This cohort study investigates the association of the Mediterranean–Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) diet with risk of dementia.
Key Points
Question
Is adherence to the Mediterranean–Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) diet associated with the risk of dementia?
Findings
In this investigation of 3 prospective cohort studies including 18 136 participants and a meta-analysis including 224 049 participants, adherence to the MIND diet was associated with a lower risk of incident dementia in middle-aged and older adults. The highest adherence was associated with approximately 17% lower risk of dementia compared with the lowest adherence.
Meaning
The MIND diet may potentially reduce dementia risk, but further investigations are needed in different populations.
Abstract
Importance
Dementia threatens the well-being of older adults, making efforts toward prevention of great importance.
Objective
To evaluate the association of the Mediterranean–Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) diet with the risk of dementia in 3 prospective studies and a meta-analysis.
Design, Setting, and Participants
Cohort analyses included the Whitehall II study (WII), the Health and Retirement Study (HRS), and the Framingham Heart Study Offspring cohort (FOS), and the meta-analysis included 11 cohort studies. Participants were middle-aged and older women and men from WII in 2002 to 2004, HRS in 2013, and FOS in 1998 to 2001 without dementia at the study baseline. Data were analyzed from May 25 to September 1, 2022.
Exposures
MIND diet score was measured using food frequency questionnaires, and scores ranged from 0 to 15, with a higher score indicating higher adherence to the MIND diet.
Main Outcome and Measures
Incident all-cause dementia, with cohort-specific definitions.
Results
Included in this study were 8358 participants (mean [SD] age, 62.2 [6.0] years; 5777 male [69.1%]) from WII, 6758 participants (mean [SD] age, 66.5 [10.4] years; 3965 female [58.7%]) from HRS, and 3020 participants (mean [SD] age, 64.2 [9.1] years; 1648 female [54.6%]) from FOS. The mean (SD) baseline MIND diet score was 8.3 (1.4) in WII, 7.1 (1.9) in HRS, and 8.1 (1.6) in FOS. Over 166 516 person-years, a total of 775 participants (220 in WII, 338 in HRS, and 217 in FOS) developed incident dementia. In the multivariable-adjusted Cox proportional hazard model, higher MIND diet score was associated with lower risk of dementia (pooled hazard ratio [HR] for every 3-point increment, 0.83; 95% CI, 0.72-0.95; P for trend = .01; I2 = 0%). The associations were consistently observed in subgroups defined by sex, age, smoking status, and body mass index. In the meta-analysis of 11 cohort studies with 224 049 participants (5279 incident dementia cases), the highest tertile of MIND diet score was associated with lower risk of dementia compared with the lowest tertile (pooled HR, 0.83; 95% CI, 0.76-0.90; I2 = 35%).
Conclusions and Relevance
Results suggest that adherence to the MIND diet was associated with lower risk of incident dementia in middle-aged and older adults. Further studies are warranted to develop and refine the specific MIND diet for different populations.
Introduction
All-cause dementia poses substantial burdens on health care systems and threatens the well-being of older adults,1,2 and lack of effective treatments makes its prevention of great importance.1,3 Among numerous potential risk factors,1,4 dietary factors have generated major interest,5,6,7,8 including specific nutrients,9,10,11,12,13 food groups,14,15 and dietary patterns.6,8,16
Devised by Morris et al,17 the Mediterranean–Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) diet has been associated with lower risk of Alzheimer disease (AD)18 and slower cognitive decline.17 The MIND diet emphasizes natural plant-based foods, limited intake of animal foods and foods high in saturated fat, and uniquely encourages consumption of berries and green leafy vegetables17 rich in vitamins and antioxidants. Previous studies have assessed its association with cognitive function and decline,17,19,20 but only a few studies have examined its association with all-cause dementia or AD, with inconclusive results. In the Memory and Aging Project (MAP), which included 923 participants for a mean follow-up of 4.5 years, higher adherence to the MIND diet was associated with lower risk of AD.18 In the Rotterdam Study,21 the association of the MIND diet with incident dementia was statistically significant in the first 7 years of follow-up but became nonsignificant afterward. In aggregate, several constituents in the MIND diet have been suggested to be beneficial for cognitive health through the promotion of cardiovascular health or independent pathways directly linked to brain health,22 but the role of the MIND diet in dementia prevention remains inconclusive.
In the current study, we assessed the associations of adherence to the MIND diet with incident all-cause dementia in 3 cohort studies,23,24,25 1 from the UK, the Whitehall II study (WII), and 2 from the US, the Health and Retirement Study (HRS) and the Framingham Heart Study Offspring cohort (FOS). Further, we conducted a meta-analysis of 11 cohort studies21,26,27,28 reported in 5 articles to understand the association of the MIND diet with the risk of dementia.
Methods
Study Population
We leveraged individual-level data from 3 prospective studies.23,24,25 The WII was aimed at understanding the causes of age-related heterogeneity in health.23 WII enrolled 10 308 civil workers aged 35 to 55 years in 1985 and administered dietary assessments29 at phases 3 (1991-1993), 5 (1997-1999), and 7 (2002-2004).16 The HRS is a nationally representative cohort study of US adults 50 years and older.24,30 In 2013, 8035 HRS participants completed the Health Care and Nutrition Study, a substudy that contains a dietary assessment,31 followed by 3 cognitive assessments in 2014, 2016, and 2018. The Framingham Heart Study is a community-based cohort study commenced in 1948.25 In 1971, children of the original cohort and their spouses formed the FOS cohort. FOS participants completed dietary assessments in examinations 5 (1991-1995), 6 (1995-1998), and 7 (1998-2001) and have undergone continuous surveillance for dementia through 2018.32 All participants provided informed consent before data collection. The WII was approved by University College London Medical School committee, the HRS was approved by University of Michigan institutional review board (IRB), and the FOS was approved by Boston University Medical Center IRB.
In this study, we followed up WII participants from baseline (phase 7, 2002-2004) to 2016, HRS participants from baseline (2013) to 2018, and FOS participants from baseline (examination 7, 1998-2001) to 2018, which was consistent with previous studies.16,33 Thus, all assessments of diet preceded the ascertainment of dementia. We included participants 45 years and older who completed 1 or more valid food frequency questionnaires ([FFQs] defined as total energy intake in 500-4500 kcal). Participants with dementia at baseline or those who developed dementia in the first 2 years of follow-up were excluded (Figure 1A-C) to account, in part, for reverse causality. All participants provided written informed consent in the cohort studies. The current study was approved by IRB of Zhejiang University School of Public Health and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines34 for reporting observational studies.
Figure 1. Flowcharts of Participant Inclusion in the Cohort Analyses and Study Inclusion in the Meta-analysis.
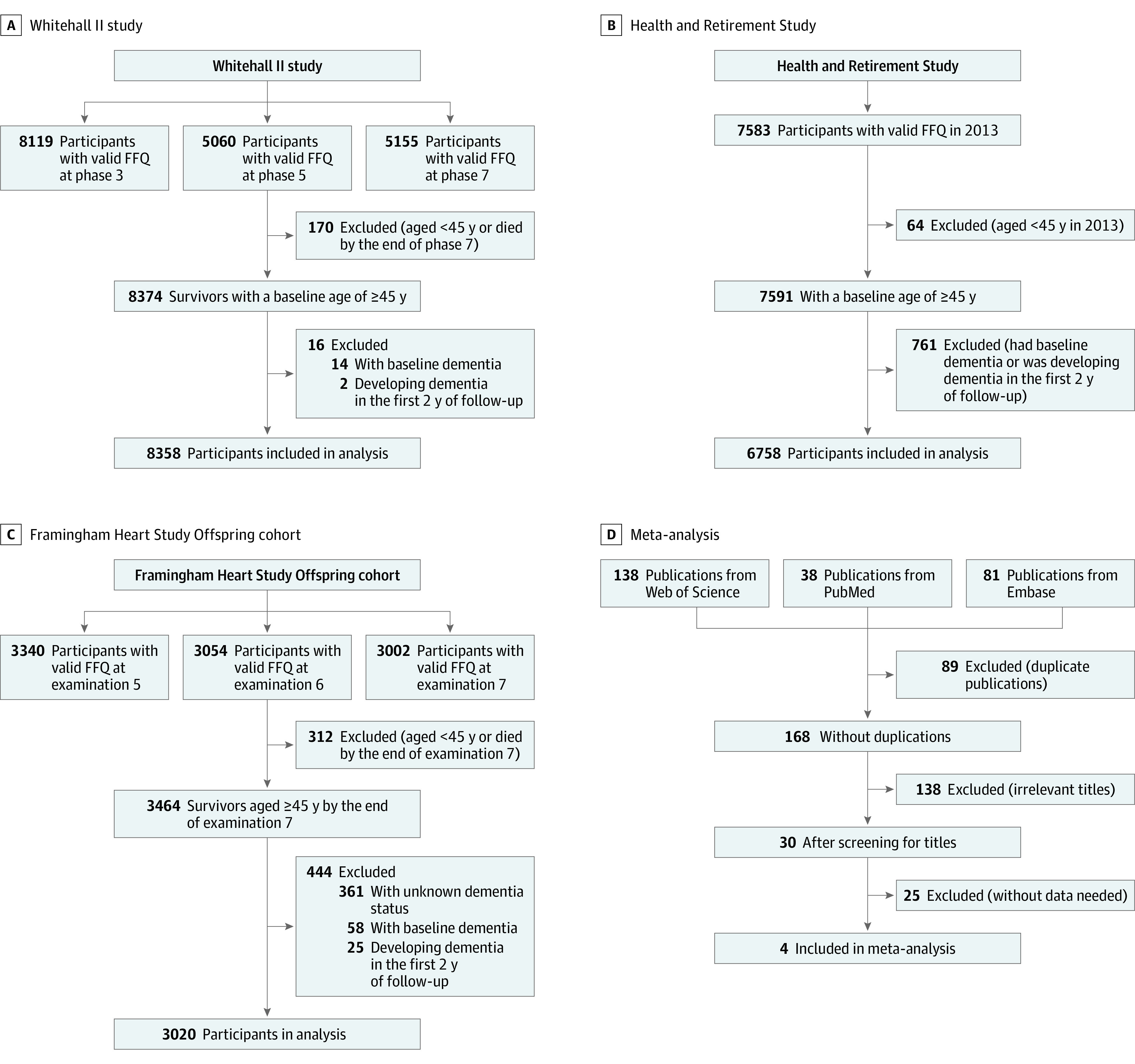
The median (IQR) follow-up durations were 12.9 (12.2-13.8) years in the Whitehall II study (A), 5.0 (3.0-5.0) years in the Health and Retirement Study (B), and 10.7 (7.5-14.3) years in Framingham Heart Study Offspring cohort (C). D, Search strategy used for the meta-analysis. FFQ indicates food frequency questionnaire.
MIND Diet Score
Participants were asked how often on average they consumed a specified amount of food in the past year using FFQs.23,24,25 Despite some cohort-specific modifications, the FFQs in all 3 cohort studies were based on the extensively validated FFQ35,36,37,38,39 devised by Willett and colleagues.35
The primary exposure was the MIND diet score, which consisted of 10 recommended food groups and 5 restricted food groups17 (eTable 1 in Supplement 1). The total score was computed by summing the 15 component scores (range, 0-15), with a higher score indicating higher adherence to the MIND diet. In the WII, which lacks information on olive oil, the original score ranged from 0 to 14 and was rescaled to a range of 0 to 15. We used the mean MIND diet scores for participants with assessments before baseline to reduce measurement error.
Ascertainment of Incident Dementia
The outcome of interest was incident all-cause dementia. The 3 cohort studies had different strategies in identifying incident dementia cases. Briefly, WII ascertained dementia using linkage to health care systems,16 HRS ascertained dementia using a validated algorithm,40 and FOS conducted panel reviews for dementia identification.32 Details are described in eMethods in Supplement 1.
Assessment of Other Covariates
We used questionnaires completed at and before baseline to collect the information on age (5-year groups), sex (male or female), race and ethnicity (White and non-White for WII and Black or African American, Hispanic, White, and Other for HRS), education level (high school diploma or not), occupational class (3 categories available in WII: administrative, professional, and supportive and clerical),16 household income (categorized into quartiles, available in the HRS and FOS), frequency of vigorous physical activity (<1 times per month, 1-4 times per month, or >1 times per week), current smoking status (yes or no), total energy intake, body mass index (BMI) categories (<25.0, 25.0-<30.0, or ≥30.0; calculated as weight in kilograms divided by height in meters squared), depressive symptom (yes or no), hypertension (yes or no), hypercholesterolemia (yes or no, not available in the HRS), diabetes (yes or no), stroke (yes or no), and cardiovascular diseases (yes or no). WII used a merged non-White racial category to protect the privacy of its participants. FOS did not take participant race into account because its racial makeup was predominantly White of European descent. Total energy was calculated from the FFQ concurrent with the MIND score. Alcohol intake was not considered a confounding variable because wine is a component of the MIND diet. Household income was assessed at examination 3 (1983-1987) for FOS participants and in 2014 for HRS participants. The assessments of health conditions are shown in eMethods in Supplement 1.
Meta-analysis
We conducted a meta-analysis using data from the current study and all other published population-based cohort studies reporting the association between the MIND diet and dementia or AD. Following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines,41 we registered the protocol on INPLASY.42 We systematically searched PubMed, Embase, and Web of Science on July 26, 2022, using strategy presented in eTable 2 in Supplement 1 and additionally screened the references of the included articles.
We included cohort studies that assessed the associations of the MIND diet and incident dementia or AD and extracted the name of the first author, publication year, cohort name, country, number of participants, age range at baseline, dietary assessment methods, and dementia ascertainment methods, risk estimates and 95% CIs from the multivariable-adjusted models and included covariates. We used the Newcastle-Ottawa scale to assess the quality of included studies. Two authors (H.C. and L.H.) independently screened the literature, extracted the data, and assessed the risk of bias. Disagreement and discordance were resolved by the third author (Y.H.).
We used random-effect models to pool the risk estimates comparing highest vs lowest MIND diet tertiles and reported the I2 statistic using R package meta (R Project for Statistical Computing).43 According to the Cochrane Handbook, an I2 statistic of 40% or less indicates minor heterogeneity, an I2 value in the range of 30% to 60% represents moderate heterogeneity, an I2 value in the range of 50% to 90% represents substantial heterogeneity; and an I2 value greater than 75% represents considerable heterogeneity.44 Publication bias was tested by using the Egger test.
Statistical Analyses
In the primary analyses, we assessed the association of MIND diet score and its components (average levels for repeated assessments) with incident dementia using Cox proportional hazard models, with adjustments for age, sex, total energy intake, education level, occupational class (only in WII), household income (only in HRS and FOS), frequency of vigorous physical activity, current smoking status, BMI categories, hypertension, diabetes, hypercholesterolemia (only in WII and FOS), stroke, and cardiovascular diseases. We calculated person-time from the study baseline (previously described) to date of incident dementia, death, loss to follow-up, or the end of follow-up, whichever occurred first. We assessed the associations of the MIND diet as a continuous (per 3-point increase) and categorical (tertiles) variable with the risk of dementia in separate models. Linearity assumption was evaluated by comparing the Akaike information criterion of a linear model with a quadratic model. We analyzed each cohort separately and pooled the estimates from the 3 cohorts using random-effects models.45 We performed prespecified stratified analyses by age (<70 years or ≥70 years), sex (female or male), current smoking status (yes or no), and BMI categories (<25.0 or ≥25.0). We tested for potential interactions using likelihood ratio tests.
We conducted several sensitivity analyses to test the robustness of our primary findings. First, we additionally adjusted the models for depression status because depression itself could be a confounding factor related to both dietary pattern and dementia, although it may be an important mediator.46 Second, we reassessed the association using the single baseline MIND score rather than the mean of multiple MIND scores. We excluded participants who developed dementia in the first 5 years of follow-up to further reduce reverse causation. We alternatively used prespecified cutoffs (7 and 9 points) of MIND diet score rather than the distribution-driven cutoffs. We restricted our analyses to participants without baseline stroke to reduce the potential confounding effect of stroke. Furthermore, we reported E values to assess robustness of the estimates to potential unmeasured confounding.47
Two-sided P values less than .05 indicate statistical significance. Statistical analyses were performed using SAS, version 9.4 (SAS Institute). We conducted multiple imputation for missing covariates using PROC MI (SAS Institute).48
Results
Cohort Analyses
In the cohort analyses, we included a total of 18 136 women and men who were free of dementia at baseline, including 8358 participants (mean [SD] age, 62.2 [6.0] years; 5777 male [69.1%]; 2581 female [30.9%]) from WII, 6758 participants (mean [SD] age, 66.5 [10.4] years; 2793 male [41.3%]; 3965 female [58.7%]) from HRS, and 3020 participants (mean [SD] age, 64.2 [9.1] years; 1372 male [45.4%]; 1648 female [54.6%]) from FOS (Table 1). The means (SDs) of baseline MIND diet scores were 8.3 (1.4) in WII, 7.1 (1.9) in HRS, and 8.1 (1.6) in FOS (eTable 3 in Supplement 1).
Table 1. Baseline Characteristics of Participants in the Whitehall II Study, the Health and Retirement Study, and the Framingham Offspring Cohort.
| Variable | No. (%) | ||
|---|---|---|---|
| Whitehall II study (n = 8358) | Health and Retirement Study (n = 6758) | Framingham Heart Study Offspring cohort (n = 3020) | |
| MIND diet score, mean (SD) | 8.3 (1.4) | 7.1 (1.9) | 8.1 (1.6) |
| Age group, y | |||
| 45-59 | 2341 (28.0) | 2077 (30.7) | 1020 (33.8) |
| 60-69 | 3896 (46.6) | 1966 (29.1) | 1093 (36.2) |
| 70-79 | 2121 (25.4) | 1897 (28.1) | 750 (24.8) |
| 80≤ | NA | 818 (12.1) | 157 (5.2) |
| Sex | |||
| Female | 2581 (30.9) | 3965 (58.7) | 1648 (54.6) |
| Male | 5777 (69.1) | 2793 (41.3) | 1372 (45.4) |
| Race and ethnicitya | |||
| Black or African American | NA | 1044 (15.4) | NA |
| Hispanic | 727 (10.8) | ||
| White | 7599 (90.9) | 4767 (70.5) | |
| Other | NA | 220 (3.3) | |
| Total energy, mean (SD), kcal/d | 2020 (584) | 1751 (678) | 1865 (553) |
| Education duration, mean (SD), y | 15.3 (4.0) | 13.1 (2.8) | 13.9 (2.5) |
| Low occupation position | 1264 (15.1) | NA | NA |
| Household income, quartile | |||
| 1 | NA | 1688 (20.3) | 742 (24.6) |
| 2 | NA | 1582 (23.4) | 740 (24.5) |
| 3 | NA | 1897 (28.1) | 731 (24.2) |
| 4 | NA | 1905 (28.2) | 807 (26.7) |
| Current smoker | 844 (10.1) | 724 (10.7) | 386 (12.8) |
| Body mass indexb | |||
| <25.0 | 3304 (39.5) | 1441 (21.3) | 928 (30.7) |
| 25.0-<30.0 | 3596 (43.0) | 2295 (34.0) | 1226 (40.6) |
| ≥30.0 | 1458 (17.4) | 3022 (44.7) | 866 (28.7) |
| Vigorous physical activity | |||
| <1 Time/mo | 4664 (55.8) | 3594 (53.2) | 1084 (35.9) |
| 1-4 Times/mo | 1713 (20.5) | 1730 (25.6) | 493 (16.3) |
| >1 Times/wk | 1981 (23.7) | 1434 (21.2) | 1443 (47.8) |
| Hypertension | 4766 (57.0) | 4073 (60.3) | 1600 (53.0) |
| Hypercholesterolemia | 7451 (89.1) | NA | 1857 (61.5) |
| Cardiovascular diseases | 2871 (34.4) | 1683 (24.9) | 450 (14.9) |
| Diabetes | 1599 (19.2) | 1580 (23.4) | 294 (9.7) |
| Stroke | 181 (2.2) | 527 (7.8) | 57 (1.9) |
| Depressive symptoms | 949 (11.4) | 863 (12.8) | 378 (12.5) |
| Follow-up duration, median (IQR), y | 12.9 (12.2-13.8) | 5.0 (3.0-5.0) | 10.7 (7.5-14.3) |
Abbreviations: DASH, Dietary Approaches to Stop Hypertension; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; NA, not applicable.
The Whitehall II study used the merged racial category of non-White to protect the privacy of its participants; the Framingham Study did not take participant race into account because its racial makeup was predominantly White of European descent.
Calculated as weight in kilograms divided by height in meters squared.
Over 166 516 person-years, 775 participants (220 of 105 949 person-years in WII, 338 of 28 934 person-years in HRS, and 217 of 31 633 person-years in FOS) developed incident all-cause dementia. In the multivariable-adjusted model (Table 2), the pooled hazard ratios (HRs) were 0.81 (95% CI, 0.67-0.98) for the highest vs lowest tertiles of MIND score and 0.83 (95% CI, 0.72-0.95; P for trend = .01) for every 3-point increment. The estimates showed moderate robustness to potential unmeasured confounders (E value, 1.70; 95% CI, 1.28-2.12). The HRs per 3-point increment were 0.97 (95% CI, 0.72-1.30) in WII, 0.82 (95% CI, 0.68-0.99) in HRS, and 0.76 (95% CI, 0.57-1.00) in FOS (I2 = 0%). Potential contributors included higher consumption of green leafy and other vegetables, berries, nuts, olive oil, and beans and lower consumptions of red meat and products (eTable 4 in Supplement 1). For example, the HRs were 0.70 (95% CI, 0.53-0.91) for nuts (≥6 vs <1 servings per week) and 1.26 (95% CI, 1.02-1.56) for red meat and products (≥7 vs <4 servings per week).
Table 2. Multivariable-Adjusted Hazard Ratios (HRs) and 95% CIs for Incident Dementia According to Tertiles of Mediterranean–Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) Diet Scorea.
| Study | Overall | Tertile 1 | Tertile 2 | Tertile 3 | Per 3-point increment | E value | P value for trend |
|---|---|---|---|---|---|---|---|
| Whitehall II study | |||||||
| Median | 8.3 | 7 | 8.3 | 9.6 | NA | NA | NA |
| Cases/total No. | 220/8358 | 53/2131 | 98/3635 | 69/2592 | NA | NA | NA |
| HR (95% CI) | NA | 1 [Reference] | 1.03 (0.73-1.45) | 0.96 (0.66-1.38) | 0.97 (0.72-1.30) | NA | .83 |
| Health and Retirement Study | |||||||
| Median | 7 | 5 | 7 | 9 | NA | NA | NA |
| Cases/total No. | 338/6758 | 137/2344 | 100/1952 | 101/2462 | NA | NA | NA |
| HR (95% CI) | NA | 1 [Reference] | 0.95 (0.73-1.25) | 0.83 (0.63-1.09) | 0.82 (0.68-0.99) | 1.73 (1.11-2.30) | .04 |
| Framingham Heart Study | |||||||
| Median | 8.2 | 6.5 | 8.2 | 9.8 | NA | NA | NA |
| Cases/total No. | 217/3020 | 73/941 | 78/1030 | 66/1049 | NA | NA | NA |
| HR (95% CI) | NA | 1 [Reference] | 0.96 (0.70-1.33) | 0.69 (0.48-0.99) | 0.76 (0.57-1.00) | 1.96 (1.00-2.90) | .04 |
| Pooledb | |||||||
| HR (95% CI) | NA | 1 [Reference] | 0.97 (0.81-1.15) | 0.81 (0.67-0.98) | 0.83 (0.72-0.95) | 1.70 (1.28-2.12) | .01 |
Abbreviations: HRS, Health and Retirement Study; NA, not applicable.
HRs (95% CIs) of the MIND diet were estimated as a continuous (per 3-point increase) and categorical (tertiles) variable with the risk of dementia in separate models. The Cox proportional hazard models were adjusted for age (5-year groups), sex (male or female), education level (high school diploma or not), occupational class (administrative, professional, and supportive; and clerical and supportive, available in the Whitehall II study), household income (as quartiles, available in the Health and Retirement Study and Framingham Heart Study Offspring cohort), frequency of vigorous physical activity (<1 times/mo, 1-4 times/mo, >1 times/week), smoking status (current, former, or never), total energy intake, body mass index categories (<25.0, 25.0-<30.0, or ≥30.0; calculated as weight in kilograms divided by height in meters squared), depressive symptom (yes or no), hypertension (yes or no), hypercholesterolemia (yes or no, not available in the Health and Retirement Study), diabetes (yes or no), stroke (yes or no), and cardiovascular diseases (yes or no).
Random-effects model was used; I2 = 0% for the estimates from the 3 cohorts.
In the subgroup analyses (eTable 5 in Supplement 1), although no significant effect modification was observed (P interactions >.10 for all tests), the MIND diet was not significantly associated with dementia risk among smokers (HR, 1.10; 95% CI, 0.68-1.75), but in nonsmokers, the association was strong and significant (HR, 0.79; 95% CI, 0.68-0.92). The associations did not vary significantly by sex, age, and BMI. For example, the HR was 0.82 (95% CI, 0.65-1.04) for nonoverweight individuals and 0.82 (95% CI, 0.68-0.97) for overweight individuals.
The primary findings persisted in the sensitivity analyses (eTable 6 in Supplement 1). When using alternative cutoffs, the HR for a score of 7.0 to 8.9 was 0.95 (95% CI, 0.80-1.13) and 0.75 (95% CI, 0.60-0.93) for a score of 9.0 to 15.0, compared with MIND score less than 7.0. Using only baseline MIND diet score rather than the mean MIND scores before the baseline, we observed similar associations (HR, 0.79; 95% CI, 0.69-0.91 for each 3-point increment in MIND score). When we further adjusted the models for depressive symptoms, the HR was 0.83 (95% CI, 0.72-0.95). When we excluded incident dementia cases within the first 5 years of follow-up, the pooled HR was 0.83 (95% CI, 0.63-1.09). Similarly, excluding participants with a stroke history, the association remained significant (HR, 0.85; 95% CI, 0.73-0.99).
Meta-analysis
We screened 257 studies and included 11 cohort studies21,26,27,28 reported in 5 articles (including this study).18,21,26,27,28 Specifically, we included the 2 largely nonoverlap subcohorts in the Rotterdam Study, and excluded the analyses on MAP by Morris et al18 because it was updated by Vu et al.26 We converted the HRs per SD increment in the Rotterdam Study to HRs comparing means in highest vs lowest tertiles assuming a linear association.49 The characteristics of the included studies were shown in eTables 7 and 8 in Supplement 1.
Pooling 11 risk estimates for 224 049 participants (5279 incident dementia cases) (Figure 2),21,26,27,28 the HR comparing the highest vs lowest tertile of the MIND diet score was 0.83 (95% CI, 0.76-0.90), and the E value was 1.70 (95% CI, 1.45-1.96). The association showed moderate heterogeneity across studies (I2 = 35%). We did not observe significant publication bias (eFigure in Supplement 1). No single study substantially affected the pooled results (data not shown).
Figure 2. Meta-analysis of Multivariable-Adjusted Hazard Ratio (HR) and 95% CI for Incident Dementia Comparing the Highest vs Lowest Tertile of Mediterranean–Dietary Approaches to Stop Hypertension (DASH) Intervention for Neurodegenerative Delay (MIND) Diet Score.
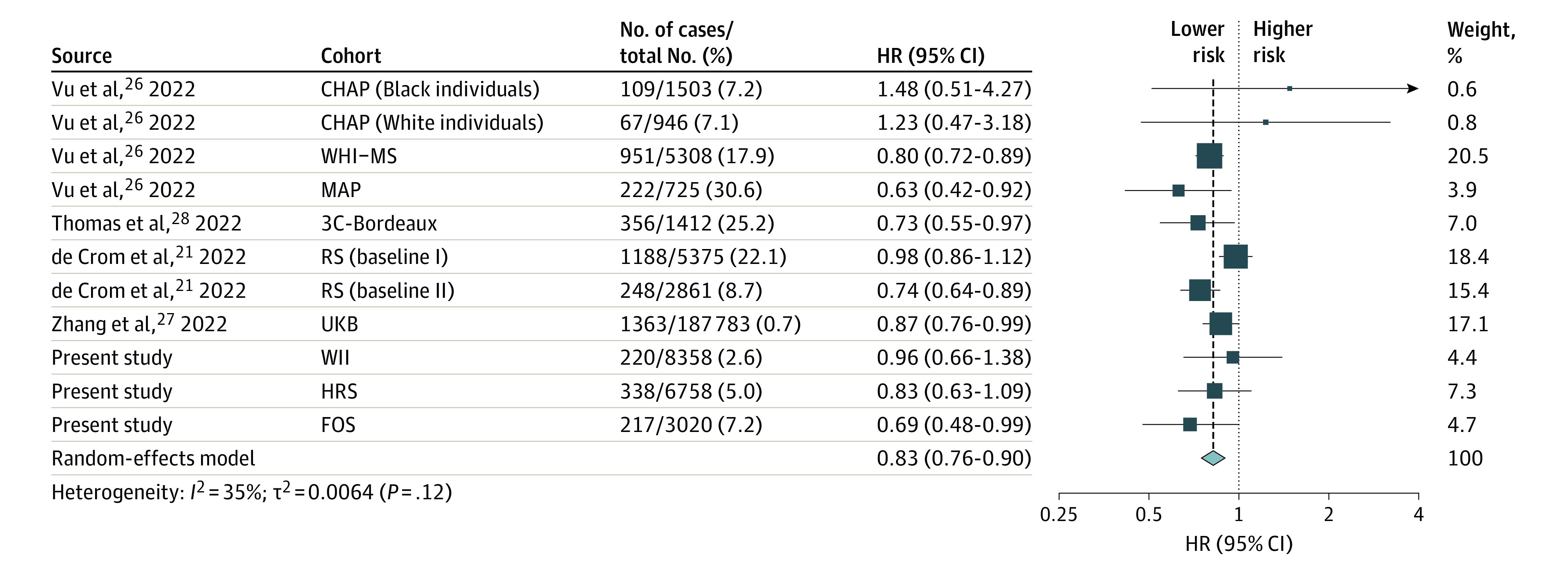
HR below 1.00 indicates a lower risk of dementia. We used random-effects models to pool the risk estimates comparing highest vs lowest MIND diet tertiles. CHAP indicates Chicago Health and Aging Project26; FOS, Framingham Heart Study Offspring cohort; HRS, Health and Retirement Study; MAP, Memory and Aging Project26; RS, Rotterdam Study21; 3C, Three-City Study28; UKB, UK Biobank27; WHI-MS, Women’s Health Initiative Memory Study26; WII, Whitehall II study.
Discussion
In the current investigation of 3 cohort studies, higher adherence to the MIND diet was associated with a lower risk of incident all-cause dementia. The associations were significant in the HRS and FOS but not in WII. We observed consistent associations across major study subgroups. In the meta-analysis, we observed that the MIND diet was associated with lower risk of dementia, although the evidence was moderately heterogeneous.
To date and to our knowledge, only a few cohort studies have examined the association of the MIND diet with dementia risk, although adherence to this diet has been linked to cognitive function and its decline.17,19 In the MAP (median follow-up = 4.5 years), higher MIND diet score was associated with substantially lower risk of incident AD18 (HR, 0.47; 95% CI, 0.29-0.76 comparing extreme tertiles). A recent study in the Netherlands reported a significant protective association of the MIND diet within the first 7 years of follow-up (HR, 0.85; 95% CI, 0.74-0.98 per SD increment), but this association was no longer significant in longer-term follow-up within the same population of baseline 1.21 Based on these previous findings, our observations in 3 well-characterized cohort studies suggest a potentially protective association. Most studies evaluating the link between the MIND diet and other cognitive outcomes (including cognitive function, its decline and incident cognitive impairment) are limited by small sample sizes or short-term follow-up, which may result in reverse causation induced by early behavioral changes resulting from preclinical dementia. Our study provided valuable information with relatively long-term follow-up, but future studies are still warranted to clarify the causal association between the MIND diet and dementia. Meanwhile, to which extent a healthy diet intervention could reduce dementia risk in specific populations and the optimal intervention time window for this intervention remain largely unknown. Currently, a 3-year, multicenter, randomized clinical trial of the MIND diet on cognitive decline is being conducted in US adults, which is expected to provide valuable evidence for dietary strategies in dementia prevention.50
Although the underlying biological mechanism remains unclear, oxidation and inflammation pathways could explain the observed associations. MIND diet emphasizes several food groups associated with brain health, such as green leafy vegetables, olive oil, and berries,50 which were confirmed in this study. These food groups may confer protection against brain aging through their antioxidant and anti-inflammatory properties and inhibition of β amyloid deposition by specific constituents, such as vitamin E,51 folate,12 flavonoids,11 and carotenoids.9 On the contrary, red and processed meat contains N-nitroso compounds, which may induce oxidative stress and inflammation and contribute to the development of dementia.52
Although the MIND diet showed potential to reduce dementia risk, we observed moderate unexplained heterogeneity in the current literature. The differences in education levels, age ranges, and duration of follow-up periods among cohorts may be the underlying reasons. For example, in the WII cohort where participants were relatively better-educated civil workers, a previous analysis found that midlife alternate healthy eating index was not associated with later-life dementia,16 and our results added that the MIND score was not associated with dementia risk. In the Rotterdam Study (baseline 1), the association of the MIND diet with incident dementia was significant in the first 7-year follow-up but was also attenuated to null in the 15.6-year follow-up.21 In addition, differences in adjustments for confounding may contribute to the heterogeneity. Furthermore, the cultural backgrounds in different populations may be an important underlying source of heterogeneity, and the current literature on the MIND diet is largely based on a Western population. Whether this pattern is beneficial for primary prevention of dementia in other populations warrants exploration.
Strengths and Limitations
The strengths of the current study include long-term follow-up in population-based cohort studies with validated approaches to assess dietary intake. Nevertheless, our findings should be interpreted with caution due to some limitations. First, we were unable to specifically assess the association of the MIND diet with subtypes of dementia because of the inconsistent definitions across cohorts. Second, confounding owing to unmeasured food items could still lead to biased estimation of the association of MIND diet with dementia. Because self-reported food consumption may be subject to measurement error and reporting bias, the MIND diet scores may not accurately reflect the actual adherence to the MIND diet. Given the long preclinical stage of dementia, the observational findings may still be subject to reverse causality because cognitive impairment may affect the ability of reporting of diet and adherence to the diet itself. Cohort studies with shorter-term follow-up may be more likely to be influenced by this, such as the HRS. Therefore, our observational findings of this study do not reflect causality. Third, the cohort studies and meta-analysis mainly consisted of a Western population; therefore, our findings may not be generalizable to populations with different cultural and ethnic backgrounds. Furthermore, misclassification of outcome may exist, especially in the cohorts relying on linkage to data with health electronic systems (eg, WII) or algorithms using data from objective cognitive tests rather than clinical diagnosis (eg, HRS). Finally, consumption of some food groups, such as olive oil, may not have been commonly consumed in specific countries at baseline, and further investigations of these food groups are warranted.
Conclusions
In this analysis of 3 cohort studies and a meta-analysis, findings suggest that adherence to the MIND diet was prospectively associated with lower dementia risk, although moderate heterogeneity was observed across studies. Future studies are needed to evaluate and refine the MIND diet in different populations and to identify the optimal intervention time window for dementia prevention.
eMethods. Ascertainment of Dementia and Other Health Conditions
eTable 1. Scoring System of the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Score
eTable 2. Searching Strategy in the Meta-analysis
eTable 3. Mean and Standard Deviation (SD) of the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet
eTable 4. Multivariable Adjusted Hazard Ratios (95% Confidence Intervals) for Incident Dementia According to Components of Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Score
eTable 5. Multivariable Adjusted Hazard Ratios (95% Confidence Intervals) for Incident Dementia According to Tertiles of Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Score in Study Subgroups
eTable 6. Multivariable Adjusted Hazard Ratios (95% Confidence Intervals) for Incident Dementia According to Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Score in Sensitivity Analyses
eTable 7. Characteristics of Included Studies in the Meta-analysis
eTable 8. Risk of Bias of the Included Studies in the Newcastle-Ottawa Scale
eFigure. Funnel Plot in the Meta-analysis
Data Sharing Statement
References
- 1.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413-446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2019 Dementia Forecasting Collaborators . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105-e125. doi: 10.1016/S2468-2667(21)00249-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015—the global impact of dementia: an analysis of prevalence, incidence, cost and trends. Accessed May 16, 2022. https://www.alzint.org/u/WorldAlzheimerReport2015.pdf
- 4.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11(6):718-726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 5.Morris MC. Nutrition and risk of dementia: overview and methodological issues. Ann N Y Acad Sci. 2016;1367(1):31-37. doi: 10.1111/nyas.13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao L, Tan L, Wang HF, et al. Dietary patterns and risk of dementia: a systematic review and meta-analysis of cohort studies. Mol Neurobiol. 2016;53(9):6144-6154. doi: 10.1007/s12035-015-9516-4 [DOI] [PubMed] [Google Scholar]
- 7.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr. 2015;6(2):154-168. doi: 10.3945/an.114.007617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YH, Gao X, Na M, Kris-Etherton PM, Mitchell DC, Jensen GL. Dietary pattern, diet quality, and dementia: a systematic review and meta-analysis of prospective cohort studies. J Alzheimers Dis. 2020;78(1):151-168. doi: 10.3233/JAD-200499 [DOI] [PubMed] [Google Scholar]
- 9.Yuan C, Chen H, Wang Y, Schneider JA, Willett WC, Morris MC. Dietary carotenoids related to risk of incident Alzheimer dementia (AD) and brain AD neuropathology: a community-based cohort of older adults. Am J Clin Nutr. 2021;113(1):200-208. doi: 10.1093/ajcn/nqaa303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000;16(4):357-363. doi: 10.1023/A:1007614613771 [DOI] [PubMed] [Google Scholar]
- 11.Cheng N, Bell L, Lamport DJ, Williams CM. Dietary flavonoids and human cognition: a meta-analysis. Mol Nutr Food Res. 2022;66(21):e2100976. doi: 10.1002/mnfr.202100976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crichton GE, Bryan J, Murphy KJ. Dietary antioxidants, cognitive function and dementia: a systematic review. Plant Foods Hum Nutr. 2013;68(3):279-292. doi: 10.1007/s11130-013-0370-0 [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Ding Y, Wu F, Li R, Hou J, Mao P. Omega-3 fatty acids intake and risks of dementia and Alzheimer’s disease: a meta-analysis. Neurosci Biobehav Rev. 2015;48:1-9. doi: 10.1016/j.neubiorev.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 14.Bakre AT, Chen R, Khutan R, et al. Association between fish consumption and risk of dementia: a new study from China and a systematic literature review and meta-analysis. Public Health Nutr. 2018;21(10):1921-1932. doi: 10.1017/S136898001800037X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ran LS, Liu WH, Fang YY, et al. Alcohol, coffee, and tea intake and the risk of cognitive deficits: a dose-response meta-analysis. Epidemiol Psychiatr Sci. 2021;30:e13. doi: 10.1017/S2045796020001183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbaraly TN, Singh-Manoux A, Dugravot A, Brunner EJ, Kivimäki M, Sabia S. Association of midlife diet with subsequent risk for dementia. JAMA. 2019;321(10):957-968. doi: 10.1001/jama.2019.1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015-1022. doi: 10.1016/j.jalz.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007-1014. doi: 10.1016/j.jalz.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berendsen AM, Kang JH, Feskens EJM, de Groot CPGM, Grodstein F, van de Rest O. Association of long-term adherence to the MIND diet with cognitive function and cognitive decline in American women. J Nutr Health Aging. 2018;22(2):222-229. doi: 10.1007/s12603-017-0909-0 [DOI] [PubMed] [Google Scholar]
- 20.Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019;15(4):581-589. doi: 10.1016/j.jalz.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 21.de Crom TOE, Mooldijk SS, Ikram MK, Ikram MA, Voortman T. MIND diet and the risk of dementia: a population-based study. Alzheimers Res Ther. 2022;14(1):8. doi: 10.1186/s13195-022-00957-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9(7):568-578. doi: 10.1038/nrn2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marmot M, Brunner E. Cohort profile: the Whitehall II study. Int J Epidemiol. 2005;34(2):251-256. doi: 10.1093/ije/dyh372 [DOI] [PubMed] [Google Scholar]
- 24.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JWR, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576-585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsao CW, Vasan RS. Cohort profile: the Framingham Heart Study (FHS): overview of milestones in cardiovascular epidemiology. Int J Epidemiol. 2015;44(6):1800-1813. doi: 10.1093/ije/dyv337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vu THT, Beck T, Bennett DA, et al. Adherence to MIND diet, genetic susceptibility, and incident dementia in 3 US cohorts. Nutrients. 2022;14(13):2759. doi: 10.3390/nu14132759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Cao X, Li X, et al. Associations of midlife diet quality with incident dementia and brain structure: findings from the UK Biobank Study. medRxiv. Preprint posted online May 2, 2022. doi: 10.1101/2022.05.06.22274696 [DOI] [PubMed]
- 28.Thomas A, Lefèvre-Arbogast S, Féart C, et al. Association of a MIND diet with brain structure and dementia in a French population. J Prev Alzheimers Dis. 2022;9(4):655-664. doi: 10.14283/jpad.2022.67 [DOI] [PubMed] [Google Scholar]
- 29.Brunner E, Stallone D, Juneja M, Bingham S, Marmot M. Dietary assessment in Whitehall II: comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br J Nutr. 2001;86(3):405-414. doi: 10.1079/BJN2001414 [DOI] [PubMed] [Google Scholar]
- 30.Juster FT, Suzman R. An overview of the Health and Retirement Study. J Hum Resour. 1995;30:S7-S56. doi: 10.2307/146277 [DOI] [Google Scholar]
- 31.Bishop NJ, Wang K. Food insecurity, comorbidity, and mobility limitations among older US adults: findings from the Health and Retirement Study and Health Care and Nutrition Study. Prev Med. 2018;114:180-187. doi: 10.1016/j.ypmed.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 32.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over 3 decades in the Framingham Heart Study. N Engl J Med. 2016;374(6):523-532. doi: 10.1056/NEJMoa1504327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pase MP, Himali JJ, Beiser AS, et al. Sugar- and artificially sweetened beverages and the risks of incident stroke and dementia: a prospective cohort study. Stroke. 2017;48(5):1139-1146. doi: 10.1161/STROKEAHA.116.016027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 35.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. doi: 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 36.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570-584. doi: 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051-1063. doi: 10.1093/aje/kwx328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Shaar L, Yuan C, Rosner B, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire in men assessed by multiple methods. Am J Epidemiol. 2021;190(6):1122-1132. doi: 10.1093/aje/kwaa280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue Y, Yuan C, Wang DD, et al. Reproducibility and validity of diet quality scores derived from food-frequency questionnaires. Am J Clin Nutr. 2022;115(3):843-853. doi: 10.1093/ajcn/nqab368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66 suppl 1(suppl 1):i162-i171. doi: 10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mediterranean–Dietary Approaches to Stop Hypertension (MIND) diet and risk of dementia: meta-analysis of cohort studies. INPLASY:INPLASY202270127. Published online July 31, 2022. https://inplasy.com/inplasy-2022-7-0127/
- 43.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40-45. [Google Scholar]
- 44.Higgins JPT, Thomas J, Chandler J, et al. , eds. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. John Wiley & Sons; 2019. [Google Scholar]
- 45.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221 [DOI] [PubMed] [Google Scholar]
- 46.Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7(6):323-331. doi: 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 48.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15. doi: 10.1177/096228029900800102 [DOI] [PubMed] [Google Scholar]
- 49.Chêne G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol. 1996;144(6):610-621. doi: 10.1093/oxfordjournals.aje.a008971 [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Morris MC, Dhana K, et al. Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) study: rationale, design and baseline characteristics of a randomized control trial of the MIND diet on cognitive decline. Contemp Clin Trials. 2021;102:106270. doi: 10.1016/j.cct.2021.106270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCleery J, Abraham RP, Denton DA, et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst Rev. 2018;11(11):CD011905. doi: 10.1002/14651858.CD011905.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de la Monte SM, Neusner A, Chu J, Lawton M. Epidemiological trends strongly suggest exposures as etiologic agents in the pathogenesis of sporadic Alzheimer’s disease, diabetes mellitus, and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;17(3):519-529. doi: 10.3233/JAD-2009-1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Ascertainment of Dementia and Other Health Conditions
eTable 1. Scoring System of the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Score
eTable 2. Searching Strategy in the Meta-analysis
eTable 3. Mean and Standard Deviation (SD) of the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet
eTable 4. Multivariable Adjusted Hazard Ratios (95% Confidence Intervals) for Incident Dementia According to Components of Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Score
eTable 5. Multivariable Adjusted Hazard Ratios (95% Confidence Intervals) for Incident Dementia According to Tertiles of Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Score in Study Subgroups
eTable 6. Multivariable Adjusted Hazard Ratios (95% Confidence Intervals) for Incident Dementia According to Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet Score in Sensitivity Analyses
eTable 7. Characteristics of Included Studies in the Meta-analysis
eTable 8. Risk of Bias of the Included Studies in the Newcastle-Ottawa Scale
eFigure. Funnel Plot in the Meta-analysis
Data Sharing Statement


