Abstract
Alpha-toxin is a major virulence factor in Staphylococcus aureus keratitis. Active or passive immunization with alpha-toxin toxoid could protect against corneal damage. Results show that either form of immunization did not kill bacteria but did significantly protect against corneal pathology, especially epithelial erosion.
Staphylococcus aureus is the leading cause of human corneal infection that may result in loss of visual acuity and blindness (3). Alpha-toxin is produced by approximately 75% of S. aureus strains (2, 8, 25) and has been shown to be the major virulence factor in Staphylococcus keratitis (6, 21). Considering the importance of alpha-toxin in S. aureus keratitis, we examined the effect of passively administered antibody to alpha-toxin and active immunization with alpha-toxin toxoid in a rabbit Staphylococcus keratitis model.
S. aureus strain 8325-4, an alpha-toxin-producing strain previously analyzed in the rabbit keratitis model (6, 21), was grown to log phase and diluted in tryptic soy broth (Difco Laboratories, Inc., Detroit, Mich.). Alpha-toxin (Sigma, St. Louis, Mo.) purity was determined by the presence of a single band at 33 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (20). Toxin was heat inactivated (80°C for 2 h) as confirmed by a loss in hemolytic activity (4, 21).
New Zealand White rabbits (2.0 to 3.0 kg; Myrtle Rabbitry, Thompson Station, Tenn.) were maintained in strict accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals (13a). Rabbits were anesthetized as described previously (6, 20, 21). For passive immunization, bacteria were mixed (1:1, volume) with either preimmune rabbit sera or rabbit sera containing antibody to alpha-toxin. Each cornea was intrastromally injected with 20 μl of the bacteria-antibody mixture containing approximately 100 CFU per cornea (6, 21). Immune sera mixed with tryptic soy broth (1:1, 20 μl) were injected into rabbit corneas to determine if the sera induced ocular inflammation. All rabbits were slit lamp examined (SLE) from 10 h postinfection (p.i.) every 5 h until time of sacrifice. SLE of rabbit eyes was performed by two masked observers (6, 20, 21). Corneal erosions were detected using fluorescein, and diameters were measured and expressed in millimeters.
Prior to experimentation, the sera of all rabbits were tested by enzyme-linked immunosorbent assay (ELISA) to ensure the absence of preexisting antibody to S. aureus alpha-toxin (5). Specific-pathogen-free rabbits (n = 15) were found to lack serum antibody to alpha-toxin. For active immunization, rabbits (n = 4) were subcutaneously injected with 50 μg of alpha-toxin toxoid mixed with complete Freund's adjuvant (CFA; Sigma) once a month over a 3-month period. Control rabbits (n = 4) were injected with CFA alone. Four weeks following each immunization, sera and tears were collected. Sera were assayed by ELISA for alpha-toxin-specific total antibody or immunoglobulin G (IgG) antibody, using as secondary antibody either anti-rabbit IgG or anti-rabbit gamma heavy chain, respectively. Total antibody and IgA antibody to alpha-toxin in tears were assayed. Sera with IgG titers of 5,000 caused neither agglutination of S. aureus nor inhibition of its growth in vitro (data not shown).
Once the serum total antibody titer (as measured by ELISA) to alpha-toxin reached 5,000, the corneas were injected intrastromally with approximately 100 CFU of S. aureus 8325-4 (10 μl). Rabbits were sacrificed when the SLE score reached 17 or at 45 h p.i.
The number of viable S. aureus organisms per cornea was determined by culturing dilutions of corneal homogenates in triplicate (6, 21). Data were analyzed statistically as described previously (6, 21, 26). P values of ≤0.05 were considered significant.
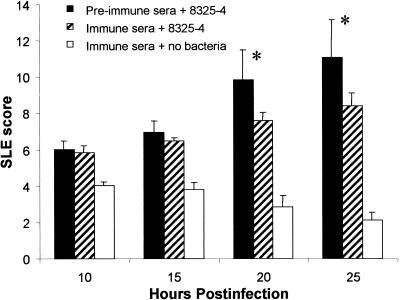
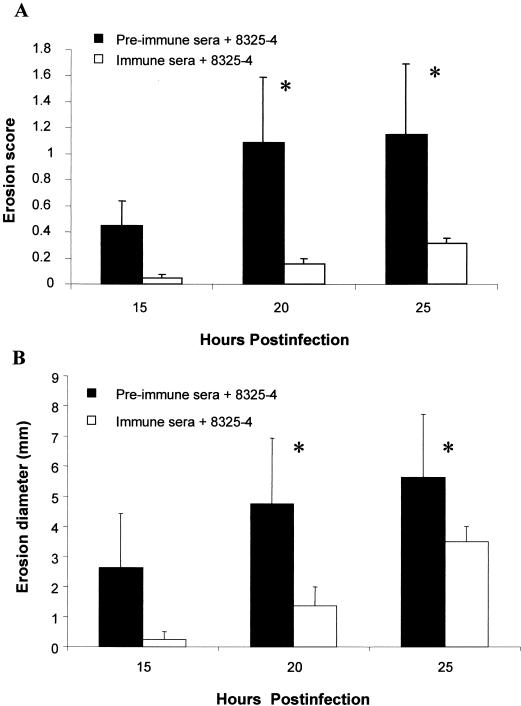
To analyze the protectiveness of passive immunization, serum containing IgG antibody to alpha-toxin (prepared as described above for active immunization) was administered at the time of infection. Passive immunization provided protection to the cornea as evidenced by significantly lower SLE scores for the antibody-treated group than for the control group at 20 and 25 h p.i. (P ≤ 0.0135) (Fig. 1). Erosions developed at 15 h p.i. in only 25% of antibody-treated eyes whereas 75% of eyes treated with preimmune sera developed erosions. There was no difference in the numbers of bacteria recovered from both groups at 15 h p.i. (immunized = 5.61 ± 0.21 and control = 5.33 ± 0.29 log CFU/cornea; P = 0.492). The erosions at 20 and 25 h p.i. were also significantly smaller in antibody-treated eyes than in the eyes of rabbits treated with preimmune sera (P ≤ 0.041) (Fig. 2).
FIG. 1.
SLE scores of passively immunized and control rabbits during Staphylococcus keratitis. Bacteria were mixed with either immune or preimmune sera at a 1:1 ratio, and the corneas (n = 4) were injected with 20 μl of the serum-bacteria mixture. A control group consisting of corneas injected only with immune sera was also included. Eyes injected with bacteria and immune sera had significantly lower SLE scores at 20 and 25 h p.i. than eyes injected with bacteria and preimmune sera. Each asterisk denotes a significant difference between groups receiving immune and preimmune sera (P ≤ 0.0135). Data are expressed as mean SLE ± the standard error of the mean (SEM).
FIG. 2.
Development of corneal erosions during Staphylococcus keratitis in passively immunized rabbits and control rabbits. Bacteria were mixed with either immune or preimmune sera at a ratio of 1:1, and the corneas (n = 4) were injected with 20 μl of the serum-bacteria mixture. Erosion score (A) is based on coverage of the cornea and ranked between 0 and 4. Erosion diameters (B) were visualized using fluorescein and measured with a ruler. Both erosion scores and diameters were significantly smaller in passively immunized eyes than in the eyes of control rabbits (treated with preimmune sera) at 20 and 25 h p.i. Each asterisk denotes a significant difference between groups treated with immune or preimmune sera (P ≤ 0.041). Data are expressed as mean erosion score/diameter ± SEM.
Active immunization with 2 to 4 injections of toxoid in CFA resulted in serum titers of 5,000 for either total antibody or IgG antibody. However, the IgA titer for alpha-toxin in tears was not substantially increased over that of the preimmune or control rabbits (titers = 300 ± 100).
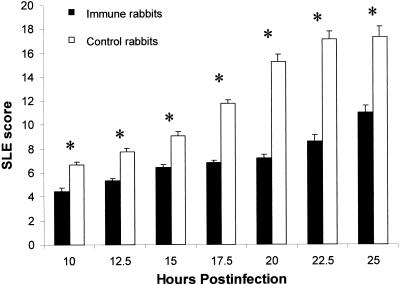
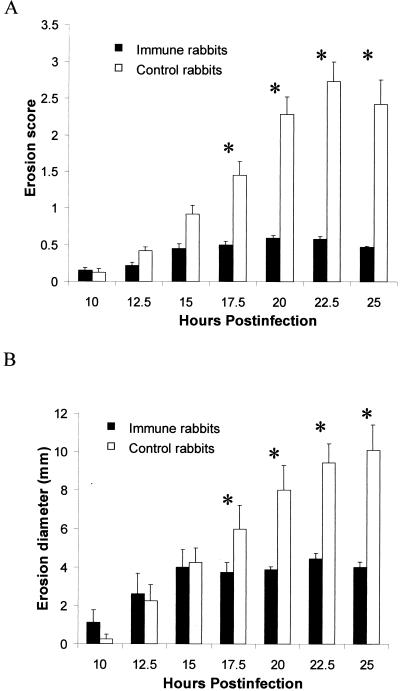
Infection of rabbits actively immunized with alpha-toxin toxoid resulted in significantly less pathology (by SLE score) throughout infection (10 to 25 h p.i.) than infection of control rabbits injected with CFA alone (P ≤ 0.0004) (Fig. 3). Epithelial erosions were not as extensive in the corneas of immune rabbits compared to those in the corneas of control rabbits from 17.5 h p.i. until time of sacrifice (P ≤ 0.0001) (Fig. 4). There was no significant difference in the numbers of bacteria (approximately 7 log CFU per cornea) obtained from the corneas of immunized or control rabbits (P = 0.3061).
FIG. 3.
SLE scores of actively immunized and control rabbits during Staphylococcus keratitis. Rabbits were immunized with either alpha-toxin toxoid mixed with CFA (immune) or CFA alone (control) and subsequently infected with approximately 100 CFU of strain 8325-4 (n = 8 per group). Immune rabbits had a significantly lower SLE score than control rabbits at all time points; each asterisk denotes a significant difference between these groups (P ≤ 0.0004). Data are expressed as mean SLE score ± SEM.
FIG. 4.
Development of corneal erosions during Staphylococcus keratitis in actively immunized rabbits and control rabbits. Rabbits were immunized with either alpha-toxin toxoid mixed with CFA (immune) or CFA adjuvant alone (control) and subsequently infected with approximately 100 CFU of strain 8325-4 (n = 8 per group). Erosion score (A) is based on coverage of the cornea ranked between 0 and 4. Erosion diameters (B) were visualized using fluorescein and measured with a ruler; values are expressed in millimeters. Erosions (both scores and diameters) in corneas of immune rabbits were significantly smaller than those in control rabbits from 17.5 to 25 h p.i. Each asterisk denotes a significant difference between groups (P ≤ 0.0001). Data expressed as mean erosion score/diameter ± SEM.
This study has shown, for the first time, that passive or active immunization to alpha-toxin protects the cornea from damage during Staphylococcus keratitis. These findings confirm the data from genetic studies (6, 21) and from histopathological studies (20) showing that alpha-toxin is largely responsible for the development of severe tissue damage and inflammation during keratitis. Passive immunization of infected individuals could be useful in limiting tissue damage, particularly in conjunction with antibiotic therapy. Active immunization for Staphylococcus infections has been extensively studied (1, 9–12, 15–19, 24) and, for those patients at risk, is a feasible proposition for controlling tissue damage. Vaccination is of increasing priority due to the broadening antibiotic resistance of Staphylococcus, including vancomycin resistance (13, 14, 22, 23, 27) and the emergence of methicillin-resistant S. aureus infections in the general population (7).
Acknowledgments
We thank Kiana Nelson for her technical assistance.
This research was supported by NIH grant RO1 EY10974.
REFERENCES
- 1.Adlam C, Ward P D, McCartney A C, Arbuthnott J P, Thorley C M. Effect of immunization with highly purified alpha- and beta-toxins on staphylococcal mastitis in rabbits. Infect Immun. 1977;17:250–256. doi: 10.1128/iai.17.2.250-256.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arvidson S O. Extracellular enzymes from Staphylococcus aureus. In: Easmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. London, England: Academic Press; 1983. pp. 745–808. [Google Scholar]
- 3.Asbell P, Stenson S. Ulcerative keratitis: survey of 30 years' laboratory experience. Arch Ophthalmol. 1982;100:77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- 4.Bernheimer A W. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- 5.Blomqvist L, Sjogren A M. Production and characterization of monoclonal antibodies against Staphylococcus aureus alpha-toxin. Toxicon. 1988;26:265–273. doi: 10.1016/0041-0101(88)90217-6. [DOI] [PubMed] [Google Scholar]
- 6.Callegan M C, Engel L S, Hill J M, O'Callaghan R J. Corneal virulence of Staphylococcus aureus: roles of alpha-toxin and protein A in pathogenesis. Infect Immun. 1994;62:2478–2482. doi: 10.1128/iai.62.6.2478-2482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. Morb Mortal Wkly Rep. 1999;32:707–710. [PubMed] [Google Scholar]
- 8.Coia J E, Browning L, Haines L, Birkbeck T H, Platt D J. Comparison of enterotoxins and haemolysins produced by methicillin-resistant (MRSA) and sensitive (MSSA) Staphylococcus aureus. J Med Microbiol. 1992;36:164–174. doi: 10.1099/00222615-36-3-164. [DOI] [PubMed] [Google Scholar]
- 9.Fattom A I, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.García V E, Iglesias M F, Cerquetti M C, Gómez M I, Sordelli D O. Interaction between granulocytes and antibodies in the enhancement of lung defenses against Staphylococcus aureus after intranasal immunization with live-attenuated bacteria. FEMS Immunol Med Microbiol. 1994;9:55–64. doi: 10.1111/j.1574-695X.1994.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg D P, Ward J I, Bayer A S. Influence of Staphylococcus aureus antibody on experimental endocarditis in rabbits. Infect Immun. 1987;55:3030–3034. doi: 10.1128/iai.55.12.3030-3034.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenberg D P, Bayer A S, Cheung A L, Ward J I. Protective efficacy of protein A-specific antibody against bacteremic infection due to Staphylococcus aureus in an infant rat model. Infect Immun. 1989;57:1113–1118. doi: 10.1128/iai.57.4.1113-1118.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet. 1997;350:1670–1673. doi: 10.1016/S0140-6736(97)07324-8. [DOI] [PubMed] [Google Scholar]
- 13a.Institute of Laboratory Animal Resources. Guide for the care and use of laboratory animals. Washington, D.C.: National Academy Press; 1996. [Google Scholar]
- 14.Kantzanou M, Tassios P T, Tseleni-Kotsovili A, Legakis N J, Vatopoulos A C. Reduced susceptibility to vancomycin of nosocomial isolates of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1999;43:729–731. doi: 10.1093/jac/43.5.729. [DOI] [PubMed] [Google Scholar]
- 15.Lee J C, Park J S, Shepherd S E, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun. 1997;65:4146–4151. doi: 10.1128/iai.65.10.4146-4151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowell G H, Colleton C, Frost D, Kaminski R W, Hughes M, Hatch J, Hooper C, Estep J, Pitt L, Topper M, Hunt R E, Baker W, Baze W B. Immunogenicity and efficacy against lethal aerosol staphylococcal enterotoxin B challenge in monkeys by intramuscular and respiratory delivery of proteosome-toxoid vaccines. Infect Immun. 1996;64:4686–4693. doi: 10.1128/iai.64.11.4686-4693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowell G H, Kaminski R W, Grate S, Hunt R, Charney C, Zimmer S, Colleton C. Intranasal and intramuscular proteosome-staphylococcal enterotoxin B (SEB) toxoid vaccines: immunogenicity and efficacy against lethal SEB intoxication in mice. Infect Immun. 1996;64:1706–1713. doi: 10.1128/iai.64.5.1706-1713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mamo W, Jonsson P, Flock J I, Lindberg M, Müller H P, Wadström T, Nelson L. Vaccination against Staphylococcus aureus mastitis: immunological response of mice vaccinated with fibronectin-binding protein (FnBP-A) to challenge with S. aureus. Vaccine. 1994;12:988–992. doi: 10.1016/0264-410x(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 19.McKenny D, Pouliot K L, Wang Y, Murthy V, Ulrich M, Döring G, Lee J C, Goldmann D A, Pier G B. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 20.Moreau J M, Sloop G M, Engel L S, Hill J M, O'Callaghan R J. Histopathological studies of staphylococcal alpha-toxin: effects on rabbit corneas. Curr Eye Res. 1997;16:1221–1228. doi: 10.1076/ceyr.16.12.1221.5022. [DOI] [PubMed] [Google Scholar]
- 21.O'Callaghan R J, Callegan M C, Moreau J M, Green L C, Foster T J, Hartford O M, Engel L S, Hill J M. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65:1571–1578. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson D L. Vancomycin-resistant Staphylococcus aureus. Infect Med. 1999;16:235–238. [Google Scholar]
- 23.Sanders C C, Sanders W E, Jr, Thomson K S. Fluoroquinolone resistance in staphylococci: new challenges. Eur J Clin Microbiol Infect Dis. 1995;14(Suppl. 1):S6–S11. [PubMed] [Google Scholar]
- 24.Schennings T, Heimdahl A, Coster K, Flock J I. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb Pathog. 1993;15:227–236. doi: 10.1006/mpat.1993.1073. [DOI] [PubMed] [Google Scholar]
- 25.Seal D, Ficker L, Ramakrishnan M, Wright P. Role of staphylococcal toxin production in blepharitis. Ophthalmology. 1990;97:1684–1688. doi: 10.1016/s0161-6420(90)32361-8. [DOI] [PubMed] [Google Scholar]
- 26.Statistical Analysis Systems Institute. Statistical analysis systems for linear models: a guide to the ANOVA and GLM procedures. In: Freund R J, Little R C, editors. SAS series in statistical applications. Cary, N.C: SAS Institute, Inc.; 1981. [Google Scholar]
- 27.Wilhelmus K R, Penland R L. Emerging resistance among ocular isolates of gram-positive cocci. Investig Ophthalmol Vis Sci. 1996;37(Suppl.):877. [Google Scholar]