Abstract
Among the deficits associated with fetal alcohol syndrome (FAS), cognitive impairments are the most debilitating and permanent. These impairments, including deficits in goal-directed behavior, attention, temporal planning, and other executive functions, could result from damage to the prefrontal cortex (PFC), an area that has not been studied sufficiently in the context of FAS. Neuronal connectivity in this area, as measured by distribution of dendritic spines and the complexity of dendritic tree structure, can be influenced by exogenous variables other than alcohol, and the neuronal connectivity in other brain regions can be affected by alcohol exposure. The goal of this study was to determine whether binge-like alcohol exposure on postnatal days (PD) 4–9 affects dendritic spine density and other dendritic tree parameters in mPFC that could possibly underlie functional damage. Rats were intubated with alcohol [5.25 g/kg/day; alcohol exposed (AE)], sham intubated (SI), or remained with the mother (SC, suckle control) on PD 4–9. Animals were sacrificed between PD 26 and PD 30 and brains were processed for Golgi-Cox staining. Apical dendrite complexity and spine density were evaluated for layer III neurons in the mPFC using NeuroLucida software (MicroBrightField, Inc.). Spine density was significantly decreased in AE animals relative to SI and SC controls, but no differences in dendritic complexity were found across experimental groups. Our findings demonstrate that neonatal alcohol exposure has a persistent effect on the spine density in mPFC that can explain functional deficits in this cortical area.
Keywords: pyramidal neurons, Golgi, prefrontal cortex, plasticity, fetal alcohol syndrome
INTRODUCTION
In humans, prenatal exposure to alcohol can result in a wide range of deficits, collectively referred to as fetal alcohol spectrum disorders (FASD) (Astley and Clarren, 2000; Stratton et al., 1996). FASD symptoms generally include growth deficiency, brain damage, and mental retardation (Astley and Clarren, 2000; Streissguth et al., 1991, 1994). Some of the most persistent deficits are cognitive in nature and are associated with damage to the frontal lobe (Spohr et al., 1993; Streissguth et al., 1991, 1994), which has been shown to have smaller volume in FASD patients (Sowell et al., 2002). Behaviorally, damage to this area is evident in humans as deficits in executive functioning, working memory, and response inhibition (Connor et al., 2000; Kodituwakku et al., 2001). Similarly, in animals, damage to this area is associated with problems in response inhibition and reversal learning (Arnsten and Li, 2005; Mihalick et al., 2001). Despite the almost certain involvement of the prefrontal cortex (PFC) in these altered behaviors, little work to date, in humans or animals, has directly observed the effect of developmental alcohol exposure on this brain region.
In humans, neurons of the neocortex are born and migrate to their final positions during the third trimester of fetal development, with the more superficial cells reaching their mature positions the latest (Goldman-Rakic et al., 1983). The third trimester of human prenatal development is a period when deep Layer III and Layer V pyramidal cortical neurons show significant dendritic and axonal growth, while superficial pyramidal cells in the same layers remain immature in appearance (Mrzljak et al., 1990). Dendritic spines also begin to appear on these neurons during this period (Mrzljak et al., 1990). Other developmental processes, including dendritic maturation and synaptogenesis, occur across pre- and postnatal development in humans, with the most rapid changes happening first few years of life (Goldman-Rakic et al., 1983; Huttenlocher and Dabholkar, 1997). In rats, neurogenesis of the most superficial cortical cells occurs just prior to birth (Bayer and Altman, 2004) with some of the latest neurons still migrating to mature positions during the first few days of postnatal life (van Eden et al., 1990). In addition, the major afferent of PFC, the mediodorsal nucleus of the thalamus, does not make contact with its target cells in PFC until a few days after birth in rodents (van Eden et al., 1990). It is also suspected that innervation of the cortex by some of the major neurotransmitter systems, including acetylcholine, dopamine (DA), serotonin, and norepinephrine, does not occur until around the time of birth in rodents and primates (Berger-Sweeney and Hohmann, 1997). In sum, it is not surprising that while in humans the appearance of six-layered frontal cortex occurs prenatally during the third trimester, in the rat it occurs postnatally during the first 10 days of life (Mrzljak et al., 1990; van Eden et al., 1990). Thus, the rat model of third trimester alcohol exposure allows for a more direct examination of alcohol’s effects on the developing frontal cortex because many important stages of development occur after the animal is born.
It is also not surprising then, that heavy exposure to alcohol, specifically within the first 10 days of postnatal life of the rat, has significant effects on one or many of these developmental processes in other cortical areas. Indeed, either acute or prolonged exposure to alcohol during this period alters the development of various cortical areas, including primary somatosensory and motor cortices (Granato et al., 2003). In this case, ethanol exposure decreased dendritic branching of Layer II/III pyramidal neurons (Granato et al., 2003). Effects of neonatal alcohol exposure also extend to the development of dendritic spines, as seen in CA1 of the hippocampus (reviewed in Berman and Hannigan, 2000).
The effect alcohol has on the structure of developing dendrites of the PFC has yet to be examined. It is known that other external stimuli, such as morphine, nicotine, caffeine, chronic stress, and maternal stress have been found to alter the dendritic structure of cells within the PFC (Brown and Kolb, 2001; Juarez-Mendez et al., 2006; Liston et al., 2006; Murmu et al., 2006; Robinson and Kolb, 1999a). Reduced dendritic complexity could be contributing to the behavioral deficits seen in FASD patients, as reduced dendritic complexity in the PFC has been correlated with poor performance on an attentional set-shifting task that is thought to rely heavily on the PFC (Liston et al., 2006).
Changes in dendritic spine density resulting from early alcohol exposure also have yet to be studied in the PFC. The majority of work with spine density and early alcohol exposure has been done in the hippocampus (reviewed in Berman and Hannigan, 2000), where third trimester equivalent alcohol exposure generally results in decreased spine density. As with dendritic complexity, the effects of other variables on spine density in the PFC have been observed, seeing changes dependent on weaning age, maternal stress, amphetamine, nicotine, and restraint stress (Brown and Kolb, 2001; Crombag et al., 2005; Ferdman et al., 2007; Murmu et al., 2006; Radley et al., 2006). Dendritic spines are the predominant sites of contact where spiny neurons like the pyramidal cell receive the majority of their stimulation, so that changes in spine distribution could have profound effects on the functioning of the cell as a whole (Ethell and Pasquale, 2005; Nimchinsky et al., 2002). Furthermore, certain phenotypes, or shapes, of the spines have been associated with either mature or immature synapses (Ethell and Pasquale, 2005; Portera-Cailliau et al., 2003; Zhang and Benson, 2000). In fact, as seen in Fragile X syndrome, disruption of dendritic spine density and spine phenotypes can be evident in a single condition (Comery et al., 1997; Irwin et al., 2000, 2001; McKinney et al., 2005).
The purpose of the current study was to examine the persistent effects of neonatal exposure to alcohol on dendritic arborization, dendritic spine density, and expression of spine phenotypes in the neurons of the PFC. Rats were given intragastric intubations of either alcohol or sham during postnatal days 4–9 and sacrificed between postnatal days 26–31. To examine the structure of neurons in PFC, dendrites were traced and spine densities and phenotypes were evaluated for Layer III pyramidal cells in animals from alcohol-exposed, sham intubated, and suckle control conditions.
MATERIALS AND METHODS
Subjects
All procedures were done in accordance with the University of Delaware Institutional Animal Care and Use Committee. Litters from timed pregnancies (Long Evans rats) were obtained by breeding in the University of Delaware animal facility. Gestational day (GD) 0 was determined by the presence of the vaginal plug, and the day of birth was nearly always GD 22. Litters were culled to 10 pups on postnatal day (PD) 3. The breeders, their suckling litters, and the weaned rats were maintained in an animal lab at the University of Delaware at 22°C with ad libitum food and water on a 12 h:12 h light-dark cycle with lights on at 09.00 h. The developmental timing of all treatments was based on gestational age; reference to ages as PD considers GD 22 as the day of birth (PD 0); hence, PD 4 is GD 26.
Alcohol exposure
At PD 4, litters were randomly assigned to the suckle control (SC) condition or to intubation treatments. The SC pups (n = 10) were weighed daily during the treatment period but otherwise were left undisturbed. Within each intubation litter, pups were randomly assigned to the alcohol exposure (AE) group and to the sham intubation (SI) group. During a single intubation session, AE and SI animals from a litter were removed together and kept on a heating pad. Intubations of an entire litter generally took between 15 and 25 min, after which all the pups were returned together to the dam. AE and SI intubations were performed by gently guiding a polyethylene tube down the pup’s esophagus and into its stomach, using vegetable oil as a lubricant. Once the stomach was reached, an alcohol or milk solution was delivered through the tube for AE animals, while for SI animals the tube was simply removed after 10–15 s. AE pups (n = 11) were given a daily dose of 5.25 g/kg of alcohol in a binge-like manner on PD 4–9. The dose was divided into two intragastric intubations each day, 2 h apart (2.625 g/kg: 11% v/v ethanol in milk formula; 0.028 ml/g body weight). A third intubation of milk (without ethanol) was given 2 h after the second alcohol dose on each day to compensate for reduced milk intake by the AE pups. In addition, on PD 4 only, AE animals were given a second intubation of milk solution 4 h after the second alcohol dose to prevent weight loss in those animals. SI pups (n = 10) were intubated on the same schedule as the AE animals, but without infusion of any solution. All pups were weaned on PD 23 and were housed in social conditions of 3–4 rats of the same sex per cage.
Blood alcohol concentrations
Blood samples for determination of blood alcohol concentrations (BACs) were collected from a tail clip of each AE pup 90 min after the second alcohol intubation on PD 4, using heparinized, 20-μl capillary tubes. BACs were assayed from the plasma of each blood sample using an Analox GL5 Alcohol Analyzer (Analox Instruments, Boston, MA), calibrated prior to each use using standards of known ethanol concentration (200 mg/dl in this case).
Tissue preparation
On PD 26–30, rats were deeply anesthetized with a ketamine-xylazine mixture and transcardially perfused with 0.9% saline. Brains were removed and placed in Golgi-Cox solution (1% potassium dichromate/1% mercuric chloride/1% potassium chromate in distilled water). The brains were left in the dark for about 3 weeks, after which they were transferred into 30% sucrose in saline. The brains were sectioned in the coronal plane using vibratome. Two hundred micrometer serial sections were cut through the entire extent of the PFC and collected in order on gelatinized slides.
Slides were processed as described by (Gibb and Kolb, 1998). In brief, slides were rinsed for 1 min in distilled water. Then they were immersed in ammonium hydroxide for 30 min in the dark, followed by another 1 min rinse in distilled water and immersion in Kodak Fix for 30 min in the dark. Lastly, the slides were dehydrated in increasing concentrations of alcohol and cleared in Safeclear. Slides were then immediately coverslipped using Permount and stored in the dark until completely dry.
Dendrite analysis
For all levels of analysis, the tissue was coded and the experimenter was blind to animal condition. Analysis of neurons was performed on the section closest to Bregma 3.7 as well as the next seven posterior sections in rostro-caudal direction, a total of eight sections per animal. A computer-based neuron tracing system (NeuroLucida; MicroBrightField, Williston, VT) was used to trace the medial prefrontal cortex (mPFC), neurons, and to perform measurements. On each section, the mPFC was identified by the experimenter at low magnification (5× objective) and outlined on the image projected on the computer screen. Using a 40× objective, the cell bodies of Layer III (LIII) pyramidal neurons were marked and traced. Layer III pyramidal neurons were identified by the experimenter, using the characteristic shape of their cell bodies at depths between 300 and 500 μm from the cortical surface (Zilles and Wree, 1995).
The identified Layer III neurons were inspected in order to be chosen for further analysis. The following criteria had to be satisfied: the apical tree’s branches were required to be contained in the section being observed; branches could not be broken or obscured; the extent of the tree should be evenly and fully impregnated, including dendritic spines. These criteria allowed for between 5 and 10 neurons to be traced per animal. At high magnification (100× oil objective), each neuron’s entire apical dendrite was traced (Fig. 1). While tracing the neuron, the software automatically assigned the branching order starting at first bifurcation of the apical dendrite. Once the entire dendritic tree had been traced, Sholl analysis was performed and spine density measurements were made. Spine density measurements consisted of marking all of the spines on each order 2 branch as well as two randomly selected order 5 branches for each cell (Fig. 1). Only branches that were over 20 lm in length were included, and branches were typically in the range of 20–100 μm. Spine density was calculated per 10 μm of dendritic length. On the order 5 branches included in spine density measures, the first 5–20 spines were chosen to be phenotyped according to shapes outlined in (Irwin et al., 2002). In this way, 100 spines were phenotyped for each animal, and each spine was further categorized as either mature or immature in shape.
Fig. 1.
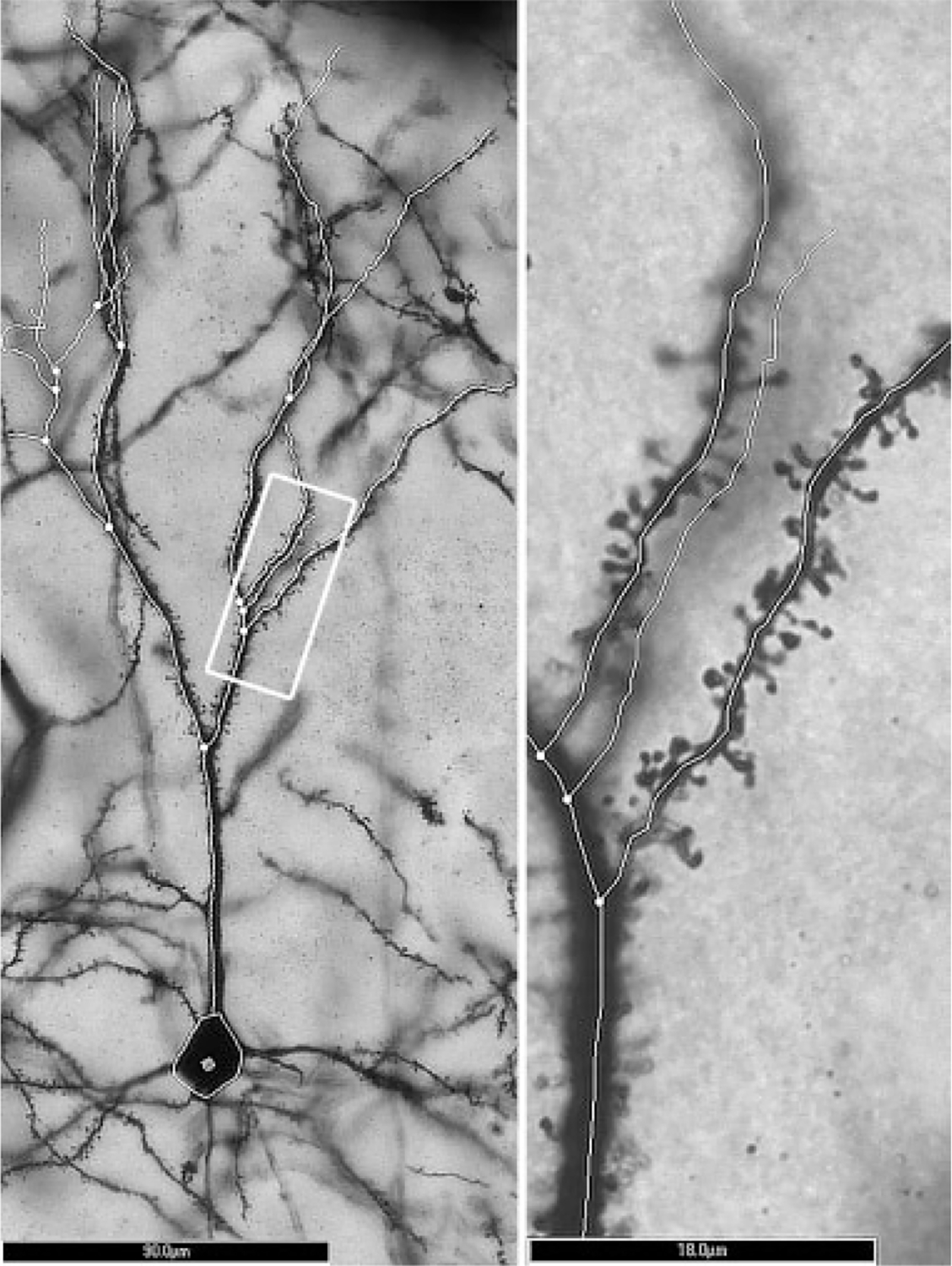
Left: Magnified (20× objective) image and tracing of a Layer III pyramidal neuron cell body and apical dendrite in mPFC. The white rectangle indicates the area magnified to the right. Scale bar = 90 μm. Right: Magnified (×100 oil lens) image and tracing of the same cell shown at left. Numerous dendritic spines are present within the focal plane. Scale bar = 18 μm.
Statistical analysis
Repeated measures analysis of variance (ANOVA) was used to compare Sholl analyses between groups. One-way ANOVA with Tukey post hoc test was used to evaluate the effect of postnatal condition on apical dendrites’ spine density in mPFC. One-way ANOVA was also used to evaluate group differences for total dendritic length and percentage of mature or immature spine phenotypes. The SPSS statistical package was used for all analyses. The level of significance was set at P < 0.05 for all tests.
RESULTS
Blood alcohol concentrations
Blood-alcohol concentrations (BAC) were measured in blood samples obtained from each AE animal 1.5 h after the second ethanol dose on PD 4. The average BAC for AE was 333.5 ± 15.0 mg/dl.
Depth and basic morphology of sampled neurons
For each sampled neuron, the distance from the cortical surface to the center of the soma and the length of the first order apical branch were measured. This allowed for a comparison of the neuronal sampling used for each group. No significant differences were found across groups for either the depth of the neurons’ location or for the length of their first order branches.
Dendritic complexity
Dendritic complexity was measured using three-dimensional Sholl analysis for each traced neuron in Layer III of mPFC (NeuroExplorer software). The mean number of intersections with each concentric sphere surface (radii 20 μm) was analyzed for each group (Fig. 2). No significant differences were found across groups for any individual sphere distance. However, SC pyramidal neurons (n = 32) did show a nonsignificant trend with more total dendritic length per neuron than the cells in SI (n = 27) and AE (n = 39) groups (F(2, 96) = 1.85, P = 0.163) (Fig. 3).
Fig. 2.
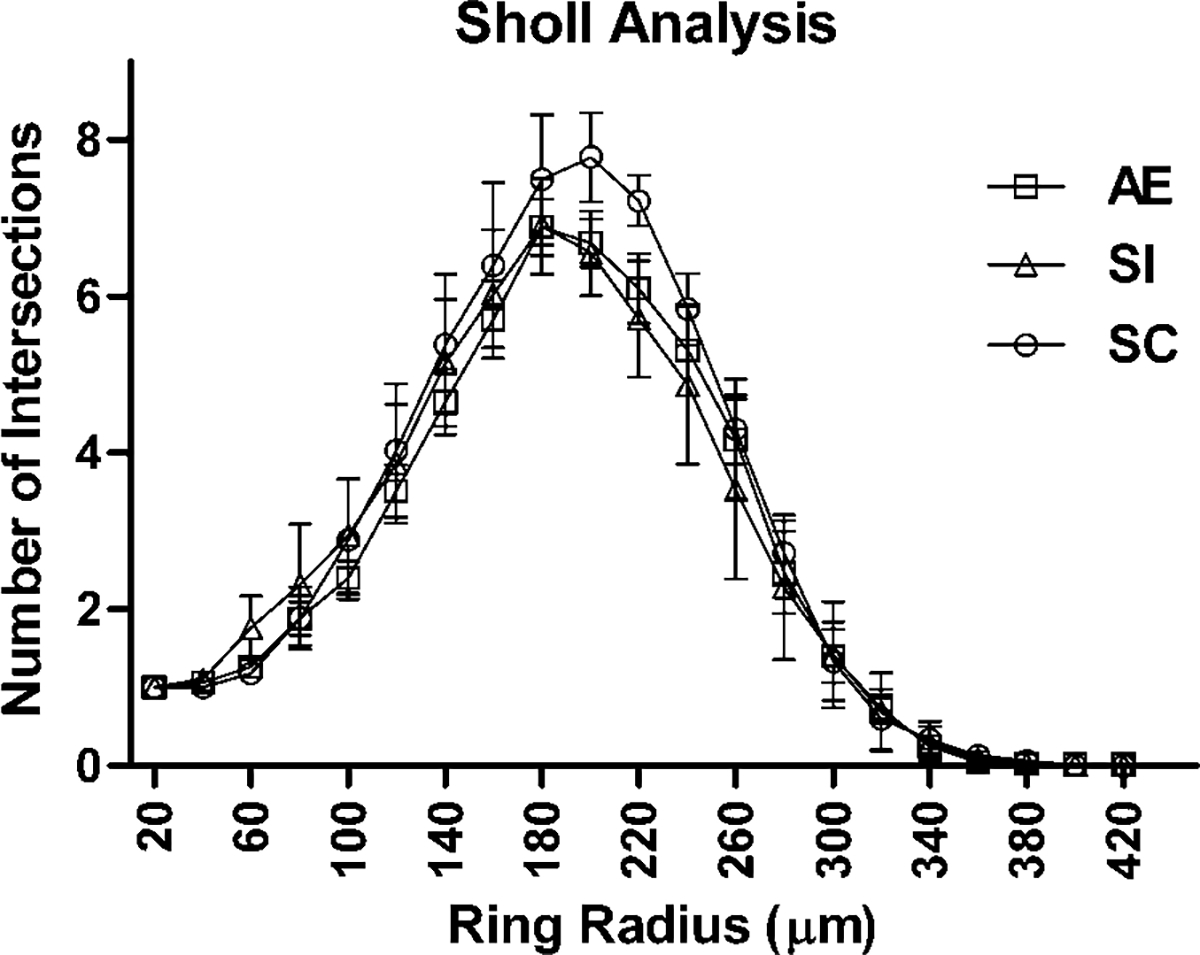
Number of dendritic tree intersections with Sholl radii in each animal group. No significant differences were found across groups (AE, alcohol exposed; SI, sham intubated; SC, suckle control). Values indicate means ± sem.
Fig. 3.
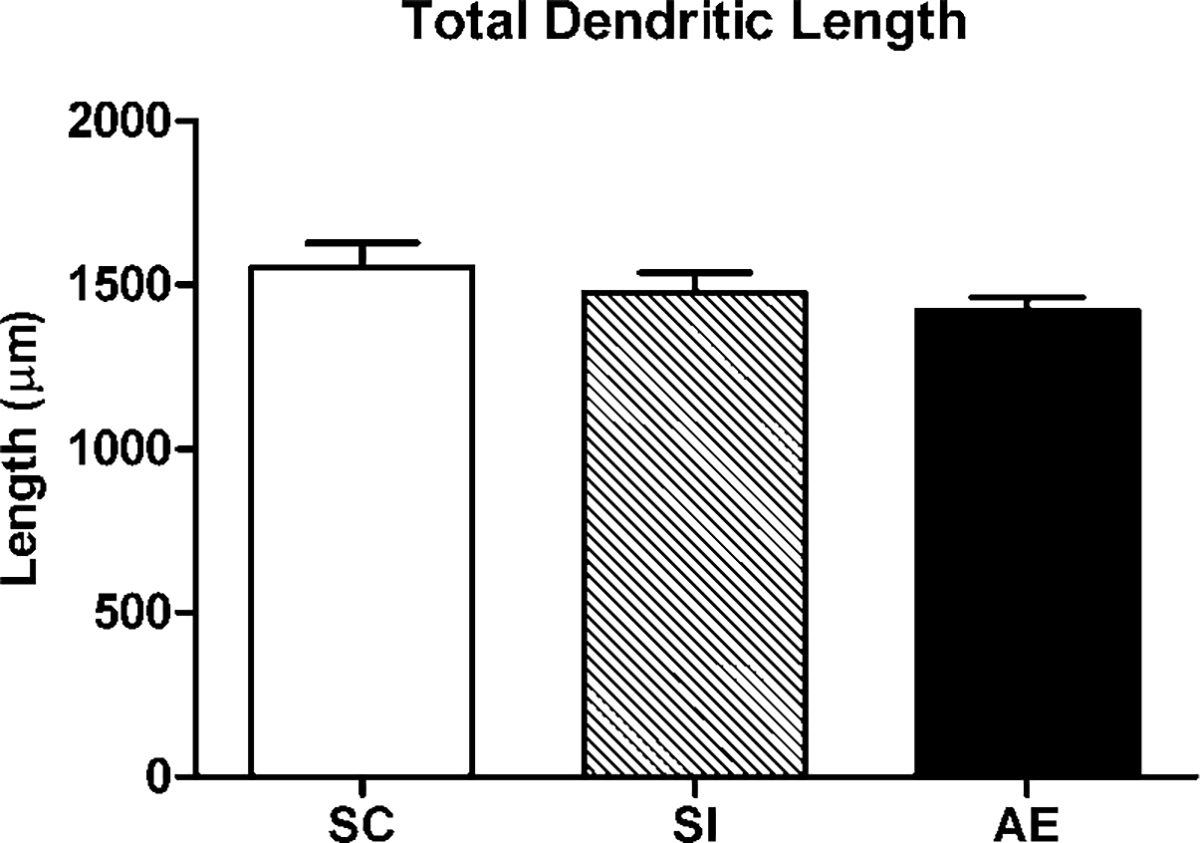
Total dendritic length per Layer III neuron in each animal group. No significant differences were found across groups (AE, alcohol exposed; SI, sham intubated; SC, suckle control). Values indicate means + sem.
Dendritic spine density
Dendritic spine density was calculated per 10 μm of dendrite length for order 2 and order 5 branches. One-way ANOVA showed significant effect of postnatal treatment on spine density for both order 2 (F(147, 2) = 3.39, P < 0.05) and order 5 (F(170, 2) = 6.03, P < 0.01). Post hoc tests demonstrated that Layer III pyramidal neurons from AE mPFC (n = 57) had reduced spine density for order 2 branches compared to SI controls (P < 0.05) and reduced spine density for order 5 branches compared to both SI (n = 49; P < 0.01) and SC (n = 65; P < 0.01) control groups (Fig. 4).
Fig. 4.
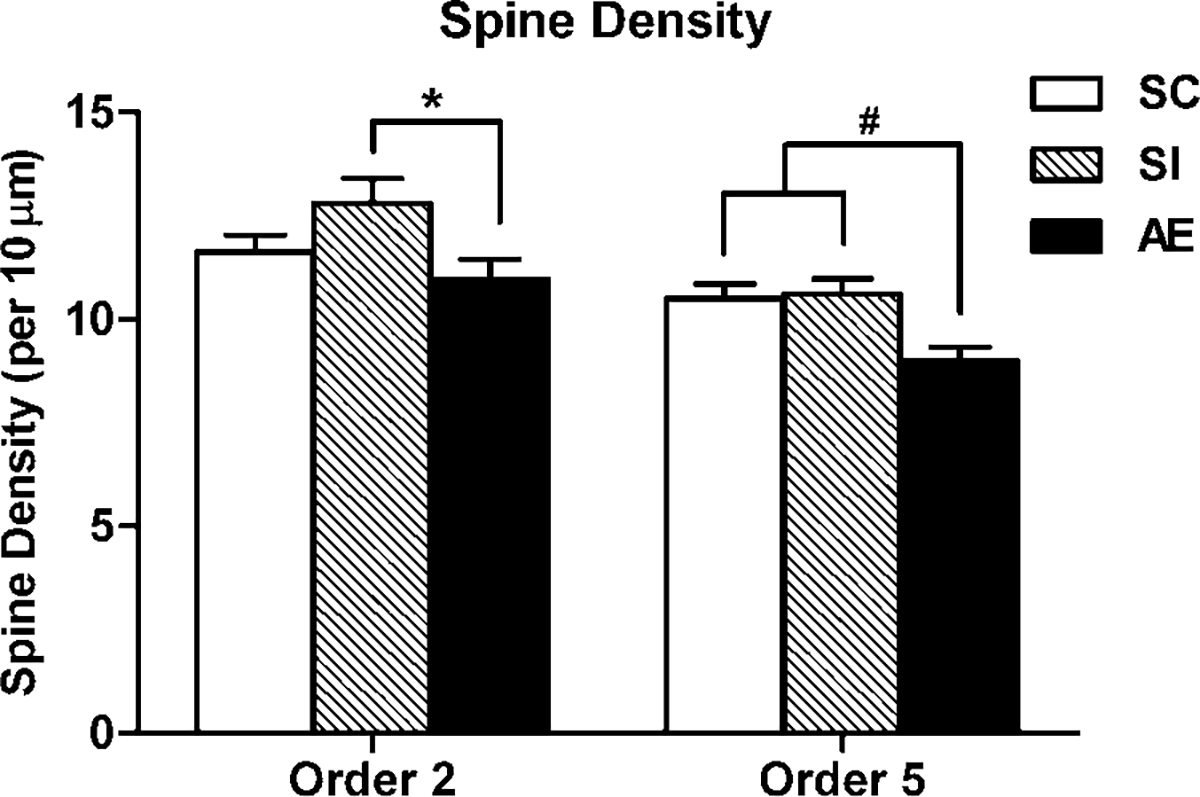
Spine density on order 2 and order 5 denritic branches of Layer III pyramidal neurons in each animal group. AE (alcohol exposed) neurons had significantly reduced density on order 2 branches compared to SI (sham intubated) controls. AE neurons had significantly lower density on order 5 branches compared to neurons from SI and SC (suckle control) groups. Values indicate means + sem (* P < 0.05; # P < 0.01).
Dendritic spine phenotypes
For each animal used in the study of dendritic spine density, one hundred order 5 dendritic spines were phenotyped. Phenotypes were defined based on principles outlined in Irwin et al. (2002) (Fig. 5, bottom). In addition, at the site of each spine included in analysis, the dendritic width was measured to ensure that the thickness of dendritic branches did not differ between any of the three animal groups. There were no significant differences between groups in the width of dendrites used for spine phenotypes. No significant differences were found between groups for any individual spine type, or for overall percentages of immature or mature spines (Fig. 5, top).
Fig. 5.
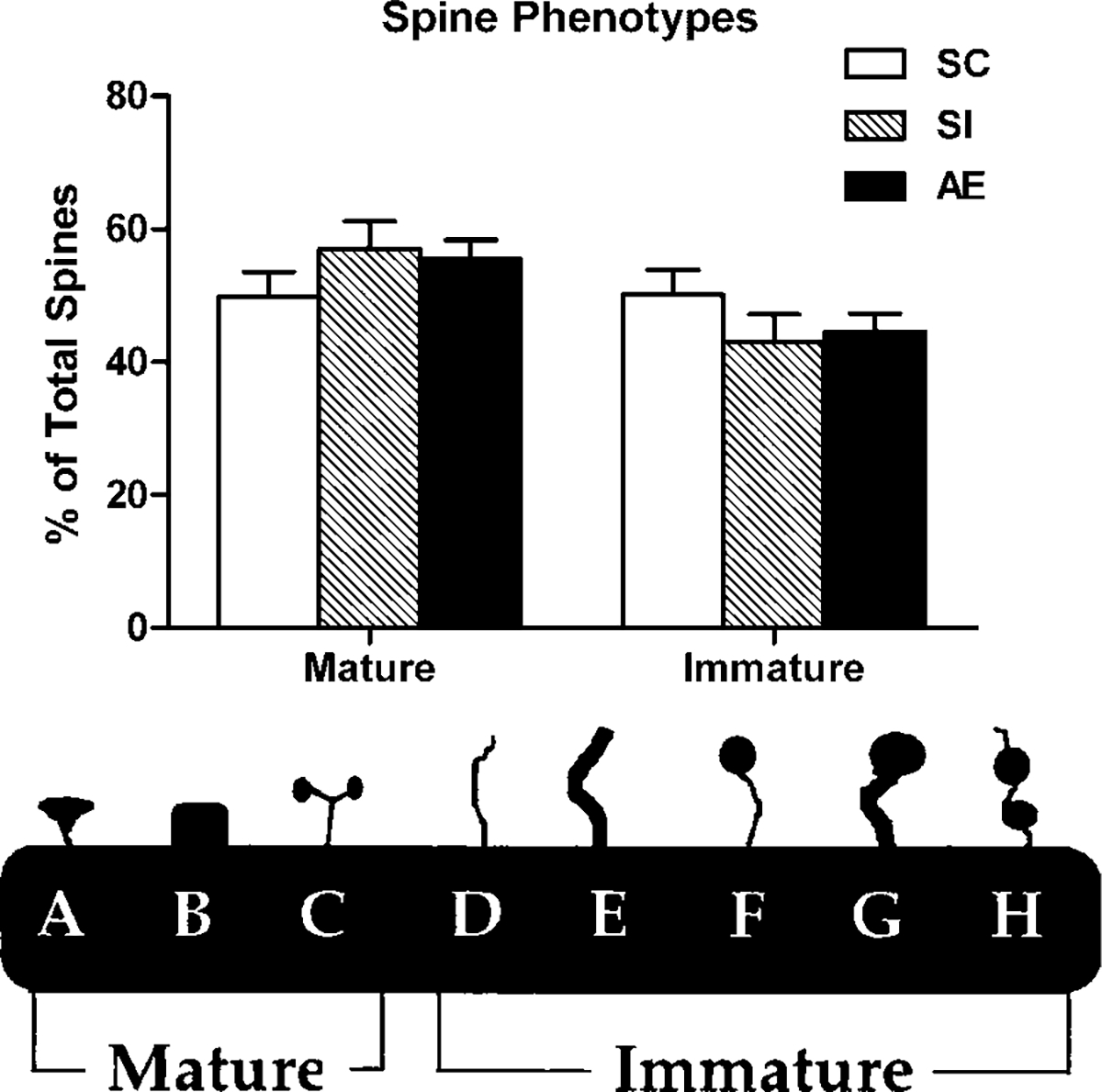
Top: Percentage of mature versus immature spine phenotypes in each animal group. No significant differences were found across groups (AE, alcohol exposed; SI, sham intubated; SC, suckle control). Values indicate means + sem. Bottom: Spine phenotype categorization. This scheme was used to categorize each spine (at least 100 per neuron) and assign it either mature or immature status. Image modified from Irwin et al. (2002).
DISCUSSION
Our results indicate that third trimester equivalent binge-like exposure to alcohol has effects on specific aspects of neuronal structure in mPFC that persist into adolescence in rats. AE animals have significantly lower spine density than controls on the apical dendrites of Layer III pyramidal neurons in mPFC. However, there is no significant evidence that cortical neurons in alcohol-exposed animals have alterations in dendritic shape or length. In addition, while the overall spine density in AE animals is diminished, the ratio of those spines that are mature or immature in appearance remains unchanged.
Decreased spine density resulting from developmental AE has been observed in other brain regions, but never before in mPFC. Gonzalez-Burgos et al. (2006) exposed pups to alcohol throughout gestation and up to PD 30. In the alcohol treated animals, they found decreased spine density in CA1 of hippocampus, as well as decreased presence of mature versus immature spines. Similarly, Berman et al. (1996) reported that pups, exposed to alcohol between gestational days 8–19 and placed in the environmental enrichment after weaning, had decreased CA1 spine density in comparison with control animals from the same environment. Outside of the hippocampus, Fabregues et al. (1985) have shown decreased spine density in somatosensory cortex resulting from gestational AE in guinea pigs. A similar result has been shown in rats with prenatal exposure to alcohol, although the decreased spine density in somatosensory cortex cannot be observed after PD 15 (Galofre et al., 1987).
Although the decrease in spine density is important in itself, the fact that it occurs in the absence of a change in dendritic shape or length, is perhaps most intriguing of all. As described previously by (Kolb et al., 2003), observing this dichotomy of results is quite rare. Various stimuli have effects on both spine density and dendritic tree structure in mPFC: stress decreases spine density and dendritic length (Radley et al., 2006), cocaine self administration increases spine density and dendritic branching (Robinson et al., 2001), repeated morphine injections decrease spine density and branching complexity (Robinson and Kolb, 1999b), and repeated amphetamine injections increase spine density and number of dendritic branches (Robinson and Kolb, 1999a).
Observation of a change, either increase or decrease, in dendritic spine density without a concurrent change in dendritic tree structure was described twice before, curiously in mPFC both times. Kolb et al. (1997b) first found this in rats recovering from frontal lesions inflicted on PD 7–10: rats recovering from lesions showed an increase in mPFC spine density, but no increase in dendritic arborization. Later, Kolb et al. (2003) found a similar result where rats exposed to environmental complexity had increased spine density without increased dendritic branching, an effect exclusive to mPFC.
Our results raise two questions. First, why do manipulations such as frontal lesions, environmental complexity, and, in our case, neonatal AE during PD 4–9 affect spine density? One possibility is that these manipulations are changing the structure of pyramidal cells in mPFC by influencing the afferents of these neurons. Two major types of afferents of pyramidal neuron apical dendrites in mPFC are glutamatergic ones, such as cortico-cortical, hippocampal-cortical, and basolateral amygdala-cortical projections, and the axons from the modulatory neurotransmitter systems, particularly dopaminergic projections from VTA. Excitatory projections from the hippocampus, as well as modulatory ones from DA centers in the midbrain, have been shown to synapse directly on dendritic spines in these neurons (Carr and Sesack, 1996, 2000; Carr et al., 1999). Indeed, inhibiting D1-receptors, norepinephrine, or muscarinic acetylcholine receptors has been shown to decrease the synaptic density in this area (Imai et al., 2004). In fact, glutamatergic and dopaminergic inputs have been found to synapse together on the same dendritic spine (Sesack et al., 2003).
Second, and perhaps more relevant for FASD, what could a reduction in spine density mean functionally? At the cellular level, a change in spine density will likely affect the functioning of mPFC neurons. Most of the excitatory inputs into the Layer III pyramidal neurons in mPFC occur on spines, so any decrease in their presence could reduce the excitability of the neuron as a whole. Cellular learning likely occurs through spine plasticity, and this is supported by the fact that long-term potentiation (LTP) alters the shape and distribution of spines in the hippocampus (Carpenter-Hyland and Chandler, 2007; Toni et al., 1999) just as synaptic plasticity in the same area has been associated with hippocampal-dependent learning (Leuner et al., 2003).
A guinea pig model of chronic prenatal AE led to deficits in both LTP induction and behavioral deficits on the Morris water maze. This effect was dose-dependent: the changes were observed when using a 4 g/kg/day dose, but not with a 3 g/kg/day dose (Byrnes et al., 2004; Richardson et al., 2002). Similarly, rats given chronic prenatal AE showed diminished hippocampal LTP in adulthood (Sutherland et al., 1997). Other than in a small preliminary study by our own collaborators, synaptic plasticity and LTP have not been examined in the context of FAS using our third trimester equivalent binge model, and no model has been used to study the same variables in mPFC. Given the connection between LTP and dendritic plasticity in the hippocampus, and the effects AE has on LTP in the hippocampus, one would expect that diminished mPFC spine density in alcohol-exposed animals accompanies a deficit in mPFC LTP. This is confirmed by our collaborators who have shown, using the exact same animal model of FAS, that cells with dendrites extending to Layer I mPFC of alcohol-treated animals have an inability to induce long-term potentiation (LTP) (Otani, preliminary data).
It is possible that the decrease of dendritic spines density prevents these cells from successful LTP induction, as is suspected to be the case in an animal model of Down syndrome (Belichenko et al., 2007). It has been demonstrated in vitro with hippocampal sections that increased dendritic spine density increases the magnitude of LTP (Collin et al., 1997). However, it is also possible that the lack of LTP prevents further growth of dendritic spines, as has been found in sensorimotor cortex (Ivanco et al., 2000). Induction of LTP in mPFC likely depends on coincident glutamatergic and dopaminergic activity (Baldwin et al., 2002). Dopaminergic afferents of mPFC largely come from the ventral tegmental area (VTA), and spontaneous activity there has been shown to decrease after prenatal exposure to alcohol (Choong and Shen, 2004).
A lack of LTP induction presumably plays an important role in complex learning behaviors that FAS patients perform poorly at. A strong correlation has been made between induction of mPFC LTP and ability to learn mPFC-dependent complex tasks in rats (Mulder et al., 2003). fMRI shows that children with fetal alcohol spectrum disorder have decreased frontal lobe activity relative to controls when performing a task sufficiently difficult enough to require the use of the frontal cortex (Malisza et al., 2005).
Our results provide evidence for a structural correlate of some of the behavioral deficits observed in FAS patients. Decreased spine density in mPFC could possibly alter the functioning of neurons in mPFC, which in turn would affect behavior. The lack of change in dendritic morphology in this animal model of FAS highlights how spines can be affected without affecting the overall structure of the dendrite. Future work with this animal model should attempt to further uncover changes in mPFC as well as identify potential ameliorative mechanisms, such as nerve growth factor, that are already known to increase dendritic spine density in this area (Kolb et al., 1997a).
ACKNOWLEDGMENT
The authors thank Courtney Schnell for her contribution to the spine density data collection.
Contract grant sponsor: NIH; Contract grant number: AA009838.
REFERENCES
- Arnsten AFT, Li B-M. 2005. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57:1377–1384. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. 2000. Diagnosing the full spectrum of fetal alcohol-exposed individuals: Introducing the 4-digit diagnostic code. Alcohol Alcohol 35:400–410. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Kelley AE. 2002. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci 22:1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer SA, Altman J. 2004. Development of the telencephalon: Neural tem cells, neurogenesis, and neuronal migration. In: Paxinos G, editor. The rat nervous system, 3rd ed. San Diego, CA: Academic Press. pp. 27–73. [Google Scholar]
- Belichenko PV, Kleschevnikov AM, Salehi A, Epstein CJ, Mobley WC. 2007. Synaptic and cognitive abnormalities in mouse models of down syndrome: Exploring genotype-phenotype relationships. J Comp Neurol 504:329–345. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Hohmann CF. 1997. Behavioral consequences of abnormal cortical development: Insights into developmental disabilities. Behav Brain Res 86:121–142. [DOI] [PubMed] [Google Scholar]
- Berman RE, Hannigan JH. 2000. Effects of prenatal alcohol exposure on the hippocampus: Spatial behavior, electrophysiology, and neuroanatomy. Hippocampus 10:94–110. [DOI] [PubMed] [Google Scholar]
- Berman RF, Hannigan JH, Sperry MA, Zajac CS. 1996. Prenatal alcohol exposure and the effects of environmental enrichment on hippocampal dendritic spine density. Alcohol 13:209–216. [DOI] [PubMed] [Google Scholar]
- Brown RW, Kolb B. 2001. Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res 899:94–100. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Richardson DP, Brien JF, Reynolds JN, Dringenberg HC. 2004. Spatial acquisition in the Morris water maze and hippocampal long-term potentiation in the adult guinea pig following brain growth spurt-prenatal ethanol exposure. Neurotoxicol Teratol 26:543–551. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. 2007. Adaptive plasticity of NMDA receptors and dendritic spines: Implications for enhanced vulnerability of the adolescent brain to alcohol addiction. Pharmacol Biochem Behav 86:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. 1996. Hippocampal afferents to the rat prefrontal cortex: Synaptic targets and relation to dopamine terminals. J Comp Neurol 369:1–15. [DOI] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. 2000. Dopamine terminals synapse on callosal projection neurons in the rat prefrontal cortex. J Comp Neurol 425:275–283. [DOI] [PubMed] [Google Scholar]
- Carr DB, O’Donnell P, Card JP, Sesack SR. 1999. Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci 19:11049–11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choong K, Shen R. 2004. Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area. Neuroscience 126:1083–1091. [DOI] [PubMed] [Google Scholar]
- Collin C, Miyaguchi K, Segal M. 1997. Dendritic spine density and LTP induction in cultured hippocampal slices. J Neurophysiol 77:1614–1623. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. 1997. Abnormal dendritic spines in fragile X knockout mice: Maturation and pruning deficits. Proc Natl Acad Sci USA 94:5401–5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. 2000. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol 18:331–354. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li YL, Kolb B, Robinson TE. 2005. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex 15:341–348. [DOI] [PubMed] [Google Scholar]
- Ethell IM, Pasquale EB. 2005. Molecular mechanisms of dendritic spine development and remodeling. Prog Neurobiol 75:161–205. [DOI] [PubMed] [Google Scholar]
- Fabregues I, Ferrer I, Gairi JM, Cahuana A, Giner P. 1985. Effects of prenatal exposure to ethanol on the maturation of the pyramidal neurons in the cerebral-cortex of the guinea-pig—A quantitative golgi-study. Neuropathol Appl Neurobiol 11:291–298. [DOI] [PubMed] [Google Scholar]
- Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. 2007. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behav Brain Res 180:174–182. [DOI] [PubMed] [Google Scholar]
- Galofre E, Ferrer I, Fabregues I, Lopeztejero D. 1987. Effects of prenatal ethanol exposure on dendritic spines of layer-v pyramidal neurons in the somatosensory cortex of the rat. J Neurol Sci 81:185–195. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. 1998. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods 79:1–4. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Isseroff A, Schwartz ML, Bugbee NM. 1983. The Neurobiology of Cognitive Development. In: Mussen PM, editor. Handbook of child psychology. New York: Wiley. pp. 281–344. [Google Scholar]
- Gonzalez-Burgos I, Alejandre-Gomez M, Olvera-Cortes ME, Perez-Vega MI, Evans S, Feria-Velasco A. 2006. Prenatal-through-postnatal exposure to moderate levels of ethanol leads to damage on the hippocampal CA1 field of juvenile rats—A stereology and Golgi study. Neurosci Res 56:400–408. [DOI] [PubMed] [Google Scholar]
- Granato A, Di Rocco F, Zumbo A, Toesca A, Giannetti S. 2003. Organization of cortico-cortical associative projections in rats exposed to ethanol during early postnatal life. Brain Res Bull 60:339–344. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. 1997. Developmental anatomy of prefrontal cortex. In: Krasnegor NA, Lyon GR, Goldman-Rakic PS, editors. Development of the prefrontal cortex: evolution, neurobiology, and behavior, 1st ed. Baltimore, MD: Paul H. Brookes Publishing Company. pp 69–83. [Google Scholar]
- Imai H, Matsukawa M, Okado N. 2004. Lamina-selective changes in the density of synapses following perturbation of monoamines and acetylcholine in the rat medial prefrontal cortex. Brain Res 1012:138–145. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Galvez R, Greenough WT. 2000. Dendritic spine structural anomalies in fragile-X mental retardation syndrome. Cereb Cortex 10:1038–1044. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Patel B, Idupulapati M, Harris JB, Crisostomo RA, Larsen BP, Kooy F, Willems PJ, Cras P, Kozlowski PB, Swain RA, Weiler IJ, Greenough WT. 2001. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: A quantitative examination. Am J Med Genet 98:161–167. [DOI] [PubMed] [Google Scholar]
- Irwin SA, Idupulapati M, Gilbert ME, Harris JB, Chakravarti AB, Rogers EJ, Crisostomo RA, Larsen BP, Mehta A, Alcantara CJ, Patel B, Swain RA, Weiler IJ, Oostra BA, Greenough WT. 2002. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. Am J Med Genet 111:140–146. [DOI] [PubMed] [Google Scholar]
- Ivanco TL, Racine RJ, Kolb B. 2000. Morphology of layer III pyramidal neurons is altered following induction of LTP in sensorimotor cortex of the freely moving rat. Synapse 37:16–22. [DOI] [PubMed] [Google Scholar]
- Juarez-Mendez S, Carretero R, Martinez-Tellez R, Silva-Gomez AB, Flores G. 2006. Neonatal caffeine administration causes a permanent increase in the dendritic length of prefrontal cortical neurons of rats. Synapse 60:450–455. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW, Kalberg W, May PA. 2001. The effects of prenatal alcohol exposure on executive functioning. Alcohol Res Health 25:192–198. [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Cote S, RibeiroDaSilva A, Cuello AC. 1997a. Nerve growth factor treatment prevents dendritic atrophy and promotes recovery of function after cortical injury. Neuroscience 76:1139–1151. [DOI] [PubMed] [Google Scholar]
- Kolb B, Stewart J, Sutherland RJ. 1997b. Recovery of function is associated with increased spine density in cortical pyramidal cells after frontal lesions and:or noradrenaline depletion in neonatal rats. Behav Brain Res 89:61–70. [DOI] [PubMed] [Google Scholar]
- Kolb B, Gorny G, Soderpalm AHV, Robinson TE. 2003. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse 48:149–153. [DOI] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. 2003. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci 23:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. 2006. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26:7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malisza KL, Allman AA, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. 2005. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: A functional magnetic resonance imaging study. Pediatr Res 58:1150–1157. [DOI] [PubMed] [Google Scholar]
- McKinney BC, Grossman AW, Elisseou NM, Greenough WT. 2005. Dendritic spine abnormalities in the occipital cortex of C57BL/6 Fmr1 knockout mice. Am J Med Genet B Neuropsychiatr Genet 136B:98–102. [DOI] [PubMed] [Google Scholar]
- Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. 2001. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicol Teratol 23:453–462. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HBM, Vaneden CG, Judas M. 1990. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Prog Brain Res 85:185–222. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Nordquist RE, Orgut O, Pennartz CMA. 2003. Learning-related changes in response patterns of prefrontal neurons during instrumental conditioning. Behav Brain Res 146:77–88. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. 2006. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci 24:1477–1487. [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, Svoboda K. 2002. Structure and function of dendritic spines. Annual Review of Physiology 64:313–353. [DOI] [PubMed] [Google Scholar]
- Portera-Cailliau C, Pan DT, Yuste R. 2003. Activity-regulated dynamic behavior of early dendritic protrusions: Evidence for different types of dendritic filopodia. J Neurosci 23:7129–7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. 2006. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16:313–320. [DOI] [PubMed] [Google Scholar]
- Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC. 2002. Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. Eur J Neurosci 16:1593–1598. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. 1999a. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 11:1598–1604. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. 1999b. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse 33:160–162. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. 2001. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 39:257–266. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. 2003. Anatomical substrates for glutamate-dopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Glutamate and disorders of cognition and motivation. Ann NY Acad Sci 1003:36–52. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. 2002. Regional Brain Shape Abnormalities Persist into Adolescence after Heavy Prenatal Alcohol Exposure. Cereb Cortex 12:856–865. [DOI] [PubMed] [Google Scholar]
- Spohr HL, Willms J, Steinhausen HC. 1993. Prenatal alcohol exposure and long-term developmental consequences. Lancet 341:907–910. [DOI] [PubMed] [Google Scholar]
- Stratton K, Howe C, Battaglia F, editors. 1996. Fetal alcohol syndrome: Diagnosis, epidemiology, prevention, and treatment. Washington, DC: National Academy Press. [Google Scholar]
- Streissguth A, Aase J, Clarren S, Randels S, LaDue R, Smith D. 1991. Fetal alcohol syndrome in adolescents and adults. J Am Med Assoc 265:1961–1967. [PubMed] [Google Scholar]
- Streissguth A, Sampson P, Carmichael Olson H, Bookstein F, Barr H, Scott M, Feldman J, Mirsky A. 1994. Maternal drinking during pregnancy: Attention and short-term memory in 14-year-old offspring—A longitudinal prospective study. Alcohol Clin Exp Res 18:202–218. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, McDonald RJ, Savage DD. 1997. Prenatal exposure to moderate levels of ethanol can have long-lasting effects on hippocampal synaptic plasticity in adult offspring. Hippocampus 7:232–238. [DOI] [PubMed] [Google Scholar]
- Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D. 1999. LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite. Nature 402:421–425. [DOI] [PubMed] [Google Scholar]
- van Eden CG, Kros JM, Uylings HBM. 1990. The development of the rat prefrontal cortex. In: Uylings HBM, van Eden CG, De Bruin JCP, Corner MA, Feenstra MGP, editors. The prefrontal cortex: Its structure, function, and pathology. New York: Elsevier. pp. 169–183. [Google Scholar]
- Zhang WD, Benson DL. 2000. Development and molecular organization of dendritic spines and their synapses. Hippocampus 10:512–526. [DOI] [PubMed] [Google Scholar]
- Zilles K, Wree A. 1995. Cortex: areal and laminar structure. In: Paxinos G, editor. The rat nervous system, 2nd ed. San Diego, CA: Academic Press. pp. 649–685. [Google Scholar]


