Abstract
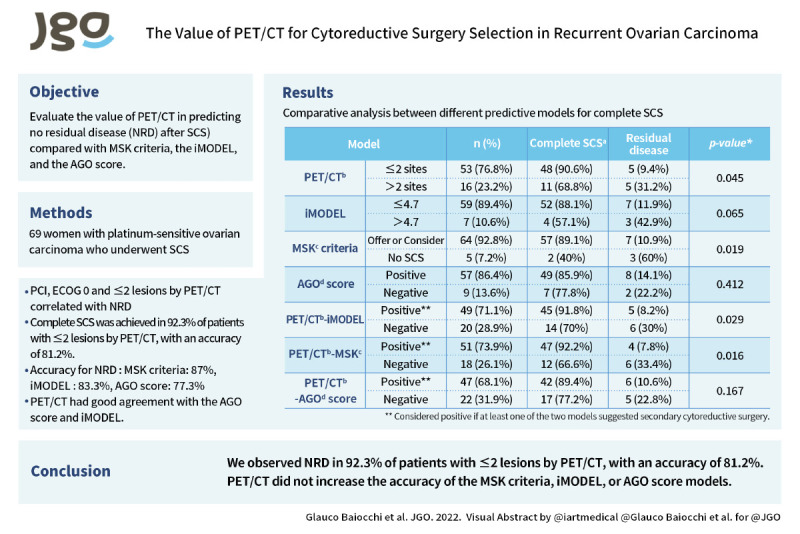
Objective
To evaluate the value of positron emission tomography/computed tomography (PET/CT) in predicting no residual disease (NRD) after secondary cytoreductive surgery (SCS) compared with MSK criteria, the iMODEL, and the AGO score.
Methods
We analyzed 112 patients with platinum-sensitive ovarian carcinoma who underwent SCS. We excluded patients for whom PET/CT was not performed, those without sufficient data, and who received chemotherapy before SCS. Ultimately, 69 patients were included.
Results
Variables that correlated with NRD were peritoneal carcinomatosis index (odds ratio [OR]=0.91; 95% confidence interval [CI]=0.83–0.99; p=0.044), European Cooperative Oncology Group Performance Status (ECOG) 0 (OR=8.0; 95% CI=1.34–47.5; p=0.022), and ≤2 lesions by PET/CT (OR=4.36; 95% CI=1.07–17.7; p=0.039). Of the patients with ≤2 lesions by PET/CT, 48 (92.3%) underwent complete SCS. The sensitivity, positive predictive value, negative predictive value, and accuracy of PET/CT for NRD were 85.7%, 92.3%, 33.3%, and 81.2%, respectively. NRD was achieved after fulfilling the MSK criteria, iMODEL and AGO Score in 89.1%, 88.1% and 85.9%, respectively. The accuracy of the MSK criteria, iMODEL, and AGO score in predicting NRD was 87%, 83.3%, and 77.3%, respectively. The PET/CT findings agreed well with the AGO score and iMODEL. The addition of PET/CT to these models increased the NRD rates (92.2%, 91.8%, and 89.4% for MSK+PET/CT, iMODEL+PET/CT, and AGO+PET/CT, respectively), but lowered their accuracy.
Conclusion
We observed NRD in 92.3% of patients with ≤2 lesions by PET/CT, with an accuracy of 81.2%. PET/CT did not increase the accuracy of the MSK criteria, iMODEL, or AGO score models.
Keywords: Ovarian Cancer, Recurrence, Cytoreductive Surgery, Clinical Decision Rules, PET-CT
Graphical Abstract
Synopsis
Complete secondary cytoreduction was achieved in 92.3% of patients with ≤2 lesions by PET/CT, with an accuracy of 81.2%. The accuracy of the MSK criteria, iMODEL, and AGO score for no residual disease was 87%, 83.3%, and 77.3%, respectively. PET/CT had good agreement with the AGO score and iMODEL.
INTRODUCTION
Ovarian carcinoma is the sixth most common cancer in women and the leading cause of death due to gynecological cancer in the western world [1]. Most cases are diagnosed as advanced disease, and the primary treatment is cytoreductive surgery and systemic chemotherapy. Despite the findings of prospective randomized controlled trials (RCTs) [2,3,4,5], primary cytoreduction remains the preferred option for most advanced-stage tumors (stage IIIC), and neoadjuvant chemotherapy should be reserved for patients with a high tumor burden in whom cytoreduction to no residual disease (NRD) is not feasible with acceptable morbidity or when the patient does not tolerate major surgery [6].
Most patients will experience a recurrence, and after the first platinum-sensitive recurrence, the value of secondary cytoreductive surgery (SCS) is still debatable. Recently, 3 RCTs addressed this issue in the recurrent setting, reporting contrasting results. The GOG-213 trial [7] showed no benefit of complete SCS compared with systemic treatment, whereas DESKTOP III/ENGOT-ov20 [8] and SOC1/SGOG-OV2 [9] reported a positive impact of SCS on disease-free survival (DFS); the DESKTOP III trial also recorded a benefit regarding overall survival (OS) [8]. A recent meta-analysis by Baek et al. [10] of 2805 patients in 80 studies concluded that despite the heterogeneity between studies, median OS increased by 9% when the rate of complete SCS increased by 10%.
Like cytoreduction in the primary setting [6], the surgical goal of SCS is to achieve NRD [11,12], and surgery might be detrimental in the recurrent setting for women who do not undergo complete SCS. Consequently, it is critical to develop a preoperative predictive tool that can select patients for SCS in a stringent manner. There are at least 3 validated models for selecting patients: the AGO score [13], iMODEL [14], and the Memorial Sloan-Kettering Cancer Center (MSK) criteria [15].
Despite the controversy over the value of imaging, combined with serum CA125 in posttreatment surveillance [16], the combination of fluorine-18 fluorodeoxyglucose positron emission tomography (PET) and computed tomography (CT) is a useful tool for assessing recurrence in ovarian carcinoma. PET/CT has a higher sensitivity and specificity than conventional techniques, such as CT and magnetic resonance imaging [17,18]. Notably, PET/CT was added to the iMODEL score in the SOC1 trial [9].
Our aim was to determine the value of PET/CT in predicting NRD after SCS and compare its performance with the MSK criteria, iMODEL, and AGO score in a cohort of patients with platinum-sensitive recurrent ovarian carcinoma.
METHODS
1. Study population
On approval by our institutional research board (#2459/17), we retrospectively analyzed a series of 112 patients with ovarian carcinoma who underwent secondary cytoreduction at AC Camargo Cancer Center between July 2008 and December 2021. All patients had platinum-sensitive recurrence (disease-free interval of over 6 months), and 43 were excluded: 15 did not undergo PET/CT before SCS, 3 cases had a second primary gynecological neoplasm, 15 were excluded due to insufficient data in their medical records, and 10 had had at least 1 cycle of chemotherapy before surgery. Ultimately, 69 patients were included.
The indications for SCS were determined at the discretion of the surgeon, the criteria for which included platinum-sensitive disease at first recurrence, ECOG 0–2, ASA I and II, and absence of extra-abdominal disease. All patients received platinum-based chemotherapy after SCS. Patients could also have undergone CT or magnetic resonance imaging before surgery.
2. Statistical analysis
Clinical and pathological data were obtained from medical records. iMODEL, AGO score, and the indications per MSK criteria were determined. The results of these models were compared with regard to the number of lesions that appeared on the PET/CT and its correlation with complete SCS. The main variable was the presence of residual disease at the end of SCS—complete SCS was defined as NRD being achieved. We defined false negatives in the PET/CT as a negative PET/CT with disease that was noted during surgery (surgery indicated after other imaging) and PET/CT images that showed a lower volume of disease than observed intraoperatively. The performance of the iMODEL, AGO score, and MSK criteria was analyzed for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy.
Chi square and Fisher’s exact tests were used to analyze correlations between categorical variables, and continuous variables were examined using independent sample t-test and Mann-Whitney U-test. For NRD, binary logistic regression was performed. Multivariate analysis was not performed due to the low number of events (residual disease). DFS was calculated as the time of SCS to the date of recurrence or last follow-up. OS was calculated as the time of SCS to the time of death or last follow-up. Patients were censored at the last follow-up if no recurrence or death occurred. A significance level of α=5% was applied for all tests. All analyses were performed in SPSS, version 24.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Patients and demographics
Table 1 summarizes the clinical and pathological variables. The median age was 55 years (range, 31–81), and the median interval between the end of the first treatment to the first relapse was 23 months (range, 6.05–104.2). Most cases had high-grade serous histology (n=54; 78.2%) and presented with advanced stages III and IV disease (n=61; 88.4%). The median CA125 level at recurrence was 421, the median surgical complexity score [19] was 2 (1–6), and the median peritoneal carcinomatosis index (PCI) score was 6 (1–39). Fifteen patients (23.4%) underwent surgery after a second recurrence (tertiary cytoreduction).
Table 1. Clinical and pathological characteristics of patients that underwent secondary cytoreductive surgery.
| Variables | Values | |
|---|---|---|
| Age [median] | 55 [31–81] | |
| PCI [median] | 6 [1–39] | |
| CA125 value at recurrence (UI/mL) [median] | 421.5 [10–17,784] | |
| Surgical Complexity Score [median] | 2 [1–6] | |
| Histology | ||
| High-grade serous | 54 (78.2) | |
| Low-grade serous | 3 (4.3) | |
| Clear cell | 4 (5.8) | |
| Endometrioid | 4 (5.8) | |
| Mixed | 3 (4.3) | |
| Carcinosarcoma | 1 (1.4) | |
| FIGO stage | ||
| IA | 1 (1.4) | |
| IC | 6 (8.7) | |
| IIB | 1 (1.4) | |
| IIIA | 4 (5.8) | |
| IIIC | 51 (73.9) | |
| IV | 6 (8.7) | |
| ECOG performance status | ||
| 0 | 63 (91.3) | |
| 1 | 6 (8.7) | |
| Ascites at recurrence | ||
| No ascites | 66 (98.5) | |
| <500 mL | 1 (1.5) | |
| >500 mL | 0 | |
| Missing data | 2 | |
| Residual disease after SCS | ||
| No | 59 (85.5) | |
| Yes | 10 (14.5) | |
| Tertiary cytoreduction | ||
| No | 54 (76.6) | |
| Yes | 15 (23.4) | |
| 0 | 5 (7.2) | |
| 1 | 31 (44.9) | |
| Number of lesions on PET/CT | ||
| 2 | 17 (24.6) | |
| 3 | 10 (14.5) | |
| 4 | 5 (7.2) | |
| 5 | 1 (1.4) | |
| BRCA status | ||
| Negative | 28 (68.3) | |
| BRCA 1 mutation | 9 (23) | |
| BRCA 2 mutation | 4 (9.7) | |
| Not tested | 28 | |
Values are presented as
ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; PCI, peritoneal carcinomatosis index; PET/CT, positron emission tomography/computed tomography.
2. Prognostic factors for residual disease after secondary cytoreduction
SCS with NRD was achieved in 59 cases (85.5%). The variables that correlated with NRD were PCI as a continuous variable (odds ratio [OR]=0.91; 95% confidence interval [CI]=0.83–0.99); p=0.044), ECOG0 (OR=8.0; 95% CI=1.34–47.5; p=0.022), and ≤2 lesions by PET/CT (OR=4.36; 95% CI=1.07–17.7; p=0.039). Table 2 shows the correlation between clinicopathological variables and NRD.
Table 2. Logistic regression of predictive factors for no residual disease after secondary cytoreductive surgery (univariate analysis).
| Variables | Category | No. | OR (95% CI) | p-value* |
|---|---|---|---|---|
| Age (yr) | Continuous | 69 | 0.98 (0.92–1.04) | 0.614 |
| PCI | Continuous | 49† | 0.90 (0.81–0.99) | 0.042 |
| Disease-free interval | Continuous | 69 | 1.01 (0.96–1.06) | 0.544 |
| CA125 at recurrence | Continuous | 63 | 0.99 (0.99–1.00) | 0.440 |
| Type 1st cytoreduction | Residual disease | 10 | Reference | 0.442 |
| No residual disease | 54 | 0.50 (0.08–2.92) | ||
| High-grade serous | No | 15 | Reference | 0.348 |
| Yes | 54 | 2.80 (0.32–24) | ||
| Stage at diagnosis | I-II | 8 | Reference | 0.865 |
| III-IV | 61 | 0.82 (0.09–7.53) | ||
| ECOG | 0 | 63 | 8.0 (1.34–47.5) | 0.022 |
| 1 | 6 | Reference | ||
| Peritoneal disease (PET) | No | 28 | Reference | 0.510 |
| Yes | 41 | 1.56 (0.40–6.0) | ||
| Number of lesions PET | ≤2 | 53 | 4.36 (1.07–17.7) | 0.039 |
| >2 | 16 | Reference | ||
| AGO score | Negative | 9 | Reference | 0.528 |
| Positive | 57 | 1.75 (0.30–9.97) | ||
| iModel | Low risk | 59 | 5.57 (1.02–30.2) | 0.047 |
| High risk | 7 | Reference | ||
| MSK criteria | No | 5 | Reference | 0.012 |
| Offer/Consider | 64 | 12.2 (1.73–86.1) | ||
| PET/CT-AGO score | Negative | 22 | Reference | 0.193 |
| Positive‡ | 47 | 0.40 (0.10–1.58) | ||
| PET/CT-iMODEL | Negative | 20 | Reference | 0.028 |
| Positive‡ | 49 | 4.82 (1.18–19.5) | ||
| PET/CT-MSK criteria | Negative | 18 | Reference | 0.014 |
| Positive‡ | 51 | 5.87 (1.42–24.1) |
AGO, Arbeitsgemeinschaft Gynäkologische Onkologie; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; MSK, Memorial Sloan Kettering; OR, odds ratio; PCI, peritoneal carcinomatosis index; PET/CT, positron emission tomography/computed tomography.
*Logistic regression; †only cases with recorded PCI>0; ‡Considered positive if at least one of the two models suggested secondary cytoreductive surgery.
3. Performance of the models
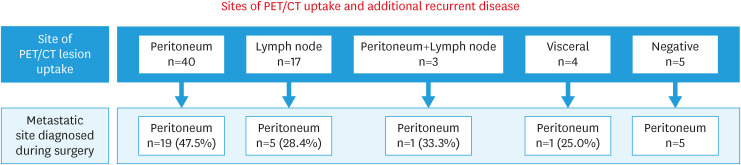
The PET/CT findings suggested recurrence on the peritoneal surface in 40 cases (58%), intra-abdominal lymph nodes in 17 cases (24.6%), hepatic parenchyma in 3 cases (4.3%), and adrenal in 1 case (1.4%) and no PET/CT uptake in 5 cases (7.2%). Patients without PET/CT uptake underwent surgery after recurrence suspected on CT or magnetic resonance imaging (MRI). Thirty-one (45%) cases presented with a false-negative PET/CT, 26 (37.7%) of whom had more peritoneal disease than reported by PET/CT (Fig. 1). Yet, when considering patients with peritoneal PET/CT uptake (n=40), 15 (37.5%) had 3 or more peritoneal implants.
Fig. 1. Sites of PET/CT uptake and site of additional lesions found during surgery.
PET/CT, positron emission tomography/computed tomography.
The median number of lesions with PET/CT uptake was 2 (range, 1–5). Of the patients with ≤2 lesions by PET/CT, 48 (92.3%) achieved complete SCS. Conversely, when >2 lesions were detected by PET/CT, 11 (68.8%) still experienced complete cytoreduction (Table 3). The sensitivity, PPV, NPV, and accuracy of PET/CT were 85.7%, 92.3%, 33.3%, and 81.2%, respectively (Table 4). Forty-nine patients (85.9%) achieved complete SCS after generating a positive AGO score (Table 3). Of those who did not have positive scores, 7 (77.8%) experienced complete cytoreduction. The AGO score had a sensitivity, PPV, NPV, and accuracy of 87.5%, 86%, 22.2%, and 77.3%, respectively (Table 4).
Table 3. Comparative analysis between different predictive models for complete secondary cytoreduction.
| Model | Values | Complete SCS | Residual disease | p-value* | |
|---|---|---|---|---|---|
| PET/CT | 0.045 | ||||
| ≤2 sites | 53 (76.8) | 48 (90.6) | 5 (9.4) | ||
| >2 sites | 16 (23.2) | 11 (68.8) | 5 (31.2) | ||
| iMODEL | 0.065 | ||||
| ≤4.7 | 59 (89.4) | 52 (88.1) | 7 (11.9) | ||
| >4.7 | 7 (10.6) | 4 (57.1) | 3 (42.9) | ||
| MSK criteria | 0.019 | ||||
| Offer or consider | 64 (92.8) | 57 (89.1) | 7 (10.9) | ||
| No SCS | 5 (7.2) | 2 (40) | 3 (60) | ||
| AGO score | 0.412 | ||||
| Positive | 57 (86.4) | 49 (85.9) | 8 (14.1) | ||
| Negative | 9 (13.6) | 7 (77.8) | 2 (22.2) | ||
| PET/CT-iMODEL | 0.029 | ||||
| Positive† | 49 (71.1) | 45 (91.8) | 5 (8.2) | ||
| Negative | 20 (28.9) | 14 (70) | 6 (30) | ||
| PET/CT-MSK | 0.016 | ||||
| Positive† | 51 (73.9) | 47 (92.2) | 4 (7.8) | ||
| Negative | 18 (26.1) | 12 (66.6) | 6 (33.4) | ||
| PET/CT-AGO score | 0.167 | ||||
| Positive† | 47 (68.1) | 42 (89.4) | 6 (10.6) | ||
| Negative | 22 (31.9) | 17 (77.2) | 5 (22.8) | ||
Values are presented as number (%).
AGO, Arbeitsgemeinschaft Gynäkologische Onkologie; MSK, Memorial Sloan Kettering; PET/CT, positron emission tomography/computed tomography; SCS, secondary cytoreductive surgery.
*Qui-square and Fisher tests; †Considered positive if at least one of the two models suggested secondary cytoreductive surgery.
Table 4. Performance of the models for no residual disease after secondary cytoreductive surgery.
| Score | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| Number of lesions PET (≤2 vs. >2) | 85.7% | 50% | 92.3% | 33.3% | 81.2% |
| AGO score (negative vs. positive) | 87.5% | 20% | 86% | 22.2% | 77.3% |
| iMODEL (low risk vs. high risk) | 92.8% | 30% | 88.1% | 42.8% | 83.3% |
| MSK criteria (no vs. offer+consider) | 96.6% | 30% | 89.1% | 60% | 87% |
| PET/CT-AGO score (negative vs. positive*) | 71.2% | 50% | 89.4% | 22.7% | 68.1% |
| PET/CT-iMODEL (negative vs. positive*) | 76.3% | 60% | 91.8% | 30% | 73.9% |
| PET/CT-MSK criteria (negative vs. positive*) | 79.7% | 60% | 92.2% | 33.3% | 76.8% |
AGO, Arbeitsgemeinschaft Gynäkologische Onkologie; MSK, Memorial Sloan Kettering; NPV, negative predictive value; PET/CT, positron emission tomography/computed tomography; PPV, positive predictive value.
*Considered positive if at least one of the two models suggested secondary cytoreductive surgery.
With regard to the MSK criteria, 57 patients (89.1%) who received a recommendation of “indication for” or “consider” underwent complete SCS. When the criteria suggested not undergoing a surgical procedure, 2 patients (40%) achieved complete cytoreduction (Table 3). The MSK criteria had a sensitivity, PPV, NPV, and accuracy of 96.6%, 89%, 60%, and 87%, respectively (Table 4).
Fifty-two patients (88.1%) underwent complete SCS after receiving a “low risk for suboptimal cytoreduction” iMODEL score (Table 3). Of those who were at “high risk,” 4 (57.1%) nevertheless achieved complete cytoreduction. iMODEL had a sensitivity, PPV, NPV, and accuracy of 92.8%, 88.1%, 42.8%, and 83.3%, respectively (Table 4).
The comparisons of the PET/CT criteria with the AGO score, MSK criteria, and iMODEL had p-values of 0.79, 0.004, and 0.109, respectively, by McNemar test, indicating that PET/CT agreed well with the AGO score and iMODEL.
Further, we added the PET/CT criteria to these models to address if it would improve the selection of patients for surgery. It was considered positive if at least one of the two models suggested SCS. For positive PET/CT-AGO, PET/CT-MSK, and PET/CT-iMODEL scores, complete SCS would be obtained in 89.4%, 92.2%, and 91.8% of patients, respectively. However, this strategy negatively impacted the accuracy of the iMODEL, MSK criteria, and AGO score, decreasing to 73.9%, 76.8%, and 68.1%, respectively (Tables 3 and 4).
4. Survival outcomes
After a median follow-up of 36 months (95% CI=29–43.1), we recorded 41 recurrences (59.4%) and 9 deaths (13%). The 5-year OS and disease-free survival rates were 75.7% (median survival not reached) and 24.4% (median, 19.8 months; 95% CI=15.4–24.4), respectively.
DISCUSSION
When PET/CT detected uptake in ≤2 sites, 92.3% of cases had a complete SCS. However, when >2 sites showed uptake, 66.7% still had a complete SCS, resulting in a sensitivity, PPV, NPV, and accuracy of 85.7%, 92.3%, 33.3% and 81.2%, respectively. The MSK criteria performed best, with an accuracy of 87%, followed by the iMODEL and AGO scores (83.3% and 77.3%, respectively). Notably, all methods had a low NPV, indicating that a better score needs to be developed—only the MSK criteria had an NPV that exceeded 50%. Yet, combining the model with PET/CT was unable to improve their performances.
We also noted that high PCI and ECOG negatively impacted the achievement of a complete SCS. Despite the high false negative rate of PET/CT in predicting the dissemination of peritoneal disease, we still obtained a high complete SCS, suggesting that unrecorded lesions do not influence the surgical goal.
Several retrospective studies support NRD as the aim of SCS, and predictive tools for NRD have been developed. The DESKTOP-I study [11] retrospectively analyzed the primary endpoint of SCS, in which NRD positively impacted survival. By multivariate analysis, the AGO score was proposed and validated in the prospective the DESKTOP-II study [13]. The iMODEL score was based on a multivariate analysis of a large retrospective multicenter study (n=1,075), in which the authors observed that complete cytoreduction was influence by FIGO stage, residual disease in primary cytoreduction, disease-free interval, ECOG performance status, CA125 level, and ascites. These findings derived a score with a cutoff point of 4.7 [14].
The third criterion was published by MSK in 2006, which suggested a survival benefit for patients with residual disease ≤0.5 cm and proposed the selection of patients based on disease-free interval and the number of sites of recurrence [15]. The MSK criteria have been described in subsequent studies, highlighting their value in selecting patients for secondary debulking [20,21,22]. Notably, the MSK group compared the MSK criteria with the iMODEL and AGO scores in 214 women who underwent SCS. They reported an NRD of 86% for the MSK criteria and concluded that there was good concordance between the MSK criteria and iMODEL but low accuracy for the AGO score (49%) [22].
The impact of surgery on survival in platinum-sensitive recurrent ovarian carcinoma was addressed in 3 recent RCTs. In the GOG study, 213 patients were randomly assigned to SCS at the discretion of the surgeon, and a second randomization evaluated the value of adding bevacizumab to chemotherapy. The authors found no difference in DFS and OS between those who were undergoing surgery followed by chemotherapy compared with those who were treated only with chemotherapy. Overall, 84% of cases had received bevacizumab [7].
In the phase III trial of the DESKTOP series, AGO DESKTOP III, on receiving a positive AGO score, patients were randomly assigned to SCS or platinum-based chemotherapy. The authors reported a better OS (median 53.7 vs. 46.2 months; HR=0.76; 95% CI=0.5–0.97; p=0.03) and a better DFS (median 18.4 vs. 14 months; HR=0.66; 95% CI=0.54–0.82; p<0.001) for patients who underwent surgery [8]. The third clinical trial, SOC-1, used iMODEL to select patients for SCS [9]. The median DFS was 17.4 months for the surgery arm and 11.9 months for the chemotherapy arm (HR=0.58; 95% CI=0.45–0.74; p<0.001). OS did not differ significantly between groups, although data are not mature. Notably, the achievement of NRD did not differ across the 3 studies, with rates of 67%, 75.5%, and 76.7% in GOG 213, DESKTOP III, and SOC-1, respectively [7,8,9].
Of these RCTs, only SOC-1 incorporated PET-CT when screening patients for the respective studies. Overall, 92.4% of cases underwent PET-CT before randomization, and 32% of cases that were subjected to surgery had up to 3 lesions that were detected by imaging. However, the criteria that were used to discriminate the resectability after the PET-CT were unclear [9].
Two issues were not addressed. Regarding the prognostic value of BRCA or homologous recombination deficiency status in the recurrent setting for selection for surgery, a recent retrospective MITO group study suggested that BRCA-mutated patients who were undergoing SCS followed by chemotherapy and maintenance with a PARP inhibitor had a better DFS and OS compared with those who did not receive surgery [23]. Moreover, our previous data have suggested a potential benefit of SCS for DFS compared with only chemotherapy, irrespective of BRCA status, in patients who are primarily not treated with a PARP inhibitor. Yet, BRCA-mutated cases were more likely to receive complete secondary cytoreduction compared with wild-type cases (89.5% vs. 65.2%; p=0.047) [24].
The second issue relates to the worse survival outcome for women who have residual disease after SCS versus those who are randomly assigned chemotherapy—eg, as evidenced by a median OS of 27.7 versus 46 months, respectively, in the DESKTOP III study [8]. Possible explanations include a worse molecular profile, peritoneal dissemination pattern, or other unknown biological and genomic features.
Nevertheless, other predictive scores for complete SCS has been published [25,26], however without either external validation or prospectively test in a RCT. In Minaguchi’s score, a complete SCS was achieved in 78% after the presence of 3–4 prognostic factors (DFS >12 months, absence of distant metastasis, solitary disease and ECOG 0) [25]. Moreover, the SeC-Score included Ca125, HE4, residual disease at primary surgery and ascites at recurrence, and the score recorded a sensitivity and specificity of 82 and 83%, respectively [26]. Notably, even artificial intelligence has been used to address predictive factors for complete SCS, being DFS considered the most important factor for no residual disease and OS [27].
Diagnostic methods for recurrence were compared in a meta-analysis from 2009. Despite the heterogeneity between 34 studies, the pooled sensitivity for PET/CT (0.91) was higher compared with MRI (0.75), CT (0.79), and CA125 (0.69). However, CA125 had a higher pooled specificity (0.93), versus 0.88 for PET/CT. The area under the receiver operating characteristic (AUC) curve was 0.92 for CA125, 0.96 for PET/CT, 0.88 for CT, and 0.8 for MRI in the detection of recurrent ovarian cancer [28]. A recent meta-analysis (2017) of 64 PET/CT studies by Xu et al. [29] confirmed the pooled sensitivity, specificity, and AUC of 0.92, 0.91, and 0.96, respectively, in the diagnosis of recurrences [29].
The sensitivity of PET/CT in detecting subcentimeter lesions of peritoneal implants is lower compared with larger lesions [30], because the spatial resolution is unable to define small-volume disease (<7 mm) due to a lack of radiotracer uptake, as with miliary or diffuse peritoneal involvement, even when suggested by CT or MRI [31,32]. However, PET/CT still detects distant metastatic disease and lymph nodes due its increased metabolic activity [17,33].
Nevertheless, PET/CT findings have been reported to alter treatment for recurrent ovarian carcinoma in 40% to 60% of patients [34,35,36,37,38], and current evidence supports the use of FDG PET/CT in patients with rising CA125 levels and negative CT or MR imaging [28,35,36]. Theoretically, PET/CT can help exclude extra-abdominal disease; select patients for site-specific treatment, such as stereotactic body radiation therapy; and identify optimal surgical candidates, recognizing otherwise undetected recurrent disease [17].
Although PET/CT has been incorporated into daily practice in the management of recurrent ovarian cancer, there are little data on the value of evaluation of resectability and the screening of patients for SCS. Moreover, data on the number of uptake lesions in PET/CT and the completeness of cytoreduction are lacking. The MSK score includes the number of lesions (1, 2, and carcinomatosis) in its criteria, and we hypothesize that PET/CT adds value to the selection of patients.
In our center, PET/CT is incorporated after a recurrence has been suspected, first aiming to rule out extra-abdominal disease. Unfortunately, we could not retrieve such data from all cases that underwent a PET/CT after recurrence and report the number of cases that did not undergo surgery due to extra-abdominal disease.
There were several limitations of the study, primarily the inherent bias of its retrospective analysis and the small number of cases. However, it is the first study to examine PET/CT as a tool to aid in the decision to perform surgery in the recurrent setting. We also evaluated and compared the 3 most important prediction models.
Notably, we had a high overall rate of complete SCS and recognize that most patients had a low tumor burden, perhaps reflecting a group of well-selected cases that are treated in a tertiary center. Despite the short median follow-up time, the favorable survival rates might also indicate that the cases were selected well. Yet, our study contributes valuable data, because studies that address novel tools to select patients and compare the 3 most recognized models remain lacking.
In conclusion, we noted NRD in 92.3% of cases after the presence of ≤2 lesions by PET/CT, with an accuracy of 81.2%. The MSK criteria performed best, with an accuracy of 87%, followed by iMODEL (83.3%) and the AGO score (77.3%). PET/CT did not increase the accuracy of the MSK criteria, iMODEL or AGO score models. Moreover, PET/CT had good agreement with the AGO score and iMODEL.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Availability of Data and Material
The data and material will be available by the authors upon reasonable request.
- Conceptualization: N.R.L., D.T.P., F.C.C., M.H., B.G.
- Data curation: N.R.L., T.F.R., D.T.P., M.H., D.A., B.G.
- Formal analysis: N.R.L., D.T.P., F.C.C., D.A., B.G.
- Investigation: N.R.L., T.F.R., F.C.C., M.H., D.A., B.G.
- Methodology: T.F.R., D.T.P., M.H., D.A., B.G.
- Project administration: B.G.
- Resources: N.R.L., T.F.R., F.C.C., B.G.
- Software: D.T.P., B.G.
- Supervision: D.A., B.G.
- Validation: N.R.L., M.H., B.G.
- Visualization: N.R.L., T.F.R., D.T.P., F.C.C., M.H., B.G.
- Writing - original draft: N.R.L., B.G.
- Writing - review & editing: F.C.C., D.A., B.G.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 3.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 4.Fagotti A, Ferrandina MG, Vizzielli G, Pasciuto T, Fanfani F, Gallotta V, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION- NCT01461850) Int J Gynecol Cancer. 2020;30:1657–1664. doi: 10.1136/ijgc-2020-001640. [DOI] [PubMed] [Google Scholar]
- 5.Onda T, Satoh T, Ogawa G, Saito T, Kasamatsu T, Nakanishi T, et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer. 2020;130:114–125. doi: 10.1016/j.ejca.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Colombo N, Sessa C, du Bois A, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RL, Spirtos NM, Enserro D, Herzog TJ, Sabbatini P, Armstrong DK, et al. Secondary surgical cytoreduction for recurrent ovarian cancer. N Engl J Med. 2019;381:1929–1939. doi: 10.1056/NEJMoa1902626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harter P, Sehouli J, Vergote I, Ferron G, Reuss A, Meier W, et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med. 2021;385:2123–2131. doi: 10.1056/NEJMoa2103294. [DOI] [PubMed] [Google Scholar]
- 9.Shi T, Zhu J, Feng Y, Tu D, Zhang Y, Zhang P, et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22:439–449. doi: 10.1016/S1470-2045(21)00006-1. [DOI] [PubMed] [Google Scholar]
- 10.Baek MH, Park EY, Ha HI, Park SY, Lim MC, Fotopoulou C, et al. Secondary cytoreductive surgery in platinum-sensitive recurrent ovarian cancer: a meta-analysis. J Clin Oncol. 2022;40:1659–1670. doi: 10.1200/JCO.21.02085. [DOI] [PubMed] [Google Scholar]
- 11.Harter P, du Bois A, Hahmann M, Hasenburg A, Burges A, Loibl S, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13:1702–1710. doi: 10.1245/s10434-006-9058-0. [DOI] [PubMed] [Google Scholar]
- 12.Zang RY, Harter P, Chi DS, Sehouli J, Jiang R, Tropé CG, et al. Predictors of survival in patients with recurrent ovarian cancer undergoing secondary cytoreductive surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105:890–896. doi: 10.1038/bjc.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harter P, Sehouli J, Reuss A, Hasenburg A, Scambia G, Cibula D, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011;21:289–295. doi: 10.1097/IGC.0b013e31820aaafd. [DOI] [PubMed] [Google Scholar]
- 14.Tian WJ, Chi DS, Sehouli J, Tropé CG, Jiang R, Ayhan A, et al. A risk model for secondary cytoreductive surgery in recurrent ovarian cancer: an evidence-based proposal for patient selection. Ann Surg Oncol. 2012;19:597–604. doi: 10.1245/s10434-011-1873-2. [DOI] [PubMed] [Google Scholar]
- 15.Chi DS, McCaughty K, Diaz JP, Huh J, Schwabenbauer S, Hummer AJ, et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer. 2006;106:1933–1939. doi: 10.1002/cncr.21845. [DOI] [PubMed] [Google Scholar]
- 16.Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol Oncol. 2017;146:3–10. doi: 10.1016/j.ygyno.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Son H, Khan SM, Rahaman J, Cameron KL, Prasad-Hayes M, Chuang L, et al. Role of FDG PET/CT in staging of recurrent ovarian cancer. Radiographics. 2011;31:569–583. doi: 10.1148/rg.312105713. [DOI] [PubMed] [Google Scholar]
- 18.Kitajima K, Murakami K, Sakamoto S, Kaji Y, Sugimura K. Present and future of FDG-PET/CT in ovarian cancer. Ann Nucl Med. 2011;25:155–164. doi: 10.1007/s12149-010-0449-8. [DOI] [PubMed] [Google Scholar]
- 19.Aletti GD, Santillan A, Eisenhauer EL, Hu J, Aletti G, Podratz KC, et al. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecol Oncol. 2007;107:99–106. doi: 10.1016/j.ygyno.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 20.Bogani G, Tagliabue E, Signorelli M, Ditto A, Martinelli F, Chiappa V, et al. A score system for complete cytoreduction in selected recurrent ovarian cancer patients undergoing secondary cytoreductive surgery: predictors- and nomogram-based analyses. J Gynecol Oncol. 2018;29:e40. doi: 10.3802/jgo.2018.29.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muallem MZ, Gasimli K, Richter R, Almuheimid J, Nasser S, Braicu EI, et al. AGO score as a predictor of surgical outcome at secondary cytoreduction in patients with ovarian cancer. Anticancer Res. 2015;35:3423–3429. [PubMed] [Google Scholar]
- 22.Cowan RA, Eriksson AG, Jaber SM, Zhou Q, Iasonos A, Zivanovic O, et al. A comparative analysis of prediction models for complete gross resection in secondary cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2017;145:230–235. doi: 10.1016/j.ygyno.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Cecere SC, Musacchio L, Bartoletti M, Salutari V, Arenare L, Lorusso D, et al. Cytoreductive surgery followed by chemotherapy and olaparib maintenance in BRCA 1/2 mutated recurrent ovarian cancer: a retrospective MITO group study. Int J Gynecol Cancer. 2021;31:1031–1036. doi: 10.1136/ijgc-2020-002343. [DOI] [PubMed] [Google Scholar]
- 24.Estati FL, Pirolli R, de Alencar VT, Ribeiro AR, Formiga MN, Torrezan GT, et al. Impact of BRCA1/2 mutations on the efficacy of secondary cytoreductive surgery. Ann Surg Oncol. 2021;28:3637–3645. doi: 10.1245/s10434-020-09366-w. [DOI] [PubMed] [Google Scholar]
- 25.Minaguchi T, Satoh T, Matsumoto K, Sakurai M, Ochi H, Onuki M, et al. Proposal for selection criteria of secondary cytoreductive surgery in recurrent epithelial ovarian, tubal, and peritoneal cancers. Int J Clin Oncol. 2016;21:573–579. doi: 10.1007/s10147-015-0910-8. [DOI] [PubMed] [Google Scholar]
- 26.Angioli R, Capriglione S, Aloisi A, Ricciardi R, Scaletta G, Lopez S, et al. A predictive score for secondary cytoreductive surgery in recurrent ovarian cancer (SeC-Score): a single-centre, controlled study for preoperative patient selection. Ann Surg Oncol. 2015;22:4217–4223. doi: 10.1245/s10434-015-4534-z. [DOI] [PubMed] [Google Scholar]
- 27.Bogani G, Rossetti D, Ditto A, Martinelli F, Chiappa V, Mosca L, et al. Artificial intelligence weights the importance of factors predicting complete cytoreduction at secondary cytoreductive surgery for recurrent ovarian cancer. J Gynecol Oncol. 2018;29:e66. doi: 10.3802/jgo.2018.29.e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu P, Pan LL, Wu SQ, Sun L, Huang G. CA 125, PET alone, PET-CT, CT and MRI in diagnosing recurrent ovarian carcinoma: a systematic review and meta-analysis. Eur J Radiol. 2009;71:164–174. doi: 10.1016/j.ejrad.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Xu B, Ma J, Jiang G, Wang Y, Ma Q. Diagnostic value of positron emission tomography (PET) and PET/computed tomography in recurrent/metastatic ovarian cancer: a meta-analysis. J Obstet Gynaecol Res. 2017;43:378–386. doi: 10.1111/jog.13222. [DOI] [PubMed] [Google Scholar]
- 30.Torizuka T, Nobezawa S, Kanno T, Futatsubashi M, Yoshikawa E, Okada H, et al. Ovarian cancer recurrence: role of whole-body positron emission tomography using 2-[fluorine-18]-fluoro-2-deoxy- D-glucose. Eur J Nucl Med Mol Imaging. 2002;29:797–803. doi: 10.1007/s00259-001-0750-9. [DOI] [PubMed] [Google Scholar]
- 31.Cho SM, Ha HK, Byun JY, Lee JM, Kim CJ, Nam-Koong SE, et al. Usefulness of FDG PET for assessment of early recurrent epithelial ovarian cancer. AJR Am J Roentgenol. 2002;179:391–395. doi: 10.2214/ajr.179.2.1790391. [DOI] [PubMed] [Google Scholar]
- 32.Sironi S, Messa C, Mangili G, Zangheri B, Aletti G, Garavaglia E, et al. Integrated FDG PET/CT in patients with persistent ovarian cancer: correlation with histologic findings. Radiology. 2004;233:433–440. doi: 10.1148/radiol.2332031800. [DOI] [PubMed] [Google Scholar]
- 33.Choi HJ, Roh JW, Seo SS, Lee S, Kim JY, Kim SK, et al. Comparison of the accuracy of magnetic resonance imaging and positron emission tomography/computed tomography in the presurgical detection of lymph node metastases in patients with uterine cervical carcinoma: a prospective study. Cancer. 2006;106:914–922. doi: 10.1002/cncr.21641. [DOI] [PubMed] [Google Scholar]
- 34.Zimny M, Siggelkow W, Schröder W, Nowak B, Biemann S, Rath W, et al. 2-[Fluorine-18]-fluoro-2-deoxy-d-glucose positron emission tomography in the diagnosis of recurrent ovarian cancer. Gynecol Oncol. 2001;83:310–315. doi: 10.1006/gyno.2001.6386. [DOI] [PubMed] [Google Scholar]
- 35.Thrall MM, DeLoia JA, Gallion H, Avril N. Clinical use of combined positron emission tomography and computed tomography (FDG-PET/CT) in recurrent ovarian cancer. Gynecol Oncol. 2007;105:17–22. doi: 10.1016/j.ygyno.2006.10.060. [DOI] [PubMed] [Google Scholar]
- 36.Soussan M, Wartski M, Cherel P, Fourme E, Goupil A, Le Stanc E, et al. Impact of FDG PET-CT imaging on the decision making in the biologic suspicion of ovarian carcinoma recurrence. Gynecol Oncol. 2008;108:160–165. doi: 10.1016/j.ygyno.2007.07.082. [DOI] [PubMed] [Google Scholar]
- 37.Fulham MJ, Carter J, Baldey A, Hicks RJ, Ramshaw JE, Gibson M. The impact of PET-CT in suspected recurrent ovarian cancer: a prospective multi-centre study as part of the Australian PET Data Collection Project. Gynecol Oncol. 2009;112:462–468. doi: 10.1016/j.ygyno.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 38.Simcock B, Neesham D, Quinn M, Drummond E, Milner A, Hicks RJ. The impact of PET/CT in the management of recurrent ovarian cancer. Gynecol Oncol. 2006;103:271–276. doi: 10.1016/j.ygyno.2006.03.004. [DOI] [PubMed] [Google Scholar]