Abstract
The large-conductance calcium-activated potassium (BK) channel is a critical regulator and potential therapeutic target of vascular tone and architecture, and abnormal expression or dysfunction of this channel is linked to many vascular diseases. Vascular remodelling is the early pathological basis of severe vascular diseases. Delaying the progression of vascular remodelling can reduce cardiovascular events, but the pathogenesis remains unclear. To clarify the role of BK channels in vascular remodelling, we use rats with BK channel α subunit knockout (BK α ‒/‒). The results show that BK α ‒/‒ rats have smaller inner and outer diameters, thickened aortic walls, increased fibrosis, and disordered elastic fibers of the aortas compared with WT rats. When the expression and function of BK α are inhibited in human umbilical arterial smooth muscle cells (HUASMCs), the expressions of matrix metalloproteinase 2 (MMP2), MMP9, and interleukin-6 are enhanced, while the expressions of smooth muscle cell contractile phenotype proteins are reduced. RNA sequencing, bioinformatics analysis and qPCR verification show that C1q/tumor necrosis factor-related protein 7 ( CTRP7) is the downstream target gene. Furthermore, except for that of MMPs, a similar pattern of IL-6, smooth muscle cell contractile phenotype proteins expression trend is observed after CTRP7 knockdown. Moreover, knockdown of both BK α and CTRP7 in HUASMCs activates PI3K/Akt signaling. Additionally, CTRP7 is expressed in vascular smooth muscle cells (VSMCs), and BK α deficiency activates the PI3K/Akt pathway by reducing CTRP7 level. Therefore, we first show that BK channel deficiency leads to vascular remodelling. The BK channel and CTRP7 may serve as potential targets for the treatment of cardiovascular diseases.
Keywords: large-conductance calcium-activated potassium channel, vascular remodelling, vascular smooth muscle cell, C1q/tumor necrosis factor-related protein 7
Introduction
The large-conductance calcium-activated potassium (BK) channel is widely distributed in the cardiovascular system [1] and can participate in the maintenance of membrane potential and regulate vascular contraction/relaxation [2]. BK channels are considered to be key players in the vascular system. The abnormal expression/activity of BK channels is related to a variety of cardiovascular diseases, such as hypertension and premature uterine artery contraction [3]. However, the roles of the BK channel in cardiovascular systems are not well understood.
Vascular remodelling is one of the early detectable parameters that predicts life-threatening cardiovascular events. This pathological feature is found in many patients with hypertension and atherosclerosis and may be involved in the development and complications of numerous vascular diseases, including myocardial ischemia, coronary heart disease, and heart failure [ 4, 5] . In fact, a high degree of arterial remodelling predicts a poorer prognosis in patients with vascular diseases [6]. However, the pathophysiological mechanisms of vascular remodelling are complicated and have not yet been elucidated. It has been demonstrated that vascular inflammation, reduced expressions of smooth muscle cell (SMC) contractile phenotype proteins, changes in extracellular matrix components and vascular function are pathological features of vascular remodelling [7]. Our previous study demonstrated that the blood vessels of BK channel α subunit-knockout (BK α ‒/‒) rats exhibited enhanced vasoconstriction [8]. Some other studies showed that BK channel deficiency stimulated an increase in the release of inflammatory factors in the blood and reduced the expressions of SMC contractile phenotype proteins [ 9, 10] , indicating that abnormal BK channel expression or function may be involved in vascular-related injury. Unexpectedly, although BK channels play a significant role in vascular structure and function, we have been unable to directly define the relationship between BK channels and vascular remodelling.
In the present study, we used BK α ‒/‒ rats to show that BK channel deficiency is involved in vascular remodelling. Meanwhile, the potential molecular mechanism of vascular remodelling was preliminarily explored in vitro, and we found that C1q/tumor necrosis factor-related protein 7 (CTRP7) was expressed in smooth muscle. Loss of the BK channel α subunit reduced CTRP7 expression, thereby activating the PI3K/Akt signaling pathway. This study revealed the mechanism of vascular remodelling, and targeting BK channels and CTRP7 might represent potential strategies for vascular disease prevention and treatment.
Materials and Methods
Animals
The animals used in this study are BK channel α subunit ( KCNMA1)-knockout and wild-type (WT) rats. In this study, the method of construction, reproductive mode and genetic identification of male BK α ‒/‒ rats were the same as previously described [11]. The animals (24 weeks) were fed in the SPF animal room of Capital Medical University. At the end of the experiment, the rats were euthanized with an intraperitoneal injection of sodium pentobarbital. All animal experiment protocols used in this study were approved by the Animal Research Ethics Committee of Capital Medical University (Licence Number: AEEI-2018-049). The heart rate and blood pressure of rats were monitored with a BP-98 A tail-cuff blood pressure measurement system (Softron, Tokyo, Japan) when the animals were in a conscious state.
Cell culture
Human umbilical artery smooth muscle cells (HUASMCs) were purchased from Shanghai Xinyu Biotechnology Company (Shanghai, China). HUASMCs were maintained in Dulbecco’s modified Eagle’s medium (DMEM; 10-014-cv; Corning, New York, USA) containing 10% fetal bovine serum (FBS; FSS500; Excell Bio, Shanghai, China) at 37°C in an incubator with 95% air and 5% CO 2 after they reached 80% confluence. To examine the effect of BK channels on HUASMCs, the medium was supplemented with the 1, 5, and 10 μM BK channel inhibitor paxilline (ab141840; Abcam, Cambridge, UK). After 24‒72 h of treatment, the cells were collected for subsequent studies.
Small interfering RNA (siRNA)-mediated gene knockdown
BK α siRNA, CTRP7 siRNA and scramble siRNA were purchased from RiboBio Company (Guangzhou, China). When SMCs reached 50% confluence, the cells were transfected with siRNA in the presence of Lipofectamine 2000 transfection reagent (11668019; Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol (final siRNA concentration: 50 nM). Successful knockdown was confirmed by western blot analysis of BK α and CTRP7 protein levels. Because BK α-1 siRNA and CTRP7-1 siRNA had high knockdown efficiency, they were selected for subsequent experiments. The siRNA sequences are shown in Table 1. The negative control (siNC; Cat. No. P201911180066) was purchase from Ribobio Company (Guangzhou, China)
Table 1 Sequence of siRNAs used in this study
|
siRNA |
Species |
Sequence (5′→3′) |
|
BK α-1 |
Homo sapiens |
GGCAGAAATACTACTTGGA |
|
BK α-2 |
Homo sapiens |
CATCGGTGCACTTGTAATA |
|
CTRP7-1 |
Homo sapiens |
GTGGAAGCATCGTGCTCAA |
|
CTRP7-2 |
Homo sapiens |
GGGCAATACCGGATAAAGA |
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was isolated from SMCs using Trizol reagent (T9424; Sigma-Aldrich, St Louis, USA). cDNA was generated using a Revert Aid First Strand cDNA Synthesis kit (K1622; Thermo Scientific, Waltham, USA) according to the manufacturer’s instructions. qRT-PCR was performed using a SYBR Green qPCR Master Mix kit (ES-QP002; ESsciense Biotech,Beijing, China), and the results were normalized to β-actin mRNA level. All amplification reactions were performed as follows: one cycle of 5 min at 95°C to activate the polymerase, followed by 40 cycles of 95°C for 10 min and 60°C for 30 s. Each sample was assayed three times. The primer sequences are shown in Table 2.
Table 2 Sequences of primers used for real-time PCR
|
Gene |
Species |
Forward primer (5′→3′) |
Reverse primer (5′→3′) |
|
β-actin |
Rattus norvegicus |
GAGACCTTCAACACCCCAGCC |
TCGGGGCATCGGAACCGCTCA |
|
FZD2 |
Rattus norvegicus |
TCGTTTTGCCCGTCTCT |
TAGCGGAATCGCTGCAT |
|
KBTBD8 |
Rattus norvegicus |
GCCGCGTCGGCAGATTT |
TCCCGTGATCCACTTCCACT |
|
PLCE1 |
Rattus norvegicus |
TGCATTCTTGCTCCAGTCTCT |
TTGAGTGTTTTCTGTTCCCTGTA |
|
CTRP7 |
Rattus norvegicus |
CCAGAGCACGAGCCCAAG |
AGTAGCTCTCCCCCTTAGCC |
|
β-actin |
Homo sapiens |
CCACGAAACTACCTTCAACTCC |
TCATACTCCTGCTGCTTGCTGATCC |
|
MCP-1 |
Homo sapiens |
CAAACTCAAGCTCGCACTC |
CATTTCCACAATAATATTTTAG |
|
MMP-9 |
Homo sapiens |
TGTCCCTTTACTGCCCTGA |
ACTCCAGGCTCTGTCCTCCTCTT |
|
MMP-2 |
Homo sapiens |
TGATCTTGACCAGAATACCATCGA |
GGCTTGCGAGGGAAGAAGTT |
|
IL-6 |
Homo sapiens |
TCCTGCAGAAAAAGGCAAAG |
GCCCAGTGGACAGGTTTCT |
|
ICAM-1 |
Homo sapiens |
GTCCCCCTCAAAAGTCATCC |
AACCCCATTCAGCGTCACCT |
|
FZD1 |
Homo sapiens |
GAGTTCGTGCCAATCCTGAC |
GTCTGTCCATCCTCCCTCTG |
|
PLCE1 |
Homo sapiens |
CACGCTCTCAGCTGTCTTGA |
TCTCACTGCATTGTAGATCTGGT |
|
KBTBD8 |
Homo sapiens |
CATGGACCCCTTCCATGCTT |
CCGCTAGTGAACATGGATCTGA |
|
CTRP7 |
Homo sapiens |
CAAGTTTTGCCATTTGTGCCAG |
GGCAAGCCAGGAATGCTGCAG |
Western blot analysis
Cell or tissue lysates were resolved by 10% SDS-PAGE and then transferred onto nitrocellulose membranes. After being blocked with 5% milk in Tris buffered solution-Tween (TBST), the membranes were incubated with primary antibodies at 4°C overnight, followed by incubation with the corresponding secondary antibodies for 1 h. The intensities protein bands were analysed using the ChemiDoc MP Imaging System (Bio-Rad, Hercules, USA). The primary antibodies used are as follows: anti-Slo1/BKAppha potassium channel (1:500; 75-022-020; Neurolab, St Louis , USA), anti-GAPDH (1:1000; TA309157; ZSGB-BIO Beijing, China), anti-MMP9 (1:1000; ab76003; Abcam), anti-MMP2 (1:1000; ab92536; Abcam), anti-IL-6 (1:500; ab9324; Abcam), anti-Calponin1 (1:1000; ab46794; Abcam), anti-SM-22α (1:1000; ab14106; Abcam), anti-CTRP7 (1:1000; A00396-01-100; Aviscera Bioscience, Santa Clara, USA), anti-FAK (1:1000; T55464; Abmart, Shanghai, China), anti-p-FAK (phospho Y397) (1:1000; ab81298; Abcam), anti-AKT (1:500; 10176-2-AP; Proteintech, Chicago, USA), anti-p-AKT (phospho S473) (1:1000; ab81283; Abcam), anti-Smad2 (1:1000; 5339T; Cell Signaling Technology, Beverly, USA), and anti-p-Smad2 (1:1000; 3108T; Cell Signaling Technology). The secondary antibodies are HRP-conjugated goat anti-rabbit IgG (1:5000; ZB-2301) or anti-mouse IgG (ZB-2305).
Histological examination
The arteries were fixed in 4% paraformaldehyde for one week and then embedded in paraffin. Three round cross-sections (approximately 4 mm in thickness) were cut from the paraffin and stained using a hematoxylin-eosin (HE) staining kit (G1120; Solarbio, Beijing, China), a Masson’s trichrome staining kit (G1345; Solarbio) and a Victoria blue staining kit (G1596; Solarbio). Aortic wall thickness, lumen/external diameters and fibrotic areas were measured and analyzed with ImageJ analysis software.
ELISA detection
The plasma samples were collected from rats, and the concentrations of TNF-α and IL-6 were assayed using ELISA kits (Invitrogen) according to the manufacturer’s recommendations.
RNA sequencing
Total RNA was extracted from the aortas using Trizol reagent (T9424; Sigma). One microgram of RNA was used to generate the sequencing library. The libraries were sequenced on a BGIseq500 platform (BGI-Shenzhen, Shenzhen, China). For RNA sequencing analysis of the aortas of BK α ‒/‒ and WT rats, clean reads were mapped to the rat reference genome using HISAT2 (v2.0.4), and gene expression was calculated using RSEM (v1.2.12). The differentially expressed genes (DEGs) were identified with the criteria of Q<0.05. KEGG enrichment analysis of the annotated DEGs was performed using Phyper ( https://en.wikipedia.org/wiki/Hypergeometric_distribution).
GEO datasets and bioinformatics analysis
Gene expression datasets were searched with the keywords KCNMA1 and aortic; eligible datasets were downloaded from the Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) database, and the RNA data were related to the human aortas with reduced KCNMA1 expression compared with the control group. We found that the GSE 7084 dataset was consistent with the above requirements. This dataset was uploaded to the GPL 570 platform. Then, we used the GEO2R online analysis tool to analyze the genes in the dataset and found the common genes between GSE7084 and the DEGs (fold change>1.5) in rats. The overlapping genes were validated by qPCR and western blot analysis.
Statistical analysis
GraphPad Prism 8.0 software was used for statistical analysis and statistical graph production. Data are expressed as the mean±SD. One-way ANOVA followed by Tukey’s test was used to compare multiple groups, and the Student’s t test was used to evaluate the comparison between two groups. P<0.05 was considered statistically significant.
Results
Deletion of the BK channel α subunit induces vascular remodelling in rats
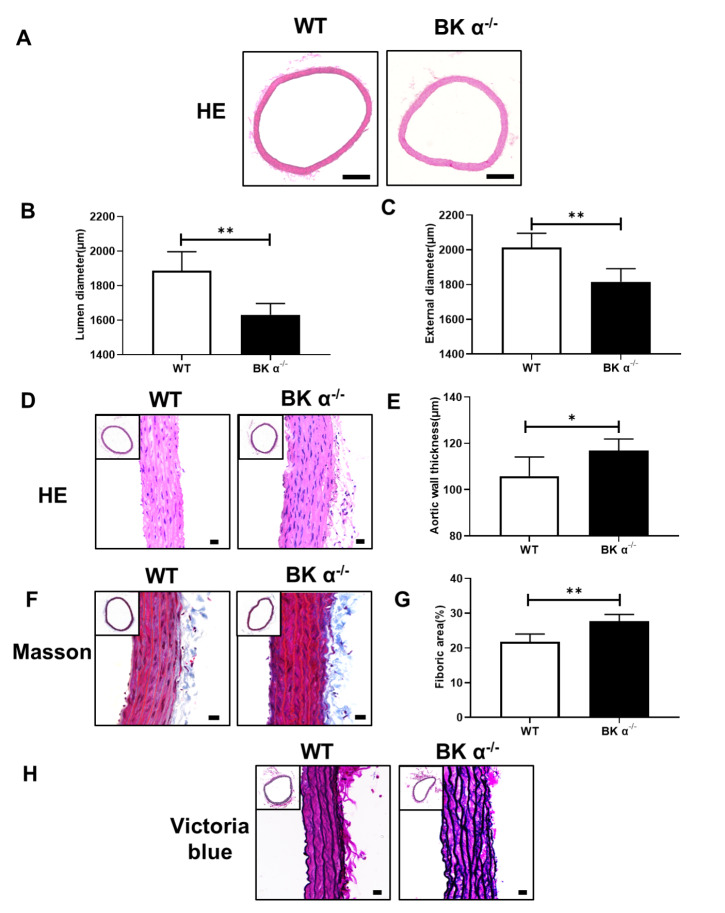
To determine the role of BK channels in vascular remodelling, BK α ‒/‒ rats were used in subsequent experiments. BK α protein was not expressed in the aortic tissues of BK α ‒/‒ rats ( Supplementary Figure S1). The body weight, heart rate and blood pressure of BK α ‒/‒ rats were lower than those of WT rats ( Supplementary Figures S2 and 3). HE and Masson’s trichrome staining showed that the lumen and external diameters of the aortas were reduced ( Figure 1A‒C), an increase in aortic wall thickness was observed ( Figure 1D, E), and aortic fibrosis was increased in BK α ‒/‒ rats compared with that in WT rats ( Figure 1F,G). Elastin staining showed a disordered elastic lamina in the aortas of BK α ‒/‒ rats ( Figure 1H). Additionally, we detected the levels of the inflammatory factors interleukin-6 (IL-6) and TNF-α in the plasma of BK α ‒/‒ and WT rats by ELISA. The concentrations of IL-6 and TNF-α were increased in the plasma of BK α ‒/‒ rats compared with those of WT rats ( Supplementary Figure S4). Overall, these results revealed that deletion of BK α contributed to vascular remodelling in rats.
Figure 1 .
The aortas of BK α ‒/‒ rats exhibited vascular remodeling
(A) Representative images of HE staining and statistical diagrams of the lumen diameter (B) and the external diameter (C) of the aortas in 6-month-old WT and BK α ‒/‒ rats. Scale bar: 500 μm. (D) Representative images of HE staining and statistical diagrams of the aortic wall thickness (E) in 6-month-old WT and BK α ‒/‒ rats. Scale bar: 20 μm. (F,G) Representative images of Masson’s trichrome staining and statistical diagrams of the fibrotic area of the aorta in 6-month-old WT and BK α ‒/‒ rats. Scale bar: 20 μm. (H) Representative images of elastin staining (Victoria blue) in the aortas of 6-month-old WT and BK α ‒/‒ rats. Scale bar: 20 μm. Data are shown as the mean±SD, n=5 per group. * P<0.05, ** P<0.01.
The deletion and functional inhibition of BK α in HUASMCs increases vascular remodelling-related markers
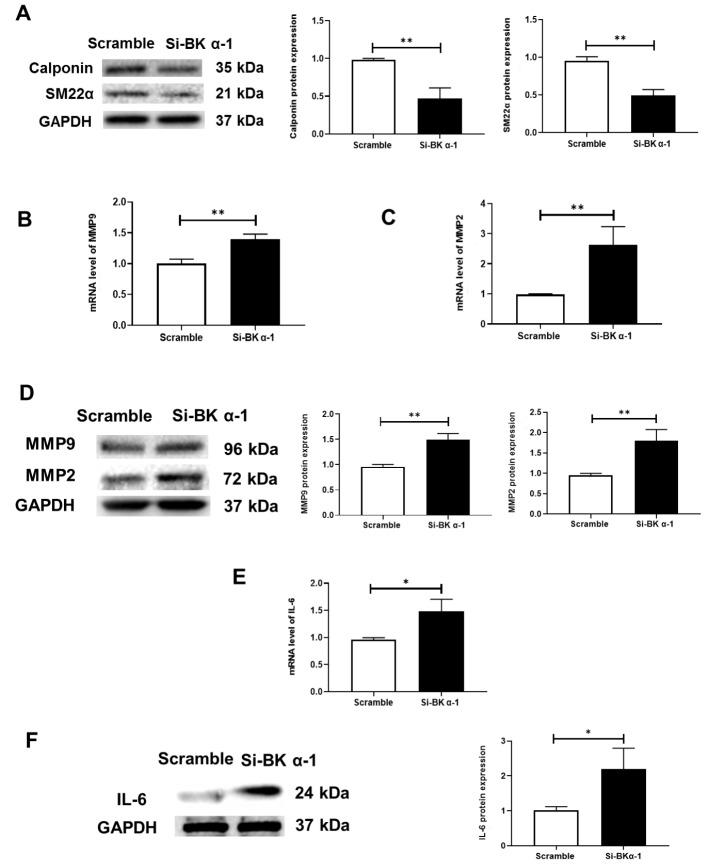
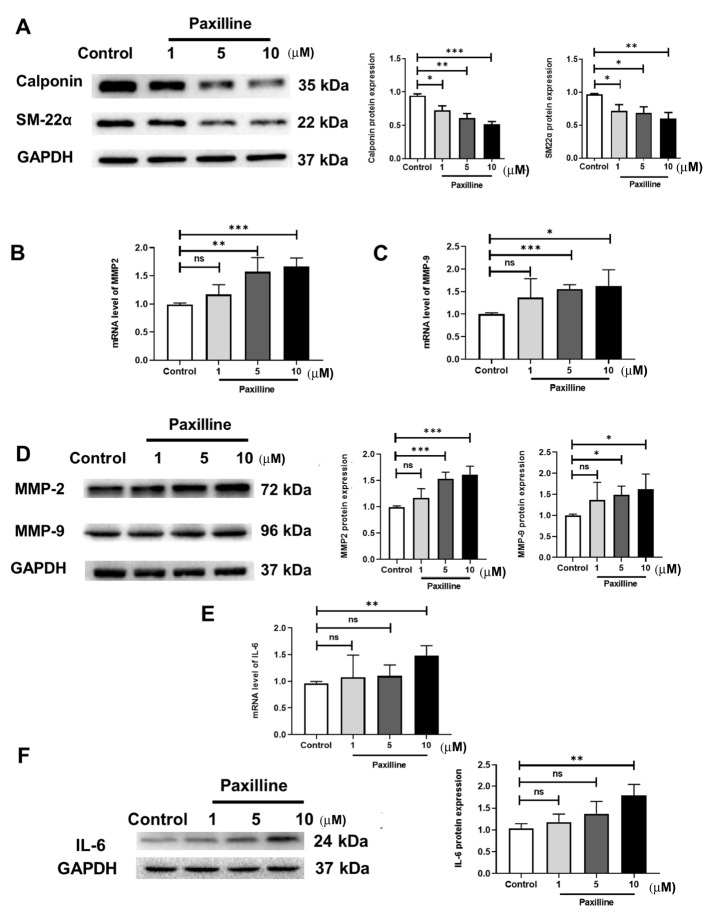
The enhanced levels of MMPs and inflammatory cytokines and the reduced expressions of SMC contractile proteins contribute to vascular remodelling. Therefore, we investigated the effect of BK α on the expression of these factors in HUASMCs. Two different approaches were employed. First, we performed an RNA interference experiment ( Supplementary Figure S5). The data showed that the expressions of the SMC contractile phenotype proteins SM-22α and Calponin were decreased ( Figure 2A), an increase in MMP2 and MMP9 was observed ( Figure 2B‒D), and the expression of IL-6 was upregulated by BK α knockdown ( Figure 2E,F). Second, to obtain more evidence supporting the involvement of BK α in the inhibition of vascular remodelling, the BK channel inhibitor paxilline was used in vitro. The data were similar to the RNA interference results ( Figure 3). These results suggested that the deletion and functional inhibition of BK α could promote vascular remodelling by increasing the release of inflammatory factors and MMPs and reducing the expressions of SMC contractile phenotype proteins.
Figure 2 .
Expressions of inflammatory cytokines, MMPs and SMC contractile phenotype proteins in vitro after BK α knockdown
(A) Representative blots and statistical diagrams showing the expressions of Calponin and SM22α, as determined by western blot analysis in vitro after BK α knockdown. (B‒D) Representative blots and statistical diagrams showing the expressions of MMP9 and MMP2, as determined by western blot analysis and qPCR in vitro after BK α knockdown. (E,F) Representative blots and statistical diagrams showing the expression of IL-6, as determined by western blot analysis and qPCR in vitro after BK α knockdown. Data are shown as the mean±SD, n=3 per group. * P<0.05, ** P<0.01.
Figure 3 .
Expressions of inflammatory cytokines, MMPs and SMC contractile phenotype proteins in vitro after paxilline treatment
(A) Representative blots and statistical diagrams showing the in vitro expressions of Calponin and SM22-α, as determined by western blot analysis after treatment with paxilline for 72 h. (B‒D) Representative blots and statistical diagrams showing the expressions of MMP9 and MMP2 in vitro after treatment with paxilline, as determined by western blot analysis (72 h) and qPCR (24 h). (E,F) Representative blots and statistical diagrams showing the expression of IL-6 in vitro after paxilline treatment, as determined by western blot analysis (72 h) and qPCR (24 h). Data are shown as the mean±SD, n=3 per group. * P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001, ns, not significant.
CTRP7 is a novel downstream target gene of BK α in HUASMCs
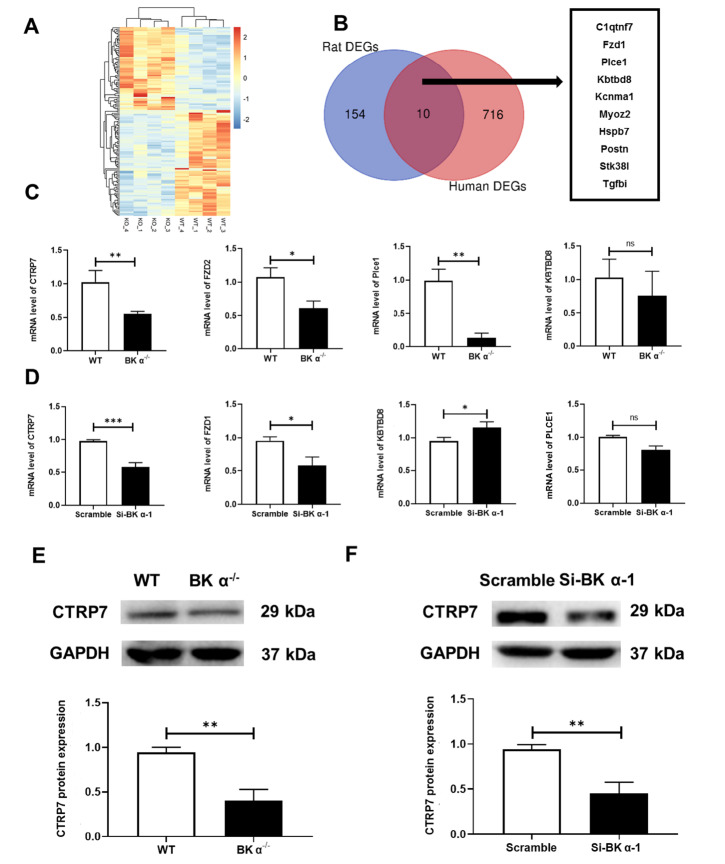
To identify the downstream target genes of BK α, aortas were obtained from BK α ‒/‒ and WT rats and used for RNA sequencing. Transcriptome analysis showed 375 differentially expressed genes (DEGs) between BK α ‒/‒ and WT rats ( P<0.05), of which 171 genes were upregulated and 204 genes were downregulated. Then, homologous comparison with human genes was performed, and 164 DEGs were selected in rats ( P<0.05, fold change>1.5). Ten common genes overlapped between the 164 previously selected genes and DEGs in the GEO database GSE 7084 ( Figure 4A,B): KCNMA1, FZD1, C1qtnf7, MYOZ2, HSPB7, PLCE1, POSTN, STK38L, KBTBD8, and TGFBI (Supplementary Table S1). Among them, KBTBD8, FZD1, C1qtnf7, PLCE1, and KCNMA1 had the same trend in the transcriptome analysis and GSE7084 dataset. Next, except for KCNMA1 (BK α), four genes were verified by qPCR ( Figure 4C,D). Among them, C1qtnf7 (CTRP7) was the most significantly altered gene in vivo and in vitro. Furthermore, western blot analysis revealed that the trend of CTRP7 expression in the aortas of BK α ‒/‒ rats and HUASMCs with BK α knockdown was consistent with that in the transcriptome analysis ( Figure 4E,F). Based on these results, we attempted to explore the role of CTRP7 in vascular remodelling.
Figure 4 .
CTRP7 is a novel downstream target gene of BK α
(A) Heatmap showing the DEGs from the transcriptome analysis. (B) Venn diagram showing the number of overlapping DEGs from the transcriptome analysis (fold change>1.5) and the GSE 7084 dataset. (C) Representative statistical diagrams showing the expressions of KBTBD8, PLCE1, FZD1, and CTRP7 determined by qPCR in the aortas of WT and BK α ‒/‒ rats, n=4 per group. (D) Representative statistical diagrams showing the expressions of KBTBD8, PLCE1, FZD1, and CTRP7 determined by qPCR in HUASMCs after BK α knockdown. n=3 per group. (E,F) Representative blots and statistical diagrams showing the expression of CTRP7 in the aortas of WT and BK α ‒/‒ rats and HUASMCs after BK α knockdown, determined by western blot analysis. n=4 per group. Data are shown as the mean±SD. * P<0.05, ** P<0.01, *** P<0.001, ns, not significant.
CTRP7 is critically involved in BK α deficiency-mediated vascular remodelling in HUASMCs
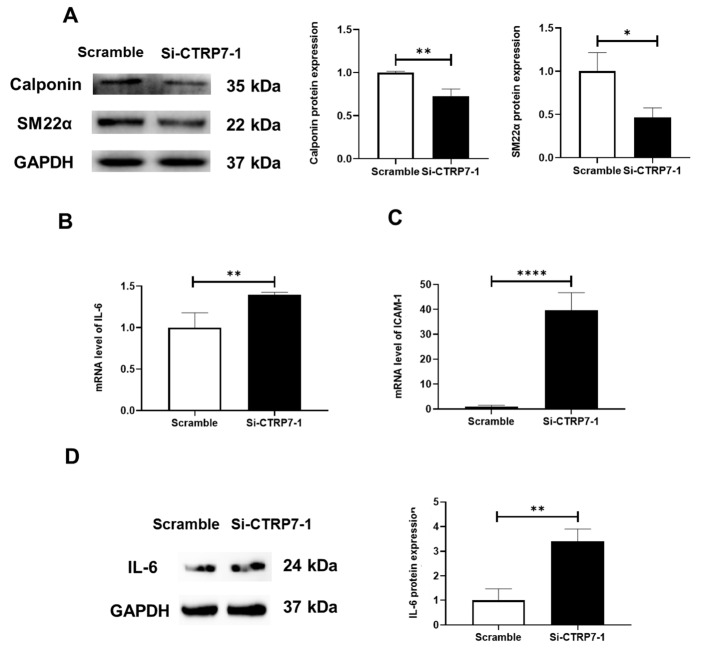
To determine the role of CTRP7 in BK α deficiency-mediated vascular remodelling, RNA interference technique was used to decrease the level of CTRP7 in HUASMCs ( Supplementary Figure S6). The data showed that decreased expressions of SMC contractile phenotype proteins were observed after CTRP7 knockdown ( Figure 5A). Simultaneously, the inhibitory effect on the mRNA and protein levels of IL-6 and ICAM-1 mRNA disappeared after CTRP7 knockdown ( Figure 5B‒D). However, CTRP7 deficiency reduced the mRNA levels of MMP2 and MMP9 (Supplementary Figure S7). Taken together, these results suggested that CTRP7 was an important mediator of vascular smooth muscle cell (VSMC) protection.
Figure 5 .
Expressions of SMC contractile phenotype proteins and inflammatory cytokines in HUASMCs after CTRP7 knockdown
(A) Representative blots and statistical diagrams showing the expressions of Calponin and SM22-α in HUASMCs after CTRP7 knockdown, as determined by western blot analysis. (B‒D) Representative blots and statistical diagrams showing the expression of IL-6, as determined by western blot analysis and qPCR in HUASMCs after CTRP7 knockdown. Data are shown as the mean±SD, n=3 per group. * P<0.05, ** P<0.01, **** P<0.0001.
The PI3K/Akt pathway is downstream of BK α/CTRP7 deficiency-mediated vascular remodelling in HUASMCs
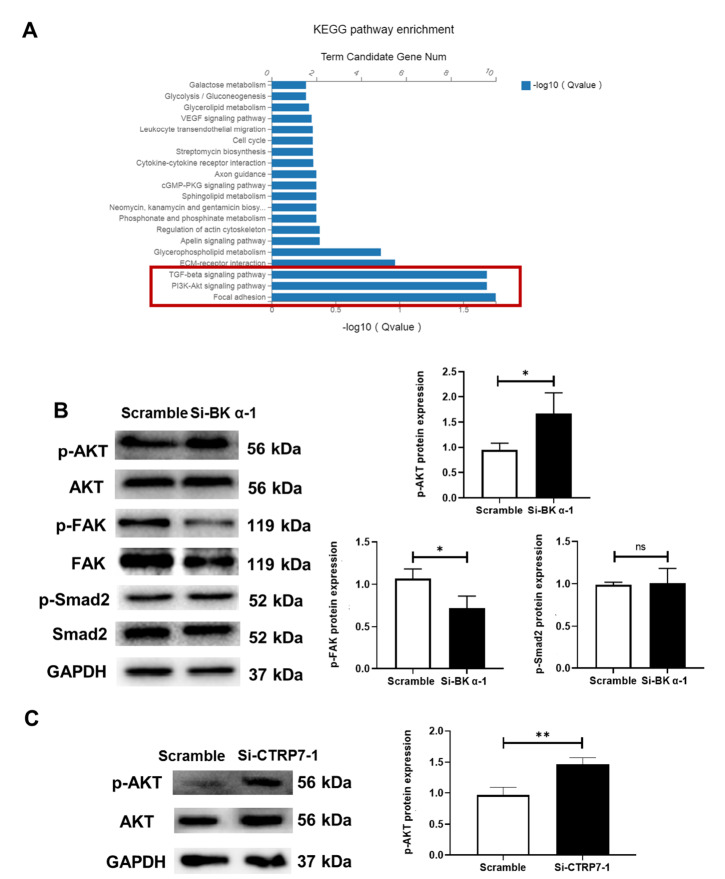
Having demonstrated that BK α and CTRP7 are involved in VSMC protection, we attempted to explore the molecular signaling pathways responsible for these effects. First, pathway analysis of the upregulated DEGs was conducted to identify the signaling pathways that were most likely responsible for BK α-mediated vascular protection. The results revealed that the focal adhesion pathway, the PI3K/Akt signaling pathway, and the TGF-β signaling pathway exhibited significant enrichment ( Figure 6A; P<0.05). We next examined these three signaling pathways in the context of BK α deficiency. Western blot analysis showed that only the PI3K/Akt signaling pathway was upregulated by BK α knockdown ( Figure 6B). Furthermore, CTRP7 deficiency activated the PI3K/Akt signaling pathway ( Figure 6C). These results suggested that the PI3K/Akt pathway was the downstream signaling pathway activated by BK α/CTRP7 deficiency signaling.
Figure 6 .
Effect of BK α deficiency on downstream signaling in the aortas of rats and HUASMCs
(A) Representative graphs showing the top enriched signaling pathways of DEGs in the aortas of WT and BK α ‒/‒ rats, as revealed by KEGG pathway analysis. (B) Representative blots and statistical diagrams showing the expressions of p-Akt, p-FAK, and p-Smad2 in HUASMCs after BK α knockdown, as determined by western blot analysis. (C) Representative blots and statistical diagrams showing the expression of p-Akt in HUASMCs after CTRP7 knockdown, as determined by western blot analysis. Data are shown as the mean±SD, n=3 per group. * P<0.05, ** P<0.01, ns, not significant.
Discussion
In the present study, we first report that the deletion of BK α promotes vascular inflammation and decreases SMC contractile phenotype proteins, thereby leading to vascular remodelling. Furthermore, this study provides evidence that CTRP7 is expressed in vascular smooth muscle and that BK deficiency activates the PI3K/Akt signaling pathway by reducing CTRP7, which results in vascular disease.
BK channels are widely distributed in the cardiovascular system. These channels are ubiquitously expressed, especially in VSMCs, and have large single channel conductance values [12]. In VSMCs, BK channels are composed of pore-forming α subunits and auxiliary subunits (β subunits and γ subunits) [13]. The α subunits regulate the concentrations of Ca 2+ and K + inside and outside the cell and vascular tension. The increase in intracellular Ca 2+ concentration or the change in voltage activates the BK channel so that the intracellular K + flows out through the pore of the channel composed of the α subunit, thereby dilating blood vessels and maintaining a balance of vascular tension [14]. Auxiliary subunits modulate the activity and opening frequency of BK channels in voltage- and calcium-dependent manners [14]. A previous study showed that blood pressure was significantly increased in β subunit knockout mice [15]. Another study showed that BKβ1 subunit-knockout mice fed with a high-fat diet had significant increases in vascular fibrosis and remodelling [16], but evidence for the effect of α subunits on vascular remodelling is lacking. In this study, we found that the blood vessels of BK α ‒/‒ rats spontaneously developed vascular remodelling, including thickening of the vessel wall, increased deposition of collagen fibers, and disordered arrangement of elastic fibers. These results indicated that the BK channel α subunit might play a crucial role in vascular remodelling.
Interestingly, it was reported that blood pressure was increased in BK α ‒/‒ mice [17]; however in the present study we found that the blood pressure and heart rate were deceased in BK α ‒/‒ rats. The reason for this discrepancy may be that different experimental animal species were used, i.e., we used rats and they used mice. Second, blood pressure is determined by cardiac ejection, blood volume, and vascular resistance [18]. In our previous studies, compared with WT rats, BK α ‒/‒ rats showed enhanced vasoconstriction; however, the left ventricular wall became thinner, and the myocardial area was smaller. At the same time, there was a decrease in left ventricular systolic function (the left ventricular ejection fraction and left ventricular short-axis shortening rate were decreased) in BK α ‒/‒ rats [8]. In addition, it was reported that blood pressure was reduced in BK channel cardiac conditional knockout mice [19]. Therefore, the reason for this discrepancy may be that whole-body BK α ‒/‒ rats showed various phenotypic disorders that may affect blood pressure, such as reduced cardiac function [8]. Apparently, the data are significant because they suggest that vascular BK channels protect against vascular remodelling and fibrosis via blood pressure-independent mechanisms.
After stimulation of inflammation and other factors, the levels of MMPs in VSMCs and macrophages were markedly increased [20]. MMPs are powerful metalloproteinases that degrade the extracellular matrix, including elastic fibers and collagen fibers, and stimulate SMC proliferation and migration [ 6, 21] . In the present study, we demonstrated for the first time that there was an increase in MMPs after the inhibition of BK α expression and activity in VSMCs. These results suggested that BK channels might have a beneficial effect on cardiovascular protection.
Abnormal expression or weakened activity of BK channels has a significant impact on the human body. Abnormal expression of BK channels, such as BK channel phosphorylation, may induce channel activity [12]. Furthermore, the α subunit can also bind to other proteins, such as focal adhesion kinase (FAK) and the protein kinase A (PKA) complex [22], and is associated with cell signaling events [1]. Therefore, we inhibited the expression and activity of BK channels in vitro to observe their effect on VSMCs. Our findings showed that inhibition of α subunit expression or function impaired VSMCs. This observation provides clues that BK channels are potential targets for the precise treatment of vascular diseases.
CTRP7 also shows a certain protective effect on VSMCs. CTRP7, which is known as C1q tumor necrosis factor-related protein 7, belongs to the complement C1q tumor necrosis factor-related protein superfamily [23]. CTRP proteins are structurally similar to adiponectin and consist of an N-terminal signal peptide, a short variable region, a collagen domain, and a C-terminal globular domain that is homologous to complement component 1q [24]. In addition, CTRPs have many functions similar to those of adiponectin, including lipid regulation, insulin sensitization and anti-inflammatory effects. The difference is that adiponectin is secreted by adipose tissue, while CTRP proteins are widely distributed in the body [25]. CTRP family members play an important role in the protection of cardiovascular disease and the regulation of inflammation. For example, CTRP1 prevents the development of pathological vascular remodelling [ 26, 27] . CTRP3 can protect cardiac function by promoting angiogenesis, and inhibiting apoptosis and fibrosis [28]. CTRP6 can inhibit postinfarction cardiac fibrosis by inhibiting fibroblast migration and transition to myofibroblasts [29]. CTRP9 is expressed at high level in the heart, and recent studies have shown that it can attenuate acute ischaemia-reperfusion injury and reduce ventricular remodelling after myocardial infarction [30] and it also has certain vasodilatory functions [31]. However, other functions of these CTRP family members are largely unknown.
At present, the function of CTRP7 is not clearly understood. In one report, the deletion of CTRP7 reduced obesity-related glucose intolerance, adipose tissue inflammation, and hepatic stress [32]. A recent study showed that circulating CTRP7 is a potential predictor of metabolic syndrome [33]. Metabolic syndrome is often accompanied by chronic inflammation [34], and elevated expression of CTRP7 may be associated with inflammation. However, we observed that CTRP7 deficiency contributed to inflammation in VSMCs. This finding seemed to contradict the proinflammatory effect of CTRP7 reported in related studies [32]. Of note, the contradiction may be attributed to different pathological models. Although there are few studies on CTRP7, multiple studies have shown that adiponectin, a homologue of the CTRP family, plays different roles in different diseases [35]. CTRP7, as a paralogue of adiponectin, may play a similar role to adiponectin, which indicates that the role of CTRP7 is complex. Another report mentioned that circulating CTRP7 level in coronary artery disease (CAD) patients was reduced [36], suggesting that CTRP7 is a potential biomarker for CAD diagnosis. We showed for the first time that CTRP7 was expressed in smooth muscle and that CTRP7 deficiency inhibited SMC contractile phenotype protein expression. These data together revealed that CTRP7 plays vital physiological and pathological roles in cardiovascular disease and may have a certain protective effect on the cardiovascular system. Considering the importance of CTRP7 in the human body, it deserves to be explored more deeply.
The PI3K/Akt pathway acts as a critical regulator of cell proliferation and nutrient metabolism. It has been shown that activation of the PI3K/Akt pathway is closely associated with vascular remodelling [37]. Notably, our study revealed that the reduced expressions of BK α and CTRP7 mediated the activation of the PI3K/Akt signaling pathway. These data identified the downstream pathway of BK α/CTRP7 deficiency-mediated vascular remodelling. Since we have only performed preliminary studies on CTRP7, the specific contribution of CTRP7 in VSMCs to vascular remodelling needs more in-depth research.
It is well known that the role of BK channels is closely related to Ca 2+. One study showed that there was no significant change in the intracellular Ca 2+ concentration of vascular smooth muscle cells from BK α ‒/‒ mice compared with that from WT mice [17]. The CTRPs are paralogues of adiponectin, and their functions are similar [25]. A previous study showed that high-density lipoprotein can promote adiponectin expression through the Ca 2+/calmodulin (CaM)-dependent protein kinase IV (CaMKIV) cascade [38]. However, the relationship between CTRP7 and Ca 2+ has not been reported. In future studies, we can measure the Ca 2+ concentration in vascular smooth muscle cells of BK α ‒/‒ rats and study the relationship between CTRP7 and Ca 2+.
In summary, we found that loss of BK channels inhibits the expressions of SMC contractile phenotype proteins and promotes the massive release of inflammatory factors, which may lead to vascular remodeling ( Figure 7). In addition, we first observed that CTRP7 is distributed in VSMCs and CTRP7 may have anti-inflammatory and other protective effects on VSMCs, suggesting that BK channels and CTRP7 may be potential targets for the prevention of vascular remodelling and other vascular diseases and for drug development.
Figure 7 .
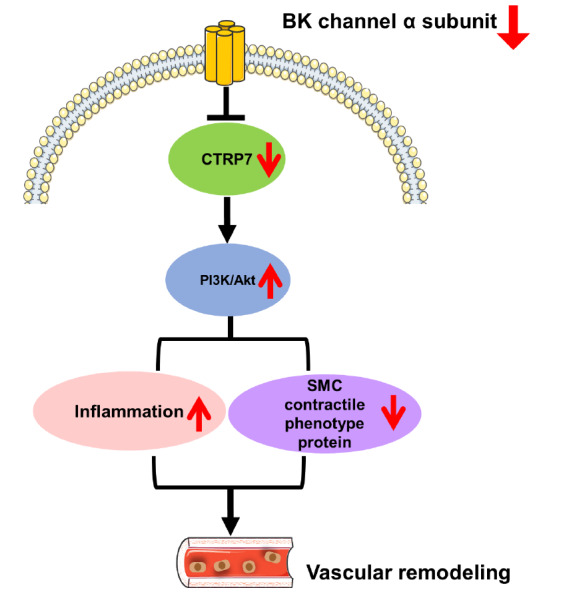
Schematic illustration of the process of vascular remodelling induced by BK channels
SMC, smooth muscle cell; CTRP7, C1q/tumor necrosis factor -related protein 7.
Supporting information
Supplementary Data
Supplementary data is available at Acta Biochimica et Biophysica Sinica online.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 91539205 and 81900415) and the Beijing Natural Science Foundation Program and Scientific Research Key Program of Beijing Municipal Commission of Education (No. KZ201810025039).
References
- 1.Toro L, Li M, Zhang Z, Singh H, Wu Y, Stefani E. MaxiK channel and cell signalling. Pflugers Arch - Eur J Physiol. . 2014;466:875–886. doi: 10.1007/s00424-013-1359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sancho M, Kyle BD. The large-conductance, calcium-activated potassium channel: a big key regulator of cell physiology. Front Physiol. . 2021;12:750615. doi: 10.3389/fphys.2021.750615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, Liu ZF, et al. Function of BK Ca channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension . Hypertension. . 2013;61:519–525. doi: 10.1161/HYPERTENSIONAHA.111.00211. [DOI] [PubMed] [Google Scholar]
- 4.Tobal R, Potjewijd J, Empel VP, Ysermans R, Schurgers LJ, Reutelingsperger CP, Damoiseaux JG, et al. Vascular remodeling in pulmonary arterial hypertension: the potential involvement of innate and adaptive immunity. Front Med. . 2021;8:806899. doi: 10.3389/fmed.2021.806899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castorena-Gonzalez JA, Staiculescu MC, Foote C, Martinez-Lemus LA. Mechanisms of the inward remodeling process in resistance vessels: is the actin cytoskeleton involved? Microcirculation. . 2014;21:219–229. doi: 10.1111/micc.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaminon A, Reesink K, Kroon A, Schurgers L. The role of vascular smooth muscle cells in arterial Remodeling: focus on calcification-related processes. Int J Mol Sci. . 2019;20:5694. doi: 10.3390/ijms20225694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteford JR, De Rossi G, Woodfin A. Mutually supportive mechanisms of inflammation and vascular Remodelingr. Int Rev Cell Mol Biol. 2016, 326: 201–278 . [DOI] [PubMed]
- 8.He C, Li X, Wang M, Zhang S, Liu H. Deletion of BK channels decreased skeletal and cardiac muscle function but increased smooth muscle contraction in rats. Biochem Biophys Res Commun. . 2021;570:8–14. doi: 10.1016/j.bbrc.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Wang Y, Garver H, Galligan JJ, Fink GD. Vascular BK channel deficiency exacerbates organ damage and mortality in endotoxemic mice. J Cardiovasc Pharmacol. . 2012;59:207–214. doi: 10.1097/FJC.0b013e31823b493b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan XJ, Zhao HC, Zhang P, Huo B, Shen BR, Yan ZQ, Qi YX, et al. Involvement of BK channel in differentiation of vascular smooth muscle cells induced by mechanical stretch. Int J Biochem Cell Biol. . 2015;59:21–29. doi: 10.1016/j.biocel.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Ren J, Cheng Y, Wen X, Liu P, Zhao F, Xin F, Wang M, et al. BK Ca channel participates in insulin‐induced lipid deposition in adipocytes by increasing intracellular calcium . J Cell Physiol. . 2021;236:5818–5831. doi: 10.1002/jcp.30266. [DOI] [PubMed] [Google Scholar]
- 12.Krishnamoorthy-Natarajan G, Koide M. BK channels in the vascular system. Int Rev Neurobiol. 2016, 128: 401–438 . [DOI] [PubMed]
- 13.Carvalho-de-Souza JL, Varanda WA, Tostes RC, Chignalia AZ. BK channels in cardiovascular diseases and aging. Aging Dis. 2013, 4: 38–49 . [PMC free article] [PubMed]
- 14.Dopico AM, Bukiya AN, Jaggar JH. Calcium- and voltage-gated BK channels in vascular smooth muscle. Pflugers Arch - Eur J Physiol. . 2018;470:1271–1289. doi: 10.1007/s00424-018-2151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plüger S, Faulhaber J̈, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, et al. Mice with disrupted BK channel β1 subunit gene feature abnormal Ca 2+ spark/STOC coupling and elevated blood pressure . Circ Res. . 2000;87:53–60. doi: 10.1161/01.RES.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Garver H, Fernandes R, Phelps JT, Harkema JJ, Galligan JJ, Fink GD. BK channel β1-subunit deficiency exacerbates vascular fibrosis and remodelling but does not promote hypertension in high-fat fed obesity in mice. J Hypertension. . 2015;33:1611–1623. doi: 10.1097/HJH.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, et al. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel–deficient mice. Circulation. . 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- 18.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. . 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankenreiter S, Bednarczyk P, Kniess A, Bork NI, Straubinger J, Koprowski P, Wrzosek A, et al. cGMP-elevating compounds and ischemic conditioning provide cardioprotection against ischemia and reperfusion injury via cardiomyocyte-specific BK channels. Circulation. . 2017;136:2337–2355. doi: 10.1161/CIRCULATIONAHA.117.028723. [DOI] [PubMed] [Google Scholar]
- 20.Cevik C, Otahbachi M, Nugent K, Warangkana C, Meyerrose G. Effect of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition on serum matrix metalloproteinase-13 and tissue inhibitor matrix metalloproteinase-1 levels as a sign of plaque stabilization. J Cardiovasc Med. . 2008;9:1274–1278. doi: 10.2459/JCM.0b013e328316912f. [DOI] [PubMed] [Google Scholar]
- 21.Ma Z, Mao C, Jia Y, Fu Y, Kong W. Extracellular matrix dynamics in vascular remodeling. Am J Physiol-Cell Physiol. . 2020;319:C481–C499. doi: 10.1152/ajpcell.00147.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian L, Coghill LS, MacDonald SHF, Armstrong DL, Shipston MJ. Leucine zipper domain targets cAMP-dependent protein kinase to mammalian BK channels. J Biol Chem. . 2003;278:8669–8677. doi: 10.1074/jbc.M211661200. [DOI] [PubMed] [Google Scholar]
- 23.Kong M, Gao Y, Guo X, Xie Y, Yu Y. Role of the CTRP family in tumor development and progression. Oncol Lett. . 2021;22:723. doi: 10.3892/ol.2021.12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ressl S, Vu BK, Vivona S, Martinelli DC, Südhof TC, Brunger AT. Structures of C1q-like proteins reveal unique features among the C1q/TNF superfamily. Structure. . 2015;23:688–699. doi: 10.1016/j.str.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Li L, Aslam M, Siegler BH, Niemann B, Rohrbach S. Comparative analysis of CTRP-mediated effects on cardiomyocyte glucose metabolism: cross talk between AMPK and Akt signaling pathway. Cells. . 2021;10:905. doi: 10.3390/cells10040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanemura N, Shibata R, Ohashi K, Ogawa H, Hiramatsu-Ito M, Enomoto T, Yuasa D, et al. C1q/TNF-related protein 1 prevents neointimal formation after arterial injury. Atherosclerosis. . 2017;257:138–145. doi: 10.1016/j.atherosclerosis.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Shen Y, Lu L, Liu ZH, Wu F, Zhu JZ, Sun Z, Zhang RY, et al. Increased serum level of CTRP1 is associated with low coronary collateralization in stable angina patients with chronic total occlusion. Int J Cardiol. . 2014;174:203–206. doi: 10.1016/j.ijcard.2014.03.205. [DOI] [PubMed] [Google Scholar]
- 28.Wu D, Lei H, Wang JY, Zhang CL, Feng H, Fu FY, Li L, et al. CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation. J Mol Med. . 2015;93:1311–1325. doi: 10.1007/s00109-015-1309-8. [DOI] [PubMed] [Google Scholar]
- 29.Lei H, Wu D, Wang JY, Li L, Zhang CL, Feng H, Fu FY, et al. C1q/tumor necrosis factor-related protein-6 attenuates post-infarct cardiac fibrosis by targeting RhoA/MRTF-A pathway and inhibiting myofibroblast differentiation. Basic Res Cardiol. . 2015;110:35. doi: 10.1007/s00395-015-0492-7. [DOI] [PubMed] [Google Scholar]
- 30.Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X, Wang Y, et al. C1q/tumor necrosis factor–related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation. . 2013;128 doi: 10.1161/CIRCULATIONAHA.112.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Q, Yuan Y, Yi W, Lau WB, Wang Y, Wang X, Sun Y, et al. C1q/TNF-related proteins, a family of novel adipokines, induce vascular relaxation through the adiponectin receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler Thromb Vasc Biol. . 2011;31:2616–2623. doi: 10.1161/ATVBAHA.111.231050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen PS, Lei X, Wolf RM, Rodriguez S, Tan SY, Little HC, Schweitzer MA, et al. CTRP7 deletion attenuates obesity-linked glucose intolerance, adipose tissue inflammation, and hepatic stress. Am J Physiol-Endocrinol Metab. . 2017;312:E309–E325. doi: 10.1152/ajpendo.00344.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu W, Zhan B, Li Q, Yang G, Yang M, Tan M, Geng S, et al. Circulating CTRP7 is a potential predictor for metabolic syndrome. Front Endocrinol. . 2021;12:774309. doi: 10.3389/fendo.2021.774309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. . 2018;36:14–20. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Jang AY, Scherer PE, Kim JY, Lim S, Koh KK. Adiponectin and cardiometabolic trait and mortality: where do we go? Cardiovasc Res. . 2022;118:2074–2084. doi: 10.1093/cvr/cvab199. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Liu C, Liu J, Guo R, Yan Z, Liu W, Lau WB, et al. Implications of C1q/TNF-related protein superfamily in patients with coronary artery disease. Sci Rep. . 2020;10:878. doi: 10.1038/s41598-020-57877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu QF, Yu HW, Sun LL, You L, Tao GZ, Qu BZ. Apelin-13 upregulates Egr-1 expression in rat vascular smooth muscle cells through the PI3K/Akt and PKC signaling pathways. Biochem Biophys Res Commun. . 2015;468:617–621. doi: 10.1016/j.bbrc.2015.10.171. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi T, Imachi H, Fukunaga K, Lyu J, Sato S, Saheki T, Ibata T, et al. HDL promotes adiponectin gene expression via the CAMKK/CAMKIV pathway. J Mol Endocrinol. . 2022;68:89–98. doi: 10.1530/JME-20-0211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.