Abstract
Objective
Although the effects and safety of transcranial direct current stimulation (tDCS) treatment in depressive patients are largely investigated, whether the self-administration of tDCS treatment at patient’s home is comparable to clinic-based treatment is still unknown.
Methods
In this single-arm, multi-center clinical trial, 61 patients with mild to moderate major depressive disorder were enrolled. tDCS treatment was delivered at the patient’s home once a day, 5 to 7 times a week for 6 weeks, and each session lasted for 30 minutes. The primary outcome was a total Beck-Depression Inventory-II score, and no concurrent antidepressants were used.
Results
The remission rates in both Full-Analysis (FA) (n = 61) and Per-Protocol (PP) (n = 43) groups were statistically significant (FA 57.4% [0.44−0.70], PP 62.8% [0.47−0.77]; percent [95% confidence interval]). The degree of depression-related symptoms was also significantly improved in 2, 4, and 6 weeks after the treatment when compared with baseline. There was no significant association between treatment compliance and remission rate in both FA and PP groups.
Conclusion
These results suggest that acute treatment of patient-administered tDCS might be effective in improving the subjective feeling of depressive symptoms in mild to moderate major depressive disorder patients.
Keywords: tDCS, Depression, At-home treatment, Patient-administered
INTRODUCTION
Transcranial direct-current stimulation (tDCS) is the type of neuromodulation that delivers low direct current through electrodes on the forehead. About 1 to 2 mA intensities of homogenous direct-current field are known to induce long-lasting changes of the cerebral cortex [1]. Electrical field across an anode and cathode shift the synaptic neurons’ resting membrane potential alters the long- term potentiation, and induce the change in the relevant brain functions [2,3]. Application of tDCS on the prefrontal cortex might affect the executive functions [4] and as the functional neuroimaging studies consistently found that the activity of the prefrontal cortex is reduced in depressive patients [5], tDCS intervention in patients with depression have been applied [6].
A recent meta-analysis showed that tDCS application for the acute treatment of major depressive patients showed a higher response when compared with sham therapy and the therapeutic effect was similar to classical non-invasive neuromodulation techniques such as repetitive transcranial magnetic stimulation [7]. The effect size of tDCS was also comparable with antidepressant drugs [8], and there was a report that tDCS therapy did not show noninferiority to a certain antidepressant (e.g., escitalopram) [9]. The combination of antidepressants (e.g., sertraline) and tDCS also increase the efficacy of treatment without the difference in safety [10]. Among the parameters of tDCS, the dosage of electrical stimulation [11], and the baseline factors of retardation might have an association with response to tDCS treatment [12].
Multiple evidence have shown that tDCS have neurobiological and clinical evidences on the treatment of patients with depressive symptoms, most of the studies have practiced at the hospital and thus patients should visit the site to have tDCS treatment. This might make it hard to properly utilize the advantages of tDCS, such as portability and few sides effects [13]. In order to elicit neuromodulation, daily treatment would be needed, but it is very difficult to visit a clinical setting every day for treat-ment. Recent pilot study have shown that trial of home- administered tDCS improved the depressive symptom which was measured with Montgomery-Asberg Depression Rating Scale [14], but the therapeutic effect of self-administered tDCS on mild to moderate depressive patients need more clinical evidence [15]. Therefore, this study aims to investigate whether the patient-application of a portable tDCS device at home might have treatment effects on the improvement of depressive symptoms.
METHODS
Trial Design
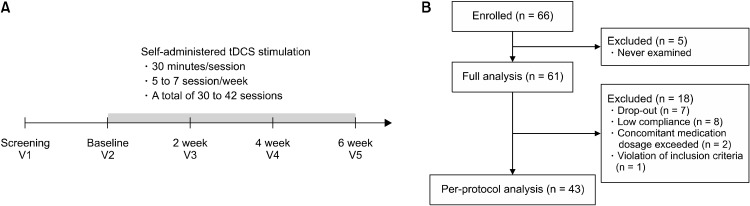
In this single-arm, multicenter clinical trial, we aimed to confirm the therapeutic effect of self-administered tDCS in patients with mild to moderate major depressive disorder. The overall process of the study is presented in Figure 1A. After the screening, participants visited the hospital and received instruction for the usage of an at-home stimulation tDCS device. Then, they self-admin-istered the tDCS device for 30 minutes a day for 6 weeks and wrote a document for the stimulation. Every 2 weeks after the first visit, all participants visited the hospital and the psychiatrist evaluated the efficacy of tDCS stimulation with a score of Beck-Depression Inventory-II score (BDI-II) [16], Hamilton Depression Scale (HAM-D) [17], and Beck Anxiety Inventory (BAI) [18]. The laboratory examination, physical examination, and vital signs were also measured at the visit.
Fig. 1.
(A) Study design and protocols. (B) Enrollment and allocation of groups and drop-out cases. tDCS, transcranial direct current stimulation.
All participants in this study provided written informed consent, and the study was approved by the Institutional Review Board of the Ethics Committee of Seoul St. Mary’s Hospital at The Catholic University of Korea (KC09FZZZ 0211).
Participants and Case Definition
Following inclusion/exclusion criteria were used to enroll the participants in the study.
Inclusion criteria
1) Males and females aged 19 to 65 years were included in this study. Those who satisfied the diagnostic criteria for the unipolar or nonpsychotic major depressive disorder based on the Diagnostic and Statistical Manual, fifth edition (DSM-5) criteria and then received a definite diagnosis thereof using the Mini-International Neuropsy-chiatric Interview.
2) Those who scored 14 to 28 points in the BDI-II.
3) Among the patients who had previously taken antidepressants, antipsychotics, or anticonvulsants for at least one week, those who underwent a sufficient wash-out period longer than five times the half-life of the drug (e.g., more than two weeks for venlafaxine hydrochloride, paroxetine hydrochloride, and fluoxetine hydrochloride).
4) Those who could read and understand the subject information sheet and the informed consent form with sufficient language skills to answer the questionnaire.
5) Those who voluntarily decided to participate in this study and gave written informed consent and were able to apply self-treatment at home throughout the entire study period.
Exclusion criteria
1) Those diagnosed with Axis I disorders other than the major depressive disorder. (However, those diagnosed with major depressive disorder with comorbidity of anxiety disorder were allowed to participate in this study. All diagnostic decisions were made based on the DSM-5).
2) Of those diagnosed with anxiety disorder as a comorbidity in consideration of the DSM-5 criteria together, patients with post-traumatic stress disorder or obsessive- compulsive disorder were excluded from recruitment.
3) Those who diagnosed with depressive disorders other than a major depressive disorder (e.g., dysthymic disorder and depressive disorder not elsewhere classified).
4) Those who are diagnosed with bipolar or psychotic major depressive disorder.
5) Those who had attempted suicide within six months from the screening date or those who were at risk of suicide sufficient to require hospitalization in a protective ward by the judgment of the clinician.
6) Those who were judged to have problems with attaching electroencephalography and direct current stimulation electrodes due to deformities of the scalp, inflam-matory reactions, or other dermatological problems.
7) Those who were judged to have other reasons for being prohibited from the use of tDCS medical devices (e.g., those with a metal plate implanted in the head, etc.).
8) Those who were taking antidepressants at the time.
9) Those who had failed to show adequate responses after administration of more than two antidepressants containing different components for more than six weeks each for each drug before the current depressive episode.
10) Those with clinically serious impairment in the cardiovascular system, digestive system, respiratory system, endocrine system, or central nervous system to the extent that would affect intellectual property application and efficacy evaluation during the study period (e.g., respiratory difficulties when sitting still, endocrine/central nervous system disorders that cannot be controlled using drugs, etc.).
11) Those who had participated in other clinical trials within 30 days from the screening date.
12) Among female subjects of childbearing potential, those who refused to agree to practice contraception by using a medically acceptable method during this study period.
13) Pregnant women.
In addition to those listed above, those who were deemed unsuitable for participating in this clinical trial by the medical judgment of the investigator due to other reasons.
Usage of Psychiatric Medication
Participants who were not taking antidepressants were screened in this study. If the participants were previously given antidepressants, antipsychotics, or anticonvulsants for more than a week, required to have a washout period of at least five drug half-lives. Concomitant usage of anxiety drugs at a dose equivalent to diazepam 20 mg per day was allowed. Zolpidem which is frequently prescribed for sleep was allowed at the maximum dosage of 10 mg per day.
Protocol and Validation of tDCS Treatment
A self-administered tDCS device was administered for six weeks. Participants were instructed to use the home-based tDCS equipment (YMS-201B; Ybrain Inc.), and all participants thereafter conducted tDCS treatment at home once a day for 30 minutes (5 to 7 times per week). A stimulation of 2 mA of current was delivered during the 30 minutes of treatment, but participants who were sensitive to stimulation were allowed to lower the current rate to 1.5 mA. This stimulus setting was automatically maintained in home care throughout the treatment period. In order to prevent overuse at home, the equipment was activated only once a day with the set treatment dose. The operating time for measuring compliance was automatically recorded. The cathode patch was attached to the right dorsolateral prefrontal area. All participants visited the designated hospital every 2 weeks (baseline, 2, 4, and 6 weeks), and the evaluation of safety and efficacy was conducted.
To check whether tDCS treatment was successful at home, the following method was used. First, when the patients visited the hospital, the log data of the at-home tDCS device was thoroughly evaluated. The pre-evaluated program guaranteed whether the treatment was applied once a day, 5 to 7 times a week, a total of 30 to 42 times. Second, the subject filled out the ‘self-feedback questionnaire’, which was distributed with pain and discomfort due to the application of the medical device at home after the first application of the medical device. Third, when the study ends, the medical staff checked the tDCS module and evaluated the history of all logs in the device, whether the participants properly used tDCS treatment at their home.
Outcome Measurements and Hypothesis
The primary outcome of this study was an improvement of a total score of BDI-II score.
We hypothesized that the response rate (P) of the patient whose BDI-II score at 6 weeks after tDCS application was less than 13 points was less than or equal to the response rate of P0 (= 12.8%).
Sample Size
The number of participants was derived from the 6-week remission rate of BDI-II, which was the primary outcome. The remission rate based on the total BDI-II score at the endpoint of 6-week tDCS treatment and the cutoff point was 12 [19].
Based on the results of the meta-analysis using repetitive transcranial magnetic stimulation [20], the target remission rate of this study was initially set to 23.7%. With the confidence interval of 95%, the lower limit of the confidence interval was set to 12.8% referring to the remission range of sham stimulation of Berlim et al. [20]’s study. Then we derived the sample size of 58 using the Power Analysis and Sample Size Software (NCSS, LLC.). Assuming a 10% dropout rate, a total of 65 participants were recruited in this study.
Assignment of Subject Identification Code
This clinical trial was designed as open, multicenter trials that confirm the therapeutic effect of self-administered tDCS on depression. As a single-group clinical trial, randomization and blinding procedures were not conducted. Therefore, all subjects who met the selection/exclusion criteria who received a subject registration number were assigned to the test group and were stimulated by tDCS devices for clinical trials.
Statistical Analysis
Descriptive statistics of a total BDI-II score at the baseline and after the 6-week treatment were presented. The frequency of remission (the number of participants who had a total score of BDI-II ≤ 12 at the 6-week point / the number of all participants) were measured. We assessed the tDCS treatment has an effect on the treatment of depression if the lower limit of the confidence interval was larger than 12.8% (two-sided test of 95%, one-sided test of 97.5%). As there was a statistically significant difference between the clinical centers, we set the ‘site of clinical center’ as a covariate and performed the generalized estimation equation analysis. The distribution was assumed to be binomial and the logit link function was used.
For the analysis of the secondary effect of tDCS treatment, paired t-test was done to the BDI-II, HAM-D, and BAI. The total score of each scale between each measurement point (2, 4, and 6 weeks) and the baseline was assessed. All statistical analyses were performed using Statistical Analysis System version 9.4 (SAS Institute Inc.).
RESULTS
A total of 66 participants enrolled in the study and 12 were drop out throughout the 6-week treatment protocol (Fig. 1B). Thus, in the full analysis group (FA) included 61 participants excluding 5 who had never rated effective variables. In the per-protocol group (PP), 43 participants were included who completed the 6-week tDCS treatment protocol. Demographic variables of all participants are presented in Supplementary Table 1 (available online).
Main Effect of Self-administered tDCS on BDI-II Score
Table 1 shows the main result of this study that examined the remission rate of FA and PP groups. At the end of the 6-week treatment, 57.5 (0.44−0.70) (ratio [95% confidence interval]) percent of the FA group and 62.8 (0.47−0.77) percent of the PP group had a total BDI-II score less than 13. As the lower limit value of confidence interval of both groups was larger than 12.8%, we concluded that the self-administered tDCS with a 6-week treatment protocol might have a significant effect on the improvement of depressive symptoms in mild to moderate major depressive disorder (MDD) patients.
Table 1.
Descriptive statistics of BDI-II total score and remission rate of treatment groups
| Groups | Measurement period | Mean ± standard deviation | Remission, number (%) | 95% confidence interval |
|---|---|---|---|---|
| Full-Analysis (n = 61) | Baseline | 24.3 ± 6.2 | 35 (57.4) | 0.44−0.70 |
| 6 weeks | 13.7 ± 7.8 | |||
| Per-Protocol (n = 43) | Baseline | 24.1 ± 6.7 | 27 (62.8) | 0.47−0.77 |
| 6 weeks | 12.6 ± 6.8 |
BDI-II, Beck-Depression Inventory-II score.
Effect of tDCS on Improvement of Depressive Symptoms
As this study was conducted in the multicenter hospital, we evaluated the association between the type of the hospital, sex, degree of depressive symptoms, concomitant usage of psychiatric medications with effective variables. The results showed that there were significant differences of remission rate across hospitals (p = 0.01, Fisher’s exact test) and thus we performed generalized estimating equations analysis. As a result, except for one hospital, the remission rate was not significantly different in 5 of 6 hospitals in both FA and PP groups (Table 2). Additional analysis showed that there was a significant correlation between BDI-II and HAM-D total scores in 5 of 6 hospitals (Supplementary Table 2; available online).
Table 2.
Results of general estimating equation analysis by site of experiment as covariates
| Site of experiment | Estimate | Standard error | Odds ratio | 95% confidence interval |
|---|---|---|---|---|
| Full-Analysis group (n = 61) | ||||
| Site 1 | Reference | Reference | 1.00 | Reference |
| Site 2 | −0.63 | 0.77 | 0.53 | 0.12−2.40 |
| Site 3 | −0.12 | 0.75 | 0.88 | 0.20−3.82 |
| Site 4 | 0.90 | 0.41 | 2.47 | 1.10−5.54 |
| Site 5 | −0.36 | 0.74 | 0.70 | 0.16−2.99 |
| Site 6 | 0.46 | 0.51 | 1.59 | 0.59−4.29 |
| Per-Protocol group (n = 43) | ||||
| Site 1 | Reference | Reference | 1.00 | Reference |
| Site 3 | −0.26 | 0.75 | 0.77 | 0.18−3.32 |
| Site 4 | 0.72 | 0.42 | 2.06 | 0.91−4.67 |
| Site 5 | −0.77 | 0.59 | 0.46 | 0.14−1.48 |
| Site 6 | 0.67 | 0.50 | 1.95 | 0.73−5.19 |
Effectiveness Validation with Secondary Variables
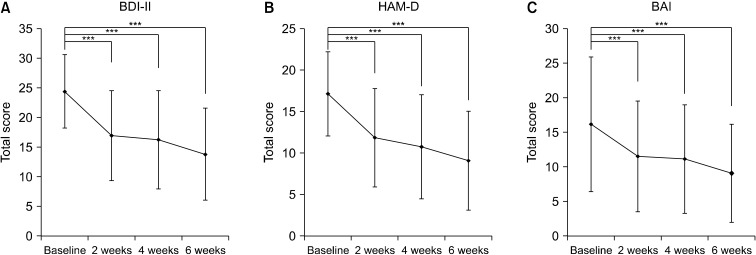
Although the primary outcome of this study was a total score of BDI-II after the 6-week tDCS treatment, other psychiatric measurements including HAM-D, BAI were also used. Similar to the findings of BDI-II score change, a total score of HAM-D was significantly decreased after the 2, 4, and 6 weeks treatment when compared to the baseline (both FA and PP groups, all p < 0.001, paired ttest; Fig. 2A, 2B). Anxiety-related symptoms in FA and PP groups were also improved as the treatment progressed, as the BAI total score was significantly decreased after the 2, 4, and 6 weeks treatment relative to baseline (both FA and PP groups, all p < 0.001, paired ttest; Fig. 2C). These results suggest that self-administered tDCS treatment might have an effect on relieving depressive symptoms rated by clinicians as well as a subjective feeling of anxiety. Numeric data representing total scores of primary and secondary variables are presented in supplementary tables (Supplementary Tables 3−5; available online).
Fig. 2.
Total score changes on psychiatric scales during the six-week treatment period. (A) Beck-Depression Inventory-II score (BDI-II), (B) Hamilton Depression Scale (HAM-D), (C) Beck Anxiety Inventory (BAI). Error bar denotes standard deviation in each point. All differences between visit and baseline were statistically significant (***paried ttest, p < 0.001).
Remission Rate according to the Treatment Compliance and Stimulus Intensity
As the 6-week treatment of self-administered tDCS showed the significant effect of relieving subjective/ob-jective depressive symptoms and anxiety, we furtherly evaluated whether the compliance to the treatment affect the remission rate of depression. When we categorized the participants into 4 groups by the number of total tDCS sessions (≤ 10 sessions [n = 5]; 11 to 20 sessions [n = 9]; 21 to 29 sessions [n = 25]; ≥ 30 sessions [n = 22]), there were no significant differences between groups and remission rate (p = 0.78) (Table 3).
Table 3.
Remission rate by treatment compliance
| Number of sessions | Remission | Non-remission | pvaluea |
|---|---|---|---|
| < 11 (n = 5) | 2 (40.0) | 3 (60.0) | 0.78 |
| 11 to 20 (n = 9) | 6 (66.7) | 3 (33.3) | |
| 21 to 29 (n = 25) | 15 (60.0) | 10 (40.0) | |
| ≥ 30 (n = 22) | 12 (54.5) | 10 (45.5) |
Values are presented as number (%).
aFischer’s exact test.
A similar result was found between stimulus intensity and remission rate; the remission rate of the 2.0 mA group was not significantly different with 1.5 mA groups (FA group, 1.5 mA [n = 36], 2.0 mA [n = 25], p = 0.48; PP group, 1.5 mA [n = 26], 2.0 mA [n = 17], p = 0.32).
Side Effects during the Self-administered tDCS Treatment
Among 66 participants who had used a self-administered tDCS device (YMS-201B) in this study, 35 participants reported 39 cases of side effects. The major side effects were burning sensation (n = 17), thermal burn (n = 6) and headache (n = 4). Most cases of reported side effects was categorized as mild (n = 35) and moderate (n = 3), severe (n = 1) cases followed. All reported side effects were reversible and there were no cases of ‘permanent discontinuation of device’.
DISCUSSION
In this study, we showed that patient-administered tDCS treatment at their home was effective in improving the subjective feeling of depression, which was measured by the total BDI-II score below 12. Six-week treatment of tDCS had a significant effect in relieving depressive symptoms, but the remission rate according to the treatment compliance and stimulus intensity was not proved.
Our results are comparable to a recent pilot study of Alonzo et al. [14] study.
In this open-label trial, 34 patients self-administered tDCS devices with 20 to 28 sessions for a month. Depressive symptoms were assessed by Montogomery- Asberg Depression Rating Scale (MADRS), and the results showed that the MADRS scale dropped to about a half after the treatment.
Similar findings were observed in the recent home-based tDCS trial of a randomized, single-blinded clinical trial [21]. With 58 participants with MDD, this study showed that a 6-week protocol of self-administered tDCS treatment significantly lowered the BDI-II score in the active tDCS group when compared to the sham group. Our study enlarged previous findings that short-term treatment of tDCS at a patient’s home might have an effect on improving subjective depressive symptoms and could be used as the acute treatment protocol in mild to moderate MDD patients.
Although participants prepared the materials and placed the electrodes on their own, the side effects were not critical. About a half of participants (35 of 66) reported mild category of side effects (e.g., burning sensation), and this is similar to a previous study of home-based tDCS treatment (61.8 % of sessions in Alonzo et al. [14]’s study). This kind of side-effects are well known in tDCS treatment [22], and a number of studies have shown that tDCS is a safe and well-tolerated treatment without evidence of risk for serious side effects [23,24]. Our findings showed that the application of tDCS at the patients’ home without the supervision of clinicians also a well-tolerated and safe procedure.
Our results should be interpreted with several limita-tions. First, as this was a single-arm study, it had no control or sham groups. Although 6 sites of hospitals independently enrolled participants and perform the study, our results of decreased BDI-II score might not represent the sole effect of tDCS treatment; mild to moderate depressive symptoms could be resolved by the natural course and the placebo effect could affect the results. Although there was a report that 40% of patients with MDD resolved their symptoms [25], it would take a year or longer to naturally resolve the depression-related symptoms. Thus, the remission rate of 57 to 63% of participants within 6 weeks of tDCS treatment might be related to both electrical stimulation and the natural course of diseases. Second, although we have observed the treatment effect of tDCS on depression, there was no association between treatment compliance (number of completed sessions) and remis-sion rate. These results might lack the evidence for the treatment effect of self-administered tDCS, but we should note that the number of participants in each group was too small to have statistical significance (i.e., n = 5 in the lowest compliance group). Further study with larger samples may delineate the dose-response of at-home-based tDCS treatment. Third, as we did not give any antidepressant medication during the treatment period, thus we could not validate the superiority or inferiority of tDCS treatment with classical antidepressant treatment. Fourth, we allowed anti-anxiety drugs (e.g., benzodiazepine) during the treatment period. It is well known that benzodiazepines could affect depressive symptoms [26]. Thus, our results of subjective symptoms of depression during the treatment partially originated from the effect of benzodiazepine drugs.
In conclusion, this study has found that 6-week self-administered tDCS treatment at home in mild to moderate MDD patients had a significant effect on the improvement of total BDI-II score. The remission rate in tDCS treatment groups was 57 to 63% (FA and PP groups, respectively), and other measurements of depression-related symptoms (e.g., HAM-D and BAI) were significantly improved as the treatment session increased. There were no severe side- effects that need halt of the protocol, and our findings suggest that self-administered tDCS treatment at the patient’s home might effectively relieve subjective depressive symptoms in mild to moderate MDD patients. The effectiveness and safety of tDCS treatment at home suggest the possibility of this treatment being widely used in patients with low compliance for hospital visit management.
Supplemental Materials
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
This research was supported by a grant from Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare of the Republic of Korea (grant no. HL19C0007 and HI17C2272).
Author Contributions
Concept and design of the study: Jeong-Ho Chae. Acquisition of data: Sekye Jeon, Tae Hyon Ha, Woojae Myung, Seung-Hwan Lee, Young-Hoon Ko, Do Hoon Kim, Hwa-Young Lee. Analysis and interpretation of data: Jihoon Oh, Jeong-Ho Chae. Drafting the manuscript: Jihoon Oh. Revising the manuscript critically for important intellectual content: Jeong-Ho Chae.
References
- 1.Paulus W. Transcranial electrical stimulation (tES - tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. 2011;21:602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- 2.Knechtel L, Thienel R, Schall U. Transcranial direct current stimulation: neurophysiology and clinical applications. Neuro-psychiatry. 2013;3:89–96. doi: 10.2217/npy.12.78. [DOI] [Google Scholar]
- 3.Khaleghi A, Mohammadi MR, Shahi K, Nasrabadi AM. Com-putational neuroscience approach to psychiatry: a review on theory-driven approaches. Clin Psychopharmacol Neurosci. 2022;20:26–36. doi: 10.9758/cpn.2022.20.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–578. doi: 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald PB, Oxley TJ, Laird AR, Kulkarni J, Egan GF, Daskalakis ZJ. An analysis of functional neuroimaging studies of dorsolateral prefrontal cortical activity in depression. Psychiatry Res. 2006;148:33–45. doi: 10.1016/j.pscychresns.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Oh J, Jang KI, Jeon S, Chae JH. Effect of self-administered transcranial direct stimulation in patients with major depressive disorder: a randomized, single-blinded clinical trial. Clin Psychopharmacol Neurosci. 2022;20:87–96. doi: 10.9758/cpn.2022.20.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu CHY, Young AH. Comparative efficacy and acceptability of non- surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. 2019;364:l1079. doi: 10.1136/bmj.l1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM, et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry. 2016;208:522–531. doi: 10.1192/bjp.bp.115.164715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, Fernandes RA, et al. Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med. 2017;376:2523–2533. doi: 10.1056/NEJMoa1612999. [DOI] [PubMed] [Google Scholar]
- 10.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–391. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 11.Lisanby SH. Noninvasive brain stimulation for depression - the devil is in the dosing. N Engl J Med. 2017;376:2593–2594. doi: 10.1056/NEJMe1702492. [DOI] [PubMed] [Google Scholar]
- 12.D'Urso G, Dell'Osso B, Rossi R, Brunoni AR, Bortolomasi M, Ferrucci R, et al. Clinical predictors of acute response to transcranial direct current stimulation (tDCS) in major depression. J Affect Disord. 2017;219:25–30. doi: 10.1016/j.jad.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Palm U, Hasan A, Strube W, Padberg F. tDCS for the treatment of depression: a comprehensive review. Eur Arch Psychiatry Clin Neurosci. 2016;266:681–694. doi: 10.1007/s00406-016-0674-9. [DOI] [PubMed] [Google Scholar]
- 14.Alonzo A, Fong J, Ball N, Martin D, Chand N, Loo C. Pilot trial of home-administered transcranial direct current stimulation for the treatment of depression. J Affect Disord. 2019;252:475–483. doi: 10.1016/j.jad.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Woo YS, Bahk WM, Seo JS, Park YM, Kim W, Jeong JH, et al. The Korean Medication Algorithm Project for Depressive Disorder 2021: comparisons with other treatment guidelines. Clin Psychopharmacol Neurosci. 2022;20:37–50. doi: 10.9758/cpn.2022.20.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu BK, Lee HK, Lee KS. Validation and factor structure of Korean version of the Beck Depression Inventory second edition (BDI-II): in a university student sample. Korean Soc Biol Psychiatry. 2011;18:126–133. [Google Scholar]
- 17.Yi JS, Bae SO, Ahn YM, Park DB, Noh KS, Shin HK, et al. Validity and reliability of the Korean version of the Hamilton Depression Rating Scale(K-HDRS) J Korean Neuropsychiatr Assoc. 2005;44:456–465. [Google Scholar]
- 18.Lee HK, Lee EH, Hwang ST, Hong SH, Kim JH. Psychometric properties of the Beck Anxiety Inventory in the community- dwelling sample of Korean adults. Korean J Clin Psychol. 2016;35:822–830. doi: 10.15842/kjcp.2016.35.4.010. [DOI] [Google Scholar]
- 19.Riedel M, Möller HJ, Obermeier M, Schennach-Wolff R, Bauer M, Adli M, et al. Response and remission criteria in major depression--a validation of current practice. J Psychiatr Res. 2010;44:1063–1068. doi: 10.1016/j.jpsychires.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44:225–239. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- 21.Oh J, Jang KI, Jeon S, Chae JH. Effect of self-administered transcranial direct stimulation in patients with major depressive disorder: a randomized, single-blinded clinical trial. Clin Psycho-pharmacol Neurosci. 2022;20:87–96. doi: 10.9758/cpn.2022.20.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5:155–162. doi: 10.1016/j.brs.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bikson M, Grossman P, Thomas C, Zannou AL, Jiang J, Adnan T, et al. Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul. 2016;9:641–661. doi: 10.1016/j.brs.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Stegenga BT, Kamphuis MH, King M, Nazareth I, Geerlings MI. The natural course and outcome of major depressive disorder in primary care: the PREDICT-NL study. Soc Psychiatry Psychiatr Epidemiol. 2012;47:87–95. doi: 10.1007/s00127-010-0317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiller JW, Schweitzer I. Benzodiazepines. Depressants or antidepressants? Drugs. 1992;44:165–169. doi: 10.2165/00003495-199244020-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.