Abstract
Post-traumatic stress disorder (PTSD) has been associated with cardiovascular disease (CVD), but the mechanisms remain unclear. Autonomic dysfunction, associated with higher CVD risk, may be triggered by acute PTSD symptoms. We hypothesized that a laboratory-based trauma reminder challenge, which induces acute PTSD symptoms, provokes autonomic dysfunction in a cohort of veteran twins. We investigated PTSD-associated real-time physiologic changes with a simulation of traumatic experiences in which the twins listened to audio recordings of a one-minute neutral script followed by a one-minute trauma script. We examined two heart rate variability metrics: deceleration capacity (DC) and logarithmic low frequency (log-LF) power from beat-to-beat intervals extracted from ambulatory electrocardiograms. We assessed longitudinal PTSD status with a structured clinical interview and the severity with the PTSD Symptoms Scale. We used linear mixed-effects models to examine twin dyads and account for cardiovascular and behavioral risk factors. We examined 238 male Veteran twins (age 68 ± 3 years old, 4% black). PTSD status and acute PTSD symptom severity were not associated with DC or log-LF measured during the neutral session, but were significantly associated with lower DC and log-LF during the traumatic script listening session. Long-standing PTSD was associated with a 0.38 (95% confidence interval, −0.83,− 0.08) and 0.79 (−1.30, −0 .29) standardized unit lower DC and log-LF, respectively, compared to no history of PTSD. Traumatic reminders in patients with PTSD lead to real-time autonomic dysregulation and suggest a potential causal mechanism for increased CVD risk, based on the well-known relationships between autonomic dysfunction and CVD mortality.
Keywords: DC-HRV, HRV, LF-HRV, PTSD, trauma stress test
1 |. INTRODUCTION
Post-Traumatic Stress Disorder (PTSD) is a chronic psychiatric disorder which may occur after experiencing or witnessing a traumatic event (Perkonigg et al., 2000). The estimated lifetime prevalence of PTSD among adult Americans is 6.8% (Kessler et al., 2005) and the number is higher in military combat exposure veterans with a lifetime prevalence between 20 and 30% (Trivedi et al., 2015; Zhu & Sano, 2021). Several previous studies have revealed an association between PTSD and cardiovascular disease (CVD) (Dursa et al., 2014; Ebrahimi et al., 2021; O’Donnell et al., 2021). In a previous study looking at PTSD and CVD association, Vaccarino et al., found an odd ratio of 2.2 for CVD incidence and a 0.21 lower coronary flow reserve on PTSD subjects as compared to those without PTSD, even after adjusting for lifestyle, CVD risk factors, and depression (Vaccarino et al., 2013). In a meta-analysis of 445 studies, Akosile et al. found a pooled hazard ratio of 1.61 for the relation between PTSD and CVD, before adjustment, and a 1.46 hazard ratio after adjustment for depression (Akosile et al., 2018). However, the mechanisms linking PTSD and CVD are not completely clear as the associations persisted despite multivariable adjustment for traditional risk factors. Therefore, more research is needed to understand the PTSD-CVD relationship and inform future cardiac prevention intervention efforts (O’Donnell et al., 2021).
Understanding acute physiological changes that occur during a traumatic reminder experience, which we have found can be induced in a closely monitored laboratory setting, may help elucidate pathways linking PTSD and CVD (Schmahl et al., 2004). One psychophysiology measure of particular interest is heart rate variability (HRV), a measure of the dynamic heart rate changes over time due to fluctuations in autonomic nervous system activity (Shaffer & Ginsberg, 2017). In a meta-analysis of 499 studies comparing autonomic nervous system function in individuals with PTSD and healthy controls, Ge et al. found a reduction of 4.86 high frequency HRV (HF) and 1.86 low frequency HRV (LF) in PTSD individuals compared to controls (Ge et al., 2020). A review paper by Hemingway et al. found six papers showing a relationship between PTSD and autonomic dysfunction, and two papers that found no relationship (Hemingway, 2021). Furthermore, Dennis et al. investigated the association between PTSD and autonomic arousal by analyzing self-reported PTSD symptoms via ecological momentary assessment and computing HRV from a 24-h Holter monitor and found a significant negative slope of 0.88 LF-HRV and 0.81 HF-HRV with momentary PTSD symptoms severity (Dennis et al., 2017).
LF-HRV reflects a combination of sympathetic and parasympathetic activity in approximately the same frequency range as the baroreflex activity (0.04–0.15 hertz) and is reduced by nearly 50% in PTSD subjects (Shah et al., 2013). This may stem from sympathetic overactivity in PTSD, which may suppress the baroreflex via the amygdala (Shah et al., 2022). High frequency HRV (HF) had also been previously associated with PTSD and some studies suggest a reduction in HF-HRV in PTSD subjects (Tucker et al., 2012; Wahbeh & Oken, 2013), however, others found no significant relationship (Hemingway, 2021; Lee et al., 2018). HF-HRV involves parasympathetic modulation and it is highly dependent on respiration (Aysin & Aysin, 2006; Quintana et al., 2016). In addition, the dependence of HF HRV with posture may help to explain conflicting results by previous studies (Mourot, 2014). Similarly, the root mean square of successive differences (RMSSD) is also associated with parasympathetic activity, and some studies suggest a significant difference of RMSSD between PTSD and non-PTSD participants. This also suggests lower resting baseline parasympathetic activity in PTSD (Schneider & Schwerdtfeger, 2020). However, some studies have found RMSSD and HF-HRV to have little to no association with CVD mortality (Hayano et al., 2021). On the other hand, the deceleration capacity (DC) of the heart rate has been proposed as a measure of vagal activity and has been found to be a more powerful prognostic measure as compared to HF-HRV (Hayano et al., 2021). An attenuation of DC was associated with a withdrawal of vagal activity after myocardial infarction and was strongly associated with an increase in mortality (Bauer, Kantelhardt, Barthel, et al., 2006). However, whether an acute traumatic reminder inducing PTSD symptoms may impact these markers of autonomic regulation has not been examined.
In this study, we measured the relationship between experimentally induced acute PTSD symptoms and HRV during a trauma reminder stress test in a highly controlled study of twin veterans with and without PTSD. We hypothesized that stress-induced autonomic dysfunction, measured by reduced LF and DC HRV during a trauma reminder stress test, is associated with PTSD symptoms severity, as well as with the duration of the PTSD diagnosis, in a dose–response manner. These physiological parameters will help us focus on possible mechanisms linking PTSD and CVD due to DC being a measure that allows study of the parasympathetic activity (Hayano et al., 2021); LF-HRV helps to study baroreflex activity indirectly (Shah et al., 2022); and Heart rate allows study of sympathovagal balance (Bootsma et al., 2003). Previous research has found all 3 of these are robust predictors of CVD mortality (Bauer, Kantelhardt, Barthel, et al., 2006; Hayano et al., 2021).
2 |. METHOD
2.1 |. Study participants
The Emory Twin Studies (ETS) project (Vaccarino et al., 2011) recruited twin pairs from the Vietnam Era Twin (VET) Registry (Eisen et al., 1987) born between 1939 and 1955. The current study includes participants of the ETS follow-up visit previously described (Huang et al., 2022; Vaccarino et al., 2022). The study was approved by the Emory Institutional Review Board, and all twins signed an informed consent form. They were recruited from the continental United States and transported to Emory University Hospital for study procedures. All data were collected on the same date for each pair.
2.2 |. Study protocol
All participants were told to hold all alcohol, caffeine, and nicotine products during the study. They ate a controlled breakfast and then began fasting until the end of the trauma reminder stress test. We did not specify to avoid strenuous exercise but as the subjects were in the clinic with the study team starting at 7 or 8 am and were also participants in an overnight sleep study the night before, they would not have been able to exercise prior to the trauma stress test. Then, each participant underwent a trauma reminder stress test in the clinical laboratory in which they sat in a room on a chair while undergoing ECG and hemodynamic monitoring. The reminder stress test involved two one-minute listening sessions of neutral recordings (neutral stimuli), followed by two one-minute listening sessions of traumatic stress recordings (psychosocial stress stimuli). The stress recordings consisted of a past traumatic experience specific to each participant that was transcribed the day prior to the session. A study experimenter not known to the participants recorded each transcription prior to the listening session. The neutral script and one trauma reminder script example are included in the Supplemental Text S1. Figure 1 shows the timeline of the study protocol.
FIGURE 1.
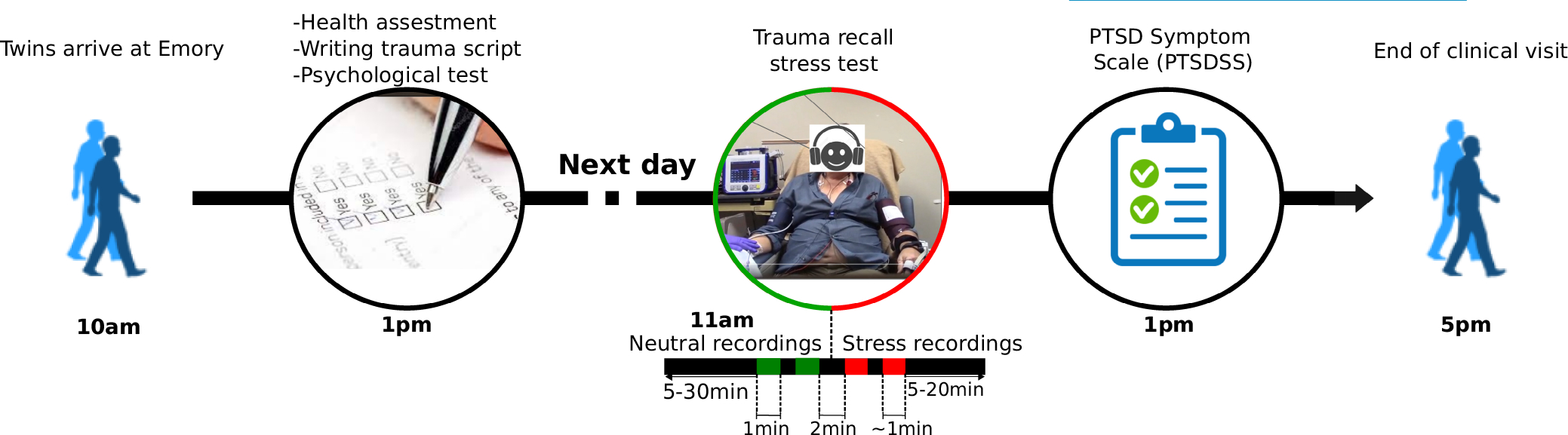
Timeline of the Emory twins study (ETS) follow-up visit with a focus on the trauma reminder stress test.
At the beginning of each recording, a researcher assigned the event timestamp by pressing a time-marker on the ECG monitor connected to the participants. At the end of the trauma reminder stress test each participant completed the PTSD symptoms scale (PTSDSS), an 18-item scale for the assessment of PTSD symptoms over time (Southwick et al., 1997). Subjects with previous trauma history assess the presence and severity of symptoms over the past 30 min.
The lifetime history of PTSD was classified as subjects that met criteria in both current and past diagnosis of PTSD assessed with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental disorder, 4th Edition (SCID) (First & Gibbon, 2004). The 4th Edition SCID was administered in-person by a trained professional interviewer on the day prior to the trauma reminder test.
In a previous study, we created a longitudinal PTSD status variable by combining PTSD diagnosis codes from a previous ETS visit (visit 1, 2002–2010) and current follow-up ETS visit (visit 2, 2015–2020) (Vaccarino et al., 2022). The twins were classified as long-standing PTSD (if they met criteria for PTSD diagnosed at both visit 1 and visit 2), late-onset PTSD (if they met criteria for PTSD diagnosed at visit 2 but not at visit 1), and never PTSD (if they did not meet criteria for PTSD diagnosed at both visit 1 and visit 2). As there was a small number of twins diagnosed with PTSD at visit 1 but not visit 2 (n = 6), we included them in the group of “never PTSD” and considered them as a measurement error similar to a previous publication (Vaccarino et al., 2022). PTSD diagnosis at both visits was assessed with the 4th Edition SCID (First & Gibbon, 2004). Although the SCID provides information on other comorbid psychiatric conditions, we only include the outcomes for PTSD and depression as other conditions were uncommon with only one subject having bipolar symptoms, one drug abuse, and two meeting criteria for anxiety.
2.3 |. Cardiovascular and behavioral risk factors measurements
We used standard procedures and questionnaires to measure potential cardiovascular and behavioral risk factors. We assessed sociodemographic characteristics (age, education, and employment), behavioral health factors (body mass index [BMI], physical activity, smoking, and alcohol drinking habits), current medication, and medical history as previously described (Vaccarino et al., 2011; Vaccarino et al., 2022). We measured education as the number of years of school, and we assessed employment based on full-time status. Weight and height were measured using standard clinical equipment. We assessed smoking status and alcohol consumption with standard questionnaires (Demirovic et al., 1993; Howard et al., 1998) and classified it as current, past, or never smokers. We measured alcohol consumption via survey as the average number of alcoholic beverages consumed in a week. We defined hypertension as current use of antihypertensive medications, systolic blood pressure >130 mmHg or diastolic blood pressure >80 mmHg (Whelton et al., 2018). We measured lifetime major depressive disorder with the SCID.
2.4 |. Heart rate variability measurements
During the trauma reminder stress test, we evaluated ECG with a single channel 1 kHz ECG module using a Biopac MP160 acquisition system (Biopac Systems, Goleta, CA) and also with a 256 Hz 12-lead ECG GE SEER-12 Holter monitor. We preferentially used the Biopac data because of the higher sampling frequency, although in cases where Biopac data were missing (33%), we used data from the Holter as a back-up as our previous unpublished evaluations showed equivalency of HRV data regardless of method with a mean absolute percentage error of 6%.
We calculated heart rate variability from ECG using the previously validated open-source PhysioNet Cardiovascular Signal Processing Toolbox (Shah et al., 2013; Vest et al., 2018). First, we computed a signal quality index (SQI) for each ECG beat (Li et al., 2008). The SQI determines if the data are of high enough quality to be analyzed. It removes noisy segments and artifacts on the ECG signals by comparing two peak detection annotation files (Li et al., 2008). Then we eliminated non-sinus rhythm and beats with SQI lower than 75% to obtain a normal to normal (NN) interval time series. We measured heart rate (HR) by calculating the mean of the NN interval time series. We measured the power spectra of the NN time series using the Lomb periodogram (Clifford et al., 2006). We calculated time and frequency domain HRV metrics on one-minute windows with 30 s sliding increments. We integrated the power spectrum throughout the low frequency (0.04–0.15 Hz) band. We performed wavelet analysis using the Haar mother wavelet function to derive the DC from the central part of the phase-rectified signal average (Bauer, Kantelhardt, Bunde, et al., 2006). We obtained mean LF and DC HRV values, our primary outcomes, during neutral- and stress-recording intervals. We also measured, as autonomic reactivity, the difference of both HRV metrics and heart rate during stress with neutral recordings.
2.5 |. Statistical analysis
We measured the cross-sectional association between the longitudinal PTSD status (never, late-onset, or long-standing) and PTSDSS (severity variable) with HRV features (outcome). We used generalized estimating equation models for the PTSD categorical variables and linear mixed-effect models for PTSDSS continuous variables that employed maximum likelihood and asymptotic tests of all the covariance parameters to account for clustering within twin pairs. We standardized the HRV metrics by rescaling them to have a mean of zero and standard deviation of one. We used standardized HR and DC HRV features, and standardized log-transformed LF HRV as the dependent variables, which allowed for unitless comparison among parameters.
First, we conducted the analysis in all subjects considering the twins as individuals. Then, we focused our analysis on the within-pair difference of standardized HRV to minimize random effects due demographic, shared familial and early environmental history. The within-pair analysis was defined as the difference of each individual from the twin-pair mean values (Carlin et al., 2005). This analysis allows the evaluation of potential influence of genetic factors on the HRV and PTSD association (Burt et al., 2009; Huang et al., 2022). For the longitudinal PTSD categories we analyzed separately those discordant for long-standing PTSD and those discordant for late-onset PTSD. However, the trend p-value includes all the additional pairs.
We created three main models to examine the impact of sociodemographic and behavioral variables. The outcome in all models was the standardized HRV metrics and both PTSD status and PTSDSS were analyzed separately as independent exposure variables in the three models. Model 1 was unadjusted. Model 2 adjusted for age, BMI, education, employment status, smoking, history of hypertension, diabetes, and beta blocker use. Model 3 adjusted for model 2 variables as well as additional psychological variables that often coexist with PTSD: alcohol abuse, lifetime depression, anti-depression medication.
In addition, we performed an analysis focused on the stress-related HRV-related results in which longitudinal PTSD status was the exposure, the standardized HRV metrics were the independent variables, and we adjusted for the same variables as Model 3 in addition to PTSDSS. In this last model, we examine the extent to which status or duration of lifetime PTSD explained any autonomic dysfunction observed, when also adjusting for acute PTSD symptoms.
3 |. RESULTS
3.1 |. Baseline characteristics
Of the 279 subjects enrolled in ETS, we successfully measured HRV and heart rate in 238 individuals that included 107 pairs of twins and 24 singletons. Reasons for missing data included incomplete (11 subjects) or poor ECG signal quality (30 subjects). The subjects with poor signal quality had a SQI lower than 75% in the 1-min neutral and/or stress segments.
Out of the 238 participants, 182 had never/remitted PTSD, 31 had late-onset PTSD, and 25 had long-standing PTSD. The mean age and years of education were similar across the three groups. However, subjects diagnosed with PTSD at visit 2 (both late-onset and long-standing) were less likely to be employed and more likely to be diagnosed with depression and have antidepressant medication (Table 1). In addition, participants that meet criteria for long-standing and late-onset PTSD had a larger score of PTSDSS as compared with participants with never/remitted PTSD.
TABLE 1.
Socio-demographics, Health factors, and medication history
| Participant characteristics | Never/remitted PTSD | Late-onset PTSD | Long standing |
|---|---|---|---|
| Sample size | 182 | 31 | 25 |
| Socio-demographics | |||
| Age | 68 (3) | 68 (2) | 69 (1) |
| Education, years | 14 (2) | 13 (2) | 13 (3) |
| Employed full time, % | 23 | 13 | 18 |
| Health factors | |||
| BMI | 29 (4) | 31 (5) | 28 (4) |
| Current smokers, % | 15 | 15 | 19 |
| Alcohol use, drinks/week | 2 (7) | 2 (5) | 2 (2) |
| History of hypertension % | 57 | 72 | 53 |
| Diabetes % | 1 | 0 | 0 |
| Depression % | 2 | 15 | 31 |
| Medication, use(%) | |||
| Antidepressant | 8 | 36 | 50 |
| Beta blockers | 26 | 33 | 7 |
| PTSDSS score | 21 (8) | 27 (11) | 33(11) |
Notes: For continuous variables, we presented the mean (SD), and for binary variables we presented the percentage prevalence.
3.2 |. Twins as individuals analysis of PTSD and HRV
When analyzing the twins as individuals we observed that subjects with long-standing PTSD status had lower LF HRV values compared with late-onset and never/remitted during the psychosocial stress stimuli but not during the neutral stimuli. Furthermore, we observed that LF values decrease when PTSDSS values increase during the psychosocial stress stimuli but not during the neutral stimuli. However, we did not find a statistically significant association between LF and longitudinal PTSD status or symptoms. The statistical association remained non- significant for the neutral task, trauma reminder task, and trauma reactivity (stress minus neutral) in all of the models previously described. Similar results were found for DC, except for a significant decrease of 0.17 (95% confidence interval, −0.38,− 0106) standardized unit lower DC values during psychosocial stress per 10 points increase of PTSDSS values for the unadjusted model (p = .04). However, it became non-significant after adjustment of socio-demographics and other psychological factors.
3.3 |. Within pair analysis of longitudinal PTSD status and HRV
Figure 2 shows the association between lifetime PTSD and within-pair log-LF during neutral stimuli, psychosocial stress stimuli, and trauma reactivity (stress minus neutral). We observed a statistically significant difference between lifetime PTSD and non-PTSD subjects during stress stimuli but not during the neutral stimuli. The association was not significant for the reactivity. We observed similar results for within pair DC values.
FIGURE 2.
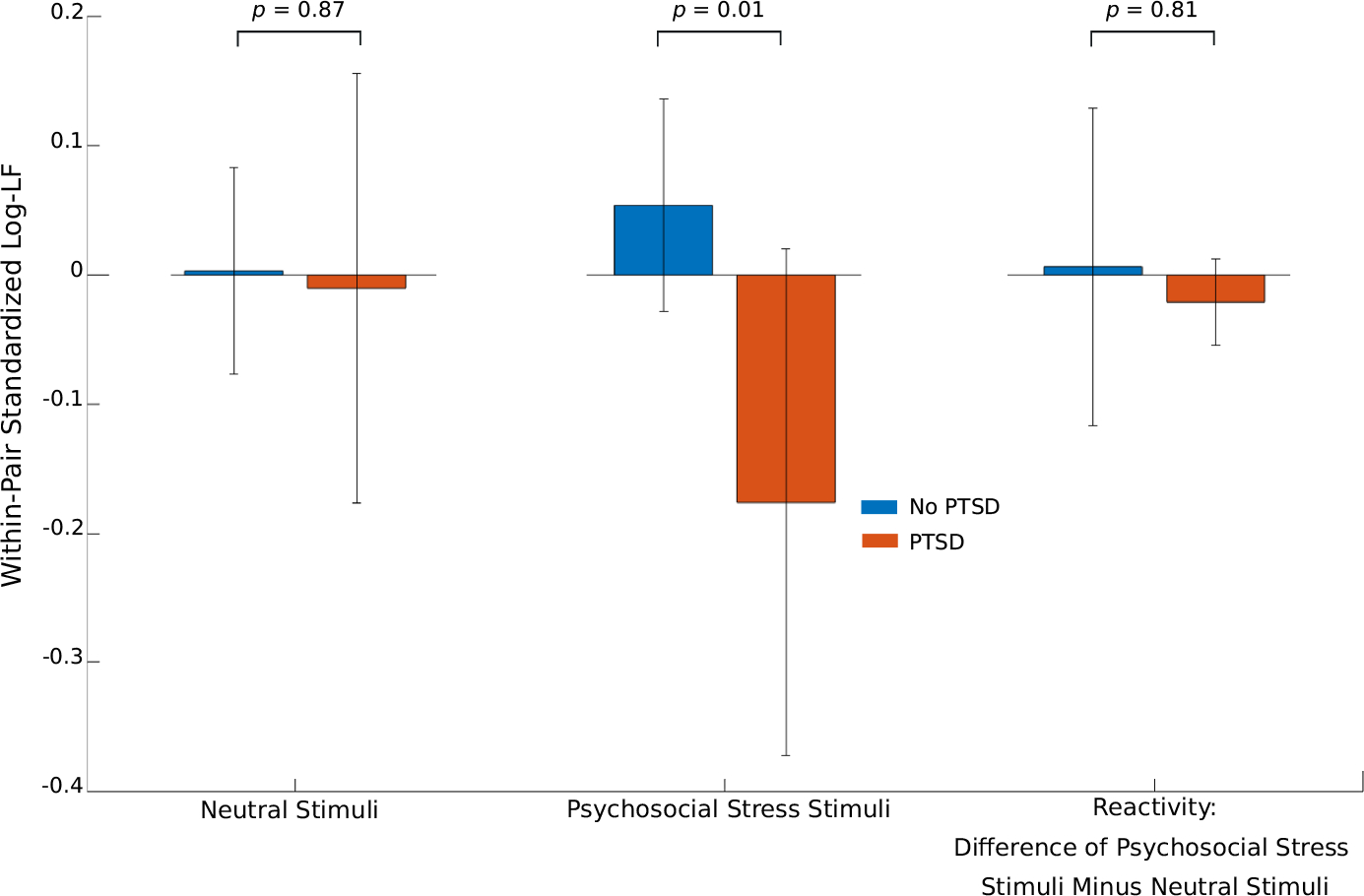
Lifetime PTSD status versus within-pair standardized log-transformed low frequency (log-LF) values during neutral stimuli (left), traumatic stress stimuli (center), and reactivity (right), which is the difference between stress and neutral phases. Error bars represent 95% confidence intervals. P-values are included for each pairwise comparison.
In Tables 2 and 3, we describe the within-pair associations between longitudinal PTSD status and mean DC, and log-LF, respectively, during the neutral task, trauma reminder task, and trauma reactivity (Supplemental Table S1 includes a similar analysis for heart rate). Models 1, 2, and 3 are sequentially adjusted for sociodemographic factors, cardiac risk factors, and psychological factors. We observed a robust association between lifetime PTSD status and stress HRV in models 1–3. Findings were similar with heart rate reactivity, such that higher heart rate changes from neutral to trauma reminder task associated with greater PTSD status. The largest difference in HR was observed in discordant twin pairs with long-standing PTSD as compared with late-onset PTSD (Supplemental Table S1). We also found a significant association between lifetime PTSD status and DC and log-LF during trauma reminder tasks. However, the differences in DC were similar for discordant twin pairs with long-standing and late-onset PTSD (Table 2). For log-LF the difference was largest in discordant long-standing PTSD twin pairs as compared with discordant late-onset PTSD twin pairs (Table 3). Supplemental Figure S2 top shows the unadjusted plots of log-LF values vs longitudinal PTSD status.
TABLE 2.
Multivariable within-pair analysis of the relationship between longitudinal PTSD status and DC during neutral stimulus, psychological stress stimulus, and its change/reactivity
| Late-Onset PTSD vs No PTSD |
Long-standing PTSD vs No PTSD |
||||
|---|---|---|---|---|---|
| Model | Mean | 95% CI | Mean | 95% CI | p for trend |
| Within-pair difference in DC during neutral stimuli | |||||
| Model 1a | −0.18 | −0.59 to 0.22 | −0.13 | −0.69 to 0.43 | .37 |
| Model 2b | −0.13 | −0.53 to 0.28 | −0.07 | −0.65 to 0.52 | .59 |
| Model 3c | 0.18 | −0.23 to 0.59 | 0.45 | −0.17 to 1.07 | .12 |
| Within-pair difference in DC during psychosocial stress stimuli | |||||
| Model 1a | −0.69 | −0.99 to -0.38 | −0.38 | −0.83 to -0.08 | <.001 |
| Model 2b | −0.62 | −0.91 to -0.34 | −0.35 | −0.80 to 0.09 | <.001 |
| Model 3c | −0.58 | −0.87 to -0.28 | −0.29 | −0.78 to 0.20 | <.01 |
| Within-pair difference in DC reactivity | |||||
| Model 1a | −0.29 | −0.68 to 0.09 | −0.12 | −0.57 to 0.32 | .11 |
| Model 2b | −0.3 | −0.69 to 0.09 | −0.13 | −0.58 to 0.33 | .10 |
| Model 3c | −0.58 | −0.96 to -0.19 | −0.38 | −0.83 to 0.07 | <.001 |
Notes: Outcome: DC (N = 106 twin pairs, 24 late-onset PTSD discordant pairs, 10 long-standing PTSD discordant pairs).
Abbreviations: BMI, body mass index; CI, confidence intervals; DC, deceleration capacity.
Base model was unadjusted for within-pair difference.
Model 2 = Model 1 + sociodemographic and traditional risk factors, including BMI, education, employment status, smoking, history of hypertension, diabetes, and beta blockers.
Model 3 = Model 2 + alcohol abuse, depression, alcohol abuse, anti-depression medication.
TABLE 3.
Multivariable within-pair analysis of the relationship between longitudinal PTSD status and log-LF during neutral and psychological stress stimulus and its change
| Late-Onset PTSD vs No PTSD |
Long-standing PTSD vs No PTSD |
||||
|---|---|---|---|---|---|
| Model | Mean | 95% CI | Mean | 95% CI | p for trend |
| Within-pair difference in log-LF during neutral stimuli | |||||
| Model 1a | −0.13 | −0.46 to 0.20 | 0.17 | −0.31 to 0.64 | .91 |
| Model 2b | −0.07 | −0.38 to 0.25 | 0.41 | −0.06 to 0.87 | .26 |
| Model 3c | 0.09 | −0.24 to 0.41 | 0.70 | 0.19 to 1.22 | .02 |
| Within-pair difference in Log-LF during psychosocial stress stimuli | |||||
| Model 1a | −0.47 | −0.82 to -0.13 | −0.79 | −1.30 to -0.29 | <.001 |
| Model 2b | −0.44 | −0.77 to -0.10 | −0.6 | −1.11 to -0.08 | <.01 |
| Model 3c | −0.40 | −0.74 to -0.07 | −0.62 | −1.17 to -0.07 | <.01 |
| Within-pair difference in Log-LF reactivity | |||||
| Model 1a | −0.05 | −0.49 to 0.39 | −0.11 | −0.74 to 0.52 | .68 |
| Model 2b | 0.07 | −0.36 to 0.49 | −0.07 | −0.70 to 0.57 | .98 |
| Model 3c | 0.04 | −0.41 to 0.49 | −0.10 | −0.81 to 0.61 | .90 |
Notes: Outcome: Log-LF (N = 106 twin pairs, 24 late-onset PTSD discordant pairs, 10 long-standing PTSD discordant pairs).
Abbreviations: BMI, body mass index; CI, confidence intervals; Log-LF, log-transformed low frequency.
Base model was unadjusted for within-pair difference.
Model 2 = Model 1 + sociodemographic and traditional risk factors, including BMI, education, employment status, smoking, history of hypertension, diabetes, and beta blockers.
Model 3 = Model 2 + alcohol abuse, depression, alcohol abuse, anti-depression medication.
Additional adjustment for PTSDSS did not result in substantial changes in the relationship of longitudinal PTSD and HRV, with the exception of log-LF HRV during the neutral script (see supplemental Table S2).
3.4 |. PTSD symptoms severity and HRV
In Table 4 we describe the within-pair associations between PTSDSS and mean HR, DC, and log-LF during the neutral task, trauma reminder task, and trauma reactivity. We found a robust relationship with most HRV outcomes in all models that included stress HRV and HRV reactivity. We also found a significant relationship with log-LF HRV during the neutral task in adjusted model 3, but not neutral DC HRV. Each 10 unit increase in PTSDSS associated with a 0.27 standard deviation (SD) decrease in DC HRV, 0.23 log-transformed standard deviation decrease in LF HRV, and 0.14 SD increase in HR during trauma reminder stress (Table 4). Supplemental Figure S1 bottom panels show the unadjusted plots of log-LF values vs longitudinal PTSD status (similar results are observed for DC values).
TABLE 4.
Within pair analysis of the association between PTSDSS 10 points increment and HRV metrics (N = 105 twins pairs)
| Model 1b | Model 2c | Model 3d | |
|---|---|---|---|
| Estimate*a (95% CI) | Estimate*a (95% CI) | Estimate*a (95% CI) | |
| HR neutral stimuli | 0.13 (0.25, 0.01)* | 0.09 (0.22, −0.03) | 0.07 (0.19, −0.05) |
| HR stress stimuli | 0.14 (0.25, 0.03)* | 0.14 (0.25, 0.03)* | 0.13 (0.24, 0.01)* |
| HR reactivity | 0.02 (0.14, −0.10) | 0.02 (0.14, −0.11) | 0.04 (0.17, −0.09) |
| DC HRV neutral stimuli | 0.02 (0.16, −0.13) | 0.03 (0.18, −0.12) | 0.10 (0.25, −0.04) |
| DC HRV stress stimuli | −0.27 (−0.14, −0.40)** | −0.26 (−0.13, −0.38)** | −0.22 (−0.09, −0.35)** |
| DC HRV reactivity | −0.19 (−0.03, −0.34)* | −0.17 (−0.01, −0.33)* | −0.20 (−0.03, −0.36) |
| log-LF HRV neutral stimuli | 0.05 (0.18, −0.07) | 0.14 (0.27, 0.01)* | 0.18 (0.31, 0.05)** |
| log-LF HRV stress stimuli | −0.23 (−0.10, −0.37)* | −0.18 (−0.04, −0.32)* | −0.12 (0.01, −0.26) |
| log-LF HRV reactivity | −0.01 (0.01, −0.04) | −0.02 (0.01, −0.05) | −0.03 (−0.01, −0.05)* |
Abbreviations: BMI, body mass index; CI, confidence intervals; DC, deceleration capacity; HR, heart rate; Log-LF, log-transformed low frequency; PTSDSS, post-traumatic stress disorder symptoms scale.
p < .05
p < .005.
Results are shown as β coefficients in the mixed models, per 10 units of increase of PTSDSS score.
Base model was unadjusted for within-pair difference.
Model 2 = Model 1 + sociodemographic and traditional risk factors, including BMI, education, employment status, smoking, history of hypertension, diabetes, and beta blockers.
Model 3 = Model 2 + alcohol abuse, depression, alcohol abuse, anti-depression medication.
4 |. DISCUSSION
In the study of Vietnam Era veteran twins with high PTSD prevalence, we observed several autonomic disturbances solicited by an in-lab trauma reminder challenge that suggest a potential causal mechanism. We demonstrate that HRV-reactivity was associated with both real-time acute PTSD symptoms, as well as status; longer duration of lifetime PTSD was associated with lower HRV stress reactivity. Our findings help to understand previous studies that reported lower heart rate variability in PTSD subjects compared to controls, and suggest a potential mechanism between acute PTSD symptoms and real-time autonomic dysfunction, which may trigger CVD events (Steptoe & Kivimäki, 2012). Because autonomic dysfunction is an important indicator of CVD risk (Fang et al., 2020; Rizas et al., 2018), these findings elucidate on previous studies which found an association of longitudinally PTSD duration with reduced coronary microcirculatory function and a greater deterioration especially among subjects with long-standing PTSD (Vaccarino et al., 2022). This expands our understanding of how PTSD might increase CVD risk (Vaccarino et al., 2013).
This study adds to previous studies on PTSD and autonomic dysfunction that examined resting or standard 24-h ambulatory assessments by describing acute autonomic changes during acute PTSD symptoms in the lab. It expands upon findings from the Shah et. al study (Shah et al., 2013) which showed a 50% reduced 24-h LF HRV in twins with PTSD (versus control brothers), as well as further examination by the Reinertsen et al. study that showed lower LF and DC HRV during quiescent periods in the same cohort (Reinertsen et al., 2017).
These studies highlight the importance of acute PTSD symptomatology, rather than other long-standing factors like underlying CVD (Shah et al., 2013), that may contribute to differences in long-term PTSD physiology. We also found that the association remained despite rigorous adjustment for confounding due to genetics, familial effects, and other traditional CVD risk factors. The independent contribution of longitudinal PTSD status to autonomic dysfunction also highlights the potential importance of neurological stress-pathways that are recurrently activated and strengthened in PTSD, leading to autonomic dysfunction (Thayer & Siegle, 2002).
Our investigation used well-established autonomic biomarkers that reflect diverse aspects of cardioneural function and suggest pleiotropic pathological effects of PTSD on autonomic dysfunction and CVD risk. DC HRV is an emerging, highly predictive autonomic biomarker of parasympathetic activity that measures the speed of short, low-latency changes in heart rate (Călburean et al., 2021). The association of DC HRV reactivity with PTSDSS after the trauma reminder challenge suggests that parasympathetic withdrawal is an important physiological change accompanying symptomatic PTSD. Low LF HRV measures the power of heart rate oscillations in the 0.1 hz range, which is the same frequency as the Mayer wave that are regulated by the baroreflex (Ghali & Ghali, 2020). The baroreflex influences both sympathetic and parasympathetic activity for the purposes of regulating blood pressure, is dampened in PTSD (Park et al., 2017), and predicts the risk of sudden cardiac death (De Ferrari et al., 1992). Heart rate is also an important prognostic marker that is reflective of sympathovagal balance (Bootsma et al., 2003); the relationship of longitudinal PTSD status with heart rate reactivity suggests altered increased sympathovagal balance during symptomatic PTSD that shift toward sympathetic activity particularly in those with long-standing PTSD.
Our findings likely stem from long-term and short-term effects of PTSD on the brain and cardioneural axis (Shah et al., 2022). Chronic PTSD may influence several key brain structures, including the hippocampus, which has been found to be smaller in PTSD due to the effects of chronic stress. Changes like these, in turn, may influence baseline autonomic activity and its reactivity to stress (Dossi et al., 2020). The associations of acute PTSD symptoms with DC and LF HRV emphasize the importance of particular structures involved in parasympathetic function (DC), such as the nucleus ambiguus and the dorsal vagal nucleus, as well as the baroreflexes, like the insular cortex, anterior cingulate cortex, medial prefrontal cortex, amygdala and cerebellum (Kimmerly, 2017). These potential mechanisms are important as we consider neuromodulation interventions such as vagal nerve stimulation, which shows some promise in PTSD treatment (Wittbrodt et al., 2020).
4.1 |. Limitations
There are several limitations. First, this was an observational study that did not have a baseline measurement of PTSD symptoms prior to the trauma reminder challenge. Therefore, we were unable to evaluate the ability of the traumatic reminder test to elicit PTSD symptoms. Nonetheless, this trauma recall stress-test has been validated to cause a significant increase in acute PTSD symptoms (Elzinga et al., 2003).
Another important limitation is the lack of generalizability to women and other racial and age groups, as most participants in our study were older white men. Nonetheless, the homogeneity in the sample and the unique twin population were also an important strength that allowed for increased precision due to the twin design and comparison within PTSD discordant pairs. The unique design of twin studies and within-pair analysis has the potential to distinguish genetics from environmental effects, as they controlled and provided a direct comparison between subjects who share potential causal factors such as unmeasured genetic and familial confounders (Carlin et al., 2005). However, the sample size of discordant pairs of longitudinal PTSD status was relatively small and limited our statistical power to examine for interaction of PTSD and zygosity within such pairs.
Furthermore, we cannot exclude the possibility of reverse causation, such that autonomic dysregulation to stress is a precedent or shared risk factor for PTSD and CVD in those exposed to trauma (Minassian et al., 2015). Also, presentation of the neutral and trauma scripts were not randomized, which makes it difficult to evaluate temporal effects related to acclimatization over time. This could not be performed because of potential long-lasting effects of the stress. The PTSD symptom scale assessment may have also been subject to bias since it occurred after the trauma recall task.
4.2 |. Clinical implications
By gaining a better understanding of the autonomic mechanisms involved in PTSD, we can apply targeted interventions such as transcutaneous vagal nerve stimulation, which has been shown to reduce the effects on behavioral, neurological, and inflammatory responses for individuals with PTSD exposed to a traumatic stress test (Bremner et al., 2020). We may also gain insight on the best biomarkers for future passively collected remote monitoring in PTSD treatment. Portable technologies such as smart watches and low-burden ambulatory ECG monitors may provide valuable insights to measure the outcome of interventions. Furthermore, this knowledge may inform appropriate cardiovascular care and prevention efforts for PTSD patients who show abnormal autonomic values indicating a higher CVD risk, and could also provide insight on potential physiology-based diagnostics and therapeutics. This includes therapies such as exercise training and vagal nerve stimulation, which are otherwise not considered standard of care, but warrant further investigation.
5 |. CONCLUSION
We found that PTSD symptoms measured after a trauma reminder challenge are associated with reduced HRV. In addition, the duration of PTSD status was independently associated with HRV and with an increase in heart rate during trauma reminder challenge. This combination of findings help to understand short-term and long-term effects of PTSD on the autonomic nervous system and may have important implications for clinical management.
Supplementary Material
FIGURE S1 Within-pair longitudinal PTSD status and PTSDSS vs HRV. Outcomes include within-paint standardized log-transformed low frequency (Log-LF) values during neutral stimuli (left panels), traumatic stress stimuli (center panels), and HRV reactivity, which is the difference between stress and neutral phases. Within-pair longitudinal PTSD status is the exposure of interest in the top panels, and Within-pair PTSDSS is the exposure of interest in the bottom panels
FIGURE S2 Within-pair longitudinal PTSD status and PTSDSS vs HRV. Outcomes include within-paint standardized deceleration capacity (DC) values during neutral stimuli (left panels), traumatic stress stimuli (center panels), and HRV reactivity, which is the difference between stress and neutral phases. Within-pair longitudinal PTSD status is the exposure of interest in the top panels, and Within-pair PTSDSS is the exposure of interest in the bottom panels
TABLE S1 Multivariable within-pair analysis of the relationship between longitudinal PTSD status and heart rate during neutral stimulus, psychological stress stimulus, and its change/reactivity
TABLE S2 Within pair analysis of the association between longitudinal PTSD Status and PTSDS with heart rate and HRV metrics in fully adjusted models that are mutually adjusted for both exposure types (PTSD duration and severity) (N = 105 Twin Pairs, 24 Discordant Pairs)
TABLE S3 Multivariable within-pair analysis of the relationship between longitudinal PTSD status and high frequency during neutral stimulus, psychological stress stimulus, and its change/reactivity
TABLE S4 Within pair analysis of the association between PTSDSS 10 points increment and HRV metrics (N = 105 Twins pairs)
ACKNOWLEDGMENTS
The Vietnam-Era Twin (VET) Registry (CSP #256) creation, ongoing development, management, and maintenance is supported by the Cooperative Studies Program (CSP) of the United States Department of Veterans Affairs (VA) Office of Research and Development. Data collection is supported by the VA and the National Institutes of Health ancillary grants, and other sponsors. The authors would like to acknowledge the past and continued participation of VET Registry members and their families. This research would not have been possible without their cooperation and contribution.
FUNDING INFORMATION
This research was supported by the National Institutes of Health (grants R01 HL68630, R01 AG026255, R01 HL125246, R01 HL136205, 2 K24 HL077506, K23 HL127251).
Footnotes
CONFLICT OF INTEREST
All authors report no biomedical financial interests or potential conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- Akosile W, Colquhoun D, Young R, Lawford B, & Voisey J (2018). The association between post-traumatic stress disorder and coronary artery disease: A meta-analysis. Australasian Psychiatry, 26, 524–530. 10.1177/1039856218789779 [DOI] [PubMed] [Google Scholar]
- Aysin B, & Aysin E (2006). Effect of respiration in heart rate variability (HRV) analysis. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2006, 1776–1779. 10.1109/IEMBS.2006.260773 [DOI] [PubMed] [Google Scholar]
- Bauer A, Kantelhardt JW, Barthel P, Schneider R, Mäkikallio T, Ulm K, Hnatkova K, Schömig A, Huikuri H, Bunde A, Malik M, & Schmidt G (2006). Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: Cohort study. Lancet, 367, 1674–1681. 10.1016/S0140-6736(06)68735-7 [DOI] [PubMed] [Google Scholar]
- Bauer A, Kantelhardt JW, Bunde A, Barthel P, Schneider R, Malik M, & Schmidt G (2006). Phase-rectified signal averaging detects quasi-periodicities in non-stationary data. Physica A: Statistical Mechanics and its Applications, 364, 423–434. 10.1016/j.physa.2005.08.080 [DOI] [Google Scholar]
- Bootsma M, Swenne CA, Janssen MJA, Cats VM, & Schalij MJ (2003). Heart rate variability and sympathovagal balance: Pharmacological validation. Netherlands Heart Journal, 11, 250–259 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2499895/ [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, Pearce BD, Vaccarino V, Shah AJ, Park J, Bikson M, & Inan OT (2020). Application of non-invasive vagal nerve stimulation to stress-related psychiatric disorders. Journal of Personalized Medicine, 10(3), 119. 10.3390/jpm10030119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, McGue M, & Iacono WG (2009). Nonshared environmental mediation of the association between deviant peer affiliation and adolescent externalizing behaviors over time: Results from a cross-lagged monozygotic twin differences design. Developmental Psychology, 45, 1752–1760. 10.1037/a0016687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Călburean PA, Osório TG, Sieira J, Ströker E, Maj R, Terasawa M, Rizzo A, Borio G, Scala O, Galli A, Brugada P, Chierchia GB, & de Asmundis C (2021). High parasympathetic activity as reflected by deceleration capacity predicts atrial fibrillation recurrence after repeated catheter ablation procedure. Journal of Interventional Cardiac Electrophysiology, 60, 21–29. 10.1007/s10840-019-00687-9 [DOI] [PubMed] [Google Scholar]
- Carlin JB, Gurrin LC, Sterne JA, Morley R, & Dwyer T (2005). Regression models for twin studies: A critical review. International Journal of Epidemiology, 34, 1089–1099. 10.1093/ije/dyi153 [DOI] [PubMed] [Google Scholar]
- Clifford G, Azuaje F, & McSharry P (2006). Advanced methods and tools for ECG data analysis. Artech books | IEEE Xplore; https://ieeexplore.ieee.org/document/9100926 [Google Scholar]
- De Ferrari GM, Vanoli E, Cerati D, & Schwartz PJ (1992). Baroreceptor reflexes and sudden cardiac death: Experimental findings and background. Giornale Italiano di Cardiologia, 22, 629–637. [PubMed] [Google Scholar]
- Demirovic J, Nabulsi A, Folsom AR, Carpenter MA, Szklo M, Sorlie PD, & Barnes RW (1993). Alcohol consumption and ultrasonographically assessed carotid artery wall thickness and distensibility. The Atherosclerosis Risk in Communities (ARIC) study investigators. Circulation, 88, 2787–2793. 10.1161/01.CIR.88.6.2787 [DOI] [PubMed] [Google Scholar]
- Dennis PA, Kimbrel NA, Sherwood A, Calhoun PS, Watkins LL, Dennis MF, & Beckham JC (2017). Trauma and autonomic dysregulation: Episodic-versus systemic-negative affect underlying cardiovascular risk in posttraumatic stress disorder. Psychosomatic Medicine, 79, 496–505. 10.1097/PSY.0000000000000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossi G, Delvecchio G, Prunas C, Soares JC, & Brambilla P (2020). Neural bases of cognitive impairments in Post-traumatic stress disorders: A mini-review of functional magnetic resonance imaging findings. Frontiers in Psychiatry, 11, 176. 10.3389/fpsyt.2020.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursa EK, Reinhard MJ, Barth SK, & Schneiderman AI (2014). Prevalence of a positive screen for PTSD among OEF/OIF and OEF/OIF-era veterans in a large population-based cohort. Journal of Traumatic Stress, 27, 542–549. 10.1002/jts.21956 [DOI] [PubMed] [Google Scholar]
- Ebrahimi R, Lynch KE, Beckham JC, Dennis PA, Viernes B, Tseng C-H, Shroyer ALW, & Sumner JA (2021). Association of posttraumatic stress disorder and incident ischemic heart disease in women veterans. JAMA Cardiology, 6, 642–651. 10.1001/jamacardio.2021.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, & Robinette CD (1987). The Vietnam era twin (VET) registry: Method of construction. Acta Geneticae Medicae et Gemellologiae, 36, 61–66. 10.1017/S0001566000004591 [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, & Bremner JD (2003). Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology, 28, 1656–1665. 10.1038/sj.npp.1300226 [DOI] [PubMed] [Google Scholar]
- Fang S-C, Wu Y-L, & Tsai P-S (2020). Heart rate variability and risk of all-cause death and cardiovascular events in patients with cardiovascular disease: A meta-analysis of cohort studies. Biological Research for Nursing, 22, 45–56. 10.1177/1099800419877442 [DOI] [PubMed] [Google Scholar]
- First MB, & Gibbon M (2004). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II). In Hilsenroth MJ & Segal DL (Eds.), Comprehensive handbook of psychological assessment. Personality assessment (Vol. 2, pp. 134–143). John Wiley & Sons Inc. [Google Scholar]
- Ge F, Yuan M, Li Y, & Zhang W (2020). Posttraumatic stress disorder and alterations in resting heart rate variability: A systematic review and meta-analysis. Psychiatry Investigation, 17, 9–20. 10.30773/pi.2019.0112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghali MGZ, & Ghali GZ (2020). Mechanisms contributing to the generation of Mayer waves. Frontiers in Neuroscience, 14, 395. 10.3389/fnins.2020.00395 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hayano J, Ueda N, Kisohara M, Yuda E, Carney RM, & Blumenthal JA (2021). Survival predictors of heart rate variability after myocardial infarction with and without low left ventricular ejection fraction. Frontiers in Neuroscience, 15, 610955. 10.3389/fnins.2021.610955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway SL (2021). Predictive value of heart rate measures on posttraumatic stress disorder: A critical review of select recent studies. International Journal of Psychological Research and Reviews, 4, 44. 10.28933/ijprr-2020-12-1905 [DOI] [Google Scholar]
- Howard G, Wagenknecht LE, Burke GL, Diez-Roux A, Evans GW, McGovern P, Nieto FJ, & Tell GS (1998). Cigarette smoking and progression of atherosclerosis: The atherosclerosis risk in communities (ARIC) study. JAMA, 279, 119–124. 10.1001/jama.279.2.119 [DOI] [PubMed] [Google Scholar]
- Huang M, Bliwise DL, Hall MH, Johnson DA, Sloan RP, Shah A, Goldberg J, Ko Y-A, Murrah N, Levantsevych OM, Shallenberger L, Abdulbagki R, Bremner JD, & Vaccarino V (2022). Association of depressive symptoms with sleep disturbance: A co-twin control study. Annals of Behavioral Medicine, 56(3), 245–256. 10.1093/abm/kaab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62, 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmerly DS (2017). A review of human neuroimaging investigations involved with central autonomic regulation of baroreflex-mediated cardiovascular control. Autonomic Neuroscience, 207, 10–21. 10.1016/j.autneu.2017.05.008 [DOI] [PubMed] [Google Scholar]
- Lee SM, Han H, Jang K-I, Huh S, Huh HJ, Joo J-Y, & Chae J-H (2018). Heart rate variability associated with posttraumatic stress disorder in victims’ families of sewol ferry disaster. Psychiatry Research, 259, 277–282. 10.1016/j.psychres.2017.08.062 [DOI] [PubMed] [Google Scholar]
- Li Q, Mark RG, & Clifford GD (2008). Robust heart rate estimation from multiple asynchronous noisy sources using signal quality indices and a Kalman filter. Physiological Measurement, 29, 15–32. 10.1088/0967-3334/29/1/002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB, & Marine Resiliency Study Team. (2015). Association of Predeployment Heart Rate Variability with Risk of Postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry, 72, 979–986. 10.1001/jamapsychiatry.2015.0922 [DOI] [PubMed] [Google Scholar]
- Mourot L (2014). Could non-linear heart rate variability analysis of short RR intervals series give clinically valuable information in heart disease? Journal of Clinical and Experimental Research in Cardiology, 104, 2394–6504. 10.13070/rs.en.1.618 [DOI] [Google Scholar]
- O’Donnell CJ, Schwartz Longacre L, Cohen BE, Fayad ZA, Gillespie CF, Liberzon I, Pathak GA, Polimanti R, Risbrough V, Ursano RJ, Vander Heide RS, Yancy CW, Vaccarino V, Sopko G, & Stein MB (2021). Posttraumatic stress disorder and cardiovascular disease: State of the science, knowledge gaps, and research opportunities. JAMA Cardiology, 6, 1207–1216. 10.1001/jamacardio.2021.2530 [DOI] [PubMed] [Google Scholar]
- Park J, Marvar PJ, Liao P, Kankam ML, Norrholm SD, Downey RM, McCullough SA, Le NA, & Rothbaum BO (2017). Baroreflex dysfunction and augmented sympathetic nerve responses during mental stress in veterans with post-traumatic stress disorder. Journal of Physiology (London), 595, 4893–4908. 10.1113/JP274269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkonigg A, Kessler RC, Storz S, & Wittchen HU (2000). Traumatic events and post-traumatic stress disorder in the community: Prevalence, risk factors and comorbidity. Acta Psychiatrica Scandinavica, 101, 46–59. 10.1034/j.1600-0447.2000.101001046.x [DOI] [PubMed] [Google Scholar]
- Quintana DS, Elstad M, Kaufmann T, Brandt CL, Haatveit B, Haram M, Nerhus M, Westlye LT, & Andreassen OA (2016). Resting-state high-frequency heart rate variability is related to respiratory frequency in individuals with severe mental illness but not healthy controls. Scientific Reports, 6, 37212. 10.1038/srep37212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen E, Nemati S, Vest AN, Vaccarino V, Lampert R, Shah AJ, & Clifford GD (2017). Heart rate-based window segmentation improves accuracy of classifying posttraumatic stress disorder using heart rate variability measures. Physiological Measurement, 38, 1061–1076. 10.1088/1361-6579/aa6e9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizas KD, Eick C, Doller AJ, Hamm W, von Stuelpnagel L, Zuern CS, Barthel P, Schmidt G, & Bauer A (2018). Bedside autonomic risk stratification after myocardial infarction by means of short-term deceleration capacity of heart rate. Europace, 20, f129–f136. 10.1093/europace/eux167 [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Elzinga BM, Ebner UW, Simms T, Sanislow C, Vermetten E, McGlashan TH, & Bremner JD (2004). Psychophysiological reactivity to traumatic and abandonment scripts in borderline personality and posttraumatic stress disorders: A preliminary report. Psychiatry Research, 126, 33–42. 10.1016/j.psychres.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Schneider M, & Schwerdtfeger A (2020). Autonomic dysfunction in posttraumatic stress disorder indexed by heart rate variability: A meta-analysis. Psychological Medicine, 50, 1937–1948. 10.1017/S003329172000207X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer F, & Ginsberg JP (2017). An overview of heart rate variability metrics and norms. Frontiers in Public Health, 5, 258. 10.3389/fpubh.2017.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AJ, Lampert R, Goldberg J, Veledar E, Bremner JD, & Vaccarino V (2013). Posttraumatic stress disorder and impaired autonomic modulation in male twins. Biological Psychiatry, 73, 1103–1110. 10.1016/j.biopsych.2013.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AJ, Wittbrodt MT, Bremner JD, & Vaccarino V (2022). Cardiovascular pathophysiology from the cardioneural perspective and its clinical applications. Trends in Cardiovascular Medicine, 32(3), 172–177. 10.1016/j.tcm.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Bremner JD, Morgan CA, Nicolaou AL, Nagy LM, Johnson DR, Heninger GR, & Charney DS (1997). Noradrenergic and serotonergic function in posttraumatic stress disorder. Archives of General Psychiatry, 54, 749–758. 10.1001/archpsyc.1997.01830200083012 [DOI] [PubMed] [Google Scholar]
- Steptoe A, & Kivimäki M (2012). Stress and cardiovascular disease. Nature Reviews. Cardiology, 9, 360–370. 10.1038/nrcardio.2012.45 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Siegle GJ (2002). Neurovisceral integration in cardiac and emotional regulation. IEEE Engineering in Medicine and Biology Magazine, 21, 24–29. 10.1109/MEMB.2002.1032635 [DOI] [PubMed] [Google Scholar]
- Trivedi RB, Post EP, Sun H, Pomerantz A, Saxon AJ, Piette JD, Maynard C, Arnow B, Curtis I, Fihn SD, & Nelson K (2015). Prevalence, comorbidity, and prognosis of mental health among US veterans. The American Journal of Public Health, 105, 2564–2569. 10.2105/AJPH.2015.302836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker P, Pfefferbaum B, Jeon-Slaughter H, Khan Q, & Garton T (2012). Emotional stress and heart rate variability measures associated with cardiovascular risk in relocated Katrina survivors. Psychosomatic Medicine, 74, 160–168. 10.1097/PSY.0b013e318240a801 [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, & Bremner JD (2013). Post-traumatic stress disorder and incidence of coronary heart disease: A twin study. Journal of the American College of Cardiology, 62, 970–978. 10.1016/j.jacc.2013.04.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Khan D, Votaw J, Faber T, Veledar E, Jones DP, Goldberg J, Raggi P, Quyyumi AA, & Bremner JD (2011). Inflammation is related to coronary flow reserve detected by positron emission tomography in asymptomatic male twins. Journal of the American College of Cardiology, 57, 1271–1279. 10.1016/j.jacc.2010.09.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Shah AJ, Moncayo V, Nye J, Piccinelli M, Ko YA, Ma X, Murrah N, Shallenberger L, Driggers E, Levantsevych OM, Hammadah M, Lima BB, Young A, O’Neal W, Alkhalaf M, Haffar A, Raggi P, Goldberg J, … Bremner JD (2022). Posttraumatic stress disorder, myocardial perfusion, and myocardial blood flow: A longitudinal twin study. Biological Psychiatry, 91(7), 615–625. 10.1016/j.biopsych.2021.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest AN, Da Poian G, Li Q, Liu C, Nemati S, Shah AJ, & Clifford GD (2018). An open source benchmarked toolbox for cardiovascular waveform and interval analysis. Physiological Measurement, 39, 105004. 10.1088/1361-6579/aae021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, & Oken BS (2013). Peak high-frequency HRV and peak alpha frequency higher in PTSD. Applied Psychophysiology and Biofeedback, 38, 57–69. 10.1007/s10484-012-9208-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, … Wright JT Jr (2018). 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College of Cardiology, 71, e127–e248. 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- Wittbrodt MT, Gurel NZ, Nye JA, Ladd S, Shandhi MMH, Huang M, Shah AJ, Pearce BD, Alam ZS, Rapaport MH, Murrah N, Ko YA, Haffer AA, Shallenberger LH, Vaccarino V, Inan OT, & Bremner JD (2020). Non-invasive vagal nerve stimulation decreases brain activity during trauma scripts. Brain Stimulation, 13, 1333–1348. 10.1016/j.brs.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CW, & Sano M (2021). Demographic, health, and exposure risks associated with cognitive loss, alzheimer’s disease and other dementias in US military veterans. Frontiers in Psychiatry, 12, 610334. 10.3389/fpsyt.2021.610334 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Within-pair longitudinal PTSD status and PTSDSS vs HRV. Outcomes include within-paint standardized log-transformed low frequency (Log-LF) values during neutral stimuli (left panels), traumatic stress stimuli (center panels), and HRV reactivity, which is the difference between stress and neutral phases. Within-pair longitudinal PTSD status is the exposure of interest in the top panels, and Within-pair PTSDSS is the exposure of interest in the bottom panels
FIGURE S2 Within-pair longitudinal PTSD status and PTSDSS vs HRV. Outcomes include within-paint standardized deceleration capacity (DC) values during neutral stimuli (left panels), traumatic stress stimuli (center panels), and HRV reactivity, which is the difference between stress and neutral phases. Within-pair longitudinal PTSD status is the exposure of interest in the top panels, and Within-pair PTSDSS is the exposure of interest in the bottom panels
TABLE S1 Multivariable within-pair analysis of the relationship between longitudinal PTSD status and heart rate during neutral stimulus, psychological stress stimulus, and its change/reactivity
TABLE S2 Within pair analysis of the association between longitudinal PTSD Status and PTSDS with heart rate and HRV metrics in fully adjusted models that are mutually adjusted for both exposure types (PTSD duration and severity) (N = 105 Twin Pairs, 24 Discordant Pairs)
TABLE S3 Multivariable within-pair analysis of the relationship between longitudinal PTSD status and high frequency during neutral stimulus, psychological stress stimulus, and its change/reactivity
TABLE S4 Within pair analysis of the association between PTSDSS 10 points increment and HRV metrics (N = 105 Twins pairs)


