Abstract
Introduction
Potentially inappropriate medications (PIMs) cause adverse events and death. We evaluate the Care Ecosystem collaborative dementia care program on medication use among community-dwelling persons living with dementia (PLWD).
Methods
Secondary analysis of a randomized clinical trial comparing Care Ecosystem to usual care on changes in PIMs, over 12 months between March 2015 and May 2020. Secondary outcomes included change in number of medications, clinically relevant PIMs and anti-dementia medications.
Results:
Of 804 PLWD, N=490 had complete medication data. Care Ecosystem resulted in significantly fewer PIMs compared to usual care (−0.35; 95% CI, −0.49 to −0.20; P <.0001). Number needed to prevent an increase in 1 PIM was 3. Total medications, PIMs for dementia or cognitive impairment, CNS-active PIMs, anticholinergics, benzodiazepines and opioids were also fewer. Anti-dementia medication regimens were modified more frequently.
Conclusion:
The Care Ecosystem medication review intervention embedded in collaborative dementia care optimized medication use among PLWD.
Trial Registration:
ClinicalTrials.gov identifier: NCT02213458
Keywords: dementia, potentially inappropriate medications, polypharmacy, anti-dementia medications, medication review, pharmacist
1. INTRODUCTION
Potentially inappropriate medication (PIM) use and polypharmacy are highly prevalent (14–74%) and dangerous among persons living with dementia (PLWD), leading to worsening cognition, adverse drug events, and death.[1–8] While safe and effective medication use is a goal for all patients, PLWD routinely require more attention and closer monitoring given complex comorbid conditions, behavioral and psychological symptoms of dementia (BPSD), and evolving symptoms with disease progression.[9] However, dementia care and medication prescribing is frequently suboptimal because it is reactive, crisis-oriented, fragmented, and focused on deficits and losses.[10] Consistent medication oversight is often lacking, while safer medication alternatives and non-drug treatments are not reliably trialed. As a result, PIMs use and polypharmacy contribute to substantial medical, psychological, and financial challenges for PLWD, their caregivers, and the healthcare system.[11–13]
Efficacy and safety of medications can be affected by comorbidities, age-related physiologic changes involving drug pharmacokinetics and pharmacodynamics, and communication challenges between providers, PLWD, and caregivers.[13] Proactive medication management requires collaboration and continuity. Pharmacist-led medication reviews with recommendations sent directly to prescribing providers have been shown to reduce medication-related problems, inappropriate prescribing, and facilitate deprescribing efforts.[14–19] Medication recommendations include minimizing or avoiding the use of anticholinergics,[6, 8, 20, 21] antipsychotics,[1, 8, 22] sedatives,[8, 20, 22] and other central nervous system (CNS)-active drugs,[8, 22] all of which can worsen cognition, increase fall-related injuries, and increase risk of mortality in PLWD.[22, 23] More research on the impact of PIMs and polypharmacy on clinical and patient important outcomes as well as successful interventions to optimize medications for PLWD are needed.[13]
The Care Ecosystem is a collaborative dementia care program that improved quality of life, reduced emergency department use among PLWD and improved caregiver well-being in a randomized clinical trial[24] and is among the most effective dementia care interventions.[25] A unique feature of the Care Ecosystem is a medication review intervention that aims to monitor and reduce inappropriate or problematic medications, enhance PLWD, caregiver, and healthcare provider knowledge about the patient’s medications, and provide strategies to optimize medication outcomes. We report for the first time the effectiveness of the Care Ecosystem medication review intervention aimed at reducing PIMs and polypharmacy among PLWD.
2. METHODS
2.1. Study Design
Secondary analysis of a multicenter, single-blind, randomized clinical trial (RCT) comparing a telephone-based collaborative dementia care program, the Care Ecosystem (CE), with usual care (UC) delivered over 12 months for community-dwelling PLWD-caregiver dyads. Design, protocol, and primary outcomes of the Care Ecosystem clinical trial have been published.[24] Enrollment occurred between March 20, 2015 and May 16, 2019, with a last follow-up date of May 16, 2020. All study procedures and consent materials were approved by the Institutional Review Boards at the University of California, San Francisco and University of Nebraska Medical Center. Dyads, including PLWD, or their legally authorized representative, and caregivers provided written informed consent prior to participating in the trial.
2.2. Study Participants
Eight hundred and four PLWD-caregiver dyads were enrolled in the Care Ecosystem trial, where 780 were initially randomized and 24 additional participants were randomized in an extension of the trial (Supplement Table 1). All participants were randomized (2:1) to receive the Care Ecosystem medication review intervention or usual care over 12 months. To be eligible, participants had to be 45 years or older; have a diagnosis of dementia; reside in California, Nebraska, or Iowa; speak English, Spanish, or Cantonese; have Medicare or Medicaid insurance; and have a primary caregiver. Demographic, medication, and survey data was collected prospectively by telephone in the participant’s preferred language (English, Spanish, or Cantonese). We excluded participants with missing medication lists (no medical record medication list was available within 6 months of baseline or 12-month follow up dates) and participants who were enrolled in hospice, died, withdrew, or no longer met eligibility criteria.
2.3. Medication Data Sources
Comprehensive medication data were obtained from retrospective chart review of routinely collected medical records. We collected data on the total number of medications listed in the medical record, including prescription and over-the-counter medications, dietary supplements and herbal products, that were scheduled or taken as needed. We collected baseline and follow-up (12-month) medication lists for participants with complete data sets. Medication lists were collected close to participants’ baseline dates (mean difference 22.1 days +/− 32.9 days) and follow-up dates (mean difference 28.6 days ± 34.6 days), and the mean time between baseline and follow-up medication lists was 12 months (mean = 12.4 +/− 1.8 months). There were no differences in the timing or method of medication list ascertainment by treatment group.
2.4. Intervention
The Care Ecosystem medication review intervention was delivered by telephone over 12 months by an interprofessional care team that included unlicensed care team navigators (CTNs) with training in dementia as the primary point of contact, and licensed dementia specialists (pharmacist, advanced practice nurse, and social worker) who provided supervision and direct consultation to the dyad as needed.
The medication review intervention was protocol-guided and supported by a cloud-based database with integrated software from mHealthCoach© and Salesforce© that was customized to support patient management and current medication information. The care team assisted with proactive medication monitoring by tracking and reducing inappropriate or problematic medication use; optimizing anti-dementia medications; enhancing participant, caregiver, and prescribing provider knowledge about the PLWD’s medications; and recommending strategies to optimize medication therapy with prescribing providers (Figure 2 and Figure 3).[24, 26]
Figure 2.
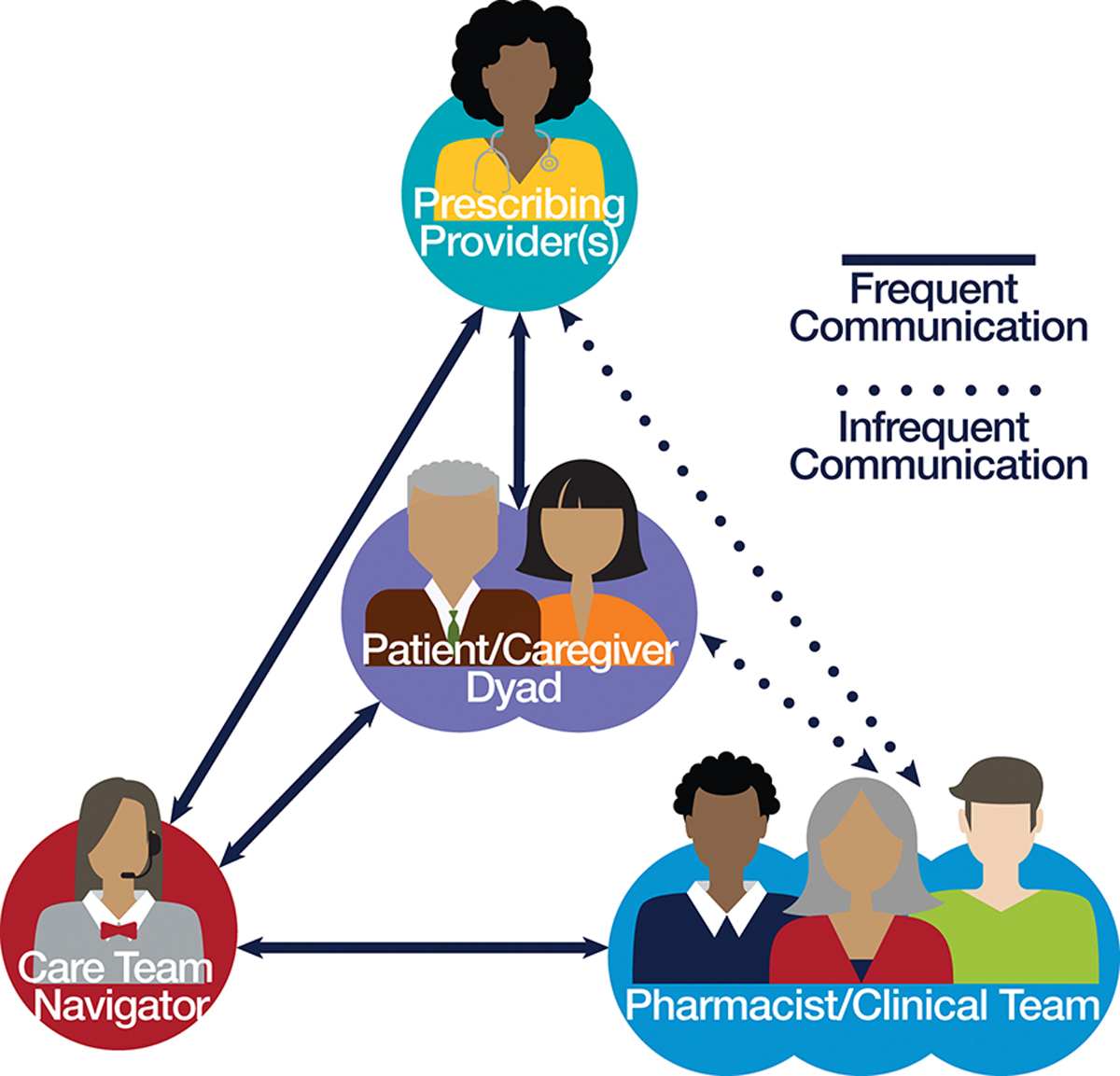
The Care Ecosystem Medication Review Intervention and Monitoring Process
Figure 3.
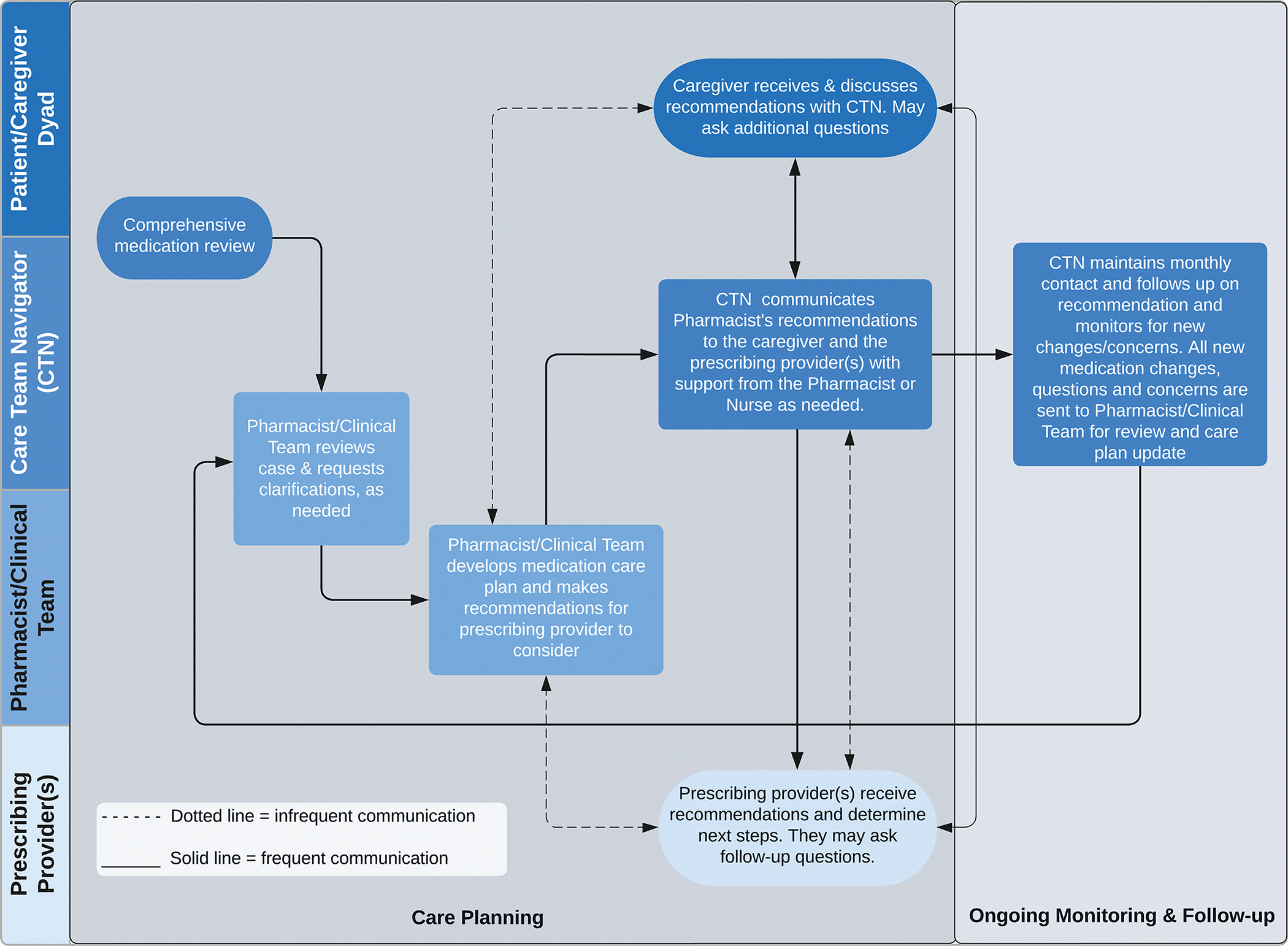
Flowchart of the Care Ecosystem Medication Review Intervention and Monitoring Process
After randomization to the Care Ecosystem, dyads were assigned a CTN who obtained a comprehensive medication history during initial care planning and recorded it in the study database. A pharmacist or trained clinician then reviewed the participant’s active medications within the database, including prescription and over-the-counter medications, dietary supplements, and herbal products. On average, CTNs followed up monthly to screen for medication changes, questions, side effects, adverse drug events, lack of efficacy, and other medication-related problems. When a PLWD experienced major care transitions, such as a hospitalization, rehabilitation stay, or long-term care placement, CTNs repeated a full comprehensive medication review.
The care team discussed medication use, medical history and relevant vitals and labs with the dyad and reviewed medical records when available. Personalized medication care plans were collaboratively developed by the care team and included a current list of medications and dosing calendar along with drug education handouts and the pharmacist’s counseling and recommendations. Drug education handouts included general information on medication adherence, safety and costs, an overview of anti-dementia medications, and medications to avoid that can worsen memory or cognition. Counseling consisted of medication use instructions, monitoring parameters for safety and efficacy of medications, and addressing adherence issues or costs. Recommendations included strategies to optimize medication therapy, such as stopping or starting medications, adjusting doses, or substituting unnecessary, potentially inappropriate, or high-risk medications for safer alternatives or non-pharmacological strategies. Medication care plans were sent by the CTNs directly to the dyad and to the PLWD’s primary care provider or other prescribing providers. The dyad was encouraged to review the personalized medication care plan with their providers. Medication reviews and care plans were completed following enrollment (months 0–3) and again when there were medication changes during transitions of care, or if the dyad reported changes or had questions about their medications. The care team did not prescribe nor deprescribe medications for PLWDs or document in the medical record.
Participants in usual care were sent quarterly newsletters with general dementia-related articles, and received contact information for national caregiver, Alzheimer’s, and aging associations. For the duration of the clinical trial, all participants continued to receive health care and services from their usual healthcare providers.
2.5. Potentially Inappropriate Medications
PIMs were defined by the American Geriatrics Society 2019 Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults,[23] an explicit tool for identifying medications where risks may outweigh benefits for adults aged 65 and over. We included 33 participants < 65 years because adverse effects and risks with PIMs are concerning among persons with dementia regardless of age (e.g., anticholinergics, benzodiazepines, antipsychotics, opioids).
PIMs were coded according to the 2020 edition of American Hospital Formulary Service Drug Information (AHFS DI®) Essentials™ therapeutic class. (A full list of PIMs drug classes is included in Supplement Table 2). We also evaluated PIMs for dementia or cognitive impairment, and CNS-active drugs to be avoided in persons with dementia, delirium, or a history of falls and fractures according to the 2019 Beers Criteria.[23] Due to an increased risk of cognitive decline, medications with anticholinergic properties were identified using the Anticholinergic Cognitive Burden (ACB) Scale.[27] The ACB Scale produced a summative score for medications with no (score of 0), possible (score of 1), and definite (score of 2 or 3) anticholinergic effects. The ACB score for each participant was computed by summing these values for each recorded medication. Some medications belonged to more than one of these categories.
Outcome Assessments
The primary outcome was the change in the number of PIMs obtained from medical records, and evaluated from baseline to follow-up. Prespecified secondary outcomes included change in the total number of medications, PIMs to be avoided in dementia or cognitive impairment[23], CNS-active drugs to be avoided in persons with dementia, delirium, or a history of falls and fractures[23], summative ACB Scale score[27] and number of prescriptions for antipsychotics, benzodiazepines, and opioids. We also included a binary indicator of whether or not a participant’s use of anti-dementia medications changed from baseline (i.e., started, stopped or changed treatment with an acetylcholinesterase inhibitor, donepezil, galantamine, rivastigmine and/or NMDA receptor antagonist, memantine).
Statistical Analysis
Descriptive statistics were used to summarize participant demographics, clinical characteristics, and medication exposures at baseline. Participants were analyzed by the group to which they had been randomized after excluding participants according to criteria above (complete case analysis, N=490). To determine if the complete case analysis sample differed from the total RCT population (N=804) at baseline in demographic (age, gender, race, ethnicity, region of residence, educational level, baseline dementia stage, number of comorbidities) and outcome variables (number of medications and PIMs), we conducted a chi-square goodness-of-fit test. Variables with continuous data were dichotomized at the mean, median, or none versus one or more. There were no significant differences between any demographic or outcome variables (all p>0.05). In order to examine whether the missing data for medications followed a pattern based on covariates or intervention groups, an analysis using Little’s MCAR test was applied.[28] The P value from this analysis, including all covariates, was not significant (P = 0.211) including all covariates for medications, demographics, and clinical data. Sensitivity analyses including separate groups of covariates all had higher P values than the overall MCAR test. This indicates that we can conclude no patterns existed and the missing data are missing completely at random.
Analysis of covariance (ANCOVA) was used to evaluate change from baseline to follow up in the mean number of PIMs prescriptions between groups for our primary and secondary outcomes. Baseline values of the medication outcome variable were included as a covariate in all analyses.[29, 30] We also adjusted for age, gender, region of residence, baseline dementia stage[31], and total number of comorbidities (Table 1). Comorbidities were defined by the Charlson Comorbidity Index[32] along with depression, hypercholesterolemia, hypertension, vascular disease, history of pneumonia, or other self-reported comorbidity.[33, 34] All P values were 2-sided. The primary analysis test of significance was conducted with a threshold of .05. P values for secondary medication outcomes analyses were unadjusted for multiple comparisons. We examined the distribution of the variables and, as a sensitivity analysis, transformed non-normally distributed variables (participant age in years squared and baseline dementia stage square rooted). There were no differences between the models with untransformed and transformed variables. Untransformed results are reported. To evaluate anti-dementia medication use, adjusted odds ratios were calculated using the proportion of participants in each group who changed their use of anti-dementia medications at follow-up. Appropriate medication adjustments can include starting or adding anti-dementia medications if indicated or stopping because of side effects/lack of benefit. Statistical analyses were carried out using STATA V.14 (StatCorp, College Station, TX, USA).
Table 1.
Baseline participant demographics, clinical characteristics, and medication exposures of persons living with dementia
| Characteristica | Care Ecosystem | Usual Care |
|---|---|---|
| Persons living with dementia | N = 304 | N = 186 |
| Age, years, mean (SD) | 77.4 (8.6) | 76.5 (9.8) |
| Female | 176 (57.9) | 90 (48.4) |
| Race | ||
| White | 243 (79.9) | 161 (86.6) |
| African American | 15 (4.9) | 5 (2.7) |
| Asian | 21 (6.9) | 10 (5.4) |
| Other or mixed | 4 (1.3) | 1 (0.5) |
| Not reported | 21 (6.9) | 9 (4.8) |
| Ethnicity | ||
| Not Hispanic or Latinx | 272 (89.4) | 167 (89.8) |
| Hispanic or Latinx | 30 (9.9) | 19 (10.2) |
| Not reported | 2 (0.7) | 0 |
| Preferred Language | ||
| English | 282 (92.8) | 180 (96.8) |
| Spanish | 14 (4.6) | 4 (2.2) |
| Cantonese | 8 (2.6) | 2 (1.0) |
| Region of Residence | ||
| California | 181 (59.5) | 106 (57.0) |
| Nebraska/Iowa | 123 (40.5) | 80 (43.0) |
| Educational Level | ||
| College graduate or higher | 156 (51.3) | 105 (56.4) |
| Some college | 61 (20.1) | 34 (18.3) |
| High school graduate | 59 (19.4) | 40 (21.5) |
| Less than high school | 28 (9.2) | 7 (3.8) |
| Dementia Stageb | ||
| Mild | 182 (59.9) | 110 (59.1) |
| Moderate | 93 (30.6) | 55 (29.6) |
| Advanced | 29 (9.5) | 21 (11.3) |
| Number of comorbiditiesc, mean (SD) | 2.8 (1.90) | 2.6 (1.8) |
| Medication exposures | ||
| Number of medicationsd, mean (SD) | 10.4 (5.2) | 10.3 (5.0) |
| ≥5 medications | 271 (89) | 167 (90) |
| ≥10 medications | 158 (52) | 92 (49) |
| Number of potentially inappropriate medicationsd, mean (SD) | 1.5 (1.6) | 1.4 (1.5) |
| PIMs for dementia or cognitive impairmente, mean (SD) | 0.4 (0.8) | 0.4 (0.7) |
| CNS-active PIMse, mean (SD) | 1.4 (1.4) | 1.3 (1.3) |
| Anticholinergic Cognitive Burden Scale scoref, mean (SD) | 1.6 (2.0) | 1.4 (1.6) |
| 0 | 114 (37.5) | 71 (38.2) |
| 1 | 75 (24.7) | 47 (25.3) |
| ≥ 2 | 115 (37.8) | 68 (36.5) |
| Antipsychoticse, mean (SD) | 0.2 (0.4) | 0.2 (0.4) |
| Benzodiazepinese, mean (SD) | 0.1 (0.3) | 0.1 (0.3) |
| Opioidse, mean (SD) | 0.2 (0.5) | 0.2 (0.4) |
Unless otherwise indicated, data are the number (percentage) of study participants in the specified category.
Dementia stage was based on the Quick Dementia Rating Scale using cut points that have been validated to correspond to Clinical Dementia Rating Scale scores of 1 or less for mild, 2 for moderate, and 3 for advanced or severe.[31]
Self-reported comorbidities were summarized by participant across 16 medical comorbidities.
Total number of medications includes prescription and over-the-counter medications, dietary supplements and herbal products, that are scheduled or taken as needed.
Potentially inappropriate medications (PIMs) were defined using American Geriatrics Society 2019 Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. CNS-active PIMs include PIMs for persons with dementia or cognitive impairment, delirium, or history of falls or fractures were also defined by the 2019 Beers Criteria and include antipsychotics, benzodiazepines and opioids.[23]
Medications with anticholinergic properties were defined using the Anticholinergic Cognitive Burden (ACB) Scale.[27] ACB scores were summarized by participant for each anticholinergic medication prescribed.
3. RESULTS
3.1. Study Participants
Among 804 PLWD enrolled in the Care Ecosystem trial, 527 were randomized to receive Care Ecosystem and 277 were randomized to receive usual care (Figure 1). A total of 490 participants (304 [58%] CE; 186 [67%] UC) had medical record medication lists available for analysis at baseline and follow-up (average 12 months). Reasons for exclusion included missing medication lists (153 [29%] CE participants; 64 [23%] UC participants) or hospice medication lists (2 [0.4%] CE; 4 [1%] UC). Of participants who had baseline medication lists available, participants who died (47 [10%] CE; 20 [8%] UC), withdrew (14 [3%] CE; 2 [0.8%] UC), and no longer met eligibility criteria (7 [1%] CE; 1 [0.4%] UC) were also excluded.
Figure 1.
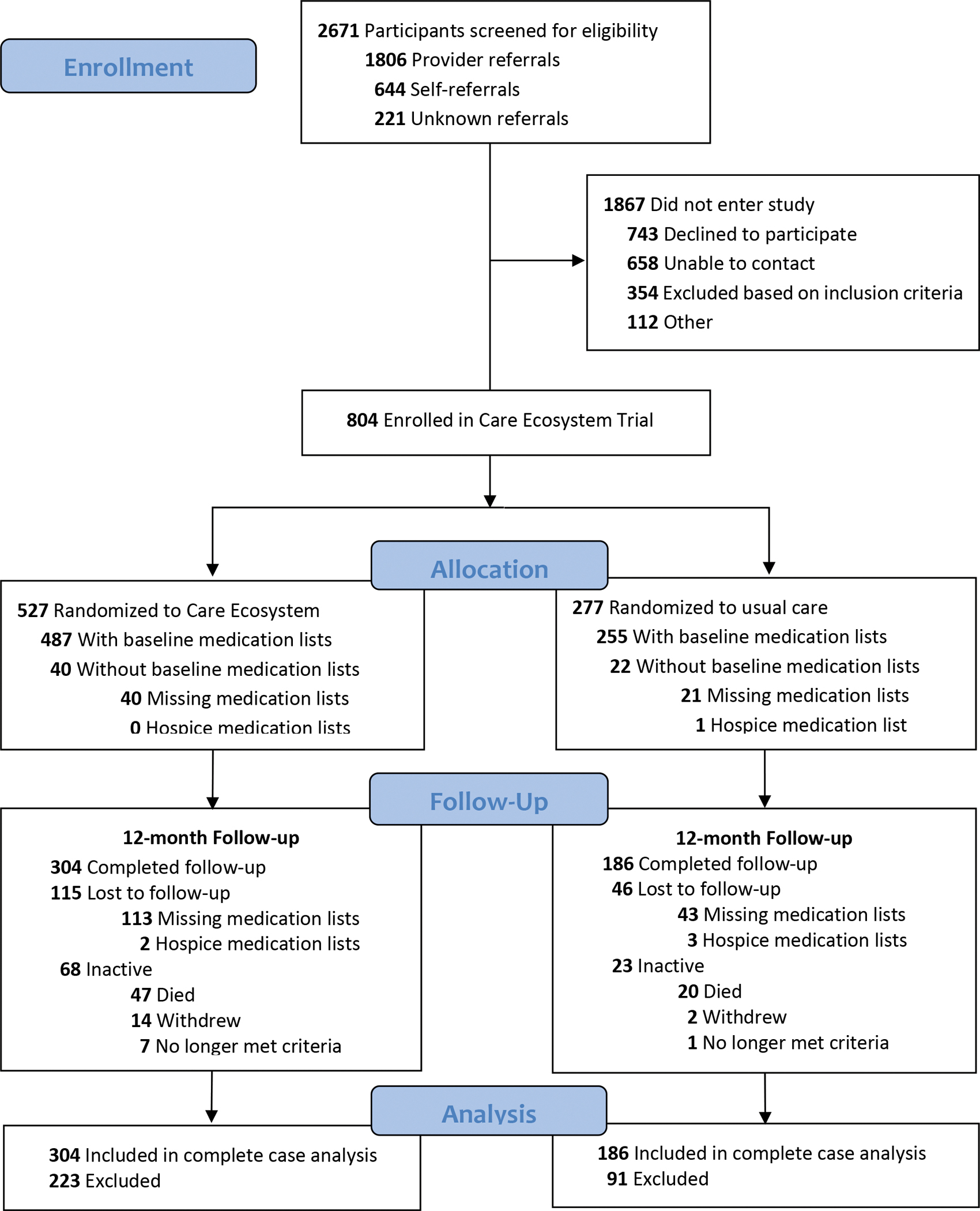
CONSORT Flow Diagram
More women were in CE vs UC (176 [57.9%] CE; 90 [48.4%] UC). All other baseline demographics, clinical characteristics, and medication exposures were similar between treatment groups (Table 1). The mean (SD) age of participants at baseline was 77.1 (9.1) years; 266 (54%) were women; 49 (10%) self-identified as being of Hispanic, Latinx, or Spanish origin; and 287 (59%) PLWD-caregiver dyads resided in California. At baseline, 66% of participants were prescribed one or more PIMs with a mean (SD) of 1.5 (1.6) PIMs, 10.4 (5.2) medications, and 1.5 (1.8) ACB Scale score.
Outcome Assessments
After adjusting for age, gender, region of residence, dementia stage, comorbidities, and baseline value of the medication outcome variable, the Care Ecosystem intervention resulted in significantly fewer PIMs prescriptions compared to UC (−0.35 PIMs; 95% confidence interval [CI], −0.49 to −0.20; P <.0001) after 12 months (Table 2). The number needed to treat to prevent an increase in one PIM was 3.[35] Similarly, the total number of medications increased significantly less in the Care Ecosystem (−0.53; 95% CI, −0.92 to −0.14; P = .008) when compared with usual care (Table 2). The percentage of PLWD in the Care Ecosystem taking 10 or more medications increased by 2% (from 52% to 54%), compared to a 5% increase (from 49% to 54%) in the usual care group.
Table 2.
Treatment effects for primary and secondary medication outcome measuresa
| Mean (SD) | ||||||
|---|---|---|---|---|---|---|
| Care Ecosystem N = 304 |
Usual Care N = 186 |
Difference between means (95% CI)b | P | |||
| Baseline | Follow-up | Baseline | Follow-up | |||
| Primary Medication Outcome | ||||||
| Number of PIMsc | 1.49 (1.59) | 1.43 (1.51) | 1.42 (1.48) | 1.72 (1.69) | −0.35 (−0.49 to −0.20) | <.0001 |
| Secondary Medication Outcomes | ||||||
| Number of medications | 10.43 (5.23) | 10.68 (5.38) | 10.28 (5.01) | 11.03 (5.42) | −0.53 (−0.92 to −0.14) | .008 |
| PIMs for dementia or cognitive impairmentc | 0.44 (0.76) | 0.45 (0.78) | 0.39 (0.74) | 0.56 (1.04) | −0.14 (−0.23 to −0.05) | .002 |
| CNS-active PIMsc | 1.40 (1.42) | 1.41 (1.36) | 1.33 (1.28) | 1.63 (1.61) | −0.28 (−0.42 to −0.14) | <.0001 |
| ACB Scale scored | 1.62 (1.98) | 1.64 (1.99) | 1.40 (1.56) | 1.69 (1.97) | −0.20 (−0.39 to −0.01) | .035 |
| Antipsychoticsc | 0.15 (0.37) | 0.17 (0.40) | 0.15 (0.40) | 0.21 (0.47) | −0.03 (−0.08 to 0.00) | .126 |
| Benzodiazepinesc | 0.13 (0.33) | 0.12 (0.34) | 0.11 (0.34) | 0.16 (0.43) | −0.05 (−0.09 to −0.01) | .008 |
| Opioidsc | 0.20 (0.50) | 0.18 (0.49) | 0.16 (0.44) | 0.23 (0.52) | −0.09 (−0.14 to −0.03) | .002 |
Abbreviations: PIMs, potentially inappropriate medications; CNS, central nervous system; ACB Scale, Anticholinergic Cognitive Burden Scale
Differences in medication outcomes at follow-up between groups using analysis of covariance. Covariates were baseline values of the medication outcome variable, age, gender, region of residence, dementia stage, and number of comorbidities.
Difference in medication outcomes means between groups using analysis of covariance. A negative value indicates that the outcome was reduced in the Care Ecosystem group when compared with the Usual Care group.
PIMs were defined using American Geriatrics Society 2019 Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. CNS-active PIMs include PIMs for persons with dementia or cognitive impairment, delirium, or history of falls or fractures were also defined by the 2019 Beers Criteria and include antipsychotics, benzodiazepines and opioids.[23]
Medications with anticholinergic properties were defined using the Anticholinergic Cognitive Burden (ACB) Scale.[27] ACB scores were summarized by participant for each anticholinergic medication prescribed.
All secondary PIMs-related medication outcomes significantly increased more in usual care than Care Ecosystem after 12 months. Care Ecosystem participants received fewer PIMs for dementia or cognitive impairment (−0.14; 95% CI, −0.23 to −0.05; P = .002) and CNS-active PIMs (−0.28; 95% CI, −0.42 to −0.14; P <.0001) than usual care participants at the end of the 12-month period (Table 2). ACB Scale score significantly increased more by 0.20 points in usual care than Care Ecosystem 95% CI, −0.39 to −0.01; P =.035) after 12 months. Additionally, compared to usual care, we found significant decreases in the number of prescriptions for benzodiazepines (−0.05; 95% CI, −0.09 to −0.01; P =.008) and opioids (−0.09; 95% CI, −0.14 to −0.03; P =.002) but not antipsychotics (−0.03; 95% CI, −0.08 to 0.00; P =.126), although a reduction was observed (Table 2). Table 3 summarizes the most common changes in PIMs for dementia or cognitive impairment between baseline and follow-up. The number of participants and the total number of PIMs for dementia or cognitive impairment decreased in the Care Ecosystem compared to increases in usual care.
Table 3.
Most common changes of PIMs for dementia or cognitive impairment between baseline and follow-upa
| N (%) | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Care Ecosystem N = 304 |
Usual Care N = 186 |
|||||
|
| ||||||
| Baseline | Follow-Up | Δ b | Baseline | Follow-Up | Δ b | |
|
| ||||||
| Persons with any PIM | 202 (66.4) | 201 (66.1) | −1 | 122 (65.6) | 135 (72.6) | +13 |
| Number of PIMs | 453 | 436 | −17 | 265 | 320 | +55 |
|
| ||||||
| Drug Class and Generic Medication Name | ||||||
|
| ||||||
| PIMs for dementia or cognitive impairment c | ||||||
|
| ||||||
| Definite Anticholinergicsd | 62 (20.4) | 64 (21.1) | +2 | 31 (16.7) | 42 (22.6) | +11 |
| Quetiapine | 27 (8.9) | 31 (10.2) | +4 | 12 (6.5) | 21 (11.3) | +9 |
| Diphenoxylate/Atropine | 0 | 1 (0.3) | +1 | 0 | 3 (1.6) | +3 |
|
|
||||||
| Diphenhydramine | 8 (2.6) | 6 (2.0) | −2 | 2 (1.1) | 0 | −2 |
| Solifenacin | 7 (2.3) | 5 (1.6) | −2 | 0 | 2 (1.1) | +2 |
| Paroxetine | 5 (1.6) | 3 (1.0) | −2 | 2 (1.1) | 4 (2.2) | +2 |
| Tolterodine | 2 (0.7) | 2 (0.7) | 0 | 2 (1.1) | 0 | −2 |
|
| ||||||
| Benzodiazepines | 38 (12.5) | 36 (11.8) | −2 | 18 (9.7) | 26 (14.0) | +8 |
| Lorazepam | 17 (5.6) | 17 (5.6) | 0 | 10 (5.4) | 19 (10.2) | +9 |
| Clonazepam | 6 (2.0) | 6 (2.0) | 0 | 6 (3.2) | 7 (3.8) | +1 |
|
|
||||||
| Alprazolam | 9 (3.0) | 7 (2.3) | −2 | 3 (1.6) | 2 (1.1) | −1 |
| Temazepam | 3 (1.0) | 3 (1.0) | 0 | 1 (0.5) | 2 (1.1) | +1 |
|
| ||||||
| Nonbenzodiazepine, benzodiazepine receptor agonist hypnotics (“Z-Drugs”) | 5 (1.6) | 4 (1.3) | −1 | 1 (0.5) | 2 (1.1) | +1 |
| Zolpidem | 4 (1.3) | 3 (1.0) | −1 | 1 (0.5) | 2 (1.1) | +1 |
|
| ||||||
| Antipsychotics | 45 (14.8) | 50 (16.4) | +5 | 24 (12.9) | 35 (18.8) | +11 |
| Quetiapine | 27 (8.9) | 31 (10.2) | +4 | 12 (6.5) | 21 (11.3) | +9 |
| Risperidone | 10 (3.3) | 12 (3.9) | +2 | 5 (2.7) | 5 (2.7) | 0 |
| Haloperidol | 0 | 2 (0.7) | +2 | 2 (1.1) | 5 (2.7) | +3 |
|
|
||||||
| Olanzapine | 4 (1.3) | 3 (1.0) | −1 | 1 (0.5) | 1 (0.5) | 0 |
| Aripiprazole | 3 (1.0) | 2 (0.7) | −1 | 4 (2.2) | 3 (1.6) | −1 |
Abbreviations: PIMs, potentially inappropriate medications
For each drug class, PIMs with the greatest increases or decreases between baseline and follow-up are listed. This list is not inclusive of all PIMs that changed.
Change in number of study participants between baseline and follow-up.
PIMs were defined using American Geriatrics Society 2019 Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults.[23]
Medications with Anticholinergic Cognitive Burden (ACB) Scale scores of 2 or higher, indicating clinical anticholinergic effect or may cause delirium.[27]
After 12 months, changes in prescriptions for anti-dementia medications were more likely to occur in the Care Ecosystem (26.3%) compared to usual care (16.7%) (adjusted odds ratio 1.82; 95% CI, 1.14 to 2.92; P =−.012). Changes included starting a new anti-dementia medication if they were not prescribed one at baseline, addition of an anti-dementia medication if previously taking one at baseline, or stopping an anti-dementia medication from baseline. Changes occurred more frequently in the Care Ecosystem for acetylcholinesterase inhibitors, memantine or both (Supplement Table 3).
4. DISCUSSION
In this 12-month trial of the Care Ecosystem, the medication review intervention embedded in a collaborative dementia care program significantly reduced the number of PIMs among community-dwelling PLWD compared to participants receiving usual care, with a number needed to treat to prevent an increase in one PIM of 3.
High rates of PIMs (66%) and polypharmacy (89%) were common in our study population, consistent with other studies.[1–7, 22] This demonstrates the need for proactive medication management and monitoring to reduce PIMs and medication burden in this vulnerable population. As expected, the total number of medications and PIMs drug classes increased over time. Yet, for those receiving Care Ecosystem medication reviews, rates of total medications and PIMs use increased significantly less compared to usual care for all PIMs drug classes we evaluated, except for antipsychotics for which we found a trend. Although the Care Ecosystem emphasizes non-pharmacological treatment for behavioral and psychological symptoms of dementia, the lack of significance for antipsychotics may reflect the difficulty in managing these symptoms. It is critical that PIMs be avoided in PLWD due to adverse effects on cognition and increased risk of falls and fractures. The Care Ecosystem medication review intervention effectively prevented an increase in the use of CNS-active and anticholinergic medications, and decreased benzodiazepines and opioids among PLWD.
While reducing or minimizing PIMs and polypharmacy are important, along with non-pharmacological interventions to help manage BPSD, prescribing anti-dementia medications may also help improve cognition and management of agitation, aggression, psychosis, depression, anxiety, delusions, and apathy.[36] A recent study examining rates of anti-dementia medications prescribed through pharmacies in Japan found the use of acetylcholinesterase inhibitors, memantine, and particularly their combination was associated with a reduction in the use of psychotropic PIMs.[37] In this trial, the Care Ecosystem medication review intervention was more likely to start, add or stop anti-dementia medications compared to usual care. Actively managing anti-dementia medications to ensure their effectiveness and safety is crucial to improving quality of life and managing BPSD in PLWD.[38]
Randomized trials of other interventions have encountered challenges in deprescribing PIMs[39] or demonstrated efficacy in improving medication use among PLWD. Moga et al implemented a patient-centered, pharmacist-physician team medication therapy management program in an Alzheimer’s clinic which decreased use of inappropriate anticholinergic medications.[14] Among nursing home patients with dementia in the Netherlands, van der Speck et al implemented a structured medication review every 6 months by pharmacists, physicians, and nurses, which improved the appropriateness of psychotropic medications for neuropsychiatric symptoms.[16] Both of these interventions targeted the discontinuation of specific medication classes (i.e., anticholinergics or psychotropics). In Germany, a home-based, nurse-led dementia care management program improved the use of anti-dementia medications, but had no effect on PIMs.[40] In contrast to prior trials, the Care Ecosystem demonstrated a broader impact by reducing multiple PIMs drug classes and optimizing anti-dementia medications, along with improving PLWD quality of life, health care use, and caregiver well-being.[24]
We believe that being embedded in a longitudinal, comprehensive dementia care program synergistically contributed to the success of our Care Ecosystem medication intervention, and may make it appealing to health systems leaders and other stakeholders who are looking to improve not only medication management but also overall dementia care. In the Care Ecosystem model, CTNs build rapport and establish ongoing relationships with dyads. Their care calls incorporate structured medication reviews and regular screening for safety or behavior concerns and medication changes. CTNs organize and clarify medications for PLWD who often have multiple prescribing providers. The care team develops a holistic view of the dyad’s health and social situation by working with dyads and providers on selecting, personalizing, and monitoring responses to pharmacological and non-pharmacological treatments. The Care Ecosystem medication review intervention provides education, expert recommendations, proactive medication monitoring, and non-pharmacological strategies to optimize dementia care. While the medication interviews were conducted by the unlicensed CTNs, medication changes and problems were always reviewed by our dementia specialists and discussed with the dyad and their providers as appropriate. The Care Ecosystem model has proven to be cost efficient[41] and among the most effective dementia care interventions with previously reported effects on patient quality of life, emergency room visit use, and caregiver well-being.[24, 25] Our study identifies another major benefit of this care model: its broad impact on medication optimization and safety through synergism between our medications reviews and collaborative dementia care.
Limitations
Our study has several limitations. First, we chose to evaluate the number of medications over 12 months using medical record medication lists. Medical record medication lists can be outdated, incomplete, or not fully accurate, and laboratory results, diagnosis codes or indications, and previously-tried medications may not be entered systematically.[42–44] We minimized bias in outcomes by standardizing our medical record data collection and review protocols for the intervention and control groups. Also, we were not able to evaluate the appropriateness of the medication regimen, the lowest effective dose, or as needed medication use. This may cause an under- or over-identification of PIMs that are clinically justifiable, such as the use of antipsychotics as second-line therapy after non-pharmacological and first-line pharmacological therapies have failed. Second, PLWD in our study continued to receive routine care from their primary providers. Medication adjustments were ultimately made at the discretion of these providers, which may differ from efficacy, safety, and cost-savings recommendations made by the Care Ecosystem team. Furthermore, this study only includes community-dwelling PLWD who identify a caregiver, and may not generalize to other dementia populations.
Conclusion
Telephone-based collaborative dementia care delivered by CTNs and dementia specialists over 12 months significantly reduced the number of PIMs among community-dwelling PLWD compared to usual care. In the Care Ecosystem medication review intervention, unlicensed CTNs periodically queried caregivers for medication lists and concerns, incorporated interprofessional teamwork with pharmacists, nurses, and social workers, and developed and kept up-to-date medication plans that were integrated into the PLWD’s overall dementia care. This personalized medication plan was communicated with dyads and prescribing clinicians along with care plans that addressed the PLWD’s medical needs, challenging behavioral symptoms, caregiver needs, complex legal and financial circumstances, and safety concerns. Given the potential for adverse cognitive effects and medication-related problems in this population, there is a growing need for proactive medication management to optimize medications and reduce PIMs for community-dwelling PLWD with the aid of their caregivers. This study provides promising insight into a PIMs reduction strategy and medication optimization intervention for this vulnerable patient population.
Supplementary Material
Research in Context.
1. Systematic review:
The authors reviewed published literature on interventions to reduce potentially inappropriate medications (PIMs) among persons living with dementia (PLWD). Specific medication classes of anticholinergics or psychotropics have decreased in some studies and one study improved the use of anti-dementia medications but had no effect on PIMs.
2. Interpretation:
Our collaborative dementia care medication review intervention significantly reduced multiple PIMs drug classes and modified anti-dementia medication regimens more frequently among community-dwelling PLWD after 12 months compared to usual care. In addition to improving PLWD quality of life, health care use, and caregiver well-being, the Care Ecosystem collaborative dementia care program with medication review has the potential to optimize medication use.
3. Future directions:
The Care Ecosystem collaborative dementia care program with medication review is being implemented at various healthcare systems. Its impact on medication use in PLWD across multiple sites will be evaluated.
Highlights.
Compared to usual care, the Care Ecosystem medication review intervention prevented increases in potentially inappropriate medications (PIMs).
Use of anticholinergics, benzodiazepines and opioids were significantly reduced, with a trend for antipsychotics.
Anti-dementia medications were adjusted more frequently.
The Care Ecosystem medication review intervention embedded in collaborative dementia care optimized medication use.
Funding/Support:
This project was funded by the Department of Health and Human Services, Centers for Medicare & Medicaid Services (1C1CMS331346), the National Institute on Aging (5R01AG056715), and the Global Brain Health Institute.
Role of the Funder/Sponsor:
The funding agencies had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures:
Dr. Liu reported receiving grant funding from the National Institute of General Medicine Sciences (T32GM007546).
Dr. Possin reported receiving grants from the National Institute of Neurological Disorders and Stroke, the National Institute on Aging, Global Brain Health Institute, Quest Diagnostics, Administration for Community Living, Rainwater Charitable Trust, and Merck Foundation; and personal fees from ClearView Health Partners and Vanguard.
Dr. Chiong reported receiving grants from the National Institute on Mental Health and the National Institute on Aging.
Dr. Guterman reported receiving grant funding from the National Institute of Neurological Disorders and Stroke (1K23NS116128-01), National Institute on Aging (5R01AG056715), and American Academy of Neurology. She has received personal compensation from Marinus Pharmaceuticals, Inc., JAMA Neurology, and Remo Health, which are unrelated the submitted work.
Disclaimer: The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the Department of Health and Human Services or any of its agencies.
Additional Contributions:
We wish to acknowledge Dr. Michael Steinman for his advice on medical record data analysis and Caroline Prioleau for assistance with figures. We also thank our Care Team Navigators and staff research coordinators for their many efforts to bring these interventions to families living with dementia and patiently measuring the effects of these interventions, and the UCSF Memory and Aging Center Family Advisory Council for their input in designing the Care Ecosystem model of care. Finally, we greatly thank our study participants for their effort and time in testing and refining our interventions.
Data Sharing Statement:
See Supplement 3.
References
- 1.Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry HE, Cooper JA, Ryan C, Passmore AP, Robinson AL, Molloy GJ, et al. Potentially Inappropriate Prescribing Among People with Dementia in Primary Care: A Retrospective Cross-Sectional Study Using the Enhanced Prescribing Database. J Alzheimers Dis. 2016;52(4):1503–13. [DOI] [PubMed] [Google Scholar]
- 3.Hukins D, Macleod U, Boland JW. Identifying potentially inappropriate prescribing in older people with dementia: a systematic review. Eur J Clin Pharmacol. 2019;75(4):467–81. [DOI] [PubMed] [Google Scholar]
- 4.Murphy C, Dyer AH, Lawlor B, Kennelly SP, Group NS. Potentially inappropriate medication use in older adults with mild-moderate Alzheimer’s disease: prevalence and associations with adverse events. Age Ageing. 2020;49(4):580–7. [DOI] [PubMed] [Google Scholar]
- 5.Delgado J, Bowman K, Clare L. Potentially inappropriate prescribing in dementia: a state-of-the-art review since 2007. BMJ Open. 2020;10(1):e029172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delgado J, Jones L, Bradley MC, Allan LM, Ballard C, Clare L, et al. Potentially inappropriate prescribing in dementia, multi-morbidity and incidence of adverse health outcomes. Age Ageing. 2021;50(2):457–64. [DOI] [PubMed] [Google Scholar]
- 7.Growdon ME, Gan S, Yaffe K, Steinman MA. Polypharmacy among older adults with dementia compared with those without dementia in the United States. J Am Geriatr Soc. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross AJ, George J, Woodward MC, Ames D, Brodaty H, Wolfe R, et al. Potentially Inappropriate Medication, Anticholinergic Burden, and Mortality in People Attending Memory Clinics. J Alzheimers Dis. 2017;60(2):349–58. [DOI] [PubMed] [Google Scholar]
- 9.Austrom MG, Boustani M, LaMantia MA. Ongoing Medical Management to Maximize Health and Well-being for Persons Living With Dementia. Gerontologist. 2018;58(suppl_1):S48–S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O’Donnell M, Sullivan R, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–62. [DOI] [PubMed] [Google Scholar]
- 12.Odenheimer G, Borson S, Sanders AE, Swain-Eng RJ, Kyomen HH, Tierney S, et al. Quality improvement in neurology: dementia management quality measures. Neurology. 2013;81(17):1545–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polypharmacy Parsons C. and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf. 2017;8(1):31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moga DC, Abner EL, Rigsby DN, Eckmann L, Huffmyer M, Murphy RR, et al. Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden. Alzheimers Res Ther. 2017;9(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a Pharmacist-Led Educational Intervention on Inappropriate Medication Prescriptions in Older Adults: The D-PRESCRIBE Randomized Clinical Trial. JAMA. 2018;320(18):1889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Spek K, Koopmans R, Smalbrugge M, Nelissen-Vrancken M, Wetzels RB, Smeets CHW, et al. The effect of biannual medication reviews on the appropriateness of psychotropic drug use for neuropsychiatric symptoms in patients with dementia: a randomised controlled trial. Age Ageing. 2018;47(3):430–7. [DOI] [PubMed] [Google Scholar]
- 17.Nakham A, Myint PK, Bond CM, Newlands R, Loke YK, Cruickshank M. Interventions to Reduce Anticholinergic Burden in Adults Aged 65 and Older: A Systematic Review. J Am Med Dir Assoc. 2020;21(2):172–80 e5. [DOI] [PubMed] [Google Scholar]
- 18.Almutairi H, Stafford A, Etherton-Beer C, Flicker L. Optimisation of medications used in residential aged care facilities: a systematic review and meta-analysis of randomised controlled trials. BMC Geriatr. 2020;20(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson LJ, Schnipper JL, Nuckols TK, Shane R, Sarkisian C, Le MM, et al. A systematic overview of systematic reviews evaluating interventions addressing polypharmacy. Am J Health Syst Pharm. 2019;76(21):1777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel T, Slonim K, Lee L. Use of potentially inappropriate medications among ambulatory home-dwelling elderly patients with dementia: A review of the literature. Can Pharm J (Ott). 2017;150(3):169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray SL, Anderson ML, Dublin S, Hanlon JT, Hubbard R, Walker R, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med. 2015;175(3):401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maust DT, Strominger J, Kim HM, Langa KM, Bynum JPW, Chang CH, et al. Prevalence of Central Nervous System-Active Polypharmacy Among Older Adults With Dementia in the US. JAMA. 2021;325(10):952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panel AGSBCUE. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2019;67(4):674–94. [DOI] [PubMed] [Google Scholar]
- 24.Possin KL, Merrilees JJ, Dulaney S, Bonasera SJ, Chiong W, Lee K, et al. Effect of Collaborative Dementia Care via Telephone and Internet on Quality of Life, Caregiver Well-being, and Health Care Use: The Care Ecosystem Randomized Clinical Trial. JAMA Intern Med. 2019;179(12):1658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butler M GJ, Talley KMC, Abdi HI, Desai PJ, Duval S, Forte ML, Nelson VA, Ng W, Ouellette JM, Ratner E, Saha J, Shippee T, Wagner BL, Wilt TJ, Yeshi L. Care interventions for people living with dementia and their caregivers. Rockville, MD: Agency for Healthcare Research and Quality; 2020. [Google Scholar]
- 26.Bernstein A, Harrison KL, Dulaney S, Merrilees J, Bowhay A, Heunis J, et al. The Role of Care Navigators Working with People with Dementia and Their Caregivers. J Alzheimers Dis. 2019;71(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boustani M CN, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–20. [Google Scholar]
- 28.Little RJ. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83(404):1198–202. [Google Scholar]
- 29.Vickers AJ, Altman DG. Statistics notes: Analysing controlled trials with baseline and follow up measurements. BMJ. 2001;323(7321):1123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang S, Paul J, Nantha-Aree M, Buckley N, Shahzad U, Cheng J, et al. Empirical comparison of four baseline covariate adjustment methods in analysis of continuous outcomes in randomized controlled trials. Clin Epidemiol. 2014;6:227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galvin JE. The Quick Dementia Rating System (Qdrs): A Rapid Dementia Staging Tool. Alzheimers Dement (Amst). 2015;1(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chaudhry S, Jin L, Meltzer D. Use of a self-report-generated Charlson Comorbidity Index for predicting mortality. Med Care. 2005;43(6):607–15. [DOI] [PubMed] [Google Scholar]
- 33.Poblador-Plou B, Calderon-Larranaga A, Marta-Moreno J, Hancco-Saavedra J, Sicras-Mainar A, Soljak M, et al. Comorbidity of dementia: a cross-sectional study of primary care older patients. BMC Psychiatry. 2014;14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa TA, Leucht S. How to obtain NNT from Cohen’s d: comparison of two methods. PLoS One. 2011;6(4):e19070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz SK, Llorente MD, Sanders AE, Tai WA, Bennett A, Shugarman S, et al. Quality improvement in dementia care: Dementia Management Quality Measurement Set 2018 Implementation Update. Neurology. 2020;94(5):210–6. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Sakakibara M, Shiraishi N, Komiya H, Akishita M, Kuzuya M. Use of Anti-Dementia Drugs Reduces the Risk of Potentially Inappropriate Medications: A Secondary Analysis of a Nationwide Survey of Prescribing Pharmacies. Dement Geriatr Cogn Disord. 2020;49(5):526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrmann N, Ismail Z, Collins R, Desmarais P, Goodarzi Z, Henri-Bhargava A, et al. CCCDTD5 recommendations on the deprescribing of cognitive enhancers in dementia. Alzheimers Dement (N Y). 2022;8(1):e12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayliss EA, Shetterly SM, Drace ML, Norton JD, Maiyani M, Gleason KS, et al. Deprescribing Education vs Usual Care for Patients With Cognitive Impairment and Primary Care Clinicians: The OPTIMIZE Pragmatic Cluster Randomized Trial. JAMA Intern Med. 2022;182(5):534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thyrian JR, Hertel J, Wucherer D, Eichler T, Michalowsky B, Dreier-Wolfgramm A, et al. Effectiveness and Safety of Dementia Care Management in Primary Care: A Randomized Clinical Trial. JAMA Psychiatry. 2017;74(10):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosa TD, Possin KL, Bernstein A, Merrilees J, Dulaney S, Matuoka J, et al. Variations in Costs of a Collaborative Care Model for Dementia. J Am Geriatr Soc. 2019;67(12):2628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milone AS, Philbrick AM, Harris IM, Fallert CJ. Medication reconciliation by clinical pharmacists in an outpatient family medicine clinic. J Am Pharm Assoc (2003). 2014;54(2):181–7. [DOI] [PubMed] [Google Scholar]
- 43.Linsky A, Simon SR. Medication discrepancies in integrated electronic health records. BMJ Qual Saf. 2013;22(2):103–9. [DOI] [PubMed] [Google Scholar]
- 44.Ernst ME, Brown GL, Klepser TB, Kelly MW. Medication discrepancies in an outpatient electronic medical record. Am J Health Syst Pharm. 2001;58(21):2072–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
See Supplement 3.


