Abstract
Purpose/Objective:
The primary objective of this study was to establish the feasibility and acceptability of an intensive data collection protocol that involves the delivery of a personalized just-in-time adaptive intervention (JITAI) in three distinct groups of care partners (care partners of persons with spinal cord injury [SCI], Huntington disease [HD], or hematopoietic cell transplantation [HCT]).
Research Methods:
70 care partners were enrolled in this study (n= 19 SCI; n = 21 HD, n= 30 HCT). This three-month (90 day) randomized control trial involved wearing a Fitbit® to track sleep and steps, providing daily reports of health-related quality of life (HRQOL), and completing end of month HRQOL surveys. Care partners in the JITAI group also received personalized pushes (i.e., text-based phone notifications that include brief tips or suggestions for improving self-care). At the end of three-months, care partners in both groups completed a feasibility and acceptability questionnaire.
Results:
98.6% of care partners completed the study, average compliance was 88% for daily HRQOL surveys, 96% for daily steps and 85% for daily sleep (from wearing the Fitbit®), and all monthly surveys were completed with the exception of one missed 3-month assessment. The acceptability of the protocol was high; ratings exceeded 80% agreement for the different elements of the study. Improvements were seen for the majority of the HRQOL measures. There was no evidence of measurement reactivity.
Conclusions/Implications:
Findings provide strong support for the acceptability and feasibility of an intensive data collection protocol that involved the administration of a JITAI. Although this trial was not powered to establish efficacy, findings indicated improvements across a variety of different HRQOL measures (~1/3 of which were statistically significant).
Keywords: Caregivers, Quality of Life, Spinal Cord Injuries, Huntington Disease, Hematopoietic Stem Cell Transplantation, Feasibility Studies, Self-management, Mobile Applications, Outcome Assessment
Introduction
While significant scientific discoveries and advances in human health are being made, the complexities of disease management and care needs are also growing substantially. As a society, these needs have always been placed on family caregivers who face an enormous and growing burden, providing care to a loved one while maintaining their own health and well-being (e.g., health-related quality of life [HRQOL]). The burden of caregiving adversely affects the well-being of the caregiver and the quality of care the primary patient receives (Applebaum et al., 2016; Aubeeluck, Buchanan, & Stupple, 2012; Blanes, Carmagnani, & Ferreira, 2007; Cox, 2012; Foxall & Gaston-Johansson, 1996; Hamidou et al., 2017; McCabe, Firth, & O’Connor, 2009; Ramkumar & Elliott, 2010; Ready, Mathews, Leserman, & Paulsen, 2008).
Despite the growing awareness of the importance of caregiving, very little action has been taken and this remains a major overlooked challenge facing the aging U.S. population (Aubeeluck, 2005; Aubeeluck et al., 2012; Hurria, Naylor, & Cohen, 2013). Thus, family caregiving is an urgent public health issue. Further, with a high risk for developing depression, insomnia, and stress-related disorders (Banaszkiewicz et al., 2012; McCabe et al., 2009; O’Connor & McCabe, 2011; Pickett, Altmaier, & Paulsen, 2007; Roscoe, Corsentino, Watkins, McCall, & Sanchez-Ramos, 2009), care partners (i.e., informal family caregivers) are an ideal population to target for early detection and intervention strategies to treat compromised well-being. While psychoeducation, skills training, or therapeutic counseling interventions are available for care partners, these interventions, even when offered remotely, are still time intensive and commonly require a face-to-face commitment (with trained personnel) which can be prohibitive for an individual that is already overwhelmed by existing caregiving responsibilities and unable to make time for self-care. Publicly available self-management apps provide a potential alternative to these more time intensive interventions, yet few have been designed for caregivers, and many are time intensive which can be prohibitive for persons that are already overwhelmed by existing responsibilities (Bidenko & Bohnet-Joschko, 2022; Park, Tracy, & Gray, 2022). Furthermore, very few of the publicly available self-management apps are evidence-based, and as such, their efficacy for improving caregiver outcomes is unknown (Agarwal et al., 2022; Park et al., 2022).
Just-in-time adaptive interventions (JITAIs) are an emerging mobile health behavior-change approach that operationalizes the selection and delivery of personalized mobile phone intervention strategies based on real-time data collection (Nahum-Shani, Hekler, & Spruijt-Metz, 2015; Nahum-Shani et al., 2016; Spruijt-Metz & Nilsen, 2014). Our newly developed CareQOL app integrates passive sensor data derived from a Fitbit Inspire 2® (e.g., accelerometer-based estimates of physical activity and sleep) with real-time self-report ratings (assessed once daily) of HRQOL to inform a personalized self-management intervention designed to improve physical and mental health outcomes. The intervention is composed of personalized “pushes” that are delivered in the form of “life insights” (empathetic feedback about behavioral patterns) or “tips” messages (a cognitive behavioral strategy with a motivational statement). These “pushes” are broadly based on Behavioral Activation (BA) theory which posits that negative life events (negative interactions between the care partner and care-recipient, increased stress due to the caregiver role, etc.) trigger negative emotional responses (depression, anxiety, etc.), which lead to negative behavioral patterns (poor sleep, decreased exercise, social withdrawal), which starts the cycle all over again (Dimidjian, Barrera, Martell, Munoz, & Lewinsohn, 2011). JITAIs have been associated with significant improvements in health outcomes including physical activity (King et al., 2013; Thomas & Bond, 2015), alcohol use (Gustafson et al., 2014; Witkiewitz et al., 2014), mental illness (Ben-Zeev et al., 2014), smoking cessation (Free et al., 2011; Riley, Obermayer, & Jean-Mary, 2008), and depression. They offer an accessible and cost-effective intervention approach that delivers personalized and adaptive interventions in a real-time, real-world context. However, we are unaware of any work that has utilized this type of JITAI in care partners of persons with significant health conditions. As such, we were interested in better understanding the feasibility and clinical utility of a JITAI to improve HRQOL for care partners of persons with significant health conditions. Also, and in line with recent work on “engagement” in mhealth interventions (Nahum-Shani, Shaw, Carpenter, Murphy, & Yoon, 2022), we were also interested in understanding if different care partner groups differed in their receptivity to this type of JITAI self-management approach, and if additional group-specific personalization’s might be warranted for different caregiver groups. That is, we wanted to know if there were important group differences among care partners of persons with a chronic condition caused by a traumatic event, care partners for persons with a fatal neurodegenerative disease, or care partners for persons with an intensive relapsing and remitting condition.
Objectives.
This study was designed to examine the acceptability and feasibility of an intensive data collection protocol that involves the delivery of the aforementioned JITAI and to identify trends for improvements in HRQOL for care partners that were randomized to the intervention group. Specifically, we examined the acceptability and feasibility of this intensive protocol design, and the impact the JITAI had on HRQOL in three distinct groups of care partners: 1) care partners for persons with a chronic condition that was caused by a traumatic event (i.e., spinal cord injury [SCI]); 2) care partners for persons with a progressive, fatal neurodegenerative disease (i.e., Huntington disease [HD]); and 3) care partners for persons with an episodic cancer condition that requires intense, prolonged inpatient and outpatient treatment (persons with hematopoietic cell transplantation [HCT]).
Methods
Participants and Setting.
A total of N=70 care partners (n=19 SCI, n=21 HD, and n=30 HCT care partners) participated in this study. Data collection occurred at the University of Michigan using hospital and community-based recruitment, as well as lab-specific research registries (of both caregivers and patients), medical record data capture systems (Hanauer, Mei, Law, Khanna, & Zheng, 2015), and a study posting on UMHealthResearch.org. Care partners were recruited directly, or through the person for whom they provide care (i.e., the individual with SCI, HD or HCT).
Eligibility.
Care partners had to be at least 18 years old, be able to read and understand English, and be caring for an individual 18 years or older with medically documented HD, SCI, or HCT. A care partner was defined as an individual that provides physical assistance, financial assistance and/or emotional support to the patient, and who is not a professional, paid caregiver; specifically, participants had to indicate a response ⩾1 on the following question: “On a scale of 0–10, where 0 is ‘no assistance’ and 10 is ‘assistance with all activities’, how much assistance does the person you care for require from you to complete activities of daily living due to problems resulting from his/her HD/SCI/HCT? Activities could consist of personal hygiene, dressing and undressing, housework, taking medications, managing money, running errands, shopping for groceries or clothing, transportation, meal preparation and cleanup, remembering things, etc.” In addition, care partners of individuals with SCI had to be caring for someone that was ⩾1 year post-injury and care partners of persons with HCT had to be caring for an individual who was receiving, had received or was scheduled to receive HCT. Care partners also had to be willing to use their own technology (i.e., smartphone/tablet and internet access) and be willing to download the CareQOL app and the Fitbit® app on their personal device.
Intervention.
The JITAI used sensor data derived from the Fitbit® (e.g., accelerometer-based estimates of physical activity and sleep) and the real-time self-report ratings (assessed once daily) of HRQOL (caregiver strain, depression, anxiety) to deliver personalized “pushes” to participants via the CareQOL app. Care partners that were randomized to the JITAI group had a 50/50 chance of receiving a personalized push notification each day during the 3-month intervention period (push notification delivery was based on a similar microrandomized trial that reported optimal delivery rates of 3.5/7 days across a 6-month intervention period (NeCamp et al., 2020)).
The JITAI push notifications were designed to promote healthy behaviors (physical activity and good sleep hygiene) and improve mood (anxiety, depression, care partner strain). Push notifications were adapted from another study designed to improve mental health outcomes among individuals in stressful work environments (details are reported in (NeCamp et al., 2020)). This adaptation process was iterative and included feedback from key stakeholders (i.e., care partners, their advocates, and healthcare providers); adaptation was focused on modifications and additional content that focused on key care partner HRQOL concepts (Carlozzi et al., 2016; Carlozzi et al., 2015). Notification content was randomly drawn from a pool of over 400 messages. Messages were composed of one or more of the following different types: 1) Data feedback (e.g., Your daily step average this week is 10,251. You have really committed to being active. Keep it up!); 2) Facts (e.g., Blue light from your devices can suppress melatonin release, which is what helps us fall asleep and achieve good quality deep sleep. When you struggle to sleep, avoid those screens before bed to feel more alert the next day.); 3) Tips (e.g., When you feel stressed, worried, or angry, notice where you feel it in your body. Maybe it’s in your neck, you’re clenching your fists, or you’re frowning. Direct your focus to that spot and try to relax. Notice any calming effect this has on your mood); and 4) Support (Everyone’s mood dips sometimes. Engaging with others is important when this happens. Consider whether a check-in with friends would help, or if a more formal support group might be a better fit. Reach out when needed.). Some messages were personalized using the participants’ data directly in the messages (e.g., Your average worry rating over the last week was [average T score from the previous week]. If you feel worried, keep in mind things can feel worse when our thoughts move to the past or future. Try being in the moment by focusing on your breathing), and the majority of the messages were personalized based on individual HRQOL data (e.g., someone with below average sadness will get a different message than someone with average sadness than someone with above average sadness; high-medium-low). Additional examples of the personalized pushes are published elsewhere (Carlozzi et al., 2021).
Study Design.
Details for the study protocol are reported elsewhere (Carlozzi et al., 2021). Briefly, this behavioral trial used a two-arm randomized controlled design. Study participation involved a 2-hour baseline virtual study visit followed by a 10 day run-in period then a 3-month (90 day) home monitoring period. The baseline visit involved obtaining informed consent, the completion of several self-report measures and instructions for the run-in period. During the 10 day run-in period, the care partners downloaded the study apps (CareQOL and Fitbit®) on their mobile device, set up the Fitbit®, and began using the Fitbit® and CareQOL app so baseline data could be gathered to inform the JITAI messages once the home monitoring period began. The home monitoring period involved continuous monitoring of steps and sleep using the Fitbit® and the collection of real-time ratings of HRQOL (3 questions each day) using the CareQOL app. Participants randomized to the JITAI (intervention) arm also received personalized push notifications delivered via the CareQOL app on ~50% of days in the home monitoring period. In addition, all care partners completed a 5–10 minute self-report survey assessment at the end of months 1, 2 and 3. Care partners for each patient group were block randomized at a 1:1 ratio to the JITAI and Control groups.
Measures.
Details for the complete schedule of assessments and study measures is reported elsewhere (Carlozzi et al., 2021). The study-specific measures that were analyzed for this report are detailed below.
Feasibility & Acceptability Questionnaire:
This study-specific questionnaire (which was based on existing measures of feasibility and acceptability (Haerens, Deforche, Vandelanotte, Maes, & De Bourdeaudhuij, 2007; Kratz, Kalpakjian, & Hanks, 2017; Vandelanotte & De Bourdeaudhuij, 2003)) was completed by participants at the 3-month assessment. Items were scaled from 1 to 5 to indicate level of agreement, where “1” indicates “strong disagreement” and “5” indicates “strong agreement.”
Baseline and End-of-Month HRQOL Assessments.
Participants completed several patient-reported outcomes at baseline at the end of months 1, 2, and 3 during the home monitoring period. HRQOL measures included the: 1) TBI-CareQOL Caregiver Strain Short Form (SF) (N.E. Carlozzi et al., 2019; N. E. Carlozzi, M. A. Kallen, P. A. Ianni, et al., 2019) which assesses perceived feelings of feeling overwhelmed, stressed and “beat-down” related to the care partner role; 2) TBI-CareQOL Caregiver-Specific Anxiety SF (N. E. Carlozzi, M. A. Kallen, R. Hanks, et al., 2019; N. E. Carlozzi, M. A. Kallen, A. M. Sander, et al., 2019) which assesses perceived feelings of worry and anxiety specific to the safety, health, and future well-being of the person with TBI; 3) PROMIS Sleep-Related Impairment SF (Cella et al., 2010; Cella et al., 2007) which evaluates the effect of poor sleep on daytime functioning; 4) PROMIS Fatigue SF (Cella et al., 2010; Cella et al., 2007) which evaluates self-reported symptoms of fatigue, ranging from mild subjective feelings of tiredness to overwhelming exhaustion that may decrease one’s ability to perform activities of daily living; 5) PROMIS Anxiety SF (Cella et al., 2010; Cella et al., 2007) which assesses self-reported feelings of fear, anxiety and hyperarousal; 6) PROMIS Depression SF (Cella et al., 2010; Cella et al., 2007) which assesses self-reported feelings of sadness and worthlessness; 7) PROMIS Anger SF (Cella et al., 2010; Cella et al., 2007) which assesses self-reported feelings of irritability and frustration; 8) NIH Toolbox Self-Efficacy SF (Salsman et al., 2013) which assesses self-reported confidence in the ability to successfully perform specific tasks or behaviors related to one’s overall functioning; 9) Neuro-QoL Positive Affect and Well-Being SF (Cella et al., 2012; Cella et al., 2011) which assesses parts of an individual’s life that are related to overall life meaning and purpose, well-being and satisfaction; 10) NIH Toolbox Perceived Stress SF (Salsman et al., 2013) which is a self-report measure designed to assess an individual’s feelings about the nature of events and individual coping resources; and 10) PROMIS Ability to Participate in Social Roles and Activities SF (Cella et al., 2010; Cella et al., 2007) which assesses involvement in one’s ability to participate in usual social roles and activities. These measures are scored on a T metric (M = 50; SD = 10). For all measures, higher scores indicate more of the named construct; thus, for positively worded concepts (i.e., Self-Efficacy, Positive Affect and well-Being, and Ability to Participate in Social Activities), higher scores indicate better HRQOL, whereas, for negatively worded constructs (i.e., Anger, Depression, etc.), higher scores indicate worse HRQOL.
Daily Real-time Assessments of Caregiver Burden, Anxiety and Depression.
The daily real-time assessments were composed of: 1 question on caregiver strain (taken from the TBI-CareQOL Caregiver Strain item bank) (N.E. Carlozzi et al., 2019; N. E. Carlozzi, M. A. Kallen, P. A. Ianni, et al., 2019), 1 question on anxiety (taken from the PROMIS Anxiety item bank) (Cella et al., 2010; Cella et al., 2007) and 1 question on depression (taken from the PROMIS Depression item bank) (Cella et al., 2010; Cella et al., 2007). The measurement development approach for these three item banks allows for a reliable score estimate even if only a single item is administered (Cappelleri, Jason Lundy, & Hays, 2014). Questions are on a five-point scale, with higher scores indicating more of the named construct. These questions were administered as a computer adaptive test, with a single item administered each day (based on the previous days response), with the CAT event resetting each week. Each of these three measures is scored on a T metric (M = 50; SD = 10), with higher scores indicating more strain, anxiety or depression, respectively.
Accelerometer Measures.
A Fitbit® with heart rate recording capabilities was used to continuously measure physical activity and sleep during the home monitoring period. The Fitbit® automatically generates accelerometer-based summary data (per established proprietary algorithms) that is based on ‘activity counts’ collected over the course of the day. This includes summary data for steps (Dominick, Winfree, Pohlig, & Papas, 2016; Reid et al., 2017; Sushames, Edwards, Thompson, McDermott, & Gebel, 2016)) and sleep (total sleep time) (Farina & Lowry, 2018; Kamper et al., 2016; Mantua, Gravel, & Spencer, 2016; Montgomery-Downs, Insana, & Bond, 2012; Sargent et al., 2018; Smith et al., 2018).
Transparency and Openness.
This study was conducted in accordance with the United States (US) Code of Federal Regulations (CFR) applicable to clinical studies (45 CFR Part 46, 21 CFR Part 50, 21 CFR Part 56, 21 CFR Part 312, and/or 21 CFR Part 812) and research best practices. Study procedures were approved by IRBMED (Application Approval HUM00184455) and is registered with ClinicalTrials.gov (NCT04556591). Caregivers provided informed consent prior to the commencement of study activities. Syntax for the analyses reported within this manuscript are provided in Appendix 3. Data and supporting materials are available by emailing the corresponding author.
Statistical Analyses.
Normally distributed continuous variables are reported using means and SD, nonnormally distributed variables are reported by medians and interquartile ranges, and categorical variables are reported by number and percentages.
Feasibility and Acceptability Analyses.
Compliance.
Percent missing data of total expected data for each person and on average was calculated to characterize the feasibility of this data collection method. We hypothesized that this intensive data collection protocol would be both feasible and acceptable for care partners (regardless of group assignment). Anticipated completion rates were based on other mobile health studies that either employed a similar JITAI intervention (NeCamp et al., 2020) or employed a similar intensive study design in a clinical population (Carlozzi, Schilling, Freedman, Kalpakjian, & Kratz, 2018; Kratz et al., 2017; Moore et al., 2017; Rogers, 2021; Wooldridge, Soriano, Harris, & Afari, 2022). Specifically, we expected: 1) ≥80% of participants to complete the study; 2) for each participant in the study, completion rates for the daily assessment questions would be ≥60%; and 3) ≥80% completion rates for the three monthly surveys. We also examined the relationship between baseline HRQOL and compliance rates using Spearman correlations.
Feasibility and Acceptability Questionnaire.
Descriptive statistics (frequencies, means, medians) of responses to individual items on the feasibility and acceptability questionnaire were calculated to characterize the relative acceptability/non-acceptability of different aspects of the study protocol and materials. We expected ≥80% of participants to indicate that they either ‘Agree’ or ‘Strongly Agree’ for the different study elements.
Preliminary Efficacy Analyses.
Care partners in each study group (JITAI and control) were compared descriptively according to Consolidated Standards of Reporting Trials Guidelines (Schulz, Altman, & Moher, 2010). T-tests/ANOVA were used to examine group differences for continuous variables (e.g., age, HRQOL outcomes). Cohen d effect sizes were computed (mean change divided by SD of the mean change). Effect sizes for the HRQOL variables were used to calculate a minimum sample size for a 2-tailed t-test study with a significance level of 0.05 and desired statistical power of 0.8. Chi-squared/Fisher exact tests were used to examine group differences for categorical variables (e.g., care partner type). Pre- and post-intervention HRQOL scores were plotted by group (JITAI, Control) for each participant and for the group mean.
Exploratory Analyses
Although this trial was not powered to examine efficacy, we conducted exploratory analyses to identify trends for an improvement in HRQOL scores (i.e., group differences between the JITAI and control group at the end of the 3-month home monitoring period). Specifically, we conducted a series of ten analyses of covariance (ANCOVAs) to determine if the JITAI had better HRQOL (separate ANCOVAs were conducted for each of the HRQOL measures: Caregiver Strain, Caregiver Anxiety, Anger, Anxiety, Depression, Fatigue, Perceived Stress, Positive Affect and Well-Being, Ability to Participate in Social Roles and Activities, Sleep-Related Impairment) relative to the control group, after controlling for baseline HRQOL of the same construct. We expected the JITAI group to report better HRQOL than the control group across the 10 different HRQOL measures. Given that these analyses were exploratory we report these results both with and without a correction for multiple comparisons (i.e., false discovery correction). We also conducted the analyses with and without controlling for work status (given a trend for differences between the JITAI and control groups on this variable); the pattern of significant findings did not change with the addition of work status as a covariate, and thus, we only present findings from the initial models (that did not control for work status). Analyses used an intention-to-treat approach where the participant will contribute data to the arm they were randomized to regardless of the amount of data contributed (i.e., the duration of participation).
We also used repeated-measures models with random effects for subject-specific intercepts to determine whether real-time ratings of Caregiver Strain, Depression or Anxiety changed over time (week) to examine measurement reactivity. Linear or curvilinear changes would suggest measurement reactivity over the course of the three months (French & Sutton, 2010). The “week” of data collection (1 – 12) served as the independent variable. Three different models were conducted for each of the three HRQOL variables (Caregiver Strain, Depression, and Anxiety). Week was treated as a linear variable and quadratic effects were examined using a Week x Week interaction term. A significant effect of week (linear) or week X week (quadratic) would indicate reactivity to the intensive protocol methodology.
Results
Seventy participants were enrolled in this study (Figure 1), with 36 randomized to the JITAI group and 34 randomized to the control group (Table 1). In general, there were very few demographic differences between groups (JITAI versus control and SCI versus HD versus HCT); the only significant differences were that: 1) care partners in the JITAI group were caring for individuals that were approximately 3 years older than care partners in the control group, Kruskal-Wallis Chi-square(df=1)= 4.37, p = .037; and HCT caregivers reported significantly fewer years in the caregiver role than the other two cohorts, Kruskal-Wallis Chi-square(df=2)= 35.15, p < .0001. There were two study-related adverse events reported in this study: 1) one participant in the JITAI group reported skin irritation from the Fitbit (this resolved when participant was sent a different type of wristband); and 2) one participant in the control group reported bruising and swelling from the Fitbit (this resolved when participant was sent a different type of wristband).
Figure 1.
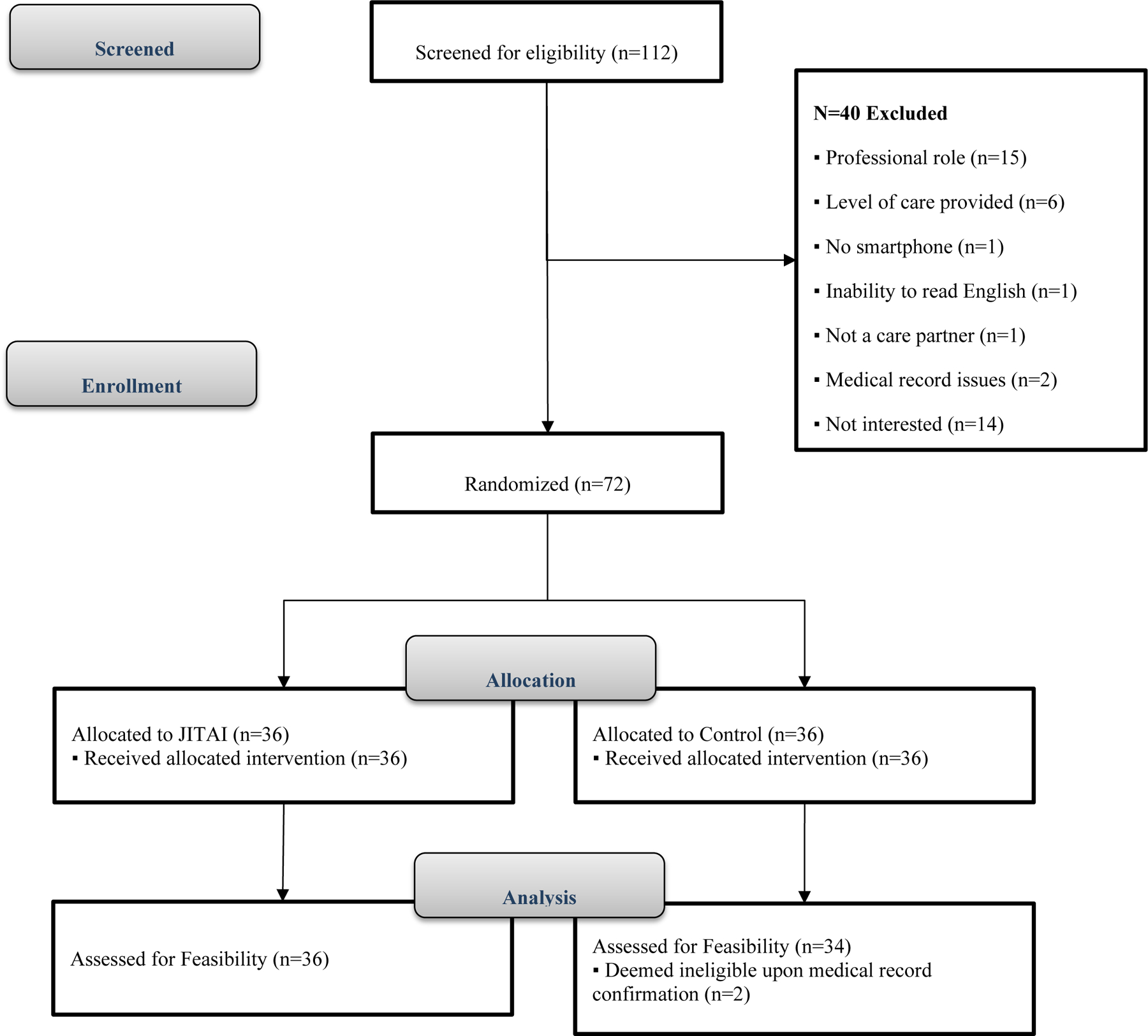
CONSORT Diagram
Table 1.
Sample Descriptive Statistics (N=70)
| Control (n=34) | JITAI (n=36) | HD (n=21) | SCI (n=19) | HCT (n=30) | |
|---|---|---|---|---|---|
| Gender (%) | |||||
| Female | 62 | 78 | 62 | 74 | 73 |
| Male | 35 | 22 | 38 | 26 | 23 |
| Not reported | 3 | 0 | 0 | 0 | 3 |
| Race (%) | |||||
| Caucasian | 97 | 81 | 100 | 74 | 90 |
| African American | 3 | 3 | 0 | 11 | 0 |
| Asian | 0 | 8 | 0 | 11 | 3 |
| More than 1 | 0 | 8 | 0 | 5 | 7 |
| Ethnicity (%) | |||||
| Non-Hispanic | 100 | 92 | 90 | 95 | 100 |
| Hispanic | 0 | 6 | 10 | 0 | 0 |
| Missing | 0 | 3 | 0 | 5 | 0 |
| Marital Status (%) | |||||
| Married/Cohabitating | 82 | 83 | 81 | 89 | 80 |
| Partnered, living apart | 3 | 0 | 0 | 5 | 0 |
| Single & divorced | 9 | 8 | 14 | 0 | 10 |
| Single & never married | 6 | 6 | 0 | 5 | 10 |
| Missing | 0 | 3 | 5 | 0 | 0 |
| Work Status (%) | |||||
| Full-time | 29 | 58 | 52 | 42 | 40 |
| Part-time | 18 | 6 | 5 | 26 | 7 |
| Homemaker | 3 | 0 | 0 | 0 | 3 |
| Student | 0 | 3 | 0 | 0 | 3 |
| Retired | 38 | 19 | 43 | 16 | 27 |
| Retired early, disability | 0 | 6 | 0 | 5 | 3 |
| Unemployed <1yr, not looking for work | 6 | 0 | 0 | 0 | 7 |
| Unemployed >1yr, looking for work | 3 | 0 | 0 | 5 | 0 |
| Unemployed >1 yr, not looking for work | 3 | 6 | 0 | 5 | 7 |
| Other | 0 | 3 | 0 | 0 | 3 |
| Age (years) | |||||
| M (SD) | 56.1 (14.48) | 54.4 (13.05) | 60.8 (9.39) | 50.4 (15.09) | 54.4 (14.33) |
| Time in caregiver role (years) | |||||
| M (SD) | 6.9 (6.76) | 11.0 (8.35) | 9.0 (7.69) | 13.0 (7.60) | 2.4 (3.54) |
| Age of the care recipient | 56.9 (13.95) | 50.3 (14.49) | 57.9 (9.61) | 48.1 (14.97) | 54.1 (16.31) |
| Relationship to care recipient (%) | |||||
| Spouse/Partner | 59 | 61 | 71 | 42 | 63 |
| Child | 6 | 8 | 0 | 21 | 3 |
| Parent | 9 | 22 | 14 | 11 | 20 |
| Sibling | 12 | 6 | 10 | 5 | 10 |
| In-law | 3 | 0 | 0 | 5 | 0 |
| Friend | 12 | 3 | 5 | 16 | 3 |
| Time caregiving (%) | |||||
| 1–2hrs/day or less | 35 | 33 | 48 | 21 | 33 |
| 3–4hrs/day (half a working day) | 35 | 36 | 38 | 32 | 37 |
| 5–8hrs/day (full working day) | 15 | 14 | 10 | 26 | 10 |
| 9–12hrs/day | 3 | 3 | 0 | 0 | 7 |
| >12hrs/day or round-the-clock care | 9 | 14 | 5 | 21 | 10 |
| Missing | 3 | 0 | 0 | 0 | 3 |
| Baseline HRQOL | |||||
| Caregiver Strain | 48.0 (7.92) | 49.7 (6.50) | 50.4 (7.02) | 49.3 (7.62) | 47.6 (7.12) |
| Caregiver-Specific Anxiety | 48.0 (6.81) | 47.4 (7.79) | 51.4 (7.19) | 44.4 (7.92) | 47.2 (5.86) |
| Anger | 49.2 (9.22) | 51.6 (11.20) | 51.3 (12.02) | 50.8 (10.59) | 49.6 (8.99) |
| Anxiety | 52.9 (7.59) | 53.2 (9.18) | 54.5 (8.03) | 50.2 (8.75) | 53.8 (8.25) |
| Depression | 48.0 (7.84) | 52.4 (8.24) | 51.4 (9.96) | 49.2 (7.91) | 50.1 (7.40) |
| Perceived Stress | 48.6 (11.68) | 49.5 (11.73) | 46.1 (13.49) | 51.6 (11.44) | 49.5 (10.22) |
| Positive Affect | 53.5 (4.93) | 53.2 (7.01) | 51.9 (7.07) | 54.2 (6.17) | 53.8 (5.16) |
| Social Role Ability | 48.1 (8.56) | 46.8 (8.47) | 49.6 (7.61) | 47.2 (10.24) | 46.0 (7.78) |
| Fatigue | 48.5 (9.82) | 51.8 (9.60) | 49.0 (9.05) | 51.3 (9.96) | 50.4 (10.38) |
| Sleep-Related Impairment | 50.2 (9.32) | 53.8 (9.53) | 51.4 (8.34) | 54.1 (8.39) | 51.2 (10.97) |
Note. HRQOL = health-related quality of life. SRAs = social roles and activities. Comparisons between JITAI and controls indicated that the JITAI group was caring for individuals that were significantly older than controls (p = .037) and there was a trend indicating that care partners in the JITAI group were more likely to be employed full-time and less likely to be retired than care partners in the control group (p < .06); there were no other significant group differences for JITAI versus controls. Comparisons among HD, SCI and HCT indicated that HCT care partners reported significant less years in the caregiver role than the other two cohorts (p < .0001) and that care partners of people with HD reported significantly greater caregiver-specific anxiety than care partners of people with HCT and SCI (p < .02); there were no other group differences among the three different caregiver groups.
Feasibility and Acceptability.
Compliance.
The vast majority (98.6%) of care partners completed the study (n=69 care partners across the three different care partner populations: HD n=21, HCT n=30, and SCI, n=18); the n=1 care partners who was lost to follow-up was a care partner of a person with SCI. Completion rates for the sample were high; Table 2 provides this data for each of the three different care partner groups (HD, SCI, and HCT) and Appendix 1 reports completion rates for the combined sample, as well as by intervention group (JITAI versus control). Compliance rates were comparable across the three care partner groups with the exception that care partners of people with SCI had lower rates of step data compliance than the other two care partner groups. In a couple of instances there were significant relationships between percentage of days with step data (compliance data for syncing and/or wearing the Fitbit provided in Table 2) and baseline HRQOL; specifically, greater levels of depression and fatigue, and lower positive affect was associated with worse compliance for daily step data (see Table 3).
Table 2.
Protocol Compliance by Caregiver Group
| Domain | N | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Daily Surveys | |||||
| • HD | 21 | 46% | 100% | 90% | 12% |
| • SCI | 19 | 51% | 100% | 86% | 14% |
| • HCT | 30 | 64% | 100% | 92% | 8% |
|
| |||||
| Daily Steps | |||||
| • HD | 21 | 92% | 100% | 98% | 2% |
| • SCI | 19 | 38% | 100% | 90% | 18% |
| • HCT | 30 | 91% | 100% | 99% | 2% |
|
| |||||
| Daily Sleep | |||||
| • HD | 21 | 8% | 100% | 82% | 29% |
| • SCI | 19 | 8% | 100% | 80% | 27% |
| • HCT | 30 | 4% | 100% | 90% | 21% |
|
| |||||
| End of Month Surveys | N | n complete | % complete | ||
|
| |||||
| Month 1 | |||||
| • HD | 21 | 21 | 100% | ||
| • SCI | 19 | 19 | 100% | ||
| • HCT | 30 | 30 | 100% | ||
| Month 2 | |||||
| • HD | 21 | 21 | 100% | ||
| • SCI | 19 | 19 | 100% | ||
| • HCT | 30 | 30 | 100% | ||
| Month 3 | |||||
| • HD | 21 | 21 | 100% | ||
| • SCI | 19 | 18 | 95% | ||
| • HCT | 30 | 30 | 100% | ||
Note. Caregiver groups included: HD = Huntington disease; SCI = spinal cord injury; HCT = hematopoietic cell transplantation. Comparisons among the three care partner groups indicated that care partners of people with SCI had lower compliance with step data than the other two care partner groups (p < .008); there were no group differences for survey or sleep compliance data.
Table 3.
Spearman correlations between baseline HRQOL and protocol compliance data (average % of days with data) for daily assessments
| Baseline HRQOL domain | Protocol Compliance | ||
|---|---|---|---|
| Daily Surveys | Daily Steps | Daily Sleep | |
| Caregiver Strain | −0.04 | −0.20 | 0.01 |
| Caregiver-Specific Anxiety | −0.02 | −0.18 | 0.10 |
| Anger | −0.09 | −0.19 | 0.05 |
| Anxiety | 0.03 | −0.15 | −0.05 |
| Depression | −0.05 | −0.24* | 0.03 |
| Perceived Stress | −0.07 | −0.19 | 0.02 |
| Positive Affect | 0.02 | 0.30* | 0.03 |
| Social Role Ability | −0.02 | 0.11 | −0.10 |
| Fatigue | −0.11 | −0.30* | 0.03 |
| Sleep-Related Impairment | −0.08 | −0.19 | 0.01 |
= p <0.05
Feasibility and Acceptability Questionnaire Results.
The Feasibility and Acceptability questionnaire responses indicate that the Fitbit, CareQOL app, and intensive study design were all acceptable according to our a priori expectations; Table 4 provides detailed data for the full sample and Appendix 2 reports the summary data for each of the three different care partner groups (HD, SCI, and HCT), as well as by intervention group (JITAI versus control).
Table 4.
Acceptability data (N=70 care partner respondents)
| Fitbit® | ||||||
|---|---|---|---|---|---|---|
| Strongly disagree | Disagree | Neither agree nor disagree | Agree | Strongly agree | Agree + Strongly Agree (>80%) | |
| Instructions for the Fitbit® were easy to understand. | 1% | 6% | 3% | 36% | 54% | 90% |
| The Fitbit® was easy to set up. | 1% | 6% | 1% | 39% | 52% | 91% |
| The Fitbit® was easy to use. | 0% | 3% | 10% | 48% | 39% | 87% |
| The Fitbit® was comfortable to wear. | 0% | 6% | 10% | 41% | 43% | 84% |
| The Fitbit® was easy to sync with my phone. | 0% | 3% | 12% | 35% | 51% | 86% |
| I was confident using the Fitbit® | 0% | 3% | 10% | 48% | 39% | 87% |
| CareQOL app | ||||||
| Strongly disagree | Disagree | Neither agree nor disagree | Agree | Strongly agree | Agree + Strongly Agree (>80%) | |
| The instructions for the CareQOL app set up were easy to understand. | 0% | 3% | 1% | 38% | 58% | 96% |
| The CareQOL app was easy to set up. | 0% | 3% | 3% | 41% | 54% | 94% |
| The CareQOL app was easy to use. | 0% | 1% | 1% | 35% | 62% | 97% |
| I was confident using the CareQOL app | 0% | 1% | 1% | 33% | 64% | 97% |
| I am confident that I was using the CareQOL app correctly. | 0% | 3% | 3% | 38% | 57% | 94% |
| Poor | Fair | Good | Very Good | Excellent | Very Good + Excellent (>80%) | |
| What is your overall rating of the design of the screens on the app, including the colors and layout? | 0% | 1% | 14% | 48% | 36% | 84% |
| Daily Questions | ||||||
| Strongly disagree | Disagree | Neither agree nor disagree | Agree | Strongly agree | Agree + Strongly Agree (>80%) | |
| The daily questions on the app were easy to understand. | 1% | 0% | 4% | 33% | 61% | 94% |
| The daily questions on the app were easy to answer. | 0% | 3% | 7% | 36% | 54% | 90% |
| Answering the daily questions fit easily into my routine. | 0% | 0% | 10% | 30% | 59% | 90% |
| The number of daily questions to answer on the app was reasonable. | 0% | 0% | 0% | 26% | 74% | 100% |
| Monthly Surveys | ||||||
| Strongly disagree | Disagree | Neither agree nor disagree | Agree | Strongly agree | Agree + Strongly Agree (>80%) | |
| The monthly surveys on the app were easy to understand. | 0% | 1% | 3% | 33% | 62% | 96% |
| The monthly surveys on the app were easy to answer. | 0% | 1% | 7% | 30% | 61% | 91% |
| Answering the monthly surveys fit easily into my routine. | 0% | 0% | 6% | 28% | 67% | 94% |
| The number of questions in the monthly surveys was reasonable. | 0% | 1% | 7% | 42% | 49% | 91% |
| Extremely unlikely | Unlikely | Neutral | Likely | Extremely likely | Likely + Extremely (>80%) | |
| How likely are you to recommend this study to another family caregiver? | 0% | 0% | 7% | 29% | 64% | 93% |
| How likely are you to recommend the CareQOL app to another family caregiver? | 0% | 0% | 19% | 29% | 52% | 81% |
| How likely would you be to participate in a study that lasted… | ||||||
| Extremely unlikely | Unlikely | Neutral | Likely | Extremely | Likely + Extremely (>80%) | |
| 1 month? | 0% | 0% | 3% | 21% | 76% | 97% |
| 3 months? | 0% | 0% | 3% | 30% | 67% | 97% |
| 6 months? | 0% | 3% | 21% | 21% | 55% | 76% |
| 9 months? | 3% | 9% | 15% | 21% | 52% | 73% |
| 1 year? | 6% | 9% | 15% | 21% | 48% | 70% |
| Study Expectations | ||||||
| A lot worse | A little worse | About the same | A little better | A lot better | About the same + A little better + A lot better (>80%) | |
| Compared to what you expected, how you would rate your experience in participating in this research study? | 0% | 1% | 35% | 26% | 38% | 99% |
Preliminary Efficacy.
Comparisons between those in the JITAI versus the control groups are indicated in Table 5. Of note, at 3-months, the JITAI group had significantly lower levels of caregiver strain, depression, and sleep-related impairment relative to the control group (after controlling for baseline rates of HRQOL); these differences held even after a false discovery rate correction was employed.
Table 5.
Univariate analysis of the effect of JITAI on HRQOL
| Control (n=34) | JITAI (n=36) | ANCOVA Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre, Mean (SD) | Post, Mean (SD) | Change, Mean (SD) | Pre, Mean (SD) | Post, Mean (SD) | Change, Mean (SD) | F | p-value | |
| Caregiver Strain | 48.0 (7.9) | 49.1 (10.9) | 1.3 (6.1) | 49.7 (6.5) | 47.1 (10.7) | −2.6 (6.8) | −4.3 | 0.0065 |
| Caregiver-Specific Anxiety | 48.0 (6.8) | 47.2 (9.0) | −0.6 (5.5) | 47.4 (7.8) | 43.7 (9.2) | −3.6 (5.3) | −3.0 | 0.026 |
| Anger | 49.2 (9.2) | 46.7 (9.1) | −2.3 (7.1) | 51.6 (11.2) | 46.8 (13.4) | −4.8 (7.6) | −2.1 | 0.23 |
| Anxiety | 52.9 (7.6) | 53.2 (7.7) | 0.5 (7.1) | 53.2 (9.2) | 53.2 (9.9) | 0.0 (7.6) | −0.4 | 0.83 |
| Depression | 48.0 (7.8) | 50.4 (7.5) | 2.6 (6.9) | 52.4 (8.2) | 50.4 (9.6) | −2.0 (6.4) | −3.4 | 0.037 |
| Perceived Stress | 48.6 (11.7) | 46.6 (12.5) | −1.6 (9.6) | 49.5 (11.7) | 46.8 (12.7) | −2.7 (7.5) | −0.9 | 0.65 |
| Positive Affect | 53.5 (4.9) | 54.2 (6.4) | 0.7 (4.2) | 53.2 (7.0) | 52.8 (9.4) | −0.4 (8.9) | −1.2 | 0.49 |
| Social Role Ability | 48.1 (8.6) | 47.5 (9.5) | −0.9 (7.6) | 46.8 (8.5) | 47.7 (10.1) | 0.9 (6.9) | 1.4 | 0.40 |
| Fatigue | 48.5 (9.8) | 50.5 (11.2) | 2.1(8.4) | 51.8 (9.6) | 51.0 (10.7) | −0.8 (5.7) | −2.4 | 0.16 |
| Sleep-Related Impairment | 50.2 (9.3) | 50.4 (9.4) | 0.3 (7.9) | 53.8 (9.5) | 49.1 (11.1) | −4.6 (6.8) | −4.2 | 0.02 |
Note. Bolding indicates significant differences (all significant differences were present both before and after the false discovery rate correction); in all cases, those in the intervention group indicated better HRQOL than those in the control group
Reactivity.
Results are detailed in Figure 2 and Table 6. For Depression, there were no significant main effects, nor were there significant interactions: there was no significant effect for change over time, no significant group differences, and no differences in the change over time between groups. Thus, for Depression, there was no evidence for either efficacy or reactivity. For Caregiver Strain, there was a significant interaction indicating a decrease in strain over time in the JITAI group relative to the control group, but there was no significant main effect for time (i.e., there was no temporal change in Caregiver Strain among controls). These results demonstrate potential efficacy—that is, the JITAI group improved over time and controls did not. For Anxiety there was a significant negative main effect for time (i.e., there was a significant decrease in Anxiety over time for both the JITAI and control groups), but no significant interactions. In other words, both groups improved over time suggesting potential reactivity of measurement. There was no evidence of any quadratic relationships.
Figure 2.
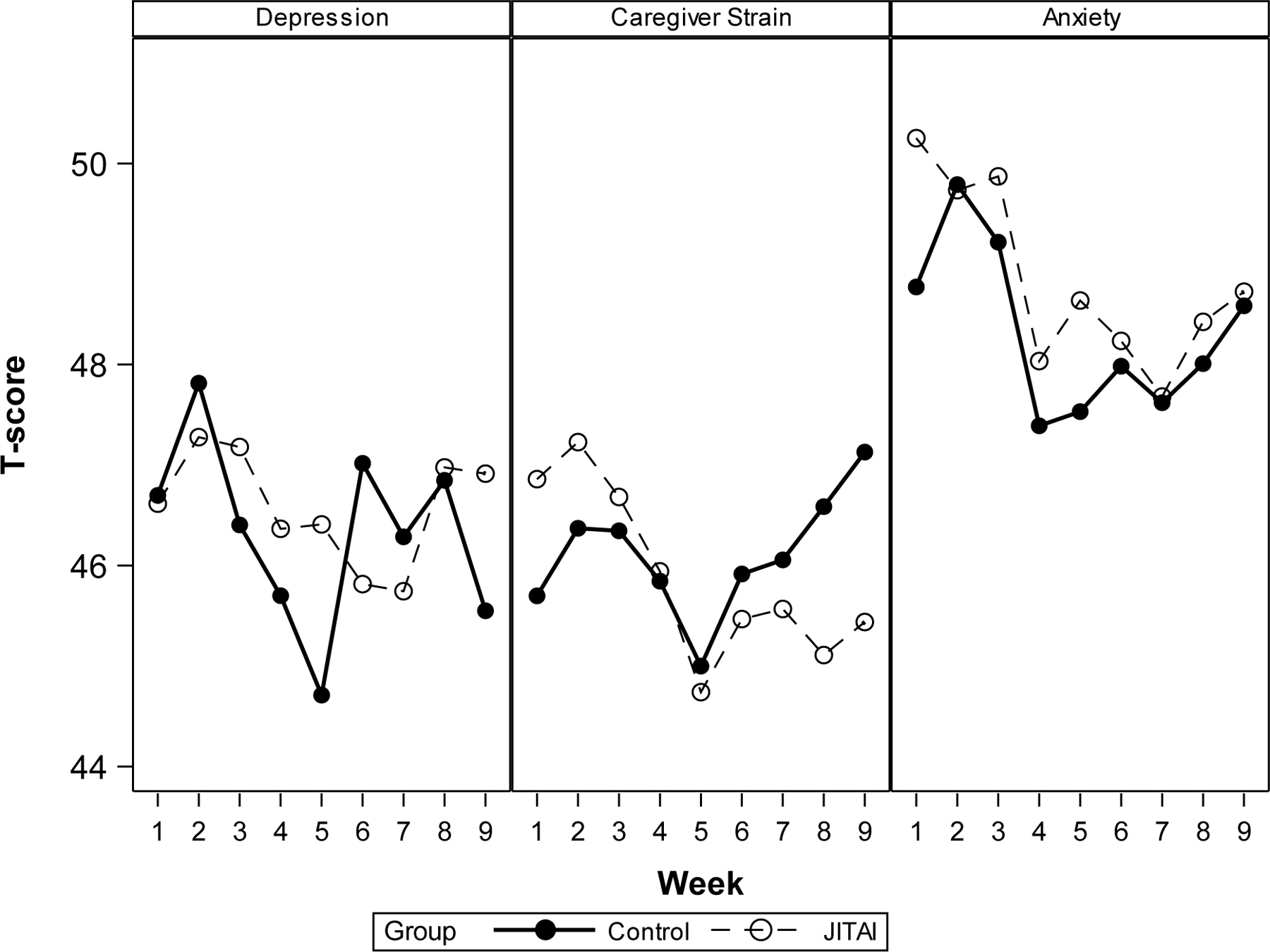
Week-by week real-time PRO data
Table 6.
Reactivity of measurement
| PROMIS Depression | Caregiver Strain | PROMIS Anxiety | ||||
|---|---|---|---|---|---|---|
| Variable | Estimate [95% CI] | p-value | Estimate [95% CI] | p-value | Estimate [95% CI] | p-value |
| Intercept | 46.80 | --- | 45.80 | --- | 49.13 | --- |
| Time (per week) | −0.09 [−0.19, 0.02] | 0.11 | 0.05 [−0.04, 0.14] | 0.28 | −0.16 [−0.27, −0.05] | 0.004 |
| JITAI vs. Control | 0.02 [−4.49, 4.54] | 0.99 | 1.17 [−4.05, 6.38] | 0.66 | 0.69 [−4.14, 5.52] | 0.78 |
| Interaction: (JITAI vs. Control) x Time (per week) | 0.04 [−0.10, 0.19] | 0.58 | −0.26 [−0.39, −0.13] | <0.001 | −0.01 [−0.17, 0.14] | 0.86 |
Discussion
The findings reported herein provide strong support for a 90-day intensive data collection protocol that involved continuous monitoring of physical activity and sleep (via a Fitbit®), daily reports of HRQOL, and end of month surveys of HRQOL. Compliance rates were in line with or exceeded those that have been reported for other studies that involve continuous monitoring via a wrist-worn device and/or the collection of daily self-report surveys (Brannon, Cushing, Crick, & Mitchell, 2016; Evans et al., 2016; Kratz et al., 2017; Moore et al., 2017; Nam et al., 2020; Rogers, 2021; Sohn et al., 2020; Stone et al., 2003; Wooldridge et al., 2022). Interestingly, compliance rates tended to be lowest for care partners of people with SCI relative to the other two cohorts. Given that care partners of people with SCI did not appear to be more distressed (i.e., have worse HRQOL) than the other two care partner groups, we postulate that this may be due to the fact that they are caring for a group that is typically characterized by more severe functional deficits than the other two care partner groups. In addition, baseline ratings of depression, fatigue and positive affect and well-being were associated with daily compliance with daily step data (more depression, more fatigue and less positive affect were associated with worse compliance).
Feasibility and acceptability ratings were also high with the vast majority of care partners indicating positive responses across the different elements of the study (i.e., using the Fitbit®, using the CareQOL app, completing the daily questions, and completing the end of month surveys). There were a couple of instances, when different subgroups were examined, that this rating fell slightly short of a priori expectations. In particular, care partners of persons with HD appeared to be less enthusiastic about the Fitbit® than the other two cohorts; only 76% indicated that it was comfortable to wear, and only 71% indicated they were confident using it, relative to the other two cohorts (where ratings all exceeded 87%). In addition, care partners of both HD and SCI were less likely to indicate they would recommend this study to others (71% for HD and 78% for SCI versus 90% for HCT care partners).
With regard to participation in future studies, 97% of care partners indicated that they would participate in a similar study of similar or less duration; this fell to 76% for a similar study that lasted 6 months, 73% for a study that lasted 9 months and 70% for a study that lasted 12 months. Care partners of people with SCI were less enthusiastic relative to the other two cohorts about participation in a similar study that lasted 6-, 9- or 12-months. Overall, care partners’ expectations about participation in this type of study were either met or were exceeded.
Taken together, overall feasibility and acceptability was extremely high across three different caregiving population. Care partners of people with neurodegenerative disease were most enthusiastic about this type of study design (e.g., HD), whereas those individuals caring for an individual with a chronic disability were the least enthusiastic (e.g., SCI). While further work is needed to explore this difference, it may be that individuals that are caring for someone with cognitive and behavioral changes (e.g., caring for someone with HD) may be more receptive to this type of study design than those caring for an individual with more substantial physical/functional impairments (e.g., SCI).
Our findings support the preliminary efficacy of this JITAI intervention. Although we were not appropriately powered to identify significant improvements in HRQOL, individuals in the JITAI group relative to the control group demonstrated significant improvements in caregiver burden (i.e., Caregiver Strain), anxiety related to the caregiver role (Caregiver-Specific Anxiety), sadness (PROMIS Depression), and sleep (PROMIS Sleep-Related Impairment). In addition, although mean important differences (MIDs) for HRQOL improvements have not yet been established in care partner populations, in other clinical populations MIDs tend to hover around a change score of 2–4 T on these HRQOL measures (Amtmann et al., 2016; Chen et al., 2018; Kroenke et al., 2020; Lapin, Thompson, Schuster, & Katzan, 2019; Lee et al., 2017; Rubery, Houck, Mesfin, Molinari, & Papuga, 2019); using this as a guide, those in the JITAI group demonstrated improvements that are potentially clinically meaningful for six of the 10 HRQOL domains that were assessed (caregiver burden, anxiety related to the caregiver role, anger, sadness, stress, and sleep). There was also some indication of reactivity of measurement for anxiety over this three-month period.
While these results are promising, we also acknowledge several study limitations. First, although we were able to identify some effects (of the intervention), future work is needed to determine if there are differential effects by care partner group and if/how type of care provided influences outcomes. In addition, the sample was predominantly Caucasian, and thus generalizability to minority participants warrants future examination. Furthermore, our sample was predominantly female, and although consistent with the caregiver literature more generally, additional work in male caregivers is also warranted. There was also a trend for group differences between the JITAI and control groups for work status; although formal inclusion of this variable in analyses did not change the overall pattern of results, future work focused on the impact that care partner work status has on care partner and care recipient outcomes is needed. Finally, it is unclear if findings would generalize to care partners of individuals that were caring for individuals with more acute conditions or other chronic conditions.
Taken together, the reported findings from this trial indicate that real-time daily assessments of symptoms and functioning, as well as compliance with passive activity monitoring (via a wrist-worn device) is both feasible and acceptable for three distinct care partner groups: care partners of individuals with chronic disability (caused by a traumatic event), care partners of persons with an insidious and progressive disease, and care partners of persons with a relapsing and remitting cancer condition. In addition, findings provide preliminary support that this JITAI can indeed improve HRQOL for these care partners over a 3-month period. Large scale efficacy trials are warranted to establish the efficacy of this low-cost, low-burden self-management JITAI.
Impact:
Although many care partners have excessive demands on their time, intensive app-based interventions are feasible and show promise for improving quality of life.
Tailored self-management messaging has the potential for improving mental, physical, and social health of care partners for people with chronic diseases.
Acknowledgements
Work on this manuscript was supported by grant numbers R01NR013658 from the National Institutes of Health (NIH), National Institute of Nursing Research, R01HL146354 and K24HL156896 from the National Heart, Lung and Blood Institute, and UL1TR002240 from the National Center for Advancing Translational Sciences. This work was also supported by the University of Michigan Institute for Healthcare Policy and Innovation. We thank the investigators, coordinators, and research associates/assistants who worked on this study, the study participants, and organizations who supported recruitment efforts.
Appendix 1
Protocol Compliance for Full Sample and by Intervention Group
| Domain | N | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Daily Surveys | |||||
| • JITAI | 34 | 59% | 100% | 91% | 9% |
| • Control | 36 | 46% | 100% | 88% | 13% |
| • Combined Sample | 70 | 46% | 100% | 90% | 11% |
|
| |||||
| Daily Steps | |||||
| • JITAI | 34 | 77% | 100% | 98% | 4% |
| • Control | 36 | 38% | 100% | 94% | 14% |
| • Combined Sample | 70 | 38% | 100% | 96% | 10% |
|
| |||||
| Daily Sleep | |||||
| • JITAI | 34 | 28% | 100% | 92% | 16% |
| • Control | 36 | 4% | 100% | 78% | 32% |
| • Combined Sample | 70 | 4% | 100% | 85% | 26% |
|
| |||||
| End of Month Surveys | N |
N
complete |
%
Complete |
||
|
| |||||
| End of Month Surveys | |||||
| Month 1 | |||||
| • JITAI | 34 | 34 | 100% | ||
| • Control | 36 | 36 | 100% | ||
| • Combined Sample | 70 | 70 | 100% | ||
| Month 2 | |||||
| • JITAI | 34 | 34 | 100% | ||
| • Control | 36 | 36 | 100% | ||
| • Combined Sample | 70 | 70 | 100% | ||
| Month 3 | |||||
| • JITAI | 34 | 34 | 100% | ||
| • Control | 36 | 35 | 97% | ||
| • Combined Sample | 70 | 69 | 99% | ||
Appendix 2
Acceptability data by care partner group
| Fitbit® | |||||
|---|---|---|---|---|---|
| JITAI Group | Control Group | HD | SCI | HCT | |
| Agree + Strongly Agree (>80%) | Agree + Strongly Agree (>80%) | ||||
| Instructions for the Fitbit® were easy to understand. | 97% | 82% | 90% | 83% | 93% |
| The Fitbit® was easy to set up. | 97% | 85% | 90% | 89% | 93% |
| The Fitbit® was easy to use. | 97% | 88% | 86% | 94% | 97% |
| The Fitbit® was comfortable to wear. | 89% | 85% | 76% | 89% | 93% |
| The Fitbit® was easy to sync with my phone. | 86% | 82% | 81% | 83% | 87% |
| I was confident using the Fitbit® | 89% | 82% | 71% | 100% | 87% |
| CareQOL app | |||||
| The instructions for the CareQOL app set up were easy to understand. | 100% | 91% | 95% | 94% | 97% |
| The CareQOL app was easy to set up. | 100% | 88% | 95% | 89% | 97% |
| The CareQOL app was easy to use. | 97% | 97% | 90% | 100% | 100% |
| I was confident using the CareQOL app | 100% | 94% | 90% | 100% | 100% |
| I am confident that I was using the CareQOL app correctly. | 94% | 94% | 90% | 100% | 93% |
| Very Good + Excellent (>80%) | Very Good + Excellent (>80%) | ||||
| What is your overall rating of the design of the screens on the app, including the colors and layout? | 81% | 88% | 76% | 89% | 87% |
| Daily Questions | |||||
| Agree + Strongly Agree (>80%) | Agree + Strongly Agree (>80%) | ||||
| The daily questions on the app were easy to understand. | 94% | 94% | 90% | 89% | 100% |
| The daily questions on the app were easy to answer. | 89% | 91% | 86% | 83% | 97% |
| Answering the daily questions fit easily into my routine. | 86% | 94% | 86% | 89% | 93% |
| The number of daily questions to answer on the app was reasonable. | 100% | 100% | 100% | 100% | 100% |
| Monthly Surveys | |||||
| The monthly surveys on the app were easy to understand. | 94% | 97% | 95% | 94% | 97% |
| The monthly surveys on the app were easy to answer. | 92% | 91% | 86% | 89% | 97% |
| Answering the monthly surveys fit easily into my routine. | 94% | 94% | 90% | 100% | 93% |
| The number of questions in the monthly surveys was reasonable. | 92% | 91% | 95% | 89% | 90% |
| Likely + Extremely (>80%) | Likely + Extremely (>80%) | ||||
| How likely are you to recommend this study to another family caregiver? | 94% | 91% | 90% | 89% | 97% |
| How likely are you to recommend the CareQOL app to another family caregiver? | 81% | 82% | 71% | 78% | 90% |
| Likely + Extremely (>80%) | Likely + Extremely (>80%) | ||||
| How likely would you be to participate in a study that lasted… | |||||
| 1 month? | 94% | 97% | 95% | 89% | 100% |
| 3 months? | 86% | 97% | 86% | 89% | 97% |
| 6 months? | 78% | 76% | 86% | 61% | 80% |
| 9 months? | 64% | 73% | 76% | 44% | 77% |
| 1 year? | 61% | 70% | 67% | 44% | 77% |
| Study Expectations | |||||
| About the same + A little better + A lot better (>80%) | About the same + A little better + A lot better (>80%) | ||||
| Compared to what you expected, how you would rate your experience in participating in this research study? | 100% | 96% | 95% | 100% | 100% |
Appendix 3
/*MODEL SYNTAX*/
/*Linear regression models were used for the ANCOVA results in Table 5. These models were performed for each HRQOL domain. The syntax below is from a macro. The macro variable
&var is for the HRQOL variable. The dataset “prepost” is a flat file (with one row per subject) with “_pre” and “_post” HRQOL variables. There is also a categorical variable “group”
for JITAI vs. control. This model tests if the “_post” scores are higher in the JITAI vs. Control group after controlling for “_pre” scores*/
proc glm data=prepost; class group(ref=“Control”)/param=ref; model &var._post=group &var._pre/solution; ods output parameterestimates=parameterestimates; quit;
/*Linear mixed-models were used for the reactivity analyses in Table 6. The dataset “fitbit_weekly2” is a long file where subjects have multiple rows for each week. This is also from a
macro and--as above--the macro variable &var is the HRQOL variable. Also--like above--there is a categorical variable “group” for JITAI vs. control. There are two identical time variables:
Week and weekn (for week “numeric”). The values are the same--this was done so that we can treat week as a categorical variable for the repeated measures portion of the model but as
a continuous/interval variable for the fixed effects portion of the model.*/
proc mixed data=fitbit_weekly2 ; class subjectid group(ref=“Control”) Week; model &var=weekn group weekn*group/s cl; random subjectid; repeated Week/type=vc subject=subjectid; ods output solutionf=solutionf; quit;
/*All other results are simple descriptive statistics comping from PROC MEANS or PROC FREQ*/
Footnotes
Trial Registration: ClinicalTrial.gov NCT04556591; https://clinicaltrials.gov/ct2/show/NCT04556591
REFERENCES
- Agarwal S, Jalan M, Wilcox HC, Sharma R, Hill R, Pantalone E, . . . Robinson KA (2022). In Evaluation of Mental Health Mobile Applications Rockville (MD). [PubMed] [Google Scholar]
- Amtmann D, Kim J, Chung H, Askew RL, Park R, & Cook KF (2016). Minimally important differences for Patient Reported Outcomes Measurement Information System pain interference for individuals with back pain. J Pain Res, 9, 251–255. doi: 10.2147/JPR.S93391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applebaum AJ, Bevans M, Son T, Evans K, Hernandez M, Giralt S, & DuHamel K (2016). A scoping review of caregiver burden during allogeneic HSCT: lessons learned and future directions. Bone Marrow Transplant, 51(11), 1416–1422. doi: 10.1038/bmt.2016.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubeeluck A (2005). Caring for the carers: quality of life in Huntington’s disease. Br J Nurs, 14(8), 452–454. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15924027 [DOI] [PubMed] [Google Scholar]
- Aubeeluck AV, Buchanan H, & Stupple EJ (2012). ‘All the burden on all the carers’: exploring quality of life with family caregivers of Huntington’s disease patients. Quality of Life Research, 21(8), 1425–1435. doi: 10.1007/s11136-011-0062-x [DOI] [PubMed] [Google Scholar]
- Banaszkiewicz K, Sitek EJ, Rudzinska M, Soltan W, Slawek J, & Szczudlik A (2012). Huntington’s disease from the patient, caregiver and physician’s perspectives: three sides of the same coin? J Neural Transm, 119(11), 1361–1365. doi: 10.1007/s00702-012-0787-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zeev D, Brenner CJ, Begale M, Duffecy J, Mohr DC, & Mueser KT (2014). Feasibility, acceptability, and preliminary efficacy of a smartphone intervention for schizophrenia. Schizophrenia bulletin, sbu033. [DOI] [PMC free article] [PubMed]
- Bidenko K, & Bohnet-Joschko S (2022). Supporting family care: a scoping app review. BMC Med Inform Decis Mak, 22(1), 162. doi: 10.1186/s12911-022-01906-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanes L, Carmagnani MIS, & Ferreira LM (2007). Health-related quality of life of primary caregivers of persons with paraplegia. Spinal Cord, 45(6), 399–403. doi:DOI 10.1038/sj.sc.3102038 [DOI] [PubMed] [Google Scholar]
- Brannon EE, Cushing CC, Crick CJ, & Mitchell TB (2016). The promise of wearable sensors and ecological momentary assessment measures for dynamical systems modeling in adolescents: a feasibility and acceptability study. Transl Behav Med, 6(4), 558–565. doi: 10.1007/s13142-016-0442-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelleri JC, Jason Lundy J, & Hays RD (2014). Overview of classical test theory and item response theory for the quantitative assessment of items in developing patient-reported outcomes measures. Clin Ther, 36(5), 648–662. doi: 10.1016/j.clinthera.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Brickell TA, French LM, Sander A, Kratz AL, Tulsky DS, . . . Lange RT (2016). Caring for our wounded warriors: A qualitative examination of health-related quality of life in caregivers of individuals with military-related traumatic brain injury. Journal of rehabilitation research and development, 53(6), 669–680. doi: 10.1682/JRRD.2015.07.0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Choi SW, Wu Z, Miner JA, Lyden AK, Graves C, . . . Sen S (2021). An App-Based Just-in-Time Adaptive Self-management Intervention for Care Partners (CareQOL): Protocol for a Pilot Trial. JMIR Res Protoc, 10(12), e32842. doi: 10.2196/32842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Kallen MA, Hanks R, Hahn EA, Brickell T, Lange R, . . . Sander AM (2019). The TBI-CareQOL Measurement System: Development and preliminary validation of health-related quality of life measures for caregivers of civilians and service members/veterans with traumatic brain injury. Archives of Physical Medicine & Rehabilitation, 100, S1–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Kallen MA, Hanks R, Hahn EA, Brickell TA, Lange RT, . . . Sander AM (2019). The TBI-CareQOL Measurement System: Development and Preliminary Validation of Health-Related Quality of Life Measures for Caregivers of Civilians and Service Members/Veterans With Traumatic Brain Injury. Arch Phys Med Rehabil, 100(4S), S1–S12. doi: 10.1016/j.apmr.2018.08.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Kallen MA, Ianni PA, Hahn EA, French LM, Lange RT, . . . Sander AM (2019). The Development of a New Computer-Adaptive Test to Evaluate Strain in Caregivers of Individuals With TBI: TBI-CareQOL Caregiver Strain. Arch Phys Med Rehabil, 100(4S), S13–S21. doi: 10.1016/j.apmr.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Kallen MA, Sander AM, Brickell TA, Lange RT, French LM, . . . Hanks R (2019). The Development of a New Computer Adaptive Test to Evaluate Anxiety in Caregivers of Individuals With Traumatic Brain Injury: TBI-CareQOL Caregiver-Specific Anxiety. Arch Phys Med Rehabil, 100(4S), S22–S30. doi: 10.1016/j.apmr.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Kratz AL, Sander AM, Chiaravalloti ND, Brickell TA, Lange RT, . . . Tulsky DS (2015). Health-related quality of life in caregivers of individuals with traumatic brain injury: development of a conceptual model. Arch Phys Med Rehabil, 96(1), 105–113. doi: 10.1016/j.apmr.2014.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlozzi NE, Schilling S, Freedman J, Kalpakjian CZ, & Kratz AL (2018). The reliability of end of day and ecological momentary assessments of pain and pain interference in individuals with spinal cord injury. Quality of Life Research. doi: 10.1007/s11136-018-1952-y [DOI] [PMC free article] [PubMed]
- Cella D, Lai JS, Nowinski C, Victorson D, Peterman A, Miller D, . . . Moy C (2012). Neuro-QOL: Brief Measures of Health-related Quality of Life for Clinical Research in Neurology. Neurology, 78, 1860–1867. doi: 10.1212/WNL.0b013e318258f744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Nowinski C, Peterman A, Victorson D, Miller D, Lai JS, & Moy C (2011). The Neurology Quality of Life Measurement (Neuro-QOL) Initiative. Arch Phys Med Rehabil, 92(Suppl 1), S28–S36. doi:doi: 10.1016/j.apmr.2011.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, . . . PROMIS Cooperative Group. (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. doi: 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, . . . Group, P. C. (2007). The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Medical Care, 45(5 Suppl 1), S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Kroenke K, Stump TE, Kean J, Carpenter JS, Krebs EE, . . . Monahan PO (2018). Estimating minimally important differences for the PROMIS pain interference scales: results from 3 randomized clinical trials. Pain, 159(4), 775–782. doi: 10.1097/j.pain.0000000000001121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox M (2012). Quality of life among carers of people with Huntington’s disease. British Journal of Neuroscience Nursing, 8(5), 288–294. Retrieved from http://search.ebscohost.com/login.aspx?direct=true&db=rzh&AN=2011739785&site=ehost-live [Google Scholar]
- Dimidjian S, Barrera M Jr., Martell C, Munoz RF, & Lewinsohn PM (2011). The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol, 7, 1–38. doi: 10.1146/annurev-clinpsy-032210-104535 [DOI] [PubMed] [Google Scholar]
- Dominick GM, Winfree KN, Pohlig RT, & Papas MA (2016). Physical Activity Assessment Between Consumer- and Research-Grade Accelerometers: A Comparative Study in Free-Living Conditions. JMIR Mhealth Uhealth, 4(3), e110. doi: 10.2196/mhealth.6281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J, Papadopoulos A, Silvers CT, Charness N, Boot WR, Schlachta-Fairchild L, . . . Ent CB (2016). Remote Health Monitoring for Older Adults and Those with Heart Failure: Adherence and System Usability. Telemed J E Health, 22(6), 480–488. doi: 10.1089/tmj.2015.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina N, & Lowry RG (2018). The Validity of Consumer-Level Activity Monitors in Healthy Older Adults in Free-Living Conditions. J Aging Phys Act, 26(1), 128–135. doi: 10.1123/japa.2016-0344 [DOI] [PubMed] [Google Scholar]
- Foxall MJ, & Gaston-Johansson F (1996). Burden and health outcomes of family caregivers of hospitalized bone marrow transplant patients. J Adv Nurs, 24(5), 915–923. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8933250 [DOI] [PubMed] [Google Scholar]
- Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, . . . Roberts I (2011). Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. The Lancet, 378(9785), 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French DP, & Sutton S (2010). Reactivity of measurement in health psychology: how much of a problem is it? What can be done about it? Br J Health Psychol, 15(Pt 3), 453–468. doi: 10.1348/135910710X492341 [DOI] [PubMed] [Google Scholar]
- Gustafson DH, McTavish FM, Chih M-Y, Atwood AK, Johnson RA, Boyle MG, . . . Dillenburg L (2014). A smartphone application to support recovery from alcoholism: a randomized clinical trial. JAMA Psychiatry, 71(5), 566–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haerens L, Deforche B, Vandelanotte C, Maes L, & De Bourdeaudhuij I (2007). Acceptability, feasibility and effectiveness of a computer-tailored physical activity intervention in adolescents. Patient Educ Couns, 66(3), 303–310. doi: 10.1016/j.pec.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Hamidou Z, Auquier P, Leroy T, Barlesi F, Salas S, Chinot O, & Baumstarck K (2017). Dyadic effects of coping strategies, time perspectives, and personality on the quality of life of cancer patients and their caregivers. Psycho-oncology. doi: 10.1002/pon.4553 [DOI] [PubMed]
- Hanauer DA, Mei Q, Law J, Khanna R, & Zheng K (2015). Supporting information retrieval from electronic health records: A report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). Journal of Biomedical Informatics, 55, 290–300. doi: 10.1016/j.jbi.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurria A, Naylor M, & Cohen HJ (2013). Improving the Quality of Cancer Care in an Aging Population. JAMA, 310(17), 1795–1796. [DOI] [PubMed] [Google Scholar]
- Kamper JE, Garofano J, Schwartz DJ, Silva MA, Zeitzer J, Modarres M, . . . Nakase-Richardson R (2016). Concordance of Actigraphy With Polysomnography in Traumatic Brain Injury Neurorehabilitation Admissions. J Head Trauma Rehabil, 31(2), 117–125. doi: 10.1097/HTR.0000000000000215 [DOI] [PubMed] [Google Scholar]
- King AC, Hekler EB, Grieco LA, Winter SJ, Sheats JL, Buman MP, . . . Cirimele J (2013). Harnessing different motivational frames via mobile phones to promote daily physical activity and reduce sedentary behavior in aging adults [DOI] [PMC free article] [PubMed]
- Kratz AL, Kalpakjian CZ, & Hanks RA (2017). Are intensive data collection methods in pain research feasible in those with physical disability? A study in persons with chronic pain and spinal cord injury. Quality of Life Research, 26(3), 587–600. doi: 10.1007/s11136-016-1494-0 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Stump TE, Chen CX, Kean J, Bair MJ, Damush TM, . . . Monahan PO (2020). Minimally important differences and severity thresholds are estimated for the PROMIS depression scales from three randomized clinical trials. J Affect Disord, 266, 100–108. doi: 10.1016/j.jad.2020.01.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapin B, Thompson NR, Schuster A, & Katzan IL (2019). Clinical Utility of Patient-Reported Outcome Measurement Information System Domain Scales. Circ Cardiovasc Qual Outcomes, 12(1), e004753. doi: 10.1161/CIRCOUTCOMES.118.004753 [DOI] [PubMed] [Google Scholar]
- Lee AC, Driban JB, Price LL, Harvey WF, Rodday AM, & Wang C (2017). Responsiveness and Minimally Important Differences for 4 Patient-Reported Outcomes Measurement Information System Short Forms: Physical Function, Pain Interference, Depression, and Anxiety in Knee Osteoarthritis. J Pain, 18(9), 1096–1110. doi: 10.1016/j.jpain.2017.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantua J, Gravel N, & Spencer RM (2016). Reliability of Sleep Measures from Four Personal Health Monitoring Devices Compared to Research-Based Actigraphy and Polysomnography. Sensors (Basel), 16(5). doi: 10.3390/s16050646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MP, Firth L, & O’Connor E (2009). A comparison of mood and quality of life among people with progressive neurological illnesses and their caregivers. J Clin Psychol Med Settings, 16(4), 355–362. doi: 10.1007/s10880-009-9168-5 [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, Insana SP, & Bond JA (2012). Movement toward a novel activity monitoring device. Sleep Breath, 16(3), 913–917. doi: 10.1007/s11325-011-0585-y [DOI] [PubMed] [Google Scholar]
- Moore RC, Kaufmann CN, Rooney AS, Moore DJ, Eyler LT, Granholm E, . . . Depp CA (2017). Feasibility and Acceptability of Ecological Momentary Assessment of Daily Functioning Among Older Adults with HIV. Am J Geriatr Psychiatry, 25(8), 829–840. doi: 10.1016/j.jagp.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Hekler EB, & Spruijt-Metz D (2015). Building health behavior models to guide the development of just-in-time adaptive interventions: A pragmatic framework. Health Psychology, 34(S), 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahum-Shani I, Shaw SD, Carpenter SM, Murphy SA, & Yoon C (2022). Engagement in digital interventions. Am Psychol doi: 10.1037/amp0000983 [DOI] [PMC free article] [PubMed]
- Nahum-Shani I, Smith SN, Spring BJ, Collins LM, Witkiewitz K, Tewari A, & Murphy SA (2016). Just-in-Time Adaptive Interventions (JITAIs) in Mobile Health: Key Components and Design Principles for Ongoing Health Behavior Support. Annals of Behavioral Medicine, 1–17. doi: 10.1007/s12160-016-9830-8 [DOI] [PMC free article] [PubMed]
- Nam S, Dunton GF, Ordway MR, Ash GI, Jeon S, Vlahov D, . . . Granger DA (2020). Feasibility and acceptability of intensive, real-time biobehavioral data collection using ecological momentary assessment, salivary biomarkers, and accelerometers among middle-aged African Americans. Res Nurs Health, 43(5), 453–464. doi: 10.1002/nur.22068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NeCamp T, Sen S, Frank E, Walton MA, Ionides EL, Fang Y, . . . Wu Z (2020). Assessing Real-Time Moderation for Developing Adaptive Mobile Health Interventions for Medical Interns: Micro-Randomized Trial. J Med Internet Res, 22(3), e15033. doi: 10.2196/15033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EJ, & McCabe MP (2011). Predictors of quality of life in carers for people with a progressive neurological illness: a longitudinal study. Quality of Life Research, 20(5), 703–711. [DOI] [PubMed] [Google Scholar]
- Park JYE, Tracy CS, & Gray CS (2022). Mobile phone apps for family caregivers: A scoping review and qualitative content analysis. Digit Health, 8, 20552076221076672. doi: 10.1177/20552076221076672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett TJ, Altmaier E, & Paulsen JS (2007). Caregiver burden in Huntington’s disease. Rehabilitation Psychology, 52(3), 311–318. [Google Scholar]
- Ramkumar NA, & Elliott TR (2010). Family caregiving of persons following neurotrauma: Issues in research, service and policy. NeuroRehabilitation, 27(1), 105–112. doi: 10.3233/nre-2010-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready RE, Mathews M, Leserman A, & Paulsen JS (2008). Patient and caregiver quality of life in Huntington’s disease. Mov Disord, 23(5), 721–726. doi: 10.1002/mds.21920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RER, Insogna JA, Carver TE, Comptour AM, Bewski NA, Sciortino C, & Andersen RE (2017). Validity and reliability of Fitbit activity monitors compared to ActiGraph GT3X+ with female adults in a free-living environment. J Sci Med Sport, 20(6), 578–582. doi: 10.1016/j.jsams.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Riley W, Obermayer J, & Jean-Mary J (2008). Internet and mobile phone text messaging intervention for college smokers. Journal of American College Health, 57(2), 245–248. [DOI] [PubMed] [Google Scholar]
- Rogers ML (2021). Feasibility and acceptability of ecological momentary assessment in a fully online study of community-based adults at high risk for suicide. Psychol Assess, 33(12), 1215–1225. doi: 10.1037/pas0001054 [DOI] [PubMed] [Google Scholar]
- Roscoe LA, Corsentino E, Watkins S, McCall M, & Sanchez-Ramos J (2009). Well-Being of Family Caregivers of Persons with Late-Stage Huntington’s Disease: Lesson in Stress and Coping. Health Communication, 24, 239–248. [DOI] [PubMed] [Google Scholar]
- Rubery PT, Houck J, Mesfin A, Molinari R, & Papuga MO (2019). Preoperative Patient Reported Outcomes Measurement Information System Scores Assist in Predicting Early Postoperative Success in Lumbar Discectomy. Spine (Phila Pa 1976), 44(5), 325–333. doi: 10.1097/BRS.0000000000002823 [DOI] [PubMed] [Google Scholar]
- Salsman JM, Butt Z, Pilkonis PA, Cyranowski JM, Zill N, Hendrie HC, . . . Cella D (2013). Emotion assessment using the NIH Toolbox. Neurology, 80(11 Suppl 3), S76–86. doi: 10.1212/WNL.0b013e3182872e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent C, Lastella M, Romyn G, Versey N, Miller DJ, & Roach GD (2018). How well does a commercially available wearable device measure sleep in young athletes? Chronobiol Int, 35(6), 754–758. doi: 10.1080/07420528.2018.1466800 [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, & Moher D (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother, 1(2), 100–107. doi: 10.4103/0976-500X.72352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MT, McCrae CS, Cheung J, Martin JL, Harrod CG, Heald JL, & Carden KA (2018). Use of Actigraphy for the Evaluation of Sleep Disorders and Circadian Rhythm Sleep-Wake Disorders: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med, 14(7), 1231–1237. doi: 10.5664/jcsm.7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn A, Speier W, Lan E, Aoki K, Fonarow GC, Ong MK, & Arnold CW (2020). Integrating remote monitoring into heart failure patients’ care regimen: A pilot study. PLoS One, 15(11), e0242210. doi: 10.1371/journal.pone.0242210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt-Metz D, & Nilsen W (2014). Dynamic models of behavior for just-in-time adaptive interventions. IEEE Pervasive Computing(3), 13–17.
- Stone AA, Broderick JE, Schwartz JE, Shiffman S, Litcher-Kelly L, & Calvanese P (2003). Intensive momentary reporting of pain with an electronic diary: reactivity, compliance, and patient satisfaction. Pain, 104(1–2), 343–351. doi: 10.1016/s0304-3959(03)00040-x [DOI] [PubMed] [Google Scholar]
- Sushames A, Edwards A, Thompson F, McDermott R, & Gebel K (2016). Validity and Reliability of Fitbit Flex for Step Count, Moderate to Vigorous Physical Activity and Activity Energy Expenditure. PLoS One, 11(9), e0161224. doi: 10.1371/journal.pone.0161224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JG, & Bond DS (2015). Behavioral response to a just-in-time adaptive intervention (JITAI) to reduce sedentary behavior in obese adults: Implications for JITAI optimization. Health Psychology, 34(S), 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandelanotte C, & De Bourdeaudhuij I (2003). Acceptability and feasibility of a computer-tailored physical activity intervention using stages of change: project FAITH. Health Educ Res, 18(3), 304–317. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12828232 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Desai SA, Bowen S, Leigh BC, Kirouac M, & Larimer ME (2014). Development and evaluation of a mobile intervention for heavy drinking and smoking among college students. Psychology of Addictive Behaviors, 28(3), 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge JS, Soriano EC, Harris DE, & Afari N (2022). Feasibility and Acceptability of Ecological Momentary Assessment of Psychosocial Factors and Self-Management Behaviors Among Veterans With Type 2 Diabetes. Diabetes Spectr, 35(1), 76–85. doi: 10.2337/ds21-0020 [DOI] [PMC free article] [PubMed] [Google Scholar]


