Abstract
Pulmonary fibrosis results from dysregulated lung repair and involves multiple cell types. The role of endothelial cells (EC) in lung fibrosis is poorly understood. Using single cell RNA-sequencing we identified endothelial transcription factors involved in lung fibrogenesis, including FOXF1, SMAD6, ETV6 and LEF1. Focusing on FOXF1, we found that FOXF1 is decreased in EC within human idiopathic pulmonary fibrosis (IPF) and mouse bleomycin-injured lungs. Endothelial-specific Foxf1 inhibition in mice increased collagen depositions, promoted lung inflammation, and impaired R-Ras signaling. In vitro, FOXF1-deficient EC increased proliferation, invasion and activation of human lung fibroblasts, and stimulated macrophage migration by secreting IL-6, TNFα, CCL2 and CXCL1. FOXF1 inhibited TNFα and CCL2 through direct transcriptional activation of Rras gene promoter. Transgenic overexpression or endothelial-specific nanoparticle delivery of Foxf1 cDNA decreased pulmonary fibrosis in bleomycin-injured mice. Nanoparticle delivery of FOXF1 cDNA can be considered for future therapies in IPF.
Subject terms: Respiratory tract diseases, Transcription
Pulmonary fibrosis results from dysregulated lung repair, but the role of endothelial cells (EC) in fibrosis is unclear. Here, the authors show that FOXF1/R-Ras signalling in EC inhibits profibrotic mediators and that ECspecific nanoparticle FOXF1 gene therapy decreases lung fibrosis in mice.
Introduction
Existing anti-fibrotic treatments for pulmonary fibrosis have not significantly improved survival. There is a critical need for new therapeutic approaches. Interstitial lung diseases and pulmonary fibrosis are characterized by injury to the lung parenchyma with varying patterns of inflammation and fibrotic remodeling1,2. The etiology and the pathogenesis of lung fibrosis are not completely understood. It has been shown that environmental, age-related, and genetic factors create an alveolar epithelium that is susceptible to injury from either endogenous or exogenous factors3,4. It is currently believed that recurrent injury of pulmonary epithelium initiates the pathology, followed by a mild inflammatory response and dysregulated repair process5. Dysregulated lung repair results in accumulation of unrestrained myofibroblasts and excessive matrix deposition leading to scarring of pulmonary parenchyma and decline of lung functions. While most studies in pulmonary fibrosis are focused on myofibroblasts, epithelial and inflammatory cells, very few studies examine the role of endothelial cells in pathogenesis of lung fibrosis. The vascular changes in human pulmonary fibrosis got much more attention after recent publications that described a decline in lung capillary cells, and the increased presence of peribronchial endothelial cells in the lung parenchyma6,7.
There is a general agreement that alterations in microvessels and vascular remodeling are involved in pulmonary fibrosis. Vascular integrity and endothelial repair are dysregulated, leading to increased vessel permeability, partial loss of capillaries, focal increase in angiogenesis and increased presence of peribronchial endothelial cells in the lung parenchyma6–8. The presence of fibrotic stimuli induces the re-programming of normal lung endothelial cells (EC) into fibrosis-associated EC. While normal EC are quiescent and provide tight endothelial barrier, fibrosis-associated EC have high permeability, form leaky endothelial barrier causing focal edema and contributing to fibrosis-associated epithelial injury and inflammation. Perfusion through fibrosis-associated pulmonary vessels is decreased, leading to increased hypoxia within fibrotic lesions and further exacerbating epithelial injury and inflammation9.
In addition to barrier functions, tissue-specific endothelium establishes specialized vascular niches that provide angiocrine growth factors necessary to maintain tissue homeostasis as well as guide organ repair and regeneration10. In the lungs, the alveolar epithelial cells and their progenitors reside in the vicinity of pulmonary capillary endothelial cells. Angiocrine factors provided by lung endothelial cells include secreted and membrane-bound growth factors, chemokines, cytokines, extracellular matrix components, exosomes that regulate homeostatic and regenerative processes in a paracrine manner10. While the changes in angiocrine factors after lung injury have been well documented, the transcriptional regulators driving these changes are not well established. There is a critical need to determine the mechanisms used by lung endothelium to maintain physiological levels of angiocrine factors and to support the normal integrity of endothelial barrier. Normalization of lung vasculature alleviates hypoxia and increases efficacy of drug delivery11. Vascular normalization also decreases endothelial permeability and improves the perfusion of blood vessels11. Restoring normal microvascular integrity and physiological levels of angiocrine factors will provide additional therapeutic approaches for treatment of pulmonary fibrosis and to promote organ repair without fibrotic scarring.
We sought to uncover the molecular mechanisms critical for vascular normalization and to determine the transcriptional regulators that control the transition of normal EC into fibrosis-associated EC. We used transcriptional profiling of endothelial cells from human and mouse fibrotic lungs and identified FOXF1 as a critical transcriptional regulator of transition from normal to fibrosis-associated EC. FOXF1 is a member of the Forkhead Box (FOX) family of transcription factors. Foxf1−/− mice are embryonic lethal12. Deletions or “loss-of-function” point mutations in FOXF1 gene locus were found in most patients with Alveolar Capillary Dysplasia with Misalignment of Pulmonary Veins (ACDMPV)13,14, a severe congenital disorder which causes mortality during the first weeks of life. FOXF1 is required for oncogenic properties of PAX3-FOXO1 in rhabdomyosarcoma15. In mice, FoxF1 deficiency in EC decreases fetal lung angiogenesis due to inability of FOXF1-deficient ECs to respond to VEGF/FLK1 and BMP9/ACVRL1 signaling16,17. While FOXF1 induces angiogenesis during embryogenesis, FOXF1 is also expressed in endothelial cells of adult lungs, where angiogenesis is inactive18. Deletion of both Foxf1 alleles in the adult lung disrupts EC adherence junctions and caused vascular leakage18. The role of FOXF1 in lung endothelial cells during pulmonary fibrosis is unknown.
In the present study, we used single cell RNA-sequencing and mouse genetics to demonstrate that EC regulate pulmonary fibrosis through FOXF1/R-Ras-dependent inhibition of profibrotic mediators. Our results support the use of FOXF1-activating therapies for vascular normalization in pulmonary fibrosis.
Results
Identification of transcription factors that regulate re-programming of normal EC into fibrosis-associated EC in IPF lungs
To identify transcriptional regulators that control re-programming of normal EC into fibrosis-associated EC, we performed single cell RNA-sequencing (10X genomics) to compare the transcriptome of endothelial cells in donor and idiopathic pulmonary fibrosis (IPF) lungs (Cincinnati Children’s Hospital Medical Center (CCHMC) datasets, Supplementary Fig. S1a, b). We also downloaded and analyzed the published scRNA-seq data19 from donor and IPF lungs (Northwestern University (NW) datasets, Supplementary Fig. S1c, d). Endothelial cell in both datasets were identified as cells expressing Pecam1 (CD31), Cdh5 (VE cadherin) and lacking Ptprc (CD45) mRNAs, consistent with published studies20. Cross-comparison of two scRNA-seq datasets identified several top differentially expressed endothelial transcription factors. Among universally downregulated transcription factors in IPF endothelial cells were FOXF1, SMAD6, HIF3A, CREB5, TBX3 and GATA2 (Fig. 1a). Universally upregulated transcription factors included ETV6, LEF1, EGR3, MEOX1 and SMAD1 (Fig. 1a). For the follow-up studies, we decided to focus on FOXF1 transcription factor because it’s expression is highly enriched in lung endothelial cells compared to endothelial cells of other organs18,21 and because the FOXF1 loss-of-function gene mutations are linked to ACDMPV, a fatal congenital lung disease with significant fibrotic remodeling13. To verify that identified decrease in FOXF1 is not limited to two datasets, we have also analyzed the publicly available datasets from Yale University and Vanderbilt University6,7. We have confirmed the decreased levels of FOXF1 mRNA in endothelial cells of patients with IPF compared to donor controls (Supplementary Fig. S2a). We sought to determine whether downregulation of FOXF1 in fibrosis-associated EC is essential for lung fibrogenesis.
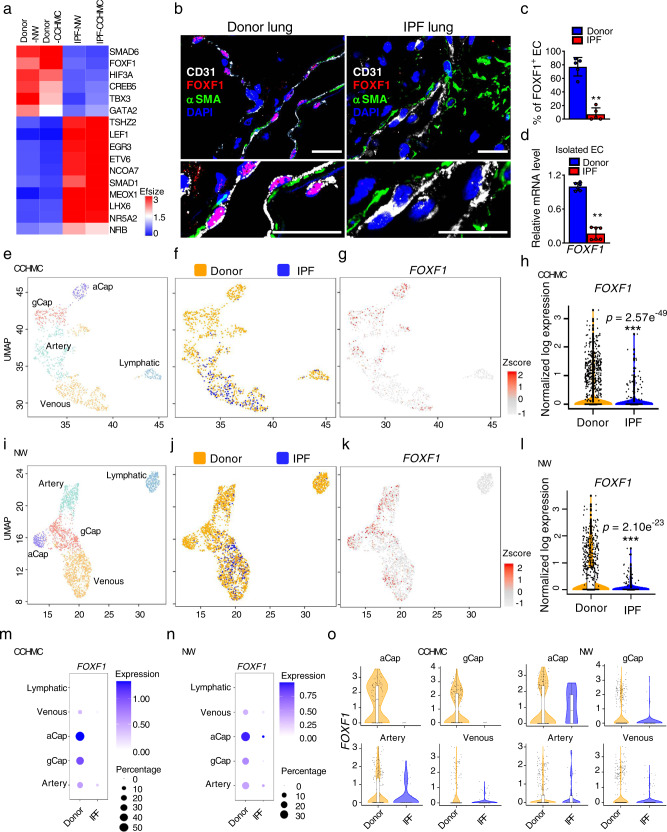
Fig. 1. FOXF1 is decreased in ECs of human IPF lung.
a Heat map shows top differentially expressed transcription factors in EC of IPF lungs compared to EC of donor lungs. scRNA-seq was performed using 3 donor (Donor-CCHMC) and 2 IPF (IPF-CCHMC) lungs. scRNA-seq datasets were also downloaded from GSE 122960 (Donor-NW, n = 8 lungs; IPF-NW, n = 4 lungs). b Co-localization for FOXF1, CD31 and αSMA show decreased FOXF1 in human EC within IPF fibrotic foci (n = 5 lungs per group). DAPI (blue) was used to stain cell nuclei. Bar = 20 μm. c Decreased percent of FOXF1-positive ECs in IPF lungs. Percentage of FOXF1+/CD31+ double positive ECs were counted in 5 random fields and presented as mean ± SD (n = 5 lungs per group), **p = 0.0079, Mann–Whitney Two-tailed test. d Decreased FOXF1 mRNA in EC isolated from human IPF lungs compared to donors (n = 6 per group) is shown by qRT-PCR. ACTB mRNA was used for normalization, **p = 0.0022, Mann–Whitney Two-tailed test. e, f Unsupervised UMAP clustering of lung EC from scRNA-seq CCHMC datasets. g UMAP plots show FOXF1 expression in IPF and donor EC clusters after Z-score normalization. h Violin plots show decreased expression of FOXF1 mRNA in ECs from IPF lungs compared to donor lungs (CCHMC datasets). i, j Unsupervised clustering of lung EC is shown using NW scRNA-seq datasets (GSE 122960). IPF samples (n = 4) were compared with donor samples (n = 8). k Expression of FOXF1 in IPF and control EC clusters after Z-score normalization. l Violin plots show decreased expression of FOXF1 mRNA in ECs from IPF lungs (NW scRNA-seq datasets). Dot-plots show the decreased FOXF1 mRNA in venous, aCap, gCap, and arterial clusters of IPF endothelial cells using CCHMC (m) and NW (n) scRNA-seq datasets. Size of the dots represent frequency of FOXF1+ cells in each cluster. Color of the dots represent the expression levels of FOXF1 in each cluster. FOXF1 expression is log normalized. o Violin plots show decreased FOXF1 mRNA in aCap, gCap, arterial and venous endothelial cells of IPF lungs compared to donor lungs in both CCHMC and NW data sets. FOXF1 expression is log normalized. Source data are provided as a Source Data file.
Expression of FOXF1 is decreased in endothelial cells within fibrotic lesions of human IPF lungs
Using immunostaining with FOXF1, CD31 and αSMA antibodies, we found that FOXF1 protein was undetectable in EC within fibrotic lesions of IPF lungs identified as αSMA-positive regions (Fig. 1b and Supplementary Fig. S2b). While in human donor lungs, FOXF1 protein was detected in approximately 80% of endothelial cells, only 5-10% of endothelial cells expressed FOXF1 in IPF lungs (Fig. 1c). We further verified by qRT-PCR that FOXF1 mRNA was decreased in FACS-sorted EC from IPF lungs compared to FACS-sorted EC from donor lungs (Fig. 1d). Thus, both mRNA and protein levels of FOXF1 are decreased in endothelial cells of IPF lungs.
To identify FOXF1-expressing lung endothelial cells in scRNA-seq CCHMC dataset, endothelial cells from donor and IPF lungs were visualized using uniform manifold approximation and projection (UMAP) after samples integration with Harmony22 (Fig. 1e–g). Both FOXF1 mRNA levels and the total number of FOXF1-expressing EC were decreased in IPF lungs compared to donor lungs (Fig. 1h). To demonstrate that these results are not limited to CCHMC scRNA-seq dataset, we analyzed the publicly available NW scRNA-seq dataset containing endothelial cells from IPF and donor lungs (GSE 12296019). Consistent with the results from CCHMC dataset, both the percentage of FOXF1-positive endothelial cells and FOXF1 mRNA levels were decreased in IPF lungs from NW dataset (Fig. 1i–l).
Based on gene expression signatures, pulmonary endothelial cells from both CCHMC and NW datasets were subdivided into five sub-clusters: arterial, general capillary (gCAP), alveolar capillary (aCAP), venous and lymphatic ECs (Fig. 1e, i, Supplementary Fig. S1a–d). Genes known to be selectively expressed in different sub-types of endothelial cells were used to annotate these sub-clusters23,24. The arterial EC sub-cluster was identified as cells expressing EFNB2, GJA5, DKK2 and HEY1, whereas the venous EC sub-cluster included cells expressing ACKR1, CLU and VWF (Supplementary Fig. S1a, b). The gCAP sub-cluster was enriched in GPIHBP1 and SLC6A4 transcripts, aCAP was enriched in EDNRB, APLN and HPGD, and lymphatic EC sub-cluster expressed PROX1, CCL21 and PDPN (Supplementary Fig. S1a–d). FOXF1 was detected in arterial, venous, and capillary sub-clusters, with the highest expression of FOXF1 in aCAPs and gCAPs (Fig. 1m, n). FOXF1 was undetectable in lymphatic EC (Fig. 1m, n). Also, FOXF1 was not expressed in the COL15+ endothelial cells that were identified in the IPF lungs, but not in the control donor lungs (Supplementary Fig. S2c), confirming the published data6. In both scRNA-seq datasets, FOXF1 expression was decreased in capillary, arterial and venous sub-clusters of IPF lungs compared to donor lungs (Fig. 1m–o). Consistent with scRNA-seq data, RNA in situ hybridization showed that both the percentage of FOXF1-positive EC and expression levels of FOXF1 transcripts were decreased in capillary, arterial and venous sub-clusters of IPF lungs compared to donor lungs (Supplementary Fig. S3).
FOXF1 is decreased in endothelial cells within fibrotic lesions of mouse bleomycin-injured lungs
We next examined expression of FOXF1 in endothelial cells in a mouse model of pulmonary fibrosis. Three weekly intratracheal (IT) injections of bleomycin were used to induce lung fibrosis in wild type mice (Supplementary Fig. S4a). Bleomycin administration caused a time-dependent increase in collagen depositions as quantified by Sircol assay (Fig. 2a) and confirmed by H&E and Sirius red/Fast green staining (Supplementary Fig. S4b). Increased accumulation of inflammatory cells in bleomycin-treated lungs was shown using flow cytometry analysis for CD45+ cells (Supplementary Fig. S4c). Next, we FACS-sorted CD31+/CD45− lung endothelial cells at different time points after bleomycin treatment and measured endothelial Foxf1 expression by qRT-PCR. A decrease in endothelial Foxf1 mRNA was observed as early as day 3 after the first bleomycin treatment (Fig. 2b). The percentage of FOXF1-positive ECs in lung tissue was also decreased as early as day 3 after the first bleomycin treatment as shown by co-localization of FOXF1 with endothelial-specific ERG transcription factor (Supplementary Fig. S4d). Immunostaining for FOXF1 and CD31 showed decreased FOXF1 protein expression in endothelial cells within fibrotic lesions identified as αSMA-positive areas (Fig. 2d, right panel). Consistent with human IPF lungs (Fig. 1b, c), the number of FOXF1+/CD31+ endothelial cells was decreased in murine fibrotic lungs (Fig. 2c).
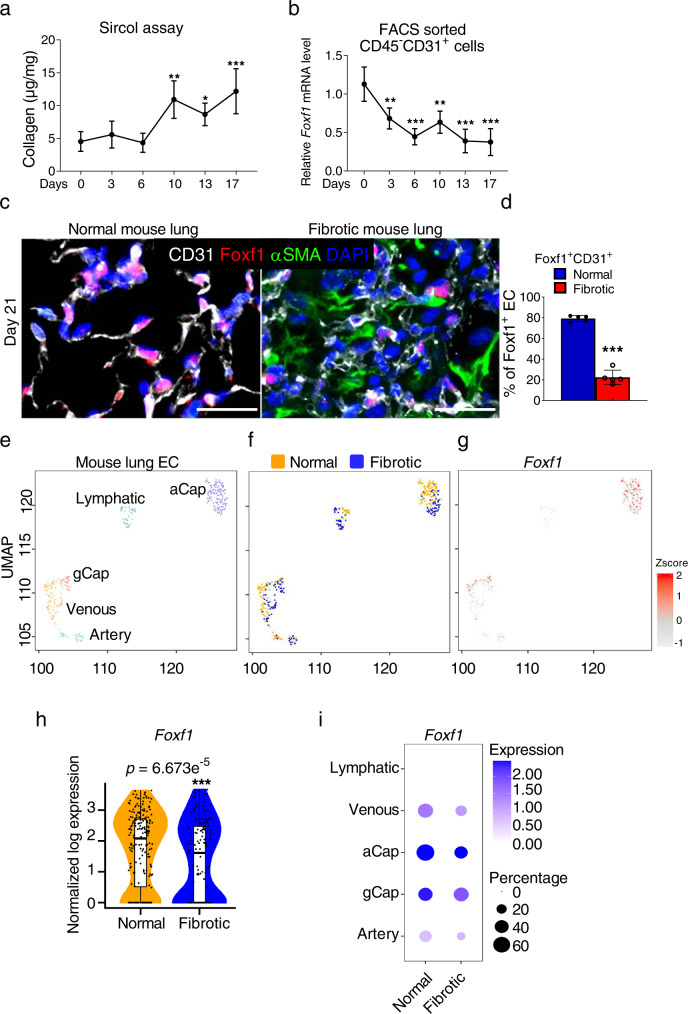
Fig. 2. Expression of FOXF1 is decreased in endothelial cells within fibrotic lesions of mouse lungs.
a Time-dependent accumulation of collagen in murine lungs after chronic bleomycin injury is quantified using Sircol collagen assay. Wild type mice were treated with three weekly IT injections of bleomycin to induce lung fibrosis (Day0, 3, 6, 10, 17, n = 4 mice per group; Day 13, n = 6 mice). b Time-dependent decrease of Foxf1 mRNA is shown in FACS-sorted lung endothelial cells during lung fibrogenesis using qRT-PCR (Day 0, 6,10, n = 3; Day 3, n = 4; Day13 n = 7; Day 17, n = 8, mice per group). c Co-localization studies show decreased FOXF1 in endothelial cells and decreased number of FOXF1+ endothelial cells within lung fibrotic foci at day 21 after bleomycin treatment. Mouse normal lungs (n = 5) and bleomycin-treated lungs (n = 5) were stained with antibodies against FOXF1 (red), CD31 (white) and αSMA (green). DAPI (blue) was used to visualize the nuclei. Bar = 25 µm. d Percent of FOXF1+/CD31+ double positive cells among CD31+ cells were counted in 5 random fields and presented as mean ± SD (n = 5 mice per group). ***p < 0.001. The integrated projection of lung EC from normal and bleomycin-treated fibrotic lungs (e, colored by cell type. f, yellow- normal lungs; blue- fibrotic lungs). Single cell RNA-seq was performed using pooled normal controls (n = 4) and bleomycin-treated (n = 6) mouse lungs. g UMAP plots show Foxf1 expression in endothelial cells of normal and fibrotic mouse lungs after Z-score normalization. h Violin plots show decreased expression of Foxf1 mRNA in ECs from fibrotic lungs. i Both Foxf1 mRNA expression and the frequency of Foxf1-positive cells are decreased in venous, aCap, gCap, and arterial endothelial cells from fibrotic lungs. Foxf1 is not expressed in lymphatic endothelial cells. Foxf1 expression is log normalized. Data presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. For two group comparisons, T-test (two-tailed) analyses were performed. For more than two group, statistical significance was determined by one-way ANOVA followed by Dunnett’s test. Source data are provided as a Source Data file.
Next, we performed a single cell RNA-sequencing of bleomycin-treated and control mouse lungs. Foxf1-expressing endothelial cells were visualized using UMAP (Fig. 2e–g). Consistent with human IPF lungs, bleomycin-treated mouse lungs had reduced numbers of Foxf1-positive endothelial cells and decreased expression of Foxf1 mRNA in these cells (Fig. 2h). Mouse lung endothelial cells were further subdivided into arterial, venous, aCAP, gCAP and lymphatic sub-clusters (Supplementary Fig. S5 and Fig. 2e). Endothelial cells expressing Foxf1 transcript were present in arterial, venous, aCAP and gCAP sub-clusters, but were completely absent in lymphatic cells (Fig. 2i). RNA in situ hybridization showed that both the percent of FOXF1-positive endothelial cells as well as the expression levels of FOXF1 transcripts were decreased in arterial, venous, aCAP and gCAP sub-clusters of bleomycin-treated lungs compared to control lungs (Supplementary Fig. S6). Altogether, FOXF1 expression is decreased in most EC types in human and mouse fibrotic lungs.
Deletion of FOXF1 in endothelial cells accelerates pulmonary fibrosis
To determine the role of FOXF1 in endothelial cells during pulmonary fibrosis, we used mice in which the Foxf1 gene was specifically deleted in endothelial cells using Pdgfb-CreER transgene, which does not target other cell types in the adult lung18,25. Since homozygous (Pdgfb-CreER /Foxf1fl/fl mice or endFoxf1−/−) mice developed lung edema and respiratory insufficiency18, we used heterozygous endFoxf1+/− mice for lung fibrosis studies (Supplementary Fig. S7a). Naïve heterozygous Tam-treated endFoxf1+/− mice were phenotypically normal, had normal lung histology and alveolar capillary density as shown by CD31 staining (Supplementary Fig. S7b and18). Since the Pdgfb-Cre transgene contain GFP18, we used flow cytometry to demonstrate that the transgene targets only CD45−CD31+ (endothelial), but CD45+CD31− (hematopoietic) or CD45−CD31− (non-endothelial, non-hematopoietic) cell types in the lung (Supplementary Fig. S7c, d). The scRNA-seq analysis showed co-expression of endothelial Foxf1 and Pdgfb mRNA transcripts in the lung EC (Supplementary Fig. S7f).
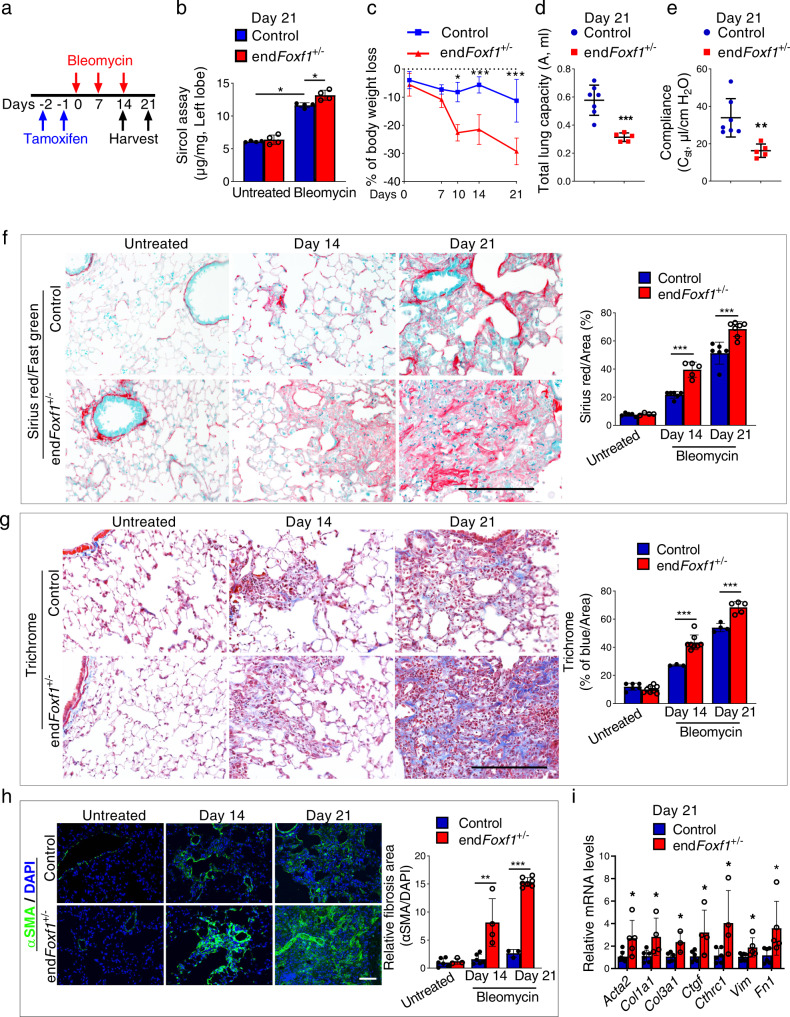
Three weekly IT injections of bleomycin were used to induce lung fibrosis in Tam-treated endFoxf1+/− and control Foxf1fl/fl mice (Fig. 3a). Using FACS-sorted lung endothelial cells, we have shown that bleomycin treatment caused more profound decrease of Foxf1 mRNA in endFoxf1+/− endothelial cells compared to control endothelial cells (Supplementary Fig. S8a). Decreased Foxf1 in endothelial cells was associated with more severe fibrosis in bleomycin-treated endFoxf1+/− mice compared to bleomycin-treated control mice, as shown by quantifying collagen depositions in the lung tissue using Sircol assay (Fig. 3b). Compared to controls, bleomycin-treated endFoxf1+/− mice had increased Ashcroft scores (Supplementary Fig. S8b, c), decreased body weights (Fig. 3c), reduced lung capacity and compliance (Fig. 3d, e), impaired lung mechanics (Supplementary Fig. S8d–j) and arterial oxygenation (Supplementary Fig. S8k). Lung fibrosis in endFoxf1+/− mice developed faster and was more severe compared to control mice, as shown by Sirius red/fast green (Fig. 3f), Trichrome (Fig. 3g), and immunostaining for αSMA (Fig. 3h). Increased fibrotic depositions in bleomycin-treated endFoxf1+/− lungs were also supported by increased Acta2, Col1a1, Col3a1, Ctgf, Cthrc1, Vim and Fn1 mRNAs (Fig. 3i). Thus, FOXF1 deficiency in murine endothelial cells exacerbates bleomycin-induced pulmonary fibrosis.
Fig. 3. Deletion of FOXF1 in endothelial cells accelerates pulmonary fibrosis.
a Schematic diagram of bleomycin administration to induce lung fibrosis and tamoxifen (TAM) administration to delete Foxf1 in endFoxf1+/− and control Foxf1+/− mice. b Lung collagen was quantified at 21 days after bleomycin administration by Sircol assay (n = 4 mice per group). Data presented as mean ± SD. *p < 0.05. Mann–Whitney Two-tailed test were performed. c Increased body weight loss in endFoxf1+/− mice is shown at different time points compared to control mice. n = 6 per group. d, e Decreased lung capacity and lung compliance in endFoxf1+/− (n = 5) mice at day 21 after bleomycin administration shown using FlexiVent. Control, n = 7. ***p = 0.0004 (d). **p = 0.0031 (e). f–h Increased severity of lung fibrosis in endFoxf1+/− mice is shown using (f) Sirius red/Fast green at Days 14 and 21 after bleomycin treatment (untreated control n = 5 and endFoxf1+/− n = 4; bleomycin treated Day 14 control n = 6 and endFoxf1+/− n = 5; Day 21 control n = 6 and endFoxf1+/− n = 7) and (g) Trichrome staining (untreated control n = 6 and endFoxf1+/− n = 8; Day 14 control n = 3 and endFoxf1+/− n = 8; Day 21 control n = 4 and endFoxf1+/− n = 5), as well as immunostaining for αSMA (green) (untreated control, n = 6; untreated endFoxf1+/− n = 3; Day 14 control, n = 6; Day 14 endFoxf1+/−, n = 4; Day 21 control, n = 3; Day 21 endFoxf1+/−, n = 7) (h). Bar = 100 µm. Sirius Red binds to all types of collagens, whereas fast green stains non-collagenous proteins. i Endothelial deletion of Foxf1 increases mRNA levels of collagen genes in total lung RNA as shown by qRT-PCR. Total lung mRNA was extracted from control and bleomycin-treated endFoxf1+/−mice on day 21. Actb mRNA was used for normalization. Control Acta2, n = 7; Control Col1a1, n = 6; Control Col3a, n = 5; Control Ctgf, n = 6; Control Cthrc1, n = 6; Control Vim, n = 7; Control Fn1, n = 6; endFoxf1+/− Acta2, n = 5; endFoxf1+/− Col1a1, n = 4; endFoxf1+/− Col3a, n = 3; endFoxf1+/− Ctgf, n = 4; endFoxf1+/− Cthrc1, n = 4; endFoxf1+/− Vim, n = 5; endFoxf1+/− Fn1, n = 5. Data presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. T-test (two-tailed) analyses were performed. Source data are provided as a Source Data file.
FOXF1-deficient endothelial cells increase myofibroblast activation
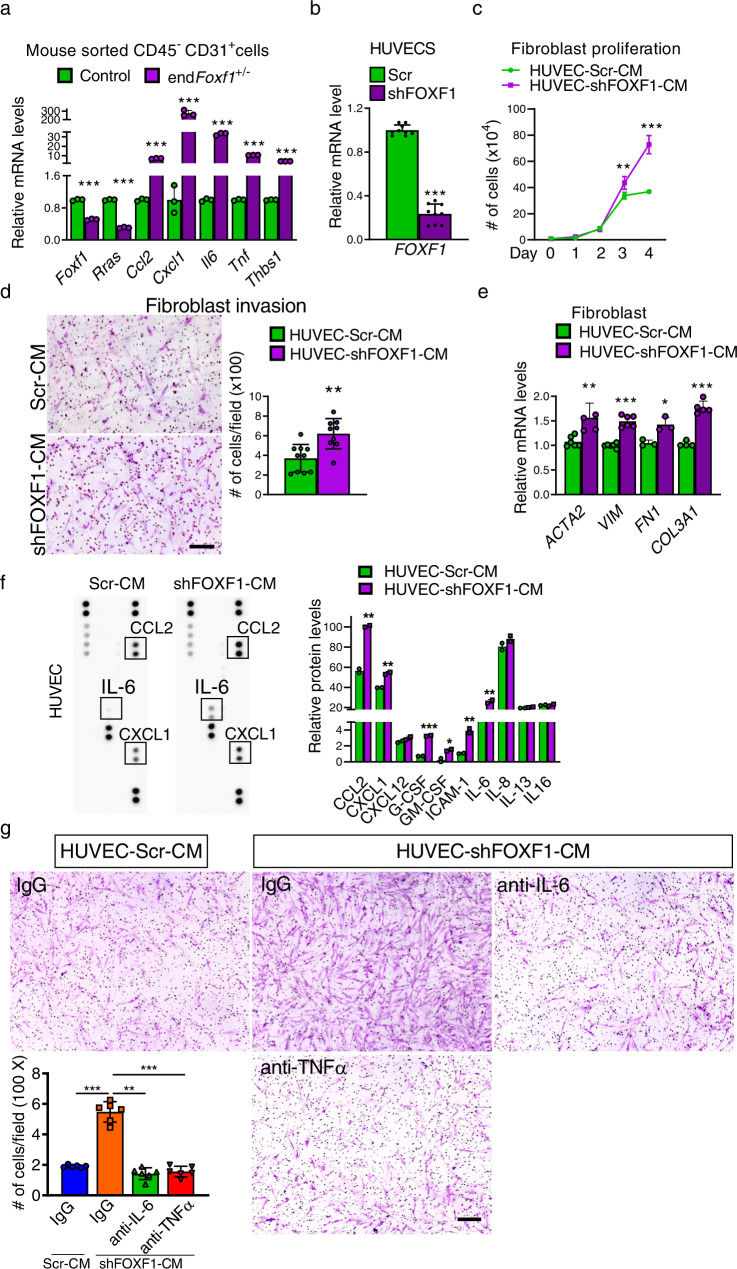
To identify molecular mechanisms by which FOXF1-deficient ECs promote lung fibrogenesis, we performed bulk RNA-seq using FACS-sorted endothelial cells from bleomycin-treated control and endFoxf1+/− lungs (Supplementary Fig. S9). Gene Set Enrichment Analysis (GSEA) of RNA-seq data showed that the most enriched functional categories in endFoxf1+/− endothelial cell were wound healing, extracellular matrix organization, cell migration, blood vessel remodeling, inflammation, myeloid leukocyte migration, and RHO GTPase signaling (Supplementary Fig. S9a–d). Consistent with inactivation of one Foxf1 allele, Foxf1 mRNA was decreased 2.1-fold in ECs of bleomycin-treated endFoxf1+/− lungs compared to control lungs (Supplementary Fig. S9a), a finding confirmed by qRT-PCR of FACS-sorted ECs (Fig. 4a). FOXF1-deficiency in endFoxf1+/− ECs was associated with increased expression of pro-fibrotic and pro-inflammatory genes, including Il6, Tnfα, Ccl2, Cxcl1 and Thbs1 (Supplementary Fig. S9a), findings validated by qRT-PCR (Fig. 4a).
Fig. 4. FOXF1-deficient endothelial cells increase myofibroblast activation.
a qRT-PCR shows increased expression of fibrosis-associated genes in FACS-sorted endothelial cells of endFoxf1+/− lungs at day 21 after bleomycin administration. Actb mRNA was used for normalization. n = 3 mice per group. b Efficient inhibition of FOXF1 expression in shFOXF1-transfected HUVEC cells is shown with qRT-PCR. ACTB mRNA was used for normalization. n = 9 samples per group. c CM from FOXF1-deficient HUVECs increases fibroblast proliferation. CCD-19Lu fibroblasts were cultured in the presence of CM from control or FOXF1-deficient HUVECs. n = 3 samples per group. Day 3, **p = 00290; Day 4 ***p < 0.0001 by two-way ANOVA test. d Conditioned media (CM) from FOXF1-deficient HUVECs increases invasion of cultured CCD-19Lu fibroblasts. Human CCD-19Lu fibroblasts were seeded on the insert of transwell chamber coated with matrigel in the presence of CM from scrambled control (Scr-CM, n = 10) or FOXF1-deficient (shFOXF1-CM, n = 9) HUVECs. Graph represents average numbers of invaded cells per field. Bar = 200 μm. **p = 0.0019. e CCD-19Lu fibroblasts cultured in CM from FOXF1-deficient HUVECs had increased expression of pro-fibrotic genes compared to fibroblasts cultured in CM from control HUVECs as shown by qRT-PCR. HUVEC-Scr-CM ACTA2, n = 6; HUVEC-Scr-CM VIM, n = 6; HUVEC-Scr-CM FN1, n = 3; HUVEC-Scr-CM COL3A1, n = 4; HUVEC-shFOXF1-CM ACTA2, n = 5; HUVEC-shFOXF1-CM VIM, n = 6; HUVEC-shFOXF1-CM FN1, n = 3; HUVEC-shFOXF1-CM COL3A1, n = 5. f CM from FOXF1-deficient HUVECs had increased levels of pro-inflammatory mediators as determined by Proteome Profiler Human Cytokine Array (n = 2). g Inhibition of IL-6 and TNFα using blocking antibodies attenuated CCD-19Lu fibroblast invasion in the presence of CM from FOXF1-deficient HUVECs. Bar = 200 μm. n = 6 samples per group. Data presented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001. CM conditioned medium. For two group comparisons, T-test (two-tailed) analyses were performed. Source data are provided as a Source Data file.
Next, we determined the effect of FOXF1 deficiency on secretion of profibrotic and proinflammatory mediators by endothelial cells in vitro. Human endothelial cells, HUVEC, were infected with lentiviruses carrying either FOXF1 shRNA or control shRNA. The shFOXF1-treated HUVECs exhibited decreased FOXF1 mRNA as shown by qRT-PCR (Fig. 4b), demonstrating approximately similar efficiency of FOXF1 inhibition compared to haploinsufficient endothelial cells from endFoxf1+/− mice (Fig. 4a). Conditioned media (CM) from control and FOXF1-deficient HUVECs were collected. Invasion and proliferation of human CCD-19Lu lung fibroblasts were measured in the presence of CM in vitro. CM from FOXF1-deficient HUVECs increased invasion and proliferation of lung fibroblasts compared to CM from control cells (Fig. 4c, d). CM from FOXF1-deficient HUVECs also increased expression of ACT2, VIM, FN1 and COL3A1 in human CCD-19Lu fibroblasts (Fig. 4e). To verify that the results are not limited to HUVECs, we also used human pulmonary arterial endothelial cells (HPAEC) and human pulmonary microvascular endothelial cells (HPMEC) to confirm that CM from FOXF1-deficient HPAEC and HPMEC cells increased invasion, proliferation as well as expression of pro-fibrotic genes in lung fibroblasts (Supplementary Figs. S10a–d and S11a–d). Thus, FOXF1-deficient endothelial cells induced the pro-fibrotic phenotype in lung fibroblasts, possibly, through secretion of soluble mediators.
To identify the differentially changed soluble mediators in the CM from FOXF1-deficient HUVECs compared to control HUVECs, we used the Proteome Profiler Human Cytokine Array. CCL2, CXCL1, G-CSF, GM-SCF, ICAM-1 and IL-6 proteins were increased in CM from FOXF1-deficient HUVECs, whereas CXCL12, IL-8, IL-13 and IL-16 were unchanged (Fig. 4f). Since TNFα and IL-6 increase activation of fibroblasts during lung fibrosis26, we used blocking antibodies to inhibit increased levels of IL-6 or TNFα in CM from FOXF1-deficient HUVECs (Fig. 4g). Inhibition of either IL-6 or TNFα significantly decreased migration of CCL-19LU fibroblasts into the bottom chamber of Transwells containing CM from FOXF1-deficient HUVECs (Fig. 4g). To verify that the results are not limited to HUVEC cells, we also used HPAEC cells and confirmed that inhibition of either IL-6 or TNFα significantly decreased migration of CCL-19LU fibroblasts into the bottom chamber of Transwells containing CM from FOXF1-deficient HPAEC (Supplementary Fig. S10e, f). Thus, FOXF1-deficient endothelial cells stimulate the migration of lung fibroblasts in vitro by secreting IL-6 and TNFα.
FOXF1 deficiency in endothelial cells increases the number of macrophages in the fibrotic lungs
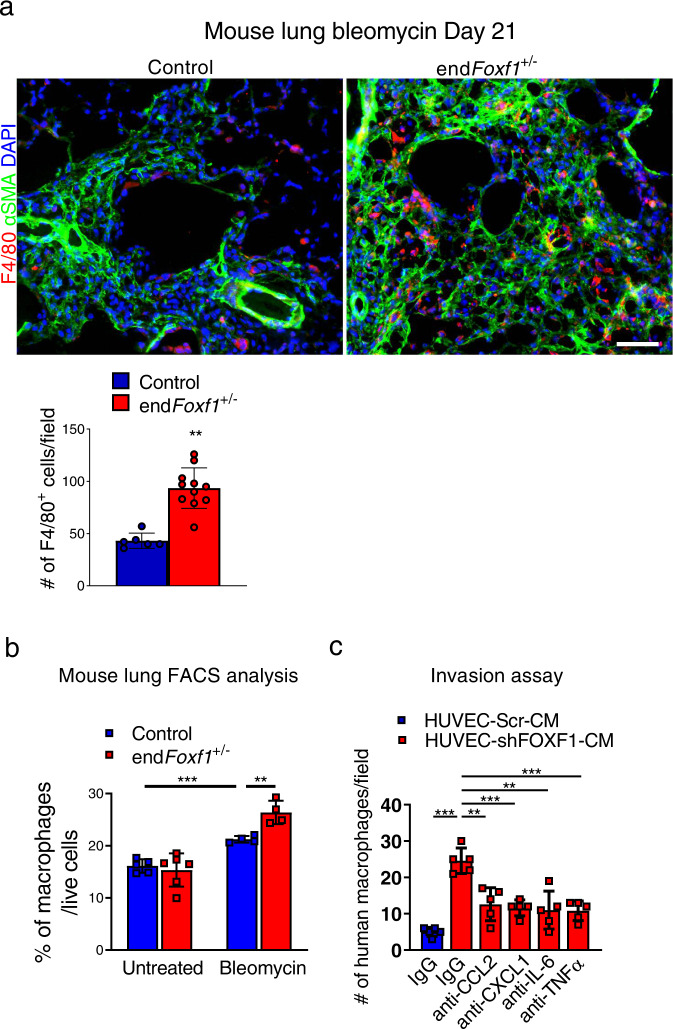
Since numerous cytokines from the protein array, including IL-6, TNFα, CCL2 and CXCL1, regulate recruitment of macrophages during lung injury26, we examined the number of macrophages in Foxf1-deficient lungs. At day 21 after bleomycin injury, accumulation of macrophages in fibrotic lesions of bleomycin-treated endFoxf1+/− lungs was increased compared to control lungs as shown by immunostaining for F4/80 (Fig. 5a) and Mac3 (Supplementary Fig. S12a). We also used flow cytometry analysis to demonstrate that the number of macrophages (CD45+ CD11clow/+ CD64+) was increased in bleomycin-injured endFoxf1+/− lungs compared to control bleomycin-injured lungs (Fig. 5b and Supplementary Fig. S12b–c). Of note, no changes in the number of inflammatory cells, including macrophages, were reported in uninjured endFoxf1+/− and control lungs18. We next determined whether increased secretion of pro-inflammatory mediators by FOXF1-deficient endothelial cells is important for macrophage migration in vitro. CM from control or FOXF1-deficient HUVECs was added to the bottom chambers of Transwells, and the migration of human macrophages from the upper chambers towards CM was assessed in the presence of blocking antibodies specific to CCL2, CXCL1, IL-6 or TNFα (Fig. 5c). Inhibition of these pro-inflammatory cytokines decreased migration of macrophages towards CM from FOXF1-deficient HUVECs compared to CM from control HUVECs (Fig. 5c and Supplementary Fig. S12d). Thus, FOXF1-deficient endothelial cells stimulate migration of macrophages by secreting multiple proinflammatory mediators.
Fig. 5. Inhibition of FOXF1 in EC increases the number of macrophages in the fibrotic lungs.
a Increased number of macrophages in the lungs of endFoxf1+/− (n = 11) mice compared to control (n = 6) mice at day 21 after bleomycin administration is shown using immunostaining for F4/80 (red) and αSMA (green) (control, n = 6 mice). Nuclei are counterstained with DAPI (blue). Bar = 100 μm. Average numbers of F4/80-positive cells were quantified using 10 random microscope fields per lung and presented as mean ± SD. **p = 0.0013. b Flow cytometry analysis shows increased percentage of macrophages in the lungs of bleomycin-treated endFoxf1+/− mice compared to control mice. Macrophages were identified as CD45+ CD64+ CD11clow/+ (Untreated Control, n = 5; untreated endFoxf1+/−, n = 6; bleomycin-treated Control and endFoxf1+/−, n = 4). Data presented as mean ± SD. c Inhibition of CCL2, CXCL1, IL-6 and TNFα in CM from FOXF1-dificient HUVECs (shFOXF1-CM) using blocking antibodies attenuated microphage invasion in transwell assay. Human macrophages were incubated in the presence of CM from scrambled control (Scr-CM) or shFOXF1-transfected (shFOXF1-CM) HUVECs. Invaded cells were counted in 5 random microscope fields and presented as mean ± SD. n = 5 samples per group. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s T test (two-tailed). CM condition medium. Source data are provided as a Source Data file.
R-Ras is a direct transcriptional target of FOXF1
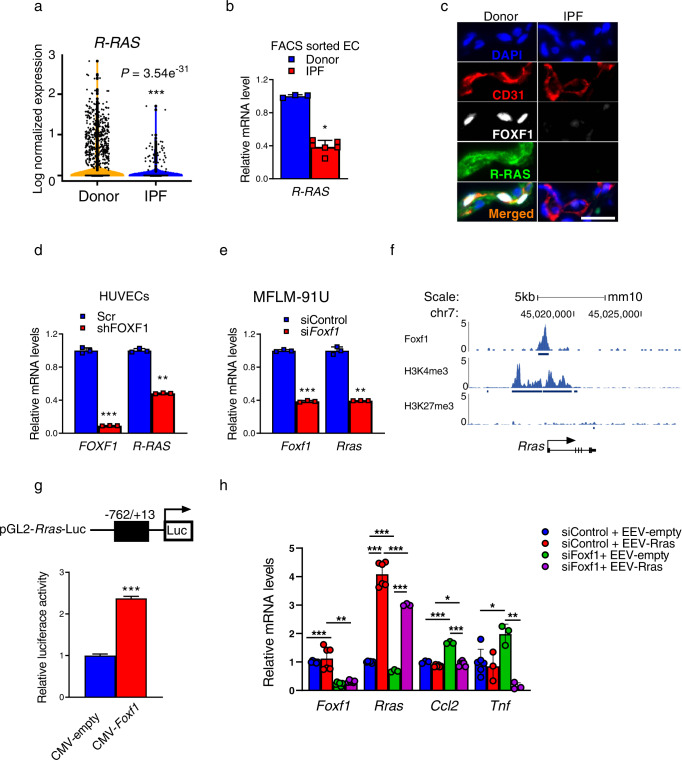
FACS-sorted endothelial cells from bleomycin-treated endFoxf1+/− lungs exhibited decreased expression of Rras (Fig. 4a), a critical mediator of endothelial barrier function and vascular repair after injury27,28. Consistent with decreased expression of Rras in endothelial cells, bleomycin-treated endFoxf1+/− lungs displayed increased endothelial permeability as determined by Evans blue dye (Supplementary Fig. S13a). Since increased endothelial permeability contributes to lung fibrosis29 and endFoxf1+/− mice had exacerbated lung fibrosis after bleomycin injury (Fig. 3), we examined whether FOXF1 regulates R-RAS in pulmonary endothelial cells. Based on scRNA-seq data analysis, R-RAS mRNA was decreased in human IPF endothelial cells compared to donor endothelial cells (Fig. 6a). We also FACS-sorted endothelial cells from IPF lungs and used qRT-PCR to demonstrate that R-RAS mRNA was decreased in IPF endothelial cells, coinciding with decreased expression of FOXF1 mRNA (Fig. 6b). Immunostaining for R-RAS, FOXF1 and CD31 showed that FOXF1 co-localized with R-RAS in endothelial cells of donor lungs, indicating that both proteins are co-expressed in normal ECs (Fig. 6c). In contrast, neither R-RAS nor FOXF1 were detected in endothelial cells within fibrotic lesions of IPF lungs (Fig. 6c). In vitro shRNA-mediated knockdown of FOXF1 in HUVECs decreased expression of R-RAS (Fig. 6d). These findings were confirmed in HPAEC and HPMEC cells (Supplementary Fig. S14a, b). In agreement with human data, shRNA-mediated knockdown of Foxf1 in mouse MFLM 91U endothelial cells also decreased expression of Rras (Fig. 6e). Next, we used publicly available ChIP-seq dataset25 to show that FOXF1 protein directly bound to the Rras promoter region in endothelial cells (Fig. 6f). The FOXF1-binding region in Rras promoter had H3K4me3 but not H3K27me3 marks (Fig. 6f), suggesting that FOXF1 transcriptionally activates Rras gene promoter. To test this hypothesis, the −762/+13 bp Rras promoter region, containing the FOXF1-binding site identified by ChIP-seq (Fig. 6f), was cloned into the pGL2 luciferase reporter plasmid (Fig. 6g, upper schematic diagram). In co-transfection experiments, CMV-Foxf1 expression vector increased transcriptional activity of the −762/+13 Rras promoter region compared to CMV-empty vector (Fig. 6g). Thus, Rras is a direct transcriptional target of FOXF1 in endothelial cells.
Fig. 6. R-Ras is a direct transcriptional target of FOXF1.
a Violin plots show decreased R-RAS mRNA in EC of IPF (n = 4 lungs) compared to donor (n = 8 lungs), GSE 122960 datasets. R-RAS expression was log normalized. b R-RAS mRNA was decreased in FACS-sorted ECs from IPF lungs (n = 6) compared to donor lungs (n = 3) as shown by qRT-PCR. Data presented as mean ± SD, *p = 0.238, Mann–Whitney Two-tailed test. c Immunostaining for R-RAS, FOXF1 and CD31 shows co-localization of FOXF1 and R-RAS in ECs of donor lungs (n = 5). Neither R-RAS nor FOXF1 are detected in ECs within IPF fibrotic lesions (n = 5). Nuclei are counterstained with DAPI. Bar = 20 μm. d qRT-PCR shows that shRNA-mediated knockdown of FOXF1 (shFOXF1) in HUVECs decreased R-RAS mRNA compared to control (Scr). ACTB mRNA was used for normalization (n = 3), **p < 0.01 and ***p < 0.001 by Student’s T test (two-tailed). e qRT-PCR shows that siRNA-mediated knockdown of Foxf1 in mouse MFLM-91U ECs decreased Rras mRNA. Actb mRNA was used for normalization (n = 3). f ChIP-seq shows direct binding of FOXF1 protein to Rras promoter region in MFLM-91U cells. Binding of FOXF1 to Rras promoter is associated with H3K4me3 marks but not H3K27me3 marks. g Schematic drawing of the pGL2-Rras-Luc construct with the −762/+13 bp Rras promoter region containing the FOXF1-binding site (top panel). In co-transfection experiments, CMV-FOXF1 expression vector increased transcriptional activity of the −762/+13 Rras promoter region compared to CMV-empty vector (bottom panel), n = 3, ***p < 0.001. h Overexpression of R-Ras decreased Ccl2 and TNFα mRNAs in mock-transfected cells and prevented upregulation of Ccl2 and TNFα in cells transfected with Foxf1-specific siRNA. MFLM-91U cells were transfected with non-targeting siRNA (siControl) or siFoxf1, and EEV-empty vector or EEV-Rras. Actb mRNA was used for normalization (siControl+EEV-empty Foxf1, n = 6; siControl+EEV-empty Rras, n = 6; siControl+EEV-empty Ccl2, n = 3; siControl+EEV-empty Tnf, n = 6; siControl+EEV-Rras Foxf1, n = 6; siControl+EEV-Rras Rras, n = 6; siControl+EEV-Rras Ccl2, n = 6; siControl+EEV-Rras Tnf, n = 3; siFoxf1+EEV-empty Foxf1, n = 6; siFoxf1+EEV-empty Rras, n = 3; siFoxf1+EEV-empty Ccl2, n = 3; siFoxf1+EEV-empty Tnf, n = 3; siFoxf1+EEV-Rras Foxf1, n = 6; siFoxf1l+EEV-Rras Rras, n = 3; siFoxf1l+EEV-Rras Ccl2, n = 6; siFoxf1+EEV-Rras Tnf, n = 3), *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test (two tailed). Source data are provided as a Source Data file.
Next, we examined whether R-RAS is involved in FOXF1-mediated regulation of CCL2, CXCL1, IL-6 and TNFα. We over-expressed R-Ras in FOXF1-deficient MFLM 91U endothelial cells in vitro (Fig. 6h and Supplementary Fig. S14a, b). Overexpression of R-Ras decreased Ccl2 and TNFα mRNAs in mock-transfected cells and prevented upregulation of Ccl2 and TNFα in cells transfected with Foxf1-specific siRNA (Fig. 6h). Overexpression of R-Ras did not prevent upregulation of Cxcl1 and IL-6 in FOXF1-deficient endothelial cells (Supplementary Fig. S14c). Altogether, our data suggest that FOXF1 in lung endothelium stimulates transcription of Rras, which inhibits expression of CCL2 and TNFα. FOXF1 inhibits CXCL1 and IL-6 independently of R-Ras.
Transgenic overexpression of FOXF1 in endothelial cells decreases lung fibrosis after bleomycin-induced injury
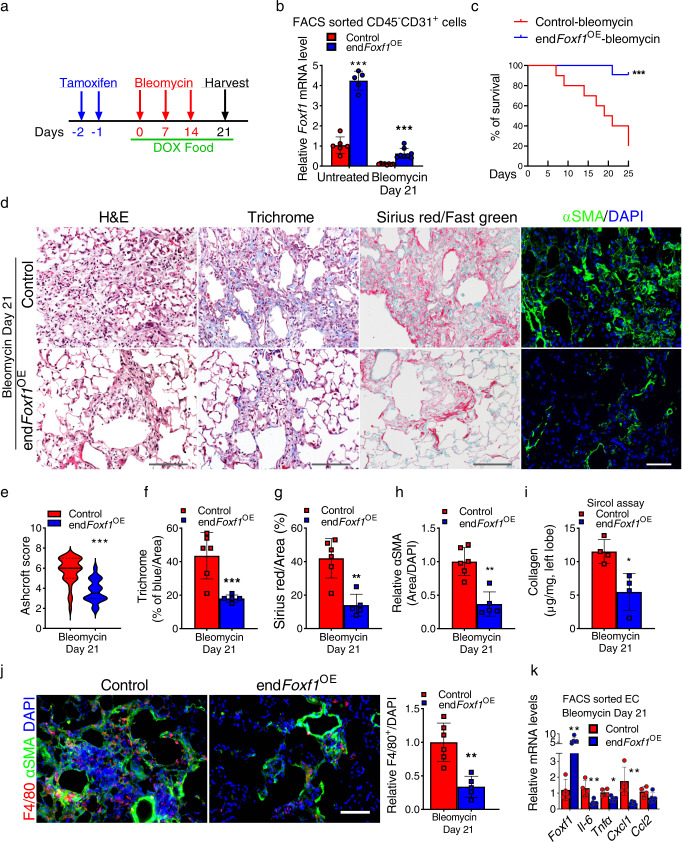
Since conditional deletion of FOXF1 in endothelial cells increased pulmonary fibrosis after chronic bleomycin injury (Fig. 3), we examined whether overexpression of FOXF1 in endothelial cells was sufficient to inhibit lung fibrosis. To test this hypothesis, we generated an inducible, EC-specific FOXF1 overexpression mouse model (Pdgfb-CreERtg/+; LSL-rtTAtg/+; TetO-Foxf1tg/+ mice; abbreviated as endFoxf1OE) (Supplementary Fig. S15a). Upon tamoxifen administration, Cre-mediated recombination of LoxP-floxed stop codon (LSL) results in rtTA expression in endothelial cells. In the presence of doxycycline, rtTA binds to and activates the TetO7-CMV promoter driving expression of the mouse Foxf1 transgene (Supplementary Fig. S15a). Thus, combined administration of tamoxifen (Tam) and doxycycline (Dox) causes increased expression of FOXF1 in endothelial cells of endFoxf1OE mice (Fig. 7a). FACS-sorted endothelial cells from Tam- and Dox-treated endFoxf1OE lungs had a 4-fold increase in Foxf1 mRNA compared to controls (Fig. 7b). Without bleomycin injury, the Tam- and Dox-treated endFoxf1OE mice had normal lung architecture and capillary density as shown by H&E staining and immunostaining for CD31 (PECAM-1) (Supplementary Fig. S15b). To induce pulmonary fibrosis, Tam- and Dox-treated endFoxf1OE and single transgenic (control) mice were given three weekly IT injections of bleomycin (Fig. 7a). While bleomycin treatment decreased Foxf1 mRNA in FACS-sorted endothelial cells from both groups of mice, Foxf1 expression was higher in endFoxf1OE ECs compared to control ECs (Fig. 7b). Transgenic overexpression of Foxf1 increased survival of mice after bleomycin injury with 95% of endFoxf1OE mice surviving the injury, but only 20% of control single-transgenic littermates being alive 25 days after the last bleomycin treatment (Fig. 7c). Fibrotic lung remodeling was decreased in bleomycin-treated endFoxf1OE mice compared to controls as demonstrated by Ashcroft score, Trichrome staining, Sirius red/Fast green staining and immunostaining for αSMA (Fig. 7d–h). Biochemical quantification of lung collagen amounts using Sircol assay was consistent with decreased fibrosis in endFoxf1OE lungs (Fig. 7i). Moreover, endothelial over-expression of Foxf1 decreased recruitment of macrophages into bleomycin-treated lungs and decreased expression of pro-inflammatory genes in total lung RNA (Fig. 7j, k). Thus, overexpression of FOXF1 in endothelial cells prior to bleomycin injury attenuates pulmonary fibrosis and improves survival after bleomycin-induced lung injury.
Fig. 7. Endothelial-specific overexpression of FOXF1 before bleomycin injury decreases pulmonary fibrosis and improves survival of mice.
a Schematic diagram shows bleomycin, tamoxifen (TAM) and doxycycline (DOX) treatments in endFoxf1OE mice. b qRT-PCR shows that Foxf1 mRNA is increased in lung ECs isolated from untreated and bleomycin-treated endFoxf1OE mice compared to control mice. Actb mRNA was used for normalization (Untreated Control, n = 6; Untreated endFoxf1OE, n = 5; Bleomycin Control and endFoxf1OE, n = 8 mice per group), ***p < 0.001. c Endothelial-specific overexpression of FOXF1 increases mouse survival after bleomycin injury (n = 10), **p = 0.001, Log-rank (Mantel–Cox) test. d Decreased fibrosis in lungs of bleomycin-treated endFoxf1OE mice is shown by H&E, Trichrome, Sirius red/fast green staining and immunostaining for αSMA (green). Mouse endFoxf1OE (n = 5) and control lungs (n = 5) were harvested at day 21 after bleomycin treatment. Bar = 100 μm. e–h Assessment of fibrotic lesions in endFoxf1OE (n = 5) lungs is presented as Ashcroft score ***p < 0.0001 (e), the percent of Trichrome-positive **p = 0.0058 (f) Sirius red-positive. **p = 0.0011 (g), and αSMA-positive areas in the lungs (Control, n = 6), **p = 0.0013. Areas positive for collagen depositions were quantified in 10 random fields per lung using Nikon’s NIS-Elements AR software (h). i Lung collagen depositions were quantified by Sircol collagen assay. Left lung lobes from endFoxf1OE (n = 4) and control mice (n = 4) were used. *p = 0.0286. t test (two tailed). j Decreased number of macrophages in the lungs of bleomycin-treated endFoxf1OE mice (n = 5) is shown using immunofluorescent staining with anti-F4/80 antibodies (red) and anti-αSMA antibodies (green) at day 21 after bleomycin administration (Control, n = 6). Nuclei are counterstained with DAPI (blue). Bar = 100 μm. Average numbers of F4/80-positive cells were quantified using 10 random microscope fields per lung and presented as mean ± SD. **p = 0.0013. t test (two tailed). k Endothelial overexpression of Foxf1 decreases mRNA levels of pro-inflammatory genes in FACS sorted EC as shown by qRT-PCR. mRNA was extracted from control and bleomycin-treated endFoxf1OE mice on day 21. Actb mRNA was used for normalization. Control, n = 4; endFoxf1OE, n = 5. Data presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test (two tailed). Source data are provided as a Source Data file.
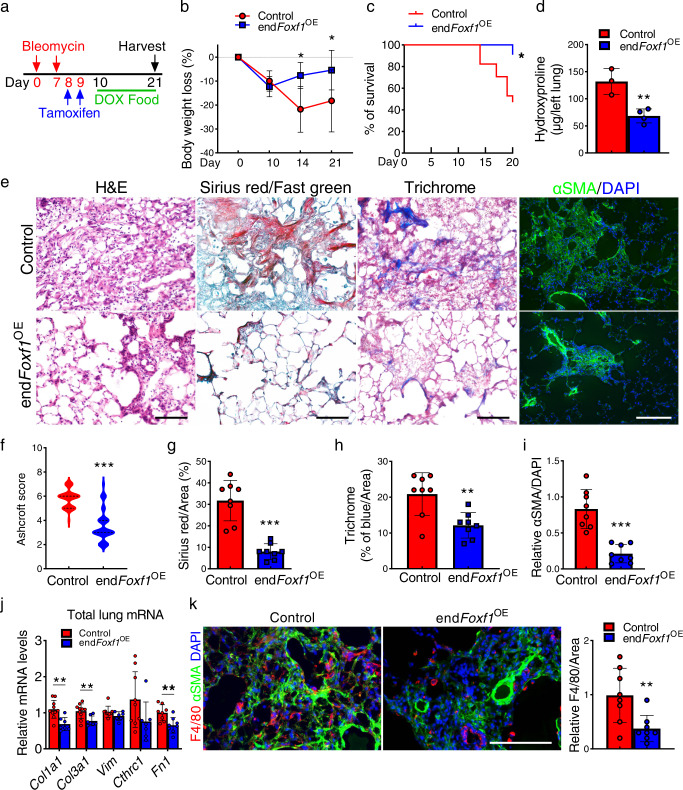
Next, we assessed whether increasing endothelial FOXF1 in already established lung fibrosis will improve the outcomes. Overexpression of FOXF1 in endothelial cells at day 10 after bleomycin injury decreased body weight loss, improved mice survival, and decreased collagen depositions in endFoxf1OE mice compared to bleomycin-injured control mice (Fig. 8a–i). Based on Flexivent measurements of lung mechanics, many functional parameters in endFoxf1OE mice were improved, including increased lung compliance, total lung capacity and decreased tissue resistance (Supplementary Fig. S16a–h). Finally, overexpression of FOXF1 in endothelial cells at day 10 after bleomycin injury, decreased expression of pro-fibrotic genes in total lung RNA and inhibited recruitment of macrophages to the lung tissue (Fig. 8j, k). Altogether, overexpression of FOXF1 in endothelial cells either prior to or after bleomycin injury attenuates pulmonary fibrosis and improves mice survival after the injury.
Fig. 8. Endothelial-specific overexpression of FOXF1 during fibrotic stage decreases lung fibrosis and increases survival of mice after bleomycin-induced injury.
a Schematic diagram shows bleomycin, tamoxifen (TAM) and doxycycline (DOX) treatment in endFoxf1OE mice. b Endothelial-specific overexpression of FOXF1 decreases bodyweight loss in bleomycin-treated endFoxf1OE mice (Control, n = 12; endFoxf1OE, n = 12 for days 0 and 21, n = 4 for day 10, n = 6 for day 14), *p < 0.05, two-way ANOVA with the Geisser–Greenhouse correction. c Endothelial-specific overexpression of FOXF1 increases mouse survival after bleomycin injury (Control, n = 11; endFoxf1OE, n = 17), *p < 0.05, Log-rank (Mantel–Cox) test. d Decreased collagen depositions in bleomycin-treated endFoxf1OE lungs is shown by Hydroxyproline assay. Left lung lobes from endFoxf1OE (n = 4) and control (n = 3) mice were used, **p = 0.0061, t test (two tailed). e Decreased fibrosis in bleomycin-treated endFoxf1OE lungs is shown by H&E, Sirius red/fast green, Trichrome, and immunostaining for αSMA. Lungs were harvested at day 21 after bleomycin treatment (n = 8 per group). Bar = 100 µm. f–i Ashcroft score of endFoxf1OE lungs (***p < 0.0001) is consistent with the percent of Sirius red-positive (***p < 0.0001), Trichrome-positive (**p = 0.0031) and αSMA-positive areas (***p < 0.0001). Areas positive for collagen depositions were quantified in 10 random fields per lung using Nikon NIE CIC Analysis Elements software. n = 8 mice per group. j Endothelial overexpression of Foxf1 at day 10 after bleomycin administration decreases mRNA levels of pro-fibrotic genes in total lung RNA as shown by qRT-PCR. Total lung mRNA was extracted from control and bleomycin-treated endFoxf1OE mice on day 21. Actb mRNA was used for normalization (Control Col1a1 and Col3a1, n = 10; Control Vim, n = 9; Control Cthrc1, n = 9; Control Fn1, n = 8; endFoxf1OE Col1a1, Col3a1, Vim and Cthrc1, n = 7; endFoxf1OE Fn1, n = 8). k Decreased number of macrophages in the lungs of bleomycin-treated endFoxf1OE mice is shown using immunofluorescent staining with anti-F4/80 antibodies (red) and anti-αSMA antibodies (green) at day 21 after bleomycin administration, ***p < 0.0001. Nuclei are counterstained with DAPI (blue). n = 8 mice per group. Data presented as mean ± SD, *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test (two tailed). Source data are provided as a Source Data file.
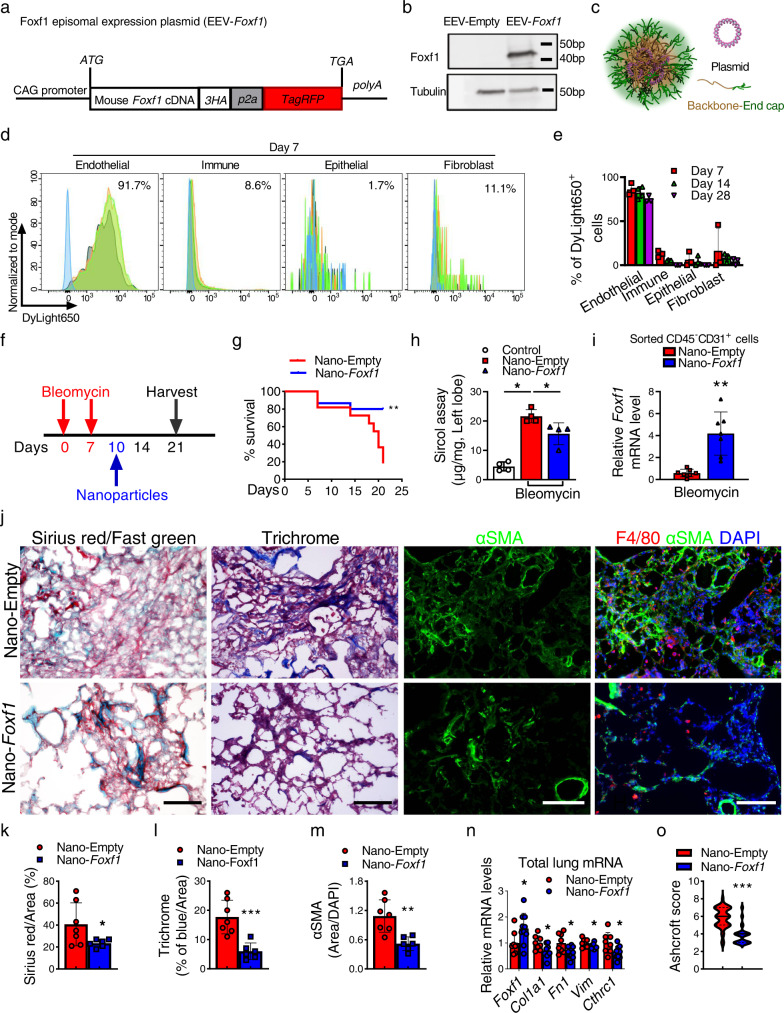
Nanoparticle delivery of non-integrating Foxf1 expression-vector into the lung endothelium attenuates pulmonary fibrosis
Since genetic overexpression of Foxf1 in endothelial cells attenuated pulmonary fibrosis, we next tested whether nanoparticle delivery of Foxf1 cDNA into endothelial cells can be a therapeutic approach to inhibit lung fibrosis. Mouse Foxf1 cDNA was cloned into non-integrating self-replicating episomal EEV vector to generate EEV-Foxf1 construct (Fig. 9a). Transfection of EEV-Foxf1 plasmid into Foxf1-negative HEK-293T cells increased the FOXF1 protein levels in vitro as shown by Western blot (Fig. 9b). To deliver EEV-Foxf1 plasmid to endothelial cells in vivo, we utilized the poly β-amino esters (PBAE) polymer30,31, which formed stable nanoparticles in complex with plasmid DNA (Fig. 9c and Supplementary Fig. S17a, b). The hydrodynamic average diameter of the PBAE nanoparticles loaded with EEV-Foxf1 plasmid DNA (Nano-Foxf1) was 146.27 ± 9.54 nm (Supplementary Fig. S17c), whereas the average surface charge of the Nano-Foxf1 was 28.3 ± 1.71 mV (Supplementary Fig. S17d). In vitro treatment of HEK-293T cells with nanoparticles containing EEV-Foxf1 plasmid resulted in the efficient expression of red fluorescent protein (RFP) in vast majority of cells (Supplementary Fig. S17e). Next, fluorescently-labeled PBAE nanoparticles with either EEV-Foxf1 or EEV-Empty (control) plasmids were delivered to bleomycin-treated mice (Fig. 9d, e and Supplementary Fig. S17f, g) via tail vein on the same day as bleomycin administration. Using FACS analysis of enzymatically digested lung tissue, nanoparticles were detected in ~92% of lung endothelial cells (CD31+/CD45−) (Fig. 9e). Nanoparticles-mediated targeting of epithelial (CD326+/CD45−/CD31−), immune (CD45+/CD31−) and mesenchymal (CD31−/CD45−/CD326−) cells was ineffective (Fig. 9d, e). Nanoparticles were still detected in ~50–70% of pulmonary endothelial cells at day 28 after single nanoparticle delivery (Fig. 9e and Supplementary Fig. S17g). Administration of EEV-Foxf1-nanoparticles increased Foxf1 mRNA in FACS-sorted lung endothelial cells as demonstrated by qRT-PCR (Fig. 9f). Treatment with nanoparticles containing pEEV-Foxf1 vector on the same day as bleomycin injury significantly increased survival of mice as shown by Kaplan–Meier curve (Supplementary Fig. S18a) and decreased collagen depositions in the lung tissue as shown by H&E and Sirius red staining (Supplementary Fig. S18b).
Fig. 9. Nanoparticle delivery of non-integrating Foxf1 expression vector into the lung endothelium attenuates pulmonary fibrosis.
a Diagram of the FOXF1 episomal plasmid (EEV-Foxf1). b Western blot shows increased FOXF1 protein after transfection of EEV-Foxf1 plasmid into FOXF1-negative HEK-293T cells. c Schematic of PBAE nanoparticles loaded with plasmid DNA. All elements of images were created using Autodesk 3ds Max 2020 (Autodesk, version 2020). d FACS analysis of mouse lungs show the presence of labeled nanoparticles in endothelial cells but not in other cell types at day 7 after I.V. administration (n = 3). e FACS analysis shows nanoparticles in lung ECs at different time-points after bleomycin injury (n = 5 mice per group). f Diagram shows nanoparticle I.V. delivery to mice at day 10 after bleomycin administration. g Kaplan–Meier analysis shows increased survival of bleomycin-injured mice after nanoparticle delivery of EEV-Foxf1 plasmid (Nano-Foxf1, n = 15) compared to EEV-Empty (Nano-Empty, n = 11), **p < 0.005, Log-rank (Mantel–Cox) test. h Sircol assay shows decreased collagen depositions in Nano-Foxf1-treated lungs (n = 4 mice per group), *p < 0.05, ***p < 0.001, Mann–Whitney Two-tailed test. i Foxf1 mRNA is increased in FACS-sorted lung ECs from Nano-Foxf1 treated mice (n = 7), *p < 0.05, **p < 0.005, Student’s t test (two tailed). j Decreased lung fibrosis in Nano-Foxf1-treated mice is shown with Sirius red/fast green, Masson’s trichrome, and immunostaining for αSMA. Decreased number of macrophages in Nano-Foxf1-treated lungs is shown by immunostaining for F4/80 and αSMA at day 21 after bleomycin administration (n = 6 mice per group). Bar = 100 μm. k–n Quantification of fibrotic lesions in mouse lungs is shown as the percent of Sirius red-positive (k), Trichrome-positive (l) and αSMA (m) areas. Areas positive for collagen depositions were quantified in 10 random microscope fields per lung (Nano-Empty, n = 7; Nano-Foxf1, n = 6). n Nanoparticle delivery of Foxf1 at day 10 after bleomycin administration decreases mRNA levels of pro-fibrotic genes in total lung RNA as shown by qRT-PCR. (Nano-Empty control, n = 8, and Nano-Foxf1-treated, n = 10, on day 21 after bleomycin administration. Actb mRNA was used for normalization. o Decreased Ashcroft score in nano-Foxf1 treated lungs (n = 10) compared to control nano-Empty (n = 8). Data presented as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 by Student’s t test (two tailed). Source data are provided as a Source Data file.
Next, we assessed the therapeutic efficacy of EEV-Foxf1-nanoparticles after lung injury. Mice were treated with EEV-Foxf1-nanoparticles at day 10 after bleomycin administration (Fig. 9f) when the fibrosis lung remodeling is already present (Fig. 2a). Nanoparticle delivery of Foxf1 into endothelial cells improved mice survival (Fig. 9g), prevented the loss of body weight after bleomycin injury (Supplementary Fig. S19a), and decreased lung collagen depositions quantified by Sircol assay (Fig. 9h). Decreased collagen amounts in EEV-Foxf1-treated lungs coincided with increased Foxf1 mRNA in FACS-sorted lung endothelial cells (Fig. 9i). Decreased fibrotic remodeling in EEV-Foxf1-treated lungs was detected and quantified based on Sirius red/Fast green staining, Trichrome staining, and immunostaining for αSMA (Fig. 9j–m). In addition, nanoparticle delivery of Foxf1 into endothelial cells at day 10 after bleomycin injury, reduced macrophage infiltration into fibrotic regions (Fig. 9j, right panels and Supplementary Fig. S19l), decreased expression of pro-fibrotic genes in total lung RNA (Fig. 9n), decreased Ashcroft score (Fig. 9o), improved lung compliance and total lung capacity in EEV-Foxf1-treated mice (Supplementary Fig. S19b–j) and arterial oxygenation (Supplementary Fig. S19k). Altogether, nanoparticle delivery of Foxf1 cDNA into endothelial cells on the day of bleomycin injury or during fibrotic stage after the injury inhibits pulmonary fibrosis and improves mice survival.
Discussion
Significant changes in gene expression among epithelial and mesenchymal cellular populations of IPF lungs have been recently identified by scRNA-seq7,19,32. Carraro et al. identified unique subclusters of lung epithelial cells that were specific to IPF lungs32. Likewise, other studies used scRNA-seq to examine gene expression signatures in distinct epithelial and mesenchymal cell types during pulmonary fibrogenesis7. Novel population of profibrotic alveolar macrophages was identified exclusively in patients with fibrosis19. Focusing on fibrosis-associated changes in pulmonary endothelial cells, we found in this study that one of the most downregulated transcription factors in human IPF and in the mouse bleomycin-treated lungs is FOXF1. The FOXF1 is highly enriched in lung endothelial cells compared to endothelial cells of other organs including liver, pancreas, brain, heart, and kidney21,33. FOXF1 is required for embryonic development of pulmonary vasculature by activating VEGF, BMP9 and TIE-2 signaling pathways12,16,17. FOXF1 stimulates lung repair and regeneration by regulating genes critical for extracellular matrix remodeling, inflammation, and endothelial barrier function18,25,34–36. FOXF1 is identified as an anti-fibrotic factor that regulates key myofibroblast functions by preventing CDH2-CDH11 cadherin switching and by reducing lung inflammation during pulmonary fibrosis37.
An important contribution of the current studies is that FOXF1 transcriptionally regulates Rras gene expression as evidenced by direct binding of FOXF1 to the Rras promoter region, leading to transcriptional activation of the Rras promoter by CMV-Foxf1 expression plasmid. The small guanosine triphosphate hydrolase (GTPase) Ras-related protein (R-Ras) regulates a lot of different cellular processes, including cell adhesion to the extracellular matrix via integrin activation38, endothelial cell adhesion via VE-cadherin stabilization27, cell survival via PI3K/AKT pathway39 and angiogenesis via inhibition of the VEGF receptor 2 (VEGFR2) internalization40,41. Published studies demonstrated that R-Ras is important for blood vessel stabilization by stimulating signaling between endothelial cells and pericytes27,42. Deletion of Rras gene in mice resulted in increased vascular permeability in various models of angiogenesis27,43. These studies are consistent with the phenotype of the mice with homozygous endothelial Foxf1 deletion that succumbed from lung hemorrhage and edema within a month after Foxf1 deletion18. FOXF1 maintains endothelial barrier function by stimulating expression of VEcadherin and S1PR118. Therefore, increased endothelial permeability can contribute to aberrant inflammation and increased fibrosis in endFoxf1+/− lungs. Since R-RAS plays an important role in blood vessel regeneration, maturation, and stability, it is important to identify molecular mechanisms regulating R-Ras signaling during lung fibrosis. We report here that overexpression of R-RAS prevents upregulation of CCL2 and TNFα in FOXF1-deficient endothelial cells, suggesting that FOXF1 inhibits CCL2 and TNFα via R-RAS. Even though, CCL2 and TNFα are produced by multiple cell types, the use of neutralizing CCL2 or TNFα antibodies in co-culture of endothelial cells and fibroblasts decreased activation of fibroblasts, indicating the importance of angiocrine functions of EC. Since both CCL2 and TNFα promote macrophage migration to the lung tissue44, decreased expression of R-RAS can contribute to aberrant accumulation of macrophages in bleomycin-treated endFoxf1+/− lungs.
Our studies support the notion that increasing expression of FOXF1 in endothelial cells through nanoparticle delivery of Foxf1 cDNA can be considered to alleviate pulmonary fibrosis. The major drawbacks of the conventional therapeutics for respiratory disorders include the insufficient drug concentrations at pathological lesions, lack of cell-specific targeting, and various bio-barriers in the conducting airways and alveoli45. To address these critical issues, various nanoparticle delivery systems have been developed to serve as carriers of specific drugs, DNA expression vectors, and RNAs46. Although intratracheal administration is an attractive method of DNA/RNA delivery to the lung tissue, specific targeting of endothelial cells from the air surface is ineffective since it requires crossing the epithelial barrier without unloading the therapeutic cargo. We found here that pulmonary endothelial cells can be efficiently and specifically deliver non-integrating Foxf1 plasmid by PBAE nanoparticles through blood circulation. The size and cationic charge of our PBAE nanoparticles are similar to the recently reported cationic polyplexes that contained PEI, fatty acids, cholesterol and PEG (PEI/PEG)47, and demonstrated an efficient targeting of pulmonary endothelial cells after intravenous administration to adult mice. Since the recent successful nanoparticle delivery of different cargos have been effective in stimulating lung repair after neonatal hyperoxic injury48 and in inhibiting fibrotic lung remodeling in mouse model of ACD/MPV49, nanoparticle gene therapy has a promise to restore endothelial function in fibrotic lung diseases. However, it is unclear at the moment whether increasing FOXF1 levels through nanoparticle gene therapy or recently discovered FOXF1-activating small molecule compound50 will have beneficial effect in human IPF. The efficacy of FOXF1 targeting in IPF can only be determined in clinical trials.
In summary, FOXF1 functions in EC to maintain R-Ras signaling and prevent secretion of pro-inflammatory mediators from EC, leading to decreased activation of lung fibroblasts. Our studies suggest the FOXF1-activating therapies can be considered to improve the long-term outcomes in IPF patients.
Methods
Ethical statement
The Institutional Review Board of the Cincinnati Children’s Hospital Medical Center (Federalwide Assurance #00002988) approved all the studies with human tissue samples (IRB protocol #2017-4321). Human lung tissue specimens were obtained from tissue repository at University of Cincinnati Medical Center that provides de-identified human biospecimen procurement and banking services in support of basic, translational, and clinical research. Total number of ten lung tissue samples were received, including six males and four females. All patients signed informed consent prior to tissues collection. All animal studies were approved by Cincinnati Children’s Research Foundation Institutional Animal Care and Use Committee and covered under our animal protocol (IACUC2016-0070). The Cincinnati Children’s Research Foundation Institutional Animal Care and Use Committee is an AAALAC and NIH accredited institution (NIH Insurance #8310801). All mice were kept under SPF (specific-pathogen free) conditions in 12/12 light/dark cycle, 18–23 °C and 40–60% humidity. Both males and females were used for studies.
Transgenic mice and bleomycin-induced fibrosis model
Generation of endothelial-cell specific Foxf1 heterozygous mice: Foxf1fl/fl mice16 were crossed with Pdgfb-CreERtg/− mice51 to generate Pdgfb-CreER/Foxf1fl/+ (abbreviated as endFoxf1+/−) mice in C57BL6 genetic background. To delete 1 allele of FOXF1 from endothelial cells, tamoxifen (3 mg; Sigma) was given as oral gavage on 2 consecutive days to 6–8 weeks old mice. Generation of TetO7-HA-mFoxf1tg/+ mice: The 5’- HA-tagged coding region of mouse Foxf1 gene in a Shuttle vector52,53 was subcloned into a TetO7-CMV vector. The 2 kb DNA fragment containing TetO7-CMV-HA-Foxf1 was microinjected into the pronucleus of fertilized mouse eggs. Transgenic mice were identified by PCR with primers flanking HA tag and exon 2 of Foxf1 gene (Supplementary Table 1). Generation of endothelial-cell specific FOXF1 overexpression mice: TetO7-HA-mFoxf1tg/+ mice were crossed with Pdgfb-CreERtg/−51 mice and Rosa26-LSL-rtTAtg/+ to generate Pdgfb-CreER/Rosa26-LSL-rtTA/TetO7-HA-mFoxf1 mice in C57BL6/129sv/FVB hybrid genetic background. To induce FOXF1 overexpression in endothelial cells, tamoxifen (3 mg; Sigma) was given as oral gavage on 2 consecutive days to 8–9 weeks old mice, followed by maintenance of mice on doxycycline chow for the duration of the experiment. To induce pulmonary fibrosis, mice were administered 2 or 2.5 U/kg of bleomycin sulfate (EMD Biosciences) intratracheally (I.T.) once a week for 3 weeks. The control group of mice (Uninjured) were administered PBS I.T. the same way as bleomycin.
10× Genomics single cell RNA-Seq data analysis
For CCHMC data, we have used donor (2 males and 1 female) and IPF (2 males) lung tissue samples. For NW data, we have re-analyzed the deposited scRNAseq data of lung tissue samples previously published by Reyfman et al.19 (Northwestern University (NW) data), focusing on individuals with IPF (3 males and 1 female) and donors without IPF (7 females and 1 male) and excluding samples from individuals with SSc-ILD, myositis and HP. Description of total number of samples was provided in Supplementary Table S4. For human lung tissue procurement, sample processing, and single-cell sequencing methods, we have used previously described methods19. Briefly, explanted lungs were acquired from donors with end-stage IPF lung disease undergoing transplant or from rejected control donor lungs. The explanted lungs were sliced and washed with cold sterile PBS. After visible vessels and airway structures were removed, lung tissues were mechanically minced, enzymatically digested, using an enzymatic cocktail containing 60 ml dispase (5 U/ml, Stemcell Technologies), 900 µl liberase (1 g/l, Sigma Aldrich), 0.03 g DNase (Sigma Aldrich) at 37 °C for 45 min. GEM generation and barcoding, and cDNA library preparation were completed by the CCHMC Gene Expression Core using the Chromium Single Cell 3’ Reagent Kit (10X Genomics version 2.0). Sequencing was performed on NovaSeq 6000 at a depth of 300–450 million reads. Read alignment and gene-expression quantification of data were performed using the CellRanger pipeline (10X Genomics version 2.1.1). Human scRNA-seq data was aligned to HG19 and mouse scRNA-seq data was aligned to mouse genome (mm10). Additional IPF scRNA-seq data was downloaded from GEO (https://www.ncbi.nlm.nih.gov/geo/; GSE122960). Cells with at least 500 expressed genes (UMI > 0) and less than 10% of UMIs mapping to mitochondrial genes were included for downstream analysis. Genes expressed in at least two cells in each dataset were included. Datasets were integrated with Harmony22 and downstream analyses were performed in R (version 3.6.1) using custom scripts, SINCERA54, and Seurat (version 3)55. The expression of a gene in a cell was measured by its UMI counts in the cell normalized by the total number of UMIs in the cell. Principal-component analysis was performed for dimension reduction. Reduced dimensions were used for cell cluster identification using the Jaccard-Louvain clustering algorithm56. Clusters were mapped to cell types based on the expression of known marker genes. Cluster-specific differentially expressed genes were identified using a binomial based differential expression test implemented in the SINCERA pipeline54. Genes with p value <0.05 and effect size >2 expressed in >20% of the cells in each cluster were considered significant. Transcription factors were mapped using “Signature comparison” in LGEA tool-box57 (https://research.cchmc.org/pbge/lunggens/tools/sigcomp.html/) and functional enrichment analyses were performed using Toppfun in ToppGene suite (https://toppgene.cchmc.org/).
Immunostaining and collagen content
Lung paraffin sections were used for H&E staining, and paraffin and frozen sections were used for immunofluorescence as previously described18. Lung tissue sections were from both female and male patients. For mouse experiments both males and females were included in equal numbers. The list of antibodies used for immunostaining is included in Supplementary Table S2. For immunofluorescence imaging, secondary antibodies conjugated with Alexa Fluor 488, Alexa Fluor 594, or Alexa Fluor 647 (Invitrogen/Molecular Probes) were used as described18,58. Cell nuclei were counterstained with DAPI. Images were obtained using a Nikon NIE upright CIC widefield microscope. Quantification of lung collagen content was performed using the Sircol collagen assay (Biocolor, Carrick Fergus, UK), Hydroxyproline assay (Jiancheng Bioengineering Institute, Nanjing, China), Sirius Red/Fast green (Chondrex, Inc. Redmond, WA, USA) and Masson’s trichome assay (Poly Scientific. Bay Shore, NY, USA) following the manufacturer’s protocols. Quantification of collagen from Sirius red and trichome staining was done using the Nikon NIE CIC Analysis Elements Workstation (NIS-Elements AR, advanced research,ver.5).
Cell lines, qRT-PCR, and western blot
Human endothelial HUVEC cells (Lonza, #C2519A) were cultured in EGM2/EBM2 (Lonza) growth medium, human Pulmonary Microvascular Endothelial Cells (HPMEC, ##CC2527) and human Pulmonary Artery Endothelial Cells (HPAEC, #CC2530) were cultured in ECM Medium (ScienCell). HUVEC, HPAEC and HPMEC cells were from pooled donors. Mouse endothelial MFLM-91U cells (Seven Hills, #AMFLM-91U) were cultured in DMEM (Gibco), human fibroblasts CCD-19Lu (ATCC, #CCL-210) were cultured in EMEM (ATCC), human macrophages (Lonza, #4W-700) were cultured in X-VIVO15,59 (Lonza). Human CCD-19Lu fibroblast cell line (ATCC) was isolated from a 20-year-old normal female lung. Mouse MFLM-91U Cell Line (Seven Hills) was isolated from murine fetal lung mesenchyme without specifying the sex. A cocktail of siRNAs was used to knockdown Foxf1 (Horizon, Cat# M-043272-00-0010) in MFLM-91U cells using Dharmafect transfection reagent 1 according to the manufacturer’s protocol (Dharmacon). A cocktail of non-targeting siRNAs was used as control (Horizon, Cat# D-001810-01-20). Cells were collected for RNA and protein extraction 48 h post transfection. In vitro rescue experiments were performed by dual transfection of siFoxf1 and CMV-Rras plasmid (Addgene plasmid # 102864) into MFLM-91U cells using Dharmafect Duo transfection reagent (Dharmacon). Empty plasmid and non-targeting siRNA were used as control. qRT-PCR was carried out using Taqman probes listed in Supplementary Table S1. Western blots were performed as previously described60 with antibodies against FOXF1 (1:500, R&D), α-Tubulin (sc-8035, 1:5000, Santa Cruz). For stable knockdown of FOXF1, the HUVEC, HPMEC and HPAEC were transduced with TRC Lentiviral Human FOXF1 shRNA (clone ID: TRCN0000013953, Horizon). GIPZ non-silencing lentiviral shRNA (Cat #: RHS4346, Horizon) was used as control.
RNAscope in situ hybridization assay
Assay was performed according to a protocol developed by Advanced Cell Diagnostics (ACD)61, using in situ probes designed by ACD, the RNAscope Multiplex Fluorescent Reagent Kit (v.2) and Opal dyes (Akoya Biosciences, 1:500 dilution for Opal 570 and 690 dyes, 1:1000 dilution for Opal 520 dyes). Nuclei were counterstain with DAPI, and tissue sections were mounted in Prolong Gold antifade reagent (Invitrogen). Proprietary (ACD) probes used were: Human, Hs-EDNRB(528301), Hs-EDN1 (459381), CLDN5-C2(517141-C2), Hs-FOXF1(505741-C3), Hs-CA4(438561), Hs-GJA5(471431), Hs-CPE(45410); Mouse, Mm-Foxf1(473051), Mm-Ednrb-C2(473801-C2), Mm-Aplnr-C2(436171-C2), Mm-Cldn5-C3(491611-C3), Mm-Car4-C2(468421-C2), Mm-Gja5-C2(518041-C2),Mm-Ptgds-C2(492781-C2). Tissue slides were photographed with a wide-field Nikon i90 or Nikon confocal microscope and quantified using the Nikon’s NIS-Elements AR (Advanced Research, ver. 5) software.
Cloning of the mouse Rras promoter region and luciferase assay
The mouse Rras promoter corresponding to the −762bp to 13bp region, was PCR amplified from C57/B6 gDNA using the following primers (5’ to 3’): Fwd. GTCTGAGCTATCAACCGCATCCTTC; Rev. TCATGTCGCCACCGCTGCTG. The promoter region was cloned into the Acc651 and XhoI sites of the pGL2 luciferase reporter plasmid (Promega). The dual-luciferase reporter assay was performed on Hek293T cells, co-transfected with the luciferase reporter and with a CMV-empty or CMV-Rras overexpression plasmid.
Flow cytometry
Flow cytometry experiments were conducted as previously described34 using antibodies listed in Supplementary Table S3. Stained cells were analyzed using FACSCanto II or LSR II (BD Biosciences). Stained cells were sorted using cell sorting (five-laser FACSAria II; BD Biosciences). Specific cell subsets were identified using flow cytometery markers recommended by American Thoracic Society:62 macrophages, CD45+ CD11clow/+ CD64+, neutrophils, CD45+ SSChi Ly6G+; monocytes, CD45+ CD11b+ CD64+ 63–65. Data analyses were performed using FlowJo Software version 10.8.0. and FACSDiva 9.0.
Transwell invasion assays
Invasion of human or mouse cells were performed as described previously37,66. Fibroblasts or macrophages were seeded on permeable transwell inserts with 8 mm pores or BioCoat Matrigel Invasion Chamber (BD), and cell invasion was measured in the presence of 10% FBS complete medium. At 48 h, the polycarbonate filters with the invaded cells were stained with crystal violet and counted in ten randomly selected fields.
Proteome profiler human cytokine array
The Proteome Profiler Human Cytokine Array kit (R&D Systems) was used. Conditioned medium was collected from scrambled (Scr) and shFOXF1 transfected HUVECs at 24 h after transfection.
RNA-seq analysis
Endothelial cells (CD45−CD31+) were FACS-sorted from bleomycin-treated control Foxf1fl/fl and endFoxf1+/− mouse lungs 14 days after bleomycin treatment. RNA was extracted and sent for sequencing. Differentially expressed genes were identified using DESeq with fold change >2 and an adjusted p value (FDR) < 0.01. The p value was calculated using Z test (Pearson’s chi-square test) in the Agilent GeneSpring GX suite. For GSEA analysis, pre-ranked gene list generated through Biowardrobe software67 and analyzed using GSEA software available from Broad Institute68.
Respiratory mechanics and arterial oxygenation
Mice were anesthetized on days 21 after first bleomycin administration. Measurements of the respiratory system mechanics were done using the flexiVent system (Scireq, Montreal, QC, Canada) as described69. Arterial oxygenation was measured using MouseOx + pulse oximeter (STARR Life Sciences) and analyzed using MouseOx Plus 1.6.X software. The MouseOx + photodiode sensor was placed on the shaved skin of the neck, and the measurements were taken for 10 min as described49,69. All data were analyzed using FlexiVent software (version 7.5).
Nanoparticle generation and delivery of plasmid
The PBAEs were synthesized using two-step Aza-Michael addition synthesis. First, to generate the PBAE backbone, the Bisphenol A glycerolate diacrylate and 6-amino-1-hexanol (Sigma Aldrich) were dissolved in DMSO at a molar of 1.15 :1 for 23 hours at 90 °C. Next, the PBAE backbone was end-capped with PEI and modified using PDFO at 40 °C for 20 h. Before use, the PBAE polymer was lyophilized to remove the DMSO and dissolved in 25 mM HEPES buffer (pH = 7.4). To label the PBAE nanoparticle, the DyLight 650 NHS ester (ThermoFisher Scientific) was mixed with the nanoparticle at a mass ratio of 1: 100. Foxf1 plasmid was generated by cloning DNA (3HA-RFP-Foxf) into the Enhanced Episomal Vectors (EEV) empty plasmid vectors (SBI). The PBAE polymer (300 µg) were used to encapsulate 40 µg of plasmid DNA (EEV-Foxf1 or EEV-empty). The size distribution and the surface potential distribution were determined by Dynamic light scattering (DLS). Nanoparticles (250 µl) were delivered to mice via tail vein or eye vein on day 0 or day 7 after bleomycin administration.
Statistics and reproducibility
Statistical significance differences in measured variables between experimental and control groups were assessed by Student’s t test (two-tailed), non-parametric Mann–Whitney U test or one-way ANOVA followed by Dunnett’s test. The two-way ANOVA test was used for two group comparison and multi-timepoint experiments. P-values <0.05 were considered significant. Values for all measurements were expressed as the mean ± standard deviation (SD). Statistical analysis was performed, and data were graphically displayed using GraphPad Prism vs 9.0 for Windows (GraphPad Software, Inc., San Diego, CA). All experiments in this study have been repeated independently with similar result more than 3 times.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work was supported by the NIH grants R01 HL132849 (T.V.K.), R01 HL158659 (T.V.K.), R01 HL141174 (V.V.K.), R01 HL149631 (V.V.K.), R01 HL152973 (V.V.K./T.V.K.), R01 HL153045 (Y.X.), U01HL148856 (Y.X.).
Source data
Author contributions
F.B. and T.V.K. conceived and designed the study. F.B., Y.W.L., J.D., S.S. and T.L. performed the in vivo experiments. F.B., A.A., D.M. and Y.W.L. completed cell culture experiments. Y.X. and S.Z. performed the sc-RNA-seq and RNA-seq bioinformatics analysis. Z.D. and D.S. synthesized nanoparticles. F.B. and T.V.K. analyzed data. F.B., Y.X., V.V.K., and T.V.K. interpreted the data. V.V.K., M.B., A.E.H., Y.T., D.S. and Y.W.C. provided critical reagents and intellectual discussions. T.V.K. wrote the paper. All authors discussed the data. T.V.K. approved the submission of the manuscript.
Peer review
Peer review information
Nature Communications thanks Omar Khan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Data availability
All the raw sequence data generated in this study is deposited in GEO database (accession number GSE213018). Single cell RNA sequencing data generated in this study from donor and IPF patient lungs (Supplementary Fig. S4) were deposited in GEO database (accession number GSE213017). Single cell RNA sequencing data generated in this study from bleomycin-treated and untreated adult mice lung were deposited in GEO database (accession number GSE213016). Single cell RNA sequencing data of Northwestern University (NW) datasets from donor and IPF lungs were retrieved from GEO database (GSE122960 https://www.ncbi.nlm.nih.gov/geo/). Bulk RNA sequencing data generated in this study from bleomycin-treated control and endFoxf1+/− mouse lung endothelial cells were also deposited in GEO database (GSM6578251 and GSM6578252). Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-38177-2.
References
- 1.Travis WD, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghu G, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 3.Crossno PF, et al. Identification of early interstitial lung disease in an individual with genetic variations in ABCA3 and SFTPC. Chest. 2010;137:969–973. doi: 10.1378/chest.09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nogee LM, et al. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N. Engl. J. Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 5.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J. Clin. Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams TS, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci. Adv. 2020;6:eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habermann AC, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv. 2020;6:eaba1972. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanumegowda C, Farkas L, Kolb M. Angiogenesis in pulmonary fibrosis: too much or not enough? Chest. 2012;142:200–207. doi: 10.1378/chest.11-1962. [DOI] [PubMed] [Google Scholar]
- 9.Plantier L, et al. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018;27:170062. doi: 10.1183/16000617.0062-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin JD, Seano G, Jain RK. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu Rev. Physiol. 2019;81:505–534. doi: 10.1146/annurev-physiol-020518-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalinichenko VV, et al. Defects in pulmonary vasculature and perinatal lung hemorrhage in mice heterozygous null for the forkhead box f1 transcription factor. Dev. Biol. 2001;235:489–506. doi: 10.1006/dbio.2001.0322. [DOI] [PubMed] [Google Scholar]
- 13.Bishop NB, Stankiewicz P, Steinhorn RH. Alveolar capillary dysplasia. Am. J. Respir. Crit. Care Med. 2011;184:172–179. doi: 10.1164/rccm.201010-1697CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dharmadhikari AV, Szafranski P, Kalinichenko VV, Stankiewicz P. Genomic and epigenetic complexity of the FOXF1 locus in 16q24.1: implications for development and disease. Curr. Genom. 2015;16:107–116. doi: 10.2174/1389202916666150122223252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milewski D, et al. FOXF1 is required for the oncogenic properties of PAX3-FOXO1 in rhabdomyosarcoma. Oncogene. 2021;40:2182–2199. doi: 10.1038/s41388-021-01694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ren X, et al. FOXF1 transcription factor is required for formation of embryonic vasculature by regulating VEGF signaling in endothelial cells. Circ. Res. 2014;115:709–720. doi: 10.1161/CIRCRESAHA.115.304382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang G, et al. Endothelial progenitor cells stimulate neonatal lung angiogenesis through FOXF1-mediated activation of BMP9/ACVRL1 signaling. Nat. Commun. 2022;13:2080. doi: 10.1038/s41467-022-29746-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai Y, et al. FOXF1 maintains endothelial barrier function and prevents edema after lung injury. Sci. Signal. 2016;9:ra40. doi: 10.1126/scisignal.aad1899. [DOI] [PubMed] [Google Scholar]
- 19.Reyfman PA, et al. Single-cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019;199:1517–1536. doi: 10.1164/rccm.201712-2410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and regenerating the lung cell by cell. Physiol. Rev. 2019;99:513–554. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalucka J, et al. Single-cell transcriptome atlas of murine endothelial cells. Cell. 2020;180:764–79.e20. doi: 10.1016/j.cell.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Korsunsky I, et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods. 2019;16:1289–1296. doi: 10.1038/s41592-019-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, et al. A census of the lung: CellCards from LungMAP. Dev. Cell. 2022;57:112–45.e2. doi: 10.1016/j.devcel.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schupp JC, et al. Integrated single-cell atlas of endothelial cells of the human lung. Circulation. 2021;144:286–302. doi: 10.1161/CIRCULATIONAHA.120.052318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolte C, et al. FOXF1 transcription factor promotes lung regeneration after partial pneumonectomy. Sci. Rep. 2017;7:10690. doi: 10.1038/s41598-017-11175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat. Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawada J, et al. Small GTPase R-Ras regulates integrity and functionality of tumor blood vessels. Cancer Cell. 2012;22:235–249. doi: 10.1016/j.ccr.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li F, Sawada J, Komatsu M. R-Ras-Akt axis induces endothelial lumenogenesis and regulates the patency of regenerating vasculature. Nat. Commun. 2017;8:1720. doi: 10.1038/s41467-017-01865-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Probst CK, Montesi SB, Medoff BD, Shea BS, Knipe RS. Vascular permeability in the fibrotic lung. Eur. Respir. J. 2020;56:1900100. doi: 10.1183/13993003.00100-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaczmarek JC, et al. Optimization of a degradable polymer-lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells. Nano Lett. 2018;18:6449–6454. doi: 10.1021/acs.nanolett.8b02917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaczmarek JC, et al. Systemic delivery of mRNA and DNA to the lung using polymer-lipid nanoparticles. Biomaterials. 2021;275:120966. doi: 10.1016/j.biomaterials.2021.120966. [DOI] [PubMed] [Google Scholar]
- 32.Carraro G, et al. Single-cell reconstruction of human basal cell diversity in normal and idiopathic pulmonary fibrosis lungs. Am. J. Respir. Crit. Care Med. 2020;202:1540–1550. doi: 10.1164/rccm.201904-0792OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paik DT, et al. Single-cell RNA sequencing unveils unique transcriptomic signatures of organ-specific endothelial cells. Circulation. 2020;142:1848–1862. doi: 10.1161/CIRCULATIONAHA.119.041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalin TV, et al. Pulmonary mastocytosis and enhanced lung inflammation in mice heterozygous null for the Foxf1 gene. Am. J. Respir. Cell Mol. Biol. 2008;39:390–399. doi: 10.1165/rcmb.2008-0044OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren X, et al. Postnatal alveologenesis depends on FOXF1 signaling in c-KIT(+) endothelial progenitor cells. Am. J. Respir. Crit. Care Med. 2019;200:1164–1176. doi: 10.1164/rccm.201812-2312OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pradhan A, et al. The S52F FOXF1 mutation inhibits STAT3 signaling and causes alveolar capillary dysplasia. Am. J. Respir. Crit. Care Med. 2019;200:1045–1056. doi: 10.1164/rccm.201810-1897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Black M, et al. FOXF1 inhibits pulmonary fibrosis by preventing CDH2-CDH11 cadherin switch in myofibroblasts. Cell Rep. 2018;23:442–458. doi: 10.1016/j.celrep.2018.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Vuori K, Wang H, Reed JC, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki J, Kaziro Y, Koide H. An activated mutant of R-Ras inhibits cell death caused by cytokine deprivation in BaF3 cells in the presence of IGF-I. Oncogene. 1997;15:1689–1697. doi: 10.1038/sj.onc.1201333. [DOI] [PubMed] [Google Scholar]
- 40.Sawada J, Li F, Komatsu M. R-Ras protein inhibits autophosphorylation of vascular endothelial growth factor receptor 2 in endothelial cells and suppresses receptor activation in tumor vasculature. J. Biol. Chem. 2015;290:8133–8145. doi: 10.1074/jbc.M114.591511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, et al. VEGFR2 pY949 signalling regulates adherens junction integrity and metastatic spread. Nat. Commun. 2016;7:11017. doi: 10.1038/ncomms11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 43.Vahatupa M, et al. Lack of R-Ras leads to increased vascular permeability in ischemic retinopathy. Invest. Ophthalmol. Vis. Sci. 2016;57:4898–4909. doi: 10.1167/iovs.16-19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gschwandtner M, Derler R, Midwood KS. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front. Immunol. 2019;10:2759. doi: 10.3389/fimmu.2019.02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Q, Guan J, Qin L, Zhang X, Mao S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Disco. Today. 2020;25:150–159. doi: 10.1016/j.drudis.2019.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Deng Z, Kalin GT, Shi D, Kalinichenko VV. Nanoparticle delivery systems with cell-specific targeting for pulmonary diseases. Am. J. Respir. Cell Mol. Biol. 2021;64:292–307. doi: 10.1165/rcmb.2020-0306TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunn AW, Kalinichenko VV, Shi D. Highly efficient in vivo targeting of the pulmonary endothelium using novel modifications of polyethylenimine: an importance of charge. Adv. Healthc. Mater. 2018;7:e1800876. doi: 10.1002/adhm.201800876. [DOI] [PubMed] [Google Scholar]
- 48.Bolte C, et al. Nanoparticle delivery of proangiogenic transcription factors into the neonatal circulation inhibits alveolar simplification caused by hyperoxia. Am. J. Respir. Crit. Care Med. 2020;202:100–111. doi: 10.1164/rccm.201906-1232OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun F, et al. Nanoparticle delivery of STAT3 alleviates pulmonary hypertension in a mouse model of alveolar capillary dysplasia. Circulation. 2021;144:539–555. doi: 10.1161/CIRCULATIONAHA.121.053980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pradhan, A. et al. Novel FOXF1-stabilizing compound tanfe stimulates lung angiogenesis in alveolar capillary dysplasia. Am. J. Respir. Crit. Care Med.207, 1042–1054 (2023). [DOI] [PMC free article] [PubMed]
- 51.Claxton S, et al. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- 52.Bolte C, et al. Forkhead box F2 regulation of platelet-derived growth factor and myocardin/serum response factor signaling is essential for intestinal development. J. Biol. Chem. 2015;290:7563–7575. doi: 10.1074/jbc.M114.609487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoggatt AM, et al. The transcription factor Foxf1 binds to serum response factor and myocardin to regulate gene transcription in visceral smooth muscle cells. J. Biol. Chem. 2013;288:28477–28487. doi: 10.1074/jbc.M113.478974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo M, Wang H, Potter SS, Whitsett JA, Xu Y. SINCERA: a pipeline for single-cell RNA-seq profiling analysis. PLoS Comput Biol. 2015;11:e1004575. doi: 10.1371/journal.pcbi.1004575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018;36:411–420. doi: 10.1038/nbt.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shekhar K, et al. Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell. 2016;166:1308–23.e30. doi: 10.1016/j.cell.2016.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Du Y, et al. Lung gene expression analysis web portal version 3: lung-at-a-glance. Am. J. Respir. Cell Mol. Biol. 2021;64:146–149. doi: 10.1165/rcmb.2020-0308LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shukla S, et al. The FOXM1 inhibitor RCM-1 decreases carcinogenesis and nuclear beta-catenin. Mol. Cancer Ther. 2019;18:1217–1229. doi: 10.1158/1535-7163.MCT-18-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goda C, et al. Loss of FOXM1 in macrophages promotes pulmonary fibrosis by activating p38 MAPK signaling pathway. PLoS Genet. 2020;16:e1008692. doi: 10.1371/journal.pgen.1008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Milewski D, et al. FOXM1 activates AGR2 and causes progression of lung adenomas into invasive mucinous adenocarcinomas. PLoS Genet. 2017;13:e1007097. doi: 10.1371/journal.pgen.1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang F, et al. RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J. Mol. Diagn. 2012;14:22–29. doi: 10.1016/j.jmoldx.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tighe RM, et al. Improving the quality and reproducibility of flow cytometry in the lung. An Official American Thoracic Society Workshop Report. Am. J. Respir. Cell Mol. Biol. 2019;61:150–161. doi: 10.1165/rcmb.2019-0191ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ren X. Forkhead Box M1 transcription factor is required for macrophage recruitment during liver repair. Mol. Cell. Biol.30, 5381–5393 (2010). [DOI] [PMC free article] [PubMed]
- 64.Ren X, et al. FOXM1 promotes allergen-induced goblet cell metaplasia and pulmonary inflammation. Mol. Cell Biol. 2013;33:371–386. doi: 10.1128/MCB.00934-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaynagetdinov R, et al. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am. J. Respir. Cell Mol. Biol. 2013;49:180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Black M, et al. FOXM1 nuclear transcription factor translocates into mitochondria and inhibits oxidative phosphorylation. Mol. Biol. Cell. 2020;31:1411–1424. doi: 10.1091/mbc.E19-07-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vallabh S, Kartashov AV, Barski A. Analysis of ChIP-Seq and RNA-Seq Data with BioWardrobe. Methods Mol. Biol. 2018;1783:343–360. doi: 10.1007/978-1-4939-7834-2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang G, et al. Generation of pulmonary endothelial progenitor cells for cell-based therapy using interspecies mouse-rat chimeras. Am. J. Respir. Crit. Care Med. 2021;204:326–338. doi: 10.1164/rccm.202003-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the raw sequence data generated in this study is deposited in GEO database (accession number GSE213018). Single cell RNA sequencing data generated in this study from donor and IPF patient lungs (Supplementary Fig. S4) were deposited in GEO database (accession number GSE213017). Single cell RNA sequencing data generated in this study from bleomycin-treated and untreated adult mice lung were deposited in GEO database (accession number GSE213016). Single cell RNA sequencing data of Northwestern University (NW) datasets from donor and IPF lungs were retrieved from GEO database (GSE122960 https://www.ncbi.nlm.nih.gov/geo/). Bulk RNA sequencing data generated in this study from bleomycin-treated control and endFoxf1+/− mouse lung endothelial cells were also deposited in GEO database (GSM6578251 and GSM6578252). Source data are provided with this paper.