Abstract
We compared the ability of Salmonella enterica serovar Typhimurium SL1344 aroA aroD (BRD509) and aroA htrA (BRD807) mutants to act as live vectors for delivery of fragment C of tetanus toxin (FrgC). FrgC was expressed in these strains from either pTETnir15 or pTEThtrA1. BRD509FrgC+ strains elicited ∼2-log-higher serum anti-FrgC antibody titers than BRD807FrgC+ strains. All mice immunized with BRD807pTEThtrA1, BRD509pTEThtrA1, and BRD509pTETnir15 (but not BRD807pTETnir15) were protected against tetanus.
Attenuated Salmonella strains are being investigated as live oral Salmonella vaccines and as vectors for delivery of heterologous antigens and DNA vaccines (2, 5, 8). The successful application of this approach requires the development of safe immunogenic strains of Salmonella spp. and efficient systems for the expression of foreign genes. Genetically defined mutants of Salmonella enterica serovar Typhi are being developed as live oral typhoid vaccines and as live vectors for use in humans (5). Because of the inability of serovar Typhi to cause typhoid-like disease in small animals, the initial characterization of attenuated Salmonella mutants has mainly been performed on Salmonella strains such as S. enterica serovar Typhimurium which can cause systemic infection in mice (8).
Inactivation of a number of different genes highly attenuates serovar Typhimurium without significantly compromising its immunogenicity. Such genes include aro, htrA, cya crp, and phoP (8). Strains with mutations in aro genes have been studied most intensively. aro mutants of serovar Typhimurium function as effective single-dose live oral serovar Typhimurium vaccines and as efficient live vectors for delivering foreign antigen to mice (8).
Recently, Dunstan et al. (3) compared the immunogenicity in mice of a number of different attenuated serovar Typhimurium mutants expressing the nontoxic C-terminal region of tetanus toxin (TT) (fragment C, FrgC). Oral immunization with serovar Typhimurium aro, htrA, cya crp, and ompR, but not purA, strains expressing FrgC all induced high titers of anti-TT serum antibodies and conferred immunity against TT challenge (3).
An aroC aroD mutant of serovar Typhi strain Ty2, CVD908, was immunogenic and well tolerated in human volunteers (11). Unfortunately, because CVD908 was detected in the blood of volunteers, although the subjects remained afebrile, this “vaccinemia” was considered undesirable (11). In an attempt to overcome this the htrA gene of CVD908 was inactivated. The approach was successful, even at a dose of 5 × 109 CFU, CVD908 htrA was undetectable in the blood of volunteers (12). Importantly, the immunogenicity of CVD908 was not significantly impaired by inactivating htrA. Phase 2 studies on CVD908 htrA have recently been reported and have confirmed the promising safety and immunogenicity of the strain (13). The success of htrA inactivation in abolishing vaccinemia can be explained from the behavior of serovar Typhimurium htrA mutants in mice. Serovar Typhimurium htrA mutants are severely compromised in their ability to translocate from the Peyer's patches to cause systemic infection (3, 4).
As mentioned above, it is hoped that live salmonella vaccine strains will also be used as live carriers for heterologous antigens. To investigate the capacity of Salmonella aroA htrA strains to act as live vectors, we compared the efficiency of isogenic serovar Typhimurium aroA htrA (BRD807 [1]) and serovar Typhimurium aroA aroD (BRD509 [10]) mutants expressing FrgC to immunize mice against tetanus and salmonella infection. Strains harboring either the pTETnir15 or the pTEThtrA1 FrgC expression plasmid was studied (9). FrgC expression is controlled by the nir15 promoter on the former plasmid and by PhtrA on the later plasmid (9). We have previously shown that a single oral immunization of mice with BRD509 harboring either of the FrgC plasmids confers complete and long-lasting protection against tetanus and serovar Typhimurium (9).
Groups of 8 to 10 mice were orally immunized once with ∼1010 CFU of BRD807, BRD807(pTETnir15), BRD807(pTEThtrA1), BRD509, BRD509(pTETnir15), or BRD509(pTEThtrA1). Serum samples were taken 42 days after immunization and assayed for anti-FrgC antibodies by enzyme-linked immunosorbent assay as described previously (9). On day 46 the mice in each group were split into two groups. One group was challenged with ∼2 × 108 CFU of wild-type serovar Typhimurium (SL1344), and the remaining mice were challenged with 50 times the 50% lethal dose of TT.
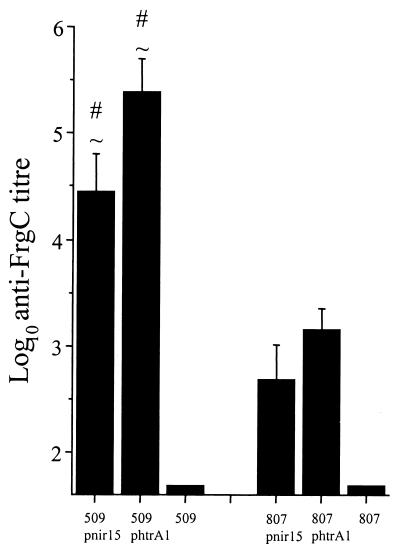
The serum anti-FrgC antibody responses are shown in Fig. 1. The mean anti-FrgC titers of mice immunized with BRD509(pTETnir15) and BRD509(pTEThtrA1) were ∼2 logs higher and were significantly greater (P < 0.05) than those of mice immunized with BRD807 constructs expressing FrgC. For both the BRD509 and the BRD807 groups, immunization with the construct possessing the pTEThtrA1 plasmid elicited higher anti-FrgC titers than did immunization with the corresponding strain possessing the pTETnir15 plasmid, as would have been expected from previous studies (7, 9).
FIG. 1.
Serum anti-FrgC antibody response. Mice were bled 42 days after oral immunization with the indicated attenuated serovar Typhimurium strains. The bars represent the mean log10 anti-FrgC titer, and the error bars indicate the standard error of the mean. Data were analyzed for statistical significance by single-factor analysis of variance. The number sign indicates that the mean titer is significantly higher (P < 0.05) than that of mice immunized with the BRD807(phtrA1). A tilde sign indicates that the mean titer is significantly higher (P < 0.05) than that of mice immunized with the BRD807(pnir15).
The results of the challenge experiments with mice immunized with the BRD509 constructs are in agreement with those from previous studies (Table 1). Namely, a single oral immunization with these strains was sufficient to induce protective immunity to tetanus and serovar Typhimurium. All mice immunized with BRD807(pTEThtrA1) were completely protected against tetanus. However, of the five TT-challenged mice that were immunized with BRD807(pTETnir15), three died 2 days after challenge and a fourth developed slight signs of tetanus 4 days after challenge and was killed. The serum anti-FrgC antibody assays were performed after the mice had been challenged. It was found that four of the mice in the BRD807(pTETnir15) group had anti-FrgC antibody titers that were very low (data not shown). Mice were randomly placed into groups to be challenged with TT or serovar Typhimurium. It is not known, but it is likely that the four mice in the BRD807pTETnir15 group with low serum anti-FrgC titers were challenged with TT. Expression of FrgC appeared to affect the ability of BRD807 to induce protective serovar Typhimurium immunity because, unlike the mice that received BRD807, not all of the animals that received BRD807 (pTETnir15) or BRD807(pTEThtrA1) were protected against serovar Typhimurium challenge. Our results indicate that serovar Typhimurium aroA htrA is an inferior live vector compared to serovar Typhimurium aro and serovar Typhimurium htrA mutants, at least for FrgC (3, 9). Despite this, a single oral immunization with BRD807(pTEThtrA1) was able to induce complete protection against tetanus. This indicates that the efficacy of Salmonella aroA htrA mutants as live vectors may be increased by using improved expression systems.
TABLE 1.
Effect of vector strain and expression system on protection against tetanus and salmonella
| Vaccine strain | Survivors (%) following challenge with:
|
|
|---|---|---|
| TT | Serovar Typhimurium SL1344 | |
| BRD509(pTETnir15) | 100 | 100 |
| BRD509(pTEThtrA1) | 100 | 100 |
| BRD509 | 0 | 100 |
| BRD807(pTETnir15) | 20 | 80 |
| BRD807(pTEThtrA1) | 100 | 75 |
| BRD807 | 0 | 100 |
| Control mice | 0 | 0 |
Using a murine intranasal immunization model, it was recently shown that CVD908 expressing FrgC from a plasmid under the control of the nir15 promoter or the lpp promoter can induce serum anti-TT antibodies (6). It was not reported if and how many of the mice were protected from tetanus or if the immunogenicity of the CVD098 htrA expressing FrgC differed from that of CVD908 expressing FrgC. We recently reported that the anti-FrgC serum antibody response elicited by immunization with the highly immunogenic BRD509 FrgC-expressing strains was severely reduced if the mice had preexisting immunity to serovar Typhimurium (7). It will be important to determine if the same holds for serovar Typhi aro htrA mutants because it is envisioned that one of the major uses of attenuated serovar Typhi strains will be as live vectors in areas of the world where typhoid is endemic.
REFERENCES
- 1.Chatfield S N, Strahan K, Pickard D, Charles I G, Hormaeche C E, Dougan G. Evaluation of Salmonella typhimurium strains harbouring defined mutations in htrA and aroA in the murine salmonellosis model. Microb Pathog. 1992;12:145–151. doi: 10.1016/0882-4010(92)90117-7. [DOI] [PubMed] [Google Scholar]
- 2.Darji A, Guzman C A, Gerstel B, Wachholz P, Timmis K N, Wehland J, Chakraborty T, Weiss S. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91:765–775. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 3.Dunstan S J, Simmons C P, Strugnell R A. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune responses against a heterologous antigen. Infect Immun. 1998;66:732–740. doi: 10.1128/iai.66.2.732-740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphreys S, Stevenson A, Bacon A, Weinhardt A B, Roberts M. The alternative sigma factor, sigmaE, is critically important for the virulence of Salmonella typhimurium. Infect Immun. 1999;67:1560–1568. doi: 10.1128/iai.67.4.1560-1568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine M M, Galen J, Barry E, Noriega F, Chatfield S, Sztein M, Dougan G, Tacket C. Attenuated Salmonella as live oral vaccines against typhoid fever and as live vectors. J Biotechnol. 1996;44:193–196. doi: 10.1016/0168-1656(95)00094-1. [DOI] [PubMed] [Google Scholar]
- 6.Pickett T E, Pasetti M F, Galen J E, Sztein M B, Levine M M. In vivo characterization of the murine intranasal model for assessing the immunogenicity of attenuated Salmonella enterica serovar Typhi strains as live mucosal vaccines and as live vectors. Infect Immun. 2000;68:205–213. doi: 10.1128/iai.68.1.205-213.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roberts M, Bacon A, Li J, Chatfield S. Prior immunity to homologous and heterologous Salmonella serotypes suppresses local and systemic anti-fragment C antibody responses and protection from tetanus toxin in mice immunized with Salmonella strains expressing fragment C. Infect Immun. 1999;67:3810–3815. doi: 10.1128/iai.67.8.3810-3815.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts M, Chatfield S N, Dougan G. Salmonella as carriers of heterologous antigens. In: O'Hagan D T, editor. Novel delivery systems for oral vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 27–58. [Google Scholar]
- 9.Roberts M, Li J, Bacon A, Chatfield S. Oral vaccination against tetanus: comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters. Infect Immun. 1998;66:3080–3087. doi: 10.1128/iai.66.7.3080-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strugnell R, Dougan G, Chatfield S, Charles I, Fairweather N, Tite J, Li J L, Beesley J, Roberts M. Characterization of a Salmonella typhimurium aro vaccine strain expressing the P.69 antigen of Bordetella pertussis. Infect Immun. 1992;60:3994–4002. doi: 10.1128/iai.60.10.3994-4002.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tacket C O, Hone D M, Curtiss R, Kelly S M, Losonsky G, Guers L, Harris A M, Edelman R, Levine M M. Comparison of the safety and immunogenicity of delta aroC delta aroD and delta cya delta crp Salmonella typhi strains in adult volunteers. Infect Immun. 1992;60:536–541. doi: 10.1128/iai.60.2.536-541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tacket C O, Sztein M B, Losonsky G A, Wasserman S S, Nataro J P, Edelman R, Pickard D, Dougan G, Chatfield S N, Levine M M. Safety of live oral Salmonella typhi vaccine strains with deletions in htrA and aroC aroD and immune response in humans. Infect Immun. 1997;65:452–456. doi: 10.1128/iai.65.2.452-456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacket C O, Sztein M B, Wasserman S S, Losonsky G, Kotloff K L, Wyant T L, Nataro J P, Edelman R, Perry J, Bedford P, Brown D, Chatfield S, Dougan G, Levine M M. Phase 2 clinical trial of attenuated Salmonella enterica serovar typhi oral live vector vaccine CVD908-htrA in U.S. volunteers. Infect Immun. 2000;68:1196–1201. doi: 10.1128/iai.68.3.1196-1201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]