Abstract
Gamma interferon (IFN-γ) is an important cytokine in host defense against chlamydial infection. An in vitro cell culture system was used to show that IFN-γ inhibition of chlamydial growth, as determined by diminished recovery of infectious elementary bodies, differed markedly among chlamydial strains. These differences in sensitivity among chlamydial strains to IFN-γ-mediated inhibition may profoundly influence the clinical outcome of infection.
Chlamydia trachomatis, a common cause of sexually transmitted disease and ocular infection in humans, is represented by serologically distinguishable variants: serovars A to K have a tropism for mucosal epithelial cells and primarily cause ocular and urogenital infections; serovars L1, L2, and L3 cause lymphogranuloma venereum and grow and replicate in the lymphatics; and strain mouse pneumonitis (MoPn) readily infects murine genital tract mucosa and has been used extensively in studies to define host immunity to chlamydial genital tract infection.
Murine models of infection, as well as studies in human patient populations, identify CD4+ T cells, specifically major histocompatibility complex class II-restricted, T helper type 1 CD4+ cells secreting gamma interferon (IFN-γ) as primary mediators of protective immunity (4, 9, 13, 14, 18). In vivo studies using IFN-γ gene knockout mice demonstrate that IFN-γ is critical for the prevention of disseminated disease following genital tract infection (8, 10, 14). Furthermore, IFN-γ- secreting T-cell clones and lines have been shown to confer a level of immune protection (9), and administration of anti-IFN-γ or recombinant IFN-γ prolongs infection or brings about the resolution of infection, respectively (16). Although the precise mechanism(s) has not been defined for in vivo infection, IFN-γ inhibits the growth and replication of chlamydiae in cell culture through an IFN-γ-inducible indoleamine 2,3-dioxygenase (IDO) pathway (5).
The differential sensitivity of some chlamydial strains to the in vivo and in vitro effects of IFN-γ has been noted previously (3, 6, 10, 15). However, because IFN-γ is critically involved in host immunity to chlamydial infection and inhibits intracellular chlamydial growth in vitro, the sensitivities of 15 serovars and one strain of C. trachomatis and two strains of C. psittaci to the growth-inhibitory effects of IFN-γ were investigated using an in vitro cell culture system.
C. trachomatis serovars A/Har-13, B/TW-5, Ba/Apa-2, C/TW-3, D/UW-31, E/Bour, F/IC-Cal-13, G/UW-57, H/UW-4, I/UW-12, J/UW-36, K/UW-31, L1/LGV-440, L2/LGV-434, and L3/LGV-404 and strain MoPn and C. psittaci strains guinea pig inclusion conjunctivitis (GPIC) and meningopneumonitis (Mn) were grown in HeLa 229 cells, and elementary bodies were purified and stored at −85°C as described previously (7). Frozen stocks of chlamydiae were thawed at 37°C, and inclusion forming units (IFU) were enumerated by titration on DEAE-dextran-treated HeLa cells. To assess the inhibitory effect of IFN-γ on chlamydial growth, HeLa cell monolayers (consisting of ∼3.0 × 105 cells) in 24-well tissue culture plates were treated with 0.5 ml of DEAE-dextran (45 μg/ml) (11, 12) for 15 min, washed twice with Hanks balanced salt solution, and inoculated with 0.2 ml of 250 mM sucrose–10 mM sodium phosphate–5 mM l-glutamic acid (pH 7.2) (SPG) containing 3× 105 IFU of the appropriate chlamydial strain. The infected monolayer was rocked at 37°C for 2 h and then washed with Hanks balanced salt solution. Minimal essential medium containing 10% fetal bovine serum and the indicated concentrations of recombinant human IFN-γ was added to infected monolayers, and incubation at 37°C in a humidified atmosphere containing 5% CO2 was continued for 48 h (for strains L1, L2, L3, GPIC, Mn, and MoPn) or 72 h (all other strains). The medium was then removed and cells were scraped into 0.2 ml of SPG and frozen at −85°C until assayed for IFU. IFU from IFN-γ-treated and nontreated monolayers were enumerated by plating dilutions of briefly sonicated samples onto DEAE-dextran-treated HeLa cell monolayers and visualizing inclusions by indirect immunofluorescent staining.
In some experiments, HeLa cell monolayers were treated with IFN-γ for 24 or 48 h prior to infection. The experimental procedure was identical to that described above except monolayers were treated with IFN-γ for the indicated time prior to infection and the medium that was removed prior to infection (i.e., the 24- or 48-h pretreatment medium) was added back to the monolayers following infection.
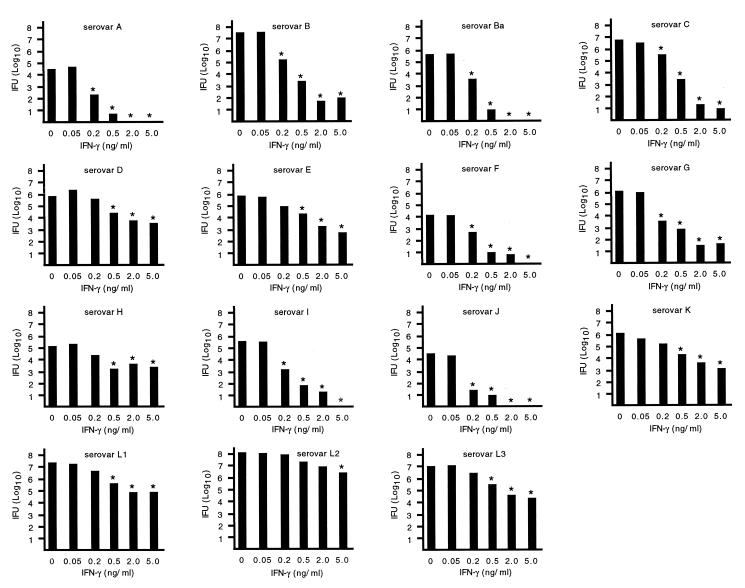
Initial experiments were performed on noninfected HeLa cells to determine IFN-γ toxicity. Concentrations of IFN-γ of up to 5 ng/ml were well tolerated by HeLa 229 cells and cell monolayers remained intact (<5% loss of cell monolayer) for at least 96 h of incubation. Concentrations of IFN-γ of >5 ng/ml were toxic and resulted in considerable loss of the cell monolayer. The differential sensitivity of chlamydial strains to IFN-γ-mediated growth inhibition was evaluated using IFN-γ at a concentration of 5 ng/ml (Fig. 1). The level of growth inhibition for the various chlamydial strains was broadly grouped into three categories: marked inhibition, characterized by a >4.0-log10 reduction in IFU; moderate inhibition, characterized by a 1.5- to 3.0-log10 reduction in IFU; and minimal inhibition, characterized by a <1.0-log10 reduction in IFU. IFN-γ inhibited the growth and markedly diminished the recovery of infectious elementary bodies (4.0- to 6.0-log10 reduction) of C. trachomatis serovars A, B, Ba, C, F, G, and I. In contrast, the growth of C. trachomatis serovars D, E, K, L1, L2, and L3 was moderately inhibited by IFN-γ (1.5- to 2.5-log10 reduction in IFU). C. psittaci strains GPIC and Mn and C. trachomatis strain MoPn were much more resistant to the inhibitory effects of IFN-γ (<1.0-log10 reduction in IFU). IFN-γ-mediated inhibition of chlamydial growth was further evaluated by examining the growth of C. trachomatis serovars in the presence of various concentrations of IFN-γ (Fig. 2). Concentrations of IFN-γ as low as 0.2 ng/ml reduced the number of IFU produced by serovars A, B, Ba, G, I, and J by >99%. An IFN-γ concentration of 0.5 ng/ml reduced the number of IFU of serovars C and F by 99%, but tenfold more IFN-γ (5 ng/ml) was needed to produce a similar reduction of IFU in the remaining serovars.
FIG. 1.
Differential sensitivity of chlamydial strains to IFN-γ. Monolayers of HeLa cells infected with the indicated strains of C. trachomatis or C. psittaci were incubated in culture medium alone or medium containing IFN-γ (5 ng/ml). Data are presented as the mean IFU (log10) of triplicate determination from three separate experiments. Error bars have been omitted for clarity. However, the standard error of the mean of any determination never exceeded 0.5 log10. Statistically significant differences (P < 0.05 [Student's t test]) between IFN-γ-treated and -untreated cultures are indicated by an asterisk.
FIG. 2.
Dose-response data of C. trachomatis serovars to IFN-γ. Data are presented as explained in the legend to Fig. 1.
C. trachomatis serovars and C. psittaci strains clearly expressed differential susceptibility to the growth-inhibitory effects of IFN-γ. One mechanism by which IFN-γ has been shown to inhibit chlamydial growth is through an IFN-γ inducible IDO pathway. IFN-γ-induced IDO activity results in the catabolism of tryptophan, which diminishes the level of intracellular pools of tryptophan available for chlamydial growth (2, 5). Perhaps those strains of chlamydiae that appeared more resistant to the inhibitory effects of IFN-γ simply outgrew the potential inhibitory effect of IFN-γ. Thus, HeLa cells were pretreated with IFN-γ for 24 or 48 h prior to infection with strains of C. trachomatis that expressed different sensitivities to the inhibitory effect of IFN-γ: serovar A, marked sensitivity; serovars D and L2, moderate sensitivity; and strain MoPn, minimal sensitivity. Pretreatment of HeLa cells with IFN-γ prior to infection significantly diminished the production of infectious chlamydiae by strains that initially appeared to be quite resistant (D, L2, and MoPn) to the growth-inhibitory effect of IFN-γ (Fig. 3).
FIG. 3.
Effect of IFN-γ on the growth of C. trachomatis when added prior to infection. Monolayers were incubated with the indicated concentrations of IFN-γ for either 24 or 48 h prior to infection with C. trachomatis serovar A, D, or L2 or strain MoPn. Data are presented as explained in the legend to Fig. 1.
Thus, Chlamydia exhibited differential sensitivities to the growth-inhibitory effects of IFN-γ. The differences in susceptibility of the various chlamydial strains to IFN-γ might be explained by differences in their ability to acquire exogenous tryptophan or to synthesize their own tryptophan. Indeed, IFN-γ-induced growth inhibition can be overcome by the addition of tryptophan to the culture medium (2), and differences exist between chlamydial species with regard to tryptophan biosynthesis genes (17). Genomic sequencing reveals the presence of tryptophan biosynthesis genes in C. trachomatis serovars D and L2 and the absence of those genes in C. pneumoniae, whose growth is also inhibited by IFN-γ (19). Serovars D and L2 were among the more resistant strains of C. trachomatis to IFN-γ-induced growth inhibition, which is consistent with the genomic sequencing data identifying tryptophan biosynthesis genes in those serovars. Perhaps serovars that are particularly sensitive to the inhibitory effect of IFN-γ (e.g., trachoma biovars) lack the tryptophan biosynthesis genes. C. trachomatis serovar B contains a 5- to 10-kb chromosomal deletion compared to serovar D (17). If the deletion contains the genes for tryptophan biosynthesis, that might explain the sensitivity of serovar B to the inhibitory effects of IFN-γ. It is noteworthy that several of the chlamydial strains that were found to be markedly sensitive to the growth-inhibiting effects of IFN-γ (notably serovars A, B, and C) are also very sensitive to tryptophan limitation (1).
The precise role of IFN-γ in immunity to chlamydial infection has not been elucidated. However, the differential susceptibility of C. trachomatis serovars to the inhibitory effects of IFN-γ may play an important role in pathogenesis of chlamydial disease as it relates to disease severity, persistence, and resolution of acute infection. For example, perhaps the C. trachomatis serovars that are more resistant to the inhibitory effects of IFN-γ (e.g., serovars D, E, and K) cause genital tract infections in humans that are characterized by increased shedding of chlamydiae, increased inflammation, and more-severe symptoms. Such an infection might resemble that of MoPn infection of the murine genital tract. Conversely, the other oculogenital serovars may cause infections that are more likely to be persistent and asymptomatic. Additional studies are necessary, however, to determine the specific role of IFN-γ in the pathogenesis of human chlamydial infections.
Acknowledgments
I thank Harlan Caldwell for providing chlamydial strains and Gerry Byrne for recombinant human IFN-γ.
This work was supported in part by grant AI-38991 from the National Institutes of Health.
REFERENCES
- 1.Allan I, Pearce J H. Amino acid requirements of strains of Chlamydia trachomatis and C. psittaci growing in McCoy cells: relationship with clinical syndrome and host origin. J Gen Microbiol. 1983;129:2001–2007. doi: 10.1099/00221287-129-7-2001. [DOI] [PubMed] [Google Scholar]
- 2.Beatty W L, Belanger T A, Desai A A, Morrison R P, Byrne G I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon-gamma mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunham R C. Human immunity to chlamydiae. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis, and immunity. Washington, D.C.: ASM Press; 1999. pp. 211–238. [Google Scholar]
- 5.Byrne G I, Lehmann L K, Landry G J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne G I, Rothermel C D. Differential susceptibility of chlamydiae to exogenous fibroblast interferon. Infect Immun. 1983;39:1004–1005. doi: 10.1128/iai.39.2.1004-1005.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 10.Ito J I, Lyons J M. Role of gamma interferon in controlling murine chlamydial genital tract infection. Infect Immun. 1999;67:5518–5521. doi: 10.1128/iai.67.10.5518-5521.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo C-C, Grayston J T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976;13:1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo C-C, Wang S-P, Grayston J T. Differentiation of TRIC and LGV organisms based on enhancement of infectivity by DEAE-dextran in cell culture. J Infect Dis. 1972;125:313–317. doi: 10.1093/infdis/125.3.313. [DOI] [PubMed] [Google Scholar]
- 13.Morrison R P, Feilzer K, Tumas D B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun. 1995;63:4661–4668. doi: 10.1128/iai.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 15.Perry L L, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell H D. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-γ-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 16.Rank R G, Ramsey K H, Pack E A, Williams D M. Effect of gamma interferon on resolution of murine chlamydial genital infection. Infect Immun. 1992;60:4427–4429. doi: 10.1128/iai.60.10.4427-4429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephens R S. Genomic autobiographies of chlamydiae. In: Stephens R S, editor. Chlamydia: intracellular biology, pathogenesis, and immunity. Washington, D.C.: ASM Press; 1999. pp. 9–27. [Google Scholar]
- 18.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Summersgill J T, Sahney N N, Gaydos C A, Quinn T C, Ramirez J A. Inhibition of Chlamydia pneumoniae growth in HEp-2 cells pretreated with gamma interferon and tumor necrosis factor alpha. Infect Immun. 1995;63:2801–2803. doi: 10.1128/iai.63.7.2801-2803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]