Version Changes
Revised. Amendments from Version 2
The difference between versions 2 and 3 of this research is in the manuscript because the author had revised the English language and grammar. There are also revisions in the result and discussion, from Table 1. The author explained the common name of P. crocatum – Red betel leaf in the introduction so that these synonyms name can be used further without confusing the reader. The explanation of the solvent used to extract secondary metabolites from P. crocatum has also been explained in Table 1, using ether 40-60 0C, chloroforms, ethanol, water, and methanol extract from the type and polarity of the solvent used. in the previous version, the author only explained the bioactive compound of P. crocatum from only one reference. For the latest version, 4 (four) new references have been added that support information about bioactive compounds in P. crocatum, becoming more complete. Several sentences have been improved which have missing verbs so that they are easier to understand. The title of Figure 3 revised also have been made as the reviewer suggested its grammar and added a new explanation regarding capital letters in Figure from A-F. The A-F explained about the differentiation of cell wall association in fungal adhesins in several fungi species, like C. albicans, C. cerevisiae, P. braziliensis, A. fumigatus, B. dermatitis, S. pombe, and C. neoformans. The narrative in Figure 3 also has been revised and broken down into 3 new sentences that are simpler than one long and unclear sentence before. The author also explained the antifungal activities by P. crocatum phytochemicals like phenols, polyphenols, tannins, saponins, and flavonoids to inhibit ergosterol by lanosterol 14 alpha demethylase inhibition. The conclusion has been revised in the grammar and added a new explanation about the relationship between ergosterol and lanosterol 14 alpha demethylase.
Abstract
Mycoses or fungal infections are a general health problem that often occurs in healthy and immunocompromised people in the community. The development of resistant strains in Fungi and the incidence of azole antibiotic resistance in the Asia Pacific which reached 83% become a critical problem nowadays. To control fungal infections, substances and extracts isolated from natural resources, especially in the form of plants as the main sources of drug molecules today, are needed. Especially from Piperaceae, which have long been used in India, China, and Korea to treat human ailments in traditional medicine. The purpose of this review is to describe the antifungal mechanism action from Piper crocatum and its phytochemical profiling against lanosterol 14a demethylase CYP51. The methods used to search databases from Google Scholar to find the appropriate databases using Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Flow Diagram as a clinical information retrieval method. From 1.150.000 results searched by database, there is 73 final results article to review. The review shows that P. crocatum contains flavonoids, tannins, terpenes, saponins, polyphenols, eugenol, alkaloids, quinones, chavibetol acetate, glycosides, triterpenoids or steroids, hydroxychavikol, phenolics, glucosides, isoprenoids, and non-protein amino acids. Its antifungal mechanisms in fungal cells occur due to ergosterol, especially lanosterol 14a demethylase (CYP51) inhibition, which is one of the main target sites for antifungal activity because it functions to maintain the integrity and function of cell membranes in Candida. P. crocatum has an antifungal activity through its phytochemical profiling against fungal by inhibiting the lanosterol 14a demethylase, make damaging cell membranes, fungal growth inhibition, and fungal cell lysis.
Keywords: Piper crocatum, antifungal, phytochemical profiling, lanosterol 14 alpha demethylase, CYP51
Introduction
Mycoses or fungal infections are a general health problem that often occurs in healthy and immunocompromised people in the community ( Ramírez et al. 2013). Fungi are divided into four classes: yeasts, filamentous, dimorphic, and dermatophytes; generally, and ubiquitous in the environment, and become pathogenic when immune cells decrease ( Howard et al. 2020). Fungal cells have essentially dynamic structure walls for morphogenesis, pathogenesis, and cell viability and act as a dynamic organelle, and need one-fifth of the yeast genome for cell wall biosynthesis ( Gow et al. 2017). The development of resistant strains in fungi and the incidence of azole antibiotic resistance in the Asia Pacific which reached 83% become a critical problem nowadays ( Whaley and Rogers 2016; Whaley et al. 2017).
The Azoles are commonly used because cheaper and have a broad spectrum of antimicrobials ( Rosam et al. 2021). To control fungal infections, substances, and extracts isolated from natural resources, especially in the form of plants as the main sources of drug molecules today, are needed ( Balouiri et al. 2016). Natural products have limited or no side effects on human and animal antifungal activity ( Tabassum and Vidyasagar 2013). Antifungal mechanisms in fungal cells occur due to ergosterol inhibition as a result of 5,6 desaturase (ERG3) downregulation which is the second final step of the ergosterol biosynthetic pathway ( Alizadeh et al. 2017). Ergosterol at the fungal plasma membrane is the most common sterol and binding at lanosterol 14α demethylase, an ergosterol-specific enzyme that can cause lanosterol demethylation ( Loeffler and Stevens 2003; Ashley et al. 2006; Emami et al. 2017).
Piperaceae plant extracts have long been used in India, China, and Korea to treat human ailments in traditional medicine ( Jeon et al. 2019). The part of the Piperaceae family which has large species of up to 1000 is the Piper genus ( Durant-Archibold et al. 2018). Piper can be found in temperate regions with tropical and sub-tropical ( Lima et al. 2020). Indonesia is located on the equator, which has a tropical climate with high humidity and many natural and biological resources ( Puspita et al. 2018). The seeds and leaves of Piper species are often cultivated and consumed for various diseases treatment such as antifungal, antibacterial, and disinfectant effects ( Astuti et al. 2014; Mgbeahuruike et al. 2017).
Isolation of several secondary metabolites of Piper species shows that therapeutically molecules like lignans, flavones, alkaloids, unsaturated amides, long and short-chain esters, aristolactams, monoterpenes, sesquiterpenes, ketones, aldehydes, arylpropanoids, chalcones, propenylphenols, and amide alkaloids as a typical constituent ( Gutierrez et al. 2013). However, no tests were found on specific compounds for antibacterial activity from Piper ( Barh et al. 2013). Based on the literature, antifungal compounds are classified into flavonoids, amides, acid derivatives, lignans, prenylated benzoic, cyclopentanedione, butenolides, and phenylpropanoids ( Xu and Li 2011). Of the various Piper species, the main constituent is an amide, which is classified as aristolactams, open-chain alkamides, amides with pyrrolidine, 4,5-dioxoaporphines, Piperidine, and Piperidone groups, ceramides, cyclohexanamid, and cyclobutanamide ( Do Nascimento et al. 2012). In this review, we summarize the antifungal activity properties, structural studies, and bio-mechanism of P. crocatum, commonly known as Red Betel leaf, which is found worldwide, against lanosterol 14 α demethylase CYP51 in fungi. This review is expected to allow us to find new alternative antifungal treatment agents from natural resources based on their active chemical compounds and activities to cure fungal infections, thus reducing the extensive and inappropriate use of antibiotics for antifungal treatment.
Methods
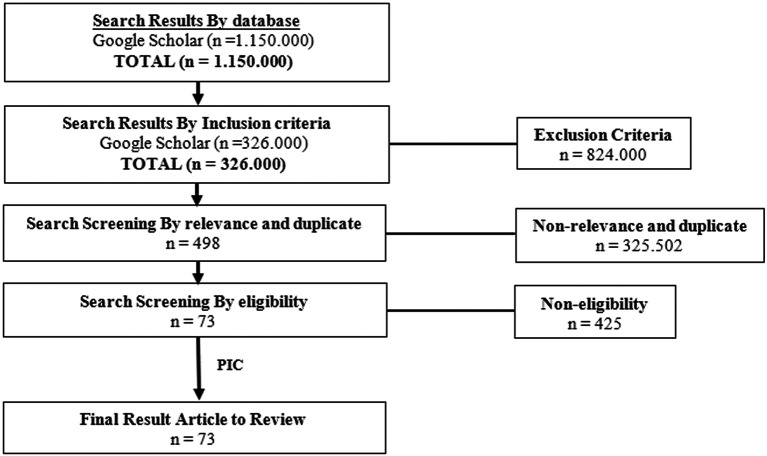
The author searches databases from Google Scholar to find the appropriate databases using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Flow Diagram as a clinical information retrieval method. The search screening occurred through four stages. The first stage was screening by the keywords, the second stage was screening by the criteria, the third stage was screening by relevance and duplicates, and the fourth stage was screening by eligibility. The keyword of this search was ‘ Piper’ which yielded 1,150,000 results. The second step was screening by inclusion criteria of the keyword ‘ Piper’ that reported articles published from 2003 until 2022, which yielded 326,000 results. For the third step, screening by relevance and duplicate with the keyword of this search is ‘ Piper crocatum’ AND ‘antifungal’, which yielded about 498 results. The final screening results included 73 articles to review. This search was conducted from February to May 2022. The criteria for this research were clinical trials in animal testing and humans, books, laboratory tests, case studies, article reviews, systematic reviews, narrative reviews, and meta-analyses. The study was conducted with a true-experimental (Double-Blind RCT), quasi-experimental, study protocol, or pilot study. Articles were published in English. The flow chart of the Literature Review showed in Figure 1.
Figure 1. The flowchart of literature reviews of P. crocatum.
Results and discussion
Ethno-botany and ethnopharmacology of P. crocatum
P. crocatum, used as spices, vegetables, and components in some ethnic’s ceremonial, and medicinal herbs by the local community in Southeast Asian Countries especially Indonesia to treat candidiasis, hypertension, hepatitis, diabetes mellitus, kidney failure, cholesterol, prevent stroke, breast cancer, hemorrhoids, inflammation, coughing up blood, nose bleed, vaginal discharge, and tuberculosis disease ( Astuti et al. 2014; Lely et al. 2021). P. crocatum, a plant native from Peru then spread to several regions in the world, and grows in temperate climates (tropical and subtropical) ( Lima et al. 2020). Red betel is a shrub that consists of trunked, 5-10 cm continuous branches, with each segment having roots growing ( Safithri and Fahma 2008). The stems of red betel are round, purplish-green heart-shaped leaves, have no flowers, green with grayish-white tapered ends, special aroma, and are very bitter ( Hermiati et al. 2013).
The taxonomy of P. crocatum is as follows ( Suri et al. 2021):
Kingdom: Plantae
Subkingdom: Tracheobionta
Division: Magnoliophyta
Class: Magnoliopsida
Order: Piperales
Family: Piperaceae
Genus: Pipers
Species: Piper crocatum Ruiz&Pav
According to ethnopharmacology data, P. crocatum leaves have anxiolytic, analgesic, anti-inflammatory, vasodilatory, immunomodulatory, antimicrobial, antifungal, antitumor, antibacterial, anti-cariogenic, antifungal, anti larva, anti protozoa, anti filaria, antiallergic, antidiabetic, antihelmintic, stress relieves, antihyperglycemic, antibiotics, platelet inhibitors, and antioxidants ( Bezerra et al. 2008; Rodrigues et al. 2009; Fadlilah 2015; Fatmawaty et al. 2019; Madhumita et al. 2020).
The phytochemical of P. crocatum
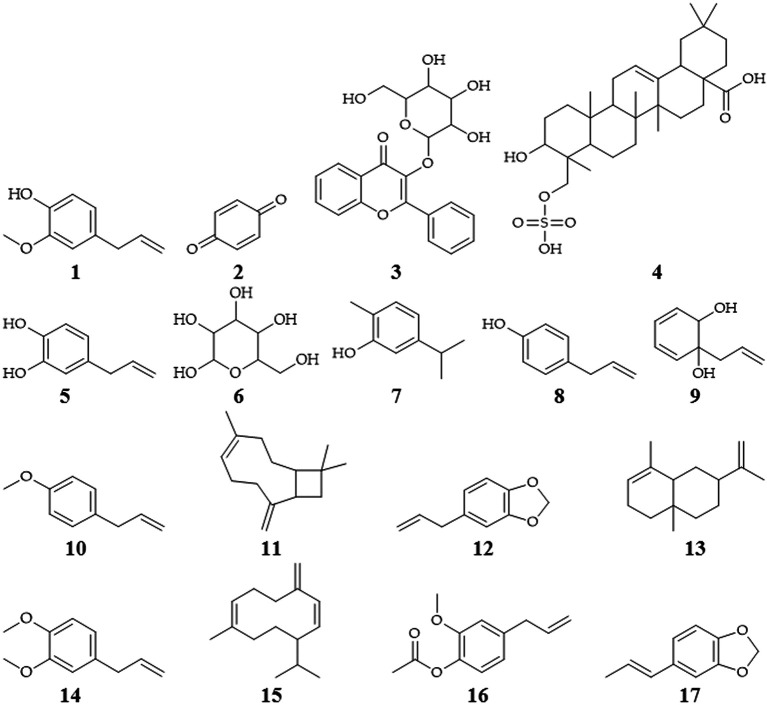
Isolated P. crocatum from 112 species contains 677 different compounds, consisting of 190 alkaloids or amides, 97 terpenes, 70 neolignans, 49 lignans, 39 phenylpropanoids, 18 kavapyrones, 17 chalcones or dihydrochalcones, 16 flavones, 15 steroids, 6 flavanones, 4 cinnamylidone butenolides or piperolides, and 146 other compounds ( Saputra et al. 2016). Isolation of several secondary metabolites of P. crocatum contains flavonoids, tannins, terpenes, saponins, polyphenols, eugenol ( 1), alkaloids, quinones ( 2), chavibetol acetate, glycosides ( 3), triterpenoids ( 4) or steroids, hydroxychavikol ( 5), phenolics, glucosides ( 6), isoprenoids, and non-protein amino acids ( Erviana 2011; Gutierrez et al. 2013; Saputra et al. 2016; Li et al. 2019; Lister et al. 2020; Safithri et al. 2020).
The bioactive compounds of P. crocatum are as follows in Table 1 and Figure 2.
Table 1. The bioactive compounds of P. crocatum.
| Extract type | Chemical compounds | ||||
|---|---|---|---|---|---|
| Used ether 40-60°C, chloroforms, ethanol, and water solvent ( Suri et al. 2021) | Used ethanol solvent ( Fatmawaty, Anggreni, Fadhil, & Prasasty 2019) | Used methanol Solvent ( Januarti, Wijayanti, Wahyuningsih, & Nisa 2019) | Used methanol solvent ( Safithri & Fahma 2008) | Used methanol extract ( Li, Yang, Kim, & Li 2019) | |
| Red Betel leaf extract | Alkaloid | Alkaloid | Saponin | Alkaloid | Pipercroside A |
| Carbohydrate | Glycosides | Tannin | Flavonoid | Pipercroside B | |
| Water | Saponin | Phenol | Tannin | 2,5-Dimethoxy-3-glucopyranosylcinnamic alcohol | |
| Tannin | Tannin | Flavonoid | Cimidahurinin | ||
| Phenol | Triterpenoid/Steroid | Erigeside II | |||
| Flavonoid | Flavonoid | Syringin | |||
| Essential oil | Essential oil | β-Phenylethyl β- D-glucoside | |||
| Methyl salicylate 2- O-β- D-Glucopyranoside | |||||
| Icariside D1 | |||||
| 4-Hydroxybenzoic acid β- D-glucosyl ester | |||||
| Benzyl β- D-Glucoside | |||||
| Phenylmethyl 6- O-α- L-Arabinofuranosyl-β- D-glucopyranoside | |||||
| Red Betel leaf essential oil (water distillation, sodium sulfate dryer) | Carvacrol (7) | ||||
| Eugenol ( 1) 28.44% | |||||
| Chavicol ( 8) | |||||
| Allylcatechol ( 9) | |||||
| Cinema | |||||
| Estragole ( 10) | |||||
| Caryophyllene ( 11) | |||||
| Pcymenedaneugenol Metil eter-19 | |||||
| Safrole ( 12) 27.48% | |||||
| Selinene ( 13) 7.32% | |||||
| Methyl eugenol ( 14) 1.46% | |||||
| Germacrene D ( 15) 0.91% | |||||
| Eugenyl Acetate ( 16) 1.72% | |||||
| Isosafrole ( 17) 1.62% | |||||
Figure 2. Chemical structures of P. crocatum ( Saputra et al. 2016; Fatmawaty et al. 2019; Lister et al. 2020).
The heated leaves are often used to relieve asthma, sore throats, coughs, and vaginal discharge, while the essential oil is used as catarrh and diphtheria inhalation and mouthwash, with antifungal activity ability against Candida sphaerospermum and Candida cladospoiroides with strong MIC of 10 μg/ml ( Safithri et al. 2020; Suri et al. 2021). The antimicrobial compounds include phenolics and flavonoids in P. crocatum by its hydroxyl group at 5 positions causing inner and outer bacterial cell membrane fluidity reduction ( Puspita et al. 2018). Alkaloid in P. crocatum by its aromatic substitution, carbon rings, and oxidation nature caused inhibition of bacterial growth and cell lysis. Tannins also have antibacterial activity by slowing the fungal cell's growth, and shrinking cell membranes thereby limiting the development of cell membrane synthesis, distorting permeability, breakdown, and cell lysis while saponins can dilute lipids (lipophilic) and then reducing cell surface pressure with their ability to attract water (hydrophilic) and caused cell damage. Terpenoids in P. crocatum cause permeability decreased and rupture of cell membranes so the nutrients and enzymes leave the cytoplasm, decrease metabolism, reduce ATP production, and inhibit bacterial growth and reproduction ( Cowan 1999; Rinanda et al. 2012).
Test methods and antifungal properties of P. crocatum
P. crocatum has been tested on C. albicans with agar diffusion method ( Kusuma et al. 2017), MIC determination with microdilution method, and MBC determination by the medium surface of Mueller Hinton Agar, and shows that extract of ethanol indicates antifungal activity, MIC value 1.25-2.5% w/v, and MBC value 0.75 min ( Kusuma et al. 2017). The study shows about ten (10) known compounds (2,5-dimethoxy-3-glucopyranosylcinnamic alcohol, cimidahurinin, erigeside II, syringin, β-phenylethyl β-D-glucoside, methyl salicylate 2- O-β-D-glucopuranoside, icariside D1, 4-hydroxybenzoic acid β-D-glucosyl ester, benzyl β-D-glucoside, and phenylmethyl 6- O-α-L-arabinofuranosyl-β-D-glucopyranoside), and two new phenolic glucosides (Pipercroside A and B) that isolated from MeOH extract of P. crocatum elucidated by spectroscopy 1D and 2D NMR, HR-ESI-MS analysis also reports erigeside II have the best antifungal activity with IC 50 value as 58.5 ( Li et al. 2019).
Polyphenol inactivates protein and inhibits enzymes on the surface of bacterial cells; flavonoids form complexes that interfere with the function of the bacterial cell wall, inactivating microbial adhesion, enzymes, and cell protein transport by binding to bacterial extracellular proteins through hydrogen and covalent bonds; saponins have hydrophilic molecules and lipid thinning molecules (lipophilic) so that they can make lower cell surface pressure; tannins functions to form complex compounds with enzymes and substrates, thereby disrupting cell membranes, and phenol has hydroxyl and carbonyl groups that can interact with fungal cells through hydrogen bonds, thereby increasing protein coagulation and fungal cell membranes which will cause fungal cells to lyse ( Januarti et al. 2019). Inhibition of fungal activity can be done by bothering cell membranes, the activity of enzymes, and fungi genetic mechanisms ( Ejele et al. 2012).
Antifungal properties and structure
Treatment of vaginal discharge due to Candida gave the best response to a combination of the intravaginal vulva and topical therapy ( Mitchell 2004). Antifungals for the treatment of vaginal discharge caused by C. albicans are fluorinated pyrimidine cytosine (5-FC) which targets RNA synthesis and DNA replication, polyenes which affect the integrity of cell membranes, azoles which affect the target of the ergosterol biosynthetic pathway, and echinocandins which affect cell wall biosynthesis, while the use of broad-spectrum antibiotics increases cases of immunocompromise ( Yücesoy and Marol 2003; Sendid et al. 2007).
Antifungals that damage cell membrane permeability work by binding to ergosterol in the polyene group, inhibiting the synthesis of ergosterol in squalene monooxygenase or epoxidase in the allylamines group, and inhibiting the synthesis of ergosterol in 14-α-demethylase or fungal cytochrome P450 in the azoles group; antifungal destroying cell walls works by inhibiting the synthesis of 1,3-β-glucan by binding to the glucan synthase enzyme which functions to form glucan in the echinocandin group; and antifungal inhibitors of DNA synthesis from fungal cells by inhibiting the synthesis of thymidylate or pyrimidine analogs in flucytosine (5-Fluorocytosine) and mitotic inhibitors in griseofulvin ( Cannon et al. 2007; Lewis 2011).
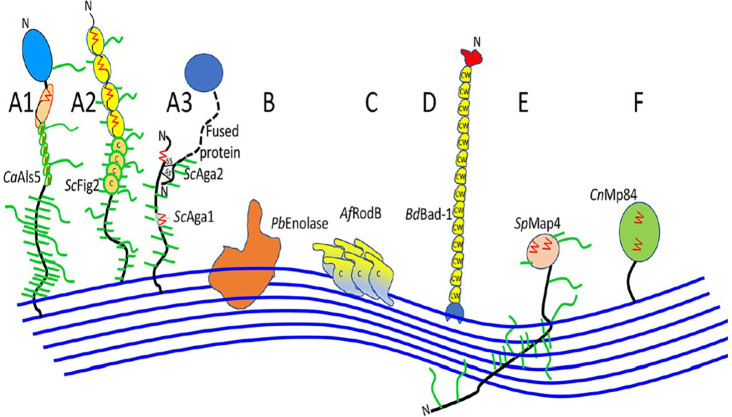
Fungal cell walls, fungal-specific, serve as protection from harmful environments and are aggressive because of its toxic and hydrolytic molecules. Ninety percent consist of polysaccharides, with Saccharomyces and Candida subphylum as the well-characterized fungal adhesins ( Figure 3) ( Latgé 2007). The core of the central fungal cell wall consists of chitin that is linked to branched β-1,3-glucan, combined with galactomannan, galactosamynoglycan, and β-1,3-1,4-glucan in A. fumigatus and β-1,6-glucan in C. albicans ( Adams 2004; Latgé 2010). Chitin, with a weight of 1-2% from dry cell wall yeast, is an important structure that consists of a homopolymer of β-1,4-linked N-acetylglucosamine that is long and linear, while disrupted chitin synthesis will cause the fungal cell wall to be lysis and unstable in osmotic ( Bowman and Free 2006).
Figure 3. An illustration of cell wall association in fungal adhesins (the blue line indicates the cell wall which is composed of glucan polymers; Ca, C. albicans; Sc, S. cerevisiae; Pb, P. braziliensis; Af, A. fumigatus; Bd, B. dermatitidis; Sp, S. pombe; Cn, C. neoformans; A, adhesins; B-E, modes of cell wall attachment; A1 and A3, have discrete ligand binding domains; A2 doesn’t have discrete ligand binding domains, Cys-rich sequences in ScFig2; C, Cys-rich sequences in AfRodB; D, Cys/Trp rich domains in Bd/Bad-1; F, attached by modified GPI anchors to the cell wall) ( Lipke 2018).
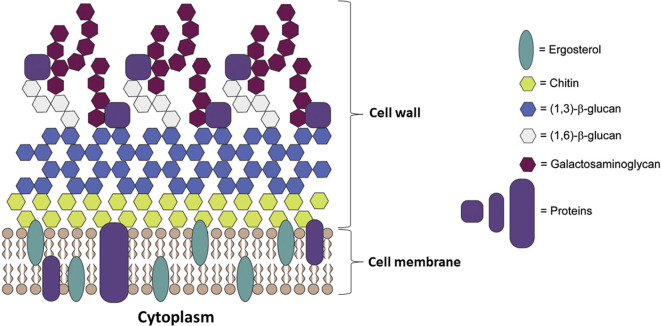
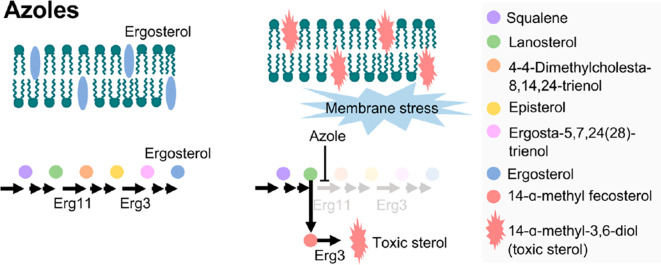
Ergosterol is a bitopian endoplasmic reticulum protein, which spans the entire length of the lipid bilayer ( Figure 4) ( Emami et al. 2017; Rosam et al. 2021). Ergosterol is a key enzyme in fungal-specific sterols, cytochrome P450 enzyme in fungi derived from S. cerevisiae, belonging to the CYP51 (lanosterol 14-α-demethylase) family. The biosynthesis inhibition of ergosterol will be caused by intermediates toxic sterol accumulation (14-α-methyl-3,6-diol) by ERG3. By binding the nitrogen atom containing heterocyclic moiety in the core ring to the iron atom of the heme domain group in the active site and preventing the formation of lanosterol demethylation, the cell membranes will get damaged, and lysis ( Arthington-Skaggs et al. 1999; Flowers et al. 2015). Ergosterol biosynthesis is one of the main target sites for antifungal activity because ergosterol functions to maintain the integrity and function of cell membranes in Candida so it can cause fungistatic effects if there is inhibition of lanosterol 14α demethylase ( Shareef et al. 2019; Kumar et al. 2020).
Figure 4. The cell wall and membrane of Candida ( Emami et al. 2017; Rosam et al. 2021).
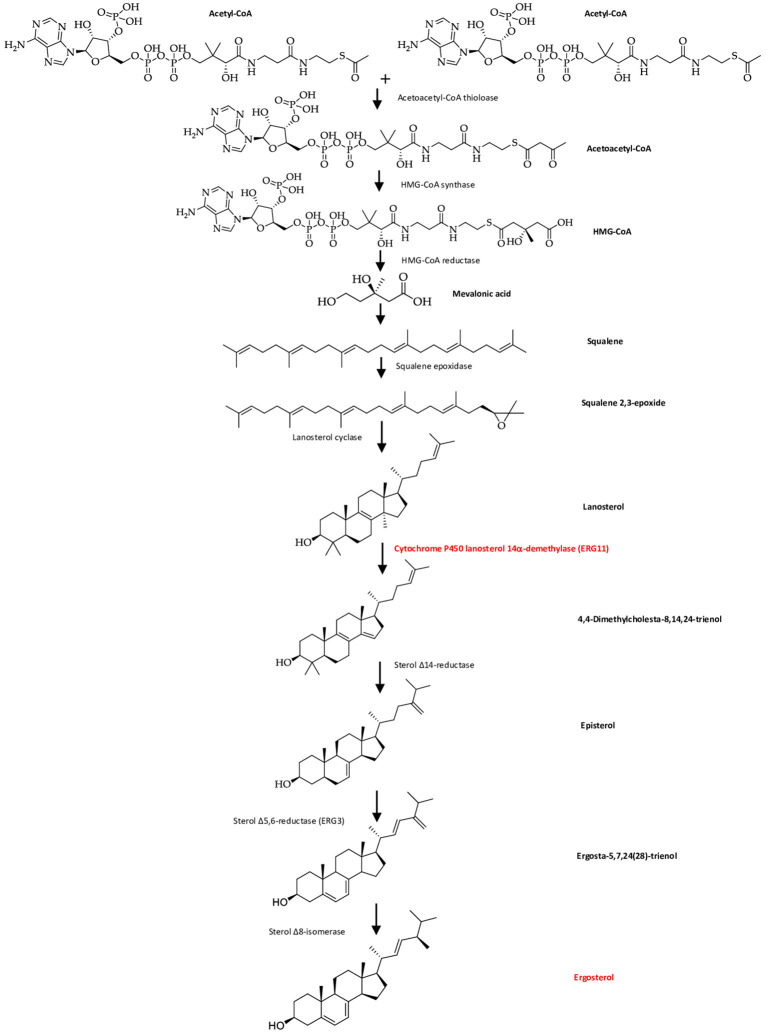
The accumulation of toxic sterol and depletion of ergosterol causes inhibition of cell growth and division, then increases pressure on the cell wall so that the cell becomes damaged ( Robbins et al. 2016). Fungal cell walls that have been damaged can cause fungal growth inhibition, morphological changes, and fungal cell lysis ( Buitimea-Cantúa et al. 2013). ERG11, the major target in the fungal membrane that is absent in the host cell membrane catalyzes C14-demethylation of lanosterol to 4,4′-dimethyl cholesta-8,14,24-triene-3-β-ol, sterol 14-reductase then reductases to episterol, which in turn is converted to ergosta-5,7,24(28)-trienol by sterol 5,6 desaturase (ERG3), and with sterol 8-isomerase converted it into ergosterol ( Figure 5). The fungistatic mechanism by inhibiting lanosterol 14α demethylase (encoded by ERG11), which leads to a block in ergosterol synthesis and the accumulation of toxic sterol intermediates, including 14-α-methyl-3,6-diol produced by Erg3 ( Lee et al. 2021). This toxic sterol gave the membrane cell heavy stress and impairs cell membrane permeability, thereby ergosterol biosynthesis becomes inhibited and cell membranes get damaged, leading to fungal lysis ( Figure 6) ( Sanglard et al. 2003; Pemán et al. 2009; Emami et al. 2017; Rosam et al. 2021).
Figure 5. The diagram of ergosterol formation in fungal cells ( Emami et al. 2017).
Figure 6. The fungal lysis process ( Lee et al. 2021).
Phenol
Phenol (carbolic acid) are secondary metabolites that can be found widely in Piper species, but rarely found in algae, fungi, and bacteria; organic compounds with low molecular weight, have one or more substituents hydroxyl group in aromatic phenyl ring, especially benzene, formed from phenylpropanoid or shikimate that produce phenylpropanoids and acetate or malonate polyketide pathway that produce simple phenols or with phenylpropanoids, identified by UV-Vis Spectra and retention times compared with the literature and reference compounds that available ( Waniska 2000; Lattanzio 2013; Ferreira et al. 2016).
Phenolic compounds have hydroxyl and carbonyl groups that can interact with fungal cells through hydrogen bonds, thereby increasing protein and cell membranes of pathogen fungal coagulation which will cause the damage and lysis of the fungal cells, and make the next fungal ergosterol growth anomaly and malformation ( Wagner and Donaldson 2014; Mohamed et al. 2017; Silva et al. 2018). Phenolic also had antifungal activities by targeting to destroy the fungal pathogenic effects by inhibiting the fungal dimorphic transition, because dimorphic nature is very important for fungal survival in the host, with making different morphology in different conditions or temperatures, both as hyphae (pathogenic) or yeast cells (non-pathogenic) ( Ansari et al. 2013).
Polyphenol
Polyphenols, known as an antifungal that has been isolated in Piper species, are water-soluble, have at least two phenolic rings, usually have 12-16 groups of phenolic hydroxyl at aromatic rings, and have a molecular weight from 500 to 3,000 (Da) ( Boulenouar et al. 2011; Cheynier 2012). Polyphenols inactivate protein and inhibit enzymes on the surface of bacterial cells; flavonoids form complexes that interfere with the function of the bacterial cell wall, inactivating microbial adhesion, enzymes, and cell protein transport by binding to bacterial extracellular proteins through hydrogen and covalent bonds ( Januarti et al. 2019).
Tannin
Tannins ([2,3-dihydroxy-5-[[(2 R,3 R,4 S,5 R,6 S)-3,4,5,6-tetrakis[[3,4-dihydroxy-5-(3,4,5-trihydroxybenzoyl)oxybenzoyl]oxy]oxan-2-yl]methoxycarbonyl]phenyl] 3,4,5-trihydroxybenzoate) are important compounds that have been isolated in the Piper species and have been classified as natural polyphenol groups, water-soluble, a molecular weight of 500-3000 daltons, condensed and hydrolyzed to polymerization that reaches high degrees and have two or three phenolic hydroxyl and carboxyl functional groups on a phenyl ring, known as antimicrobial, against various types of microorganisms, including bacteria, yeasts, fungi, and virus ( Chung et al. 1998; de Jesus et al. 2012; Kardel et al. 2013; Delimont et al. 2017; Ge et al. 2020).
Tannins inhibit the chitin growth in the fungi cell wall, which will cause fungal growth inhibition and cell metabolism disruption ( Ridzuan et al. 2021) . With a high affinity to polysaccharides and proteins, tannins function to form complex compounds with enzymes and substrates, thereby disrupting cell membranes ( Hoste et al. 2006). Plasma membrane and cell wall disruption that being tannin targeted will cause intracellular contents leakage ( Zhu et al. 2019). Tannins have the best antifungal activity in C. albicans at a concentration above 7.80 mg/L, similar to nystatin, slightly lower than fluconazole, by shoots increasing, substrate and metal ion reduction, germ tube formation inhibition, and wall ultrastructure changing ( Ishida et al. 2006).
Saponins
Saponins (beta-Escin), sapo in Latin, are one of the important secondary metabolites in plant, insects, and marine organisms. They are amphiphilic, have surfactant, soap-like foams, and are heat-stable ( Melzig et al. 2001; Haralampidis et al. 2002; Shi et al. 2004), used as herbs and known as antifungal and antibacterial ( Francis et al. 2002; Zhang et al. 2006). Divided into triterpenoid and steroidal saponins based on their aglycones structure and biochemistry (in plants, the core structures-27 carbon atoms-as furostan (16β, 22-epoxy-cholestan) and spirostan (16α, 22:22α, 26-diepoxy-cholestan), they usually have a hydroxyl group at C-3 position for monodesmosidik, and at C-26 at saponin furastanol bidesmosidik or C-28 at triterpene bidemosidik, sometimes also reported at C-2, C-15, C-16, C-21, lyobipolar so can affect to lower aqueous surface tension and cell membranes.
Saponins destroy the cell membrane by binding in the cell wall with sterol components so that the pores are formed ( Ridzuan et al. 2021). Saponins have hydrophilic molecules and lipid thinning molecules (lipophilic) so that they can make lower cell surface pressure ( Januarti et al. 2019). It is reported that 64 μg/ml saponin extract can inhibit C. albicans growth and development, by mycelium inhibition, inhibits yeast transition to filamentous, inhibits surface polystyrene adhesion and phospholipase production secretion, and endogenous reactive species oxygen (ROS) induce and the high activity showed by A. minutiflorum saponin (minutoside B) against fungal attack ( Barile et al. 2007; Jiang et al. 2015; Yang et al. 2018).
Flavonoid
Flavonoids (bioflavonoids), flavus in Latin, yellow powder, low molecular weight, are secondary metabolites that are very useful as antimicrobials by making bacterial damage, contain diphenylpropane (C 6-C 3-C 6), and have a three-carbon bridge with phenyl groups, as the core structure of 2-phenylbenzopyra, less toxic and low cost ( Baum et al. 2001; Galeotti et al. 2008; Can et al. 2015; Kurnia et al. 2018; Khalid et al. 2019; Herdiyati et al. 2020). As an important polyphenol class, flavonoids divided by C-ring oxidation degree, with the three-carbon segment oxidation degree and unsaturation degree, and the major classes are flavonols (3-hydroxy with the different site at OH group of phenolic), flavanones (C-4-keto-group with double-blind of C-2 and C-3), flavanols or flavan-3-ols (C 3-hydroxyl group and carbon ring that fully saturated), isoflavones (act like phytoestrogens), aurones, chalcones (two aromatic rings by three-unit carbons to make the group of α,β unsaturated carbonyl), and anthocyanidins ( Pourcel et al. 2007; Corradini et al. 2011; Seleem et al. 2017). Flavonoids have activities such as antibacterial, antioxidant, anti-inflammation, and antifungal properties through its ability to form complexes with extracellular proteins and interfere with microbial membrane activity because of its lipophilic properties ( Candiracci et al. 2011).
Research showed that flavonoids isolated, like 3,4-dihydroxy-5,6,7-trimethoxyflavone, cirsiliol, cirsimaritin, and hispidulin showed antifungal activity to C. sphaerospermum ( Alcerito et al. 2002). Research also showed that flavonoids in honey have an antifungal activity to C. albicans growth inhibition, but not kill the yeasts ( Candiracci et al. 2011), prenylated flavanones indicated that high antifungal activity to Trichophyton spp with 1.95 g/ml MIC value ( Jin 2019), and flavonoids inhibit the growth of fungal that increased every concentrations level against Aspergillus niger van Tieghem, Aspergillus fumigatus Fresenius, Altenaria alternata (Fr.) Keissler, Penicillium citrii, and Macrophomia phaseolina (Tassi) Goid ( Kanwal et al. 2010).
This literature review summarizes the phytochemicals and antifungal mechanisms of P. crocatum that are commonly found worldwide, as also other important activities of this Piper. Fungal infections are a problem that often occurs and becomes an ongoing and serious threat to public health ( Kathiravan et al. 2012). Polyene amphotericin B is one of the antibiotics that still often used for the treatment of life-threatening fungal diseases, even though it is known to have toxic side effects ( Odds 2003). Fluconazole is also known to have resistance to Candida species due to pressure continuous exposure, drug interactions, and side effects like visual impairment ( Canuto and Rodero 2002; Chen and Sorrell 2007). The increasing use of antifungal treatment causes resistance to antibiotics that are used commonly, even in patients who have never used the drug ( Arif et al. 2009; Arendrup 2014). The development of strains that are starting to become resistant to antibiotics from fungal species nowadays is a critical problem that must be addressed immediately in therapeutic problems in society by providing new antifungal agents ( Johnson et al. 2004; Jianhua and Hai 2009; Moghadamtousi et al. 2014). Natural resources are very important to developing new active molecules and properties, with the utilization of natural ingredients as antifungal treatment having a greater level of safety if used appropriately and correctly in terms of dose, time, and method of use ( Vengurlekar et al. 2012). Plants have a lot of bioactive secondary metabolites such as flavonoids, terpenoids, alkaloids, tannins, saponins, and other compounds as antifungal agents ( Arif et al. 2009). Due to the high rate of antibiotic resistance in the treatment of antifungal because of long-term use, new antifungal treatment agents are needed. The new antifungal should be safer, have minimal side effects, be cheaper, easier to get, and more potent against fungal infections. Based on the results of the review, it was found that P. crocatum contains compounds that have antifungal activity. By its secondary metabolites, P. crocatum has the opportunity to become a new antifungal agent as an alternative non-pharmacological antifungal treatment.
Conclusions
Natural products are important resources in the discovery and development of new medicinal raw materials. P. crocatum has antifungal activities that are against fungal by its compounds and inhibit ergosterol as a key enzyme in fungal-specific sterols. The inhibition of ergosterol can be induced by the inhibition of lanosterol 14 α demethylase in the biosynthesis which will cause integrity and function damage to fungal cell membranes. Damaged cell membranes will cause fungal growth inhibition, morphological changes, and fungal cell lysis. Based on the review data, it is hoped that it can be used as a reference regarding information of new potential bioactive compounds as an alternative treatment for fungal infections by their lanosterol 14 α demethylase CYP51 inhibition effect other than the use of antibiotics or currently used drugs.
Data availability
No data are associated with this article.
Acknowledgments
The authors are grateful to the Universitas Padjadjaran, Indonesia; Badan Pengembangan dan Pemberdayaan Sumber Daya Manusia (Badan PPSDM) Kementerian Kesehatan, Indonesia, and Poltekkes Kemenkes Pontianak, Indonesia.
Funding Statement
This research was funded by Article Review Grant – 2022, Academic Leadership Grant (ALG) and Penelitian Disertasi Doktoral (PDD) Grant Universitas Padjadjaran, Bandung, Indonesia with contract letter no 2203/UN6.3.1/PT.00/2022.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 3; peer review: 2 approved]
References
- Adams DJ: Fungal Cell Wall Chitinases and Glucanases. Microbiology. 2004;150(7):2029–2035. 10.1099/mic.0.26980-0 [DOI] [PubMed] [Google Scholar]
- Alcerito T, Barbo FE, Negri G, et al. : Foliar epicuticular Wax of Arrabidaea brachypoda: Flavonoids and Antifungal Activity. Biochem. Syst. Ecol. 2002;30(7):677–683. 10.1016/S0305-1978(01)00149-1 [DOI] [Google Scholar]
- Alizadeh F, Khodavandi A, Zalakian S: Quantitation of ergosterol content and gene expression profile of ERG11 gene in fluconazole-resistant Candida albicans. Curr. Med. Mycol. 2017;3(1):13–19. 10.29252/cmm.3.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Anurag A, Fatima Z, et al. : Natural Phenolic Compounds: A Potential Antifungal Agent. Microb. Pathog. Strateg. Combat. Them. Sci. Technol. Educ. 2013,January 2013. [Google Scholar]
- Arendrup MC: Update on Antifungal Resistance in Aspergillus and Candida. Clin. Microbiol. Infect. 2014;20(6):42–48. 10.1111/1469-0691.12513 [DOI] [PubMed] [Google Scholar]
- Arif T, Bhosale JD, Kumar N, et al. : Natural Products - Antifungal Agents Derived from Plants. J. Asian Nat. Prod. Res. 2009;11(7):621–638. 10.1080/10286020902942350 [DOI] [PubMed] [Google Scholar]
- Arthington-Skaggs BA, Jradi H, Desai T, et al. : Quantitation of Ergosterol content: Novel Method for Determination of Fluconazole Susceptibility of Candida albicans. J. Clin. Microbiol. 1999;37(10):3332–3337. 10.1128/JCM.37.10.3332-3337.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley ESD, Lewis R, Lewis JS, et al. : Pharmacology of systemic antifungal agents. Clin. Infect. Dis. 2006;43(SUPPL. 1):S28–S39. 10.1086/504492 [DOI] [Google Scholar]
- Astuti P, Wahyono, Nababan OA: Antimicrobial and Cytotoxic Activities of Endophytic Fungi Isolated From Piper crocatum Ruiz & Pav. Asian Pac. J. Trop. Biomed. 2014;4(2):S592–S596. 10.12980/APJTB.4.2014APJTB-2014-0073 Reference Source [DOI] [Google Scholar]
- Augustin JM, Kuzina V, Andersen SB, et al. : Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry. 2011;72(6):435–457. 10.1016/j.phytochem.2011.01.015 [DOI] [PubMed] [Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK: Methods For in vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016;6(2):71–79. 10.1016/j.jpha.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barh D, Barve N, Gupta K, et al. : Exoproteome and Secretome Derived Broad Spectrum Novel Drug and Vaccine Candidates in Vibrio cholerae Targeted by Piper betel Derived Compounds. PLoS One. 2013;8(1):e52773. 10.1371/journal.pone.0052773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile E, Bonanomi G, Antignani V, et al. : Saponins from Allium minutiflorum With Antifungal Activity. Phytochemistry. 2007;68(5):596–603. 10.1016/j.phytochem.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Baum EZ, Montenegro DA, Licata L, et al. : Identification and Characterization of New Inhibitors of the Escherichia coli MurA Enzyme. Antimicrob. Agents Chemother. 2001;45(11):3182–3188. 10.1128/AAC.45.11.3182-3188.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra DP, Pessoa C, Moraes MO, et al. : In vivo Growth Inhibition of Sarcoma 180 by Piperlonguminine, an Alkaloid Amide from the Piper Species. J. Appl. Toxicol. 2008;28:599–607. 10.1002/jat.1311 [DOI] [PubMed] [Google Scholar]
- Boulenouar N, Marouf A, Cheriti A: Antifungal Activity and Phytochemical Screening of Extracts from Phoenix dactylifera L. Cultivars. Nat. Prod. Res. 2011;25(20):1999–2002. 10.1080/14786419.2010.536765 [DOI] [PubMed] [Google Scholar]
- Bowman SM, Free SJ: The Structure and Synthesis of The Fungal Cell Wall. BioEssays. 2006;28(8):799–808. 10.1002/bies.20441 [DOI] [PubMed] [Google Scholar]
- Buitimea-Cantúa GV, Rosas-Burgos EC, Cinco-Moroyoqui FJ, et al. : In Vitro Effect of Antifungal Fractions from the Plants Baccharis glutinosa and Jacquinia macrocarpa on Chitin and β-1,3-glucan Hydrolysis of Maize Phytopathogenic Fungi and on the Fungal β-1,3-glucanase and Chitinase Activities. J. Food Saf. 2013;33(4):526–535. 10.1111/jfs.12085 [DOI] [Google Scholar]
- Can Z, Yildiz O, Sahin H, et al. : An Investigation of Turkish Honeys: Their Physico-Chemical Properties, Antioxidant Capacities and Phenolic Profiles. Food Chem. 2015;180:133–141. 10.1016/j.foodchem.2015.02.024 [DOI] [PubMed] [Google Scholar]
- Candiracci M, Citterio B, Diamantini G, et al. : Honey Flavonoids, Natural Antifungal Agents Against Candida albicans. Int. J. Food Prop. 2011;14(4):799–808. 10.1080/10942910903453355 [DOI] [Google Scholar]
- Cannon RD, Lamping E, Holmes AR, et al. : Candida albicans Drug Resistance - Another Way to Cope with Stress. Microbiology. 2007;153(10):3211–3217. 10.1099/mic.0.2007/010405-0 [DOI] [PubMed] [Google Scholar]
- Canuto MM, Rodero FG: Antifungal Drug Resistance to Azoles and Polyenes. Lancet Infect. Dis. 2002;2(9):550–563. 10.1016/S1473-3099(02)00371-7 [DOI] [PubMed] [Google Scholar]
- Chen SCA, Sorrell TC: Antifungal Agents. New Drugs, Old Drugs. 2007;187(7):404–409. 10.5694/j.1326-5377.2007.tb01313.x [DOI] [PubMed] [Google Scholar]
- Cheynier V: Phenolic Compounds: From Plants to Foods. Phytochem. Rev. 2012;11(2–3):153–177. 10.1007/s11101-012-9242-8 [DOI] [Google Scholar]
- Chung KT, Wong TY, Wei CI, et al. : Tannins and Human Health: A Review. Crit. Rev. Food Sci. Nutr. 1998;38(6):421–464. 10.1080/10408699891274273 [DOI] [PubMed] [Google Scholar]
- Corradini E, Foglia P, Giansanti P, et al. : Flavonoids: Chemical Properties and Analytical Methodologies of Identification and Quantitation in Foods and Plants. Nat. Prod. Res. 2011;25(5):469–495. 10.1080/14786419.2010.482054 [DOI] [PubMed] [Google Scholar]
- Cowan MM: Plant Products as Antimicrobial agents. Clin. Microbiol. Rev. 1999 Oct;12(4):564–582. 10.1128/CMR.12.4.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delimont NM, Haub MD, Lindshield BL: The Impact of Tannin Consumption on Iron Bioavailability and Status: A Narrative Review. Curr. Dev. Nutr. 2017;1(2):1–12. 10.3945/cdn.116.000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant-Archibold AA, Santana AI, Gupta MP: Ethnomedical Uses and Pharmacological Activities of Most Prevalent Species of Genus Piper in Panama: A Review. J. Ethnopharmacol. 2018;217:63–82. 10.1016/j.jep.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Ejele AE, Duru IA, Ogukwe CE, et al. : Phytochemistry and Antimicrobial Potential of Basic Metabolites of Piper umbellatum, Piper gratissimium and Newbouldia laevis Extracts. J. Emerg. Trends. Eng. Appl. Sci. 2012;3(2). [Google Scholar]
- Emami S, Tavangar P, Keighobadi M: An overview of Azoles Targeting Sterol 14α-Demethylase for Antileishmanial Therapy. Eur. J. Med. Chem. 2017;135:241–259. 10.1016/j.ejmech.2017.04.044 [DOI] [PubMed] [Google Scholar]
- Erviana R: Active Compounds Isolated From Red Betel ( Piper Crocatum Ruiz & Pav) Leaves Active Against Streptococcus mutans Through Its Inhibition Effect on Glucosyltransferase Activity. J. Med. Sci. 2011 June;43(2). [Google Scholar]
- Fadlilah M: Benefit of Red Betel (Piper Crocatum Ruiz & Pav.) as Antibiotics. J. Major. 2015;4(3):71–75. [Google Scholar]
- Fatmawaty F, Anggreni NGM, Fadhil N, et al. : Potential in Vitro and in Vivo Antioxidant Activities from Piper crocatum and Persea americana Leaf Extracts. Biomed. Pharmacol. J. 2019;12(2). 10.13005/bpj/1686 [DOI] [Google Scholar]
- Ferreira V, Fernandes F, Pinto-Carnide O, et al. : Identification of Vitis vinifera L. Grape Berry Skin Color Mutants and Polyphenolic Profile. Food Chem. 2016;194:117–127. 10.1016/j.foodchem.2015.07.142 [DOI] [PubMed] [Google Scholar]
- Flowers SA, Colón B, Whaley SG, et al. : Contribution of Clinically Derived Mutations in ERG11 to Azole Resistance in Candida albicans. Antimicrob. Agents Chemother. 2015;59(1):450–460. 10.1128/AAC.03470-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis G, Kerem Z, Makkar HPS, et al. : The Biological Action of Saponins in Animal Systems: a Review. Br. J. Nutr. 2002;88(6):587–605. 10.1079/BJN2002725 [DOI] [PubMed] [Google Scholar]
- Galeotti F, Barile E, Curir P, et al. : Flavonoids from Carnation ( Dianthus caryophyllus) and Their Antifungal Activity. Phytochem. Lett. 2008;1(1):44–48. 10.1016/j.phytol.2007.10.001 [DOI] [Google Scholar]
- Ge D, Dong Y, Zhang W, et al. : A Novel Fe2+/Persulfate/Tannic Acid Process with Strengthened Efficacy on Enhancing Waste Activated Sludge Dewaterability and Mechanism Insight. Sci. Total Environ. 2020;733:139146. 10.1016/j.scitotenv.2020.139146 [DOI] [PubMed] [Google Scholar]
- Gow NAR, Latge JP, Munro CA: The Fungal Cell Wall: Structure, Biosynthesis, and Function. Microbiol. Spectr. 2017;5. 10.1128/microbiolspec.FUNK-0035-2016 [DOI] [PubMed] [Google Scholar]
- Guclu-Ustundag Ö, Mazza G: Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007;47(3):231–258. 10.1080/10408390600698197 [DOI] [PubMed] [Google Scholar]
- Gutierrez RMP, Gonzalez AM, Neira, et al. : Alkaloids from Piper: A Review of its Phytochemistry and Pharmacology. Mini Rev. Med. Chem. 2013;13(2):163–193. 10.2174/1389557511313020001 [DOI] [PubMed] [Google Scholar]
- Haralampidis K, Trojanowska M, Osbourn AE: Biosynthesis of Triterpenoid Saponins in Plants. Adv. Biochem. Eng. Biotechnol. 2002;75. 10.1007/3-540-44604-4_2 [DOI] [PubMed] [Google Scholar]
- Herdiyati Y, Atmaja HE, Satari MH, et al. : Potential Antibacterial Flavonoid from Buah Merah ( Pandanus conodieus Lam.) Against Pathogenic Oral Bacteria of Enterococcus faecalis ATCC 29212. Open. Dent. J. 2020;14(1):433–439. 10.2174/1874210602014010433 [DOI] [Google Scholar]
- Hermiati NY, Manalu MS, Sinaga: Ekstrak Daun Sirih Hijau Dan Merah Sebagai Antioksidan Pada Minyak Kelapa. J. Tek. Kim. USU. 2013;2(1):37–43. 10.32734/jtk.v2i1.1425 [DOI] [Google Scholar]
- Hoste H, Jackson F, Athanasiadou S, et al. : The Effects of Tannin-Rich Plants on Parasitic nematodes in Ruminants. Trends Parasitol. 2006;22(6):253–261. 10.1016/j.pt.2006.04.004 [DOI] [PubMed] [Google Scholar]
- Howard KC, Dennis EK, Watt DS, et al. : A Comprehensive Overview of The Medicinal Chemistry of Antifungal Drugs: Perspectives and Promise. Royal Society of Chemistry;2020. [DOI] [PubMed] [Google Scholar]
- Ishida K, Palazzo de Mello JC, Garcia Cortez DA, et al. : Influence of Tannins from Stryphnodendron adstringens on Growth and Virulence Factors of Candida albicans. J. Antimicrob. Chemother. 2006;58(5):942–949. 10.1093/jac/dkl377 [DOI] [PubMed] [Google Scholar]
- Januarti IB, Wijayanti R, Wahyuningsih S, et al. : Potensi Ekstrak Terpurifikasi Daun Sirih Merah ( Piper crocatum Ruiz &Pav) Sebagai Antioksidan Dan Antibakteri. JPSCR J. Pharm. Sci. Clin. Res. 2019;4(2):60. 10.20961/jpscr.v4i2.27206 [DOI] [Google Scholar]
- Jeon HJ, Kim K, Kim YD, et al. : Naturally Occurring Piper Plant Amides Potential in Agricultural and Pharmaceutical Industries: Perspectives of Piperine and Piperlongumine. Appl. Biol. Chem. 2019;62. 10.1186/s13765-019-0471-z [DOI] [Google Scholar]
- Jesus NZT, Souza FH, Gomes IF, et al. : Tannins, Peptic Ulcers and Related Mechanisms. Int. J. Mol. Sci. 2012;13(3):3203–3228. 10.3390/ijms13033203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Feng K, Yang X: In Vitro Antifungal Activity and Mechanism of Action of Tea Polyphenols and Tea Saponin Against Rhizopus stolonifer. J. Mol. Microbiol. Biotechnol. 2015;25(4):269–276. 10.1159/000430866 [DOI] [PubMed] [Google Scholar]
- Jianhua W, Hai W: Antifungal Susceptibility Analysis of Berberine, Baicalin, Eugenol and Curcumin on Candida albicans. J. Med. Coll. PLA. 2009;24(3):142–147. 10.1016/S1000-1948(09)60030-7 [DOI] [Google Scholar]
- Jin YS: Recent Advances in Natural Antifungal Flavonoids and Their Derivatives. Bioorganic Med. Chem. Lett. 2019;29(19):126589. 10.1016/j.bmcl.2019.07.048 [DOI] [PubMed] [Google Scholar]
- Johnson MD, MacDougall C, Ostrosky-Zeichner L, et al. : Combination Antifungal Therapy. Antimicrob. Agents Chemother. 2004;48(3):693–715. 10.1128/AAC.48.3.693-715.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal Q, Hussain I, Latif Siddiqui H, et al. : Antifungal Activity of Flavonoids Isolated from Mango ( Mangifera indica L.) Leaves. Nat. Prod. Res. 2010;24(20):1907–1914. 10.1080/14786419.2010.488628 [DOI] [PubMed] [Google Scholar]
- Kardel M, Taube F, Schulz H, et al. : Different Approaches to Evaluate Tannin Content and Structure of Selected Plant Extracts - Review and New Aspects. J. Appl. Bot. Food Qual. 2013;86(1). [Google Scholar]
- Kathiravan MK, Salake AB, Chothe AS, et al. : The Biology and Chemistry of Antifungal Agents: A Review. Bioorg. Med. Chem. 2012;20(19):5678–5698. 10.1016/j.bmc.2012.04.045 [DOI] [PubMed] [Google Scholar]
- Khalid M, Saeed-ur-Rahman, Bilal M, et al. : Role of Flavonoids in Plant Interactions with The Environment and Against Human Pathogens - A Review. J. Integr. Agric. 2019;18(1):211–230. 10.1016/S2095-3119(19)62555-4 [DOI] [Google Scholar]
- Kreis W, Müller-Uri F: Biochemistry of Sterols, Cardiac Glycosides, Brassinosteroids, Phytoecdysteroids and Steroid saponins. 2010.
- Kumar A, Singh PP, Gupta V, et al. : Assessing the antifungal and aflatoxin B1 inhibitory efficacy of nanoencapsulated antifungal formulation based on combination of Ocimum spp. essential oils. Int. J. Food Microbiol. 2020;330: 108766. 10.1016/j.ijfoodmicro.2020.108766 [DOI] [PubMed] [Google Scholar]
- Kurnia D, Apriyanti E, Soraya C, et al. : Antibacterial Flavonoids Against Oral Bacteria of Enterococcus faecalis from Sarang Semut ( Myrmecodia pendans) and Its Inhibitor Activity Against Enzyme MurA. Curr. Drug Discov. Technol. 2018;16(3):290–296. 10.2174/1570163815666180828113920 [DOI] [PubMed] [Google Scholar]
- Kusuma SAF, Hendriani R, Genta A: Antimicrobial Spectrum of Red Piper Betel Leaf Extract ( Piper crocatum Ruiz & Pav) as Natural Antiseptics Against Airborne Pathogens. J. Pharm. Sci. Res. 2017;9(5). [Google Scholar]
- Kusuma SAF, Manan WS, Budiman F: Inhibitory effect of red piper betel leaf ethanol extract ( Piper crocatum Ruiz and Pav.) against Trichomonas vaginalis trophozoites in vitro. Asian J Pharm. Clin. Res. 2017;10(11):311. 10.22159/ajpcr.2017.v10i11.20778 [DOI] [Google Scholar]
- Latgé JP: The Cell Wall: A Carbohydrate Armour for the Fungal Cell. Mol. Microbiol. 2007;66(2):279–290. 10.1111/j.1365-2958.2007.05872.x [DOI] [PubMed] [Google Scholar]
- Latgé JP: Tasting The Fungal Cell Wall. Cell. Microbiol. 2010;12(7):863–872. 10.1111/j.1462-5822.2010.01474.x [DOI] [PubMed] [Google Scholar]
- Lattanzio V: Phenolic Compounds: Introduction. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Berlin Heidelberg: Springer-Verlag;2013. [Google Scholar]
- Lee Y, Puumala E, Robbins N, et al. : Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021;121(6):3390–3411. 10.1021/acs.chemrev.0c00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lely N, Arifin H, Aldi Y, et al. : Anti-inflammatory Effects of Methanol Extract, Hexane, Ethyl Acetate, and Butanol Fraction of Piper crocatum Ruiz & Pav Leaves on Lipopolysaccharide-Induced RAW 264.7 Cells. Pharmacogn. J. 2021;13(6):1341–1346. 10.5530/PJ.2021.13.169 [DOI] [Google Scholar]
- Lewis RE: Current Concepts in Antifungal Pharmacology. Mayo Clin. Proc. 2011;86(8):805–817. 10.4065/mcp.2011.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HX, Yang SY, Kim YH, et al. : Isolation of Two New Compounds and other Constituents from Leaves of Piper crocatum and Study of Their Soluble Epoxide Hydrolase Activities. Molecules. 2019;24(3):489. 10.3390/molecules24030489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima CNF, Lima LF, Correia DB, et al. : Systematic Review: Medicinal Use and Scientific Elucidation of the Piper Genus for the Treatment of Symptoms and Inflammatory Diseases. J. Med. Plants Res. 2020;14(2):62–72. 10.5897/jmpr2019.6855 [DOI] [Google Scholar]
- Lipke PN: What We Do Not Know About Fungal Cell Adhesion Molecules. J. Fungi. 2018;4(2). 10.3390/jof4020059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister INE, Ginting CN, Girsang E, et al. : Hepatoprotective Properties of Red Betel ( Piper crocatum Ruiz and Pav) Leaves Extract Towards H2O2-induced HepG2 Cells via Anti-Inflammatory, Antinecrotic. Antioxidant Potency. Saudi Pharm. J. 2020;28(10):1182–1189. 10.1016/j.jsps.2020.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler J, Stevens DA: Antifungal Drug Resistance Mechanisms. Clin. Infect. Dis. 2003;36(1):S31–S41. 10.1086/344658 [DOI] [PubMed] [Google Scholar]
- Madhumita M, Guha P, Nag A: Bio-actives of Betel Leaf (Piper betle L.): A Comprehensive Review on Extraction, Isolation, Characterization, and Biological Activity. Phyther. Res. 2020;34(10):2609–2627. 10.1002/ptr.6715 [DOI] [PubMed] [Google Scholar]
- Melzig MF, Bader G, Loose R: Investigations of the Mechanism of Membrane Activity of Selected Triterpenoid Saponins. Planta Med. 2001;67(1):43–48. 10.1055/s-2001-10632 [DOI] [PubMed] [Google Scholar]
- Mgbeahuruike EE, Yrjönen T, Vuorela H, et al. : Bioactive Compounds from Medicinal Plants: Focus on Piper Species. South African J. Bot. 2017;112:54–69. 10.1016/j.sajb.2017.05.007 [DOI] [Google Scholar]
- Mitchell H: Vaginal Discharge-Causes, Diagnosis, and Treatment. Br. Med. J. 2004;328(7451):1306–1308. 10.1136/bmj.328.7451.1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadamtousi SZ, Kadir HA, Hassandarvish P, et al. : A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed. Res. Int. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MSM, Saleh AM, Abdel-Farid IB, et al. : Growth, Hydrolases and Ultrastructure of Fusarium oxysporum as Affected by Phenolic Rich Extracts from Several Xerophytic Plants. Pestic. Biochem. Physiol. 2017;141:57–64. 10.1016/j.pestbp.2016.11.007 [DOI] [PubMed] [Google Scholar]
- Do Nascimento JC, De Paula VF, David JM, et al. : Occurrence, Biological Activities and 13C NMR Data of Amides from Piper (Piperaceae). Quim. Nova. 2012;35(11):2288–2311. 10.1590/S0100-40422012001100037 [DOI] [Google Scholar]
- Odds FC: Antifungal Agents: Their Diversity and Increasing Sophistication. Mycologist. 2003;17(2):51–55. 10.1017/S0269915X03002064 [DOI] [Google Scholar]
- Pemán J, Cantón E, Espinel-Ingroff A: Antifungal Drug Resistance Mechanisms. Expert Rev. Anti-Infect. Ther. 2009;7(4):453–460. 10.1586/eri.09.18 [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Diekema DJ, Turnidge JD, et al. : Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species from 1997-2016. Open Forum Infect. Dis. 2019;6(Suppl 1):S79–S94. 10.1093/ofid/ofy358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourcel L, Routaboul JM, Cheynier V, et al. : Flavonoid Oxidation in Plants: From Biochemical Properties to Physiological Functions. Trends Plant Sci. 2007;12(1):29–36. 10.1016/j.tplants.2006.11.006 [DOI] [PubMed] [Google Scholar]
- Puspita PJ, Safithri M, Sugiharti NP: Antibacterial Activities of Sirih Merah ( Piper crocatum) Leaf Extracts. Curr. Biochem. 2018;5(3):1–10. 10.29244/cb.5.3.1-10 [DOI] [Google Scholar]
- Ramírez J, Cartuche L, Morocho V, et al. : Antifungal Activity of Raw Extract and Flavanons Isolated from Piper ecuadorense from Ecuador. Rev. Bras Farmacogn. 2013;23(2):370–373. 10.1590/S0102-695X2013005000012 [DOI] [Google Scholar]
- Ridzuan PM, Aisyah B, Kausar A, et al. : In Vitro Antimicrobial Inhibitory Effect of Piper aduncum. Leaves Extracts on Bacteria and Fungi. 2021;4(2). [Google Scholar]
- Rinanda T, Zulfitri ADM: Antibacterial activity of red betel (Piper crocatum) leaf methanolic extracts aginst methicillin resistant Staphylococcus aureus. Proc. Annu. Int. Conf. Syiah Kuala Univ. 2012;2(1):22–24. http://www.jurnal.unsyiah.ac.id/AICS-SciEng/article/view/1781 [Google Scholar]
- Robbins N, Wright GD, Cowen LE: Antifungal Drugs: The current Armamentarium and Development of New Agents. Microbiology. 2016:1–20. 10.1128/9781555819583.ch44 [DOI] [PubMed] [Google Scholar]
- Rodrigues RV, Lanznaster D, Longhi Balbinot DT, et al. : Antinociceptive Effect of Crude Extract, Fractions and Three Alkaloids Obtained from Fruits of Piper tuberculatum. Biol. Pharm. Bull. 2009;32(10):1809–1812. 10.1248/bpb.32.1809 [DOI] [PubMed] [Google Scholar]
- Rosam K, Monk BC, Lackner M: Sterol 14α-demethylase Ligand-Binding Pocket-Mediated Acquired and Intrinsic Azole Resistance in Fungal Pathogens. J. Fungi. 2021;7(1):1–22. 10.3390/jof7010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safithri M, Fahma F: Potency of Piper crocatum Decoction as an Antihiperglycemia in Rat Strain Sprague Dawley. Hayati J. Biosci. 2008;15(1):45–48. 10.4308/hjb.15.1.45 [DOI] [Google Scholar]
- Safithri M, Indariani S, Yuliani R: Effect of Microencapsulation Techniques on Physical and Chemical Characteristics of Functional Beverage Based on Red Betel Leaf Extract ( Piper crocatum). J. Kim. Sains Apl. 2020;23(8):276–282. 10.14710/jksa.23.8.276-282 [DOI] [Google Scholar]
- Sanglard D, Ischer F, Parkinson T, et al. : Candida albicans Mutations in The Ergosterol Biosynthetic Pathway and Resistance to Several Antifungal Agents. Antimicrob. Agents Chemother. 2003;47(8):2404–2412. 10.1128/AAC.47.8.2404-2412.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saputra A, Andayani S, Nursyam H: Total Quantity of Phenol and Isolation Methanol Tannin Extract of Red Betel Leaf ( Piper crocatum). Int. J. PharmTech. Res. 2016;9(7):146–153. [Google Scholar]
- Seleem D, Pardi V, Murata RM: Review of Flavonoids: A Diverse Group of Natural Compounds with Anti- Candida albicans Activity In Vitro. Arch. Oral Biol. 2017;76:76–83. 10.1016/j.archoralbio.2016.08.030 [DOI] [PubMed] [Google Scholar]
- Sendid B, François N, Standaert A, et al. : Prospective Evaluation of the New Chromogenic Medium CandiSelect 4 for Differentiation and Presumptive Identification of the Major Pathogenic Candida Species. J. Med. Microbiol. 2007;56(4):495–499. 10.1099/jmm.0.46715-0 [DOI] [PubMed] [Google Scholar]
- Shareef MA, Sirisha K, Khan I, et al. : Design, Synthesis, and Antimicrobial Evaluation of 1,4-dihydroindeno[1,2- c] Pyrazole Tethered Carbohydrazide Hybrids: Exploring Their In silico ADMET, Ergosterol Inhibition and ROS Inducing Potential. MedChemComm. 2019;10(5):806–813. 10.1039/c9md00155g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Arunasalam K, Yeung D, et al. : Saponins from Edible Legumes: Chemistry, Processing, and Health Benefits. J. Med. Food. 2004;7(1):67–78. 10.1089/109662004322984734 [DOI] [PubMed] [Google Scholar]
- Silva V, Igrejas G, Falco V, et al. : Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry By-Products. Food Control. 2018;92:516–522. 10.1016/j.foodcont.2018.05.031 [DOI] [Google Scholar]
- Suri MA, Azizah Z, Asra R: A Review: Traditional Use, Phytochemical And Pharmacological Review Of Red Betel Leaves ( Piper crocatum Ruiz & Pav). Asian J. Pharm. Res. Dev. 2021;9(1):159–163. 10.22270/ajprd.v9i1.926 [DOI] [Google Scholar]
- Tabassum N, Vidyasagar GM: Antifungal Investigations on Plant Essential Oils. A Review. Int. J. Pharm. Pharm. Sci. 2013;5(SUPPL. 2). [Google Scholar]
- Vengurlekar S, Sharma R, Trivedi P: Efficacy of Some Natural Compounds as Antifungal Agents. Pharmacogn. Rev. 2012;6(12):91–99. 10.4103/0973-7847.99942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Donaldson L: Metabolic Engineering of Wood Formation. Appl. Plant Cell Biol. 2014;22. 10.1007/978-3-642-41787-0_12 [DOI] [Google Scholar]
- Waniska RD: Structure, Phenolic Compounds, and Antifungal Proteins of S orghum caryopses. Int Crop Res Inst Semi-Arid Trop. 2000. [Google Scholar]
- Whaley SG, Berkow EL, Rybak JM, et al. : Azole Antifungal Resistance in Candida albicans and Emerging Non-Albicans Candida Species. Front. Microbiol. 2017;7(Jan). 10.3389/fmicb.2016.02173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley SG, Rogers PD: Azole Resistance in Candida glabrata. Curr. Infect. Dis. Rep. 2016;18(12). 10.1007/s11908-016-0554-5 [DOI] [PubMed] [Google Scholar]
- Xu W-H, Li X-C: Antifungal Compounds from Piper Species. Curr. Bioact. Compd. 2011;7(4):262–267. 10.2174/157340711798375822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Liu X, Zhuang X, et al. : Antifungal Effects of Saponin Extract from Rhizomes of Dioscorea panthaica Prain et Burk against Candida albicans. Evidence-based Complement Altern. Med. 2018;2018:1–13. 10.1155/2018/6095307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yücesoy M, Marol S: Performance of CHROMAGAR Candida and BIGGY Agar for Identification of Yeast Species. Ann. Clin. Microbiol. Antimicrob. 2003;2:1–7. 10.1186/1476-0711-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JD, Xu Z, Cao YB, et al. : Antifungal Activities and Action Mechanisms of Compounds from Tribulus terrestris L. J. Ethnopharmacol. 2006;103(1):76–84. 10.1016/j.jep.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Zhu C, Lei M, Andargie M, et al. : Antifungal Activity and Mechanism of Action of Tannic Acid against Penicillium digitatum. Physiol. Mol. Plant Pathol. 2019;107(January):46–50. 10.1016/j.pmpp.2019.04.009 [DOI] [Google Scholar]