Abstract
Objective
Lower extremity lymphedema (LEL) is a well-known adverse effect related to cervical and endometrial cancer (CEC); however, very few studies have elucidated the clinicopathologic risk factors related to LEL. We investigated the incidence and risk factors in patients who received primary surgery and/or adjuvant radiotherapy (RT) or chemotherapy for CEC.
Methods
We retrospectively reviewed 2,565 patients who underwent primary surgery following CEC diagnosis between January 2007 and December 2020. LEL diagnosis was based on objective and subjective assessments by experts. We identified important predictors of LEL to construct a nomogram predicting individual risks of LEL. For internal validation of the nomogram, the original data were separated using the split-sample method in a 7:3 ratio of training data and test data.
Results
Overall, 858 patients (33.5%) received RT, 586 received external beam RT (EBRT), and 630 received intracavitary RT. During follow-up period, LEL developed in 331 patients, with an overall cumulative 5-year incidence of 13.3%. In multivariate analysis, age at primary treatment, use of docetaxel-based chemotherapy, type of hysterectomy, type of surgical pelvic lymph node (LN) assessment, number of dissected pelvic and para-aortic LNs, and EBRT field were the independent predictors of LEL. We subsequently developed the nomogram showing excellent predictive power for LEL.
Conclusion
LEL is associated with various treatment modalities, and their interactions may increase the possibility of occurrences. De-escalation strategies for treatment modalities should be considered to reduce LEL in patients with CEC.
Keywords: Radiotherapy, Cervical Cancer, Endometrial Cancer, Lymphedema, Risk Factor
Synopsis
Lower extremity lymphedema (LEL) is an adverse effect associated with cervical and endometrial cancer (CEC). We identified risk factors of LEL occurrence following primary surgery and/or adjuvant radiotherapy. Based on the identified risk factors, we developed a nomogram showing excellent predictive power for LEL.
INTRODUCTION
Radiotherapy (RT) plays an important role as an adjuvant treatment for gynecological cancer. Following upfront radical hysterectomy, cervical cancer is classified into low-, intermediate-, and high-risk groups, according to pathological findings. Evidence-based guidelines recommend no adjuvant treatment for low-risk groups, adjuvant RT for intermediate-risk groups, and adjuvant RT and chemotherapy for high-risk groups [1]. RT, chemotherapy, or both are used for resected endometrial cancer, depending on the tumor stage and histologic grade.
Lymphedema of the lower extremities (LELs) is a well-known adverse effect of surgical treatment in gynecological cancer, with a reported incidence of up to 47.0%–78.7% [2,3]. It is crucial that physicians address LEL promptly since LEL not only hinders motor function but also has the potential risk to progress to cellulitis, ultimately lowering the quality of life in some patients. Therefore, it is important to identify the relevant risk factors to prevent LEL. Among factors related to surgery, pelvic LN dissection significantly increases the number of removed lymph nodes (LNs) [4,5]. External beam RT (EBRT) targeting the entire pelvis is also a significant factor compared to intracavitary RT (ICR) in the development of LEL in patients with cervical and endometrial cancer (CEC) [3,6,7]. However, the risk factors and incidence of LEL in patients who undergo primary surgery for CEC with or without RT are not precisely known. In particular, few studies have investigated the association between the development of LEL and recent treatment modalities, such as sentinel LN biopsy (SLNB) or intensity-modulated RT (IMRT).
In this study, we investigated the incidence of and related risk factors for LEL in patients who underwent primary surgery and/or adjuvant RT for CEC.
MATERIALS AND METHODS
1. Study design and patient selection
We retrospectively reviewed 3,222 patients who underwent primary treatment for newly diagnosed localized CEC between January 2007 and December 2020 at the Yonsei Cancer Center. The exclusion criteria include patients who did not undergo primary surgery (n=645), those with insufficient RT-related records (n=12), and those with a history of previous pelvic RT (n=26). A total of 2,565 patients were finally included in the cohort. This study was approved by the Severance Hospital institutional review board (number: 4-2022-0100), and the requirement for informed consent was waived because of the retrospective nature of this study.
2. Treatment
All patients underwent hysterectomies such as simple, modified radical, or radical hysterectomy, as their initial therapy based on the preoperative diagnosis or the surgeon’s discretion. En bloc resection of the pelvic LNs is often performed in conjunction with hysterectomy. SLNB was recently performed instead of pelvic LN dissection by injecting indocyanine green directly into the cervix and removing stained pelvic LNs [8]. In addition, para-aortic or inguinal LN dissection was selectively performed according to patients’ disease status. In particular, inguinal LN dissection is performed when inguinal LN metastasis is suspected on images or diagnosed on histological examination. Adjuvant chemotherapy was administered sequentially or concurrently with RT. For sequential chemotherapy, a docetaxel-based regimen was mainly used, and most patients received cisplatin for concurrent chemoradiotherapy.
Adjuvant RT was categorized based on the tumor bed and LN involvement. Whole-pelvis irradiation covered the tumor bed, external and internal iliac LN chains, and bifurcation of the common iliac LN. Additionally, para-aortic LN irradiation was considered prophylactically when multiple pelvic LNs were involved or during pathological para-aortic LN metastasis. Prophylactic para-aortic LN irradiation was conducted in a semi-extended field to cover the region below the renal vessel level. An extended field up to the T12/L1 level was used for para-aortic LN metastasis. EBRT to the whole-pelvis, semi-extended field, or extended field was delivered at a total dose ranging from 41.4 Gy/23 fractions to 50.4 Gy/28 fractions. Inguinal LN irradiation is performed under multidisciplinary discussion in patients with inguinal LN metastasis confirmed by LN dissection or imaging. For patients who needed inguinal RT, the radiation field was defined such that it would encompass both the pelvic RT field and the inguinal area; thus, the same radiation dose also irradiated the inguinal area when pelvic irradiation was performed. For ICR, either an ovoid or cylinder applicator was used, depending on the margin status or grade. ICR was delivered at a total dose of 8.0–21.0 Gy with 3.0–8.0 Gy per fraction in combination with EBRT, and ICR alone was delivered at a total dose of 30.0 Gy/6 fractions or 24.0 Gy/3 fractions. The most commonly used dose schedules were 10 Gy/2 fractions in combination with EBRT and 30 Gy/6 fractions in ICR alone. The total pelvic dose was calculated as the sum of the doses irradiated to the pelvic wall during EBRT and ICR in each patient. The biologically effective dose (BED) was calculated by adding different EBRT and ICR fractionation regimens and comparing the total pelvic dose on a patient-by-patient basis. The BED was calculated using the following equation: BED=nD[1+D/(α/β)], where n is the fraction number, D is the dose per fraction, and α/β=3 and 10 for normal tissues and tumors, respectively. According to the cancer type, EBRT was performed in all cases of cervical cancer, and if the vaginal resection margin was positive or there was vaginal involvement, ICR using an ovoid or cylinder applicator was also performed, depending on the extent of the cancer. In cases of endometrial cancer, ICR using an ovoid applicator was performed, and EBRT was also performed in some cases, depending on histologic grade, risk factors (age over 60 years, lymphovascular space invasion, tumor size over 4 cm, and lower uterine involvement), and depth of myometrial invasion.
The treatment characteristics are presented in Table 1. In our study, 806 patients (31.4%) underwent radical hysterectomy for the primary lesion. Overall, 2,152 patients (83.9%) underwent surgical LN assessment for pelvic LN, 562 underwent SLNB alone, while the remaining 1,590 underwent pelvic LN dissection. The number of patients who underwent LN dissection for para-aortic and inguinal LN were 1,076 (41.9%) and 570 (22.2%), respectively. The median numbers of removed LNs in patients with surgical LN assessment were 12 pelvic LNs (interquartile range [IQR], 6–18), 5 para-aortic LNs (IQR, 3–10), and 3 inguinal LNs (IQR, 2–5).
Table 1. Baseline characteristics of all patients.
| Characteristics | Value (n=2,565) | ||||
|---|---|---|---|---|---|
| No. or Median | % or IQR | ||||
| Age at primary treatment (yr) | 52 | 44–60 | |||
| BMI at primary treatment (kg/m2) | 23.4 | 21.2–26.3 | |||
| Type of malignancy | |||||
| Cervical cancer | 1,120 | 43.7 | |||
| Endometrial cancer | 1,445 | 56.3 | |||
| LN metastasis at diagnosis | 344 | 13.4 | |||
| Type of hysterectomy | |||||
| Simple hysterectomy | 1,637 | 63.8 | |||
| Modified radical hysterectomy | 122 | 4.8 | |||
| Radical hysterectomy | 806 | 31.4 | |||
| Surgical LN assessment | 2,182 | 85.1 | |||
| Pelvic LN | 2,152 | 98.6 | |||
| Surgical procedure | |||||
| Sentinel LN biopsy | 562 | 26.1 | |||
| LN dissection | 1,590 | 73.9 | |||
| No. of removed LNs | 12 | 6–18 | |||
| Para-aortic LN | 1,076 | 49.3 | |||
| No. of removed LNs | 5 | 3–10 | |||
| Inguinal LN | 570 | 26.1 | |||
| No. of removed LNs | 3 | 2–5 | |||
| Use of RT | 858 | 33.5 | |||
| External beam RT | 586 | 68.3 | |||
| RT field | |||||
| Whole pelvis | 456 | 77.8 | |||
| Semi-extended field | 45 | 7.7 | |||
| Extended field | 85 | 14.5 | |||
| RT to inguinal area | 12 | 2.0 | |||
| RT modality | |||||
| 3D-CRT | 302 | 51.5 | |||
| IMRT | 284 | 48.5 | |||
| Intracavitary irradiation | 630 | 73.4 | |||
| Applicator type | |||||
| Ovoid | 569 | 90.3 | |||
| Cylinder | 61 | 9.7 | |||
| Radiation dose (BED, Gy) | |||||
| Total pelvic dose | 72.0 | 3.3–74.2 | |||
| Inguinal dose | 72.0 | 66.7–72.0 | |||
| Use of chemotherapy | 989 | 38.6 | |||
| Sequential | 864 | 87.4 | |||
| Concurrent chemo-RT | 146 | 14.8 | |||
| Docetaxel-based | 505 | 51.1 | |||
| Hypertension | 581 | 22.7 | |||
| Diabetes mellitus | 323 | 12.6 | |||
| Congestive heart failure | 41 | 1.6 | |||
| Smoking history | 100 | 3.9 | |||
3D-CRT, 3-dimensional conformal radiotherapy; BED, biologically effective dose; BMI, body mass index; IMRT, intensity-modulated radiotherapy; IQR, interquartile range; LN, lymph node; RT, radiotherapy.
A total of 858 patients (33.5%) received adjuvant RT. Of the patients who received RT, 586 (68.3%) received EBRT, and 630 (73.4%) received ICR. Among the patients who received EBRT, the most common RT field was the whole pelvis (456/586 patients, 77.8%), followed by the extended field (85/586 patients, 14.5%) and semi-extended field (45/586 patients, 7.7%). Twelve patients received RT to the inguinal area, with a median total BED of 72.0 Gy (IQR, 66.7–72.0 Gy). Three-dimensional conformal RT and IMRT were administered to 302 and 284 patients, respectively. The total median pelvic BED was 72.0 Gy (IQR, 3.3–74.2 Gy).
Chemotherapy was administered to 146 patients concurrently with RT and to 864 patients sequentially after primary local treatment. A docetaxel-based regimen was administered to 505 patients.
3. LEL evaluation
During the follow-up, the patients were regularly evaluated by gynecologic and radiation oncologists once every 3 months for the first 2 years, every 6 months for 2 years, and once a year thereafter. Patients suspected of having LEL were referred to cancer rehabilitation specialists, and the presence of LEL was assessed through objective and subjective assessments, including patients’ perception of leg swelling and circumference measurement and lymphoscintigraphy [9]. Objective evaluation of LEL was mostly performed with comparative circumferential measurements comparing one limb to the other at 4 specific points (10 cm above and below the patella, 2 cm up to the medial malleolus, and metatarsophalangeal joint). Although it does not have a standard definition, LEL was diagnosed when there is a difference in circumference of 2–2.5 cm at various levels between the 2 legs. Severity assessment and staging were conducted using the International Society of Lymphology criteria [10].
To differentiate LEL from generalized edema in the acute phase related to cancer treatment or vascular complications, it was diagnosed when it persisted for ≥4 weeks without postoperative conditions, drugs that induce edema, or mechanical causes.
4. Statistical analysis
The cumulative incidence of LEL was calculated from the date of primary treatment to the date of the first diagnosis of LEL and was analyzed using the Kaplan-Meier method. Patients who experienced recurrence or died before the development of LEL were censored at the time of their recurrence or death. For nomogram construction and validation, two-thirds of the patients were randomly assigned to the training set (n=1,796) and one-third to the validation set (n=769). The baseline characteristics of the training and validation set patients were compared using the independent t-test for continuous data and Pearson χ2 test for categorical data. Univariate and multivariate Cox regression analyses were performed to identify the risk factors associated with the development of LEL. To determine the optimal combination of risk factors in multivariate analysis, multicollinearity was checked by the variance inflation factor, and the relative importance of each variable was identified through random survival forest. A nomogram was built based on the results of multivariable analyses using the concordance index (C-index). The nomogram was internally validated by measuring discrimination and calibration plots. Discrimination, measured by the Harrell C-index and Heagerty integrated area under the receiver operating characteristic curve (iAUC), was performed using the bootstrap method with 1,000 resamples in the validation set. Calibration plots for 2-, 3-, and 5-year LEL-free survival probabilities were obtained graphically by dividing the patients into quintiles according to their predicted LEL-free survival probabilities at each time point, and the actual probabilities for each group were subsequently plotted against the corresponding Kaplan-Meier estimates. A p-value <0.05 was considered statistically significant. All statistical analyses were performed using R software (version 4.0.3; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
1. Incidence and risk factors of LEL
The median age was 52 years (IQR, 44–60 years) at the time of the primary surgery. The median body mass index was 23.4 kg/m2 (IQR, 21.2–26.3 kg/m2). Of 2,565 patients, 1,120 (43.7%) and 1,445 (56.3%) were treated for CECs, respectively. The baseline patient characteristics are presented in Table 1.
The median follow-up time was 54.5 months (IQR, 27.0–97.3 months) among all patients. During the follow-up period, LEL developed in 331 patients, and the overall cumulative 3-and 5-year incidences of LEL were 11.0% and 13.3%, respectively. The median interval to the development of LEL was 10.1 months after the first date of primary treatment (IQR, 3.2–31.1 months). Twenty patients were diagnosed with LEL by lymphoscintigraphy. Among the patients with LEL, the stage of lymphedema was I in 28 (8.5%) patients, II in 286 (86.4%) patients, and III in 17 (5.1%) patients. There were 214 (64.7%), 104 (31.4%), and 13 (3.9%) patients with mild, moderate, and severe LEL, respectively. Ten patients underwent lymphovenous anastomosis for LEL, and the remaining patients were treated with rehabilitation or medication.
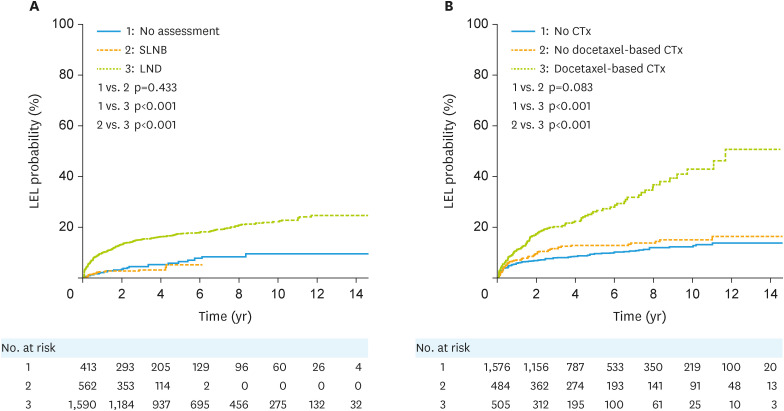
In the analysis of all patients, among the factors related to surgery, the risk of LEL was significantly higher in patients who underwent radical hysterectomy than in those who underwent modified radical hysterectomy or simple hysterectomy (p<0.001; Fig. S1). Patients who underwent SLNB for pelvic LNs had a significantly lower risk of developing LEL than those who underwent pelvic LN dissection (p<0.001; Fig. 1A), and there was no statistically significant difference from those who did not undergo surgical LN assessment for pelvic LN (p=0.433; Fig. 1A). A subgroup analysis of 509 patients with 1 to 5 pelvic LNs removed showed significantly lower risk of developing LEL in patients who underwent SLNB than those who underwent LN dissection (p=0.008; Fig. S2). The LEL risk increased as the number of removed LNs increased for pelvic, para-aortic, and inguinal LNs (Fig. S3).
Fig. 1. Cumulative probabilities of LEL according to the (A) type of surgical assessment of pelvic lymph nodes and (B) chemotherapy regimen in all patients.
CTx, chemotherapy; LEL, lymphedema of the lower extremity; LND, lymph node dissection; SLNB, sentinel lymph node biopsy.
Among the factors related to chemotherapy in all patients, the risk of LEL was significantly higher in patients receiving docetaxel-based chemotherapy than in those receiving other chemotherapy or those not receiving chemotherapy (p<0.001; Fig. 1B).
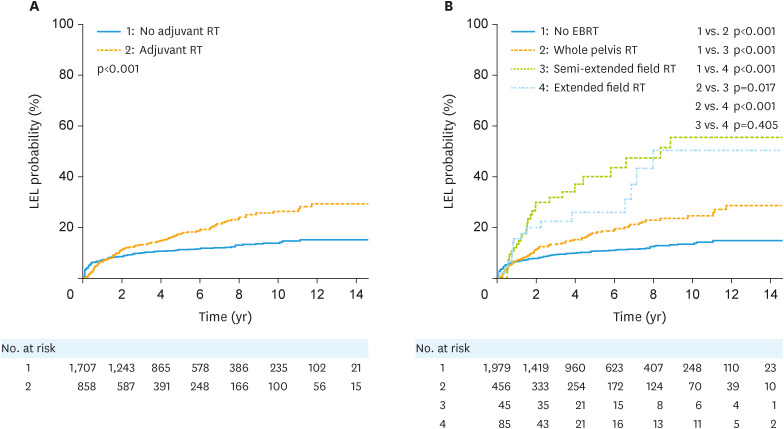
Regarding RT, the incidence of LEL was significantly higher in patients receiving RT than those not receiving RT among all patients (p<0.001; Fig. 2A). Patients who received RT for para-aortic LNs had a higher risk of developing LEL than those who received whole pelvic irradiation (p=0.017 and p<0.001, respectively; Fig. 2B). The risk of LEL was greater in patients with a higher total pelvic dose than in those with a lower total pelvic dose (p<0.001). There was no statistically significant difference in the risk of LEL with or without ICR (p=0.568; Fig. S4).
Fig. 2. Cumulative probabilities of LEL according to (A) whether adjuvant RT was administered or not and (B) the EBRT field in all patients.
EBRT, external beam radiotherapy; LEL, lymphedema of the lower extremity; RT, radiotherapy.
Of the 344 patients with LN metastasis at the time of diagnosis, all but eighteen (94.8%) had pelvic LN metastasis, and the median number of metastatic LNs was 3. Para-aortic LN metastasis and inguinal LN metastasis were observed in 96 (27.9%) and 12 (3.5%) patients, respectively (Table S1). In patients with LN metastasis at the time of diagnosis, there was no statistical difference in the incidence of LEL according to location of LN metastasis.
The baseline characteristics of the 1,796 patients in the training set and the 769 patients in the validation set are presented in Table S2. There was no significant difference in the patient and treatment characteristics between the 2 groups. In the univariate analysis performed on the training data, older age at primary treatment; use of docetaxel-based chemotherapy; radical hysterectomy; LN dissection (vs. SLNB); greater number of removed or metastatic pelvic, para-aortic, and inguinal LNs; larger EBRT field; and higher total pelvic dose were significantly associated with the risk of LEL (Table 2). Multivariate analysis of these factors was performed on the training data. The total pelvic dose was excluded from the analysis owing to multicollinearity with the EBRT field and relatively low importance as a result of random survival forest (Fig. S5). The number of metastatic LNs at each location were also excluded from the analysis owing to multicollinearity with the number of removed LNs at each location and relatively low importance as results of random survival forest, respectively (Fig. S5). Age at primary treatment, use of docetaxel-based chemotherapy, type of hysterectomy, type of surgical pelvic LN assessment, number of dissected pelvic and para-aortic LNs, and EBRT field were found to be independent predictors of LEL (Table 2).
Table 2. Results of univariate and multivariate Cox regression analyses of the training data for predicting the development of lower extremity lymphedema.
| Characteristics | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (yr)* | 1.01 (1.00–1.02) | 0.028 | 1.05 (1.00–1.03) | 0.016 | |
| Body mass index (kg/m2)* | 1.01 (0.98–1.05) | 0.402 | |||
| Smoking history (No vs. Yes) | 0.72 (0.32–1.62) | 0.429 | |||
| Hypertension (No vs. Yes) | 1.19 (0.89–1.59) | 0.250 | |||
| Diabetes mellitus (No vs. Yes) | 1.18 (0.82–1.72) | 0.377 | |||
| Congestive heart failure (No vs. Yes) | 1.74 (0.82–3.69) | 0.150 | |||
| Type of malignancy (Cervical vs. Endometrial) | 1.17 (0.90–1.52) | 0.254 | |||
| Docetaxel-based CTx (No vs. Yes) | 2.76 (2.10–3.62) | <0.001 | 1.77 (1.30–2.42) | <0.001 | |
| Concurrent CTx with RT (No vs. Yes) | 1.42 (0.89–2.27) | 0.143 | |||
| Type of hysterectomy (No radical vs. Radical) | 1.45 (1.11–1.89) | 0.007 | 1.45 (1.08–1.93) | 0.012 | |
| Surgical pelvic LN assessment | |||||
| Sentinel LN biopsy vs. LN dissection | 5.15 (2.80–9.49) | <0.001 | 2.85 (1.51–5.38) | 0.001 | |
| Sentinel LN biopsy vs. No surgical assessment | 1.95 (0.92–4.13) | 0.082 | 2.36 (1.10–5.04) | 0.027 | |
| No. of removed pelvic LNs* | 1.05 (1.04–1.06) | <0.001 | 1.02 (1.01–1.04) | 0.002 | |
| No. of removed para-aortic LNs* | 1.06 (1.05–1.08) | <0.001 | 1.04 (1.02–1.05) | <0.001 | |
| No. of removed inguinal LNs* | 1.12 (1.08–1.17) | <0.001 | 1.03 (0.98–1.08) | 0.338 | |
| No. of metastatic pelvic LNs* | 1.05 (1.03–1.06) | <0.001 | |||
| No. of metastatic para-aortic LNs* | 1.06 (1.06–1.07) | <0.001 | |||
| No. of metastatic inguinal LNs* | 1.09 (1.08–1.12) | <0.001 | |||
| EBRT field | |||||
| No EBRT vs. Whole pelvis RT | 1.42 (1.03–1.96) | 0.036 | 1.02 (0.73–1.42) | 0.924 | |
| No EBRT vs. Semi-extended field RT | 3.77 (2.09–6.80) | <0.001 | 2.32 (1.26–4.27) | 0.007 | |
| No EBRT vs. Extended field RT | 3.95 (2.49–6.24) | <0.001 | 1.82 (1.09–3.04) | 0.021 | |
| Total pelvic dose (BED, Gy)* | 1.01 (1.01–1.01) | <0.001 | |||
| Inguinal RT dose (BED, Gy)* | 1.02 (1.00–1.04) | 0.123 | |||
| Intracavitary RT (No vs. Yes) | 1.07 (0.79–1.46) | 0.659 | |||
BED, biologically effective dose; CI, confidence interval; CTx, chemotherapy; EBRT, external beam radiotherapy; HR, hazard ratio; LN, lymph node; RT, radiotherapy.
*Age; body mass index; number of removed or metastatic pelvic, para-aortic, and inguinal LNs; total pelvic dose; and inguinal RT dose were treated as continuous variables.
2. Nomogram for predicting LEL
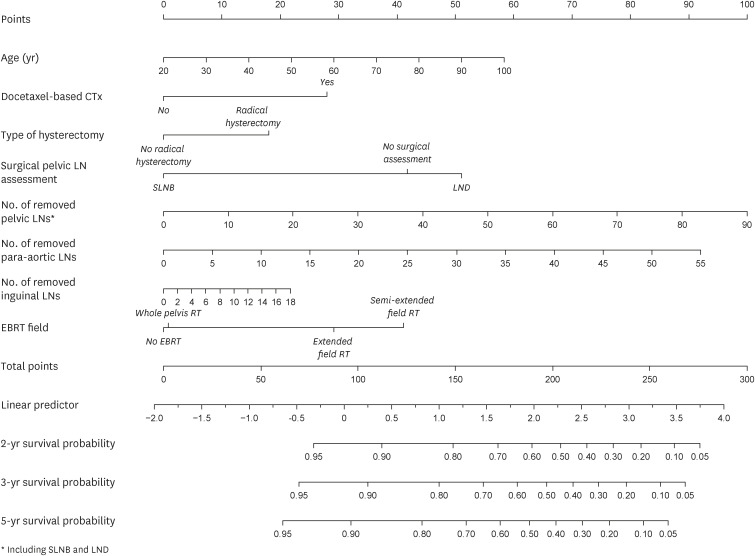
A nomogram to estimate the 2-, 3-, and 5-year survival probability of LEL was constructed using the 8 predictive factors used in the multivariate analysis that were derived from the training data (1,796 patients; Fig. 3). In the training data, the C-index and iAUC for the entire study period were 0.730 and 0.704, respectively (Table S3).
Fig. 3. Nomogram for predicting the 2-, 3-, and 5-year survival probability of LEL.
CTx, chemotherapy; EBRT, external beam radiotherapy; LEL, lymphedema of the lower extremity; LN, lymph node; LND, lymph node dissection; RT, radiotherapy; SLNB, sentinel lymph node biopsy.
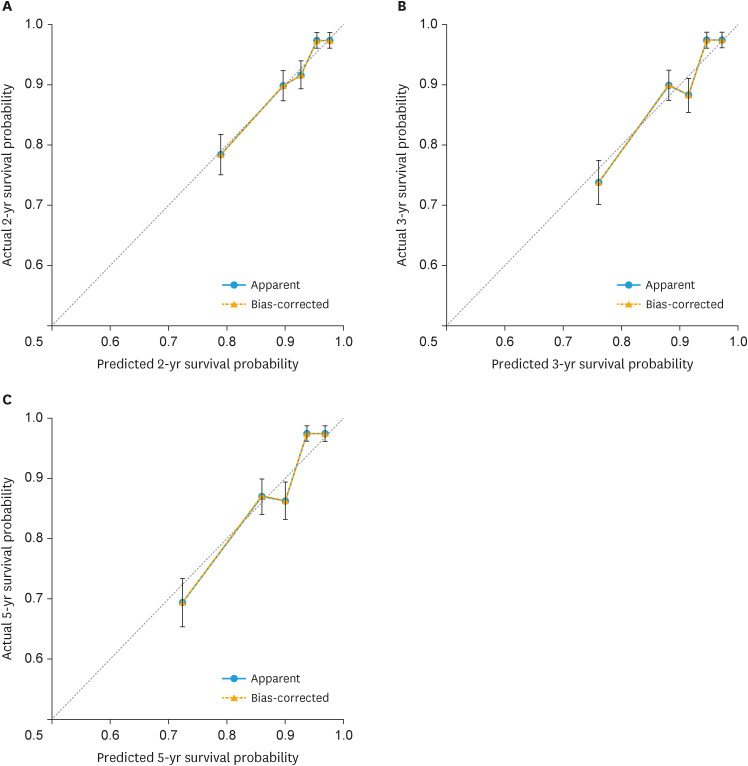
For internal validation, discrimination and calibration were performed on 769 patients using the test data. The C-index for the entire period and at 2, 3, and 5 years were 0.714 (95% confidence interval [CI]=0.667–0.762), 0.710 (95% CI=0.654–0.769), 0.713 (95% CI=0.660–0.767), and 0.713 (95% CI=0.662–0.764), respectively. The iAUC for the entire period and at 2, 3, and 5 years were 0.695 (95% CI=0.658–0.729), 0.705 (95% CI=0.655–0.753), 0.709 (95% CI=0.662–0.754), and 0.704 (95% CI=0.661–0.743), respectively (Table S4). Calibration plots (in quintiles) of the nomogram for the test data at 2, 3, and 5 years revealed that the observed and predicted rates were in fair agreement (Fig. 4).
Fig. 4. Calibration plots in quintiles of the nomogram at (A) 2 years, (B) 3 years, and (C) 5 years for the test data.
DISCUSSION
This study showed that the cumulative 3- and 5-year incidences of LEL for all patients who received primary treatment for newly diagnosed CEC were 11.0% and 13.3%, respectively. Based on the analysis of risk factors for LEL after CEC treatment in a cohort of >2,000 patients, age at primary treatment, use of docetaxel-based chemotherapy, type of hysterectomy, type of surgical pelvic LN assessment, number of dissected pelvic and para-aortic LNs, and EBRT fields were found to be important predictors. Using these predictors, we developed a nomogram to predict individual risk of LEL. Internal validation showed that the nomogram had excellent predictive power for LEL.
Our study focused on the association between the combination of surgery, RT, and LEL in CEC treatments. Recently, several therapeutic approaches have been proposed to reduce treatment-related toxicity in gynecological cancers. Surgeons perform SLNB instead of LN dissection to reduce treatment-related toxicities while preserving oncological outcomes. As the number of patients in our study who received SLNB was greater than a quarter of the total, the number of removed LNs was relatively small compared to the numbers in other institutions; this may have contributed to the low incidence of LEL. More precise and accurate IMRT treatment reduced unnecessary radiation exposure to the surrounding normal tissues [11] (Fig. S6). Combining these 2 novel treatments, such as SLNB and pelvic IMRT, rather than conventional radical LN dissection and/or adjuvant RT can dramatically reduce treatment-related toxicities, including LEL.
In our study, more than half of the important predictors of LEL were surgery-related factors (the type of hysterectomy, type of surgical pelvic LN assessment, and number of dissected pelvic and para-aortic LNs). Among surgical factors, LN dissection is the most well-known of the factors related to LEL, and both the number of LN dissections and the location of resected LNs are related to LEL [6,12,13]. Radical hysterectomy was also found to be an important predictor of LEL, as analyzed in our study. Although there are a few related studies, radical hysterectomy is more likely to cause LEL than simple hysterectomy because of the wider excision [14].
Several studies have shown that RT increases LEL levels after gynecological cancer treatment. Although both are part of RT, EBRT and ICR differ significantly in the incidence of LEL; EBRT showed a higher incidence rate than ICR [7]. The reason for this difference is that in the case of ICR, radiation only affects the vicinity of the applicator, reducing the radiation dose to nearby organs or LNs in the pelvis.
The relationship between the EBRT field and LEL in gynecological cancers was analyzed for the first time in our study. LEL was more developed when para-aortic areas were included, which was likely related to the relationship between para-aortic LN removal and LEL. It seems that extended/semi-extended field RT had a more extensive effect on lymphatic drainage than whole pelvic irradiation, from both lower extremities to the retroperitoneal area, and thus had a greater effect on the incidence of LEL. RT to the inguinal area was also expected to increase LEL by affecting lymphatic drainage, but this was not found to be a significant factor in our study. The presumed reason is that only 12 patients underwent RT in the inguinal area; hence, it is thought that it was difficult to obtain statistical results.
The relationship between chemotherapy and LEL in gynecological cancers has not been clearly established. In our study, docetaxel-based chemotherapy was an independent predictor of LEL. A recent study showed that the use of docetaxel-based chemotherapy significantly increased lymphedema in breast cancer [15]. It is thought that docetaxel-based chemotherapy increased LEL by the same mechanism as in breast cancer [16]. Similar to gene expression testing in breast cancer [17], an innovative methodology is required to avoid unnecessary chemotherapy to reduce treatment-related toxicities, such as LEL, in gynecological cancer.
To our knowledge, this is the first study to develop a nomogram to predict LEL in gynecological cancer. Using our nomogram, clinicians could assess the risk of developing LEL. Close and meticulous observation can help prevent LEL in high-risk patients.
Our study has some limitations owing to its retrospective nature. First, the incidence of LEL might have been underestimated based on the medical records. Second, SLNB was introduced during the study period, so patients who received SLNB may have had less LEL owing to a short follow-up period. Third, there is a possibility of statistical inaccuracy due to the relatively small number of patients who received extended (45/2,565 patients, 1.8%) or semi-extended field RT (85/2,565 patients, 3.3%). For this reason, in our study results, the semi-extended field RT scored larger than the extended field RT in the nomogram. Finally, because the predictive power evaluation of the nomogram was performed through internal validation, the patient characteristics of the training and test sets were similar, which may have resulted in a high predictive rate. To compensate for these limitations, our nomogram should be validated in different institutions where treatment schemes and the criteria for diagnosing LEL may differ. Despite these limitations, our study analyzed the largest number of patients among the studies on LEL in gynecological cancer, and the nomogram was developed and tested using the largest number of patients.
In conclusion, LEL is associated with various treatment modalities, and interactions among modalities might increase the possibility of development. De-escalation strategies should be considered to reduce LEL in CEC patients, such as the application of SLNB, development of genomic tests, and individualized use of advanced RT.
Footnotes
Presentation: This study was presented at the Annual meeting of the Korean Society of Radiation Oncology, Republic of Korea, 2021.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: K.Y.B.
- Data curation: L.J., B.H.K., K.Y.B.
- Formal analysis: L.J., B.H.K., S.W.J., R.Y.H.
- Investigation: B.H.K., I.S.H., S.W.J., R.Y.H., K.Y.B.
- Methodology: K.Y.B.
- Project administration: K.Y.B.
- Resources: L.J., B.H.K., I.S.H.
- Software: L.J.
- Supervision: I.S.H., K.Y.B.
- Validation: L.J., B.H.K., I.S.H., S.W.J., R.Y.H.
- Visualization: L.J., R.Y.H.
- Writing - original draft: L.J.
- Writing - review & editing: K.Y.B.
SUPPLEMENTARY MATERIALS
Location and number of lymph node metastases in patients with lymph node metastases at the time of diagnosis
Comparison of baseline characteristics of patients between the training and validation set
Predictive performance of the model in training data
Predictive performance of the model in test data
Cumulative probabilities of LEL according to the type of hysterectomy.
Cumulative probabilities of LEL according to the type of surgical assessment of pelvic LNs in patients with 1 to 5 pelvic LNs removed.
Cumulative probabilities of LEL according to number of removed (A) pelvic, (B) para-aortic, and (C) inguinal LNs.
Cumulative probabilities of LEL according to whether intracavitary radiotherapy was administered or not.
Variable importance resulted by random survival forest of the training data.
Comparison of isodose line and normal organ irradiation when irradiating pelvic lymph node in (A) 3-dimensional conformal radiotherapy and (B) IMRT. Bold lines correspond to the following normal organs: Yellow, small and large bowel; Blue, bladder; Yellow-green, cauda equina. Bold red line indicates the isodose line of 95% of the prescription dose. IMRT results in lesser irradiation to the small bowel.
References
- 1.Marth C, Landoni F, Mahner S, McCormack M, Gonzalez-Martin A, Colombo N, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv72–iv83. doi: 10.1093/annonc/mdx220. [DOI] [PubMed] [Google Scholar]
- 2.Yost KJ, Cheville AL, Al-Hilli MM, Mariani A, Barrette BA, McGree ME, et al. Lymphedema after surgery for endometrial cancer: prevalence, risk factors, and quality of life. Obstet Gynecol. 2014;124:307–315. doi: 10.1097/AOG.0000000000000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim JH, Choi JH, Ki EY, Lee SJ, Yoon JH, Lee KH, et al. Incidence and risk factors of lower-extremity lymphedema after radical surgery with or without adjuvant radiotherapy in patients with FIGO stage I to stage IIA cervical cancer. Int J Gynecol Cancer. 2012;22:686–691. doi: 10.1097/IGC.0b013e3182466950. [DOI] [PubMed] [Google Scholar]
- 4.Füller J, Guderian D, Köhler C, Schneider A, Wendt TG. Lymph edema of the lower extremities after lymphadenectomy and radiotherapy for cervical cancer. Strahlenther Onkol. 2008;184:206–211. doi: 10.1007/s00066-008-1728-3. [DOI] [PubMed] [Google Scholar]
- 5.Mitra D, Catalano PJ, Cimbak N, Damato AL, Muto MG, Viswanathan AN. The risk of lymphedema after postoperative radiation therapy in endometrial cancer. J Gynecol Oncol. 2016;27:e4. doi: 10.3802/jgo.2016.27.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Todo Y, Yamamoto R, Minobe S, Suzuki Y, Takeshi U, Nakatani M, et al. Risk factors for postoperative lower-extremity lymphedema in endometrial cancer survivors who had treatment including lymphadenectomy. Gynecol Oncol. 2010;119:60–64. doi: 10.1016/j.ygyno.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Chang WI, Kang HC, Wu HG, Kim HJ, Jeon SH, Lee M, et al. Lower extremity lymphedema in gynecologic cancer patients: propensity score matching analysis of external beam radiation versus brachytherapy. Cancers (Basel) 2019;11:1471. doi: 10.3390/cancers11101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frumovitz M, Plante M, Lee PS, Sandadi S, Lilja JF, Escobar PF, et al. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): a randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018;19:1394–1403. doi: 10.1016/S1470-2045(18)30448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho KH, Han EY, Lee SA, Park H, Lee C, Im SH. Feasibility of bioimpedance analysis to assess the outcome of complex decongestive therapy in cancer treatment-related lymphedema. Front Oncol. 2020;10:111. doi: 10.3389/fonc.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013;46:1–11. [PubMed] [Google Scholar]
- 11.Klopp AH, Yeung AR, Deshmukh S, Gil KM, Wenzel L, Westin SN, et al. Patient-reported toxicity during pelvic intensity-modulated radiation therapy: NRG Oncology-RTOG 1203. J Clin Oncol. 2018;36:2538–2544. doi: 10.1200/JCO.2017.77.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beesley VL, Rowlands IJ, Hayes SC, Janda M, O’Rourke P, Marquart L, et al. Incidence, risk factors and estimates of a woman’s risk of developing secondary lower limb lymphedema and lymphedema-specific supportive care needs in women treated for endometrial cancer. Gynecol Oncol. 2015;136:87–93. doi: 10.1016/j.ygyno.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Kunitake T, Kakuma T, Ushijima K. Risk factors for lower limb lymphedema in gynecologic cancer patients after initial treatment. Int J Clin Oncol. 2020;25:963–971. doi: 10.1007/s10147-019-01608-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung SG, Im SH, Kim M, Choi MC, Joo WD, Song SH, et al. The association between the number of retrieved pelvic lymph nodes and ipsilateral lower limb lymphedema in patients with gynecologic cancer. J Invest Surg. 2022;35:978–983. doi: 10.1080/08941939.2021.1980160. [DOI] [PubMed] [Google Scholar]
- 15.Byun HK, Chang JS, Im SH, Kirova YM, Arsene-Henry A, Choi SH, et al. Risk of lymphedema following contemporary treatment for breast cancer: an analysis of 7617 consecutive patients from a multidisciplinary perspective. Ann Surg. 2021;274:170–178. doi: 10.1097/SLA.0000000000003491. [DOI] [PubMed] [Google Scholar]
- 16.Guastalla JP, 3rd, Diéras V. The taxanes: toxicity and quality of life considerations in advanced ovarian cancer. Br J Cancer. 2003;89(Suppl 3):S16–S22. doi: 10.1038/sj.bjc.6601496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Location and number of lymph node metastases in patients with lymph node metastases at the time of diagnosis
Comparison of baseline characteristics of patients between the training and validation set
Predictive performance of the model in training data
Predictive performance of the model in test data
Cumulative probabilities of LEL according to the type of hysterectomy.
Cumulative probabilities of LEL according to the type of surgical assessment of pelvic LNs in patients with 1 to 5 pelvic LNs removed.
Cumulative probabilities of LEL according to number of removed (A) pelvic, (B) para-aortic, and (C) inguinal LNs.
Cumulative probabilities of LEL according to whether intracavitary radiotherapy was administered or not.
Variable importance resulted by random survival forest of the training data.
Comparison of isodose line and normal organ irradiation when irradiating pelvic lymph node in (A) 3-dimensional conformal radiotherapy and (B) IMRT. Bold lines correspond to the following normal organs: Yellow, small and large bowel; Blue, bladder; Yellow-green, cauda equina. Bold red line indicates the isodose line of 95% of the prescription dose. IMRT results in lesser irradiation to the small bowel.