Abstract
Objectives
Implementing medical cannabis (MC) into a child’s daily routine can be challenging and there is a lack of guidance for its therapeutic use in schools in Canada. Our objective was to learn about the experiences of caregivers of school-aged children who require MC.
Methods
Qualitative description was used and caregivers were interviewed about MC in schools and in general. The transcripts were entered into Dedoose software for qualitative analysis and content analysis was performed. Sentences and statements were ascribed line by line into meaning units and labelled with codes, and organized according to categories and subcategories.
Results
Twelve caregivers of school-aged children who take MC participated. The most common reasons for treatment were drug-resistant epilepsy (DRE), autism, or other developmental disorders. Approximately half of the participants’ children (n = 6) took MC during the school day and most (5/6) perceived their experiences to be positive or neutral but reported a lack of knowledge about MC. While data saturation was not reached regarding MC in schools, rich dialogues were garnered about MC in general and three categories were identified: challenges (subcategories stigma, finding an authorizer, cost, dosing, and supply); parents as advocates (subcategories required knowledge, attitudes, skills, and sources of information); and caregiver relief for positive outcomes.
Conclusions
Caregivers demonstrate remarkable tenacity despite the many challenges associated with MC use. Education and practice change are needed to ensure that children using MC can benefit from or continue to experience its positive outcomes within the school environment and beyond.
Keywords: Caregiver, Education, Medical cannabis, School
The awareness of cannabis use for medical purposes has increased drastically in recent years (1). Research advancements suggest that medical cannabis (MC) has a legitimate therapeutic role in some children or youth, particularly to decrease the frequency of seizures in certain types of drug-resistant epilepsy (DRE), alleviate chemotherapy-induced nausea and vomiting or chronic pain, or ameliorate behavioural symptoms in conditions such as autism spectrum disorder (2–4). MC is typically reserved for patients who experience severe symptoms that are resistant to traditional treatments, and is often a last resort treatment option for families (5,6). It should be noted that there is an urgent need for more prospective studies to examine safety and efficacy of MC of children.
Caregivers and clinicians who provide care for these children attest to the need to treat MC like any other medication (5,7). However, unlike other prescription medications in Canada, MC does not follow the regular drug review process or have a drug identification number (8). As such, MC is not placed on provincial or territorial drug formularies and insurance health plans do not consistently cover MC, additionally caregivers report barriers with access to supply (9). With a complicated history due to prohibition and a policy emphasis on recreational use, misconceptions about MC remain prevalent and a there is a lack of knowledge among the general population and health care providers (7,9–11). As such, implementing MC into a child’s daily routine can be challenging, especially for those who require a dosage to be administered outside of the home (7). In a recent scoping review, we identified few publications and policies on MC use in Canadian schools (12). A qualitative study of clinicians who authorize MC to children concluded that significant enhancements are needed to improve support for caregivers and children who require it in schools and in general (7). The present study aimed to learn about the experiences of caregivers of school-aged children who require MC.
MATERIALS AND METHODS
Methodology and recruitment
The study followed best practices for undertaking and reporting qualitative research (13,14). We used qualitative description, a methodology which aims to explore a phenomenon of interest using participants in a particular situation and describes a rich description of the experience in an easily understood language (15,16). Advertisements and invitation letters were shared through various Canadian websites and social medical channels to reach the target audience of caregivers for school-aged children and youth that require MC. Potential participants were encouraged to share recruitment materials. A Survey Monkey link remained open from August 2021 to February 2022 for interested participants to provide their contact information to the study team.
Interviews
A semi-structured interview guide (Supplementary Appendix) was developed by The Canadian Collaborative for Childhood Cannabinoid Therapeutics (C4T) ‘Medical Cannabis in Schools’ working group members, which consists of parents of children who take MC, physicians who authorize MC for children, community health nurses, and a pharmacist (n = 10) (17,18). ZZ, a researcher with previous experience in qualitative research, conducted the interviews, which were continued until all open-ended questions were answered and the participant had nothing else to add. The interview guide was structured around questions about MC in schools, but allowed for flexibility to explore topical trajectories about MC in general as they emerged within the dialogue. A $25 gift card was provided to the participants in appreciation of their time.
Data analysis
Eleven interviews (transcribed verbatim) and the notes of one interview (which was not recorded by request of the participant) were analyzed by three researchers experienced in qualitative analysis (HM, SM, and TK). Qualitative content analysis (19) was used to analyze the data using Dedoose (20) to organize the data. All transcripts were reviewed initially by the researchers. Sentences and statements were ascribed line by line into meaning units and labelled with codes (19). The codes were subsequently organized according to categories and subcategories. The researchers met multiple times throughout to iteratively review for category relabeling and refinement until the results were collated into a final manuscript. Participants were sent a copy of the report with an opportunity to provide feedback prior to publication.
Ethics approval
The Behavioural Ethics Board at the University of Saskatchewan (Beh# 2804) approved the study and consent was obtained from participants.
RESULTS
The research team followed up with 30 individuals who provided their contact information to learn more about the study and 12 were reached and agreed to be interviewed. The interviews lasted between 18 and 40 minutes, and 10 were conducted by phone and two by video conference. Participants included parents of children who were taking cannabis oil or pills authorized by a health care provider (either cannabidiol [CBD] alone or CBD and tetrahydrocannabinol) primarily for DRE and/or autism and developmental disorders (Supplementary Table 1).
MC for children at schools
Approximately half of the parents who participated had children (n = 6) who took MC during the school day. Of those who did not, most (n = 4) indicated that the schools were aware their child was on MC and didn’t perceive any barriers should school-dosing be necessary. Participant 1, however, anticipated resistance from the school, while participant 6 said “There’s a zero-tolerance marijuana policy at his school. I’ve just been kind of too scared to bring it up with them because I don’t want it to be an issue. We have already gone through so many issues with school already.”
Five families that required MC administration at school perceived their experiences to be positive or neutral and did not report encountering barriers. The requirements for MC (e.g., paperwork, packaging, labelling) and person responsible for administering the dosage (teacher, educational assistant, or health care provider) varied with the institution. One school, however, administered all medications except MC, which necessitated the parent to drive to the school at lunch hour to administer the mid-day dosage to their child. Caregivers commented on some of the additional fears surrounding MC. Participant 11: “There seems to be a real push to keep the cannabis under lock and key. Some kids can just keep their medications in their backpack or perhaps at the teacher’s desk, or in the office, but the cannabis needs to be put away, away, away. I think that’s just silly at this point, especially an oil… I think a lot of the fear is making it harder for families just to go out on a day trip or to school.”
In general, participants did not perceive teachers and administrators to be well-informed about MC as they learned about it only on a case-by-case basis. According to participant 3, “It was pretty much completely on me to inform them about it and educate them about it, which is fine. I didn’t expect them to be knowledgeable about it. But yeah, I would say their prior knowledge to it was close to zero.” Participants were queried about what they believed schools should know, and whether they had advice for others in their situation (Supplementary Table 2). According to participant 1 “cannabis should be treated the same way as any other medication,” and this sentiment was echoed by the others.
Medical cannabis for children in general
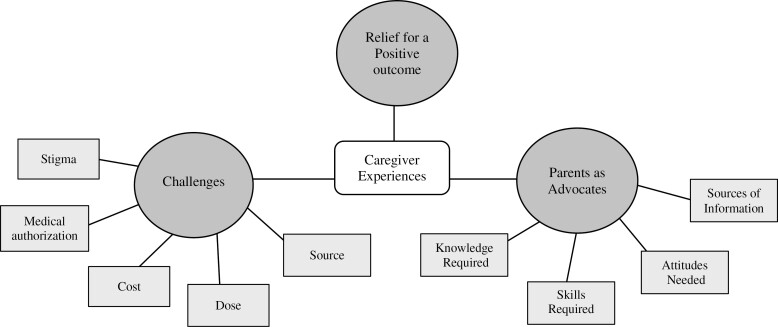
Three main topics emerged consistently throughout the dialogue about MC in general: (1) challenges; (2) parents as advocates; and (3) caregiver relief for positive outcomes. Additional supporting quotes are found in Supplementary Tables 3 and 4. Figure 1 provides an overview of the categories and subcategories.
Figure 1.
Overview of categories and subcategories pertaining to caregiver experiences with medical cannabis.
Challenges
Caregivers encountered numerous challenges related to MC. The majority (n = 8) recounted stigma with physicians, school administrators and staff, and/or family, but many acknowledged improvements as MC use has become more common. Some participants described how stigma decreased once the benefits were realized. For example, participant 2 said, “My family physician went from absolutely I’m not touching that to wow, he’s doing incredibly. She did a 180 based on my son’s experience.”
Caregivers were often met with resistance when discussing MC with their physician. Finding a suitable authorizer was perceived to be a major barrier (n = 7) and they had to seek out multiple health care providers to achieve a positive outcome. Participant 7 shared, “We had been asking to try CBD oil with our neurologist for a year and it was a strict no. They felt like there wasn’t enough research for its use and that we should be using these other medications that have pretty terrible side effects.”
Parents described other challenges, such as finding a place to obtain the MC and determining the appropriate dose for their child’s needs (n = 5). Some highlighted their use of trial and error to find the correct dose. Participant 6, “The doctor only did the set up. Everything else, like the dosage and everything, that’s for me to figure out by myself. I find it really hard to find information on dosage because if you ask on the Facebook group, everyone will always tell you it’s different for everyone. You can’t just have a set dose.”
The high cost associated with acquiring MC was voiced by nearly all participants (n = 10). For some this was “about a mortgage payment per month.” In other instances, MC suppliers subsidized the cost. As stated by Participant 10, “we did have to pay like $1200, $1000 for the first couple prescriptions. But then we found [supplier] where they give the youth program… And now it’s $540.” Participants expressed the need for insurance coverage to include MC as cost is barrier for many families.
Parents as advocates
Parents described having to continuously advocate to access MC. To overcome obstacles, they acknowledged the need for themselves to be well informed and possess key skills to ensure their child’s sustained MC use. According to participant 2, “Parents must take on the task of being well-educated and well-read and then advocating on their child’s behalf in a big way.” Extensive knowledge about cannabis and physiology was perceived to be important, with an ongoing commitment to keep informed about research and to act as an educator (when needed) to their health care providers and others within their circle. Effective communication was perceived to be essential, including the ability to tailor conversations and speak carefully and articulately to change mindsets. The need for a ‘thick skin’ to manage criticism and stigma; tenacity and confidence to ‘speak up’ in advocacy; and therapeutic management were skills and attitudes required to ‘sustain the journey’.
Parents described accessing information and support from several sources. These included online websites, social media, support groups, dispensaries, word of mouth, TedTalks, television and movies, supervising physicians, interprofessional teams, perceived experts, and other parents who have been successful in securing MC for positive outcomes. They were eager to gain information from the lived experiences of others and to support other parents encountering challenges. Clear support from an interprofessional team and working collaboratively was perceived to contribute to success.
Caregiver relief for positive outcomes
Significant improvements in health were attributed to MC, and the caregiver’s relief for these positive outcomes was evident. Participant 5 said, “His prescription has been life changing. He is able to now attend school and play with friends… We’ve had no meltdowns at school, zero. That would be not possible without the cannabis. Not possible.” According to participant 2, “We’re just most grateful for a less violent kid.”
Caregivers expressed gratitude for health care providers that helped navigate the process. According to participant 3, “We were lucky enough to have a really amazing paediatrician and that doctor was very receptive.” Participant 4 said “And it’s just by divine intervention, luck, that she [the physician]’s passionate about cannabis and kids. It was fate that we tried it again.” Participant 2: “And so this gentleman was available to us by email… and I have thanked him and thanked him and thanked him to this day for doing that for us.”
DISCUSSION
Some children taking MC require dose administration at school. Since administrative policies to support children taking MC and their families vary across Canada and some schools prohibit its use altogether (12), we sought to characterize perspectives of caregivers. Except for two parents who faced barriers with MC administration, most described positive or neutral experiences with the schools. Consistent with our previous study of clinicians who prescribe MC (7), caregivers encountered a lack of knowledge about MC within the school system. However, in the present study, only 6 of 12 participants had direct experience with MC in schools, most because it was dosed twice daily around school hours. Hence, data saturation was not reached and more study on this topic is warranted.
A semi-structured interview process provided flexibility to continue the interview with a focus on MC in general and saturation was achieved in this domain. The tenacity of participants to advocate for their children was highlighted and the categories of ‘challenges’, ‘parents as advocates’, and ‘caregiver relief for a positive outcome’ remained consistent throughout the cohort. Identifying a suitable authorizer and cost were among the top challenges, which agrees with two recent Canadian studies of parents of children taking MC for epilepsy (n = 19) (6), and cancer and epilepsy (n = 10) (5). Both studies also describe the relentless pursuit of caregivers to acquire knowledge and navigate the medical system to achieve a positive outcome for their child with a refractory condition.
Our study is unique in that in addition to describing the experiences, we queried caregivers about the facilitators that allowed them to successfully advocate for their child despite significant obstacles. A variety of knowledge, skills, and attitudes were deemed important, such as the ability to synthesize information and keep up to date on cannabis pharmacology, communicate effectively and act as an educator, be proactive and practice resilience. They provided their perspective on what was important for schools and other caregivers to know. The participants in this study were well educated and articulate and 50% were health care providers; we surmise that caregivers who lack such confidence and self-efficacy—especially within the health care system—may not be successful with navigating MC for their children. Efforts should be undertaken to support all caregivers of children who require MC, so the burden does not fall exclusively to the family. For example, creating accessible, evidence-based education that could be shared with teachers, families, or friends may help decrease stigma and alleviate the need for caregivers to continuously articulate ‘the science’ behind MC. This study has several limitations. We recruited participants by advertising through Canadian websites and social medical channels. Although 30 participants provided contact information, we could schedule appointments only for 12. Selection bias could have played a role, whereby participants who felt confident and strongly about the topic, and had positive experiences with MC, were more likely to respond to follow-up communications and participate in the study. We set out explore the perceptions of MC in schools but only six participants had direct experience and data saturation was not achieved on this topic, since many MC regimens are administered on a twice daily basis, negating the need for administration in school hours. Nevertheless, rich dialogue ensued on the experiences of MC in general. While we aimed to learn from caregivers across Canada, the majority of participants were from Ontario and British Columbia. We acknowledge that regional differences exist and may influence caregiver’s experiences with respect to MC.
CONCLUSION
Caregivers demonstrate remarkable tenacity despite the many challenges associated with MC use, but education and practice change are needed to ensure that children using MC can benefit from or continue to experience its positive outcomes within the school environment and beyond.
Supplementary Material
Contributor Information
Holly Mansell, College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Zina Zaslawski, Department of Pediatrics and Child Health, University of Manitoba, Winnipeg, Manitoba, Canada.
Sophia Mbabaali, Centre for Healthcare Innovation, University of Manitoba, Winnipeg, Manitoba, Canada.
Patricia M King, College of Nursing, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Lauren E Kelly, Departments of Pharmacology and Therapeutics and Community Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada; Children’s Hospital Research Institute of Manitoba, Winnipeg, Manitoba, Canada.
Taylor Lougheed, Department of Family Medicine, University of Ottawa, Ottawa, Ontario, Canada; Section of Emergency Medicine, Northern Ontario School of Medicine, Sudbury, Ontario, Canada.
Jennifer Anderson, Department of Family Medicine, University of Manitoba, Winnipeg, Manitoba, Canada.
Richard J Huntsman, Department of Pediatrics, College of Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
Jane Alcorn, College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, Saskatchewan, Canada.
ETHICAL APPROVAL
The Behavioural Ethics Board at the University of Saskatchewan approved the study (Beh#2804).
FUNDING
This study was funded by a seed grant from the College of Pharmacy and Nutrition, University of Saskatchewan and the Canadian Collaborative for Childhood Cannabinoid Therapeutics (C4T).
POTENTIAL CONFLICTS OF INTEREST
Dr. Kelly holds funding from the Canadian Institutes of Health Research, the Canadian Cancer Society, and the SickKids Foundation for C4T. She holds a Mitacs Accelerate grant for a separate project in partnership with Canopy Growth who played no role in the design or funding provided to this project. Dr. Kelly has no financial conflicts of interest. Dr. Lougheed has received speaker’s honoraria and/or consulting fees from Spectrum Therapeutics, Children’s Hospital of Eastern Ontario, University of Ottawa, Syqe Medical, Aleafia Health, and Sanofi. Dr. Alcorn has participated on a advisory board for Zyus Life Sciences Inc. Dr. Anderson has received a speaker’s honoraria and/or consulting fees from Spectrum Therapeutics and C4T, and has participated on the Medcann Advisory Board. Dr. Huntsman is a co-chair of Health Canada’s Scientific Advisory Committee on Health Products Containing Cannabis whose views are not reflected in this manuscript. The other authors have no relevant conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Simonian JS, Varanasi S, Nguyen AV, Diaz-Fong JP, Richards GJ, Le J.. A critical narrative review of medical cannabis in pediatrics beyond epilepsy, part I: background. Pediatr Med 2020;3:14 [Google Scholar]
- 2. Aran A, Cayam-Rand D.. Medical cannabis in children. Rambam Maimonides Med J 2020;11:e0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pawliuk C, Chau B, Rassekh SR, et al. Efficacy and safety of paediatric medicinal cannabis use: A scoping review. Paediatr Child Health 2020;26:228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huntsman RJ, Tang-Wai R, Alcorn J, et al. Dosage related efficacy and tolerability of cannabidiol in children with treatment-resistant epileptic encephalopathy: Preliminary results of the CARE-E study. Front Neurol 2019;10:716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibbard M, Mount D, Rassekh SR, Siden HH.. Family attitudes about and experiences with medical cannabis in children with cancer or epilepsy: An exploratory qualitative study. CMAJ Open 2021;9:E563–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elliott J, DeJean D, Potter BK, et al. Barriers in accessing medical cannabis for children with drug-resistant epilepsy in Canada: A qualitative study. Epilepsy Behav 2020 Oct;111:107120. doi: 10.1016/j.yebeh.2020.107120. [DOI] [PubMed] [Google Scholar]
- 7. Mansell H, Zaslawski Z, Kelly LE, Loughheed T, Brace T, Alcorn J.. Medical cannabis in schools: A qualitative study on the experiences of clinicians. 2022. College of Pharmacy and Nutrition, University of Saskatchewan; [Unpublished manuscript]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Government of Canada. Health products containing cannabis or for use with cannabis: Guidance for the Cannabis Act, the Food and Drugs Act, and related regulations. Cannabis for medical purposes under the Cannabis Act: information and improvements. July 2018. https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/guidance-cannabis-act-food-and-drugs-act-related-regulations/document.html. Accessed April 10, 2022.
- 9. Elliott J, DeJean D, Potter BK, et al. Neurologists’ perspectives on medical cannabis for pediatric drug-resistant epilepsy in Canada: A qualitative interview study. Seizure 2020;78:118–26. [DOI] [PubMed] [Google Scholar]
- 10. Bélanger R, Grant C, Côté M, et al. Canadian pediatricians’ views and knowledge about cannabis use for medical purposes among children and adolescents. Paediatr Child Health 2018;23:e53–4. [Google Scholar]
- 11. Kruger DJ, Mokbel MA, Clauw DJ, Boehnke KF.. Assessing health care providers’ knowledge of medical cannabis. Cannabis Cannabinoid Res 2021;7(4):501–7. doi: 10.1089/can.2021.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansell H, Awal M, Kelly LE, et al. Medical cannabis in Canadian schools; A scoping review of existing policies. Cannabis Cannabinoid Res 2022. Accepted. [DOI] [PubMed] [Google Scholar]
- 13. Tong A, Sainsbury P, Craig J.. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. [DOI] [PubMed] [Google Scholar]
- 14. O’Brien BC, Harris IB, Beckman TJ, Reed DA, Cook DA.. Standards for reporting qualitative research: A synthesis of recommendations. Acad Med 2014;89:1245–51. [DOI] [PubMed] [Google Scholar]
- 15. Bradshaw C, Atkinson S, Doody O.. Employing a qualitative description approach in health care research. Glob Qual Nurs Res 2017;4. doi: 10.1177/2333393617742282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hyejin K, Sefcik J, Bradway C.. Characteristics of qualitative descriptive studies: a systematic review. Res Nurs Health 2017;40:23–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. C4T Working Groups. Canadian Collaborative for Childhood Cannabinoid Therapeutics (C4T). https://www.c4trials.org/subgroups. Accessed May 19, 2021.
- 18. About Us. Canadian Collaborative for Childhood Cannabinoid Therapeutics (C4T). https://www.c4trials.org/aboutus1. Accessed May 19, 2021.
- 19. Elo S, Kyngäs H.. The qualitative content analysis process. J Adv Nurs 2008;62:107–15. [DOI] [PubMed] [Google Scholar]
- 20. Dedoose Version 9.0.19, web application for managing, analyzing, and presenting qualitative and mixed method research data 2021. Los Angeles, CA: SocioCultural Research Consultants, LLC; www.dedoose.com. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.