Abstract
Since their approval by the Food and Drug Administration (FDA) in 1989, proton pump inhibitors (PPIs) have become one of the most highly utilized drugs in the United States, assuming a position as one of the top 10 most prescribed medications in the country. The purpose of PPIs is to limit the amount of gastric acid secreted by the parietal cells via irreversible inhibition of the H+/K+-ATPase pump, therefore maintaining an elevated gastric acid pH of greater than 4 for 15–21 h. Even though PPIs have many clinical uses, they are not without their adverse effects, mimicking achlorhydria. Besides electrolyte abnormalities and vitamin deficiencies, long-term use of PPIs has been linked to acute interstitial nephritis, bone fractures, poor COVID-19 infection outcomes, pneumonia, and possibly an increase in all-cause mortality. The causality between PPI use and increased mortality and disease risk can be questioned since most studies are observational. Confounding variables can greatly affect an observational study and explain the wide-ranging associations with the use of PPIs. Patients on PPIs are generally older, obese, sicker with a higher number of baseline morbidities, and on more medications than the compared PPI non-users. These findings suggest that PPI users are at a higher risk of mortality and complications based on pre-existing conditions. This narrative review aims to update readers on the concerning effects that proton pump inhibitor use can have on patients and give providers a resource to create informed decisions on appropriate PPI use.
Keywords: Proton pump inhibitors, AKI, PPIs, Mortality, Adverse events
Key Summary Points
| One of the most often prescribed drug types in the world is proton pump inhibitors (PPIs). For several gastrointestinal disorders like gastroesophageal reflux disease (GERD) and peptic ulcer disease, they provide significant, affordable relief. |
| Although PPIs are quite efficient in treating various hypersecretory illnesses, it is clear that their usage should be carefully supervised and that treatment should be stopped when no longer required to prevent side effects. |
| It should be emphasized that acid rebound hypersecretion is a possibility, and PPI tapering is advised. Public access to PPIs has rapidly increased due to their over-the-counter availability, which permits their long-term usage without direct supervision from a doctor. This has allowed patients to frequently receive prescriptions for these medications for "gut protection" or for other unspecified reasons with no goals for discontinuation. |
| Long-term PPI use has its drawbacks, and has been linked to a number of unfavorable clinical consequences. Long-term use of PPIs can cause malabsorption in the small intestine, increased risk of infection, bone fracture, and negative effects on the kidney. |
| The relatively recent side effect of hypomagnesemia can necessitate periodic magnesium infusions to lessen negative effects. Patients on long-term PPI therapy, particularly those with pre-existing comorbidities, are also more likely to die. |
| PPI use can also result in worse clinical results when combined with other viral diseases like COVID-19. PPIs should, in the end, only be prescribed with prudence and with the development of adverse events in mind. |
Introduction
Since their approval by the Food and Drug Administration (FDA) in 1989, proton pump inhibitors (PPIs) have become one of the most highly utilized drugs in the United States, assuming a position as one of the top 10 most prescribed medications in the country [1, 2]. PPIs work to cause a substantial decrease in acid production in the upper gastrointestinal system, which has allowed this medication to be utilized in a wide variety of conditions, including gastroesophageal reflux disease (GERD) and peptic ulcer disease. Furthermore, the side effect profile of PPIs has been regarded to most as both minimal and tolerable, with headache, diarrhea, constipation, and abdominal discomfort being the most widely reported adverse effects [3]. Nearly 7% of adults have a prescription for any type of PPI due to the medication's wide range of uses and apparent manageable side effects; this does not account for the large number of people who use over-the-counter medicines [3].
Although PPIs are regarded as being effective and safe, there may be serious concerns about their long-term effects. PPIs are most effective for treating several chronic illnesses, such as GERD, which necessitate long-term use by the patient [4]. Other types of gastrointestinal conditions associated with long-term use of PPIs are Zollinger–Ellison Syndrome and Barrett's esophagus. This type of extended exposure to PPIs can increase the risk for serious adverse effects [3]. Long-term exposure to PPIs as a form of medication therapy can potentially impact numerous organ systems in the body, resulting in electrolyte disturbances, such as hypomagnesemia and hypocalcemia, which could cause tetany, convulsions, and cardiac arrhythmias [5]. Kidney disease, increased risk of infection, and pneumonia, especially in the elderly, along with many other abnormalities, are other documented possible adverse effects. [3]. Among these, the renal manifestations of chronic PPI use are particularly concerning. This, and other manifestations of prolonged PPI use, need to be studied further to ensure that informed and appropriate administration of PPIs is ensured.
The present investigation, therefore, provides a focused literature analysis on the usage of PPIs, and how they may mediate or modulate kidney damage, other side effects, and mortality. This narrative review intends to enlighten readers about the potentially harmful consequences of PPI use on patients, and to provide healthcare professionals with resources and to optimize safe usage of PPIs. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Proton Pump Inhibitors Overview
PPIs have been approved by the FDA for the use and treatment of erosive esophagitis, GERD, Helicobacter pylori infection, Zollinger–Ellison syndrome, and its hypersecretory effects, Barrett’s esophagus, and peptic ulcer disease, and for reducing the risk of ulcer development with non-steroidal anti-inflammatory drugs (NSAIDs) [6]. PPIs have a non-FDA-approved use as an adjunct therapy for patients on anticoagulants who are planning to undergo procedures with a high risk of post-procedure hemorrhagic complications [7]. Interestingly, PPIs are also currently being examined as a potential treatment of melanomas, multiple myeloma, and colorectal cancer due to their hypothesized antitumor effects [8].
The PPIs currently available for use in the United States include omeprazole, lansoprazole, pantoprazole, rabeprazole, and esomeprazole [7]. In 2006, Zegrid (omeprazole/sodium bicarbonate) was approved as an extended-release PPIs [9]. Dexlansoprazole was approved for use in the United States in 2009 for healing all grades of erosive esophagitis in ages 12<years and older. Deprescribing has been suggested over the years due to concerns regarding the effects acid suppression can cause. These drugs are benzimidazole derivatives and cause steroid synthesis and cortisol release in addition to their effects on the H+/K+-ATPase pump [10]. PPIs cause acid suppression by irreversibly inhibiting the H+/K+-ATPase proton pump in gastric parietal cells. PPIs bind to one or more cysteine residues on the H+/K+-ATPase pump; causing the pump to become nonfunctional [11–14].
Historically, the gold standard for treating a wide variety of gastric conditions was the histamine-2 (H2) receptor blocker class. Although the development of H2 receptor blockers significantly advanced the treatment of diseases of the gastrointestinal tract, H2 receptor blockers have been found to have a few drawbacks. H2 receptor blockers have a short duration of action, are subject to tolerance development, and are not as effective as PPIs because most acid secretion is stimulated by gastrin, an enzyme upon which H2 receptor blockers have no effect [15]. PPIs were thought to be safer compared to H2 blockers, as there were studies linking central nervous system reactions, such as agitation, hallucinations, disorientations, delirium, and irritability [16]. Ranitidine has fallen out of favor, as it was found to have a significantly increased risk of ductal carcinoma [17]. Another H2 blocker, cimetidine, inhibits numerous CYP 450 enzymes, which may increase both the levels and, therefore, the effects of many hepatically metabolized drugs [18]. PPIs are more effective at treating GERD, and are the better choice for accelerating peptic ulcer wound healing, thus explaining their replacement of H2 receptor blockers as the gold standard treatment for these conditions [19–21]. Omeprazole was the first PPI introduced in 1989, revolutionizing the treatment of the aforementioned gastrointestinal tract diseases, and has become one of the most widely used drugs worldwide [4, 22]. Although numerous studies claim one PPI may be more effective than another, studies show that there may be no significant difference in efficacy between the members of the PPI class [22].
Aside from their use in diseases related to acid secretion, PPIs are also used to treat a variety of other diseases, including eosinophilic esophagitis, viral infection, H. pylori infection, and respiratory system diseases [4]. PPI utilization has been beneficial in a specific subset of patients with eosinophilic esophagitis, called PPI-responsive esophageal eosinophilia, and case studies have shown that patients with eosinophilic esophagitis were able to achieve remission after an 8-week course of PPIs [23, 24]. The mechanism of PPI action in the treatment of esophageal eosinophilia may involve both acid suppression and unique anti-inflammatory actions of the PPIs [25]. PPIs are not only effective in combination with other therapies in treating H. pylori by directly suppressing bacterial growth but also by increasing the pH in the stomach to allow the other drugs to function more efficiently. Most drugs cannot function properly in the high-PH setting in the stomach and, therefore, have shorter retention times [26]. A study by Sasaki et al. demonstrated that 15 mg per day of lansoprazole reduced the frequency of common colds and other upper respiratory diseases, such as chronic obstructive pulmonary disease [27]. PPIs are thought to be effective in treating these viral and respiratory diseases by decreasing the production of proinflammatory cytokines (e.g., IL-6, IL-8, and TNF-alpha) in airway epithelial cells [28], and by inhibiting virus-specific serine proteases [29].
Generally, PPIs are FDA-approved for short-term use [30]. However, PPIs are becoming increasingly widespread, and these drugs are not always discontinued after just a few weeks of use. Research has shown that over 50% of PPIs are prescribed inappropriately in hospital medicine wards [15, 31]. PPIs are also misused in the clinical setting, as patients are continued on PPI therapy after being discharged from the hospital, and physicians do not always review the necessity of the drug in the patients taking them [32]. PPIs are also available for sale over the counter in many countries, allowing patients to use these drugs without the direct supervision of a physician [33]. Since PPIs have been proven to be very effective in treating gastrointestinal diseases, it is often initiated to “protect” the gastrointestinal lining; this is often why PPIs are erroneously prescribed [34].
Proton Pump Inhibitor Gastrointestinal Adverse Effects
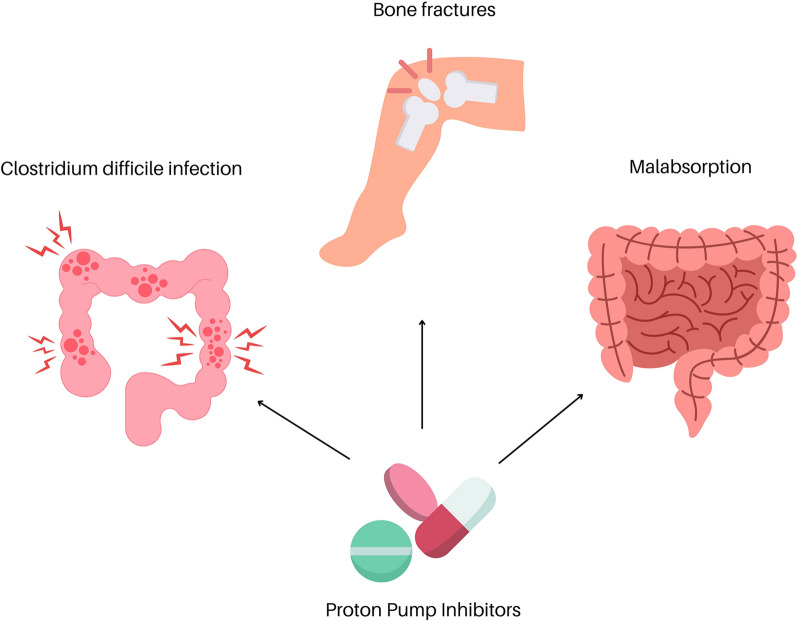
Although PPIs are effective and well tolerated by patients, there are increasing concerns about misuse, over-prescription, and the development of adverse events with long-term use of these drugs. Patients suffering from GERD require long-term therapy with PPIs, and are often prescribed these drugs at large doses for several months [35]. The most common adverse effects of PPI use are gastrointestinal-related: abdominal pain, diarrhea, constipation, nausea, and vomiting [4]. However, continued use of PPIs has been associated with other serious adverse events, such as an increased risk of gastrointestinal infection by Clostridium difficile, bone fractures, and malabsorption in the gut [36] (see Table 1).
Table 1.
Proton pump inhibitor gastrointestinal adverse effects
| Common adverse events | Abdominal pain, diarrhea, constipation, nausea, and vomiting [4] |
| Serious adverse events | Increased risk of gastrointestinal infection by Clostridium difficile, bone fractures, and malabsorption in the gut [34] |
| Clostridium difficile infection | A study performed on 136 subjects showed that one of the major risk factors for developing C. difficile infection was the long-term use of PPIs [36] |
| The increased risk of infection can be explained by three proposed mechanisms: (1) the reduction in gastric acid may allow infectious organisms to reach the intestine easily, (2) antineutrophilic effect, and (3) alterations in the tight junctions between cells, allowing bacterial organisms to more freely pass from one organ to another [34] | |
| Inhibition of acid secretion perpetuated by PPI use can allow vegetative forms of the bacterium to survive in this less acidic environment and propagate infection [37] | |
| Acid secreted by parietal cells into the gastrointestinal tract serves as an innate immunological barrier; inhibition of this acid secretion by PPIs allows for increased bacterial colonization and increased opportunity for infection [38] | |
| PPIs also cause hypochlorhydria, which causes changes in bacterial homeostasis and growth in the GI tract, and allows for overgrowth of bacteria and perpetuation of enteric infections [39] | |
| Bone fractures | Yang et al. showed that PPI users had a 1.5-fold increased risk of developing hip fractures compared to control groups [40] |
| Hypochlorhydria caused by PPIs leads to a decrease in calcium absorption in the small intestine and, ultimately, lower calcium levels in the blood [41] | |
| Decreased serum calcium not only leads to disruption of bone formation performed by osteoblasts but also promotes osteoclast function and increased bone resorption [43] | |
| Higher gastric pH has also been hypothesized to decrease the absorption of magnesium, leading to the formation of large hydroxyapatite crystals. These crystals decrease osteoblast function and increase the number of osteoclasts [44] | |
| Lewis et al. showed an increased risk of fracture in patients who were taking PPIs for 1 year or more [46] | |
| Brozek et al. demonstrated that use of PPIs is associated with an increased risk of subsequent hip fracture after sustaining a hip fracture before initiation of a PPI; this risk was found to be greater in male PPI users than in female PPI users [47] | |
| Malabsorption | Acquired hypochlorhydria has been found to decrease the absorption of multiple micronutrients, including vitamin B12 and iron [48] |
| The suppression of gastric acid from PPI use can lead to vitamin B12 deficiency [49, 50] | |
| Hamano et al. demonstrated that there is an increased risk of iron deficiency anemia with PPI and H2 receptor blockers [54] |
Clostridium difficile Infection
Clostridium difficile infection is one of the most recognized causes of infectious diarrhea in healthcare settings and is associated with high mortality [37]. A study performed on 136 subjects showed that one of the major risk factors for developing C. difficile infection was the long-term use of PPIs [38]. However, a 2021 scoping review of 59 studies found that the definition of long-term PPI use varied from > 2 weeks to > 7 years [39]. The increased risk of infection can be explained by three proposed mechanisms: (1) the reduction in gastric acid may allow infectious organisms to reach the intestine easily, (2) antineutrophilic effect, and (3) alterations in the tight junctions between cells, allowing bacterial organisms to more freely pass from one organ to another [36]. Clostridium difficile spores are normally resistant to the acid environment in the intestine. However, inhibition of acid secretion perpetuated by PPI use can allow vegetative forms of the bacterium to survive in this less acidic environment and propagate infection [40]. The acid secreted by parietal cells into the gastrointestinal tract serves as an innate immunological barrier; inhibition of this acid secretion by PPIs allows for increased bacterial colonization and increased opportunity for infection [41]. PPIs also cause hypochlorhydria, which causes changes in bacterial homeostasis and growth in the GI tract, and allows for overgrowth of bacteria and perpetuation of enteric infections [42].
Bone Fractures
The association between PPI use and bone fractures was first reported by Yang et al. who showed that PPI users had a 1.5-fold increased risk of developing hip fractures compared to control groups [43]. Research has shown that the hypochlorhydria caused by PPIs leads to a decrease in calcium absorption in the small intestine and, ultimately, to lower calcium levels in the blood [44]. Under physiologic conditions, osteoblast and osteoclast actions remain balanced to maintain optimal bone structure [45]. Decreased serum calcium not only leads to disruption of bone formation performed by osteoblasts but also promotes osteoclast function and increased bone resorption [46]. The higher gastric pH has also been hypothesized to decrease the absorption of magnesium, leading to the formation of large hydroxyapatite crystals, which decrease osteoblast function and increase the number of osteoclasts [47].
Hip fractures are one of the most severe types of osteoporotic fractures, and are associated with an increased risk of mortality in geriatric populations [48]. A study by Lewis et al. on Australian women showed an increased risk of fracture in patients who were taking PPIs for 1 year or more [49]. In an observational cohort study, Brozek et al. demonstrated that use of PPIs is associated with an increased risk of subsequent hip fracture after sustaining a hip fracture before initiation of a PPI; this risk was found to be greater in male PPI users than in female PPI users [50].
Malabsorption
The associated acquired hypochlorhydria has been found to decrease the absorption of multiple micronutrients, including vitamin B12 and iron [51]. Gastric acid is necessary to release vitamin B12 from food in the digestive tract, while the suppression of gastric acid from PPI use can lead to vitamin B12 deficiency [52, 53], which in turn, can lead to neurologic abnormalities, such as neuropathy, depression, dementia, and spinal cord degeneration. These conditions can be reversed if they are diagnosed early on in the clinical course [54].
Iron absorption mainly occurs in the small intestine through the actions of ferroportin, and is regulated via the actions of hepcidin, which increases iron sequestration into cells, thereby decreasing the amount of iron in the systemic circulation [55, 56]. Through a large-scale database analysis of the FDA Adverse Event Reporting System database, Hamano et al. demonstrated that there is an increased risk of iron deficiency anemia with PPI and H2 receptor blockers [57]. This group also conducted in vitro experiments and concluded that PPIs upregulated hepcidin activity, leading to decreased iron absorption in the gut (see Fig. 1).
Fig. 1.
Use of PPIs has been associated with other serious adverse events, such as an increased risk of gastrointestinal infection by Clostridium difficile, bone fractures, and malabsorption in the gut
Mortality with PPIs
There is an association between PPI use and increased mortality in patients with preexisting comorbidities. In 2018, Shiraev et al. examined all-cause mortality data from a study of Danish patients which followed the clinical course of patients after they suffered from their first myocardial infarction [58]. Their systematic review showed that the mortality rate was higher among PPI users compared to patients who were not on PPI therapy. Ghebremariam et al. demonstrated that this increase in morbidity and mortality with cardiovascular events could be associated with the effects of PPIs on endothelium and the clotting cascade. PPIs cause a reduction in endothelial nitric oxide synthase, which ultimately leads to a decrease in nitric oxide production. Nitric oxide plays a protective role by decreasing platelet interactions with the endothelium, while reduction in any of the precursors to nitric oxide formation can lead to inflammation and thrombosis, which could lead to an increase in adverse cardiovascular events [59, 60]. Of interest, it has been hypothesized that this increase in adverse cardiovascular events can be attributed to physician error if a physician misinterprets angina symptoms for gastric symptoms [61].
There also exists documentation of increased mortality with PPI use independent of other comorbidities. A longitudinal study based on data from the United States Department of Veteran Affairs reported an association between PPI use and mortality [62]. A few studies have demonstrated that the risk of 1-year mortality associated with PPI use was increased in groups of older patients discharged from hospitals or other long-term facilities [63].
It has been hypothesized that using PPIs is associated with an increased risk of mortality among patients with diseases other than cardiovascular disease. A well-documented relationship exists between gut bacteria and hepatic encephalopathy [64]. Gastrointestinal bacteria release ammonia and endotoxins, two substances that play a critical role in developing hepatic encephalopathy. Nardelli et al. found that patients with cirrhosis who used PPIs had an increased risk of both minimal and overt hepatic encephalopathy and lower survival rates, when compared to patients who did not use PPIs [65]. This study also concluded that PPI use is associated with increased mortality independent of overt hepatic encephalopathy and age.
Furthermore, there is an association between PPI use and coronavirus disease 2019 (COVID-19), which is caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Previously published data have demonstrated that pH values higher than 3 do not inactivate the virus and may increase its survival and infectivity [66]. The H+/K+-ATPase proton pump is also present in the upper and lower intestinal tract, and inhibition of the pump in this area can alter the respiratory flora and lead to an increased risk of acute respiratory distress syndrome and mortality from COVID-19 [67].
These findings suggest that it may be useful to carefully monitor the prolonged use of PPIs in specific patient populations and to discontinue PPI therapy when it is no longer necessary.
Clinical Studies
PPI Effects on Organ Systems in Adults
Numerous observational studies have shown an association between the use of PPIs and increased disease risk and poor outcomes affecting multiple organ systems, such as renal and gastrointestinal systems. PPIs have been linked in many studies to an increased risk of developing renal disease and disorders such as acute kidney injury (AKI), acute interstitial nephritis (ANI), and chronic kidney disease (CKD) [68–70]. AKI is becoming a larger burden in healthcare, with rapid growth from 11.6 to 18.3% in the adult population and 19.6 to 26.9% in the pediatric population [71–73].
A retrospective cohort study investigated the relationship between PPI use and the risk of developing AKI and CKD [70]. A total of 192,935 individuals, 86,000 CKD patients and 93,335 AKI patients, 18 years or older without pre-existing renal disease, between 1993 and 2008, were included in the study from Western New York HMO claim data. Inpatient, outpatient, laboratory, and prescription data were used to follow PPIs and kidney function. Based on this study, PPIs were associated with a fourfold increased risk for developing an AKI [odds ratio (OR) 4.35, 95% confidence interval (CI) 3.14–6.04, p < 0.0001] compared to non-users in the cohort. The data were adjusted for potential confounders, such as demographics; however, it should be noted that race was not included. The Western New York study concluded that using PPIs was associated with a 20% higher risk of CKD after adjustment for confounders (OR 1.20, 95% CI 1.12–1.28, p < 0.0001). Individuals who used PPIs and H2 antagonists (H2A) had higher AKI and CKD risks; however, PPIs seem to have had a more significant risk than H2As [70, 74, 75].
A case–control study was performed in 2004 to investigate the connection between community-acquired pneumonia and acid suppression therapy, including PPIs and H2A. After adjusting for confounding variables, current PPI users had a 1.89-fold risk for pneumonia versus non-users. Additionally, acid suppression therapy can lead to microbiome disruption in the intestine, causing pyogenic liver abscesses and enteric infections, such as C. difficile enterocolitis, as already discussed in this manuscript. A study exploring the associations between PPI use and mortality in patients with pyogenic liver abscesses resulted in a 1.5-fold risk of mortality in patients on PPIs versus patients not using PPIs [76]. These findings suggest that PPI treatment should not be started unless indications are present.
Electrolytes, vitamin absorption, and other micronutrients in the gastrointestinal tract can be disrupted by the acid suppression caused by PPI use. Magnesium is passively diffused in the small intestine, and magnesium deficiencies can affect multiple organ systems. Hypomagnesemia can be linked to poor renal function, and can lead to severe recurrent infection due to impaired immune function with decreased CD8 + T cells, CD19 + B cells, Natural Killer cells, and dendritic cells [77]. Additionally, hypomagnesemia can lead to poor bone metabolism, leading to low bone density, hip fracture, and osteoporosis, as already discussed. However, one study showed that the 90-day mortality rate was significantly reduced in patients with hip fractures who received PPIs before and after fracture; longer-term mortality (> 6 months) increased in the patients who received PPIs before fracture [78].
PPIs Combined with Medications in Adults
Several studies have observed whether increased risks of morbidity and mortality were solely associated with PPIs or other medications. One retrospective cohort study from 2021 examined the association of hypomagnesemia with PPIs and tacrolimus in patients with connective tissue disease [77]. A total of 284 patients with connective tissue disease were included in the study, with 63 (22.2%) having hypomagnesemia; 141 of the patients (49.6%) used PPIs, and 68 (23.9%) used tacrolimus. The study showed that patients using PPIs (OR 1.48; p = 0.01) and tacrolimus (OR 6.14; p < 0.01) had associated hypomagnesemia leading to poor renal outcomes and severe infection in patients with connective tissue disease. Additionally, renal deterioration was observed when PPIs were used in combination with tacrolimus in connective tissue disease patients.
PPIs are often administered with NSAIDs to suppress stomach acid production and to lower the possibility of forming peptic ulcers from prostaglandin inhibition by NSAIDs. In addition, NSAIDs are nephrotoxic and cause renal vessel vasoconstriction by prostaglandin inhibition. A nested case–control study investigated the combined use of PPIs and NSAIDs or antibiotics associated with an increased AKI risk using data from health insurance claims healthcare constructed by the Japan Medical Data Center [79]. The combined use of PPIs and NSAIDs significantly increased the risk of AKI. The OR for increased AKI risks associated with combined PPI and NSAIDs use was 3.12 (95% CI 1.84–5.37) after adjusting for confounding variables. The incident rate of AKI in persons using combined NSAIDs and PPIs was 38.5 per 10,000 person-years. These findings suggest that combined PPI and NSAID use has a higher risk of adverse renal outcomes.
The antibiotics examined in the case–control study included macrolides, fluoroquinolones, penicillin, and cephalosporins [79]. Cephalosporins and fluoroquinolones had increased AKI risks when used in current PPI users; the adjusted ORs for cephalosporins and fluoroquinolones were 1.88 (95% CI 1.02–3.47) and 2.35 (95% CI 1.12–4.95). However, in combination with PPIs, macrolides and penicillin had reduced AKI risks with OR 0.47 (95% CI 0.21–0.96) and 0.83 (0.42–1.59). The difference in the severity of systemic illness of the macrolide group versus the NSAID or other antibiotic groups may explain these results.
PPIs can also significantly interact with the commonly prescribed antiplatelet agent, clopidogrel. In a review, omeprazole was thought to decrease the effectiveness of clopidogrel [80]. The authors note that the studies in their review were observational; therefore, causation is hard to pinpoint because the studies were not randomized. Another review of the literature in 2014 came to the same conclusion [81]. In the analyzed trials, a lower efficacy with the concurrent use of a PPI and clopidogrel was detected; however, the studies were observational and no obvious causality was identified. Earlier reviews have noted that, due to CYP interactions, it is felt that PPIs decrease blood levels of clopidogrel due to the increase in its metabolism, but make no mention of how the observational, nonrandomized nature of the studies could make these studies not as valid as the authors would have hoped [81]. Co-prescribing these two drugs seems appropriate to protect people who need clopidogrel's protection.
PPI Dosing and Adverse Effects
As previously stated, PPIs are among the most prescribed medications globally, and they are available over the counter, making it difficult to assess the PPI dose patients were taking in various studies. Two studies included the effects of different PPI doses on adverse outcomes.
A study investigating PPI use and mortality in kidney transplant recipients includes the following study criteria: low-dose omeprazole < 20 mg/day, high-dose omeprazole > 20 mg/day, and no PPI dose [82]. In the study, high-dose omeprazole (> 20 mg/day) led to an increase in premature mortality risk compared with low-dose omeprazole (< 20 mg/day). Mortality risk was increased for both high and low doses compared to the no PPI dose group. These findings suggest that the PPI dose, in general, may be a factor in adverse outcomes, since there was an increased mortality risk in comparison to not taking PPIs in this study group.
Adverse renal effects and PPI dosing were explored in a study investigating PPI use and hospital-acquired AKI in pediatric patients [73]. AKI risk was found to be increased at 1 mg/kg of body weight (children recommended dose 1–4 mg/kg/day) after only 1 day of use, based on a dose–response curve. The risk of AKI plateaued on the curve at 6 mg/kg, meaning that an increased risk exists for adverse renal outcomes at the recommended PPI dose.
PPI Use and All-Cause Mortality and Specific-Cause Mortality
Several observational studies have investigated the relationship between PPI use and all-cause mortality and specific-cause mortality; however, the results conflicted. Some studies supported the association between death and PPI use, whereas others rejected this idea.
PPI use was strongly associated with all-cause mortality and specific-cause mortality in a study involving US Veterans [83]. Over the observation period in this longitudinal observational cohort study, 80,062 (37.33%) of 214,467 veterans taking PPIs died, with the leading cause being circulatory disease (12.45%) followed by neoplasms (9.72%) and respiratory disease (4.8%). PPI use was strongly associated with specific-cause mortality, including death due to cardiovascular disease, chronic kidney disease, and upper GI cancer. There was a higher burden on upper GI cancer patients who did not have indications for PPI use. Two negative controls were tested in this study to help strengthen the association between PPI use and mortality: transportation-related death and peptic ulcer disease death. PPI use was unaffected by transportation-related death (− 0.21, 95% CI − 2.96 to 2.48) and peptic ulcer disease (− 0.46, 95% CI − 2.43 to 0.27). These findings suggest that a connection exists between PPI use and specific causes of mortality.
One cohort study examined the association of all-cause mortality and specific-cause mortality with two groups: new PPI users versus new H2A users and new PPI users versus non-users of acid suppression therapy [84]. Generally, PPI users had increased mortality risks compared to H2A users for both all-cause and specific-cause mortality. This study concluded that PPI use versus non-use strongly correlates with all-cause mortality [hazard ratio (HR) 1.96 95% CI 1.94–1.99]. Additionally, PPI use was associated with increased specific-cause mortality in neoplasms (HR 3.74, 95% CI 3.63–3.84), liver cirrhosis (HR 4.10, 95% CI 3.36–5.01), and gastric cancer (HR 14.59, 95% CI 11.16–19.08). There was no association between PPI use and neurological diseases, musculoskeletal diseases, or mental/behavior disorders. Even though PPI use was associated with mortality, the individuals who used PPIs had an increased risk of death and were generally sicker than the non-users. When PPI use was compared to both groups (H2A and non-users), the HRs decreased towards null as the data were adjusted for confounding, making residual confounding likely.
Another prospective study found no association between PPI use and all-cause and specific-cause mortality [85]. In this study, the authors felt that confounding variables played a large role in the results. The unadjusted HR for PPI use and overall mortality was 1.37 (95% CI 1.29–1.46). However, when adjusted, the HR of 1.05 (95% CI 0.97–1.13) decreased drastically to null, showing no association between mortality and PPI use. In addition, there was no connection between PPI use and specific-cause mortality, such as neoplasms, cardiovascular disease, respiratory disease, gastrointestinal disease, and external causes.
The studies investigating mortality and PPI use were not randomized, limiting the ability to eliminate all residual confounding variables. Randomized trials must be performed for causality and to control for possible confounding factors.
PPI Effect on Renal Transplant Recipients
Renal transplant is the desired outcome for individuals with end-stage renal disease. The short-term outcomes of renal transplants have improved over time; however, long-term renal graft survivability continues to be poor, with minimal signs of change [86]. Kidney transplant recipients have a sixfold higher death risk than the general population. Due to lifelong usage of immunosuppressants to prevent renal graft rejection, kidney transplant recipients have significant cardiovascular mortality, nutrition deficit, and recurring infections [82].
A prospective cohort study investigated the relationship between PPI use and mortality after kidney transplantation [82]. The study followed 703 kidney transplant recipients in the outpatient setting at the University Medical Center Groningen, Netherlands, between 2008 and 2011. The study's primary endpoint was to examine all-cause mortality, while secondary endpoints included graft failure incidents and specific-cause mortality (cardiovascular disease, malignancy, infections, and miscellaneous causes). A total of 398 patients were using PPIs during the study, and 132 (33.2%) died during follow-up, whereas 62 (20.3%) of the 305 patients not on PPIs died during follow-up (p < 0.001). Kidney transplant recipients who used PPIs had nearly a twofold increased risk of mortality when compared to PPI non-users (HR 1.86, 95% CI 1.38–2.52, p < 0.001). The results were adjusted for confounders, such as age, sex, BMI, comorbidities, medications, eGFR, deceased/alive donor, proteinuria, and primary renal disease type, without a considerable change in risk.
Specific-cause mortality in kidney transplant recipients and graft failure were explored in this study as secondary endpoints. Cardiovascular disease was the primary cause of death in these patients (37.1%), followed by infectious disease (24.2%), miscellaneous causes (20.1%), and malignancy (18.6%). Kidney transplant recipients with PPI use had over double the increased risk of cardiovascular death (HR 2.42, 95% CI 1.43–4.08, p < 0.001); the relationship remained considerable after the data were adjusted for confounding factors. In addition, kidney transplant recipients with PPI use had increased mortality risk from infectious disease (HR 1.89, 95% CI 1.02–3.49, p = 0.04); however, the associations between PPI use and malignancy and miscellaneous causes were not significant. Moreover, a higher risk of graft failure did not appear to be strongly associated with PPI use (HR 1.20, 95% CI 0.82–1.75, p = 0.4).
This study was replicated with a renal transplant cohort from Leuven, Belgium. The replicated study had similar results showing an increased risk of all-cause and specific-cause mortality in kidney transplant recipients who used PPIs. Both results were independent of confounders, such as donor characteristics, immunological factors, eGFR, lifestyle, age, sex, and comorbidities.
As previously mentioned, PPIs can potentially disrupt micronutrients, electrolytes, and vitamin absorption in the gastrointestinal tract by making the environment less acidic. Magnesium is an important electrolyte in a healthy, functioning immune system. Hypomagnesemia can further limit the effectiveness of an individual’s immune system who is already on immunosuppressive therapy. In addition, high-pH environments will allow harmful bacterial growth and colonization in the gastrointestinal tract. These factors can lead to susceptibility to recurrent gastrointestinal infections, pneumonia, and increased mortality risk in kidney transplant recipients.
PPI Effects on Pediatric Population
Data on the association between PPIs and AKI risk in the pediatric population are limited. One study examined the association between hospital-acquired AKI risks and PPI use in China [73]. This retrospective study used data from 2013 to 2015 on 42,232 children ranging from 1 month old to 18 years old from 25 tertiary hospitals (16 general hospitals and 9 children’s hospitals). The results showed that PPI pediatric users in the hospital setting had a 37% greater risk of developing AKI versus PPI non-users, and that the risk was evident even with the recommended dose (OR 1.37; 95% CI 1.23–1.53]. Additionally, the study investigated the associations between H2A, specifically cimetidine, and AKI risk, indicating a 35% increased risk in H2A users versus non-users. However, there was a 24% greater risk in PPI users when compared to H2A users (OR 1.24; 95% CI 1.01–1.52). Seriously ill children with intensive care needs and those with CKD have a higher risk of developing AKI with PPI use; children with CKD have a 2.37-fold increased PPI-related AKI risk (OR 3.37; 95% CI 2.46–4.62).
Confounding variables were adjusted for in this study with a propensity score match analysis, and an elevated PPI-related AKI remained for the pediatric population. The association remained consistent across different ages, gender, PPI subtypes, and PPI administration methods. It is possible that residual confounding still existed. In addition, this study was limited to children in China. Therefore, more evaluation would be needed to generalize these results to other populations.
Limitations of Clinical Studies
The causality between PPI use and increased mortality and disease risk can be questioned, since most studies were observational. Confounding variables can greatly affect an observational study and explain the wide-ranging associations with the use of PPIs. This can be seen in the three studies examining PPI use and all-cause and specific-cause mortality. Two studies determined that mortality was associated with PPI use and one study rejected the association [83–85]. Many studies have tried to adjust for confounding variables; however, residual confounding can still impact the overall results. Randomized trials can assist in determining PPI safety and whether causality exists in the relationship between PPI use and increased mortality and disease risk.
Patients on PPIs were generally older, obese, sicker with a higher number of baseline morbidities, and on more medications than PPI non-users [82–84]. One study showed increased all-cause and specific-cause mortality associated with PPI use; however, PPIs were given to people with an increased risk of death [84]. These findings suggest that PPI users are at a higher risk of mortality and with complications based on pre-existing conditions. Many studies have tried to adjust for confounders to account for these types of patients.
Conclusions
PPIs are one of the most widely prescribed medications globally. They offer significant, cost-effective relief for many diseases of the gastrointestinal tract, such as GERD and peptic ulcer disease. While PPIs are extremely effective in these hypersecretory diseases, it is evident that their use should be closely monitored, and that therapy should be discontinued when no longer needed to avoid adverse effects. It should be noted that there could be rebound hypersecretion of acid, and tapering of the PPI is suggested. Related to PPIs' over-the-counter availability, which permits their long-term use without direct supervision from a doctor, public access to them has rapidly increased, allowing patients to have often prescribed these drugs for “protection of the gut” or for other unspecified reasons with no goals for discontinuation. Long-term PPI use does not come without stipulations, as its use is associated with several adverse clinical outcomes, such as adverse effects on the kidney, increased risk of infection, bone fracture, and malabsorption in the small intestine. Hypomagnesemia is a relatively new side effect that can lead to routine magnesium infusions to decrease adverse effects. Patients on long-term PPI therapy are also at an increased risk of mortality, especially those with pre-existing comorbidities. PPI use can also lead to poorer clinical outcomes in the setting of other viral illnesses, such as COVID-19. Ultimately, PPIs should be prescribed with caution and intention to avoid misuse and the development of adverse events.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Amber N. Edinoff, Natalie W. Wu, Katelyn Parker, Edwin Dudossat, Lauren Linquest, Chelsi J. Flanagan, Anam Dharani, Hirni Patel, Olga Willett, Elyse M. Cornett, Adam M. Kaye and Alan D. Kaye contributed to the study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and statistical analysis.
Disclosures
Amber N. Edinoff, Natalie W. Wu, Katelyn Parker, Edwin Dudossat, Lauren Linquest, Chelsi J. Flanagan, Anam Dharani, Hirni Patel, Olga Willett, Elyse M. Cornett, Adam M. Kaye and Alan D. Kaye have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Contributor Information
Amber N. Edinoff, Email: aedinoff@mgh.harvard.edu
Natalie W. Wu, Email: natalie.wu@lsuhs.edu
Katelyn Parker, Email: kdp001@lsuhs.edu.
Edwin Dudossat, Email: ead002@lsuhs.edu.
Lauren Linquest, Email: lal002@lsuhs.edu.
Chelsi J. Flanagan, Email: cjflanag@student.uiwtx.edu
Anam Dharani, Email: adharani@student.uiwtx.edu.
Hirni Patel, Email: Hirni.Patel@lsuhs.edu.
Olga Willett, Email: olga.willett@lsuhs.edu.
Elyse M. Cornett, Email: elyse.bradley@lsuhs.edu
Adam M. Kaye, Email: akaye@PACIFIC.EDU
Alan D. Kaye, Email: alan.kaye@lsuhs.edu
References
- 1.Ahmed A, Clarke JO. Proton Pump Inhibitors (PPI). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK557385/. Accessed 15 May 2022.
- 2.Kherad O, Restellini S, Martel M, Barkun A. Proton pump inhibitors for upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019;1(42–43):101609. doi: 10.1016/j.bpg.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Spechler S. Proton pump inhibitors. Med Clin N Am. 2019;103(1):1–14. doi: 10.1016/j.mcna.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Yu LY, Sun LN, Zhang XH, Li YQ, Yu L, Yuan ZQY, et al. A review of the novel application and potential adverse effects of proton pump inhibitors. Adv Ther. 2017;34(5):1070–1086. doi: 10.1007/s12325-017-0532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabras P, Anedda M, Caddeo L, Francesco M, Antonella M. Hypomagnesemia and hypocalcemia caused by proton-pump inhibitors long-term therapy. Am J Ther. 2020;27(6):e676. doi: 10.1097/MJT.0000000000001041. [DOI] [PubMed] [Google Scholar]
- 6.Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153(1):35–48. doi: 10.1053/j.gastro.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed A, Clarke JO. Proton Pump Inhibitors (PPI). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. http://www.ncbi.nlm.nih.gov/books/NBK557385/. Accessed 5 Dec 2022.
- 8.Strand DS, Kim D, Peura DA. 25 Years of proton pump inhibitors: a comprehensive review. Gut Liver. 2017;11(1):27–37. doi: 10.5009/gnl15502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SANTARUS GETS FDA APPROVAL FOR ZEGERID CAPSULES | FDAnews [Internet]. https://www.fdanews.com/articles/84918-santarus-gets-fda-approval-for-zegerid-capsules. Accessed 11 Dec 2022.
- 10.Gau JT, Heh V, Acharya U, Yang YX, Kao TC. Uses of proton pump inhibitors and serum potassium levels. Pharmacoepidemiol Drug Saf. 2009;18(9):865–871. doi: 10.1002/pds.1795. [DOI] [PubMed] [Google Scholar]
- 11.Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6):528–534. doi: 10.1007/s11894-008-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearns GL, Winter HS. Proton pump inhibitors in pediatrics: relevant pharmacokinetics and pharmacodynamics. J Pediatr Gastroenterol Nutr. 2003;37(Supplement 1):S52–S59. doi: 10.1097/00005176-200311001-00011. [DOI] [PubMed] [Google Scholar]
- 13.El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. 2018;14(4):447–460. doi: 10.1080/17425255.2018.1461835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+, K+ ATPase. Annu Rev Pharmacol Toxicol. 1995;35(1):277–305. doi: 10.1146/annurev.pa.35.040195.001425. [DOI] [PubMed] [Google Scholar]
- 15.Savarino V, Marabotto E, Zentilin P, Furnari M, Bodini G, De Maria C, et al. Proton pump inhibitors: use and misuse in the clinical setting. Expert Rev Clin Pharmacol. 2018;11(11):1123–1134. doi: 10.1080/17512433.2018.1531703. [DOI] [PubMed] [Google Scholar]
- 16.Cantú TG, Korek JS. Central nervous system reactions to histamine-2 receptor blockers. Ann Intern Med. 1991;114(12):1027–1034. doi: 10.7326/0003-4819-114-12-1027. [DOI] [PubMed] [Google Scholar]
- 17.White CM. Ranitidine’s N-nitrosodimethylamine Problem May be Tip of the Iceberg. JAMA Netw Open. 2021;4(1):e2035158. doi: 10.1001/jamanetworkopen.2020.35158. [DOI] [PubMed] [Google Scholar]
- 18.Cimetidine—an overview | ScienceDirect Topics [Internet]. https://www.sciencedirect.com/topics/neuroscience/cimetidine. Accessed 11 Dec 2022.
- 19.Bell NJV, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51(1):59–67. doi: 10.1159/000200917. [DOI] [PubMed] [Google Scholar]
- 20.Howden CW, Burget DW, Hunt RH. Appropriate acid suppression for optimal healing of duodenal ulcer and gastro-oesophageal reflux disease. Scand J Gastroenterol. 1994;29(sup201):79–82. doi: 10.3109/00365529409105369. [DOI] [PubMed] [Google Scholar]
- 21.Hunt RH. Importance of pH control in the management of GERD. Arch Intern Med. 1999;159(7):649. doi: 10.1001/archinte.159.7.649. [DOI] [PubMed] [Google Scholar]
- 22.Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16(6):800–808.e7. doi: 10.1016/j.cgh.2017.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-Cervera J, Aj L. Eosinophilic esophagitis: an evidence-based approach to therapy. J Investig Allergol Clin Immunol. 2016;26(1):8–18. doi: 10.18176/jiaci.0002. [DOI] [PubMed] [Google Scholar]
- 24.Lipka S, Muhammad A, Champeaux A, Richter JE. Case report of proton pump inhibitor responsive esophageal eosinophilia: why 2 months of proton pump inhibitors is required: PPI responsive esophageal eosinophilia. Dis Esophagus. 2016;29(6):700–703. doi: 10.1111/dote.12237. [DOI] [PubMed] [Google Scholar]
- 25.Asher Wolf W, Dellon ES. Eosinophilic esophagitis and proton pump inhibitors: controversies and implications for clinical practice. Gastroenterol Hepatol. 2014;10(7):427–432. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z. Influence of efflux pump inhibitors on the multidrug resistance of Helicobacter pylori. World J Gastroenterol. 2010;16(10):1279. doi: 10.3748/wjg.v16.i10.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki T, Nakayama K, Yasuda H, Yoshida M, Asamura T, Ohrui T, et al. A randomized, single-blind study of lansoprazole for the prevention of exacerbations of chronic obstructive pulmonary disease in older patients: the proton pump inhibitor and exacerbation of COPD. J Am Geriatr Soc. 2009;57(8):1453–1457. doi: 10.1111/j.1532-5415.2009.02349.x. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki T, Nakayama K, Yasuda H, Yamaya M. A new strategy with proton pump inhibitors for the prevention of acute exacerbations in COPD. Ther Adv Respir Dis. 2011;5(2):91–103. doi: 10.1177/1753465810392264. [DOI] [PubMed] [Google Scholar]
- 29.Method of using (H+ /K+)ATPase inhibitors as antiviral agents-Patent US-5945425-A-PubChem [Internet]. https://pubchem.ncbi.nlm.nih.gov/patent/US5945425. Accessed 9 Feb 2023.
- 30.Ben-Eltriki M, Green CJ, Maclure M, Musini V, Bassett KL, Wright JM. Do proton pump inhibitors increase mortality? A systematic review and in-depth analysis of the evidence. Pharmacol Res Perspect. 2020;8:5. doi: 10.1002/prp2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19–24. doi: 10.1016/j.ejim.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Ther Adv Gastroenterol. 2012;5(4):219–232. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Zhan J, Bao Q, Lu J, Tan L. Possible dementia risk of proton pump inhibitors and H2 receptor blockers use in the treatment of Helicobacter pylori: a meta-analysis study. Med Hypotheses. 2020;1:44. doi: 10.1016/j.mehy.2020.109989. [DOI] [PubMed] [Google Scholar]
- 34.Lanas A. We are using too many PPIs, and we need to stop: a European perspective. Am J Gastroenterol. 2016;111(8):1085–1086. doi: 10.1038/ajg.2016.166. [DOI] [PubMed] [Google Scholar]
- 35.Raghunath AS, O’Morain C, Mcloughlin RC. Review article: the long-term use of proton-pump inhibitors. Aliment Pharmacol Ther. 2005;22(s1):55–63. doi: 10.1111/j.1365-2036.2005.02611.x. [DOI] [PubMed] [Google Scholar]
- 36.Castellana C, Pecere S, Furnari M, Telese A, Matteo MV, Haidry R, et al. Side effects of long-term use of proton pump inhibitors: practical considerations. Pol Arch Intern Med. 2021;131(6):541–549. doi: 10.20452/pamw.15997. [DOI] [PubMed] [Google Scholar]
- 37.McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin Infect Dis. 2018;66(7):e1–48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogielska M, Lanotte P, Le Brun C, Valentin AS, Garot D, Tellier AC, et al. Emergence of community-acquired Clostridium difficile infection: the experience of a French hospital and review of the literature. Int J Infect Dis. 2015;37:36–41. doi: 10.1016/j.ijid.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Haastrup PF, Jarbøl DE, Thompson W, Hansen JM, Søndergaard J, Rasmussen S. When does proton pump inhibitor treatment become long term? A scoping review. BMJ Open Gastroenterol. 2021;8(1):e000563. doi: 10.1136/bmjgast-2020-000563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jump RLP, Pultz MJ, Donskey CJ. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: a potential mechanism to explain the association between proton pump inhibitors and C difficile-associated diarrhea? Antimicrob Agents Chemother. 2007;51(8):2883–2887. doi: 10.1128/AAC.01443-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yibirin M, De Oliveira D, Valera R, Plitt AE, Lutgen S. Adverse Effects Associated with Proton Pump Inhibitor Use. Cureus [Internet]. 2021. https://www.cureus.com/articles/50133-adverse-effects-associated-with-proton-pump-inhibitor-use. Accessed 20 Nov 2022. [DOI] [PMC free article] [PubMed]
- 42.Lewis SJ, Franco S, Young G, O’Keefe SJD. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10(4):557–561. doi: 10.1046/j.1365-2036.1996.d01-506.x. [DOI] [PubMed] [Google Scholar]
- 43.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 44.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: a randomized crossover trial. Am J Med. 2005;118(7):778–781. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Hadjidakis DJ, Androulakis II. Bone remodeling. Ann N Y Acad Sci. 2006;1092(1):385–396. doi: 10.1196/annals.1365.035. [DOI] [PubMed] [Google Scholar]
- 46.Serfaty-Lacrosniere C, Wood RJ, Voytko D, Saltzman JR, Pedrosa M, Sepe TE, et al. Hypochlorhydria from short-term omeprazole treatment does not inhibit intestinal absorption of calcium, phosphorus, magnesium or zinc from food in humans. J Am Coll Nutr. 1995;14(4):364–368. doi: 10.1080/07315724.1995.10718522. [DOI] [PubMed] [Google Scholar]
- 47.Castiglioni S, Cazzaniga A, Albisetti W, Maier J. Magnesium and osteoporosis: current state of knowledge and future research directions. Nutrients. 2013;5(8):3022–3033. doi: 10.3390/nu5083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernlund E, Svedbom A, Ivergård M, Compston J, Cooper C, Stenmark J, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA) Arch Osteoporos. 2013;8(1–2):136. doi: 10.1007/s11657-013-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lewis JR, Barre D, Zhu K, Ivey KL, Lim EM, Hughes J, et al. Long-term proton pump inhibitor therapy and falls and fractures in elderly women: a prospective cohort study: proton pump inhibitors and falls. J Bone Miner Res. 2014;29(11):2489–2497. doi: 10.1002/jbmr.2279. [DOI] [PubMed] [Google Scholar]
- 50.Brozek W, Reichardt B, Zwerina J, Dimai HP, Klaushofer K, Zwettler E. Higher dose but not low dose proton pump inhibitors are associated with increased risk of subsequent hip fractures after first hip fracture: a nationwide observational cohort study. Bone Rep. 2019;10:100204. doi: 10.1016/j.bonr.2019.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lanas-Gimeno A, Hijos G, Lanas Á. Proton pump inhibitors, adverse events and increased risk of mortality. Expert Opin Drug Saf. 2019;18(11):1043–1053. doi: 10.1080/14740338.2019.1664470. [DOI] [PubMed] [Google Scholar]
- 52.Carmel R. Cobalamin, the stomach, and aging. Am J Clin Nutr. 1997;66(4):750–759. doi: 10.1093/ajcn/66.4.750. [DOI] [PubMed] [Google Scholar]
- 53.Jung SB, Nagaraja V, Kapur A, Eslick GD. Association between vitamin B12 deficiency and long-term use of acid-lowering agents: a systematic review and meta-analysis: Vitamin B12 deficiency. Intern Med J. 2015;45(4):409–416. doi: 10.1111/imj.12697. [DOI] [PubMed] [Google Scholar]
- 54.Werder Cobalamin deficiency, hyperhomocysteinemia, and dementia. Neuropsychiatr Dis Treat. 2010;20:159. doi: 10.2147/NDT.S6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Severance S, Hamza I. Trafficking of heme and porphyrins in metazoa. Chem Rev. 2009;109(10):4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 57.Hamano H, Niimura T, Horinouchi Y, Zamami Y, Takechi K, Goda M, et al. Proton pump inhibitors block iron absorption through direct regulation of hepcidin via the aryl hydrocarbon receptor-mediated pathway. Toxicol Lett. 2020;318:86–91. doi: 10.1016/j.toxlet.2019.10.016. [DOI] [PubMed] [Google Scholar]
- 58.Shiraev TP, Bullen A. Proton pump inhibitors and cardiovascular events: a systematic review. Heart Lung Circ. 2018;27(4):443–450. doi: 10.1016/j.hlc.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 59.Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT, Cooke JP. Proton pump inhibitors accelerate endothelial senescence. Circ Res. 2016;118:12. doi: 10.1161/CIRCRESAHA.116.308807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitridge R, Thompson M. Mechanisms of vascular disease: a reference book for vascular specialists [Internet]. 1st ed. University of Adelaide Press; 2011. https://www.cambridge.org/core/product/identifier/9781922064004/type/book. Accessed 25 Nov 2022. [PubMed]
- 61.Juurlink DN, Dormuth CR, Huang A, Hellings C, Paterson JM, Raymond C, et al. Proton pump inhibitors and the risk of adverse cardiac events. PLoS One. 2013;8(12):e84890. doi: 10.1371/journal.pone.0084890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of proton pump inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open. 2017;7(6):e015735. doi: 10.1136/bmjopen-2016-015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bell JS, Strandberg T, Teramura-Gronbland M. Use of proton pump inhibitors and mortality among institutionalized older people. Arch Intern Med. 2010;170(17):1604. doi: 10.1001/archinternmed.2010.304. [DOI] [PubMed] [Google Scholar]
- 64.Rai R, Saraswat VA, Dhiman RK. Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S29–36. doi: 10.1016/j.jceh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nardelli S, Gioia S, Ridola L, Farcomeni A, Merli M, Riggio O. Proton pump inhibitors are associated with minimal and overt hepatic encephalopathy and increased mortality in patients with cirrhosis. Hepatology. 2019;70(2):640–649. doi: 10.1002/hep.30304. [DOI] [PubMed] [Google Scholar]
- 66.Darnell MER, Subbarao K, Feinstone SM, Taylor DR. Inactivation of the coronavirus that induces severe acute respiratory syndrome. SARS-CoV J Virol Methods. 2004;121(1):85–91. doi: 10.1016/j.jviromet.2004.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corsonello A, Lattanzio F, Bustacchini S, Garasto S, Cozza A, Schepisi R, et al. Adverse events of proton pump inhibitors: potential mechanisms. Curr Drug Metab. 2018;19(2):142–154. doi: 10.2174/1389200219666171207125351. [DOI] [PubMed] [Google Scholar]
- 68.Brewster UC, Perazella MA. Proton pump inhibitors and the kidney: critical review. Clin Nephrol. 2007;68(2):65–72. doi: 10.5414/CNP68065. [DOI] [PubMed] [Google Scholar]
- 69.Arora P, Gupta A, Golzy M, Patel N, Carter RL, Jalal K, et al. Proton pump inhibitors are associated with increased risk of development of chronic kidney disease. BMC Nephrol. 2016;17(1):112. doi: 10.1186/s12882-016-0325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hart E, Dunn TE, Feuerstein S, Jacobs DM. Proton pump inhibitors and risk of acute and chronic kidney disease: a retrospective cohort study. Pharmacotherapy. 2019;39(4):443–453. doi: 10.1002/phar.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol CJASN. 2014;9(1):12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11–20. doi: 10.1056/NEJMoa1611391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Y, Xiong M, Yang M, Wang L, Nie S, Liu D, et al. Proton pump inhibitors and the risk of hospital-acquired acute kidney injury in children. Ann Transl Med. 2020;8(21):1438. doi: 10.21037/atm-20-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Y, George KC, Shang WF, Zeng R, Ge SW, Xu G. Proton-pump inhibitors use, and risk of acute kidney injury: a meta-analysis of observational studies. Drug Des Devel Ther. 2017;11:1291–1299. doi: 10.2147/DDDT.S130568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheema E. Investigating the association of proton pump inhibitors with chronic kidney disease and its impact on clinical practice and future research: a review. J Pharm Policy Pract. 2019;12:6. doi: 10.1186/s40545-019-0167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bettinger D, Martin D, Rieg S, Schultheiss M, Buettner N, Thimme R, et al. Treatment with proton pump inhibitors is associated with increased mortality in patients with pyogenic liver abscess. Aliment Pharmacol Ther. 2018;47(6):801–808. doi: 10.1111/apt.14512. [DOI] [PubMed] [Google Scholar]
- 77.Hanaoka H, Kikuchi J, Kaneko Y, Seki N, Tsujimoto H, Chiba K, et al. Proton pump inhibitor and tacrolimus uses are associated with hypomagnesemia in connective tissue disease: a potential link with renal dysfunction and recurrent infection. Front Pharmacol. 2021;12:616719. doi: 10.3389/fphar.2021.616719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brozek W, Reichardt B, Zwerina J, Dimai HP, Klaushofer K, Zwettler E. Use of proton pump inhibitors and mortality after hip fracture in a nationwide study. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2017;28(5):1587–1595. doi: 10.1007/s00198-017-3910-x. [DOI] [PubMed] [Google Scholar]
- 79.Ikuta K, Nakagawa S, Momo K, Yonezawa A, Itohara K, Sato Y, et al. Association of proton pump inhibitors and concomitant drugs with risk of acute kidney injury: a nested case–control study. BMJ Open. 2021;11(2):e041543. doi: 10.1136/bmjopen-2020-041543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Juel J, Pareek M, Jensen SE. The clopidogrel-PPI interaction: an updated mini-review. Curr Vasc Pharmacol. 2014;12(5):751–757. doi: 10.2174/157016111205140926161509. [DOI] [PubMed] [Google Scholar]
- 81.D’Ugo E, Rossi S, De Caterina R. Proton pump inhibitors and clopidogrel: an association to avoid? Intern Emerg Med. 2014;9(1):11–22. doi: 10.1007/s11739-013-1000-4. [DOI] [PubMed] [Google Scholar]
- 82.Douwes RM, Gomes-Neto AW, Eisenga MF, Van Loon E, Schutten JC, Gans ROB, et al. The association between use of proton-pump inhibitors and excess mortality after kidney transplantation: a cohort study. PLoS Med. 2020;17(6):e1003140. doi: 10.1371/journal.pmed.1003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie Y, Bowe B, Yan Y, Xian H, Li T, Al-Aly Z. Estimates of all cause mortality and cause specific mortality associated with proton pump inhibitors among US veterans: cohort study. BMJ. 2019;365:l1580. doi: 10.1136/bmj.l1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brown JP, Tazare JR, Williamson E, Mansfield KE, Evans SJ, Tomlinson LA, et al. Proton pump inhibitors and risk of all-cause and cause-specific mortality: a cohort study. Br J Clin Pharmacol. 2021;87(8):3150–3161. doi: 10.1111/bcp.14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He Q, Xia B, Meng W, Fan D, Kuo ZC, Huang J, et al. No associations between regular use of proton pump inhibitors and risk of all-cause and cause-specific mortality: a population-based cohort of 0.44 million participants. Am J Gastroenterol. 2021;116(11):2286–2291. doi: 10.14309/ajg.0000000000001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lamb KE, Lodhi S, Meier-Kriesche HU. Long-term renal allograft survival in the United States: a critical reappraisal. Am J Transplant. 2011;11(3):450–462. doi: 10.1111/j.1600-6143.2010.03283.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.