Abstract
Background
Securing sufficient blood perfusion to the anastomotic area after low-anterior resection is a crucial factor in preventing anastomotic leakage (AL). Intra-operative indocyanine green fluorescent imaging (ICG-FI) has been suggested as a tool to assess perfusion. However, knowledge of inter-observer variation among surgeons in the interpretation of ICG-FI is sparse. Our primary objective was to evaluate inter-observer variation among surgeons in the interpretation of bowel blood-perfusion assessed visually by ICG-FI. Our secondary objective was to compare the results both from the visual assessment of ICG and from computer-based quantitative analyses of ICG-FI between patients with and without the development of AL.
Method
A multicenter study, including patients undergoing robot-assisted low anterior resection with stapled anastomosis. ICG-FI was evaluated visually by the surgeon intra-operatively. Postoperatively, recorded videos were anonymized and exchanged between centers for inter-observer evaluation. Time to visibility (TTV), time to maximum visibility (TMV), and time to wash-out (TWO) were visually assessed. In addition, the ICG-FI video-recordings were analyzed using validated pixel analysis software to quantify blood perfusion.
Results
Fifty-five patients were included, and five developed clinical AL. Bland–Altman plots (BA plots) demonstrated wide inter-observer variation for visually assessed fluorescence on all parameters (TTV, TMV, and TWO). Comparing leak-group with no-leak group, we found no significant differences for TTV: Hazard Ratio; HR = 0.82 (CI 0.32; 2.08), TMV: HR = 0.62 (CI 0.24; 1.59), or TWO: HR = 1.11 (CI 0.40; 3.11). In the quantitative pixel analysis, a lower slope of the fluorescence time-curve was found in patients with a subsequent leak: median 0.08 (0.07;0.10) compared with non-leak patients: median 0.13 (0.10;0.17) (p = 0.04).
Conclusion
The surgeon’s visual assessment of the ICG-FI demonstrated wide inter-observer variation, there were no differences between patients with and without AL. However, quantitative pixel analysis showed a significant difference between groups.
Trial Registration
ClinicalTrials.gov Identifier: NCT04766060.
Keywords: Anastomotic leakage, Fluorescence, Inter-observer variation, Colorectal surgery, q-ICG
Anastomotic leakage (AL) is a serious complication after low anterior resection for rectal cancer and is associated with an increased risk of postoperative mortality and morbidity, cancer recurrence, and impaired functional outcome [1–3]. In the last 3 years, the rate of clinical ALs registered in the nationwide Danish Colorectal Cancer Group’s database has been around 9% [4].
Among several factors, sufficient blood supply and oxygen delivery to the anastomotic area is crucial to ensure optimal conditions for anastomotic healing [5]. Traditionally the evaluation of blood supply has been based on the surgeon’s subjective surrogate measures such as tissue colour, mesenteric pulsation, and marginal arterial bleeding. Clinical studies have demonstrated that the surgeon’s intraoperative judgment in predicting AL, based on these parameters, has a low sensitivity and specificity [6]. Several other different techniques have been evaluated for a more objective evaluation of the blood supply including laser Doppler flowmetry [7, 8], near-infrared spectroscopy [9, 10], intra-mucosal pH measurements [11], and tissue oxygen tension [12] as the most common, but none of these methods has become routine in clinical use.
Clinical studies in robot-assisted and laparoscopic colorectal surgery confirm the feasibility of using indocyanine green fluorescent imaging (ICG-FI) intra-operatively [13–19], and cohort studies have shown that when taking into account whether to re-do the anastomosis or not it may reduce the AL rate by 54–67% [14, 16, 20, 21]. Two randomized clinical trials have been published [22, 23]. One of the studies showed a signifcant difference in grade A anastomotic leakage in those who had undergone a perioperative ICG evaluation compared to a control group and no difference in the other study. Software-based pixel analyses to quantify the fluorescence signal have been developed [24–26], but no cut-off values for anastomotic re-do have been provided. Animal experimental studies have shown that quantifying indocyanine green fluorescent imaging (q-ICG-FI) can be used as a surrogate measure of local bowel blood perfusion. In addition, a correlation between relative flow in the anastomotic area and anastomotic strength as assessed by stretch-tension and histological healing has been demonstrated [27–29].
Different doses of ICG have been used [30, 31]. However, there is no consensus on the optimal dose or whether this has any importance for the visual interpretation of the ICG-FI.
The primary aim of the present study was to investigate inter-observer variation among surgeons regarding their visual assessment of ICG-FI and based on this assessment, whether they would recommend a re-do of the anastomosis to prevent AL. Our secondary aim was to compare the results from both the visual and the quantitative assessment of ICG-FI in relation to the development of AL, and to investigate whether the dose of indocyanine green (ICG) had an influence on the assessment.
Material and methods
This was a prospective study including patients undergoing robot-assisted low anterior resection with stapled anastomosis. We included patients from five different colorectal centers in Denmark from April 2017 to November 2018. Each center included between six and 22 patients.P
After bowel resection and placement of the circular stapler anvil in the oral end, the bowel segment was placed intraabdominally to obtain full visibility of the bowel serosa and a distance to the camera tip of 5 cm (Fig. 1). The perfusion was only assessed at this time before connecting the anvil to the base of the stapler and performing the anastomosis.
Fig. 1.
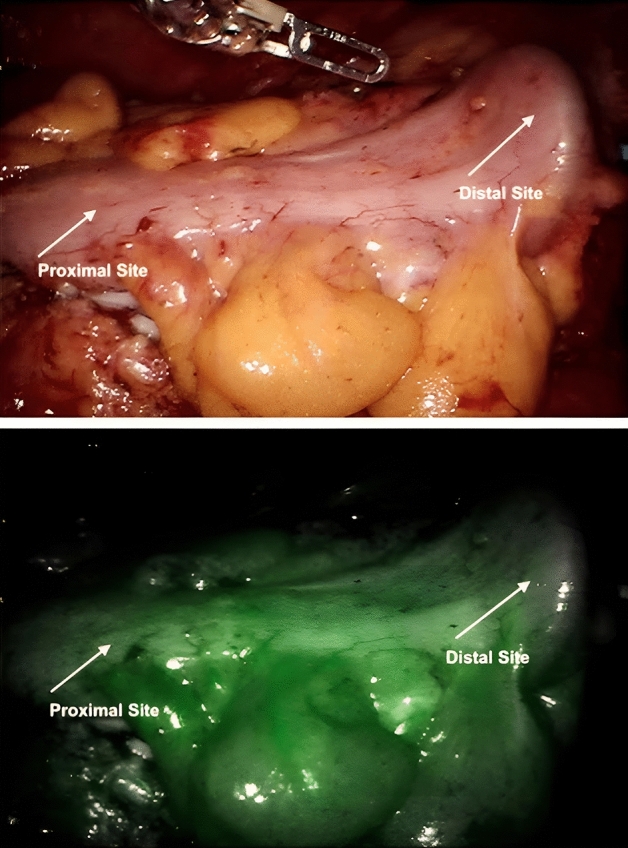
Prepared bowel in white and infrared light + ICG, respectively
To investigate whether dosage impacted our assessment, patients randomly received either 7.5 mg or 15 mg ICG (VERDYE; Diagnostic Green GmbH, Germany) as an i.v. bolus. At the same time, the camera was switched to near-infrared light (Firefly, da Vinci Robotic Assisted Surgical System, Intuitive Inc, CA, USA.), and a stopwatch was started. With ICG injection as time zero, the fluorescence perfusion in the proximal part of the anastomosis was evaluated by the following parameters: “Time to visibility” (TTV), “time to maximum visibility” (TMV), and “time to wash-out” (TWO) as judged visually by the surgeon and recorded intraoperatively. In addition, the surgeons were asked to decide whether they considered blood supply to be sufficient to perform an anastomosis or whether they would do a re-resection.
The ICG-FI was video recorded, and anonymized videos were exchanged between centers and surgeons for interobserver variation analysis and software analysis. None of the surgeons analyzed videos from their own department.
The videos from the ICG-FI procedures were analysed using a validated pixel analysis software (q-ICG) as previously described [32–36]. This software was developed to quantify the fluorescence using the normalized slope from the fluorescence intensity time curve as an indirect measure of blood flow. This method showed a good correlation to another method of measuring tissue blood flow with the injection of radioactive marked microspheres [32]. The software program was developed on basis of experimental studies on pigs.
The normalized slope is defined as fluorescence increase over time divided by maximum fluorescence minus baseline fluorescence (∆Fluorescence intensity/∆time)/(maximum intensity–baseline intensity) (Fig. 2). Regions of interest (ROI) near the anvil (distal) and 5 cm orally (proximal) to the edge of the anvil were chosen (Fig. 1), and pixel analysis was performed in these ROIs.
Fig. 2.
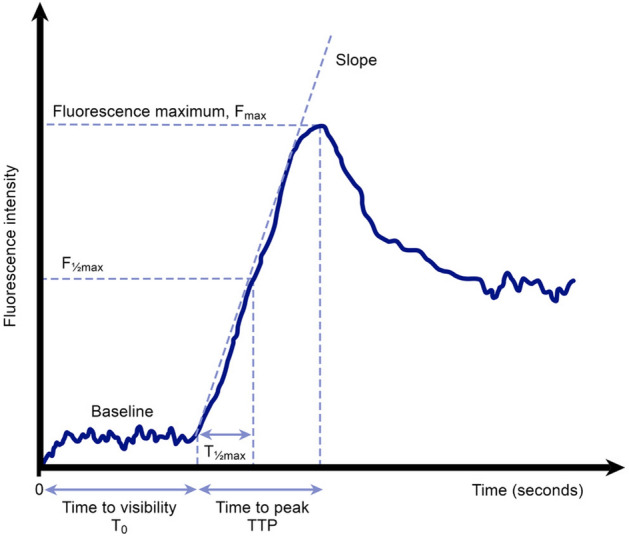
Fluorescence time curve. Normalized Slope is defined as fluorescence increase over time divided by maximum fluorescence minus baseline fluorescence
Data sources
Clinical baseline characteristics were obtained pre-operatively from the patient record. Intra-operatively the surgeon and nurse recorded the requested data for the ICG-FI in collaboration. Mean arterial blood pressure during the procedure was recorded. Complications within 30 days post-operatively were recorded and graded according to the Clavien-Dindo classification. In addition, any recognized clinical AL, grades B, and C were recorded and defined according to the definition given by the International Study Group of Rectal Cancer [37].
Postoperatively, the videos were, to simulate real-life, presented only once and continuously by peer surgeons.
All data were entered into a RedCap database, provided by OPEN, Open Patient data Explorative Network. Data were recorded by single entry.
Statistics
Bland–Altman (BA) plots were used to evaluate interobserver variation. The two-sample t-test for numerical variables and Fisher's exact test for categorical variables were used to compare the background variables between the groups with AL and non-AL. Values for TTV, TMV, and TWO in the two groups were presented as Kaplan–Meier curves and compared by log-rank test. The time differences were evaluated by estimating hazard ratios (HR) comparing the AL group with the non-AL group by Cox regression, both unadjusted as well as adjusted for age, comorbidity, performance status, use of tobacco and alcohol, mean arterial blood pressure, and ICG dosage.
A Wilcoxon rank-sum test was used for the pixel analysis to compare the groups with AL and non-AL and presented by median and interquartile range (IQR).
Statistical calculations were performed using STATA software (version 16; Stata Corp LP, College Station, Texas, USA). P-values less than 0.05 were considered statistically significant.
Ethics and approvals
The study was approved by the Regional Scientific Ethical Committees of Southern Denmark (Project-ID: S-20160137) and the Danish Data Protection Agency (jr.no. 16/30618).
All patients received written and orally information about the project and written consent was obtained from each patient.
Results
Fifty-five patients were included, and five patients developed clinical AL (9.1%). We found significantly more patients with heart disease (p = 0.018), diabetes (p = 0.001), and lymph node metastases (p = 0.048) in the AL group. There were no significant differences in other baseline characteristics between the AL and non-AL group (Table 1).
Table 1.
Baseline characteristics for patients with and without anastomotic leakage (AL)
| No leak = 50 | Leak = 5 | |
|---|---|---|
|
Male Female |
31 (62%) 19 (38%) |
5 (100%) 0 (0%) |
| Age, mean (SD) | 63.9 (8.6) | 70 (6.4) |
| BMI, mean (SD) | 26.0 (4.0) | 26.1 (3.3) |
|
ASA 1 ASA 2 |
19 (38%) 31 (62%) |
0 (0%) 5 (100%) |
|
Heart disease (p = 0.018): No Yes Unknown |
45 (90%) 4 (8%) 1 (2%) |
2 (40%) 3 (60%) 0 (0%) |
|
Diabetes (p = 0.001) No Yes Unknown |
48 (96%) 1 (2%) 1 (2%) |
2 (40%) 3 (60%) 0 (0%) |
|
cT0 cT1 cT2 cT3 cT4 cTx |
1 (2%) 6 (12%) 16 (32%) 19 (38%) 4 (8%) 4 (8%) |
0 (0%) 0 (0%) 2 (40%) 2 (40%) 1 (20%) 0 (0%) |
|
Positive Lymph Nodes (p = 0.048) cN0 cN1 cN2 cNx |
21 (42%) 13 (26%) 7 (14%) 9 (18%) |
0 (0%) 4 (80%) 1 (20%) 0 (0%) |
| Operation time, mean (SD), minutes | 234.40 (84.81) | 284.00 (72.66) |
| Bleeding ml, mean (SD) | 59.91 (62.37) | 60 (22.36) |
| Anastomotic distance from the anal verge, mean, cm (SD) | 7.93 (4.75) | 5.60 (2.70) |
|
Drain No Yes |
6 (70%) 15 (30%) |
2 (40%) 3 (60%) |
|
Diverting loop-ileostomy No Yes |
30 (60%) 20 (40%) |
2 (40%) 3 (60%) |
|
No. of transverse staplings 1 2 3 Unknown |
17 (34%) 26 (52%) 4 (8%) 3 (6%) |
1 (20%) 3 (60%) 0 (0%) 1 (20%) |
SD Standard Deviation, BMI Body Mass Index, ASA American Society of Anesthesiologists, cT clinical Tumor stage, cN clinical Lymph Node metastasis
Visual assessment
For the inter-observer investigation, 11 of 55 patients were excluded due to difficulty in video transfer. Due to missing observer data, only 43, 40, and 40 recordings were included in the analysis of TTV, TMV, and TWO, respectively. Missing data occurred both in the intra-operative group and in the observer group. One patient in the observer group developed AL.
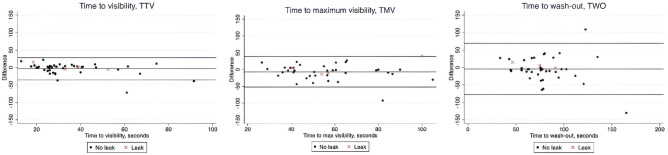
We found poor agreement for all three parameters, TTV, TMV, and TWO, and the observations by the operating surgeon and the blinded observer differed by up to 70 s. The inter-observer variation was lowest for TTV and highest for TWO (Fig. 3).
Fig. 3.
Bland Altman plots illustrating interobserver variation in the leak and no-leak patients assessed intra-operatively by surgeons and postoperatively by peer surgeons (video recordings). The closer dots are to the centerline, the more agreement between the primary surgeon and blinded observer
In all the operated cases the surgeon considered the blood perfusion to be sufficient to construct an anastomosis based on the ICG-FI, and did not consider a re-resection. Forty-four videos were available for postoperative assessment of whether a re-resection would be recommended, and in seven cases the observer would recommend a re-resection based on the visual assessment of the ICG-FI, but only two of these patients developed an AL. Thus, the postoperative visual assessment of ICG-FI could predict AL came out with a sensitivity of 40% (95% CI: 0.05;0.85) and a specificity of 87% (95% CI 0.73;0.96).
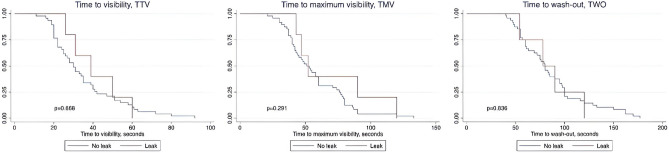
The number of patients included in the intraoperative subjective analyses of ICG for TTV, TMV, and TWO was 52, 53, and 52 s, respectively. Comparing time intervals between AL and non-AL we obtained HRs for TTV 0.82 (95% CI: 0.32, 2.08), for TMV 0.62 (95% CI 0.24, 1.59), and 1.11 (95% CI 0.40, 3.11) for TWO. There was no significant difference between the leak and the no-leak group (Fig. 4). Adjusting for confounders the HR for leakage was 1.69 (95% CI 0.57, 5.08), 1.09 (95% CI 0.36, 3.35), and 2.15 (95% CI 0.64, 7.16) for TTV, TMV, and TWO, respectively. Adjusting for the number of cross staples did not change the HRs.
Fig. 4.
Kaplan–Meier curves illustrating the subjective analysis in the leak and no-leak patients. Number of patients included in the subjective analyses of ICG for TTV, TMV, and TWO was 52, 53, and 52, respectively
Quantitative assessment
Quantitative pixel analysis with q-ICG showed a significant difference between the leak and the no-leak group when looking at the results from the ICG-FI 5 cm proximal from the edge of the anvil. The normalized slope was median 0.08 (0.07;0.10) and 0.13 (0.10;0.17), respectively (p = 0.04) (Table 2). We included 31 patients without AL and 5 patients with AL in this analysis. Thirteen videos were excluded from this analysis due to poor pixel quality, which compromised the software analysis.
Table 2.
Quantitative perfusion analyses, normalized slope (∆Fluorescence intensity/∆time)/(maximum intensity – baseline intensity)
| No Leak | Leak n = 5 | p-value | Dosage 7.5 mg n = 20 |
Dosage 15 mg | p-value | |
|---|---|---|---|---|---|---|
|
Perfusion distally Median (IQR) |
n = 31 0.13 (0.10;0,15) |
0.07 (0.05;0.13) | 0.10 | 0.14 (0.11;0.16) |
n = 16 0.11 (0.07;0.14) |
0.07 |
|
Perfusion proximally Median (IQR) |
n = 30 0.13 (0.10;0.17) |
0.08 (0.07;0.10) | 0.04 | 0.13 (0.10;0.16) |
n = 15 0.12 (0.09;0.16) |
0.53 |
Distally = anastomotic site (anvil), proximally = 5 cm proximal to the anastomotic site; Statistics: Wilcoxon Rank sum test. Bold value is statistically significant (p < 0.05)
ICG dose
There was no difference in TTV, TMV, or TWO when comparing patients receiving low or high doses of ICG (Table 3), regardless of whether the subjective evaluation was intraoperative or based on video recordings. Similarly, we found no difference in the normalized slope/fluorescence intensity when using a low or high ICG dose (Table 2).
Table 3.
Subjective evaluation by surgeon and observer of ICG-FI in time to visibility (TTV), time to maximum visibility (TMV) and time to wash-out (TWO) from the two different ICG dosages
| Dose of ICG | Primary surgeon | Observer | ||||
|---|---|---|---|---|---|---|
| 7.5 mg | 15 mg | p-value | 7.5 mg | 15 mg | p-value | |
| TTV | 32 (26–38) | 33 (26–40) | 0.71 | 29 (23–34) | 40 (30–39) | 0.08 |
| TMV | 51 (42–59) | 57 (45–69) | 0.44 | 51 (54–78) | 66 (54–78) | 0.11 |
| TWO | 70 (59–81) | 82 (69–95) | 0.2 | 69 (57–82) | 85 (72–98) | 0.09 |
Values are mean 95% confidence limits in brackets
Discussion
In this study, we chose to assess the perfusion after resection and placement of stapler anvil before creating the anastomosis to mimic the clinical situation where the surgeon must decide whether the blood flow is sufficient, or a further resection is necessary before creating the anastomosis. A post-anastomotic analysis may be relevant but meets the problem with the washout period of ICG which may impair interpretation. At least for the non-software-based visual analysis.
In our study we found no difference in the subjective assessment of ICG-FI or qICG using 7,5 mg of ICG or 15 mg of ICG (Table 2). Different dosages have been used varying from a bolus of 6 mg to a bolus of 0.5 mg/kg. No consensus on dosage has been achieved, but titration according to weight might be reasonable.
Most studies exploring fluorescence imaging in gastro-intestinal surgery have been based on the assessment of perfusion on subjective appraisal [30]. A recent meta-analysis [38] reported and found overall frequency of AL on 6.7%, with 4.2% in the ICG group compared to 11.3% in the control group and the difference was highly significant. Due to the heterogeneity of the included studies, they carefully conclude that ICG fluorescence imaging appears to be a promising tool to reduce the rate of AL. In our study we were not able to predict AL from the subjective assessment of ICG-FI.
We found wide inter-observer variation in all parameters chosen for subjective visual evaluation of ICG-FI. None of the patients had a re-resection performed based on the results of ICG-FI, but five of the patients developed AL. In seven patients, the blinded observer would recommend re-resection, but only two of these patients developed AL. Based on this, we found poor sensitivity (40%) of the visual ICG-FI in identifying patients who develop AL, and we suggest that subjective semiquantitative evaluation of ICG-FI should be explored and refined further.
A randomized study [22] including 377 patients, found an overall incidence of AL of 12.7%, with 9.1% in the ICG group and 16.3% in the non-ICG group (p = 0.04). There was no difference in AL rate for high anastomoses (9–15 cm from the anal verge), but a significant difference in low anastomoses (14.4% vs 25.7%). This difference consisted of more grade A leakages in the non-ICG group. Perfusion was assessed visually and defined as good if there were uniform fluorescence within 2–3 min.
Another randomized study [23] including 240 patients from three hospitals AL occurred in 7% in total with 5% in the ICG group, and 9% in the non-ICG group. Perfusion was evaluated visually as good, poor, or absent within 1 minute after ICG injection.
In our study, surgeon-observer agreement was better in the early phase of fluorescence, when intensity was highest and became more divergent as time passed (Fig. 2). This emphasizes the importance of fluorescence assessment over a short time-period, for example 1 min.
In the two studies, impaired perfusion in the ICG groups was seen in 19.2% [22] and 11% [23], respectively, leading to further resection. In none of the 55 patients in our study the surgeons considered further resection. The risk of futile resections is an important problem, which hasn't been devoted much attention in any of the previous studies.
Software programs have been developed and validated for the quantification of bowel blood perfusion with ICG-FI [27, 32–36]. By utilizing our quantification software (q-ICG), we found a significant difference between the leak and no-leak patients when measuring 5 cm proximal to the anastomotic site. Due to our small sample size with only five events of anastomotic leakages, this difference should be interpreted with caution and evaluated further in a larger setup.
Strengths and limitations
The present study has several limitations. The patient cohort was limited to 55 patients, of whom only five had AL.
We performed the ICG-FI after the anvil was placed, which might entail temporary compression on blood vessels at the anastomotic site, with the risk of a false low fluorescence intensity. This compression ceases once the anastomosis is performed, and a second fluorescence evaluation at this time might have given other results.
Conventional ICG-FI as used in our study only reflects the serosa side of the bowel. It has been shown that the mucosa is more susceptible to ischaemia than the serosa [39]. Therefore, it would be interesting to assess the fluorescent intensity from the mucosal side with trans-anal ICG-FI [40].
The strength of our study was the systematic approach towards evaluating the ICG-FI and its comparison with computer-based pixel analysis. We did not find any relationship between the results and the infusion of 7,5 mg or 15 mg.
All the qICG values reported were from a postoperative examination of video recordings of the ICG angiography during the operation. We did not have real-time pixel analysis software available at the time of the study. Since then, on table qICG pixel analysis is now feasible on a touch screen tablet, where the surgeon can define areas of interest intraoperatively, for example, anastomotic site [41]. In the future it will be essential to establish quantitative fluorescence cut-off values, where AL due to poor perfusion might be prevented by re-resection [42].
Acknowledgements
We thank the Department of Surgery and surgeons at Odense University Hospital, Vejle Hospital, Aarhus University Hospital, University Hospital of Zeeland, and Herlev Hospital for participating in this study. Furthermore, we thank OPEN, Open Patient Data Explorative Network, Region of Southern Denmark, for providing research assistance, database software and statistic support.
Funding
None.
Declarations
Disclosures
Pernille Oehlenschlager Larsen, Nikolaj Nerup, Mads Falk Klein, Jesper Andersen, Soren Salomon, Per Vadgaard Andersen, Steffen Brisling, Niclas Dohrn, Soren Möller, Morten Bo Sondergaard Svendsen, Lene Hjerrild Iversen, Ismail Gögenur, Hans Bjarke Rahr, Niels Qvist and Mark Bremholm Ellebaek have no conflicts of interest or financial ties to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.den Dulk M, Marijnen CA, Collette L, Putter H, Pahlman L, Folkesson J, Bosset JF, Rodel C, Bujko K, van de Velde CJ. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg. 2009;96:1066–1075. doi: 10.1002/bjs.6694. [DOI] [PubMed] [Google Scholar]
- 2.Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest. 2007;11:8–15. doi: 10.1007/s11605-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 3.Nesbakken A, Nygaard K, Lunde OC. Outcome and late functional results after anastomotic leakage following mesorectal excision for rectal cancer. Br J Surg. 2001;88:400–404. doi: 10.1046/j.1365-2168.2001.01719.x. [DOI] [PubMed] [Google Scholar]
- 4.DCCG (2019) National Yearly Report 2018.
- 5.Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, Margolin DA, Martz JE, McLemore EC, Molena D, Newman MI, Rafferty JF, Safar B, Senagore AJ, Zmora O, Wexner SD. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest. 2016;20:2035–2051. doi: 10.1007/s11605-016-3255-3. [DOI] [PubMed] [Google Scholar]
- 6.Karliczek A, Harlaar NJ, Zeebregts CJ, Wiggers T, Baas PC, van Dam GM. Surgeons lack predictive accuracy for anastomotic leakage in gastrointestinal surgery. Int J Colorectal Dis. 2009;24:569–576. doi: 10.1007/s00384-009-0658-6. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosetti P, Robert J, Mathey P, Rohner A. Left-sided colon and colorectal anastomoses: doppler ultrasound as an aid to assess bowel vascularization. A prospective evaluation of 200 consecutive elective cases. Int J Colorectal Dis. 1994;9:211–214. doi: 10.1007/BF00292253. [DOI] [PubMed] [Google Scholar]
- 8.Vignali A, Gianotti L, Braga M, Radaelli G, Malvezzi L, Di Carlo V. Altered microperfusion at the rectal stump is predictive for rectal anastomotic leak. Dis Colon Rectum. 2000;43:76–82. doi: 10.1007/BF02237248. [DOI] [PubMed] [Google Scholar]
- 9.Hirano Y, Omura K, Tatsuzawa Y, Shimizu J, Kawaura Y, Watanabe G. Tissue oxygen saturation during colorectal surgery measured by near-infrared spectroscopy: pilot study to predict anastomotic complications. World J Surg. 2006;30:457–461. doi: 10.1007/s00268-005-0271-y. [DOI] [PubMed] [Google Scholar]
- 10.Karliczek A, Benaron DA, Baas PC, Zeebregts CJ, Wiggers T, van Dam GM. Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis. 2010;12:1018–1025. doi: 10.1111/j.1463-1318.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 11.Millan M, Garcia-Granero E, Flor B, Garcia-Botello S, Lledo S. Early prediction of anastomotic leak in colorectal cancer surgery by intramucosal pH. Dis Colon Rectum. 2006;49:595–601. doi: 10.1007/s10350-006-0504-7. [DOI] [PubMed] [Google Scholar]
- 12.Sheridan WG, Lowndes RH, Young HL. Tissue oxygen tension as a predictor of colonic anastomotic healing. Dis Colon Rectum. 1987;30:867–871. doi: 10.1007/BF02555426. [DOI] [PubMed] [Google Scholar]
- 13.Hellan M, Spinoglio G, Pigazzi A, Lagares-Garcia JA. The influence of fluorescence imaging on the location of bowel transection during robotic left-sided colorectal surgery. Surg Endosc. 2014;28:1695–1702. doi: 10.1007/s00464-013-3377-6. [DOI] [PubMed] [Google Scholar]
- 14.Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ, Pigazzi A. The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc. 2013;27:3003–3008. doi: 10.1007/s00464-013-2832-8. [DOI] [PubMed] [Google Scholar]
- 15.Jafari MD, Wexner SD, Martz JE, McLemore EC, Margolin DA, Sherwinter DA, Lee SW, Senagore AJ, Phelan MJ, Stamos MJ. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): a multi-institutional study. J Am Coll Surg. 2015;220:82–92.e81. doi: 10.1016/j.jamcollsurg.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Kudszus S, Roesel C, Schachtrupp A, Hoer JJ. Intraoperative laser fluorescence angiography in colorectal surgery: a noninvasive analysis to reduce the rate of anastomotic leakage. Langenbeck’s Arch surg. 2010;395:1025–1030. doi: 10.1007/s00423-010-0699-x. [DOI] [PubMed] [Google Scholar]
- 17.Ris F, Hompes R, Cunningham C, Lindsey I, Guy R, Jones O, George B, Cahill RA, Mortensen NJ. Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg Endosc. 2014;28:2221–2226. doi: 10.1007/s00464-014-3432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Protyniak B, Dinallo AM, Boyan WP, Jr, Dressner RM, Arvanitis ML. Intraoperative indocyanine green fluorescence angiography–an objective evaluation of anastomotic perfusion in colorectal surgery. Am Surg. 2015;81:580–584. doi: 10.1177/000313481508100621. [DOI] [PubMed] [Google Scholar]
- 19.Grone J, Koch D, Kreis ME. Impact of intraoperative microperfusion assessment with Pinpoint Perfusion Imaging on surgical management of laparoscopic low rectal and anorectal anastomoses. Colorectal Dis. 2015;17(Suppl 3):22–28. doi: 10.1111/codi.13031. [DOI] [PubMed] [Google Scholar]
- 20.Boni L, David G, Dionigi G, Rausei S, Cassinotti E, Fingerhut A. Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc. 2015;30(7):2736–2742. doi: 10.1007/s00464-015-4540-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James DR, Ris F, Yeung TM, Kraus R, Buchs NC, Mortensen NJ, Hompes RJ. Fluorescence angiography in laparoscopic low rectal and anorectal anastomoses with pinpoint perfusion imaging- a critical appraisal with specific focus on leak risk reduction. Colorectal Dis. 2015;17(Suppl 3):16–21. doi: 10.1111/codi.13033. [DOI] [PubMed] [Google Scholar]
- 22.Alekseev M, Rybakov E, Shelygin Y, Chernyshov S, Zarodnyuk I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: results of the FLAG randomized trial. Colorectal Dis. 2020;22:1147–1153. doi: 10.1111/codi.15037. [DOI] [PubMed] [Google Scholar]
- 23.De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, Fumagalli U, Gardani M, De Pascale S, Parise P, Vignali A, Rosati R. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. 2020;34:53–60. doi: 10.1007/s00464-019-06730-0. [DOI] [PubMed] [Google Scholar]
- 24.Hayami S, Matsuda K, Iwamoto H, Ueno M, Kawai M, Hirono S, Okada K, Miyazawa M, Tamura K, Mitani Y, Kitahata Y, Mizumoto Y, Yamaue H. Visualization and quantification of anastomotic perfusion in colorectal surgery using near-infrared fluorescence. Tech Coloproctol. 2019;23:973–980. doi: 10.1007/s10151-019-02089-5. [DOI] [PubMed] [Google Scholar]
- 25.Son GM, Kwon MS, Kim Y, Kim J, Kim SH, Lee JW. Quantitative analysis of colon perfusion pattern using indocyanine green (ICG) angiography in laparoscopic colorectal surgery. Surg Endosc. 2019;33:1640–1649. doi: 10.1007/s00464-018-6439-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wada T, Kawada K, Takahashi R, Yoshitomi M, Hida K, Hasegawa S, Sakai Y. ICG fluorescence imaging for quantitative evaluation of colonic perfusion in laparoscopic colorectal surgery. Surg Endosc. 2017;31:4184–4193. doi: 10.1007/s00464-017-5475-3. [DOI] [PubMed] [Google Scholar]
- 27.Diana M, Agnus V, Halvax P, Liu YY, Dallemagne B, Schlagowski AI, Geny B, Diemunsch P, Lindner V, Marescaux J. Intraoperative fluorescence-based enhanced reality laparoscopic real-time imaging to assess bowel perfusion at the anastomotic site in an experimental model. Br J Surg. 2015;102:e169–176. doi: 10.1002/bjs.9725. [DOI] [PubMed] [Google Scholar]
- 28.Toens C, Krones CJ, Blum U, Fernandez V, Grommes J, Hoelzl F, Stumpf M, Klinge U, Schumpelick V. Validation of IC-VIEW fluorescence videography in a rabbit model of mesenteric ischaemia and reperfusion. Int J Colorectal Dis. 2006;21:332–338. doi: 10.1007/s00384-005-0017-1. [DOI] [PubMed] [Google Scholar]
- 29.Gosvig K, Jensen SS, Qvist N, Agnus V, Jensen TS, Lindner V, Marescaux J, Diana M, Ellebaek MB. Remote computer-assisted analysis of ICG fluorescence signal for evaluation of small intestinal anastomotic perfusion: a blinded, randomized, experimental trial. Surg Endosc. 2019;34(5):2095–2102. doi: 10.1007/s00464-019-06990-w. [DOI] [PubMed] [Google Scholar]
- 30.Degett TH, Andersen HS, Gogenur I. Indocyanine green fluorescence angiography for intraoperative assessment of gastrointestinal anastomotic perfusion: a systematic review of clinical trials. Langenbeck,s Arch Surg. 2016;401(6):767–775. doi: 10.1007/s00423-016-1400-9. [DOI] [PubMed] [Google Scholar]
- 31.Blanco-Colino R, Espin-Basany E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: a systematic review and meta-analysis. Tech Coloproctol. 2018;22:15–23. doi: 10.1007/s10151-017-1731-8. [DOI] [PubMed] [Google Scholar]
- 32.Nerup N, Andersen HS, Ambrus R, Strandby RB, Svendsen MBS, Madsen MH, Svendsen LB, Achiam MP. Quantification of fluorescence angiography in a porcine model. Langenbeck’s Arch Surg. 2017;402:655–662. doi: 10.1007/s00423-016-1531-z. [DOI] [PubMed] [Google Scholar]
- 33.Nerup N, Knudsen KBK, Ambrus R, Svendsen MBS, Thymann T, Ifaoui IBR, Svendsen LB, Achiam MP. Reproducibility and reliability of repeated quantitative fluorescence angiography. Surg Technol Int. 2017;31:35–39. [PubMed] [Google Scholar]
- 34.Nerup N, Ring LL, Strandby RB, Egeland C, Svendsen MBS, Hasselby JP, Willemoe GL, Hartmann B, Svendsen LB, Achiam MP. Quantitative perfusion assessment of intestinal anastomoses in pigs treated with glucagon-like peptide 2. Langenbeck’s Arch Surg. 2018;403:881–889. doi: 10.1007/s00423-018-1718-6. [DOI] [PubMed] [Google Scholar]
- 35.Rønn JH, Nerup N, Strandby RB, Svendsen MBS, Ambrus R, Svendsen LB, Achiam MP. Laser speckle contrast imaging and quantitative fluorescence angiography for perfusion assessment. Langenbeck’s Arch Surg. 2019;404:505–515. doi: 10.1007/s00423-019-01789-8. [DOI] [PubMed] [Google Scholar]
- 36.Osterkamp J, Strandby R, Nerup N, Svendsen M, Svendsen L, Achiam M. Quantitative fluorescence angiography detects dynamic changes in gastric perfusion. Surg Endosc. 2021;35:6786–6795. doi: 10.1007/s00464-020-08183-2. [DOI] [PubMed] [Google Scholar]
- 37.Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Buchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Arezzo A, Bonino MA, Ris F, Boni L, Cassinotti E, Foo DCC, Shum NF, Brolese A, Ciarleglio F, Keller DS, Rosati R, De Nardi P, Elmore U, Fumagalli Romario U, Jafari MD, Pigazzi A, Rybakov E, Alekseev M, Watanabe J, Vettoretto N, Cirocchi R, Passera R, Forcignanò E, Morino M. Intraoperative use of fluorescence with indocyanine green reduces anastomotic leak rates in rectal cancer surgery: an individual participant data analysis. Surg Endosc. 2020;34:4281–4290. doi: 10.1007/s00464-020-07735-w. [DOI] [PubMed] [Google Scholar]
- 39.Nikolic AL, Keck JO. Ischaemic colitis: uncertainty in diagnosis, pathophysiology and management. ANZ J Surg. 2018;88:278–283. doi: 10.1111/ans.14237. [DOI] [PubMed] [Google Scholar]
- 40.Sherwinter DA, Gallagher J, Donkar T. Intra-operative transanal near infrared imaging of colorectal anastomotic perfusion: a feasibility study. Colorectal Dis. 2013;15:91–96. doi: 10.1111/j.1463-1318.2012.03101.x. [DOI] [PubMed] [Google Scholar]
- 41.Nerup N, Svendsen MBS, Svendsen LB, Achiam MP. Feasibility and usability of real-time intraoperative quantitative fluorescent-guided perfusion assessment during resection of gastroesophageal junction cancer. Langenbeck’s Arch Surg. 2020;405:215–222. doi: 10.1007/s00423-020-01876-1. [DOI] [PubMed] [Google Scholar]
- 42.Lütken CD, Achiam MP, Svendsen MB, Boni L, Nerup N. Optimizing quantitative fluorescence angiography for visceral perfusion assessment. Surg Endosc. 2020;34:5223–5233. doi: 10.1007/s00464-020-07821-z. [DOI] [PubMed] [Google Scholar]