Abstract
Breast cancer is the most commonly diagnosed cancer worldwide. Previously, we reported that calycosin, a typical isoflavone phytoestrogen, triggers apoptosis and is associated with lncRNA HOTAIR in the estrogen receptor (ER)-positive breast cancer MCF-7-cell line. In the present study, we aim to uncover the mechanism of lncRNA HOTAIR in the inhibitory effect induced by calycosin in both ER-positive and ER-negative breast cancer cell lines. Results show that calycosin significantly inhibits proliferation and triggers apoptosis in both ER-positive (MCF-7 and T47D) and ER-negative (MDA-MB-231 and SK-BR-3) breast cancer cell lines, accompanied by downregulation of lncRNA HOTAIR expression. Accordingly, knockdown of lncRNA HOTAIR promotes the anti-tumor effect of calycosin, while overexpression of lncRNA HOTAIR attenuates this effect. Meanwhile, the expression levels of HuR and IGF2BP1 are also reduced by calycosin. More importantly, calycosin facilitates the downregulation of HuR and IGF2BP1 caused by decreasing lncRNA HOTAIR expression, and the upregulation of HuR and IGF2BP1 caused by overexpression of lncRNA HOTAIR is weakened by calycosin. These results demonstrate that downregulating HuR and IGF2BP1 by suppressing lncRNA HOTAIR results in inhibited growth of breast cancer cells by calycosin. In addition, the binding of HuR and IGF2BP1 to lncRNA HOTAIR is detected by RIP assay, implying an interaction between these two proteins and lncRNA HOTAIR. Together, lncRNA HOTAIR may play a carcinogenic role in breast cancer development and has the potential to be a novel therapeutic target for breast cancer in the future, especially in isoflavone phytoestrogen therapy.
Keywords: lncRNA HOTAIR, HuR, IGF2BP1, calycosin, breast cancer
Introduction
Breast cancer is the most commonly diagnosed malignant tumor, with an estimated 2.26 million new cases in 2020 worldwide. It is also the leading cause of cancer death among women [1], and the incidence and mortality rates of breast carcinoma are expected to increase significantly in the coming years. The therapy of breast cancer patients includes surgery, chemotherapy, neoadjuvant and endocrine therapy. Improvements have been made in the diagnosis and therapeutic schemes in recent decades, but the situation remains severe due to the complexity and heterogeneity of breast carcinoma [2].
The incidence rate of breast cancer in Asians is remarkably lower than that in individuals from the USA or Europe. Previous studies have shown that higher consumption of soybeans and soy products, which are rich in phytoestrogens such as isoflavones, is associated with a decreased incidence rate of breast cancer in Asian regions [ 3‒ 5] . There is a general consensus that long-term massive exposure to estrogen is related to breast cancer initiation and progression, including endogenous and exogenous estrogens [6]. Isoflavones are similar to estrogen in molecular structure and act as estrogen antagonists in a high estrogen environment [7]. Calycosin (C 16H 12O 5) is a natural isoflavone extracted from the Chinese traditional herbal medicine Astragali radix. Calycosin has a wide range of pharmacological activities, including anti-tumor, anti-inflammatory, antioxidant, anti-osteoporosis, antidiabetic, neuroprotective, hepatoprotective and cardioprotective activities [ 8‒ 16] . Meanwhile, its application in treating estrogen-dependent cancers (breast, endometrial, and ovarian) is of interest, especially in treating breast cancer [17]. According to the status of estrogen receptor (ER), breast cancers are classified into two subtypes in the clinic: ER-positive breast cancer and ER-negative breast cancer. Our previous data suggested that calycosin triggers cell apoptosis in ER-positive breast cancer MCF-7 cells by decreasing the expression of long noncoding RNA (lncRNA) HOX antisense intergenic RNA (HOTAIR) [18].
LncRNA is a class of RNA transcripts with a length greater than 200 nucleotides, which are unable to encode proteins [19]. Accumulating evidence indicates that lncRNAs play a crucial role in the differentiation, progression and pathogenesis of numerous diseases, especially cancers [ 20, 21] . Although dysregulated expressions of lncRNAs have been identified in many laboratory studies, more investigations are needed to elucidate their mechanism. The lncRNA HOTAIR has been introduced as an important oncogenic factor that contributes to the initiation and progression of various kinds of cancer, such as cervical cancer and gastric cancer [ 22, 23] . However, whether the lncRNA HOTAIR is involved in the inhibitory effect induced by the phytoestrogen calycosin in breast cancer cell progression remains largely unclear, particularly in the case of ER-negative breast cancer cells. Therefore, the question of the downstream molecular mechanism of lncRNA HOTAIR in the inhibitory effect induced by calycosin in both ER-positive and ER-negative breast cancer cells is still open.
In the present study, we aim to uncover the mechanism of lncRNA HOTAIR in the inhibitory effect induced by calycosin in both ER-positive and ER-negative breast cancer cell lines.
Materials and Methods
Cell culture and calycosin treatment
The human breast cancer cell lines MCF-7, T47D, MDA-MB-231 and SK-BR-3 were obtained from the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China), and cultured in Dulbecco’s modified Eagle’s medium (DEME; Gibco, Carlsbad, USA) supplemented with 10% fetal bovine serum (FBS; Gibco) and 1% penicillin-streptomycin (Solarbio Science & Technology, Beijing, China) within a humidified atmosphere with 5% CO 2 at 37°C. Calycosin (purity≥98%) was purchased from Shanghai Yuanye Bio-Technology Ltd. (Shanghai, China). Calycosin was dissolved in dimethylsulfoxide (DMSO; Sigma Aldrich, St Louis, USA) and diluted with fresh DMEM to the required concentration. The final concentration of DMSO in the fresh medium did not exceed 0.1%, and DMSO had no significant effect on the cell viability at this concentration.
Cell transfection
The pcDNA-NC, siRNA-NC, overexpression plasmids (pcDNA-HOTAIR, pcDNA-HuR, and pcDNA-IGF2BP1) and siRNAs (si-HOTAIR, si-HuR, and si-IGF2BP1) were constructed by GenePharma Inc. (Shanghai, China). The sequences of the siRNAs are listed in Table 1. Transient transfection was performed using Lipofectamine 3000 (Thermo Fisher, Waltham, USA) according to the manufacturer’s instructions. The transfected cells with or without calycosin treatment were harvested for subsequent experiments after 48 h of incubation.
Table 1 Sequences of siRNAs used in this study
|
Name |
Sequence |
|
si-HOTAIR-1162 |
Sense: 5′-CAAUAUAUCUGUUGGGCGUTT-3′ Antisense: 3′-ACGCCCAACAGAUAUAUUGTT-5′ |
|
si-HOTAIR-536 |
Sense: 5′-GCCUUCCUUAUAAGCUCGUTT-3′ Antisense: 3′-ACGAGCUUAUAAGGAAGGCTT-5′ |
|
si-HOTAIR-1597 |
Sense: 5′-GGAAGCUCUUGAAGGUUCATT-3′ Antisense: 3′-UGAACCUUCAAGAGCUUCCTT-5′ |
|
si-HuR |
Sense: 5′-GGUUUGGGCGGAUCAUCAATT-3′ Antisense: 5′-UUGAUGAUCCGCCCAAACCTT-3′ |
|
si-IGF2BP1 |
Sense: 5′-CCAGGUUAAGCAGCAGCAUTT-3′ Antisense: 5′-AUGCUGCUGCUUAACCUGGTT-3′ |
|
si-NC |
Sense: 5′-UUCUCCGAACGUGUCACGUTT-3′ Antisense: 5′-ACGUGACACGUUCGGAGAATT-3′ |
CCK-8 cell assay
Breast cancer cells were seeded in 96-well plates at 5000 cells per well and treated with different concentrations of calycosin (0, 20, 40, and 80 μM) at 37°C with 5% CO 2 for 0, 24, 48, and 72 h. At each time point, the cells were incubated with 10% CCK-8 solution (Dojindo, Kumamoto, Japan) in fresh medium for another 2 h. Subsequently, the optical density (OD) value was measured at a wavelength of 450 nm with a microplate reader.
MTT assay
Transiently transfected breast cancer cells were plated into 96-well plates and treated with 80 μM calycosin at 37°C with 5% CO 2 for 48 h. After incubation, 20 μL MTT (5 mg/mL; Sigma Aldrich) was added to each well and incubated for another 4 h. Then, the medium was removed, and 100 μL DMSO was added to each well to dissolve the sediment. The OD value of each well was measured at 490 nm with a microplate reader after shaking for 15 min at room temperature.
Flow cytometry
Calycosin-treated cells were harvested and washed twice with cold PBS. Then, an Annexin V-FITC/propidium iodide (PI) detection kit (BD Pharmingen, San Jose, USA) was used to analyze the apoptosis of breast cancer cells. In each group, approximately 1×10 5 cells were resuspended in 100 μL of binding buffer. After incubation with 5 μL of Annexin V-FITC staining solution and 5 μL of PI staining solution for 15 min in the dark, 400 μL of binding buffer was added to each group, and the stained cells were immediately analyzed by flow cytometry (Invitrogen, Carlsbad, USA).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated using Trizol reagent (Invitrogen) and reverse-transcribed into cDNA using a FastKing RT kit (TIANGEN, Beijing, China) according to the manufacturer’s instructions. The relative expression levels of Bcl-2, Bax, lncRNA HOTAIR, HuR and IGF2BP1were quantified by qRT-PCR using specific primers ( Table 2) and SYBR Green PCR Master Mix (Monad, Wuhan, China). Relative mRNA expressions were calculated by the standard 2 –ΔΔCt method. GAPDH was used as the internal control for normalization.
Table 2 Sequences of primers used in this study
|
Name |
Primer sequence (5′→3′) |
|
GAPDH |
Forward: ATGGGTGTGAACCATGAGAA |
|
Reverse: GTGCTAAGCAGTTGGTGGTG | |
|
Bcl-2 |
Forward: TGTCCTTTGACCTTGTTTCT |
|
Reverse: TCATTTGCCATCTGGATTTT | |
|
Bax |
Forward: GCCCTTTGCTTCAGGGTTTC |
|
Reverse: CACTCGCTCAGCTTCTTGGT | |
|
HOTAIR |
Forward: TAAGCTCGTTGGGGCCTAAG |
|
Reverse: CATTTCTCTGCGTGGTTCGC | |
|
HuR |
Forward: CAGCAGCATTGGTGAAGTTG |
|
Reverse: AGCGTGTTGATCGCTCTCTC | |
|
IGF2BP1 |
Forward: CAGGAGATGGTGCAGGTGTT |
|
Reverse: GTGTTTCGGGTGGTGCAATC |
Western blot analysis
Proteins were extracted from cells using RIPA buffer (Beyotime, Shanghai, China), and the protein concentration of samples was quantified using a BCA assay kit (Beyotime). Equal amounts of proteins were separated by SDS-PAGE, and transferred onto polyvinylidene difluoride membranes (PVDF; Solarbio). The membranes were blocked with 5% skim milk powder and incubated with specific primary antibodies against the following proteins: anti-β-actin (1:1000; ZSGB-BIO, Beijing, China), anti-Bcl-2 (1:2000; Proteintech Group, Wuhan, China), anti-Bax (1:2000; Proteintech Group), anti-HuR (1:1000; Proteintech Group) and anti-IGF2BP1 (1:1000; Proteintech Group) at 4°C overnight. The membranes were then incubated with the appropriate secondary antibodies (ZSGB-BIO) and visualized with enhanced chemiluminescence reagents (ECL; Beyotime). Band intensities were quantified using Image-Pro Plus 5.02 software (Media Cybernetics, Bethesda, USA). β-Actin was used as the loading control.
RNA binding protein immunoprecipitation (RIP)
An RNA-binding protein immunoprecipitation kit (BersinBio, Guangzhou, China) was used to determine the relationship between HuR, IGF2BP1 and lncRNA HOTAIR according to the manufacturer’s protocol. The antibodies used for the RIP assay included anti-AGO2 and control IgG (ZSGB-BIO), and the coprecipitated RNAs were used for cDNA synthesis and evaluated by qRT-PCR.
Hoechst 33258 staining
Cells were seeded into 6-well plates at 2×10 5 cells per well, incubated for 12 h, and then untreated or treated with calycosin for 48 h. Cells were fixed in 4% paraformaldehyde for 10 min at room temperature, washed with PBS twice and stained with Hoechst 33258 (Beyotime) for 5 min in the dark. The stained cells were visualized from randomly selected fields under a fluorescence microscope (Olympus, Tokyo, Japan). Nuclear fragmentation and nuclear condensation were identified as cell apoptosis.
Statistical analysis
Data are presented as the mean±standard deviation (SD). Statistical analyses were performed by SPSS 18.0 (IBM Corp., Armonk, USA) and GraphPad Prism 8.0.2 statistical software (GraphPad Software, La Jolla, USA). Student’s t tests were used to compare the differences between two groups. One-way ANOVA was used for the comparison among multiple groups, and P<0.05 was considered statistically significant.
Results
Calycosin inhibits proliferation and induces apoptosis in both ER-positive and ER-negative breast cancer cells
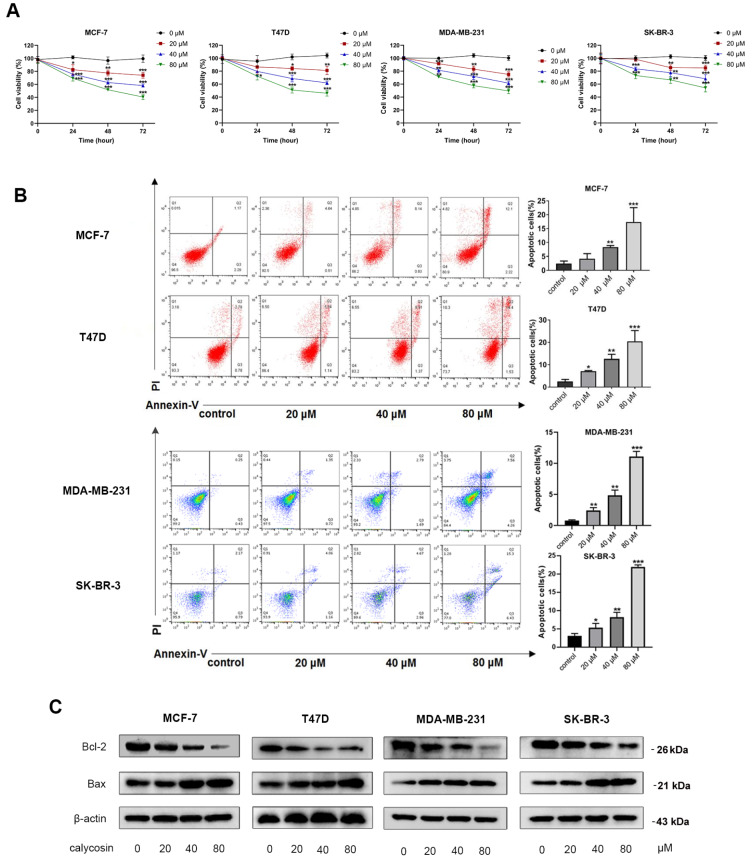
The viability of ER-positive breast cancer cell lines (MCF-7 and T47D) was prominently decreased by calycosin in a concentration- and time-dependent manner ( Figure 1A), and the rate of apoptosis in MCF-7 and T47D cells was gradually increased with the increasing dose of calycosin in comparison with the control group ( Figure 1B). Likewise, for ER-negative MDA-MB-231 and SK-BR-3 cells, the effects of calycosin on inhibiting proliferation and promoting apoptosis were similar to those of ER-positive MCF-7 and T47D cells ( Figure 1A,B). Calycosin-induced apoptosis was further confirmed by the modulation of the Bcl-2/Bax ratio. The transcriptional and protein levels of the apoptotic protein Bax were increased, whereas those of the antiapoptotic protein Bcl-2 and the ratio of Bcl-2/Bax were downregulated significantly in all four cell lines ( Figure 1C and Supplementary Figure S1A,B). These data indicated that calycosin inhibited proliferation and induced apoptosis in both ER-positive breast cancer cells and ER-negative breast cancer cells.
Figure 1 .
Inhibition of calycosin in breast cancer cell progression in both ER-positive and ER-negative breast cancer cell lines
Different concentrations of calycosin (0, 20, 40 and 80 μM) were used to treat ER-positive breast cancer cells (MCF-7 and T47D) and ER-negative breast cancer cells (MDA-MB-231 and SK-BR-3). (A) The viability of cells was detected by CCK-8 assays. (B) Flow cytometry was performed to detect the apoptotic rate after 48 h of calycosin treatment. (C) Western blot analysis was applied to detect the protein expression levels of Bcl-2 and Bax after 48 h of calycosin treatment. β-Actin was used as an internal control. Data are presented as the mean±SD ( n=3). * P<0.05, ** P<0.01 and *** P<0.001 compared with the control group.
Expression of lncRNA HOTAIR is reduced by calycosin in both ER-positive and ER-negative breast cancer cells
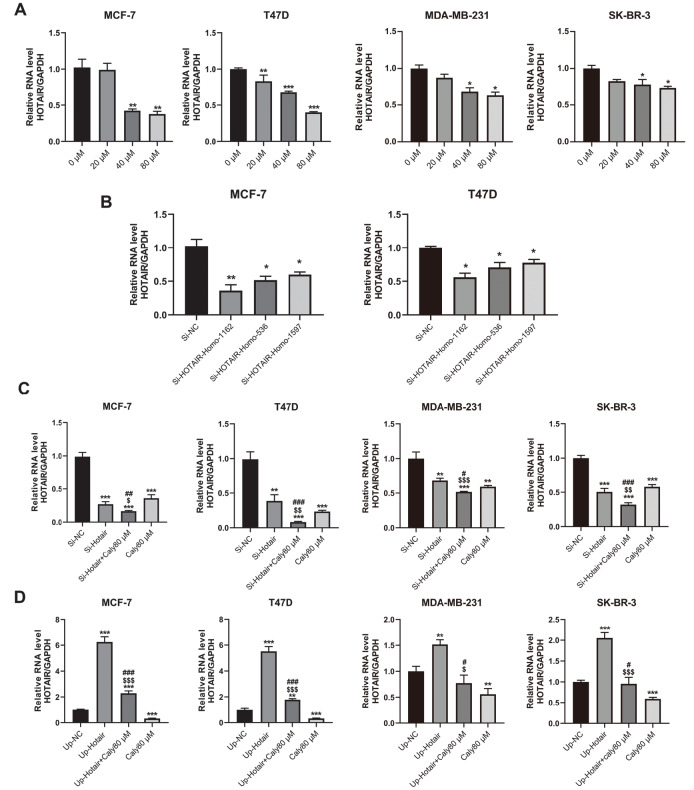
Previously, we reported that lncRNA HOTAIR is related to calycosin-induced cell apoptosis in ER-positive breast cancer MCF-7 cells [18], and thus we wondered whether calycosin could also inhibit cell growth by regulating lncRNA HOTAIR expression in ER-negative breast cancer cells. As shown in Figure 2A, the changes in lncRNA HOTAIR expression were measured by qRT-PCR in MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells after treatment with calycosin. Results showed that calycosin downregulated the transcriptional expression of lncRNA HOTAIR in both ER-positive and ER-negative breast cancer cell lines in a dose-dependent manner. Subsequently, to further investigate the role of lncRNA HOTAIR in calycosin-treated breast cancer cells, short interference RNAs (siRNAs; HOTAIR-Homo1162, HOTAIR-Homo536, HOTAIR-Homo1597) against human lncRNA HOTAIR were applied to downregulate the expression of lncRNA HOTAIR, and the efficiency of downregulation was confirmed by qRT-PCR ( Figure 2B). The si-HOTAIR-Homo1162 sequence exhibited the highest efficiency of knockdown and was selected for the subsequent experiments. Compared with the negative control (NC) group, the expression of lncRNA HOTAIR was significantly reduced in siHOTAIR-knockdown cells and further decreased by calycosin ( Figure 2C). In contrast, lncRNA HOTAIR expression was upregulated by transfection with the pcDNA-HOTAIR plasmid, while this upregulation was diminished by exposure to calycosin ( Figure 2D). Taken together, these results strongly proved that calycosin could regulate lncRNA HOTAIR levels in both ER-positive and ER-negative breast cancer cells in a concentration-dependent manner.
Figure 2 .
Reduced expression of lncRNA HOTAIR by calycosin
Different concentrations of calycosin (0, 20, 40 and 80 μM) were used to treat ER-positive breast cancer cell lines (MCF-7 and T47D) and ER-negative breast cancer cell lines (MDA-MB-231 and SK-BR-3), followed by qRT-PCR (A) to detect changes in lncRNA HOTAIR expression. The knockdown efficiency of three siRNA sequences against lncRNA HOTAIR in MCF-7 and T47D cell lines was measured by qRT-PCR (B). MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells were treated for 48 h with empty vector, HOTAIR-Homo1162, HOTAIR-Homo1162 and 80 μM calycosin, 80 μM calycosin or pcDNA-HOTAIR, pcDNA-HOTAIR and 80 μM calycosin. The transcription levels of lncRNA HOTAIR after knockdown (C) and overexpression (D) were determined by qRT-PCR. The expression level of lncRNA HOTAIR was normalized to GAPDH as the internal control. Data are presented as the mean±SD ( n=3). * P<0.05, ** P<0.01 and *** P<0.001 compared with the control group; $ P<0.05, $$ P<0.01 and $$$ P<0.001 compared with the model group; and # P<0.05, ## P<0.01 and ### P<0.001 compared with the 80 μM calycosin group.
Calycosin inhibits proliferation and induces apoptosis of breast cancer cells by downregulating lncRNA HOTAIR
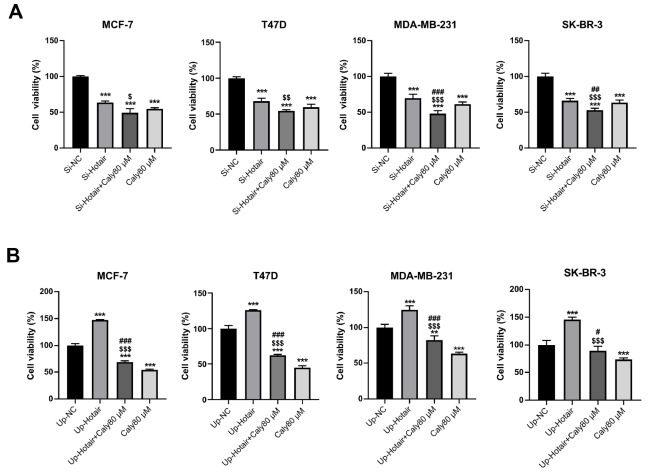
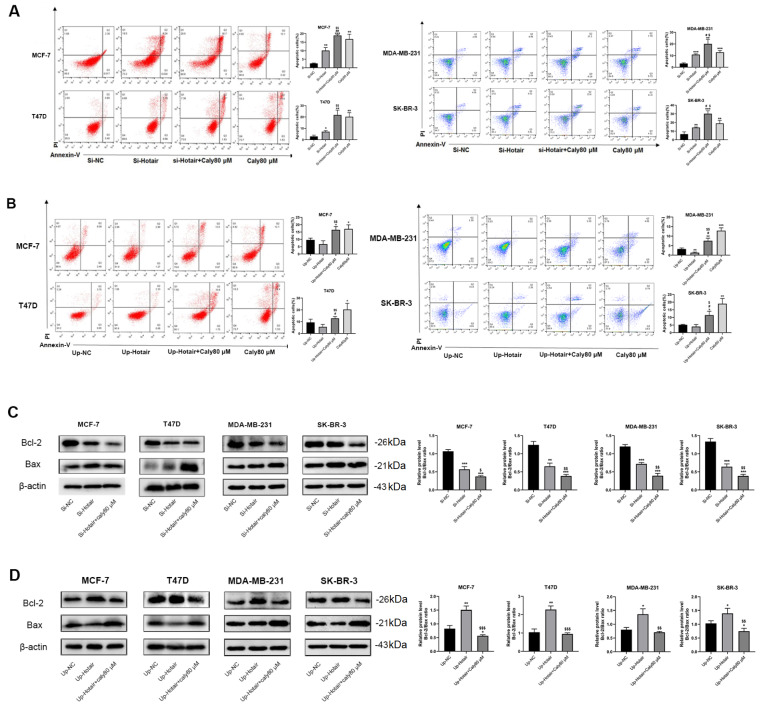
To further investigate the role of lncRNA HOTAIR in the inhibitory effect of calycosin in breast cancer cells, CCK-8 and MTT assays were performed to measure the viability of transfected breast cancer cells. Knockdown of lncRNA HOTAIR significantly inhibited the proliferation of all four breast cancer cell lines, and the inhibition was more prominent after incubation with calycosin ( Figure 3A). In contrast, overexpression of lncRNA HOTAIR greatly promoted cell proliferation, which was reversed by calycosin treatment ( Figure 3B). Additionally, flow cytometry analysis showed that knockdown of lncRNA HOTAIR facilitated cell apoptosis, and the highest rate of apoptosis was observed in lncRNA HOTAIR-knockdown cells incubated with calycosin ( Figure 4A). Meanwhile, knockdown of lncRNA HOTAIR reduced the ratio of Bcl-2/Bax, and the ratio reached maximum in lncRNA HOTAIR-knockdown cells incubated with calycosin, which is consistent with flow cytometry data ( Figure 4C). On the contrary, overexpression of lncRNA HOTAIR significantly reduced the apoptotic rate in MDA-MB-231 cells but not MCF-7, T47D and SK-BR-3 cells. However, calycosin increased the total apoptotic rate in the four lncRNA HOTAIR-overexpressing cell lines ( Figure 4B). Accordingly, the ratio of Bcl-2/Bax was promoted by lncRNA HOTAIR overexpression, and this promotion was attenuated by calycosin ( Figure 4D). These data demonstrated that lncRNA HOTAIR was likely to play a carcinogenic role in breast cancer cells and that calycosin inhibited the progression of both ER-positive and ER-negative breast cancer cells by targeting lncRNA HOTAIR.
Figure 3 .
Calycosin inhibited cell proliferation by regulating lncRNA HOTAIR in breast cancer cells
After knockdown (A) or overexpression (B) of lncRNA HOTAIR, MTT and CCK-8 assays were performed to detect the viability of MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells with or without calycosin treatment. Data are presented as the mean±SD ( n=3). ** P<0.01 and *** P<0.001 compared with the control group; $ P<0.05, $$ P<0.01 and $$$ P<0.001 compared with the model group; and # P<0.05, ## P<0.01 and ### P<0.001 compared with the 80 μM calycosin group.
Figure 4 .
Calycosin promoted cell apoptosis by regulating lncRNA HOTAIR in breast cancer cells
After the knockdown (A) or overexpression (B) of lncRNA HOTAIR, the apoptotic rate of MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells with or without calycosin treatment was detected by flow cytometry. Western blot analysis was applied to detect the protein expression levels of Bcl-2 and Bax after the knockdown (C) or overexpression (D) of lncRNA HOTAIR in transfected MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells. β-Actin was used as an internal control. Data are presented as the mean±SD ( n=3). * P<0.05, ** P<0.01 and *** P<0.001 compared with the control group; $ P<0.05 and $$ P<0.01 compared with the model group; and # P<0.05 and ## P<0.01 compared with the 80 μM calycosin group.
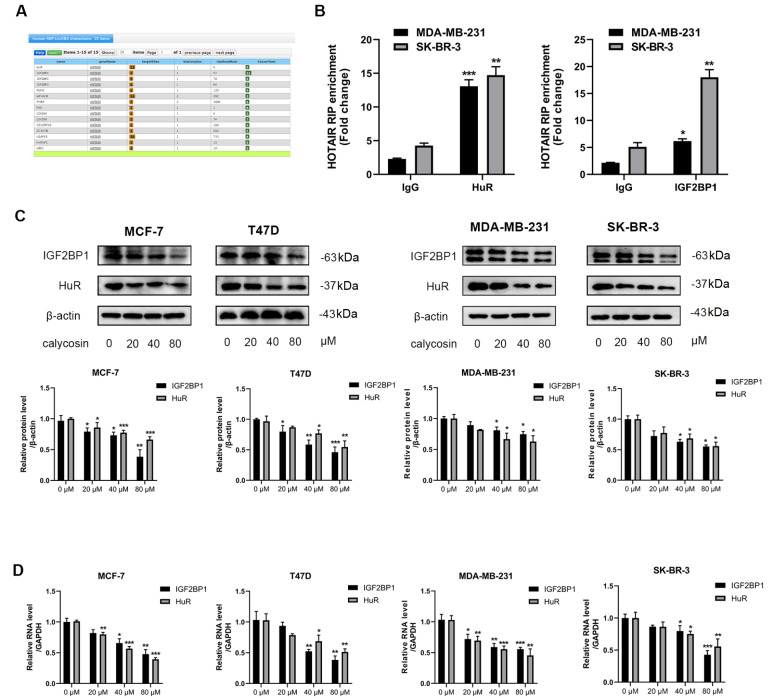
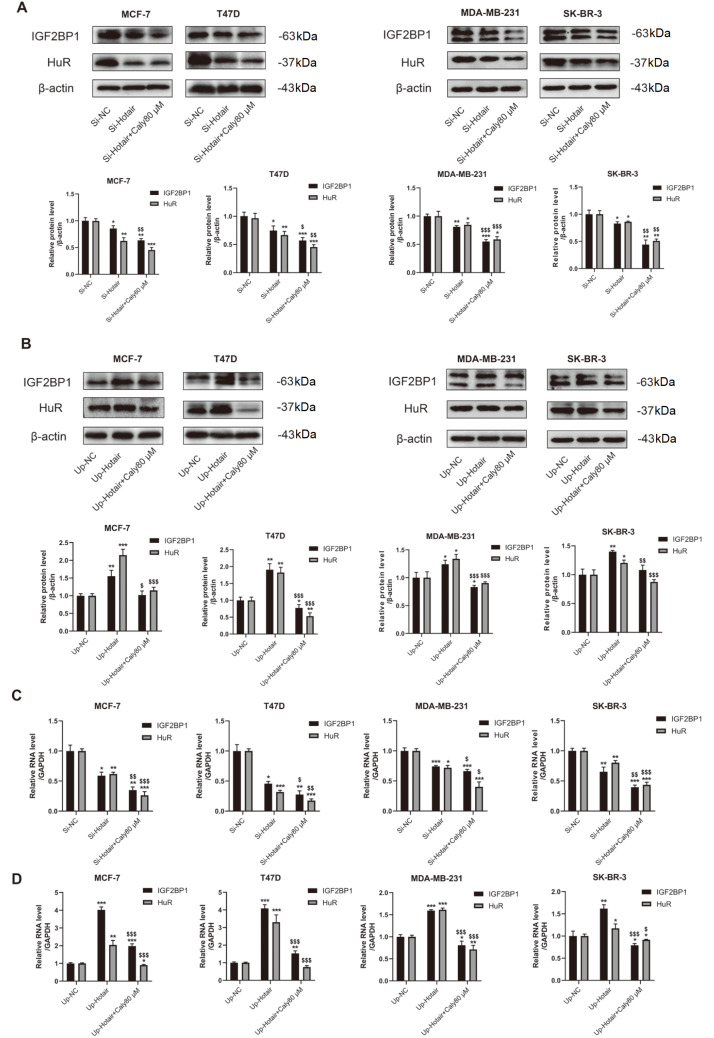
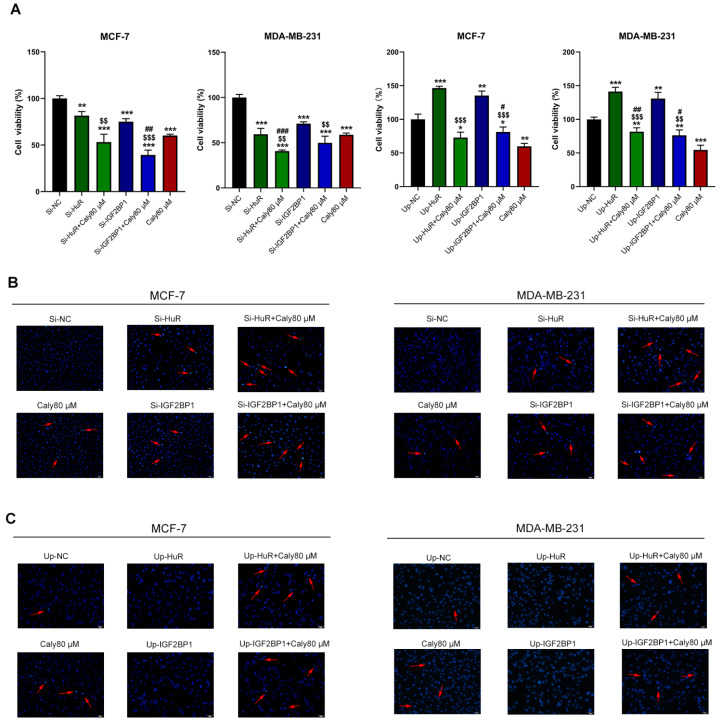
Calycosin inhibits the progression of breast cancer cells by downregulating lncRNA HOTAIR and its downstream targets HuR and IGF2BP1
RNA binding proteins (RBPs) human antigen R (HuR) and insulin-like growth factor-2 mRNA-binding protein 1 (IGF2BP1) were predicted to be the candidate downstream targets of lncRNA HOTAIR through StarBase database prediction ( Figure 5A). The protein and transcriptional expression levels of HuR and IGF2BP1 were reduced by calycosin in a dose-dependent manner ( Figure 5C,D). Furthermore, HuR and IGF2BP1 expressions were reduced in cells with siRNA-mediated knockdown of lncRNA HOTAIR at the protein and transcriptional levels, and a greater reduction was observed after exposure to calycosin ( Figure 6A,C). In contrast, overexpression of lncRNA HOTAIR promoted the expression of HuR and IGF2BP1, and this promotion was attenuated by calycosin ( Figure 6B,D). In addition, knockdown of HuR or IGF2BP1 inhibited cell proliferation and induced apoptosis, and this knockdown enhanced the calycosin-induced inhibitory effect on the growth of breast cancer cells. On the contrary, overexpression of HuR or IGF2BP1 promoted cell proliferation and reduced apoptosis, which partly blocked the inhibition by calycosin ( Figure 7A,B). These phenomena strongly illustrated that both HuR and IGF2BP1 are implicated in breast carcinogenesis, and their expression levels are positively associated with lncRNA HOTAIR. Moreover, calycosin suppressed the expression of HuR and IGF2BP1 by regulating lncRNA HOTAIR. Meanwhile, the binding of HuR and IGF2BP1 to lncRNA HOTAIR was validated via RNA binding protein immunoprecipitation ( Figure 5B), suggesting a possible feedback regulatory loop between HuR and IGF2BP1 to lncRNA HOTAIR in breast cancer cells. In conclusion, calycosin inhibited proliferation and induced apoptosis by downregulating the RNA binding proteins HuR and IGF2BP1 by suppressing lncRNA HOTAIR expression in both ER-positive breast cancer cell lines (MCF-7 and T47D) and ER-negative breast cancer cell lines (MDA-MB-231 and SK-BR-3).
Figure 5 .
Calycosin decreased the expressions of HuR and IGF2BP1 in breast cancer cells
(A) The potential targets of lncRNA HOTAIR were identified by bioinformatics analysis using StarBase ( https://starbase.sysu.edu.cn/starbase2/). (B) The targeted relationships between lncRNA HOTAIR, HuR and IGF2BP1 were verified by RNA binding protein immunoprecipitation in MDA-MB-231 and SK-BR-3 cells. IP/input% enrichment efficiency obtained by qRT-PCR (input stands for internal reference). The protein level (C) and transcription level (D) of HuR and IGF2BP1 were detected in MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells after treatment with calycosin (0, 20, 40 and 80 μM). β-Actin and GAPDH were used as internal controls. Data are presented as the mean±SD ( n=3). * P<0.05, * * P<0.01 and *** P<0.001 compared with the control group.
Figure 6 .
Calycosin suppressed the expression levels of HuR and IGF2BP1 by downregulating lncRNA HOTAIR in breast cancer cells
After knockdown or overexpression of lncRNA HOTAIR, western blot analysis (A,B) and qRT-PCR (C,D) were performed to detect the protein levels and transcription levels of HuR and IGF2BP1 in MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells with or without 80 μM calycosin treatment. β-Actin and GAPDH were used as internal controls. Data are presented as the mean±SD ( n=3). * P<0.05, ** P<0.01 and *** P<0.001 compared with the control group; and $ P<0.05, $$ P<0.01 and $$$ P<0.001 compared with the model group.
Figure 7 .
Calycosin inhibited cell progression by suppressing the expressions of the lncRNAs HOTAIR, HuR and IGF2BP1
After the knockdown or overexpression of HuR or IGF2BP1, the viability and apoptosis of transfected MCF-7, T47D, MDA-MB-231 and SK-BR-3 cells were detected by CCK-8 assay (A) and Hoechst 33258 assay (B), respectively. Data are presented as the mean±SD ( n=3). ** P<0.01 and *** P<0.001 compared with the control group; $$ P<0.01 and $$$ P<0.001 compared with the model group; and # P<0.05, ## P<0.01 and ### P<0.001 compared with the 80 μM calycosin group.
Discussion
Breast cancer is the most threatening cancer worldwide and treatments directed to specific targets have hardly achieved significant improvements in the clinic. Breast cancer is generally classified into two subtypes in the clinic: ER-positive and ER-negative breast cancer based on the status of ER. ER plays a central role in the development of hormone-dependent cancers, such as breast, ovarian and prostate cancers [ 24, 25] . Endocrine therapies reduce mortality and recurrence in ER-positive breast cancer at the beginning. However, recurrence of breast cancer or lack of response to endocrine therapy could lead to the inevitable progression to incurable metastatic cancer [26]. ER-negative breast cancer is mainly characterized by aggressive clinicopathological features and poorer survival than ER-positive breast cancer. Little progress has been made in the treatment of ER-negative breast cancer for decades due to its aggressive features, lack of targeted therapies and insensitivity to endocrine therapy and chemotherapy [27]. Novel potential treatments for breast cancer have been reported, but significant clinical achievements are rare [ 28, 29] . Therefore, the search for novel potent therapeutic options and targets for breast cancer is of great importance.
In the present study, we demonstrated that calycosin, a typical isoflavone phytoestrogen, significantly inhibited proliferation and induced apoptosis in both ER-positive breast cancer cell lines (MCF-7 and T47D) and ER-negative breast cancer cell lines (MDA-MB-231 and SK-BR-3). Our preliminary experimental data illustrated that lncRNA HOTAIR is likely to be the target of calycosin to inhibit breast cancer cell development, but the involved mechanism is still unclear. Hence, we designed a series of experiments to elaborate the specific role of lncRNA HOTAIR in calycosin-induced inhibition of both ER-positive and ER-negative breast cancer progression. Our results showed that the expression of lncRNA HOTAIR was decreased by calycosin in a dose-dependent manner. Then, gain-of-function and loss-of-function models were constructed by using the pcDNA-HOTAIR plasmid and siRNAs against lncRNA HOTAIR. LncRNA HOTAIR knockdown promoted the inhibitory effect of calycosin on breast cancer cell progression, while lncRNA HOTAIR overexpression attenuated the inhibitory effect of calycosin on cell progression. These results indicated that lncRNA HOTAIR acts as a tumor promoter and that calycosin inhibits cell development by downregulating the expression of lncRNA HOTAIR in both ER-positive breast cancer cell lines (MCF-7 and T47D) and ER-negative breast cancer cell lines (MDA-MB-231 and SK-BR-3).
LncRNA HOTAIR, located on chromosome 12q13.13 between HOXC11 and HOXC12 genes [30], is a prime example of an oncogenic trans-binding regulatory lncRNA. The lncRNA HOTAIR has been reported to contribute to cancer initiation, progression, metastasis, drug resistance and poor prognosis, and is aberrantly expressed in breast cancer [ 31‒ 34] . Functionally, lncRNA HOTAIR promotes or inhibits the function of other molecules by constructing a complex structure of RNA-DNA, RNA-RNA and RNA-protein interactions in the cell [31]. For breast cancer, it was reported that lncRNA HOTAIR promotes breast cancer progression by regulating miRNA-mediated control of specific gene expression [ 35, 36] . However, the downstream molecular mechanism of lncRNA HOTAIR in the calycosin-induced inhibition effect of breast cancer cells is still unknown. Then, we searched for the targets of lncRNA HOTAIR. HuR and IGF2BP1 were identified as potential downstream targets of lncRNA HOTAIR through bioinformatics analysis using the StarBase database. Western blot analysis verified that the siRNA-mediated knockdown of lncRNA HOTAIR downregulated the expression of HuR and IGF2BP1 and that overexpression of lncRNA HOTAIR upregulated the expression of HuR and IGF2BP1.
RBPs are a class of proteins that bind to the corresponding RNA specifically to regulate RNA metabolism and function. Protein-RNA interactions are key components of many cellular processes, e.g., splicing, stability, transport and translation, and are of crucial importance in almost every aspect of the gene expression cascade [ 37‒ 39] . RBPs are aberrantly expressed in cancers in comparison to those of the adjacent normal tissues, and this expression is associated with patient prognosis. HuR is a well-studied posttranscriptional regulator of cytoplasmic RNAs that belongs to the embryonic lethal abnormal vision (ELAV) family. HuR mediates the cellular response to proliferation, apoptosis, senescence, differentiation, inflammation and immune stimuli [40]. The expression of HuR was found to be usually increased in cancer tissues compared with that in the normal tissue counterparts in a global survey of healthy and cancerous tissue specimens [ 40, 41] . Studies focusing on cancers reported a correlation between the expression levels of HuR and advancing stages of tumor malignancy, indicating the significance of HuR in cancer development and progression [42]. In breast carcinoma, high level of cytoplasmic HuR is correlated with tumor grade and bleak prognosis [43]. IGF2BP1 is another widely expressed RBP that is highly expressed in various cancers, including breast carcinoma. IGF2BP1 has the potential to facilitate cancer proliferation, migration, invasion and chemotherapy resistance by impairing mRNA decay and elevating mRNA stability and translation [ 44, 45] . Zhu et al. [46] reported that lncRNAs recruit IGF2BP1 to form a structure that retains the stability of c-Myc mRNA and facilitates self-renewal and tumorigenesis in breast cancer stem cells. HuR/IGF2BP1 complexes promote breast cancer metastasis by increasing the stability of keratin 7 (KRT7) mRNA [47].
Consistent with previous studies demonstrating that HuR or IGF2BP1 could stimulate the growth of breast cancer cells [48], we found that their overexpressions both promoted cell proliferation and reduced apoptosis. Conversely, knockdown of HuR or IGF2BP1 inhibited cell proliferation and induced apoptosis. More importantly,such effects could be enhanced or attenuated by calycosin. As expected, the expression levels of HuR and IGF2BP1 in breast cancer cells were decreased by calycosin treatment. In addition, calycosin facilitated the downregulation of HuR and IGF2BP1 caused by decreasing lncRNA HOTAIR expression, while the upregulation of HuR and IGF2BP1 caused by increasing lncRNA HOTAIR expression was weakened by calycosin treatment. These results suggested that by downregulating lncRNA HOTAIR and its downstream targets HuR and IGF2BP1, calycosin successfully inhibits proliferation and triggers apoptosis in both ER-positive and ER-negative breast cancer cells ( Figure 8).
Figure 8 .
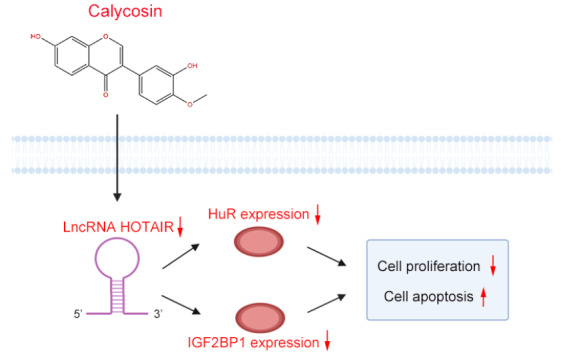
Schematic diagram of the lncRNA HOTAIR-dependent pathway whereby calycosin leads to the inhibition of breast cell proliferation and survival
Calycosin inhibited breast cancer proliferation and induced apoptosis by downregulating lncRNA HOTAIR and its binding protein: HuR and IGF2BP1.
Actually, a positive feedback loop between HuR and lncRNA HOTAIR has been demonstrated in some types of cancer ( e. g., neck squamous cell carcinoma and bladder cancer) but not in breast cancer. There is evidence that the expression levels of lncRNA HOTAIR and HuR are mutually regulated during tumor progression and metastasis [49]. LncRNA HOTAIR positively controls HuR expression by sponging miRNA molecules, and HuR in turn specifically binds to lncRNA HOTAIR, regulating its stability and thus promoting lncRNA HOTAIR expression. However, the interaction between IGF2BP1 and lncRNA HOTAIR is unknown. In the present study, we demonstrated the direct binding of HuR and IGF2BP1 to lncRNA HOTAIR in breast cancer cells, while further research is needed to validate the relationship between HuR, IGF2BP1 and lncRNA HOTAIR in breast tumors.
Here, we demonstrated that lncRNA HOTAIR and its binding proteins HuR and IGF2BP1 were involved in calycosin-induced inhibition of breast cancer progression in vitro. However, it is still unclear whether lncRNA HOTAIR-dependent regulation of HuR and IGF2BP1 also exists in vivo, although we previously showed that calycosin inhibited tumor growth in mice bearing MCF-7 or SK-BR-3 xenografts [50]. Therefore, in our future study, we will evaluate the in vivolncRNA HOTAIR-mediated control of growth in human breast carcinoma cells, along with its role in the treatment of breast cancer by calycosin.
In summary, our data provide a further understanding of the role of lncRNA HOTAIR in breast cancer development and progression. Therefore, lncRNA HOTAIR may serve as a potential therapeutic target and prognostic predictor in both ER-positive breast cancer and ER-negative breast cancer, especially when treated with isoflavone phytoestrogens.
Supporting information
Supplementary Data
Supplementary data is available at Acta Biochimica et Biophysica Sinicaonline.
COMPETING INTERESTS
The authors declare that they have no conflict of interest.
Funding Statement
This work was supported by the grants from the Natural Science Foundation of Guangxi (No. 2019GXNSFFA245001) and the Innovation Project of Guangxi Graduate Education (No. YCSW2022383).
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. . 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Barzaman K, Karami J, Zarei Z, Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, Safari E, et al. Breast cancer: Biology, biomarkers, and treatments. Int Immunopharmacol. . 2020;84:106535. doi: 10.1016/j.intimp.2020.106535. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo G, Baroni L. Soy, soy foods and their role in vegetarian diets. Nutrients. . 2018;10:43. doi: 10.3390/nu10010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houghton L, Ganmaa D, Rosenberg P, Davaalkham D, Stanczyk F, Hoover R, Troisi R. Associations of breast cancer risk factors with premenopausal sex hormones in women with very low breast cancer risk. Int J Environ Res Public Health. . 2016;13:1066. doi: 10.3390/ijerph13111066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucuk O. Soy foods, isoflavones, and breast cancer. Cancer. . 2017;123:1901–1903. doi: 10.1002/cncr.30614. [DOI] [PubMed] [Google Scholar]
- 6.Sun YS, Zhao Z, Yang ZN, Xu F, Lu HJ, Zhu ZY, Shi W, et al. Risk factors and preventions of breast cancer. Int J Biol Sci. . 2017;13:1387–1397. doi: 10.7150/ijbs.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, Hur HG, et al. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. . 2006;101:246–253. doi: 10.1016/j.jsbmb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Deng M, Chen H, Long J, Song J, Xie L, Li X. Calycosin: a review of its pharmacological effects and application prospects. Expert Rev Anti-infective Ther. . 2021;19:911–925. doi: 10.1080/14787210.2021.1863145. [DOI] [PubMed] [Google Scholar]
- 9.Sun H, Yin M, Qian W, Yin H. Calycosin, a phytoestrogen isoflavone, induces apoptosis of estrogen receptor-positive MG-63 osteosarcoma cells via the phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway. Med Sci Monit. . 2018;24:6178–6186. doi: 10.12659/MSM.910201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Ren Q, Zhang X, Lu H, Chen J. Neuroprotective mechanisms of calycosin against focal cerebral ischemia and reperfusion injury in rats. Cell Physiol Biochem. . 2018;45:537–546. doi: 10.1159/000487031. [DOI] [PubMed] [Google Scholar]
- 11.Kong X, Wang F, Niu Y, Wu X, Pan Y. A comparative study on the effect of promoting the osteogenic function of osteoblasts using isoflavones from Radix Astragalus. PhytoTher Res. . 2018;32:115–124. doi: 10.1002/ptr.5955. [DOI] [PubMed] [Google Scholar]
- 12.Quan GH, Wang H, Cao J, Zhang Y, Wu D, Peng Q, Liu N, et al. Calycosin suppresses RANKL-mediated osteoclastogenesis through inhibition of MAPKs and NF-κB. Int J Mol Sci. . 2015;16:29496–29507. doi: 10.3390/ijms161226179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y, Dong X, Li Z, Wang W, Tian J, Chen J. Downregulated RASD1 and upregulated miR-375 are involved in protective effects of calycosin on cerebral ischemia/reperfusion rats. J Neurol Sci. . 2014;339:144–148. doi: 10.1016/j.jns.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Deng T, Liu J, Zhang M, Wang Y, Zhu G, Wang J. Inhibition effect of phytoestrogen calycosin on TGF-β 1-induced hepatic stellate cell activation, proliferation, and migration via estrogen receptor β . Can J Physiol Pharmacol. . 2018;96:1268–1275. doi: 10.1139/cjpp-2018-0474. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Zhang J, Liu W, Liu N, Fu X, Kwan H, Liu S, et al. Calycosin inhibits oxidative stress-induced cardiomyocyte apoptosis via activating estrogen receptor-α/β. BioOrg Medicinal Chem Lett. . 2016;26:181–185. doi: 10.1016/j.bmcl.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Zhao X, Li X, Wu Y. Calycosin induces apoptosis by the regulation of ERβ/miR-17 signaling pathway in human colorectal cancer cells. Food Funct. . 2015;6:3091–3097. doi: 10.1039/C5FO00374A. [DOI] [PubMed] [Google Scholar]
- 17.Gong G, Zheng Y, Yang Y, Sui Y, Wen Z. Pharmaceutical values of calycosin: one type of flavonoid isolated from astragalus. EVID-BASED COMPL ALT. 2021, 2021: 1‒9 . [DOI] [PMC free article] [PubMed]
- 18.Chen J, Lin C, Yong W, Ye Y, Huang Z. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol Biochem. . 2015;35:722–728. doi: 10.1159/000369732. [DOI] [PubMed] [Google Scholar]
- 19.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, Lajoie BR, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. . 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. . 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 21.Zhu L, Wang F, Fan W, Jin Z, Teng C, Zhang J. lncRNA NEAT1 promotes the Taxol resistance of breast cancer via sponging the miR-23a-3p-FOXA1 axis. Acta Biochim Biophys Sin. . 2021;53:1198–1206. doi: 10.1093/abbs/gmab098. [DOI] [PubMed] [Google Scholar]
- 22.Huang L, Liao LM, Liu AW, Wu JB, Cheng XL, Lin JX, Zheng M. Overexpression of long noncoding RNA HOTAIR predicts a poor prognosis in patients with cervical cancer. Arch Gynecol Obstet. . 2014;290:717–723. doi: 10.1007/s00404-014-3236-2. [DOI] [PubMed] [Google Scholar]
- 23.Endo H, Shiroki T, Nakagawa T, Yokoyama M, Tamai K, Yamanami H, Fujiya T, et al. Enhanced expression of long non-coding RNA HOTAIR is associated with the development of gastric cancer. PLoS ONE. . 2013;8:e77070. doi: 10.1371/journal.pone.0077070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Arch Toxicol. . 2012;86:1491–1504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayala B, Zoure AA, Baron S, de Joussineau C, Simpore J, Lobaccaro JMA. Pharmacological modulation of steroid activity in hormone-dependent breast and prostate cancers: effect of some plant extract derivatives. Int J Mol Sci. . 2020;21:3690. doi: 10.3390/ijms21103690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. . 2020;37:496–513. doi: 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin L, Duan JJ, Bian XW, Yu S. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. . 2020;22:61. doi: 10.1186/s13058-020-01296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou Y, Cai S, Yu S, Lin H. Metformin induces ferroptosis by targeting miR-324-3p/GPX4 axis in breast cancer. Acta Biochim Biophys Sin. . 2021;53:333–341. doi: 10.1093/abbs/gmaa180. [DOI] [PubMed] [Google Scholar]
- 29.Yu Y, Wu X, Liu S, Zhao H, Li B, Zhao H, Feng X. Piezo1 regulates migration and invasion of breast cancer cells via modulating cell mechanobiological properties. Acta Biochim Biophys Sin. . 2021;53:10–18. doi: 10.1093/abbs/gmaa112. [DOI] [PubMed] [Google Scholar]
- 30.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. . 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mozdarani H, Ezzatizadeh V, Rahbar Parvaneh R. The emerging role of the long non-coding RNA HOTAIR in breast cancer development and treatment. J Transl Med. . 2020;18:152. doi: 10.1186/s12967-020-02320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding W, Ren J, Ren H, Wang D. Long noncoding RNA HOTAIR modulates miR-206-mediated bcl-w signaling to facilitate cell proliferation in breast cancer. Sci Rep. . 2017;7:17261. doi: 10.1038/s41598-017-17492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai MC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. . 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W, Geng D, Li S, Chen Z, Sun M. LncRNA HOTAIR influences cell growth, migration, invasion, and apoptosis via the miR-20a-5p/ HMGA2 axis in breast cancer . Cancer Med. . 2018;7:842–855. doi: 10.1002/cam4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Lozano-Romero A, Astudillo-de la Vega H, Terrones-Gurrola MCR, Marchat LA, Hernández-Sotelo D, Salinas-Vera YM, Ramos-Payan R, et al. HOX transcript antisense RNA HOTAIR abrogates vasculogenic mimicry by targeting the angiomiR-204/FAK axis in triple negative breast cancer cells. ncRNA. . 2020;6:19. doi: 10.3390/ncrna6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Gong G, Xu J, Zhang Y, Wu S, Wang S. Long noncoding RNA HOTAIR promotes breast cancer development by targeting ZEB1 via sponging miR-601. Cancer Cell Int. . 2020;20:320. doi: 10.1186/s12935-020-01410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh R. RNA-protein interactions that regulate pre-mRNA splicing. Gene expression. 2002, 10: 79‒92 . [PMC free article] [PubMed]
- 38.Kishore S, Luber S, Zavolan M. Deciphering the role of RNA-binding proteins in the post-transcriptional control of gene expression. Briefings Funct Genomics. . 2011;9:391–404. doi: 10.1093/bfgp/elq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrè F, Colantoni A, Helmer-Citterich M. Revealing protein–lncRNA interaction. Brief Bioinform. . 2016;17:106–116. doi: 10.1093/bib/bbv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srikantan S, Gorospe M. HuR function in disease. Front Biosci. . 2012;17:189–205. doi: 10.2741/3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Silanes IL, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, Gorospe M. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene. . 2003;22:7146–7154. doi: 10.1038/sj.onc.1206862. [DOI] [PubMed] [Google Scholar]
- 42.Lopez DSI, Lal A, Gorospe M. HuR: post-transcriptional paths to malignancy. RNA Biol. . 2005;2:11–13. doi: 10.4161/rna.2.1.1552. [DOI] [PubMed] [Google Scholar]
- 43.Heinonen M, Bono P, Narko K, Chang SH, Lundin J, Joensuu H, Furneaux H, et al. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res. . 2005;65:2157–2161. doi: 10.1158/0008-5472.CAN-04-3765. [DOI] [PubMed] [Google Scholar]
- 44.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H, Zhao BS, et al. Recognition of RNA N6-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. . 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Müller S, Glaß M, Singh AK, Haase J, Bley N, Fuchs T, Lederer M, et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. . 2019;47:375–390. doi: 10.1093/nar/gky1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu P, He F, Hou Y, Tu G, Li Q, Jin T, Zeng H, et al. A novel hypoxic long noncoding RNA KB-1980E6.3 maintains breast cancer stem cell stemness via interacting with IGF2BP1 to facilitate c-Myc mRNA stability. Oncogene. . 2021;40:1609–1627. doi: 10.1038/s41388-020-01638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen F, Chen Z, Guan T, Zhou Y, Ge L, Zhang H, Wu Y, et al. N6-methyladenosine regulates mRNA stability and translation efficiency of KRT7 to promote breast cancer lung metastasis . Cancer Res. . 2021;81:2847–2860. doi: 10.1158/0008-5472.CAN-20-3779. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Gardashova G, Lan L, Han S, Zhong C, Marquez RT, Wei L, et al. Targeting the interaction between RNA-binding protein HuR and FOXQ1 suppresses breast cancer invasion and metastasis. Commun Biol. . 2020;3:193. doi: 10.1038/s42003-020-0933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu CZ, Jiang C, Wu Q, Liu L, Yan X, Shi R. A feed-forward regulatory Loop between HuR and the long noncoding RNA HOTAIR promotes head and neck squamous cell carcinoma progression and metastasis. Cell Physiol Biochem. . 2016;40:1039–1051. doi: 10.1159/000453160. [DOI] [PubMed] [Google Scholar]
- 50.Tian J, Wang Y, Zhang X, Ren Q, Li R, Huang Y, Lu H, et al. Calycosin inhibits the in vitro and in vivo growth of breast cancer cells through WDR7-7-GPR30 Signaling . J Exp Clin Cancer Res. . 2017;36:153. doi: 10.1186/s13046-017-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.