Abstract
Endometrial cancer (EC) is the most common gynecological malignancy in developed countries. The present study aimed to determine the frequency of germline pathogenic variants (PV) in patients with EC. In this multicenter retrospective cohort study, germline genetic testing (GGT) was performed in 527 patients with EC using a next generation sequencing panel targeting 226 genes, including 5 Lynch syndrome (LS) and 14 hereditary breast and ovarian cancer (HBOC) predisposition genes, and 207 candidate predisposition genes. Gene-level risks were calculated using 1,662 population-matched controls (PMCs). Patients were sub-categorized to fulfill GGT criteria for LS, HBOC, both or none. A total of 60 patients (11.4%) carried PV in LS (5.1%) and HBOC (6.6%) predisposition genes, including two carriers of double PV. PV in LS genes conferred a significantly higher EC risk [odds ratio (OR), 22.4; 95% CI, 7.8-64.3; P=1.8×10−17] than the most frequently altered HBOC genes BRCA1 (OR, 3.9; 95% CI, 1.6-9.5; P=0.001), BRCA2 (OR, 7.4; 95% CI, 1.9-28.9; P=0.002) and CHEK2 (OR, 3.2; 95% CI, 1.0-9.9; P=0.04). Furthermore, >6% of patients with EC not fulfilling LS or HBOC GGT indication criteria carried a PV in a clinically relevant gene. Carriers of PV in LS genes had a significantly lower age of EC onset than non-carriers (P=0.01). Another 11.0% of patients carried PV in a candidate gene (the most frequent were FANCA and MUTYH); however, their individual frequencies did not differ from PMCs (except for aggregated frequency of loss-of-function variants in POLE/POLD1; OR, 10.44; 95% CI, 1.1-100.5; P=0.012). The present study demonstrated the importance of GGT in patients with EC. The increased risk of EC of PV carriers in HBOC genes suggests that the diagnosis of EC should be included in the HBOC GGT criteria.
Keywords: uterine malignancies, EC, multigene panel testing, germline mutations
Introduction
Endometrial cancer (EC) is the most common gynecological malignancy in the developed countries (1). Its rate of incidence per 100,000 people in Europe was 32 and in the Czech Republic was 39 in the year 2020 (https://ecis.jrc.ec.europa.eu/). Most EC cases are diagnosed post-menopausally (with a peak incidence between 65–69 years) and in early stages with relatively favorable prognosis (2). EC mortality is approximately four times lower than EC incidence (<20%; www.svod.cz). However, the mortality may vary based on geography and race (3).
Many non-genetic factors modify EC risk. While excess of endogenous estrogens, obesity, insulin resistance, and tamoxifen use increase EC risk, oral contraceptives and sufficient physical activity have protective effects (4).
The risk of EC development is also affected by genetic factors. Germline pathogenic variants (PV) in known EC-predisposition genes are considered the most clinically important [reviewed in (5)]. Germline variants in EC patients were studied by several next generation sequencing (NGS) based studies, dominantly using limited gene panels (21–84 genes) (6–15). These studies reported variable prevalence of germline variants in EC patients ranging from 4.5 to 23%. Majority of hereditary EC cases are associated with Lynch syndrome (LS; also known as hereditary nonpolyposis colorectal cancer), which is caused by germline PV in mismatch repair genes (MMR; MLH1, MSH2, MSH6, PMS2, and structural alterations of 3′ end of EPCAM) (16). Guidelines for clinical follow-up of carriers of germline PV in LS genes include specific management of increased EC risk. Modest increase of EC risk has been suggested in BRCA1 and BRCA2 PV carriers (most notably the serous-like EC subtype), and other hereditary breast and ovarian cancer genes (HBOC; ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, NF1, PALB2, PTEN, RAD51C, RAD51D, STK11, TP53) (17). Other noteworthy candidate EC-predisposition genes include e.g. POLD1 and POLE (18). Germline missense PV affecting proofreading capabilities of POLE/POLD1 are associated with increased EC risk as a part of polymerase proofreading-associated polyposis, but the importance of germline POLD1/POLE truncating variants remains rather elusive (18). Importantly, the genetic basis of most EC cases has not been explained yet as the diagnosis itself is not a criterion for germline genetic testing unless fulfilling LS criteria (5).
We aimed to evaluate germline genetic background of 527 patients with uterine tumors to identify genes associated with EC risk in our population, and to evaluate clinicopathological features in germline PV carriers.
Materials and methods
Patients
For this retrospective cohort study, we collected 527 patients with uterine malignancies diagnosed at nine Czech health care centers (General University Hospital in Prague, Masaryk Memorial Cancer Institute, AGEL Laboratories, Gennet, GHC Genetics, University Hospital Pilsen, Pronatal, Palacky University Olomouc) and the Bank of Clinical Samples (First Faculty of Medicine). The full list of all participating institutions is provided in the Table SI. Patients were enrolled between 2011–2021 and were Caucasians of the Czech origin. The clinicopathological characteristics (Table I) revealed that endometrial cancers (EC; 89.7%) were the dominant type of collected uterine malignances, therefore the whole cohort of patients with uterine malignancies will be hereafter referred to as ‘EC patients’. Deficient MMR, microsatellite instability and MLH1 hypermethylation statuses were not available. We divided patients according to national indication criteria for germline genetic testing of LS and/or HBOC patients:
Table I.
Clinicopathological characteristics of 527 patients with EC.
| Variables | All patients with EC (N=527) | LS only (N=151) | HBOC only (N=16) | LS + HBOC (N=82) | Non-indicated (N=278) |
|---|---|---|---|---|---|
| Age at EC diagnosis | |||||
| Mean, years | 59.1 | 50.8 | 59.0 | 51.3 | 65.8 |
| Median, years | 60.5 | 47.8 | 57.0 | 49.0 | 65.3 |
| Range, years | 24-92 | 24-91 | 51-73 | 29-82 | 50-92 |
| <50 years, n (%) | 120 (23.2) | 79 (53.4) | 0 | 41 (51.3) | 0 |
| ≥50 years, n (%) | 397 (76.8) | 69 (46.6) | 15 (100.0) | 39 (48.8) | 274 (100.0) |
| N.A., n | 10 | 3 | 1 | 2 | 4 |
| Histology of uterine malignances, n (%) | |||||
| Endometrial carcinoma | 349 (89.7) | 76 (85.4) | 8 (72.7) | 48 (100.0) | 217 (90.0) |
| Endometrioid adenocarcinoma | 284 (73.0) | 65 (73.0) | 7 (63.6) | 44 (91.7) | 168 (69.7) |
| Serous | 35 (9.0) | 4 (4.5) | 1 (9.1) | 3 (6.3) | 27 (11.2) |
| Clear cell | 7 (1.8) | 2 (2.2) | 0 | 0 | 5 (2.1) |
| Undifferentiated | 3 (0.8) | 0 | 0 | 0 | 3 (1.2) |
| Mixed (endometroid/serous) | 3 (0.8) | 0 | 0 | 0 | 3 (1.2) |
| Mixed (endometroid/serous/clear cell) | 1 (0.3) | 1 (1.1) | 0 | 0 | 0 |
| Mixed (endometroid/clear cell) | 4 (1.0) | 3 (3.4) | 0 | 0 | 1 (0.4) |
| EIN | 8 (2.1) | 1 (1.1) | 0 | 1 (2.1) | 6 (2.5) |
| Unspecified | 4 (1.0) | 0 | 0 | 0 | 4 (1.7) |
| Sarcoma | 40 (10.3) | 13 (14.6) | 3 (27.3) | 0 | 24 (10.0) |
| Leiomyosarcoma | 32 (8.2) | 9 (10.1) | 2 (18.2) | 0 | 21 (8.7) |
| Undifferentiated | 2 (0.5) | 0 | 0 | 0 | 2 (0.8) |
| Endometrial stromal sarcoma | 3 (0.8) | 2 (2.2) | 0 | 0 | 1 (0.4) |
| Unspecified | 3 (0.8) | 2 (2.2) | 1 (9.1) | 0 | 0 |
| Unknown malignant tumor of corpus uteri | 138 | 62 | 5 | 34 | 37 |
| FIGO grade, n (%) | |||||
| 1 | 123 (35.9) | 35 (48.6) | 4 (40.0) | 16 (45.7) | 68 (30.1) |
| 2 | 100 (29.2) | 15 (20.8) | 3 (30.0) | 12 (34.3) | 70 (31.0) |
| 3 | 120 (35.0) | 22 (30.6) | 3 (30.0) | 7 (20.0) | 88 (38.9) |
| N.A. | 184 | 79 | 6 | 47 | 52 |
| FIGO stage, n (%) | |||||
| 0 | 8 (2.8) | 1 (2.1) | 0 | 1 (4.2) | 6 (2.8) |
| I | 176 (60.9) | 33 (68.8) | 4 (66.7) | 17 (70.8) | 122 (57.8) |
| II | 38 (13.1) | 5 (10.4) | 1 (16.7) | 2 (8.3) | 30 (14.2) |
| III | 48 (16.6) | 8 (16.7) | 1 (16.7) | 2 (8.3) | 37 (17.5) |
| IV | 19 (6.6) | 1 (2.1) | 0 | 2 (8.3) | 16 (7.6) |
| N.A. | 238 | 103 | 10 | 58 | 67 |
| Multiple primary tumors in personal history, n (%) | |||||
| Present | 214 (40.6) | 69 (45.7) | 16 (100.0) | 82 (100.0) | 47 (16.9) |
| Absent | 313 (59.4) | 82 (54.3) | 0 | 0 | 231 (83.1) |
| Multiple primary tumors in personal history, n (%) | |||||
| CRC | 31 (5.9) | 31 (20.5) | 0 | 0 | 0 |
| OC | 59 (11.2) | 0 | 1 (6.3) | 58 (70.7) | 0 |
| BC | 80 (15.2) | 14 (9.3) | 15 (93.8) | 13 (15.9) | 38 (13.7) |
| Triple primary EC+(BC/OC/CRC) | 13 (2.5) | 2 (1.3) | 0 | 11 (13.4) | 0 |
| Other | 31 (5.9) | 22 (14.6) | 0 | 0 | 9 (3.2) |
| None | 313 (59.4) | 82 (54.3) | 0 | 0 | 231 (83.1) |
| Family cancer history (first/second degree), n (%) | |||||
| Positive | 353 (69.8) | 120 (81.6) | 13 (100.0) | 56 (73.7) | 164 (60.7) |
| Negative | 153 (30.2) | 27 (18.4) | 0 | 20 (26.3) | 106 (39.3) |
| Unknown | 21 | 4 | 3 | 6 | 8 |
| Tumors in family history, n (%) | |||||
| EC | 35 (6.9) | 14 (9.5) | 1 (7.7) | 6 (7.9) | 14 (5.2) |
| CRC | 88 (17.4) | 39 (26.5) | 4 (30.8) | 15 (19.7) | 30 (11.1) |
| OC | 15 (3.0) | 7 (4.8) | 1 (7.7) | 5 (6.6) | 2 (0.7) |
| BC | 60 (11.9) | 14 (9.5) | 3 (23.1) | 9 (11.8) | 34 (12.6) |
| Multiple (EC/OC/CRC) | 10 (2.0) | 10 (6.8) | 0 | 0 | 0 |
| Other | 145 (28.7) | 36 (24.5) | 4 (30.8) | 21 (27.6) | 84 (31.1) |
| None | 153 (30.2) | 27 (18.4) | 0 | 20 (26.3) | 106 (39.3) |
| Unknown | 21 | 4 | 3 | 6 | 8 |
Percentages were calculated from the overall number of patients with known characteristics. BC, breast cancer; CRC, colorectal cancer; EC, endometrial cancer; EIN, endometrial intraepithelial neoplasia; FIGO, The International Federation of Gynecology and Obstetrics; HBOC, hereditary breast and ovarian cancer; LS, Lynch syndrome; N, number; N.A., not available; OC, ovarian cancer.
Breast cancer or ovarian cancer (C50/C56)-national indication criteria for germline genetic testing [HBOC criteria; cancer diagnoses (C##) correspond to the International Classification of Diseases 10; available at https://icd.who.int/browse10/2019/en#/C00-C97]. Personal history: i) patient is diagnosed with C50 <45 years or <50 years, if family history is unknown; ii) patient has bilateral C50 with the age of diagnosis of the first one <50 years and of both <60 years; iii) patient is diagnosed with triple negative C50 ≤60 years; iv) patient is a male diagnosed with C50; v) patient is diagnosed with either C56, C57 or C48; vi) patient has a duplicity od C50 and C25 regardless of age. Family history: i) patient and two relatives are diagnosed with C50; ii) patient and one relative are diagnosed with C50 <50 years or both C50 <60 years (patient included); iii) patient and a direct relative (parent, sibling, child, alternatively mother or father's sister) are diagnosed with either ovarian cancer, fallopian tube or primary peritoneal tumor, triple negative C50/medullar C50, male relative diagnosed with C50, pancreatic cancer, prostate cancer with Gleason score ≥7 or primary metastatic C61.
Colorectal cancer or EC-national indication criteria for germline genetic testing (LS criteria): i) Age of diagnosis <50 years; ii) proven microsatellite instability <60 years; iii) patient has a concurrent diagnosis linked to LS (colorectal cancer, stomach cancer, pancreatic cancer, ovarian cancer, small intestine cancer, ureter cancer, renal pelvis cancer, bile tract cancer, glioblastoma); iv) patient and one first degree relative have diagnoses linked to LS <50 years; v) patient and two second degree relatives have diagnoses linked to LS regardless of the age of diagnosis; and vi) patients with colorectal cancer and more than ten adenomas/polyps.
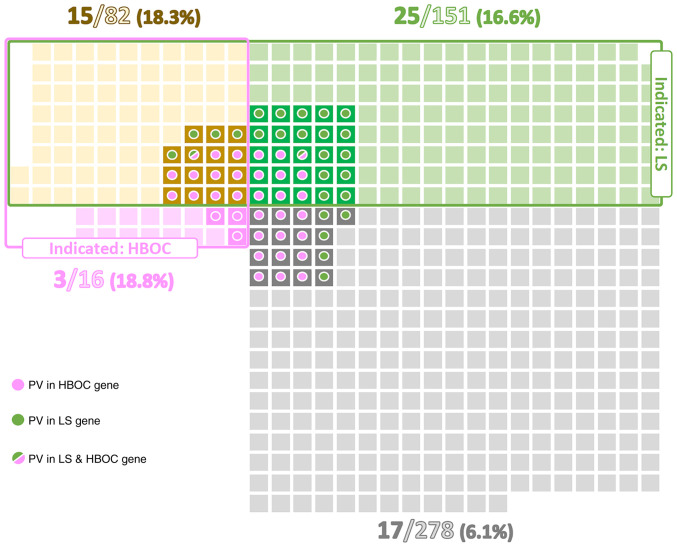
Of all patients 151/527 (28.7%) met only LS genetic testing criteria, 16/527 (3.0%) met only HBOC criteria, and 82/527 (15.6%) met both these criteria. A total of 278/527 (52.7%) patients would not be indicated for germline genetic testing.
The study was approved by Ethics Committees of participating institutions. Written consent for the research analysis was obtained from all participants. Clinicopathological information was collected during genetic counselling or retrieved from patients' record.
Two sets of population-matched controls (PMC) were used for comparisons with analyzed EC patients. First, used as a reference for genetic variant prioritization, included 777 non-cancer volunteers aged >60 years that were analyzed identically with EC patients as described previously (19). Second group, used in case-control analyses, included 1662 PMC analyzed as described previously (20). Briefly, the unselected controls (1,170 males and 492 females; median age 57 years, range 18–88 years) were unrelated individuals analyzed by whole-exome sequencing by National Center for Medical Genomics (https://ncmg.cz/) for various noncancer conditions.
Genetic testing using panel NGS
Genomic DNA was isolated from peripheral blood collected at the time of enrollment in each respective center. DNA samples were analyzed by NGS using a custom-designed CZECANCA panel as described previously (21) with minor modifications reflecting recent technological updates. These modifications included a new probe synthesis HyperDesign (Roche) improving target coverage for all 226 genes (the sequence capture panel development is shown in detail on the panel web page: http://www.czecanca.cz/eng/panel.html, and full list of genes targeted in this project is described in Table SII). Further modifications included usage of cheaper and faster enzymatic fragmentation replacing ultrasound DNA fragmentation, preparation of DNA libraries using recently introduced KAPA HyperPlus Library Preparation kit (Roche; according to the manufacturer's instruction) and Illumina NextSeq500 sequencing. Resulting NGS data were processed by an in-house bioinformatics pipeline as we described previously (21). Briefly, SAM files were generated from FASTQ using NovoAlign v2.08.03 (http://www.novocraft.com/products/novoalign/) and transformed into BAM by Picard tools v1.129 (https://broadinstitute.github.io/picard/). The Genome Analysis Toolkit v3.8.1 (https://software.broadinstitute.org/gatk/) (22) was used to prepare variant-call format, annotated by SnpEff v4.3 (http://pcingola.github.io/SnpEff/). Identification of medium size indels was performed by Pindel v0.2.5a7 (http://gmt.genome.wustl.edu/packages/pindel/) and copy number variations (CNV) were detected using CNV kit v0.7.4 (https://pypi.python.org/pypi/CNVkit).
All 226 analyzed genes were divided into 19 known EC-predisposition genes described by NCCN guidelines or reviewed by Spurdle et al (5) and 207 other ‘candidate’ genes. Five genes associated with LS (MLH1, MSH2, MSH6, PMS2, EPCAM) and 14 genes associated with HBOC (ATM, BARD1, BRCA1, BRCA2, BRIP1, CDH1, CHEK2, NF1, PALB2, PTEN, RAD51C, RAD51D, STK11, TP53) were considered as the EC-predisposition genes. The remaining 207 candidate cancer-susceptibility genes included those that have been episodically associated with EC predisposition (incl. APC, MUTYH, NBN, POLD1, POLE; Table SII) (5).
Variant prioritization
Genetic variants found in patients were filtered, excluding variants: i) with low sequencing quality (q<150); ii) with a high minor allele frequency (MAF >0.001) in population databases (gnomAD https://gnomad.broadinstitute.org/, Exome Sequencing Project https://evs.gs.washington.edu/EVS/, 1000 Genomes Project http://www.internationalgenome.org/) (23–25) unless classified as pathogenic/likely pathogenic (P/LP) in the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/) (26); iii) present with frequency higher than 0.5% in a group of 777 PMC, except for variants with P/LP ClinVar classification; iv) in untranslated region, intronic outside of consensus splice sites, synonymous and insertion/deletions not resulting in a frameshift unless classified as pathogenic/likely pathogenic (P/LP) in the ClinVar database; v) classified as benign/likely benign in ClinVar with at least two-star rating; vi) low risk variants in BRCA2 (c.9976A>T; p.Lys3326Ter) and in CHEK2 (c.470T>C; p.Ile157Thr).
Resulting set of variants was evaluated according to the ACMG (American College of Medical Genetics) recommendations (27). Variants mentioned in ClinVar as a single submitter or with a conflicting interpretation of pathogenicity were categorized as variants of uncertain significance (VUS). Whole gene duplication and truncating variants localized in the last exon were considered VUS, unless they were classified as P/LP in ClinVar. All PV were inspected in Integrative Genomics Viewer or confirmed using Sanger sequencing or multiplex ligation-dependent probe amplification analysis (MRC Holland). Confirmed PV were submitted to ClinVar database.
Statistical analysis
The frequencies of PV in EC patients were compared with the frequencies of PV in a group of 1662 unselected PMC. Odds ratios (OR) with 95% confidence intervals (CI) were calculated for EC patients carrying found germline PV using 2×2 contingency table. The χ2 or Fisher's exact tests were used for the calculation of P-values (considered significant when P<0.05). Differences in age at diagnosis were analyzed by one-way ANOVA followed by Tukey-Kramer's test. Statistical analysis was performed using the R language v4.1.
Results
Germline PV in patients with uterine malignances
We performed germline genetic testing in 527 Czech EC patients including 249 individuals fulfilling LS, HBOC, LS + HBOC indication criteria and 278 individuals not fulfilling any criteria for germline genetic testing. Germline PV were significantly more frequent in patients (118/527; 22.4%) than in population-matched controls (290/1662; 17.4%; P=0.011).
Germline PV in EC-predisposition genes
PV were found in 12 (out of 19 tested) EC-predisposition genes (Table II). Frequency of these variants was more than four-times higher in EC patients (60/527; 11.4%; Table SIII) than in PMC (46/1662; 2.8%; P=9.7×10−16). PV in LS genes were found in 27/527 (5.1%) patients (half of them were MSH6 PV carriers) and in 4/1662 (0.25%) controls and they represented the strongest genetic risk factor for EC development (OR=22.4, P=1.8×10−17). Interestingly, no PMS2 PV were observed among patients. BRCA1, BRCA2 and CHEK2 were the most frequently mutated HBOC genes, their PVs conferred significantly increased EC risk for female carriers. However, this risk was lower in comparison to LS genes (ranging from high EC risk in BRCA2 to moderate EC risk in BRCA1 and CHEK2, respectively). PV in the remaining 11 HBOC genes were not identified or did not differ significantly from PMC (Table II). Two carriers harbored coincidental mutations in MLH1/BRCA1 and MSH2/ATM, respectively.
Table II.
Frequencies of germline PV in 19 ec-predisposition genes.
| Indication for germline genetic testing | All patients with EC vs. PMC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Gene group | Germline PV | LS, n (%) (N=151) | HBOC, n (%) (N=16) | LS+HBOC, n (%) (N=82) | Non-indicated, n (%) (N=278) | All patients with EC, n (%) (N=527) | PMC, n (%) (N=1662) |
|
|
| OR (95% CI) | P-value | ||||||||
| LS | MLH1a | 3 (2.0) | 0 | 2a (2.4) | 1 (0.4) | 6a (1.1) | 1 (0.1) | 19.1 | 1.3×10−4 |
| (2.3-159.1) | |||||||||
| MSH2b | 6b (4.0) | 0 | 2 (2.4) | 0 | 8b (1.5) | 0 | N.A. | ||
| MSH6 | 8 (5.3) | 0 | 1 (1.2) | 4 (1.4) | 13 (2.4) | 0 | N.A. | ||
| PMS2 | 0 | 0 | 0 | 0 | 0 | 3 (0.2) | N.A. | ||
| EPCAM | 0 | 0 | 0 | 0 | 0 | 0 | N.A. | ||
| All LS | 17 | 0 | 5 (6.1) | 5 (1.8) | 27 (5.1) | 4 (0.2) | 22.4 | 1.8×10−17 | |
| genes | (11.3) | (7.8-64.3) | |||||||
| HBOC | ATMb | 1b (0.7) | 0 | 1 (1.2) | 3 (1.1) | 5b (1.0) | 7 (0.4) | 2.3 | 0.2 |
| (0.2-7.2) | |||||||||
| BARD1 | 0 | 0 | 1 (1.2) | 0 | 1 (0.2) | 0 | N.A. | ||
| BRCA1a | 2 (1.3) | 2 (12.5) | 6a (7.3) | 1 (0.4) | 11a (2.1) | 9 (0.5) | 3.9 | 1.0×10−3 | |
| (1.6-9.5) | |||||||||
| BRCA2 | 1 (0.7) | 1 (6.3) | 0 | 5 (1.8) | 7 (1.3) | 3 (0.2) | 7.4 | 2.0×10−3 | |
| (1.9-28.9) | |||||||||
| BRIP1 | 0 | 0 | 0 | 1 (0.4) | 1 (0.2) | 3 (0.2) | 1.1 | >0.9 | |
| (0.1-10.1) | |||||||||
| CDH1 | 0 | 0 | 0 | 0 | 0 | 0 | N.A. | ||
| CHEK2 | 3 (2.0) | 0 | 1 (1.2) | 2 (0.7) | 6 (1.1) | 6 (0.4) | 3.2 | 4.0×10−2 | |
| (1.0-9.9) | |||||||||
| NF1 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | N.A. | ||
| PALB2 | 1 (0.7) | 0 | 0 | 0 | 1 (0.2) | 8 (0.5) | 0.4 | 0.4 | |
| (0.1-3.1) | |||||||||
| PTEN | 1 (0.7) | 0 | 0 | 0 | 1 (0.2) | 1 (0.1) | 3.2 | 0.4 | |
| (0.2-50.5) | |||||||||
| RAD51C | 0 | 0 | 2 (2.4) | 0 | 2 (0.4) | 2 (0.1) | 3.2 | 0.2 | |
| (0.4-22.5) | |||||||||
| RAD51D | 0 | 0 | 0 | 0 | 0 | 0 | N.A. | ||
| STK11 | 0 | 0 | 0 | 0 | 0 | 0 | N.A. | ||
| TP53 | 0 | 0 | 0 | 0 | 0 | 2 (0.1) | N.A. | ||
| All | 9 (6.0) | 3 (18.8) | 11 (13.4) | 12 (4.3) | 35 (6.6) | 42 (2.5) | 2.7 | 7.9×10−5 | |
| HBOC | (1.7-4.3) | ||||||||
| All genes | All PV | 26 | 3 | 16 | 17 | 62 | 46 | ||
| All genes | All | 25b | 3 (18.8) | 15a (18.3) | 17 (6.1) | 60a,b (11.4) | 46 (2.8) | ||
| carriers | (16.6) | ||||||||
Double PV carrier in MLH1/BRCA1.
Double PV carrier in MSH2/ATM. Frequencies of germline PV found in a subgroup of patients fulfilling criteria for germline genetic testing for LS, HBOC, LS + HBOC, individuals not fulfilling any criteria (non-indicated), an aggregated group of all EC patients, and a group of PMC, respectively. CI, confidence interval; EC, endometrial cancer; HBOC, hereditary breast and ovarian cancer; LS, Lynch syndrome; N, number; N.A., not available; OR, odds ratio; PMC, population-matched controls; PV, pathogenic variant.
Indication criteria for identification of PV carriers
Among EC patients indicated for germline genetic testing according to the above-mentioned criteria, the proportions of PV carriers fulfilling criteria for LS, HBOC, and both conditions were similar (16.6, 18.8, and 18.3%, respectively). These proportions were approximately three-times higher than in EC patients not fulfilling any criteria for germline genetic testing (6.1%; Fig. 1). As expected, the highest proportion of PV in LS genes (11.3%) was detected in a subgroup of patients fulfilling criteria only for LS testing. Similarly, patients meeting solely the HBOC testing criteria had the highest frequency (18.8%) of PV in HBOC genes. Even though the overall percentage of PV carriers differed between subgroups of patients meeting both LS + HBOC genetic testing criteria (18.3%) and not fulfilling any criteria (6.1%), the ratio of carriers of PV in LS:HBOC genes in these two subgroups was similar (5:11 vs. 5:12; Table II, Fig. 1). On the other hand, highly penetrant genes (MLH1, MSH2, BRCA1) were predominantly affected in the subgroup fulfilling both criteria, whereas the subgroup of non-indicated patients was characterized by PV in less penetrant genes (MHS6, ATM).
Figure 1.
Distribution of PV carriers in patient subgroups based on criteria for germline genetic testing for LS and HBOC. Squares colored in green, pink, yellow and grey represent individual patients fulfilling criteria for LS only, criteria for HBOC only, both criteria or not fulfilling any criteria, respectively. Circles denote carriers of PV in LS genes (green), HBOC genes (pink) or both (green/pink). HBOC, hereditary breast and ovarian cancer; LS, Lynch syndrome; PV, pathogenic variant.
Moreover, among non-indicated patients we found 2 PVs in HBOC genes in subset of 41 patients with double primary EC and breast cancer (BC; 1×ATM, 1×BRCA1; 2/41; 4.9%) and 3 PVs in HBOC genes in subset of 31 patients with EC and BC in family cancer history (2×BRCA2, 1×CHEK2; 3/31; 9.7%).
Germline PV in other candidate cancer predisposition genes
The overall prevalence of PV in remaining candidate genes (identified in 48 out of 207 genes) was significantly higher in EC patients (66/527; 12.5%) compared to controls (139/1662; 8.4%; P=0.004; Table SIV). Eight EC patients (and no PMC) carried a coincidental PV in EC-predisposition and candidate genes. Excluding all 60 carriers of PV in EC-predisposition genes, the frequency of PV carriers in other candidate genes was still significantly higher in 467 EC patients (N=58; 12.4%) in comparison to 1616 PMC (N=139; 8.6%; P=0.01). The most frequent PV were found in MUTYH (monoallelic PV in 5/467, 1.1%) and FANCA (4/467; 0.8%). Their frequencies, however, did not differ from that in PMC (MUTYH−18/1616, 1.1%; FANCA−10/1616, 0.6%).
Interestingly, three patients carried germline truncating variant in the genes coding for DNA polymerases (two in POLE and one in POLD1) that have been linked to EC-predisposition previously (5). In contrast, only one POLE and no POLD1 mutation was detected in PMC. Thus, the overall frequency of PV in DNA polymerases was significantly higher in EC-predisposition gene negative patients (3/467; 0.6%) than in PMC (1/1616; 0.06%; OR=10.44; 95% CI 1.08-100.51; P=0.012).
Regarding subgroups of patients based on indication criteria for genetic testing, the frequency of PV in candidate predisposition genes (after excluding the carriers of PV in EC-predisposition genes) was significantly higher in patients fulfilling both indication criteria for LS + HBOC (14/67; 20.9%) in comparison to subgroup of patients fulfilling no genetic testing criteria (28/261; 10.7%; P=0.04, Table SIV). The frequencies of PV in patients meeting indication criteria for LS only and HBOC only did not differ significantly (14/126; 11.1 and 2/13; 15.4%, respectively).
Clinicopathological characteristics in germline PV carriers
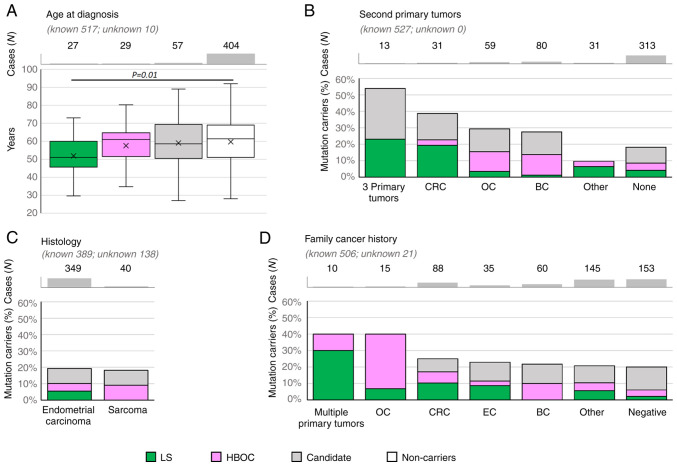
The median age at EC onset was significantly lower only in patients with PV in LS genes compared to non-carriers (51.0 vs. 61.4 years, P=0.01, Fig. 2A).
Figure 2.
Relative proportion of mutation carriers in clinicopathological subgroups, including (A) age at diagnosis, (B) second primary tumors, (C) histology and (D) family cancer history in 527 patients. Error bars in (A) indicate the first and the fourth quartile. BC, breast cancer; CRC, colorectal cancer; EC, endometrial cancer; HBOC, hereditary breast and ovarian cancer; LS, Lynch syndrome; OC, ovarian cancer.
Concerning the histology subtypes (Fig. 2C), the overall frequency of PV in EC-predisposition genes was similar in patients with endometrial carcinoma to those with sarcoma subtypes (39/349, 11.2% and 4/40, 10.0%; respectively); however, no carrier of PV in LS gene was diagnosed with sarcoma. Interestingly, two out of eight patients diagnosed with precancerous EIN (endometrial intraepithelial neoplasia) carried a PV in BRCA1. Unfortunately, the histologic subtypes of endometrial carcinomas other than endometrioid were rarely represented, thus the frequencies of PVs in these subgroups cannot be calculated and compared.
Analysis of patients with second primary tumors (Fig. 2B) revealed that the highest frequency of PV in EC-predisposition genes was found in patients with 3 primary tumors and in patients with second primary colorectal cancer (CRC). The proportion between carriers of PV in LS and HBOC genes respected the corresponding indication criteria: the carriers of LS gene variants were enriched in patients with EC + CRC and 3 primary tumors. Accordingly, all 13 patients with 3 primary tumors developed either CRC (N=5) and/or ovarian cancer (OC; N=10). The carriers of PV in HBOC genes were more frequent in patients with EC + OC and EC + BC.
When considering family cancer history (Fig. 2D), the highest frequency (reaching 40%) of PV in EC-predisposition genes were found in small subgroups of patients with family history of multiple primary tumors and family history of ovarian tumors. Not surprisingly, predominant tumor types in a family were in concordance with the elevated frequencies of PV in LS or HBOC genes.
The prevalence of carriers of PV in candidate predisposition genes did not differ from that of non-carriers in any of the clinicopathological categories.
The information about immunohistochemistry and microsatellite instability in EC tumor specimens was unavailable.
Discussion
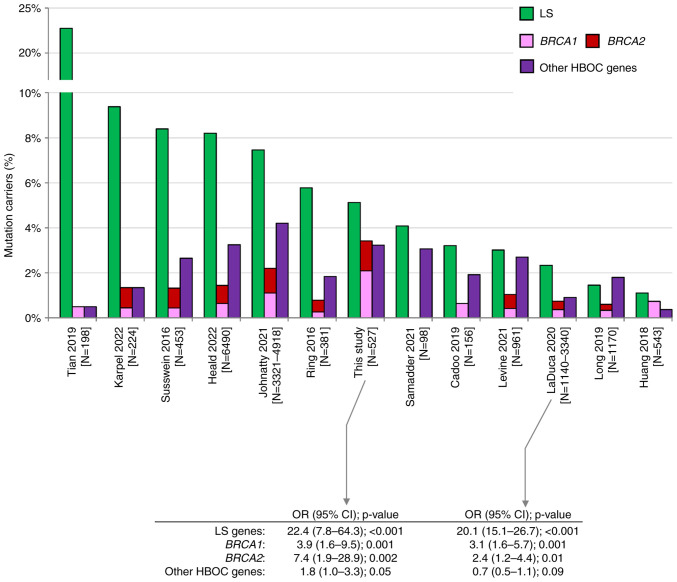
Pathogenic germline alterations in LS genes are considered the most significant genetic risk factor for EC predisposition (5). In our study, the carriers of PV in LS genes represented 5.1% of all analyzed EC patients. This frequency is approximately in the middle of frequencies reported by other studies (Fig. 3). Variable frequencies result from inconsistent patients' enrollment criteria. Studies reporting the highest frequency (Tian et al (7), Karpel et al (13), Susswein et al (14), Heald et al (15) with 22.7, 9.4, 8.4, and 8.2% of LS PV carriers, respectively) analyzed high-risk EC patients enriched in individuals with familial LS criteria or in patients with positive MMR gene immunohistochemistry [Tian et al (7)]. In contrast, the lowest frequency of PV in LS genes was reported by studies with unselected EC cases, including Huang et al (28) (1.1%), a study of EC samples from The Cancer Genome Atlas (TCGA). We have found similar differences as we identified 22/233 (9.4%) vs. 5/294 (1.7%) carriers of PV in LS genes in LS-indicated vs. LS non-indicated patients, respectively (Fig. 1). Interestingly, despite differences in frequencies of PV in EC patients, the risk of EC development in LS PV carriers was similar in our and LaDuca et al (29) study (OR 22.4 and 20.1, respectively; Fig. 3), the only study among those previously published that quantified the EC risk associated with PV in LS genes.
Figure 3.
Comparison among previously published studies describing germline PV in patients with endometrial cancer (6–15,28,29). Green, pink, red and purple bars represent the prevalence of PV in LS genes, BRCA1, BRCA2 and other HBOC genes (ATM, BARD1, BRIP1, CDH1, CHEK2, NF1, PALB2, PTEN, RAD51C, RAD51D, STK11 and TP53), respectively. CI, confidence interval; HBOC, hereditary breast and ovarian cancer; LS, Lynch syndrome; N, number; OR, odds ratio; PV, pathogenic variant.
Even though only less than 20% of analyzed EC patients (98/527, 18.6%) met the HBOC germline genetic testing criteria, the overall frequencies of PV carriers in BRCA1/BRCA2 were unusually high in contrast to other studies (Fig. 3). We identified 11 PVs in BRCA1 (2.1%) and 7 PVs in BRCA2 (1.3%). Compared to frequencies of PVs in controls we calculated the risks OR=3.9 for BRCA1 and OR=7.4 for BRCA2 (Table II). The risk of EC development associated with BRCA1 and BRCA2 mutations was substantially lower than in LS carriers, and similar to EC risk reported previously by LaDuca et al (29). Our results suggest that PV in BRCA1/BRCA2 are associated with at least moderate EC risk. Among 16 EC patients meeting only the HBOC criteria, three harbored BRCA1/BRCA2 mutation. This was also documented by results of a small study by Vietri et al (30), who identified PV in BRCA1/BRCA2 in 9/21 hereditary EC patients fulfilling HBOC testing criteria. In the group of 82 patients meeting both LS and HBOC testing criteria, BRCA1 PV were more frequent than PV in LS genes. Moreover, up to 5 and 10% of PVs in HBOC genes were identified in non-indicated EC patients with BC in personal or family cancer history, respectively. This further implies that the diagnosis of EC should be considered as a part of indication criteria for HBOC germline genetic testing irrespective to EC histology subtype. Among PV carriers in other HBOC genes, PV in CHEK2 and ATM were the most frequent. Importantly, PV in CHEK2 were associated with moderately increased risk (OR=3.2, P=0.04). Mutations in CHEK2 were associated with predisposition to EC in several studies previously (31).
Our analysis of other candidate genes showed that only PVs in POLD1 and POLE (three truncating variants, one in POLD1, two in POLE) were significantly associated with EC risk. Germline truncating variants in DNA polymerase genes in our EC patients conferred about 10-times increased risk of EC development. Germline missense PV in both DNA polymerase genes affecting exonuclease domains were previously linked to EC predisposition (5) and their specific somatic missense PV represent important predictive markers for favorable prognosis and/or immune checkpoint therapy in EC patients (32–34). However, the exact risk as well as the overall role of germline truncating variants needs to be further validated in larger cohorts due to the low frequency of POLD1 and POLE mutation carriers.
Analysis of clinicopathological characteristics confirmed an earlier age at disease onset in carriers of LS gene mutations in comparison to non-carriers as referred in other studies (6,7,9,10). The age at EC onset varied even among the carriers of PV in particular LS genes: the carriers of PV in MSH6 had later age at onset (56 years) compared to the MLH1/MSH2 PV carriers (48 years), as previously described by Tian et al (7). Interestingly, the age at EC onset in carriers of PV in HBOC genes did not differ from non-carriers.
As expected, other differences in clinicopathological characteristics largely corresponded to subgroups of patients classified according to the germline genetic testing criteria. PV in LS genes were most frequently identified in patients with ≥3 primary tumors or second primary CRC in personal cancer history, or multiple primary tumors/CRC in family cancer history. Similarly, carriers of PV in HBOC genes recruited in majority from individuals with BC/OC in personal or family cancer history. On the other hand, clinicopathological characteristics did not differ in carriers of candidate EC-predisposition genes and non-carriers.
Generally and as expected, we have identified the majority of PV in the groups of patients fulfilling genetic testing criteria for LS or HBOC with majority of PV in genes related to a corresponding cancer syndrome. Overall, 43/60 PV (71.7%) carriers were indicated for germline genetic testing. Importantly, remaining 17 PV carriers, who would not be indicated for genetic testing using current indication criteria, still represent a significant proportion (28.3%) of cases carrying a germline PV in the LS (MLH1, MSH6) or the HBOC (ATM, BRCA1, BRCA2, BRIP1, CHEK2) genes. Of these, two had double primary tumors and an additional 10 had a positive family cancer history. The frequency of PV carriers among EC patients with double primary tumors was 15.4% (33/214). While we have found eight PV carriers in HBOC genes and four carriers in LS genes (including a patient with co-occurrence of BRCA1 and MLH1 PV and diagnosed with EC, OC, and BC) in the group of 69 patients with EC and OC (11.6%), we have identified eight carriers of LS genes mutation and only one additional carrier of the CHEK2 gene mutation (a patient with EC, CRC, and melanoma) in the group of 34 patients with EC and CRC (26.5%). This suggests that the presence of double primary tumors could potentially represent a sole indication criterion for germline genetic testing, as indicated by previous studies (19,20,35,36).
Strengths of our study include homogeneity of studied population consisting of Caucasians, Slavs of the Czech origin and inclusion of PMC that allowed calculation of overall/gene-level risks for EC development. Study limitations include retrospective design and unavailability of EC tumors immunohistochemistry, microsatellite instability and mutation status of POLE, which prevented us from correlating presence of germline mutations with different molecular subtypes of EC. Moreover, as approximately half of the analyzed EC patients (292/527, 55.4%) were recruited from the CZECANCA consortium (focused on analyses of genetic cancer predisposition), we cannot exclude a potential bias toward enriched prevalence of PV carriers. To minimize this bias, we divided all enrolled patients according to the testing criteria and analyzed them independently.
In conclusion, over 11% of EC patients carried a germline PV in genes associated with established germline cancer predisposition. EC patients fulfilling LS criteria had five-times higher chance to carry a LS gene PV than EC patients not fulfilling criteria for germline genetic testing. Presence of PV in LS gene increases the EC risk 20-fold when compared with non-carriers. However, 28.3% of PV carriers in clinically relevant genes would not be indicated for germline genetic testing using current indication criteria. Therefore, we believe that EC as a second primary tumor in proband or occurrence of EC in a family cancer history should be considered within the indication criteria for germline genetic testing. This is of particular importance for countries where reflex testing is not routinely performed in EC patients.
Supplementary Material
Acknowledgements
The authors would like to thank Professor Stanislav Kmoch and Dr Viktor Stranecky (National Center for Medical Genomics, Prague, Czech Republic) (LM2018132) for performing whole-exome sequencing of PMC (project CZ.02.1.01/0.0/0.0/18_046/0015515).
Glossary
Abbreviations
- ACMG
American College of Medical Genetics
- BC
breast cancer
- CI
confidence interval
- CNV
copy number variation
- CRC
colorectal cancer
- EC
endometrial cancer
- EIN
endometrial intraepithelial neoplasia
- FIGO
The International Federation of Gynecology and Obstetrics
- GGT
germline genetic testing
- HBOC
hereditary breast and ovarian cancer
- LS
Lynch syndrome
- MMR
mismatch repair
- N
number
- N.A.
not available
- NCCN
National Comprehensive Cancer Network
- NGS
next generation sequencing
- OC
ovarian cancer
- OR
odds ratio
- P/LP
pathogenic/likely pathogenic
- PMC
population-matched controls
- PV
pathogenic variant
- VUS
variant of uncertain significance
Funding Statement
This work was supported by the Ministry of Health of the Czech Republic (grant no. NU20-03-00285), institutional support of the Ministry of Health of the Czech Republic (grant nos. DRO VFN 64165 and DRO MMCI 00209805), the Ministry of Education, Youth, and Sports of the Czech Republic (grant nos. BBMRI-CZ LM2023033, LM2023067 and LX22NPO5102), the Charles University (grant no. GAUK902120), and the Charles University institutional programs (grant nos. SVV260516 and COOPERATIO, research area DIAG).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to restrictions imposed by national regulatory authorities but are available from the corresponding author on reasonable request.
Authors' contributions
JK, SJ, MB, LC, MC, PD, KH, MH, SC, MaKo, MoKo, VK, EM, BO, AP and ST performed the experiments. MV, LD, LF, OH, MaKa, JN, MS, JiS, VS, IS and JaS provided additional data, and participated in data analysis and interpretation. PZ, PN and MZ participated in data analysis and interpretation, as well as revision of the manuscript. PK, ZK, TZ and MJ designed the study and were involved in drafting and editing of the manuscript. JK and MJ confirm the authenticity of all the raw data. All authors agreed to be accountable for all aspects of the work. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All patients provided written informed consent. The study was conducted in line with the Helsinki Declaration and was approved by The Ethics Committee of General University Hospital in Prague, Prague, Czech Republic under reference number 11/20 Grant VFN IGP.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978–2013. J Natl Cancer Inst. 2018;110:354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- 2.Arend RC, Jones BA, Martinez A, Goodfellow P. Endometrial cancer: Molecular markers and management of advanced stage disease. Gynecol Oncol. 2018;150:569–580. doi: 10.1016/j.ygyno.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E, Martin-Hirsch P, Tsilidis KK, Kyrgiou M. Risk factors for endometrial cancer: An umbrella review of the literature. Int J Cancer. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 5.Spurdle AB, Bowman MA, Shamsani J, Kirk J. Endometrial cancer gene panels: Clinical diagnostic vs research germline DNA testing. Mod Pathol. 2017;30:1048–1068. doi: 10.1038/modpathol.2017.20. [DOI] [PubMed] [Google Scholar]
- 6.Levine MD, Pearlman R, Hampel H, Cosgrove C, Cohn D, Chassen A, Suarez A, Barrington DA, McElroy JP, Waggoner S, et al. Up-front multigene panel testing for cancer susceptibility in patients with newly diagnosed endometrial cancer: A multicenter prospective study. JCO Precis Oncol. 2021;5:1588–1602. doi: 10.1200/PO.21.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tian W, Bi R, Ren Y, He H, Shi S, Shan B, Yang W, Wang Q, Wang H. Screening for hereditary cancers in patients with endometrial cancer reveals a high frequency of germline mutations in cancer predisposition genes. Int J Cancer. 2019;145:1290–1298. doi: 10.1002/ijc.32389. [DOI] [PubMed] [Google Scholar]
- 8.Cadoo KA, Mandelker DL, Mukherjee S, Stewart C, DeLair D, Ravichandran V, Srinivasan P, Hurley D, Kemel Y, Arnold AG, et al. Understanding inherited risk in unselected newly diagnosed patients with endometrial cancer. JCO Precis Oncol. 2019;3 doi: 10.1200/PO.18.00338. PO.18.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long B, Lilyquist J, Weaver A, Hu C, Gnanaolivu R, Lee KY, Hart SN, Polley EC, Bakkum-Gamez JN, Couch FJ, Dowdy SC. Cancer susceptibility gene mutations in type I and II endometrial cancer. Gynecol Oncol. 2019;152:20–25. doi: 10.1016/j.ygyno.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ring KL, Bruegl AS, Allen BA, Elkin EP, Singh N, Hartman AR, Daniels MS, Broaddus RR. Germline multi-gene hereditary cancer panel testing in an unselected endometrial cancer cohort. Mod Pathol. 2016;29:1381–1389. doi: 10.1038/modpathol.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samadder NJ, Riegert-Johnson D, Boardman L, Rhodes D, Wick M, Okuno S, Kunze KL, Golafshar M, Uson PLS, Jr, Mountjoy L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230–237. doi: 10.1001/jamaoncol.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnatty SE, Pesaran T, Dolinsky J, Yussuf A, LaDuca H, James PA, O'Mara TA, Spurdle AB. Case-case analysis addressing ascertainment bias for multigene panel testing implicates BRCA1 and PALB2 in endometrial cancer. Hum Mutat. 2021;42:1265–1278. doi: 10.1002/humu.24256. [DOI] [PubMed] [Google Scholar]
- 13.Karpel HC, Chern JY, Smith JM, Smith AJ, Pothuri B. Utility of germline multi-gene panel testing in patients with endometrial cancer. Gynecol Oncol. 2022;165:546–551. doi: 10.1016/j.ygyno.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Susswein LR, Marshall ML, Nusbaum R, Vogel Postula KJ, Weissman SM, Yackowski L, Vaccari EM, Bissonnette J, Booker JK, Cremona ML, et al. Pathogenic and likely pathogenic variant prevalence among the first 10,000 patients referred for next-generation cancer panel testing. Genet Med. 2016;18:823–832. doi: 10.1038/gim.2015.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heald B, Mokhtary S, Nielsen SM, Rojahn S, Yang S, Michalski ST, Esplin ED. Unexpected actionable genetic variants revealed by multigene panel testing of patients with uterine cancer. Gynecol Oncol. 2022;166:344–350. doi: 10.1016/j.ygyno.2022.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Daniels MS, Lu KH. Genetic predisposition in gynecologic cancers. Semin Oncol. 2016;43:543–547. doi: 10.1053/j.seminoncol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 17.de Jonge MM, de Kroon CD, Jenner DJ, Oosting J, de Hullu JA, Mourits MJE, Gómez Garcia EB, Ausems MGEM, Margriet Collée J, van Engelen K, et al. Endometrial cancer risk in women with germline BRCA1 or BRCA2 mutations: Multicenter cohort study. J Natl Cancer Inst. 2021;113:1203–1211. doi: 10.1093/jnci/djab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mur P, Garcia-Mulero S, Del Valle J, Magraner-Pardo L, Vidal A, Pineda M, Cinnirella G, Martin-Ramos E, Pons T, López-Doriga A, et al. Role of POLE and POLD1 in familial cancer. Genet Med. 2020;22:2089–2100. doi: 10.1038/s41436-020-0922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieme G, Kral J, Rosseel T, Zemankova P, Parton B, Vocka M, Van Heetvelde M, Kleiblova P, Blaumeiser B, Soukupova J, et al. Prevalence of germline pathogenic variants in cancer predisposing genes in Czech and Belgian pancreatic cancer patients. Cancers (Basel) 2021;13:4430. doi: 10.3390/cancers13174430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lhotova K, Stolarova L, Zemankova P, Vocka M, Janatova M, Borecka M, Cerna M, Jelinkova S, Kral J, Volkova Z, et al. Multigene panel germline testing of 1333 Czech patients with ovarian cancer. Cancers (Basel) 2020;12:956. doi: 10.3390/cancers12040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soukupova J, Zemankova P, Lhotova K, Janatova M, Borecka M, Stolarova L, Lhota F, Foretova L, Machackova E, Stranecky V, et al. Validation of CZECANCA (CZEch CAncer paNel for clinical application) for targeted NGS-based analysis of hereditary cancer syndromes. PLoS One. 2018;13:e0195761. doi: 10.1371/journal.pone.0195761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.1000 Genomes Project Consortium, corp-author. Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, Kang HM, Marth GT, McVean GA. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Exome Variant Server NGESPE, corp-author; Available online:, corp-author http://evs.gs.washington.edu/EVS/ [ March 15; 2022 ]; [Google Scholar]
- 26.Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: Public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014;42:D980–D985. doi: 10.1093/nar/gkt1113. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller DT, Lee K, Chung WK, Gordon AS, Herman GE, Klein TE, Stewart DR, Amendola LM, Adelman K, Bale SJ, et al. ACMG SF v3.0 list for reporting of secondary findings in clinical exome and genome sequencing: A policy statement of the American college of medical genetics and genomics (ACMG) Genet Med. 2021;23:1381–1390. doi: 10.1038/s41436-021-01278-8. [DOI] [PubMed] [Google Scholar]
- 28.Huang KL, Mashl RJ, Wu Y, Ritter DI, Wang J, Oh C, Paczkowska M, Reynolds S, Wyczalkowski MA, Oak N, et al. Pathogenic germline variants in 10,389 adult cancers. Cell. 2018;173:355–370.e14. doi: 10.1016/j.cell.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaDuca H, Polley EC, Yussuf A, Hoang L, Gutierrez S, Hart SN, Yadav S, Hu C, Na J, Goldgar DE, et al. A clinical guide to hereditary cancer panel testing: Evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22:407–415. doi: 10.1038/s41436-019-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vietri MT, D'Elia G, Caliendo G, Casamassimi A, Federico A, Passariello L, Cioffi M, Molinari AM. Prevalence of mutations in BRCA and MMR genes in patients affected with hereditary endometrial cancer. Med Oncol. 2021;38:13. doi: 10.1007/s12032-021-01454-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stolarova L, Kleiblova P, Janatova M, Soukupova J, Zemankova P, Macurek L, Kleibl Z. CHEK2 germline variants in cancer predisposition: Stalemate rather than checkmate. Cells. 2020;9:2675. doi: 10.3390/cells9122675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen TT, Wang TL, Fader AN, Shih IM, Gaillard S. Molecular classification and emerging targeted therapy in endometrial cancer. Int J Gynecol Pathol. 2020;39:26–35. doi: 10.1097/PGP.0000000000000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.León-Castillo A, Britton H, McConechy MK, McAlpine JN, Nout R, Kommoss S, Brucker SY, Carlson JW, Epstein E, Rau TT, et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J Pathol. 2020;250:323–335. doi: 10.1002/path.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Green AK, Feinberg J, Makker V. A review of immune checkpoint blockade therapy in endometrial cancer. Am Soc Clin Oncol Educ Book. 2020;40:1–7. doi: 10.1200/EDBK_280503. [DOI] [PubMed] [Google Scholar]
- 35.Bychkovsky BL, Lo MT, Yussuf A, Horton C, Richardson M, LaDuca H, Garber JE, Rana HQ. Prevalence and spectrum of pathogenic variants among patients with multiple primary cancers evaluated by clinical characteristics. Cancer. 2022;128:1275–1283. doi: 10.1002/cncr.34056. [DOI] [PubMed] [Google Scholar]
- 36.Stolarova L, Jelinkova S, Storchova R, Machackova E, Zemankova P, Vocka M, Kodet O, Kral J, Cerna M, Volkova Z, et al. Identification of germline mutations in melanoma patients with early onset, double primary tumors, or family cancer history by NGS analysis of 217 genes. Biomedicines. 2020;8:404. doi: 10.3390/biomedicines8100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to restrictions imposed by national regulatory authorities but are available from the corresponding author on reasonable request.