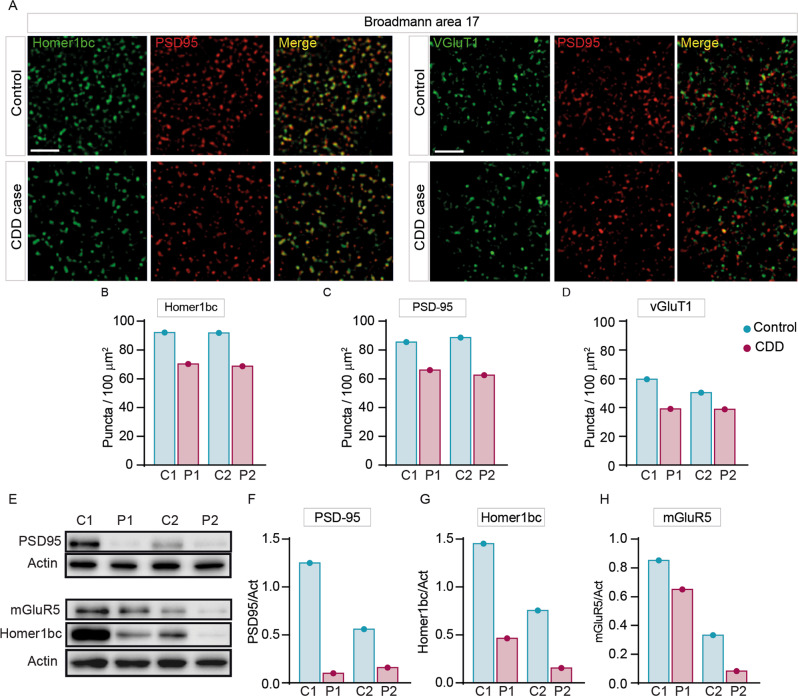
The BA17 cortex of CDD patients recapitulates the synaptic defects shown by Cdkl5−/y mice
To assess the translational potential of our findings, we examined excitatory synaptic structures in the 2 postmortem CDD patient brains available worldwide that were obtained from the Harvard Brain Tissue Resource Center (Belmont; USA). These experiments were performed on sections from the primary visual cortex (BA17) of CDD cases and age/sex-matched neurotypical controls. Intriguingly, the results showed a clear reduction of both postsynaptic proteins PSD-95+ and Homer1bc+ as well as of the presynaptic marker VGluT1+, irrespective of case age (5 and 30 years old), compared to NTs (Fig. 5A). Moreover, although a statistical comparison was not performed with only 2 cases, the analysis of immunopuncta revealed a reduction in the cortices of CDD patients with respect to controls (Fig. 5A–D), indicative of an overall reduction of glutamatergic synapses. We next evaluated Homer1bc, PSD-95 and mGluR5 expression by western blotting on BA17 cortical lysates. Intriguingly, as shown in Fig. 5E–H, the BA17 area from CDD samples showed a robust reduction of their expression compared to controls. Although derived from a limited dataset, these results suggest that both structural and molecular signatures of CDKL5 loss in the cerebral cortex largely overlap between mice and humans and support the translational potential of a mGluR5-directed therapeutic strategy.
Fig. 5. Aberrant expression of excitatory synaptic proteins in the BA17 cortex of CDD patients.
A Illustrative confocal images taken from layers II-III of the BA17 cortex. (A) PSD-95+(red), Homer1bc+(green), VGluT1+(green) immunofluorescence puncta. Note the virtually complete overlapping of PSD-95 and Homer1bc immunofluorescence (scale bar: 5 µm). B–D Bar graphs showing the analysis of puncta density in layers II-III of BA17 cortices. E Western blotting showing the expression of PSD-95, Homer1bc and mGluR5 in lysates from BA17 cortices. F–H Bar graphs displaying the optical density (O.D.) analysis of PSD-95 (F), Homer1bc (G) and mGluR5 (H) expression. Student’s t-test, *p < 0.05, **p < 0.01 (C1 = F, 4 years old; P1 = F, 5.7 years old; C2 = F, 29 years old; P2 = F, 30 years old).
Discussion
It is urgent to find therapeutic targets that shall be rapidly translated into treatments for CDD, a devastating condition without corrective options. In this study, we focus our attention on mGluR5, a group I metabotropic glutamate receptor highly expressed in the cerebral cortex of both mice and humans [48]. To properly function, mGluR5 requires binding with Homer1bc [32], a scaffolding protein that is severely downregulated in the cerebral cortex of both Cdkl5−/y mice and CDD patients [18, 21]; Fig. 5A] as well as in iPSCs-derived neurons from CDD patients [49].
We show for the first time that CDKL5 plays a role in the expression of mGluR5 in the cerebral cortex of both CDD patients and CDKL5 mutant mice, an effect likely produced by the defective formation of mGluR5-Homer1bc complexes at synapses as indicated by our data. Moreover, we revealed that synaptic transmission, both basal and NMDA-mediated, is altered in S1 neurons lacking CDKL5 and that it is unresponsive to the modulation normally produced by the selective mGluR5 agonist DHPG. Because Shank1, by forming complexes with Homer1bc, PSD-95 and NMDAR, promotes the cooperation between NMDAR and mGluR5 signaling machineries [29, 50, 51], our electrophysiological evidence strongly suggest that CDKL5 loss tampers with the synergistic cooperation between these glutamatergic receptors. This effect is likely produced by a reduced amount of Homer1bc recruited in the postsynaptic density in the absence of CDKL5 which, in turn, results in an atypical postsynaptic localization/stabilization of mGluR5. Interestingly, aberrant NMDARs signaling have been previously reported by Okuda and colleagues [13] in the hippocampus of a different CDKL5 mutant mouse line showing severe NMDA-dependent epileptic seizures due to the incorrect postsynaptic accumulation of GluN2B-containing NMDARs [13]. Similar results have been obtained in the hippocampus of the Cdkl5R59X knock-in CDD mouse model [52]. Altogether, although with some differences, these findings further support the idea that CDKL5 plays a crucial role in the correct localization/function of glutamate receptors, both ionotropic and metabotropic, at the synapse. Remarkably, an aberrant expression and function of mGluR5 has been reported in several neurodevelopmental diseases such as Fragile X, Phelan McDermid syndrome, Tuberous sclerosis (TSC) and Rett syndrome [32, 38, 39, 53] further supporting the primary role of mGluR5 signaling as a common deranged pathway in monogenic neurodevelopmental disorders.
The reduced expression/function of mGluR5, combined with relevant synaptic and behavioral signs shown by CDKL5 mutants, provided us with solid bases for attempting the first preclinical assessment of mGluR5 PAMs efficiency for treating CDD that we report in this study. Intriguingly, our results revealed that an acute treatment with CDPPB is effective in restoring several endophenotypes and behavioral signs produced by CDKL5 loss. Our data show that in primary cortical neuronal cultures, CDPPB can restore mGluR5-mediated potentiation of NMDA currents in Cdkl5−/y pyramidal neurons. Considering the negative response of NMDA current to DHPG treatment that we report in mutant neurons, the effect of CDPPB is surprising and still without a clear pharmacological explanation, although it closely replicates what has been found previously in Shank3-KO neurons [38]. Furthermore, the present findings suggest that CDPPB treatment can facilitate the functional maturation of excitatory contacts in the absence of CDKL5. The effect of CDPPB is likely due to the following mechanisms: (i) increased synaptic expression of both Homer1bc and mGluR5, two essential molecular determinants of dendritic spine formation and stabilization [54, 55], as revealed by our immunofluorescence experiments; (ii) restored mGluR5-Homer1bc interaction, a crucial mechanism for the normal activity of mGluR5 [27, 32], as suggested by our electrophysiology results.
Interestingly, as shown by our results, these synaptic effects are reflected by beneficial outcomes in terms of cortical activation and behavioral response in mutant animals. Indeed, the results showing that cortical activation in response to visual stimulation can be rescued by CDPPB treatment in Cdkl5−/y mice, strengthens the translational value of our preclinical results, confirming that CVI can be used in the clinic as a solid biomarker for CDD [5, 17].
Interestingly, our findings indicate that mGluR5 signaling greatly suffers from the lack of CDKL5, but it does not become completely non-functional. In support of this idea, our data show that a 5-days treatment with CDPPB in CDKL5-null mice produces an effect on both the density of Homer1bc+ excitatory synapses and cortical IEG expression (i.e.: c-Fos and ARC) in the cerebral cortex as well as on behavioral/visual defects. Thus, although further studies are needed to dissect out the mechanisms of CDPPB action on excitatory synapse signaling, our results encourage further testing of mGluR5 PAMs in CDD models and offer hope for a future use of these compounds in the clinic. Our data set obtained with another mGluR5 PAM, the RO68 compound, further strengthens this idea. Considerably, RO68 has the clinically relevant advantage that it can be dissolved in salina with an extremely low percentage of detergent (i.e.,: Tween-80) and has a higher potency compared to other mGluR5 PAMs. Remarkably, RO68 is efficacious even at very low concentrations (i.e., 0.3 mg/kg), thus reducing the risk of toxicity, as we show in this study where this compound was able to rescue neuroanatomical and functional signs of CDKL5 mutants, and as it was previously shown in a TSC mouse model [47].
The positive action of mGluR5 PAMs on the molecular organization of postsynaptic structures is encouraging in view of the data we have obtained from two post-mortem CDD brains. Remarkably, we show for the first time that CDKL5 mutation robustly affects the excitatory synaptic compartment in the human BA17 cortex. Our data show that both the localization and the expression of several synaptic molecules (i.e., VGluT1, Homer1bc, PSD-95 and mGluR5) could be negatively affected, as we and others previously reported in CDKL5-null mice [5, 12, 18]. These results, when confirmed on a larger group CDD brains, shall contribute to disclose connectivity impairments of the primary visual cortex underlying CVI in these patients [15] and, consequently, strengthen the face-validity of Cdkl5−/y mice in modeling CDD. Importantly, our data indicate that the synaptic abnormalities and mGluR5 downregulation occurring in human CDD patients are potentially rescuable by positive allosteric modulation of mGluR5. Positively, we did not detect the insurgence of any spontaneous seizures in our study using two different drugs, thus heightening our confidence about the safety of mGluR5-PAM treatment for CDD. Importantly, previous studies reported that mGluR5 positive modulation does not exacerbate seizure incidence under both acute and subchronic treatment regimens [56, 57]. Nevertheless, it is known that the modulation of the NMDARs by the activation of the mGluR5 might be associated with epileptic phenotypes [58] thus prompting us to further explore this issue.
In further support of our findings, Negraes et al. (2021) have found similar synaptic defects in iPSCs-derived cortical neurons from CDD patients [49]. In apparent contrast from our observation, they disclosed an increased mGluR5-PanHomer association in CDD human organoids [49] while we revealed that mGluR5-Homer1bc binding, an association crucial for this receptor function, is decreased in CDKL5 mutant mice. The most parsimonious explanation of this discrepancy arises primarily either the different technical approaches or the experimental models used (i.e., mice brain vs CDD human organoids). Moreover, no discrimination between different Homer isoforms was attempted by Negraes et al. although it is known that the binding between mGluR5 and Homer1bc or Homer1a produces opposite effects on mGluR5 membrane expression and function [44, 59, 60]. Hence, the enhanced mGluR5-PanHomer interaction could be produced by an increased association with Homer1a, thus not ruling out a decrease of mGluR5-Homer1bc binding as revealed by our study.
In conclusion, we believe that our findings on the efficacy of mGluR5 activation pave the way for including these receptors as a promising therapeutic target for CDD. Our results also suggest that an early-onset and prolonged regime of mGluR5 activation has the potential to stably revert the morphofunctional defects shown by adult CDKL5 mutants, without inducing epileptic seizures. Finally, this study further supports previous indications that abnormalities of mGluR5 signaling represents a convergent pathway for multiple neurodevelopmental diseases, a solid hallmark now including CDD.
Supplementary information
Author contributions
AG and MG conceived and designed the study. AG performed biochemical experiments. LL, GS, RM, and EP performed IOS experiments. SG performed experiments on human tissues, AG, RP, NM, and FP performed behavioural experiments, AG, RP, SD and DC performed immunofluorescence experiments. AM and GC performed electrophysiological experiments. CS, AN synthesized and provided RO6807794; AG, RP, AR, SD, TP, AM, and MG analyzed the data. AG and MG wrote the manuscript.
Funding
This work was supported by research grants from: University of Pennsylvania Orphan Disease Center on behalf of LouLou Foundation (CDKL5 PILOT GRANT PROGRAM n. CDKL5 - 17 - 106 – 01) and from Associazione CDKL5 Insieme verso la cura (Italy) to MG and TP; The International Foundation for CDKL5 Research, Associazione Albero di Greta and Fondazione CRT (n. 2018.0889) and by Fondazione Telethon-Italy (Grants nn. GGP15098 and GGP19045) to MG.
Competing interests
The authors declare no competing interests.
Ethical approval
The study was conducted in accordance with European Community Council Directive 2010/63/UE for care and use of experimental animals with protocols approved by the Italian Minister for Scientific Research (Authorization number 38/2020-PR) and the Bioethics Committee of the University of Torino, Italy.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-022-01412-3.
References
- 1.Rusconi L, Salvatoni L, Giudici L, Bertani I, Kilstrup-Nielsen C, Broccoli V, et al. CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail. J Biol Chem. 2008;283:30101–11. doi: 10.1074/jbc.M804613200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baltussen LL, Negraes PD, Silvestre M, Claxton S, Moeskops M, Christodoulou E, et al. Chemical genetic identification of CDKL5 substrates reveals its role in neuronal microtubule dynamics. EMBO J. 2018;37:e99763. doi: 10.15252/embj.201899763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muñoz IM, Morgan ME, Peltier J, Weiland F, Gregorczyk M, Cm Brown F, et al. Phosphoproteomic screening identifies physiological substrates of the CDKL5 kinase. EMBO J. 2018;37:e99559. doi: 10.15252/embj.201899559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nawaz MS, Giarda E, Bedogni F, La Montanara P, Ricciardi S, Ciceri D, et al. CDKL5 and Shootin1 interact and concur in regulating neuronal polarization. PLoS One. 2016;11:e0148634. doi: 10.1371/journal.pone.0148634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trazzi S, De Franceschi M, Fuchs C, Bastianini S, Viggiano R, Lupori L, et al. CDKL5 protein substitution therapy rescues neurological phenotypes of a mouse model of CDKL5 disorder. Hum Mol Genet. 2018;27:1572–92. doi: 10.1093/hmg/ddy064. [DOI] [PubMed] [Google Scholar]
- 6.Kameshita I, Sekiguchi M, Hamasaki D, Sugiyama Y, Hatano N, Suetake I, et al. Cyclin-dependent kinase-like 5 binds and phosphorylates DNA methyltransferase 1. Biochem Biophys Res Commun. 2008;377:1162–7. doi: 10.1016/j.bbrc.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 7.Mari F, Azimonti S, Bertani I, Bolognese F, Colombo E, Caselli R, et al. CDKL5 belongs to the same molecular pathway of MeCP2 and it is responsible for the early-onset seizure variant of Rett syndrome. Hum Mol Genet. 2005;14:1935–46. doi: 10.1093/hmg/ddi198. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y-C, Li D, Wang L, Lu B, Zheng J, Zhao S-L, et al. Palmitoylation-dependent CDKL5-PSD-95 interaction regulates synaptic targeting of CDKL5 and dendritic spine development. Proc Natl Acad Sci USA. 2013;110:9118–23. doi: 10.1073/pnas.1300003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricciardi S, Ungaro F, Hambrock M, Rademacher N, Stefanelli G, Brambilla D, et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol. 2012;14:911–23. doi: 10.1038/ncb2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbiero I, Peroni D, Tramarin M, Chandola C, Rusconi L, Landsberger N, et al. The neurosteroid pregnenolone reverts microtubule derangement induced by the loss of a functional CDKL5-IQGAP1 complex. Hum Mol Genet. 2017;26:3520–30. doi: 10.1093/hmg/ddx237. [DOI] [PubMed] [Google Scholar]
- 11.Tramarin M, Rusconi L, Pizzamiglio L, Barbiero I, Peroni D, Scaramuzza L, et al. The antidepressant tianeptine reverts synaptic AMPA receptor defects caused by deficiency of CDKL5. Hum Mol Genet. 2018;27:2052–63. doi: 10.1093/hmg/ddy108. [DOI] [PubMed] [Google Scholar]
- 12.Amendola E, Zhan Y, Mattucci C, Castroflorio E, Calcagno E, Fuchs C, et al. Mapping pathological phenotypes in a mouse model of CDKL5 disorder. PLoS One. 2014;9:5–16. doi: 10.1371/journal.pone.0091613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuda K, Kobayashi S, Fukaya M, Watanabe A, Murakami T, Hagiwara M, et al. CDKL5 controls postsynaptic localization of GluN2B-containing NMDA receptors in the hippocampus and regulates seizure susceptibility. Neurobiol Dis. 2017;106:157–70.. doi: 10.1016/j.nbd.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang I-TJ, Allen M, Goffin D, Zhu X, Fairless AH, Brodkin ES, et al. Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proc Natl Acad Sci USA. 2012;109:21516–21. doi: 10.1073/pnas.1216988110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demarest ST, Olson HE, Moss A, Pestana‐Knight E, Zhang X, Parikh S, et al. CDKL5 deficiency disorder: Relationship between genotype, epilepsy, cortical visual impairment, and development. Epilepsia. 2019;60:1733–42. doi: 10.1111/epi.16285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang HT, Zhu ZA, Li YY, Lou SS, Yang G, Feng X, et al. CDKL5 deficiency in forebrain glutamatergic neurons results in recurrent spontaneous seizures. Epilepsia. 2021;62:517–28. doi: 10.1111/epi.16805. [DOI] [PubMed] [Google Scholar]
- 17.Mazziotti R, Lupori L, Sagona G, Gennaro M, Sala GD, Putignano E, et al. Searching for biomarkers of CDKL5 disorder: early-onset visual impairment in CDKL5 mutant mice. Hum Mol Genet. 2017;26:2290–8. doi: 10.1093/hmg/ddx119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pizzo R, Lamarca A, Sassoè-Pognetto M, Giustetto M. Structural Bases of Atypical Whisker Responses in a Mouse Model of CDKL5 Deficiency Disorder. Neuroscience. 2020;445;130–43. [DOI] [PubMed]
- 19.Della Sala G, Putignano E, Chelini G, Melani R, Calcagno E, Michele Ratto G, et al. Dendritic spine instability in a mouse model of CDKL5 disorder is rescued by insulin-like growth factor 1. Biol Psychiatry. 2016;80:302–11. doi: 10.1016/j.biopsych.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Lupori L, Sagona G, Fuchs C, Mazziotti R, Stefanov A, Putignano E, et al. Site-specific abnormalities in the visual system of a mouse model of CDKL5 deficiency disorder. Hum Mol Genet. 2019;28:2851–61. doi: 10.1093/hmg/ddz102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pizzo R, Gurgone A, Castroflorio E, Amendola E, Gross C, Sassoè-Pognetto M, et al. Lack of Cdkl5 disrupts the organization of excitatory and inhibitory synapses and parvalbumin interneurons in the primary visual cortex. Front Cell Neurosci. 2016;10:261. doi: 10.3389/fncel.2016.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballester-Rosado CJ, Sun H, Huang JY, Lu HC. mGluR5 exerts cell-autonomous influences on the functional and anatomical development of layer IV cortical neurons in the mouse primary somatosensory cortex. J Neurosci. 2016;36:8802–14. doi: 10.1523/JNEUROSCI.1224-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C-C, Lu H-C, Brumberg JC. mGluR5 knockout mice display increased dendritic spine densities. Neurosci Lett. 2012;524:65–68. doi: 10.1016/j.neulet.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edfawy M, Guedes JR, Pereira MI, Laranjo M, Carvalho MJ, Gao X, et al. Abnormal mGluR-mediated synaptic plasticity and autism-like behaviours in Gprasp2 mutant mice. Nat Commun. 2019;10:1431. doi: 10.1038/s41467-019-09382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piers TM, Kim DH, Kim BC, Regan P, Whitcomb DJ, Cho K. Translational concepts of mglur5 in synaptic diseases of the brain. Front Pharm. 2012;3:1–7. doi: 10.3389/fphar.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, et al. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive Homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–16. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ronesi JA, Collins KA, Hays SA, Tsai N-P, Guo W, Birnbaum SG, et al. Disrupted Homer scaffolds mediate abnormal mGluR5 function in a mouse model of fragile X syndrome. Nat Neurosci. 2012;15:431–40, S1. doi: 10.1038/nn.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scheefhals N, MacGillavry HD. Functional organization of postsynaptic glutamate receptors. Mol Cell Neurosci. 2018;91:82–94. doi: 10.1016/j.mcn.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, et al. Coupling of mGluR/Homer and PSD-95 complexes by the shank family of postsynaptic density proteins. Neuron. 1999;23:583–92. doi: 10.1016/S0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 30.Morello N, Schina R, Pilotto F, Phillips M, Melani R, Plicato O, et al. Loss of Mecp2 causes atypical synaptic and molecular plasticity of parvalbumin-expressing interneurons reflecting rett syndrome-like sensorimotor defects. eNeuro. 2018;5:ENEURO.0086–18.2018. doi: 10.1523/ENEURO.0086-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- 32.Aloisi E, Le Corf K, Dupuis J, Zhang P, Ginger M, Labrousse V, et al. Altered surface mGluR5 dynamics provoke synaptic NMDAR dysfunction and cognitive defects in Fmr1 knockout mice. Nat Commun. 2017;8:1103. doi: 10.1038/s41467-017-01191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kammermeier PJ, Worley PF. Homer 1a uncouples metabotropic glutamate receptor 5 from postsynaptic effectors. Proc Natl Acad Sci USA. 2007;104:6055–60. doi: 10.1073/pnas.0608991104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, et al. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–54. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verpelli C, Dvoretskova E, Vicidomini C, Rossi F, Chiappalone M, Schoen M, et al. Importance of Shank3 protein in regulating metabotropic glutamate receptor 5 (mGluR5) expression and signaling at synapses. J Biol Chem. 2011;286:34839–50. doi: 10.1074/jbc.M111.258384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiner A, Levitz J. Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron. 2018;98:1080–98. doi: 10.1016/j.neuron.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcantoni A, Cerullo MS, Buxeda P, Tomagra G, Giustetto M, Chiantia G, et al. Amyloid Beta42 oligomers up-regulate the excitatory synapses by potentiating presynaptic release while impairing postsynaptic NMDA receptors. J Physiol. 2020;598:2183–97. doi: 10.1113/JP279345. [DOI] [PubMed] [Google Scholar]
- 38.Vicidomini C, Ponzoni L, Lim D, Schmeisser M, Reim D, Morello N, et al. Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice Europe PMC Funders Group. Mol Psychiatry. 2017;22:689–702. doi: 10.1038/mp.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–8. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Goudet C, Pin J-P, Conn PJ. N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol. 2008;73:909–18. doi: 10.1124/mol.107.040097. [DOI] [PubMed] [Google Scholar]
- 41.Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, et al. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat Protoc. 2009;4:1560–4. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- 42.Komotar RJ, Kim GH, Sughrue ME, Otten ML, Rynkowski MA, Kellner CP, et al. Neurologic assessment of somatosensory dysfunction following an experimental rodent model of cerebral ischemia. Nat Protoc. 2007;2:2345–7. doi: 10.1038/nprot.2007.359. [DOI] [PubMed] [Google Scholar]
- 43.Fuchs C, Trazzi S, Torricella R, Viggiano R, De Franceschi M, Amendola E, et al. Loss of CDKL5 impairs survival and dendritic growth of newborn neurons by altering AKT/GSK-3beta signaling. Neurobiol Dis. 2014;70:53–68. doi: 10.1016/j.nbd.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terzic B, Davatolhagh MF, Ho Y, Tang S, Liu YT, Xia Z, et al. Temporal manipulation of Cdkl5 reveals essential postdevelopmental functions and reversible CDKL5 deficiency disorder-related deficits. J Clin Invest. 2021;131:e143655. [DOI] [PMC free article] [PubMed]
- 45.Ménard C, Quirion R. Successful cognitive aging in rats: A role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PLoS One. 2012;7:e28666. doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Zhuo M. Group I metabotropic glutamate receptor-mediated gene transcription and implications for synaptic plasticity and diseases. Front Pharm. 2012;3:189. doi: 10.3389/fphar.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly E, Schaeffer SM, Dhamne SC, Lipton JO, Lindemann L, Honer M, et al. MGluR5 modulation of behavioral and epileptic phenotypes in a mouse model of tuberous sclerosis complex. Neuropsychopharmacology. 2018;43:1457–65. doi: 10.1038/npp.2017.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 49.Negraes PD, Trujillo CA, Yu N-K, Wu W, Yao H, Liang N, et al. Altered network and rescue of human neurons derived from individuals with early-onset genetic epilepsy. Mol Psychiatry. 2021;26:7047–68. [DOI] [PMC free article] [PubMed]
- 50.Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, et al. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by Homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–6. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hering H, Sheng M. Dendritic spines: Structure, dynamics and regulation. Nat Rev Neurosci. 2001;2:880–8. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 52.Yennawar M, White RS, Jensen FE. AMPA receptor dysregulation and therapeutic interventions in a mouse model of CDKL5 deficiency disorder. J Neurosci. 2019;39:4814–28. doi: 10.1523/JNEUROSCI.2041-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gogliotti RG, Senter RK, Rook JM, Ghoshal A, Zamorano R, Malosh C, et al. mGlu5 positive allosteric modulation normalizes synaptic plasticity defects and motor phenotypes in a mouse model of Rett syndrome. Hum Mol Genet. 2016;25:1990–2004. doi: 10.1093/hmg/ddw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh WC, Hill TC, Zito K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc Natl Acad Sci USA. 2013;110:E305–12. doi: 10.1073/pnas.1214705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sala C, Futai K, Yamamoto K, Worley PF, Hayashi Y, Sheng M. Inhibition of dendritic spine morphogenesis and synaptic transmission by activity-inducible protein Homer1a. J Neurosci. 2003;23:6327–37. doi: 10.1523/JNEUROSCI.23-15-06327.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pacey LK, Tharmalingam S, Hampson DR. Subchronic administration and combination metabotropic glutamate and GABAB receptor drug therapy in fragile X syndrome. J Pharm Exp Ther. 2011;338:897–905. doi: 10.1124/jpet.111.183327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanak TJ, Libbey JE, Doty DJ, Sim JT, DePaula-Silva AB, Fujinami RS. Positive modulation of mGluR5 attenuates seizures and reduces TNF-α+ macrophages and microglia in the brain in a murine model of virus-induced temporal lobe epilepsy. Exp Neurol. 2019;311:194–204. doi: 10.1016/j.expneurol.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hanada T. Ionotropic glutamate receptors in epilepsy: A review focusing on AMPA and NMDA receptors. Biomolecules. 2020;10:464. doi: 10.3390/biom10030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bertaso F, Roussignol G, Worley P, Bockaert J, Fagni L, Ango F. Homer1a-dependent crosstalk between NMDA and metabotropic glutamate receptors in mouse neurons. PLoS ONE. 2010;5:e9755. doi: 10.1371/journal.pone.0009755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.