Key Points
Question
What is the association between intensive blood pressure control targeting less than 120 mm Hg and incident left ventricular conduction disease?
Findings
In this post hoc analysis of the Systolic Blood Pressure Intervention Trial (SPRINT), randomization to intensive blood pressure control was associated with lower risk of left ventricular conduction disease, including fascicular and left bundle-branch block, compared with standard blood pressure control targeting less than 140 mm Hg. The risk of right bundle-branch block did not differ between treatment groups.
Meaning
Targeting intensive blood pressure control was associated with lower risk of left ventricular conduction disease, which suggests that conduction disease may be a modifiable outcome susceptible to prevention strategies.
This post hoc analysis of the SPRINT randomized clinical trial evaluates the association of intensive vs standard blood pressure control with incident left ventricular conduction disease.
Abstract
Importance
Left ventricular conduction disease predicts heart failure and death, and the only strategies to mitigate its effects involve implantation of a permanent pacemaker. There are currently no proven preventive strategies for this common condition.
Objective
To determine the association between targeting intensive blood pressure (BP) control and the risk of developing left ventricular conduction disease.
Design, Setting, and Participants
This was a post hoc analysis of the 2-arm multicenter Systolic Blood Pressure Intervention Trial (SPRINT), which recruited participants from 102 sites in the US and Puerto Rico and was conducted from November 2010 until August 2015. Adults 50 years and older with hypertension and at least 1 other cardiovascular risk factor were included. Participants with baseline left ventricular conduction disease, ventricular pacing, or ventricular pre-excitation were excluded for the current analysis. Data were analyzed from November 2021 to November 2022.
Intervention
Participants were randomly assigned to a systolic BP target of less than 140 mm Hg (standard treatment group) or less than 120 mm Hg (intensive treatment group).
Main Outcome
The primary outcome was incident left ventricular conduction disease, including any fascicular or left bundle-branch block, assessed by serial electrocardiography. Incident right bundle-branch block was examined as a negative control.
Results
Among 3918 participants randomized to standard treatment and 3956 to intensive treatment (mean [SD] age, 67.6 [9.2] years; 2815 [36%] female) monitored for a median [IQR] 3.5 (0.02-5.2) years, 203 developed left ventricular conduction disease. Older age (hazard ratio per 10-year increase [HR], 1.42; 95% CI, 1.21-1.67; P < .001), male sex (HR, 2.31; 95% CI, 1.63-3.32; P < .001), and cardiovascular disease (HR, 1.46; 95% CI, 1.06-2.00; P = .02) were associated with a higher risk of left ventricular conduction disease. Assignment to intensive treatment was associated with a 26% lower risk of left ventricular conduction disease (HR, 0.74; 95% CI, 0.56-0.98; P = .04). These results persisted when incident ventricular pacing was included in the outcome and when considering all-cause death as a competing risk. In contrast, no association between randomization assignment and right bundle-branch block was observed (HR, 0.95; 95% CI, 0.71-1.27; P = .75).
Conclusions and Relevance
In this study, targeting intensive BP control was associated with lower risk of left ventricular conduction disease in a randomized clinical trial, suggesting that clinically relevant conduction disease may be preventable.
Trial Registration
ClinicalTrials.gov Identifier: NCT01206062
Introduction
Cardiac conduction disease can lead to life-threatening rhythm disturbances, heart failure, and death.1,2 Systolic dysfunction that occurs due to left bundle-branch block can only be rectified with implantation of a biventricular pacing device,3 and progression to complete heart block can only be treated with implantation of some form of a ventricular pacemaker.4 There are no proven preventive strategies to reduce the risk of this common disorder. Observational studies have found hypertension to be associated with left ventricular conduction disease,5,6 suggesting that blood pressure (BP) control might be an effective target in pursuing a prevention strategy.
Methods
This is a post hoc analysis of the Systolic Blood Pressure Intervention Trial (SPRINT), a randomized clinical open-label trial in which individuals with hypertension at high risk of cardiovascular disease were recruited from 102 clinical centers in the US, including Puerto Rico, from November 2010 to August 2015. The design and rationale of SPRINT have been published previously.7 Institutional Review Boards at all participating institutions approved the study. All participants provided informed consent. The trial protocol can be found in Supplement 1.
Participants were required to meet all of the following criteria: age 50 years or older, systolic BP of 130 to 180 mm Hg, and an increased risk of cardiovascular events. A total of 9361 eligible participants underwent randomization. For the purpose of the present analysis, we excluded participants with insufficient electrocardiographic (ECG) data, including those without available ECG data or fewer than 2 serial study ECG scans, prevalent left ventricular conduction disease, ventricular pacing, or ventricular pre-excitation detected on the baseline ECG (eFigure 1 in Supplement 2). Eligible participants were randomly assigned in a 1:1 ratio to a systolic BP target of less than 140 mm Hg (standard treatment group) or less than 120 mm Hg (intensive treatment group) for prevention of cardiovascular disease events.
Race and ethnicity data were collected to assess representativeness of the general population and because of previously described differences in risks associated with conduction disease. Race was self-reported and categorized as Asian, Black, multiracial, White, or other (including Hawaiian or Pacific Islander, Native American, and other race, consolidated because of relatively small numbers in each of these groups). Self-reported ethnicity was categorized as Hispanic or non-Hispanic. Definitions of comorbidities can be found in the eMethods in Supplement 2.
Standard 12-lead ECG scans were obtained at baseline, 2 years, 4 years, and the closeout visit (year 5). ECG abnormalities, including evidence of conduction disease, were classified using the standards of the Minnesota ECG Classification or in accordance with previous literature (eTable 1 in Supplement 2).1 Incident left ventricular conduction disease was defined as the development of left anterior fascicular block, left posterior fascicular block, left bundle-branch block, or nonspecific intraventricular conduction delay. Incident right bundle-branch block was assessed as a negative control.
Incidence rate ratios between treatment groups were assessed using Poisson regression. Cox proportional hazards regression models were used to assess the association between randomization assignment and incident outcomes. Participants were censored at the time of their first relevant ECG-based diagnosis or at the time of their last ECG scan, whichever came first.
Three sensitivity analyses were performed. First, incident ventricular pacing detected by study ECG scans were included in the outcome. Second, analyses adjusting for time-updated myocardial infarction and congestive heart failure, respectively, were assessed. Third, an analysis accounting for the competing risk of all-cause death was performed.8
Interaction analyses were ascertained to determine whether the association of targeting intensive BP lowering with left ventricular conduction disease risk differed with respect to baseline demographics and comorbidities. A 2-tailed P < .05 was considered statistically significant. Statistical analyses were performed using Stata version 17 (StataCorp). Data were analyzed from November 2021 to November 2022.
Results
A total of 7874 participants (mean [SD] age, 67.6 [9.2] years; 2815 [36%] female) were included in the analysis: 3918 in the standard treatment group and 3956 in the intensive treatment group. By self-report, 45 participants were Asian, 2465 were Black, 43 were multiracial, 5117 were White, and 183 were of another race; 833 were Hispanic and 7039 were non-Hispanic. Baseline characteristics are presented in Table 1.
Table 1. Baseline Characteristics by Randomization Assignment.
| Variable | No. (%) | P value | |
|---|---|---|---|
| Standard treatment (n = 3918) | Intensive treatment (n = 3956) | ||
| Age, mean (SD), y | 67.6 (9.2) | 67.6 (9.2) | .88 |
| Female | 1385 (35.3) | 1430 (36.1) | .46 |
| Male | 2533 (64.7) | 2526 (63.9) | |
| Racea | |||
| Asian | 24 (0.6) | 21 (0.5) | .30 |
| Black | 1242 (31.7) | 1223 (30.9) | |
| Multiracial | 22 (0.6) | 21 (0.5) | |
| White | 2546 (65.0) | 2571 (65.0) | |
| Otherb | 84 (2.1) | 99 (2.5) | |
| Ethnicitya | |||
| Hispanic | 409 (10.4) | 424 (10.7) | .69 |
| Non-Hispanic | 3508 (89.6) | 3531 (89.3) | |
| Body mass index, mean (SD)c | 30.5 (5.7) | 30.7 (5.8) | .13 |
| Smoking | |||
| Never | 1742 (44.5) | 1755 (44.4) | .31 |
| Ever | 2140 (54.7) | 2154 (54.4) | |
| Physical vigorous activity | |||
| Rarely or never | 1008 (25.8) | 1035 (26.2) | .52 |
| 1-3 Times/mo | 661 (16.9) | 636 (16.1) | |
| 1 Time/wk | 409 (10.5) | 437 (11.1) | |
| 2-4 Times/wk | 1310 (33.5) | 1280 (32.5) | |
| >5 Times/wk | 518 (13.3) | 555 (14.1) | |
| Daily use of aspirin | 1957 (50.1) | 2034 (51.5) | .20 |
| Clinical or subclinical cardiovascular disease | 738 (18.8) | 768 (19.4) | .51 |
| Atrial fibrillation | 275 (7.0) | 299 (6.9) | >.99 |
| Congestive heart failure | 112 (2.9) | 124 (3.1) | .47 |
| Chronic kidney disease | 1036 (26.4) | 1099 (27.4) | .18 |
Self-reported race and ethnicity data were collected to assess representativeness of the general population and because of previously described differences in risks associated with conduction disease.
Other included Hawaiian or Pacific Islander, Native American, consolidated owing to small numbers.
Calculated as weight in kilograms divided by height in meters squared.
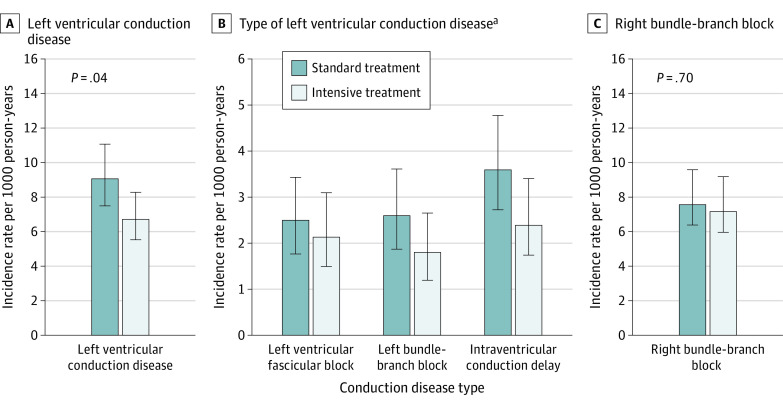
During a median (IQR) follow-up of 3.5 (0.02-5.2) years, 203 participants exhibited incident left ventricular conduction disease (eTable 2 in Supplement 2). The incidence rate of left ventricular conduction disease was significantly lower in the intensive treatment group compared with the standard treatment group (hazard ratio per 10-year increase [HR], 0.74; 95% CI, 0.56-0.98; P = .04), whereas no differences in incidence rates for right bundle-branch block were observed between the treatment groups (HR, 0.95; 95% CI, 0.71-1.27; P = .75) (Figure).
Figure. Incidence Rates of Left Ventricular Conduction Disease and Right Bundle-Branch Block According to Randomization Assignment.
P values indicate the comparisons of the respective incidence rates between treatment groups.
aIncidence rates for left posterior fascicular block are not shown due to insufficient numbers of events.
Random assignment to intensive BP treatment was associated with a significantly lower risk of left ventricular conduction disease both before and after multivariable adjustment, compared with standard BP treatment (Table 2). This remained significant across all sensitivity analyses (eTables 3, 4, and 5 in Supplement 2). In the negative control analysis, no significant association between randomization assignment and right bundle-branch block was observed. No significant interactions by demographic characteristics or comorbidities were detected between randomization assignment and the risk of incident left ventricular conduction disease (eFigure 2 in Supplement 2). After multivariable adjustment, older age, male sex, and clinical or subclinical cardiovascular disease remained significantly associated with a higher risk of incident left ventricular disease (older age: HR, 1.42; 95% CI, 1.21-1.67; P < .001; male sex: HR, 2.31; 95% CI, 1.63-3.32; P < .001; cardiovascular disease: HR, 1.46; 95% CI, 1.06-2.00; P = .02) (eTable 6 and eFigure 3 in Supplement 2).
Table 2. Risk of Incident Left Ventricular Conduction Disease, Each Type of Left Ventricular Conduction Disease, and Right Bundle-Branch Block in Intensive Treatment Groupa.
| Variableb | No. of events | Modelc | Hazard ratio (95% CI) | P value |
|---|---|---|---|---|
| Left ventricular conduction disease | 203 | Unadjusted | 0.75 (0.57-0.99) | .05 |
| Adjusted | 0.74 (0.56-0.98) | .04 | ||
| Left anterior fascicular block | 64 | Unadjusted | 0.89 (0.54-1.45) | .64 |
| Adjusted | 0.91 (0.56-1.49) | .72 | ||
| Left bundle-branch block | 61 | Unadjusted | 0.70 (0.42-1.16) | .17 |
| Adjusted | 0.68 (0.41-1.13) | .14 | ||
| Intraventricular conduction delay | 84 | Unadjusted | 0.68 (0.44-1.05) | .09 |
| Adjusted | 0.66 (0.43-1.02) | .06 | ||
| Right bundle-branch block | 184 | Unadjusted | 0.96 (0.71-1.28) | .77 |
| Adjusted | 0.95 (0.71-1.27) | .75 |
The standard treatment group was the reference group.
Risk of left posterior fascicular block left is not shown due to insufficient number of events.
Adjusted models included age, sex, race, clinical or subclinical cardiovascular disease, congestive heart failure, and chronic kidney disease.
Discussion
Among individuals with hypertension, random assignment to intensive BP control was associated with a significantly lower risk of developing left ventricular conduction disease compared with standard BP control. No differences in the risk of right bundle-branch block were observed by randomization assignment.
While the long-term prognosis of left bundle-branch block is relatively well known, recent studies investigating nonspecific intraventricular conduction delay as predictor have revealed similar adverse outcomes, including heightened risks of atrial fibrillation and mortality.2,9 Progression of left ventricular conduction disease may lead to heart failure or complete heart block, either requiring implantation of a permanent device or eventually progressing to death.1,3 Ideally, the disease might be prevented, negating the need for such devices.
The notion that chronic hypertension might promote cardiac conduction disease has biological plausibility. In hypertension, left ventricular pressure overload may lead to interstitial fibrosis, with subsequent downregulation of gap junctions and impairment in normal electrical cell coupling.10 Higher systemic blood pressure may also be associated with activation of neurohormonal, cytokine, inflammatory, and oxidative stress signaling pathways that may similarly lead to such fibrosis.11,12
The present study did not detect an association between intensive BP control and risk of right bundle-branch block. The negative finding of right bundle-branch block risk demonstrates that the association between intensive BP lowering and lower risk of left ventricular conduction disease was less likely a chance finding or that the changes in left ventricular conduction disease were epiphenomena reflective of some other unknown factor that differed between the randomization groups.
Limitations
Several limitations should be acknowledged. Our study was limited to patients with hypertension, without diabetes, and at an increased risk of cardiovascular disease, which may decrease the generalizability of our findings to other populations. SPRINT was a treatment strategy trial, examining the effect of different levels of systolic BP as a general approach rather than individual antihypertensive drugs per se and we are therefore unable to comment on the associations between specific medications and conduction disease rates. Our study did not examine whether the risk of left ventricular conduction disease varied with continuous BP lowering, nor were we able to identify a specific threshold effect at any particular level of systolic BP. In addition, left ventricular conduction disease was not a primary end point of SPRINT, and this was a post hoc analysis.
Conclusions
Compared with standard BP treatment, intensive BP control in individuals with hypertension was associated with lower risk of developing left ventricular conduction disease. In comparison, intensive blood pressure lowering was not associated with the development of right ventricular conduction disease. These findings suggest that cardiac conduction disease may be a modifiable outcome susceptible to prevention strategies.
Trial protocol
eMethods. Definition of comorbidities
eTable 1. Electrocardiographic Findings by Minnesota codes
eTable 2. Type of Incident Left-Ventricular Conduction Disease First Detected According to Randomization Assignment Group
eTable 3. Risk of Left-Ventricular Conduction Disease or Incident Ventricular Pacing in the Intensive Treatment Group Compared with the Standard Treatment Group
eTable 4. Risk of Left-Ventricular Conduction Disease in the Intensive Treatment Group Comparedwith Standard Treatment Group when Adjusting for Time-Updated Myocardial Infarction and Congestive Heart Failure
eTable 5. Competing Risk Regression with All-Cause Death as a Competing Risk
eTable 6. Baseline Characteristics Associated with Incident Left Ventricular Conduction Disease in Unadjusted Models
eFigure 1. Study Population
eFigure 2. Interaction Analyses
eFigure 3. Blood Pressure Control Group and Baseline Characteristics Associated with Incident Left-Ventricular Conduction Disease After Multivariable Adjustment
Data sharing statement
References
- 1.Mandyam MC, Soliman EZ, Heckbert SR, Vittinghoff E, Marcus GM. Long-term outcomes of left anterior fascicular block in the absence of overt cardiovascular disease. JAMA. 2013;309(15):1587-1588. doi: 10.1001/jama.2013.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aro AL, Anttonen O, Tikkanen JT, et al. Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol. 2011;4(5):704-710. doi: 10.1161/CIRCEP.111.963561 [DOI] [PubMed] [Google Scholar]
- 3.Sze E, Daubert JP. Left bundle branch block-induced left ventricular remodeling and its potential for reverse remodeling. J Interv Card Electrophysiol. 2018;52(3):343-352. doi: 10.1007/s10840-018-0407-2 [DOI] [PubMed] [Google Scholar]
- 4.Kusumoto FM, Schoenfeld MH, Barrett C, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019;140(8):932-987. doi: 10.1161/CIR.0000000000000628 [DOI] [PubMed] [Google Scholar]
- 5.Schneider JF, Thomas HE Jr, Kreger BE, McNamara PM, Kannel WB. Newly acquired left bundle-branch block: the Framingham study. Ann Intern Med. 1979;90(3):303-310. doi: 10.7326/0003-4819-90-3-303 [DOI] [PubMed] [Google Scholar]
- 6.Eriksson P, Hansson PO, Eriksson H, Dellborg M. Bundle-branch block in a general male population: the study of men born 1913. Circulation. 1998;98(22):2494-2500. doi: 10.1161/01.CIR.98.22.2494 [DOI] [PubMed] [Google Scholar]
- 7.Ambrosius WT, Sink KM, Foy CG, et al. ; SPRINT Study Research Group . The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11(5):532-546. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 9.Uhm JS, Lee Y, Roh YH, et al. Nonspecific intraventricular conduction delay is associated with future occurrence of atrial fibrillation in patients with structurally normal heart. Eur J Intern Med. 2020;72:67-72. doi: 10.1016/j.ejim.2019.11.006 [DOI] [PubMed] [Google Scholar]
- 10.Spadaccio C, Rainer A, Mozetic P, et al. The role of extracellular matrix in age-related conduction disorders: a forgotten player? J Geriatr Cardiol. 2015;12(1):76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wikstrand J. Left ventricular function in early primary hypertension. functional consequences of cardiovascular structural changes. Hypertension. 1984;6(6 Pt 2):III108-III116. doi: 10.1161/01.HYP.6.6_Pt_2.III108 [DOI] [PubMed] [Google Scholar]
- 12.Carlson SH, Wyss JM. Neurohormonal regulation of the sympathetic nervous system: new insights into central mechanisms of action. Curr Hypertens Rep. 2008;10(3):233-240. doi: 10.1007/s11906-008-0044-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods. Definition of comorbidities
eTable 1. Electrocardiographic Findings by Minnesota codes
eTable 2. Type of Incident Left-Ventricular Conduction Disease First Detected According to Randomization Assignment Group
eTable 3. Risk of Left-Ventricular Conduction Disease or Incident Ventricular Pacing in the Intensive Treatment Group Compared with the Standard Treatment Group
eTable 4. Risk of Left-Ventricular Conduction Disease in the Intensive Treatment Group Comparedwith Standard Treatment Group when Adjusting for Time-Updated Myocardial Infarction and Congestive Heart Failure
eTable 5. Competing Risk Regression with All-Cause Death as a Competing Risk
eTable 6. Baseline Characteristics Associated with Incident Left Ventricular Conduction Disease in Unadjusted Models
eFigure 1. Study Population
eFigure 2. Interaction Analyses
eFigure 3. Blood Pressure Control Group and Baseline Characteristics Associated with Incident Left-Ventricular Conduction Disease After Multivariable Adjustment
Data sharing statement