Abstract
The spine is the most common site of bone metastases. Many cancer patients will ultimately develop spinal metastatic disease with symptomatic epidural spinal cord compression. At present, the main treatment for cervical spine tumors is surgical resection combined with postoperative radiotherapy. Implant materials for cervical spine anterior column reconstruction need to meet amounts of different properties, such as biocompatibility, bioactivity and the ability to maintain long‐term mechanical strength. The selection of different materials determines the surgical efficacy and prognosis of patients to a certain extent. This article provides an overview of a variety of implant materials used for anterior column reconstruction after cervical spine tumor resection, introduces and analyzes their properties, advantages, disadvantages, derivatives, and applications in clinical practice, and looks forward to the future development of implant materials.
Keywords: 3D‐printed, Allogenic bone, Autogenous bone, Bioactive ceramics, Bone cement, Cervical spine reconstruction, Cervical spine tumor, Implant materials, PEEK, TMC
This article provides an overview of a variety of implant materials used for reconstruction after cervical spine tumor resection, introduces and analyzes their properties, advantages, disadvantages, derivatives, and applications in clinical practice. They all play an essential role in maintaining the mechanical stability of cervical spine and improving patient outcomes after surgery and we cannot determine which one is best without a specific condition. The best implant material is still being explored.

Introduction
In clinical practice, primary spine tumors are rare, while most spine tumors are metastatic. Bone is the third most common site for tumor metastasis, next only to the lung and liver, and the spine is the most common site of bone metastases. 1 , 2 , 3 Primary cervical spine tumors mainly include osteoid osteoma, osteoblastoma, chordoma, and plasmacytoma. 4 The most common primary tumors that metastasize to the cervical spine are breast, lung, and prostate tumors. 5 There are many differences in the pathological features of different types of cervical spine tumors. Approximately 40% of patients with cancer will develop spinal metastatic diseases, and 5%–10% will finally develop symptomatic epidural spinal cord compression. 6 When the tumor compresses the spinal cord, it is possible to cause a series of adverse neurological symptoms, 7 such as paralysis, pain, and even death in severe cases. 8 , 9 At this point, surgical intervention is usually required. 10 In the past, the main surgical treatment for cervical spine tumors with spinal cord compression was decompressive laminectomy. 11 In recent years, it has gradually evolved into a more direct anterior approach called anterior cervical corpectomy, 12 involving removal of the vertebral body, reconstruction and stabilization. It offers a more direct approach for decompression of neural elements, tumor excision, and reconstruction of the weight‐bearing vertebral column for the majority of cervical spine tumors. 13 Although surgical treatment may not cure the disease, it can relieve pain, restore stability, improve ambulatory function, and preserve neurologic function. 14 Therefore, scientists are exploring in the field of implant materials for anterior column reconstruction.
The implant material must allow the surrounding bone to grow and adapt to its new adjacent structure. Ideally, it should have properties including biocompatibility, elastic modulus similar to that of bone, imaging and radiotherapy compatibility, optimal biomechanics, low artifacts on imaging, etc., 15 , 16 but this is not the case in practice. For example, autogenous bone may cause many complications in the donor site; metal materials such as titanium mesh cage (TMC) may generate artifacts, which may hinder optimal tumor evaluation; polyetheretherketone (PEEK) is not conducive to osteoblast adhesion due to bioinertia. Therefore, more suitable implant materials are still being explored. Currently, implant materials frequently used for anterior column reconstruction include autogenous and allogenic bone, bone cement, TMC, expandable cervical cage (ECC), PEEK, or a combination of the above.
Methods
Literature was identified by searching the PubMed and Google Scholar databases. The following MeSH (Medical Subject Heading) terms were included in our search strategy: “spinal neoplasms,” “surgery,” “bone cement,” “polymethyl methacrylate,” “titanium,” “polyetheretherketone,” “carbon fiber,” “porosity,” “elastic modulus,” “weight‐bearing,” “artifacts,” and “osseointegration.” The free text words included “cervical spine tumor,” “implants,” “bone graft,” “autogenous bone,” “allogeneic bone,” “bioactive ceramics,” “PMMA,” “titanium mesh cage,” “TMC,” “PEEK,” “3D‐printed,” “biocompatibility,” “bioactivity,” “porous,” “surface coating,” “subsidence,” “fatigue strength,” and “stress shielding.” The literature we reviewed included the following article types: review articles, systematic reviews, case reports, and original research studies. Among them, articles that were related to our topic and involved implant materials and/or cervical spine tumor were considered. Literature that involved implant materials but did not elaborate on the application to a specific field was excluded from consideration. Articles not relevant to our topic or in which the full text were not available were also excluded. Additionally, we reviewed the shortlisted literature from the search results to extract potentially suitable articles for this review. All publications were written in English. We also made a table for comparison of the advantages and disadvantages of different implant materials (Table 1).
TABLE 1.
A summary of the advantages and disadvantages of different implant materials
| Implant materials | Advantages | Disadvantages |
|---|---|---|
| Autogenous bone | Easy sampling | Donor site pain |
| Good biocompatibility | Infection | |
| Complete histocompatibility | Long operative time and bleeding | |
| Without immune rejection | Subsidence | |
| High fusion rate | Pseudoarthrosis | |
| Insufficient supply | ||
| Allogenic bone | Sufficient supply | Poor bioactivity |
| Shorter operative time | Immune rejection | |
| Less bleeding (compared to autogenous bone) | Transmission of infectious disease | |
| High fusion rate | Poor healing | |
| Relatively inexpensive | Aseptic loosening | |
| Bioactive ceramics (CS, HAP, TCP) | Excellent bioactivity | Lack plasticity |
| Good biodegradability | ||
| Good biocompatibility | ||
| Bone cement (mainly PMMA) | Good plasticity | Toxicity |
| Short setting time | Graft dislodgement | |
| Relatively inexpensive | Esophageal perforation | |
| Thermal damage to the spinal cord | ||
| Compress surrounding tissues | ||
| TMC | Maintain the height of vertebral body | cage migration |
| Immediate stability | Subsidence | |
| Good biocompatibility | High elastic modulus | |
| High fusion rate | Stress shielding | |
| Artifacts during imaging | ||
| PEEK | Moderate modulus of elasticity | Bioinert |
| Optimal loading | Micromotion | |
| Without stress shielding | Relatively expensive | |
| Good chemical resistance | ||
| Radiolucent | ||
| Suitable mechanical property | ||
| 3D‐printed vertebral body | Personalized customization | Relatively expensive |
| Low subsidence | ||
| Low pseudoarthrosis | ||
| Maintain intervertebral height | ||
| Maintain cervical physiological curvature |
Abbreviations: CS, calcium silicate; HAP, hydroxyapatite; PEEK, polyetheretherketone; PMMA, polymethylmethacrylate; TCP, tricalcium phosphate; TMC, titanium mesh cage.
Autogenous Bone and Allogenic Bone
In orthopedics, a bone graft is usually used to provide an osteoconductive, osteoinductive, or osteogenic environment to promote bone repair and fusion. The clinical indications for the use of a bone graft mainly include malunion, nonunion, tumors that cause bone defects, avascular necrosis, etc.
Autogenous Bone
Autogenous and allogenic bone grafts were among the first bone graft types used and are still frequently used today. Autologous bone graft means transplanting bone from one anatomical site of the patient to another site. It is considered an excellent bone graft material for cervical spine anterior column reconstruction due to its advantages of easy sampling, good biocompatibility, excellent osseointegration, etc. It was even the gold standard for anterior cervical spine surgery. Ilium bone is the most common site.
Autogenous bone is osteogenic, retains complete histocompatibility, provides structural support, and has no risk of disease transmission. 17 Compared with allogenic bone, immune rejection will not occur as well. In fresh autogenous bone, surviving cells including osteocytes, osteoblasts, and mesenchymal cells can form new bone, increasing the probability of successful fusion, which is the most prominent advantage. Park et al. 18 performed double‐segmental anterior cervical decompression and fusion with autogenous bone in 32 patients and recorded fusion rates, which were 28.1%, 68.8%, 93.8%, and 93.8% at 3, 6, 12, and 24 months respectively.
However, autogenous bone grafts may also cause donor site pain (the most common complication), infection, increased operative time and bleeding, and gait disturbance. 19 Complications related to autogenous iliac bone graft include bone resorption, vertebral body subsidence, and pseudoarthrosis formation. 20 Sayama et al. 21 found that harvesting the iliac crest for grafting significantly resulted in postoperative pain and morbidity, affecting patients' life quality. They also claimed that using autogenous bone for anterior column reconstruction should be limited to patients with an expected survival of more than 6 months. Roberts et al. 22 created a trephine harvest technique that provided ample autogenous bone without any complications or obvious pain at the harvest site during either short‐ or long‐term follow‐up, while their study was subject to historical controls for comparison. In contrast, the avoidance of donor site complications can be avoided by using allogenic bone.
Allogenic Bone
Allogenic bone graft was first applied to clinical practice in 1881. It solves the major problem of insufficient autogenous bone supply in patients. Initially, the development of clinical application of allograft bone graft was limited due to poor bioactivity, immune rejection of the recipient, transmission of infectious diseases, poor healing, aseptic loosening, and insufficient donors. 23 The above problems have been solved with the progress of allogenic bone processing and preservation technology, the development of bone bank construction, and the establishment of relevant management systems. According to different treatment methods, allogenic bone can be classified as fresh allogenic bone, deep‐frozen bone, fresh frozen allograft (FFA), and demineralized bone matrix (DBM). Fresh allogenic bone refers to the direct graft of the donor after removal without any treatment. Its disadvantages include easy to cause immune rejection of the recipient, and increased risk of transmission of infectious disease. Therefore, it has been eliminated in clinical surgery. Deep‐frozen bone refers to a bone graft processed from allogenic bone, trimmed into different shapes as needed, and then stored at low temperatures to retain the original mechanical strength. FFA is obtained by separating and thawing frozen bone to dehydrate it sufficiently and keep the water content in bone tissue below 5%, while the corresponding stress intensity is lost to some extent. DBM is obtained by using a series of chemical methods to decalcify and decrease allogenic bone to reduce immunogenicity while retaining a variety of osteogenic factors such as bone morphogenetic proteins to induce osteogenesis. It has a porous structure and is easier to combine with cytokines thus promoting bone regeneration. 24 Although the structural strength decreases and the bearing capacity of bone is lost, it has unique clinical advantages in the repair and filling of bone defects.
Compared with autogenous bone, allogenic bone has advantages in terms of operative time and bleeding control, and donor site complications when using autogenous bone will not occur, just as we mentioned before. Allogenic bone is plentiful in supply and relatively inexpensive. 25 Sun et al. 26 reconstructed a patient's cervical spine with a mesh cage filled with allogenic bone graft to treat primary leiomyosarcoma of the cervical spine invading the vertebra. There were no recurrences 6 months after operation, and the reconstruction effect was good. Martin et al. 27 used a freeze‐dried fibula allograft for reconstruction after anterior cervical discectomy and found that the allogenic fibula revealed a high fusion rate with minimal complications.
Bioactive Ceramics
With the development of biomechanics and materials science, bone grafting methods become increasingly diverse. The implantation of artificial bone substitutes to repair bone defects and reconstruct spine has been a research hotspot. Artificial biomaterials that can replace human bone mainly consist of polymer synthetic materials, such as bioactive ceramics. Bioactive ceramics such as calcium silicate (CS), hydroxyapatite (HAP), and tricalcium phosphate (TCP) have been profusely studied for their ability to form direct bonds with living bone after implantation in bone defects. 28 CS is the major ingredient of natural bone with excellent bioactivity and can bond to living bone and soft tissue. 29 HAP can form new bone tissue binding to bone tissues through chemical bonds. 30 TCP can enhance stem cell proliferation capacity and has good biodegradability, biocompatibility, and bioactivity. 31 However, it is sintered at high temperatures in lumpy or granular form and lacks plasticity. During operation, the doctor cannot arbitrarily shape the material according to the bone defect of patients, and cannot completely fill the abnormal bone cavity. Therefore, these materials are usually used as surface coatings to improve the properties of PEEK, such as osseointegration and bioactivity. 32 Frankenberger et al. 33 found that the HA/SiO2‐based bioactive coating with interfacial composites on PEEK implants provided a lasting bone‐implant interface, and may be conducive to improving the property of bioinert surface of PEEK‐based implants. Addai et al. 34 successfully prepared the reduced graphene oxide hydroxyapatite composite coating, significantly improving the hydrophilicity and bioactivity of PEEK.
Bone Cement
As a cementing material, bone cement can be used in artificial joint replacement surgery and vertebral reconstruction surgery. It solves the problem of lack of plasticity in bioactive materials and can change from viscous state to solid state in approximately 15 min. Additionally, the short setting time enables patients to get out of bed earlier and reduce the time of bed rest. Bone cement can be divided into two categories: one is polymethylmethacrylate (PMMA) cement, which is a non‐degradable bioinert material, and the other is calcium phosphate cement (CPC), which is degradable. PMMA is used more widely in clinical practice. 35 PMMA‐assisted reconstruction achieves immediate stabilization after radical tumor resection and is an advisable bone graft option for patients with limited life expectancy requiring further treatment. PMMA negates the need for postoperative external orthosis, avoids donor site complications, is unaffected by tumor invasion, and is inexpensive. Anchoring PMMA to the vertebral body securely can achieve better results.
However, PMMA has toxicity, together with the possibility of graft dislodgement, esophageal perforation, or even thermal damage to the spinal cord. Once it leaks, it is possible to compress the surrounding tissues. Allergic reaction to the components of bone cement may occur if patients have an allergic constitution. In severe cases, adverse effects such as shock may also occur. Long‐term problems include tumor regrowth that may necessitate repeat surgery. To solve thermal damage to the spinal cord, Xia et al. 36 developed Paraffin/P(MMA‐MBA) PCM using the method of emulsion polymerization. They found that doping PMMA with microcapsules decreases its Tmax and thus avoids the adverse effects of strong exothermicity during the PMMA setting process. Additionally, other techniques with PMMA have also been developed to prevent thermal injury to the underlying spinal cord. Cooper et al. 37 first reported the use of PMMA‐filled silicone rubber tubing for thoracic and lumbar spine reconstruction. The technique placed key holes into the adjacent vertebral bodies and inserted a chest tube (impregnated with PMMA) into these holes. Shaaya et al. 38 performed a C6‐C7 partial vertebrectomy for a patient with follicular thyroid carcinoma metastasized to the cervical spine using a posterior approach with posterior decompression and fusion from C2 to T2. The anterior column support was provided by a chest tube/PMMA construct, allowing the implant to be placed within the anterior column during the posterior approach without sacrificing the nerve roots. The patient tolerated it well, and no postoperative neurological deficit was noted. Later, Miller et al. 39 used this PMMA reconstruction technique for patients with metastasized cervical spine tumors. A total of 29 patients underwent the coaxial double‐lumen PMMA technique followed by a subsequent anterior cervical plate, and the spinal cord of patients were protected well.
Titanium Mesh Cage
TMC is one of the most common metal implants for cervical spine anterior column reconstruction. The cage constructs can restore the height of vertebral body and correct lordosis without obtaining autogenous bone from other sites. In anterior cervical corpectomy decompression and fusion surgery, using TMC combined with a local bone graft avoids bone donor site complications, and maintains immediate stability of the anterior column, together with biocompatibility and high fusion rate. TMC combined with autogenous bone graft has been generally accepted and used. Thalgott et al. 40 also achieved a 100% fusion rate using TMC combined with a local bone graft in multilevel cervical corpectomy fusion. Additionally, Kang et al. 41 proposed a novel method: a flanged TMC, which is also an effective graft for cervical spine anterior column reconstruction.
TMC has shown good efficacy in corpectomy, but placing the corpectomy defect in situ has proven difficult. Non‐expandable TMC may result in excessive distraction and weak compression. Excessive distraction may lead to segmental nerve stretch, injury, placing excessive pressure on the endplate, and increasing the risk of subsidence. Weak compression may lead to cage migration. An expandable TMC, which determines the height of the cage with proper compression but without excessive distraction of the adjacent vertebral body can solve these problems. 42 Expandable cage directly applies and maintains the distraction force with a simple one‐step kyphosis‐correcting device without dangerous impaction (necessary with single‐height devices) on the spinal cord, and can also span multiple segments. Other types of expandable cages such as Synex ECC, have a self‐locking ratchet expansion mechanism for easy insertion into the vertebral body defect, and provide different heights and endplate sizes for the cervical, thoracic, or lumbar spine. And it was used for a case of cervical spine tumor by Auguste et al. 43 who found that ECC was suitable for cervical spine anterior column reconstruction and correction of sagittal malalignment.
Other disadvantages of TMC include the potential of cage migration, fracture, and subsidence. Severe subsidence may cause symptoms recurring, neurological function deterioration, internal fixation failure, and cervical kyphosis. 44 The incidence of postoperative TMC subsidence was reported to be over 28.3%. 20 TMC is embedded in the endplate with a dentate edge once implantation, while the adhesiveness is poor. The contact area between TMC and the vertebral endplate is point‐to‐face and limited, resulting in uneven stress distribution and relatively concentrated stress, thus leading to postoperative TMC subsidence. However, the definition of TMC subsidence is controversial. Van Jonbergen et al. 45 suggested that due to measurement errors, a decrease in postoperative intervertebral height of over 3 mm is defined as TMC subsidence. Chen et al. 46 classified TMC subsidence as mild subsidence (1–3 mm) and severe subsidence (>3 mm). In an 8‐year follow‐up study, the TMC subsidence of >3 mm was approximately 40.4%. 47 Wang et al. 48 proposed a novel type of anatomical TMC (Fig. 1) with a curved structure of the upper and lower edges completely conforming to the upper and lower end of the cervical disc and achieving face‐to‐face contact of the cervical spine, and it was reported to greatly improve the anti‐subsidence performance, 51.3% higher than that of traditional TMC.
Fig. 1.
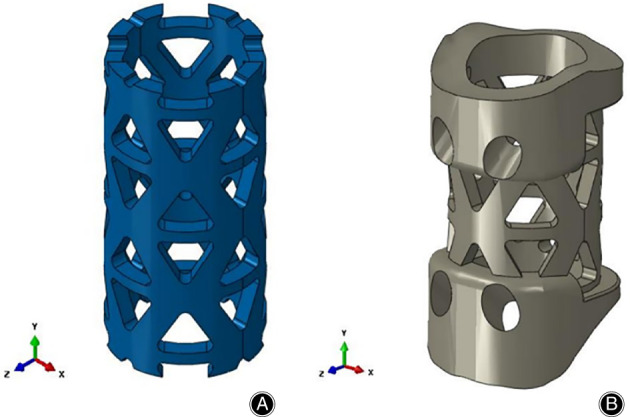
Two titanium mesh cage models. (A) Traditional titanium mesh cage (TTMC); (B) novel titanium mesh cage (NTMC) (Solidworks 2016, SOLIDWORKS, Co, USA) 48 (Taken from Wang et al. 48 )
Apart from subsidence, TMC, as a metal material, also causes artifacts and stress shielding. Artifacts affect doctors' judgment on tumor recurrence and efficacy after tumor resection. The higher elastic modulus of TMC than that of cortical bone is the main reason for stress shielding. According to Wolf's law, 49 high elastic materials result in a decrease in bone density in the peri‐implant area. Tytgat et al. 50 reported that bone remodeling is highly sensitive to dynamic loading. Due to stress shielding, developing materials with lower elastic modulus and mechanical resistance in short‐ and long‐term becomes necessary.
Peek
As a high‐performance thermoplastic, PEEK solved the problem of high elastic modulus of metal implants and was commercially supplied from April 1998. 51 The first application of PEEK as spinal implant was as an intervertebral cage, which overcame two problems of traditional metal intervertebral cages: high elastic modulus and stress shielding. The elastic modulus of PEEK (approximately 3.6 GPa) is smaller than that of cortical bone (17–21 GPa), and carbon fiber can be added into PEEK to make its elastic modulus closer to that of cortical bone. 52 This property allows optimal loading of the bone and prevents stress shielding. Moreover, with good chemical resistance, 53 PEEK can resist the body's natural oxidative environment, thus minimizing the chance of local tissue reactions. Moreover, PEEK is radiolucent and resistant to radiation damage, 54 which is convenient for facilitating radiographic assessment of implants fusion results, 55 and suitable for postoperative radiotherapy. 44
However, PEEK is a kind of bioinert material due to its hydrophobic chemistry of the surface, which is not conducive to osteoblast adhesion. Creating an osteogenic environment by modifying the surface structure can promote bone formation and implant stability without adding exogenous growth factors. Therefore, HAP whiskers, HAP microparticles, and other ceramic particulates have been incorporated into PEEK to enhance the bioactivity such as osteocyte adhesion and mechanical stiffness. 56 We also mention this when we introduce bioactive ceramics. On the other hand, PEEK cannot fuse well with the surrounding bone and might form a fibrous junction interface, 57 so subsequent micromotion may occur, causing reconstruction failure. To improve the properties of PEEK, a variety of PEEK composites such as Nano‐TiO2/PEEK, 58 , 59 and Nano‐HAp/PEEK, 60 , 61 have also been developed to promote cell adhesion, proliferation and osteogenesis. Moreover, PEEK/Carbon improves the mechanical property and crystallization rate of PEEK. 62 , 63 , 64 Boriani et al. 65 used composite PEEK/carbon fiber rods to treat cervical spine tumor and reported that hybrid implants were an effective solution for cervical and cervicothoracic segment reconstruction. And the implants did not produce artifacts in postoperative images, alleviating the execution of postoperative radiotherapy. Pipola et al. 66 reported a case of sclerosing epithelioid fibrosarcoma at C7 involving the right posterior cervical region from C5 to right lung apex. To ensure the stability of the cervical spine and the effect of particle treatment, they proposed a new technology of carbon fiber reinforced peek preformed rods with a sublaminar belt to fix the cervicothoracic junction. During a 2‐year follow‐up, the implant was stable, and no local recurrence or mechanical failure were noted. However, currently, the properties of PEEK‐based implant materials affecting bioactivity and postoperative stability have not been completely optimized.
3D‐Printed Vertebral Body
3D‐printed technology is an innovative technology and has undergone substantial development in the field of spine surgery. 67 Its unique production process allows for customization and rapid prototyping of complex geometries, which are impossible for traditional manufacturing to achieve. Engineers can reconstruct the damaged bone of patients through computer modeling using CT, MRI, or other medical images, and create prostheses with mechanical and biological properties that better match to the bones, which may produce lower subsidence and pseudoarthrosis rate. 68 3D printed vertebral body customized according to different patients is a breakthrough in implant materials for anterior column reconstruction. Due to the unique process of 3D‐printed technology, the surface of 3D‐printed vertebral body is relatively rough, creating a more ideal condition for early cell adhesion.
Compared with TMC, 3D‐printed vertebral body has better performance in maintaining intervertebral height and cervical physiological curvature. 69 Yang et al. 70 used a personalized 3D‐printed vertebral body produced from titanium alloy for cervicothoracic reconstruction of a six‐segment recurrent chordoma and claimed that it had a better load‐bearing capacity against subsidence or dislocation, which effectively reduced the rate of revision in the long‐ term. Xu et al. 71 employed a personalized 3D‐printed vertebral body (Fig, 2) 72 fabricated through computer model using titanium alloy powder to reconstruct the upper cervical spine of a teenager with Ewing sarcoma. The microstructure was optimized through the designs of optimized pore diameter and pore density of pore metal structure to gain better biomechanical stability and enhance bone healing. This was the first successful case of the clinical application of 3D‐printed titanium alloy orthopedic implant.
Fig. 2.
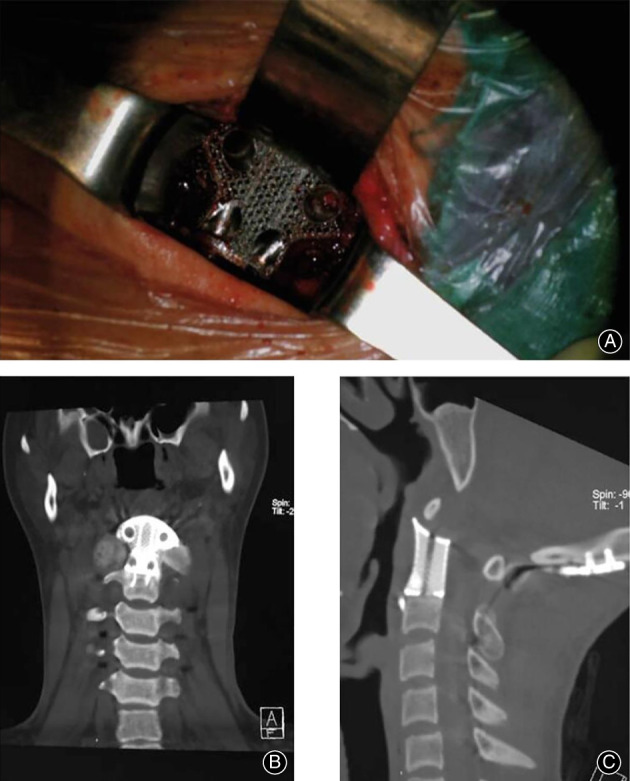
(A) Intraoperative view of the 3D‐printed artificial vertebral body in place during the anterior procedure. (B) Coronal CT reconstruction of the 3D‐printed artificial vertebral body at the 3‐month follow‐up visit. (C) Sagittal CT reconstruction of the 3D‐printed artificial vertebral body at the 3‐month follow‐up visit. 72 (Taken from Cai et al. 72 )
Additionally, the mechanical rigidity and elasticity of 3D‐printed PEEK are close to those of human bone, 73 and it is transparent to X‐rays. Given PEEK's bioinertia and thus limited interaction with bone, porous PEEK perfectly solves this problem. Controlling porosity can significantly improve the material osteoconduction. Godlewski et al. 74 thought that producing 3‐dimensional porous‐surfaced implants opens up considerable prospects for this technique in the production of modern interbody implants. 3D‐printed porous PEEK is related to increased preosteoblast activity compared with 3D‐printed solid controls. 75 , 76 Vijayavenkataraman et al. 77 found that in biological testing, the size of the pores can affect the response of cells, while for bone ingrowth, a size gradient is recommended. Spece et al. 75 successfully fabricated porous PEEK scaffolds with pore size and porosity similar to trabecular bone using an extrusion‐based and commercialized 3D printing technique, but they did not conduct animal or human experiments to further verify the bone ingrowth ability of 3D‐printed porous PEEK. And other literature related to 3D‐printed porous PEEK has no similar reports yet. In the future, if the bone ingrowth of 3D‐printed porous PEEK is verified efficiently through animal or human experiments, it will make a major breakthrough in the field of implant materials for cervical spine anterior column reconstruction.
Conclusion
Surgery combined with postoperative radiotherapy has become the main treatment modality for cervical spine tumors. In recent years, implant materials for cervical spine anterior column reconstruction after tumor resection have been extensively studied and used with varying degrees of success in orthopedic surgery. Implant materials have undergone a constant evolution and developed from original autogenous bone to bioactive materials, metal materials, organic polymer materials, and now personalized 3D‐printed vertebral body. We cannot easily determine which one is the best without a specific condition. They all play an essential role in maintaining the mechanical stability of the cervical spine and improving patient outcomes after surgery. Currently, the most popular implant materials are TMC and PEEK. The best implant material is still being explored. Looking ahead, continued in‐depth research on implant materials will be beneficial to the further development of the field of cervical spine anterior column reconstruction. Spine surgeons should keep an eye on the current literature on implant materials.
Author Contributions
All authors contributed to the review conception and design. Xiaoguang Liu and Zhongjun Liu decided the content and framework of the article. Xiao Liu, Yan Li, and Panpan Hu were the guarantors of the overall content. Jiasheng Chen, Shuheng Zhai, and Hua Zhou wrote the first draft of the manuscript. Zihe Li and Feng Wei revised the manuscript.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Jiasheng Chen and Shuheng Zhai contributed to the work equally and should be regarded as co‐first authors.
Contributor Information
Zihe Li, Email: ziheli@bjmu.edu.cn.
Feng Wei, Email: mountwei@163.com.
References
- 1. Böhm P, Huber J. The surgical treatment of bony metastases of the spine and limbs. J Bone Joint Surg Br. 2002;84(4):521–9. [DOI] [PubMed] [Google Scholar]
- 2. Kumar N, Malhotra R, Zaw AS, Maharajan K, Naresh N, Kumar A, et al. Evolution in treatment strategy for metastatic spine disease: presently evolving modalities. Eur J Surg Oncol. 2017;43(9):1784–801. [DOI] [PubMed] [Google Scholar]
- 3. Rothrock RJ, Barzilai O, Reiner AS, Lis E, Schmitt AM, Higginson DS, et al. Survival trends after surgery for spinal metastatic tumors: 20‐year cancer center experience. Neurosurgery. 2021;88(2):402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdu WA, Provencher M. Primary bone and metastatic tumors of the cervical spine. Spine. 1998;23(24):2767–77. [DOI] [PubMed] [Google Scholar]
- 5. Vazifehdan F, Karantzoulis VG, Igoumenou VG. Surgical treatment for metastases of the cervical spine. Eur J Orthop Surg Traumatol. 2017;27(6):763–75. [DOI] [PubMed] [Google Scholar]
- 6. Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327:614–9. [DOI] [PubMed] [Google Scholar]
- 7. Kato S, Demura S, Shinmura K, Yokogawa N, Shimizu T, Murakami H, et al. Surgical Metastasectomy in the spine: a review article. Oncologist. 2021;26(10):e1833–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laufer I, Bilsky MH. Advances in the treatment of metastatic spine tumors: the future is not what it used to be. J Neurosurg Spine. 2019;30(3):299–307. [DOI] [PubMed] [Google Scholar]
- 9. Roser S, Maharaj MM, Taylor MA, Kuru R, Hansen MA, Ferch R. Vertebrectomy in metastatic spinal tumours: a 10 year, single‐Centre review of outcomes and survival. J Clin Neurosci. 2019;68:218–23. [DOI] [PubMed] [Google Scholar]
- 10. Bond MR, Versteeg AL, Sahgal A, Rhines LD, Sciubba DM, Schuster JM, et al. Surgical or radiation therapy for the treatment of cervical spine metastases: results from the epidemiology, process, and outcomes of spine oncology (EPOSO) cohort. Global Spine J. 2020;10(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galgano M, Fridley J, Oyelese A, Telfian A, Kosztowski T, Choi D, et al. Surgical management of spinal metastases. Expert Rev Anticancer Ther. 2018;18(5):463–72. [DOI] [PubMed] [Google Scholar]
- 12. Shaaya E, Fridley J, Barber SM, Syed S, Xia J, Galgano M, et al. Posterior nerve‐sparing multilevel cervical corpectomy and reconstruction for metastatic cervical spine tumors: case report and literature review. World Neurosurg. 2019;122:298–302. [DOI] [PubMed] [Google Scholar]
- 13. Liu JK, Apfelbaum RI, Chiles BW 3rd, Schmidt MH. Cervical spinal metastasis: anterior reconstruction and stabilization techniques after tumor resection. Neurosurg Focus. 2003;15(5):E2–7. [PubMed] [Google Scholar]
- 14. Fehlings MG, David KS, Vialle L, Vialle E, Setzer M, Vrionis FD. Decision making in the surgical treatment of cervical spine metastases. Spine (Phila Pa 1976). 2009;34(22 Suppl):S108–17. [DOI] [PubMed] [Google Scholar]
- 15. Kumar N, Lopez KG, Alathur Ramakrishnan S, Hallinan JTPD, Fuh JYH, Pandita N, et al. Evolution of materials for implants in metastatic spine disease till date—have we found an ideal material? Radiother Oncol. 2021;163:93–104. [DOI] [PubMed] [Google Scholar]
- 16. Warburton A, Girdler SJ, Mikhail CM, Ahn A, Cho SK. Biomaterials in spinal implants: a review. Neurospine. 2020;17(1):101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fillingham Y, Jacobs J. Bone grafts and their substitutes. Bone Joint J. 2016;98‐b(1 Suppl A):6–9. [DOI] [PubMed] [Google Scholar]
- 18. Park JS, Park SJ, Lee CS, Chung SS, Park HJ. Is allograft a more reliable treatment option than autograft in 2‐level anterior cervical discectomy and fusion with plate fixation? Medicine. 2019;98(32):e16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13(1):77–86. [PubMed] [Google Scholar]
- 20. Weber MH, Fortin M, Shen J, Tay B, Hu SS, Berven S, et al. Graft subsidence and revision rates following anterior cervical corpectomy: a clinical study comparing different interbody cages. Clin Spine Surg. 2017;30(9):E1239–e1245. [DOI] [PubMed] [Google Scholar]
- 21. Sayama CM, Schmidt MH, Bisson EF. Cervical spine metastases: techniques for anterior reconstruction and stabilization. Neurosurg Rev. 2012;35(4):463‐74; discussion 475. [DOI] [PubMed] [Google Scholar]
- 22. Mclain RF, Techy F. Trephine technique for iliac crest bone graft harvest: long‐term results. Spine. 2021;46(1):41–7. [DOI] [PubMed] [Google Scholar]
- 23. Sternheim A, Drexler M, Kuzyk PR, Safir OA, Backstein DJ, Gross AE. Treatment of failed allograft prosthesis composites used for hip arthroplasty in the setting of severe proximal femoral bone defects. J Arthroplasty. 2014;29(5):1058–62. [DOI] [PubMed] [Google Scholar]
- 24. Al‐Jandan BA, Al‐Harkan A, Pompura J, Lim LZ, Guerrero JS, Marei HF, et al. Evaluation of deproteinized bone mineral (DBM) as an onlay bone‐graft in the rabbit mandible. Saudi J Dent Res. 2015;6(2):133–9. [Google Scholar]
- 25. Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8(4):114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sun H, Zhuang M, Cheng D, Zhu C, Liu Z, Qiu X. Primary leiomyosarcoma of cervical spine invading the vertebra without obvious osteoclasia: case report and literature review. J Spinal Cord Med. 2022;45:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin GJ Jr, Haid RW Jr, Macmillan M, Rodts GE Jr, Berkman R. Anterior cervical discectomy with freeze‐dried fibula allograft. Overview of 317 cases and literature review. Spine (Phila Pa 1976). 1999;24(9):852–8. discussion 858‐9. [DOI] [PubMed] [Google Scholar]
- 28. Liu F‐H. Synthesis of bioceramic scaffolds for bone tissue engineering by rapid prototyping technique. J Solgel Sci Technol. 2012;64(3):704–10. [Google Scholar]
- 29. Liu X, Morra M, Carpi A, Li B. Bioactive calcium silicate ceramics and coatings. Biomed Pharmacother. 2008;62(8):526–9. [DOI] [PubMed] [Google Scholar]
- 30. Zhou H, Lee J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011;7(7):2769–81. [DOI] [PubMed] [Google Scholar]
- 31. Mirtchi AA, Lemaitre J, Terao N. Calcium phosphate cements: study of the beta‐tricalcium phosphate–monocalcium phosphate system. Biomaterials. 1989;10(7):475–80. [DOI] [PubMed] [Google Scholar]
- 32. Ma J, Li ZJ, Xue YZB, Liang XY, Tan ZJ, Tang B. Novel PEEK/nHA composites fabricated by hot‐pressing of 3D braided PEEK matrix. Adv Compos Hybrid Mater. 2020;3(2):156–66. [Google Scholar]
- 33. Frankenberger T, Graw CL, Engel N, Gerber T, Frerich B, Dau M. Sustainable surface modification of Polyetheretherketone (PEEK) implants by hydroxyapatite/silica coating‐an In vivo animal study. Materials. 2021;14(16):4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Addai Asante N, Wang Y, Bakhet S, Kareem S, Owusu KA, Hu Y, et al. Ambient temperature sulfonated carbon fiber reinforced PEEK with hydroxyapatite and reduced graphene oxide hydroxyapatite composite coating. J Biomed Mater Res B Appl Biomater. 2021;109(12):2174–83. [DOI] [PubMed] [Google Scholar]
- 35. Garnon J, Meylheuc L, Jennings J, Koch G, Cazzato R, Bayle B, et al. PMMA bone cement in interventional oncology. Crit Rev Biomed Eng. 2021;49(1):35–50. [DOI] [PubMed] [Google Scholar]
- 36. Xia X, Shi R, Huang J, Li Y, Zuo Y, Li J. Development of a phase change microcapsule to reduce the setting temperature of PMMA bone cement. J Appl Biomater Funct Mater. 2020;18:2280800020940279. [DOI] [PubMed] [Google Scholar]
- 37. Cooper PR, Errico TJ, Martin R, Crawford B, DiBartolo T. A systematic approach to spinal reconstruction after anterior decompression for neoplastic disease of the thoracic and lumbar spine. Neurosurgery. 1993;32(1):1–8. [DOI] [PubMed] [Google Scholar]
- 38. Shaaya E, Fridley J, Barber SM, Syed S, Xia J, Galgano M, et al. Posterior nerve‐sparing multilevel cervical corpectomy and reconstruction for metastatic cervical spine tumors: case report and literature review. World Neurosurg. 2019;122:298–302. [DOI] [PubMed] [Google Scholar]
- 39. Miller DJ, Lang FF, Walsh GL, Abi‐Said D, Wildrick DM, Gokaslan ZL. Coaxial double‐lumen methylmethacrylate reconstruction in the anterior cervical and upper thoracic spine after tumor resection. J Neurosurg. 2000;92(2 Suppl):181–90. [DOI] [PubMed] [Google Scholar]
- 40. Thalgott JS, Xiongsheng C, Giuffre JM. Single stage anterior cervical reconstruction with titanium mesh cages, local bone graft, and anterior plating. Spine J. 2003;3(4):294–300. [DOI] [PubMed] [Google Scholar]
- 41. Kang JH, Im SB, Yang SM, Chung M, Jeong JH, Kim BT, et al. Surgical reconstruction using a flanged mesh cage without plating for cervical Spondylotic myelopathy and a symptomatic ossified posterior longitudinal ligament. J Korean Neurosurg Soc. 2019;62(6):671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Burkett CJ, Baaj AA, Dakwar E, Uribe JS. Use of titanium expandable vertebral cages in cervical corpectomy. J Clin Neurosci. 2012;19(3):402–5. [DOI] [PubMed] [Google Scholar]
- 43. Auguste KI, Chin C, Acosta FL, Ames CP. Expandable cylindrical cages in the cervical spine: a review of 22 cases. J Neurosurg Spine. 2006;4(4):285–91. [DOI] [PubMed] [Google Scholar]
- 44. Ji C, Yu S, Yan N, Wang J, Hou F, Hou T, et al. Risk factors for subsidence of titanium mesh cage following single‐level anterior cervical corpectomy and fusion. BMC Musculoskelet Disord. 2020;21(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Jonbergen HP, Spruit M, Anderson PG, et al. Anterior cervical interbody fusion with a titanium box cage: early radiological assessment of fusion and subsidence. Spine J. 2005;5(6):645–9. discussion 649. [DOI] [PubMed] [Google Scholar]
- 46. Chen Y, Chen D, Guo Y, Wang X, Lu X, He Z, et al. Subsidence of titanium mesh cage: a study based on 300 cases. J Spinal Disord Tech. 2008;21(7):489–92. [DOI] [PubMed] [Google Scholar]
- 47. Hu B, Wang L, Song Y, Hu Y, Lyu Q, Liu L, et al. A comparison of long‐term outcomes of nanohydroxyapatite/polyamide‐66 cage and titanium mesh cage in anterior cervical corpectomy and fusion: a clinical follow‐up study of least 8 years. Clin Neurol Neurosurg. 2019;176:25–9. [DOI] [PubMed] [Google Scholar]
- 48. Wang Y, Zhan Y, Yang H, Guo H, Zhang H, Zhao Q, et al. A novel anatomic titanium mesh cage for reducing the subsidence rate after anterior cervical corpectomy: a finite element study. Sci Rep. 2021;11(1):15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Balla VK, Tadimeti J, Kate KH, Satyavolu J. 3D printing of modified soybean hull fiber/polymer composites. Mater Chem Phys. 2020;254:123452. [Google Scholar]
- 50. Tytgat L, Van Damme L, Ortega Arevalo MDP, Declercq H, Thienpont H, Otteveare H, et al. Extrusion‐based 3D printing of photo‐crosslinkable gelatin and κ‐carrageenan hydrogel blends for adipose tissue regeneration. Int J Biol Macromol. 2019;140:929–38. [DOI] [PubMed] [Google Scholar]
- 51. Pakhaliuk V, Poliakov A. Simulation of wear in a spherical joint with a polymeric component of the total hip replacement considering activities of daily living. Facta Univ Ser: Mech Eng. 2018;16(1):51–63. [Google Scholar]
- 52. Kumar N, Ramakrishnan SA, Lopez KG, Madhu S, Ramos MRD, Fuh JYH, et al. Can polyether ether ketone dethrone titanium as the choice implant material for metastatic spine tumor surgery? World Neurosurg. 2021;148:94–109. [DOI] [PubMed] [Google Scholar]
- 53. Sharma M, Sharma H, Shannigrahi S. 17‐Tribology of advanced composites/biocomposites materials. In: Ambrosio L, editor. Biomedical Composites. Second ed. Elsevier/Woodhead Publishing; 2017. p. 413–29. [Google Scholar]
- 54. Buck E, Li H, Cerruti M. Surface modification strategies to improve the osseointegration of poly(etheretherketone) and its composites. Macromol Biosci. 2020;20(2):e1900271. [DOI] [PubMed] [Google Scholar]
- 55. Mcgilvray KC, Waldorff EI, Easley J, Seim HB, Zhang N, Linovitz RJ, et al. Evaluation of a polyetheretherketone (PEEK) titanium composite interbody spacer in an ovine lumbar interbody fusion model: biomechanical, microcomputed tomographic, and histologic analyses. Spine J. 2017;17(12):1907–16. [DOI] [PubMed] [Google Scholar]
- 56. Kurtz SM, Devine JN. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials. 2007;28(32):4845–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liao C, Li Y, Tjong SC. Polyetheretherketone and its composites for bone replacement and regeneration. Polymers. 2020;12(12):2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kong F, Nie Z, Liu Z, Hou S, Ji J. (2018) developments of nano‐TiO(2) incorporated hydroxyapatite/PEEK composite strut for cervical reconstruction and interbody fusion after corpectomy with anterior plate fixation. J Photochem Photobiol B. 2018;187:120–5. [DOI] [PubMed] [Google Scholar]
- 59. Wu X, Liu X, Wei J, Ma J, Deng F, Wei S. Nano‐TiO2/PEEK bioactive composite as a bone substitute material: in vitro and in vivo studies. Int J Nanomedicine. 2020;7:1215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou L, Qian Y, Zhu Y, Liu H, Gan K, Guo J. The effect of different surface treatments on the bond strength of PEEK composite materials. Dent Mater. 2014;30(8):e209–15. [DOI] [PubMed] [Google Scholar]
- 61. Ren Y, Sikder P, Lin B, Bhaduri SB. Microwave assisted coating of bioactive amorphous magnesium phosphate (AMP) on polyetheretherketone (PEEK). Mater Sci Eng C. 2018;85:107–13. [DOI] [PubMed] [Google Scholar]
- 62. Bodunde OP, Ikumapayi OM, Akinlabi ET, Oladapo BI, Adeoye AO, Fatoba SO. A futuristic insight into a “nano‐doctor”: a clinical review on medical diagnosis and devices using nanotechnology. Mater Today: Proc. 2021;44:1144–53. [Google Scholar]
- 63. Trungu S, Ricciardi L, Forcato S, Scollato A, Minniti G, Miscusi M, et al. Anterior corpectomy and plating with carbon‐PEEK instrumentation for cervical spinal metastases: clinical and radiological outcomes. J Clin Med. 2021;10(24):5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Trungu S, Ricciardi L, Forcato S, Scollato A, Minniti G, Miscusi M, et al. Percutaneous carbon‐PEEK instrumentation for spine tumors: a prospective observational study. J Neurosurg Sci. 2021. [DOI] [PubMed] [Google Scholar]
- 65. Boriani S, Pipola V, Cecchinato R, Ghermandi R, Tedesco G, Fiore MR, et al. Composite PEEK/carbon fiber rods in the treatment for bone tumors of the cervical spine: a case series. Eur Spine J. 2020;29(12):3229–36. [DOI] [PubMed] [Google Scholar]
- 66. Pipola V, Boriani S, Ghermandi R, Tedesco G, Evangelisti G, Girolami M, et al. Composite peek/carbon fiber pre‐shaped rods and sublaminar bands for posterior stabilization of cervico‐thoracic junction: a novel technique. J Clin Neurosci. 2020;72:429–33. [DOI] [PubMed] [Google Scholar]
- 67. Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014;86(7):3240–53. [DOI] [PubMed] [Google Scholar]
- 68. Wallace N, Schaffer NE, Aleem IS, Patel R. 3D‐printed patient‐specific spine implants: a systematic review. Clin Spine Surg. 2020;33(10):400–7. [DOI] [PubMed] [Google Scholar]
- 69. Fang T, Zhang M, Yan J, Zhao J, Pan W, Wang X, et al. Comparative analysis of 3D‐printed artificial vertebral body versus titanium mesh cage in repairing bone defects following single‐level anterior cervical corpectomy and fusion. Med Sci Monit. 2021;27:e928022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yang X, Wan W, Gong H, Xiao J. Application of individualized 3D‐printed artificial vertebral body for cervicothoracic reconstruction in a six‐level recurrent chordoma. Turk Neurosurg. 2020;30(1):149–55. [DOI] [PubMed] [Google Scholar]
- 71. Xu N, Wei F, Liu X, Jiang L, Cai H, Li Z, et al. Reconstruction of the upper cervical spine using a personalized 3D‐printed vertebral body in an adolescent with Ewing sarcoma. Spine. 2016;41(1):E50–4. [DOI] [PubMed] [Google Scholar]
- 72. Cai H, Liu Z, Wei F, Yu M, Xu N, Li Z. 3D printing in spine surgery. Adv Exp Med Biol. 2018;1093:345–59. [DOI] [PubMed] [Google Scholar]
- 73. Landy BC, Vangordon SB, Mcfetridge PS, et al. Mechanical and in vitro investigation of a porous PEEK foam for medical device implants. J Appl Biomater Funct Mater. 2013;11(1):e35–44. [DOI] [PubMed] [Google Scholar]
- 74. Godlewski B, Dominiak M. Advantages and disadvantages of the use of various types of interbody implants in cervical spine surgery. Critical review of the literature. Ortop Traumatol Rehabil. 2020;22(4):213–20. [DOI] [PubMed] [Google Scholar]
- 75. Spece H, Yu T, Law AW, Marcolongo M, Kurtz SM. 3D printed porous PEEK created via fused filament fabrication for osteoconductive orthopaedic surfaces. J Mech Behav Biomed Mater. 2020;109:103850. [DOI] [PubMed] [Google Scholar]
- 76. Torstrick FB, Lin ASP, Potter D, Safranski DL, Sulchek TA, Gall K, et al. Porous PEEK improves the bone‐implant interface compared to plasma‐sprayed titanium coating on PEEK. Biomaterials. 2018;185:106–16. [DOI] [PubMed] [Google Scholar]
- 77. Vijayavenkataraman S, Kuan LY, Lu WF. 3D‐printed ceramic triply periodic minimal surface structures for design of functionally graded bone implants. Mater Des. 2020;191:108602. [Google Scholar]


