Abstract
Aims:
Although atrial fibrillation (AF) frequently coexists with heart failure with preserved ejection fraction (HFpEF), few data are available evaluating AF-specific care patterns and post-discharge outcomes in patients hospitalized for HFpEF. We evaluated AF-specific medical therapies and post-discharge outcomes among patients hospitalized for heart failure with mildly reduced ejection fraction (HFmrEF) or HFpEF by AF history.
Methods and results:
Trends in AF prevalence were evaluated among patients hospitalized for HFmrEF or HFpEF in the Get With The Guidelines-Heart Failure Registry from 2014 to 2020. Among those with linked Centers for Medicare & Medicaid Services post-discharge data, we assessed associations of AF with 12-month outcomes and determined trends in post-discharge prescriptions. Among 429 464 patients (median age 76 years [interquartile range 65–85], 57% women), 216 486 (50%) had a history of AF. Over time, the proportion of patients with AF increased slightly. Among the 79 895 patients with post-discharge data, AF was independently associated with higher risk of mortality and all-cause readmissions at 12 months, with stronger associations in HFpEF than in HFmrEF (mortality hazard ratio [HR] 1.13, 95% confidence interval [CI] 1.09–1.16 vs. HR 1.03, 95% CI 0.97–1.10; pinteraction = 0.009). Anti-arrhythmic drug use after heart failure hospitalization was low (18%) and increased modestly over time. Amiodarone accounted for 71% of total anti-arrhythmic drug prescriptions. Overall use of anticoagulants after heart failure hospitalization has significantly increased from 52% in 2014 to 61% in 2019, but remained modest.
Conclusion:
Prevalence of AF is rising among patients hospitalized with HFpEF. Those with comorbid AF face elevated post-discharge risks of death and rehospitalization. Current use of pharmacological rhythm control is low.
Keywords: Anti-arrhythmic drug, Atrial fibrillation, Clinical outcomes, Heart failure with preserved ejection fraction, Hospitalization, Registry
Graphical Abstract

Atrial fibrillation in heart failure with mildly reduced and preserved ejection fraction. AF, atrial fibrillation; CI, confidence interval; GWTG-HF, Get With The Guidelines-Heart Failure; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio.
Introduction
Over 60% of individuals with chronic heart failure (HF) with preserved ejection fraction (HFpEF) will have comorbid atrial fibrillation (AF) during their life time,1 and the presence of comorbid AF in chronic HFpEF is associated with worse long-term clinical outcomes across both trial and cohort-based populations.2–7 In fact, AF-predominant HFpEF may represent a distinct high-risk phenotype, characterized by left atrial cardiomyopathy with mechanical derangement, poor haemodynamic performance, and increased congestion.8, 9 Indeed, the presence of AF is more strongly associated with incident HFpEF as compared with HF with reduced ejection fraction (HFrEF).1 Management of AF is now prioritized as one of the few high-level recommendations in latest clinical practice guidelines for HFpEF.10 Despite both the frequency and high-risk nature of the AF-HFpEF syndrome, several questions remain unanswered. First, trends in prevalence of AF in contemporary, hospitalized HFpEF cohorts are not well-defined, and care patterns of AF-specific therapies in this cohort are lacking. Furthermore, the relationships between AF, its timing (i.e. new onset vs. previously diagnosed), and post-discharge clinical outcomes among hospitalized HFpEF populations, as opposed to chronic HFpEF cohorts, are not well understood. Finally, the influence of AF on clinical outcomes by HF subtype, including HF with mildly reduced ejection fraction (HFmrEF; ejection fraction 41–49%) and HFpEF (ejection fraction ≥50%), is not clear. Detailed understanding of AF in hospitalized HFpEF may identify current care gaps and potential population subsets that may benefit most from targeted therapies. As such, we evaluated clinical profiles, AF-specific care, and post-discharge clinical outcomes among patients hospitalized for HFmrEF or HFpEF by AF history in the Get With The Guidelines-Heart Failure (GWTG-HF) Registry (NCT02693509).
Methods
GWTG-HF study design
The GWTG-HF Registry is a hospital-based quality improvement registry of the American Heart Association. Its design and objectives have been previously described.11 GWTG-HF prospectively collects information from participating US centres regarding hospitalizations for HF. Trained personnel abstract data on standardized case report forms that include demographics, comorbidities, vital signs, laboratory data, left ventricular ejection fraction (LVEF), hospital characteristics, and patient disposition. Data were collated using a web-based Patient Management Tool (IQVIA Inc.) through the American Heart Association. All deidentified data are aggregated by the Duke Clinical Research Institute and are independently monitored for quality assurance on an ongoing basis. A waiver for patient informed consent is granted under the Common Rule given that the primary purpose of the registry is for quality improvement. The institutional review boards of each participating hospital have approved the GWTG-HF protocol. This analysis was approved by the Duke Clinical Research Institute Institutional Review Board.
Study population
The study population for the primary analyses consisted of adults hospitalized for a primary diagnosis of HF between 1 January 2014 and 31 December 2020 across 680 fully participating GWTG-HF sites. Patients were excluded based on the following criteria: (i) missing demographic, medical history, LVEF, patient disposition, or common site characteristic data; (ii) diagnosis of HFrEF (LVEF ≤40%); (iii) discharged against medical advice, transferred to acute care facility, or discharged to hospice care; or (iv) history of heart transplant, left ventricular assist device, or listed for heart transplant.
Associations between AF history and post-discharge outcomes and medication patterns for AF-specific therapies were evaluated. To facilitate post-discharge data linkage, the analysis focused on Medicare beneficiaries ≥65 years discharged after hospitalization between 1 January 2014 and 30 September 2019 with available centers for medicare & medicaid (CMS) inpatient claims and Medicare Part A and B Fee-for-Service eligibility at month of discharge and Part D eligibility within 90 days of discharge. If multiple hospitalizations existed for a patient, the first hospitalization was kept as the index hospitalization and other hospitalizations were excluded. AF history was abstracted from case report forms as either history of AF/atrial flutter (AFL), or AF/AFL at presentation or during hospitalization.
Outcomes
Twelve-month outcomes after discharge were assessed in the GTWG-HF cohort with linked CMS claim data. Outcomes of interest were all-cause mortality, all-cause rehospitalization, and cause-specific hospitalization (HF, stroke, primary bleeding readmission, readmission with bleeding as a secondary diagnosis). International Classification of Diseases codes for cause-specific hospitalizations are listed in online supplementary Table Appendix S1.
Ascertainment of atrial fibrillation-specific medical therapy prescription
Among individuals with Medicare Part D data linkage, we identified prescription claims for the following medications from index HF admission to 3 months post-discharge: anticoagulants (apixaban, dabigatran, edoxaban, rivaroxaban, and warfarin) and anti-arrhythmic drugs (amiodarone, dofetilide, dronedarone, flecainide, propafenone, and sotalol). Search terms for each medical therapy are listed in online supplementary Table S2.
Statistical analysis
Demographics, medical history, and laboratory data at hospitalization among individuals with LVEF >40% (HFmrEF and HFpEF cohorts) were compared by AF status using standardized differences, in which standardized difference >10 constitutes meaningful difference. Trends in prevalence of AF in HFmrEF and HFpEF patients from 2014 to 2020 and trends in prevalence of AF-specific medical therapies from 2014 to 2019, were assessed using Cochran–Armitage trend tests. We additionally assessed trends in prevalence of AF in the HFrEF (≤40%) cohort from 2014 to 2020 using Cochran–Armitage trend tests.
Among Medicare Fee-for-Service beneficiaries with linked data, associations between history of AF and unadjusted and adjusted risk of 12-month mortality, 12-month all-cause readmission, and 12-month cause-specific hospitalization (HF, AF, stroke, primary bleeding readmission, readmission with bleeding as a secondary diagnosis) were assessed. For mortality, incidence was calculated based on Kaplan–Meier estimates and test for differences between groups used log-rank tests. Readmission incidence was calculated based on estimates from cumulative incidence function, which accounts for the competing risks of deaths. We also analysed the unadjusted and adjusted associations between AF status and 12-month endpoints in HFmrEF and HFpEF patients using Cox proportional hazards models. Models were adjusted for prespecified patient- and hospital-level clinical and sociodemographic covariates: age, sex, race/ethnicity, insurance, systolic blood pressure at discharge, heart rate at discharge, body mass index, LVEF, anaemia, ischaemic aetiology of HF, prior cerebrovascular accident/transient ischaemic attack, diabetes mellitus, hypertension, hyperlipidaemia, chronic obstructive pulmonary disease/asthma, peripheral vascular disease, dialysis, chronic kidney disease, smoking history, discharge medical therapies (angiotensin-converting enzyme inhibitors/angiotensin receptor blockers/angiotensin receptor–neprilysin inhibitors, beta-blockers, mineralocorticoid receptor antagonists), admission year, hospital geographic region, hospital teaching status, hospital size (# of beds), and hospital rural location. In sensitivity analysis, we evaluated the association of newly diagnosed AF at HF hospitalization with all 12-month outcomes compared with no history of AF using similar covariate adjustments. Finally, we determined whether AF status differentially predicted outcomes by HF subtype (HFmrEF and HFpEF) using interaction terms. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Two-tailed testing was performed and p < 0.05 was considered statistically significant.
Results
Study cohort selection
Of 921 374 hospitalizations between 1 January 2014 and 31 December 2020, a primary analytic cohort consisted of 429 464 hospitalizations from 644 sites (Figure 1). Of the 429 464 hospitalizations, 79 895 patients from 597 sites had Medicare Fee-for-Service linked claims files for analyses of post-discharge outcomes and AF care patterns.
Figure 1.

STROBE diagram for patient inclusion. AMA, against medical advice; EF, ejection fraction; GWTG-HF, Get With The Guidelines-Heart Failure; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LVAD, left ventricular assist device; UTD, unable to determine.
Temporal trends and clinical profiles by atrial fibrillation status
Among 429 464 patients (median age 76 years [interquartile range: 65–85], 57% women, median LVEF 57% [interquartile range: 51–63]), 216 486 (50%) had a history of AF. Prevalence of AF increased from 48.5% in Q1 2014 to 52.1% in Q4 2020 among the 76 886 patients with HFmrEF and modestly increased from 49.7% to 50.1% among the 352 578 patients with HFpEF (both p-trend <0.001; Table 1). Although the overall prevalence of AF was lower in the HFrEF cohort, AF prevalence also increased in a consistent fashion (online supplementary Table S3). In the HFmrEF/HFpEF cohort, patients with a history of AF were older, more likely White, and more likely to have coronary artery disease and ischaemic aetiology of HF (Table 2). Additionally, patients with AF history were less likely to have diabetes and chronic kidney disease. Those with a history of AF had lower systolic blood pressure and body mass index, but had higher N-terminal pro-B-type natriuretic peptide levels and estimated glomerular filtration rate compared with patients without previous AF history. Participants with AF history were less likely to be prescribed angiotensin receptor–neprilysin inhibitors/angiotensin receptor blockers/angiotensin-converting enzyme inhibitors, but were more likely to be prescribed mineralocorticoid receptor antagonists and digoxin. Of patients with AF, 24 053 (11.1%) had newly diagnosed AF at the time of HF hospitalization. Among 105 680 patients with AF and in-hospital procedural data available, 2703 (2.6%) underwent cardioversion and 428 (0.4%) underwent AF ablation during their HF hospitalization.
Table 1.
Trend in atrial fibrillation prevalence in hospitalized heart failure with mildly reduced or preserved ejection fraction from 2014 to 2020
| year | Quarter | Overall | HFmrEF | HFpEF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| AF | Overall | % | AF | Overall | % | AF | Overall | % | ||
|
| ||||||||||
| 2014 | Q1 | 5303 | 10 725 | 49.4% | 950 | 1959 | 48.5% | 4353 | 8766 | 49.7% |
| Q2 | 5033 | 10 303 | 48.8% | 914 | 1847 | 49.5% | 4119 | 8456 | 48.7% | |
| Q3 | 4730 | 9601 | 49.3% | 881 | 1793 | 49.1% | 3849 | 7808 | 49.3% | |
| Q4 | 5468 | 11 096 | 49.3% | 1002 | 2043 | 49.0% | 4466 | 9053 | 49.3% | |
| 2015 | Q1 | 6504 | 12 962 | 50.2% | 1183 | 2350 | 50.3% | 5321 | 10 612 | 50.1% |
| Q2 | 6188 | 12 425 | 49.8% | 1118 | 2253 | 49.6% | 5070 | 10 172 | 49.8% | |
| Q3 | 5712 | 11 377 | 50.2% | 1062 | 2076 | 51.2% | 4650 | 9301 | 50.0% | |
| Q4 | 6321 | 12 751 | 49.6% | 1138 | 2293 | 49.6% | 5183 | 10 458 | 49.6% | |
| 2016 | Q1 | 7740 | 15 418 | 50.2% | 1347 | 2697 | 49.9% | 6393 | 12 721 | 50.3% |
| Q2 | 7163 | 14 428 | 49.6% | 1361 | 2673 | 50.9% | 5802 | 11 755 | 49.4% | |
| Q3 | 6872 | 13 999 | 49.1% | 1188 | 2437 | 48.7% | 5684 | 11 562 | 49.2% | |
| Q4 | 7860 | 15 796 | 49.8% | 1405 | 2896 | 48.5% | 6455 | 12 900 | 50.0% | |
| 2017 | Q1 | 8884 | 17 555 | 50.6% | 1586 | 3134 | 50.6% | 7298 | 14 421 | 50.6% |
| Q2 | 8449 | 16 607 | 50.9% | 1546 | 2990 | 51.7% | 6903 | 13 617 | 50.7% | |
| Q3 | 7967 | 15 752 | 50.6% | 1439 | 2784 | 51.7% | 6528 | 12 968 | 50.3% | |
| Q4 | 8539 | 17 002 | 50.2% | 1548 | 3070 | 50.4% | 6991 | 13 932 | 50.2% | |
| 2018 | Q1 | 9365 | 18 263 | 51.3% | 1672 | 3292 | 50.8% | 7693 | 14 971 | 51.4% |
| Q2 | 8589 | 17 383 | 49.4% | 1548 | 3119 | 49.6% | 7041 | 14 264 | 49.4% | |
| Q3 | 8089 | 16 064 | 50.4% | 1410 | 2816 | 50.1% | 6679 | 13 248 | 50.4% | |
| Q4 | 9021 | 17 916 | 50.4% | 1628 | 3254 | 50.0% | 7393 | 14 662 | 50.4% | |
| 2019 | Q1 | 9924 | 19 320 | 51.4% | 1741 | 3426 | 50.8% | 8183 | 15 894 | 51.5% |
| Q2 | 9604 | 18 691 | 51.4% | 1806 | 3419 | 52.8% | 7798 | 15 272 | 51.1% | |
| Q3 | 9228 | 18 146 | 50.9% | 1639 | 3179 | 51.6% | 7589 | 14 967 | 50.7% | |
| Q4 | 10 094 | 19 401 | 52.0% | 1737 | 3337 | 52.1% | 8357 | 16 064 | 52.0% | |
| 2020 | Q1 | 9996 | 19 402 | 51.5% | 1798 | 3498 | 51.4% | 8198 | 15 904 | 51.5% |
| Q2 | 7616 | 14 922 | 51.0% | 1424 | 2721 | 52.3% | 6192 | 12 201 | 50.7% | |
| Q3 | 8314 | 16 473 | 50.5% | 1458 | 2834 | 51.4% | 6856 | 13 639 | 50.3% | |
| Q4 | 7913 | 15 686 | 50.4% | 1405 | 2696 | 52.1% | 6508 | 12 990 | 50.1% | |
| Trend p | <0.0001 | <0.0001 | <0.0001 | |||||||
| Total | 216 486 | 429 464 | 50.4% | 38 934 | 76 886 | 50.6% | 177 552 | 352 578 | 50.4% | |
AF, atrial fibrillation; HFmrFF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction
Table 2.
Characteristics of patients hospitalized for heart failure with mildly reduced or heart preserved ejection fraction by atrial fibrillation status
| Variable | AF history (n = 216 486) |
No AF (n = 212 978) |
% Std. diff. |
|---|---|---|---|
|
|
|
|
|
| Demographics | |||
| Age, years, median (25th– 75th) | 80 (71 – 87) | 71 (60 – 81) | 62.4 |
| Female sex | 120 743 (55.8) | 124 681 (58.5) | |
| Race/ethnicity | 46.6 | ||
| White | 173 889 (80.3) | 128 219 (60.2) | |
| Black | 22 824 (10.5) | 53 174 (25.0) | |
| Hispanic (any race) | 10 599 (4.9) | 19 952 (9.4) | |
| Asian | 4265 (2.0) | 5024 (2.4) | |
| Other | 4909 (2.3) | 6609 (3.1) | |
| Insurance status | 26.4 | ||
| Medicare– private/HMO/other | 44 589 (20.6) | 37 021 (17.4) | |
| Medicare | 81 509 (37.7) | 67 814 (31.8) | |
| Medicaid | 22 262 (10.3) | 39 146 (18.4) | |
| Private/HMO/other | 57 460 (26.5) | 54 129 (25.4) | |
| No insurance/ND/UTD/missing | 10 666 (4.9) | 14 868 (7.0) | |
| Ejection fraction | |||
| EF, %, median (25th– 75th) | 57 (50 – 62) | 58 (52 – 63) | 7.1 |
| EF group | 0.4 | ||
| HFmrEF (EF 41 – 49%) | 38 934 (18.0) | 37 952 (17.8) | |
| HFpEF (EF >50%) | 177 552 (82.0) | 175 026 (82.2) | |
| AF profile | |||
| Medical history of AF | 187 794 (86.7) | – | – |
| Medical history of atrial flutter | 17 661 (8.2) | – | – |
| Medical history of AF or atrial flutter | 192 433 (88.9) | – | – |
| AF (at presentation or during hospitalization) | 149 178 (69.3) | – | – |
| Atrial flutter (at presentation or during hospitalization) | 19 806 (9.2) | – | – |
| AF (at presentation or during hospitalization) | 156 305 (72.7) | – | – |
| History of AF but no AF at presentation or during hospitalization | 60 181 (27.8) | – | – |
| New-onset AF (AF at presentation or during hospitalization but no history of AF) | 24 053 (11.1) | – | – |
| Medical history | |||
| COPD or asthma | 85 286 (39.4) | 82 426 (38.7) | 1.4 |
| Diabetes mellitus | 93 918 (43.4) | 119 639 (56.2) | 25.8 |
| Hyperlipidaemia | 133 060 (61.5) | 123 754 (58.1) | 6.9 |
| Hypertension | 189 441 (87.5) | 190 232 (89.3) | 5.7 |
| Peripheral vascular disease | 27 359 (12.6) | 25 229 (11.8) | 2.4 |
| CAD | 101 665 (47.0) | 89 211 (41.9) | 10.2 |
| Prior MI | 35 300 (16.3) | 36 195 (17.0) | 1.8 |
| CVA/TIA | 42 042 (19.4) | 33 737 (15.8) | 9.4 |
| Anaemia | 61 524 (28.4) | 60 304 (28.3) | 0.2 |
| Dialysis, chronic | 7070 (3.3) | 16 207 (7.6) | 19.2 |
| Renal insufficiency, chronic (SCr >2.0 mg/dl) | 54 996 (25.4) | 66 336 (31.1) | 12.8 |
| Ischaemic aetiology of HF | 109 540 (50.6) | 97 133 (45.6) | 10.0 |
| Smoking | 18 292 (8.5) | 36 059 (17.1) | 25.9 |
| Discharge vital signs, laboratory measurements, and medications | |||
| Heart rate, bpm | 75 (66 – 86) | 74 (66 – 84) | 5.6 |
| SBP, mmHg | 124 (111 – 139) | 132 (118–148) | 36.2 |
| BMI, kg/m2 | 29.4 (24.6 – 36.1) | 31.2 (25.5 – 38.9) | 17.6 |
| Sodium, mEq/L | 139 (136 – 141) | 139 (136 – 141) | 1.1 |
| eGFR, ml/min/1.73 m2 | 49.8 (33.5 – 69.6) | 46.2 (25.9 – 70.8) | 12.4 |
| Potassium, mEq/L | 4.0 (3.7 – 4.3) | 4.1 (3.8 – 4.4) | 16.4 |
| NT-proBNP, pg/ml | 3938 (2006 – 7972) | 3321 (1285 – 8913) | 13.2 |
| SCr, mg/dl | 1.3 (0.9 – 1.7) | 1.4 (1.0–2.2) | 18.8 |
| Haemoglobin, g/dl (admission data only) | 11.3 (9.9 – 12.8) | 11.1 (9.6 – 12.7) | 8.2 |
| ACEi/ARB or ARNI | 76 729 (37.6) | 90 795 (44.8) | 15.4 |
| Beta-blocker | 161 183 (77.8) | 157 677 (76.2) | 8.4 |
| Aldosterone antagonist | 30 068 (14.7) | 24 275 (11.9) | 10.4 |
| Digoxina | 8267 (9.9) | 903 (1.1) | 39.3 |
| Hospital characteristics | |||
| No. of beds, median (25th– 75th) | 361 (226 – 537) | 376 (232 – 539) | 7.5 |
| Geographic region | 12.6 | ||
| West | 31 784 (14.7) | 33 003 (15.5) | |
| South | 62 672 (28.9) | 72 134 (33.9) | |
| Midwest | 55 291 (25.5) | 51 828 (24.3) | |
| Northeast | 66 739 (30.8) | 56 013 (26.3) | |
| Rural location | 7088 (3.3) | 7206 (3.4) | 0.6 |
| Teaching status | 165 740 (76.6) | 163 807 (76.9) | 0.8 |
Values are mean ± standard deviation, n (%), or median (interquartile range).
ACEi, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor– neprilysin inhibitor; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cardiovascular accident; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HMO, Health Maintenance Organization; HFpEF, heart failure with preserved ejection fraction; MI, myocardial infarction; ND, not defined; NT-proBNP, N-terminal pro-B-type natriuretic peptide; SBP, systolic blood pressure; SCr, serum creatinine; TIA, transient ischaemic attack; UTD, unable to determine.
Data available in 265 810 (61.9%) of participants.
Atrial fibrillation status and 1-year clinical outcomes
Of the 79 895 patients with linked post-discharge data (median age 81 years [interquartile range: 74–87], 63% women, median LVEF 58% [interquartile range: 53–63]), 45 104 patients (56%) had a history of AF. The variation in demographics, clinical characteristics, and laboratory markers by AF status were consistent with the primary analytic cohort (online supplementary Table S4).
At 12-month follow-up, the cumulative incidence of all-cause mortality was higher in the AF group compared with those without AF history (15 121 [35%] vs. 9715 [29%], p < 0.0001; Figure 2). Similarly, the cumulative incidence of all-cause readmissions and readmission subtypes (HF, AF, stroke, primary and secondary bleeding) were higher among individuals with AF history compared with those without AF (Figure 2), and after accounting for key patient- and hospital-level clinical and sociodemographic covariates, history of AF was independently associated with higher all-cause mortality, all-cause readmissions, and all cause-specific readmissions (Figure 2). The associations of AF with all-cause mortality, HF readmission, and readmission for bleeding were stronger in the HFpEF compared with the HFmrEF subgroup (Figure 3; pinteraction = 0.009 for all-cause mortality, pinteraction = 0.01 for HF readmission, pinteraction = 0.006 for secondary bleeding readmission). Associations of AF with all-cause readmission, AF readmission, stroke readmission, and primary bleeding readmission were consistent across LVEF subgroups. In sensitivity analysis, the associations of newly diagnosed AF at time of HFmrEF/HFpEF hospitalization with all-cause mortality, all-cause readmission, and cause-specific readmissions compared with no history of AF were consistent with the main outcome analyses (online supplementary Table S5).
Figure 2.
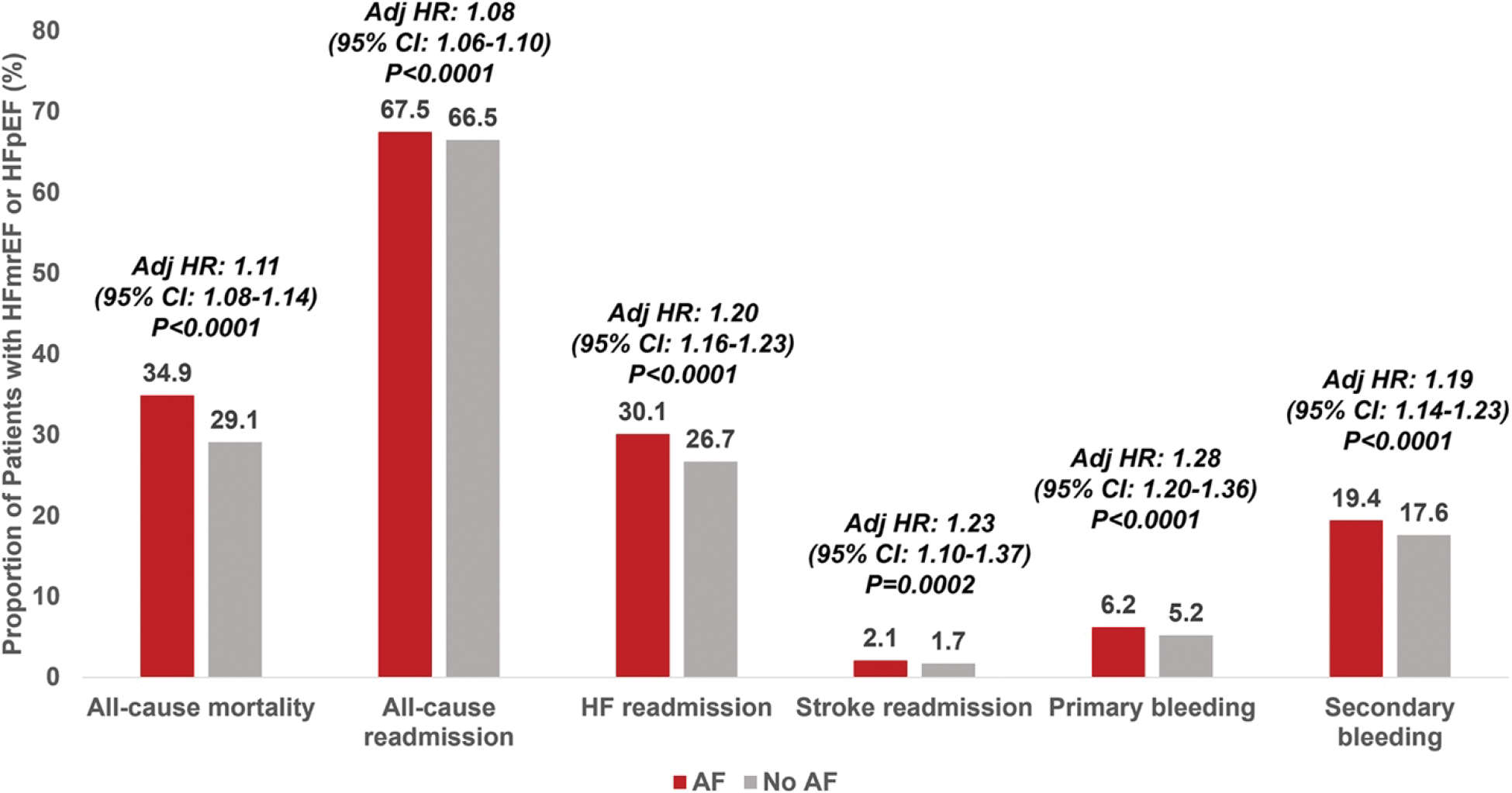
Associations of atrial fibrillation (AF) with 12-month clinical outcomes after hospitalization for heart failure with mildly reduced ejection fraction (HFmrEF) or heart failure with preserved ejection fraction (HFpEF). CI, confidence interval; HF, heart failure; HR, hazard ratio.
Figure 3.
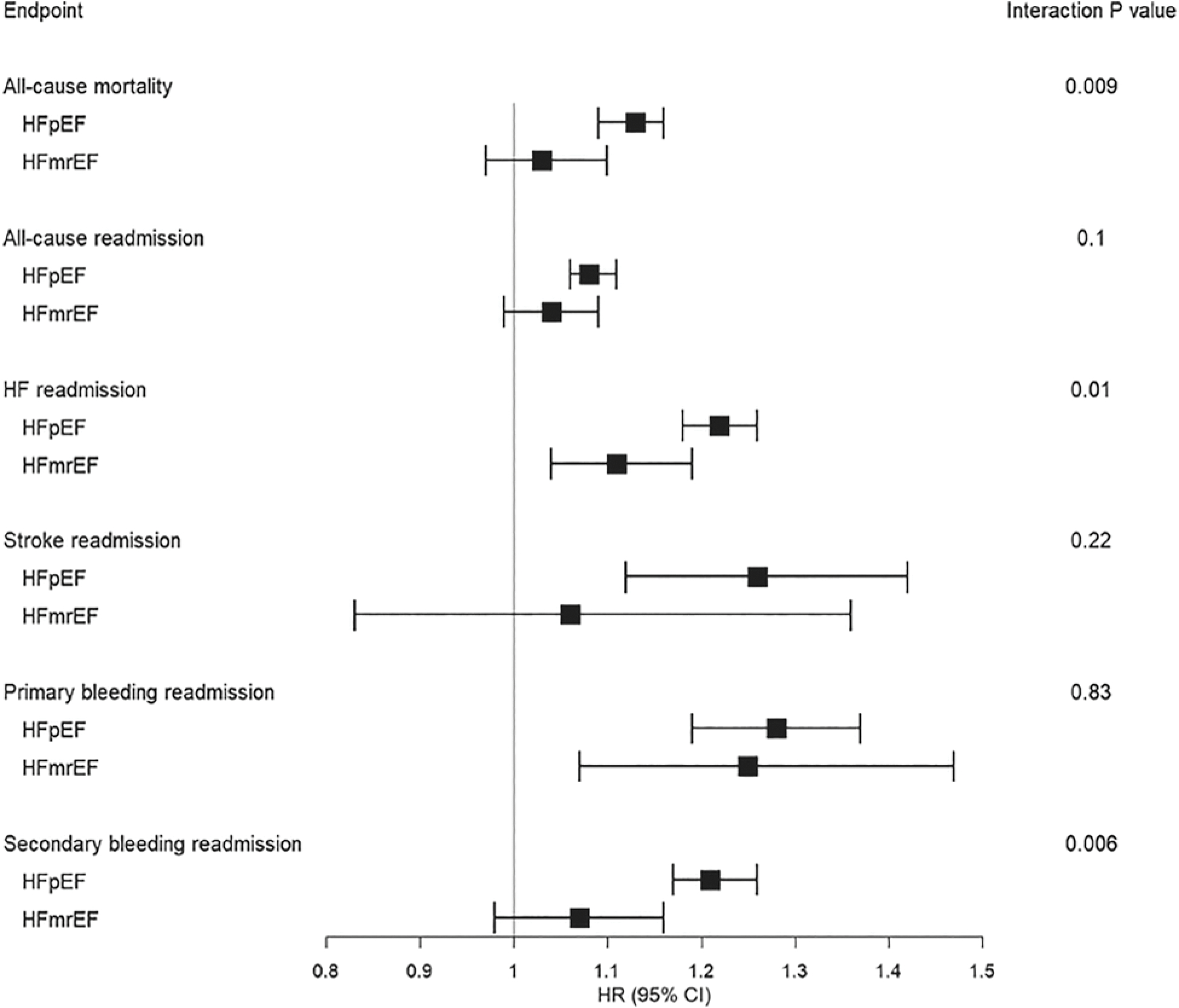
Associations of atrial fibrillation with clinical outcomes by heart failure (HF) subtype. CI, confidence interval; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio.
Trends in post-hospitalization atrial fibrillation-specific medical prescriptions
From 2014 to 2019, 18% of patients were prescribed an anti-arrhythmic drug within 3 months after hospitalization for HFmrEF or HFpEF (Figure 4). Amiodarone accounted for 71% of total anti-arrhythmic drug prescriptions. There were statistically significant increases in prescriptions for amiodarone, dofetilide, and flecainide from 2014 to 2019, but overall use remained low. Use of dronedarone, sotalol, and propafenone was low and remained stable over the study period. There were more marked changes in prescriptions of oral anticoagulants over the study period. Overall use of any oral anticoagulant increased significantly from 2014 to 2019 (52% vs. 61%, p < 0.01). From 2014 to 2019, the use of warfarin significantly decreased, which was mirrored by an increase in prescriptions for factor Xa inhibitors during the same timeframe (Figure 5). Direct thrombin inhibitor use was low in 2014, with a significant decrease in use over time.
Figure 4.

Trends in prescriptions for atrial fibrillation-specific medical therapies after heart failure hospitalization from 2014 to 2019. HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction.
Figure 5.

Trends in prescriptions for oral anticoagulants after heart failure hospitalization from 2014 to 2019. HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction.
Discussion
In this contemporary, US-based analysis of over 400 000 hospitalizations for HFmrEF or HFmrEF across over 600 hospitals, we identify a number of key findings (Graphical Abstract): (i) approximately 50% of patients have AF, which was consistent across HF subtypes; (ii) prevalence of AF at or during the time of HFmrEF/HFpEF hospitalization is rising slightly over time; (iii) history of AF and new-onset AF are significantly and independently associated with mortality and a broad-range of clinical events requiring readmission, particularly for HFpEF; (iv) anti-arrhythmic drug use in patients with AF after HFmrEF/HFpEF hospitalization is relatively low, has increased only modestly over time, and is largely limited to amiodarone; and (v) overall use of anticoagulants among patients with AF after HF hospitalization has significantly increased from 2014 to 2019, but remain only modest in current care; these patterns have been driven by increased prescription of factor Xa inhibitors, despite decreases in warfarin and direct thrombin inhibitor use.
Atrial fibrillation and HFpEF frequently co-exist due to both shared risk factors and bi-directional pathophysiologic links, in which each syndrome may mechanistically contribute to risk for the other. Previous investigations have demonstrated that the prevalence of AF in chronic HFpEF populations ranges from ∼30% to >60%.1, 6, 7, 12, 13 Similarly, in studies of hospitalized HFpEF, the prevalence of AF in HFpEF is approximately 40–60%.14–16 These estimates vary depending upon the population under study (i.e. clinical trial, prospective cohort, or hospital-based) and their respective clinical profiles. AF has also been associated with worse long-term clinical outcomes in both chronic HFpEF cohorts and smaller cohorts of hospitalized HFpEF.5, 6, 14, 16 Despite these existing data, previous studies of AF in hospitalized HFpEF were of smaller sample size, less contemporary, and lacked data surrounding both care patterns of AF-specific medical therapies and timing of AF diagnosis. As such, our current investigation furthers the understanding of the burden and implications of AF in hospitalized HFpEF in a large, contemporary registry that includes granular information regarding AF medical therapy use over time, cause-specific readmission data, and timing of AF diagnosis.
In the present study, AF history was associated with increased risk of several long-term, post-discharge clinical outcomes, particularly for the HFpEF cohort. Newly diagnosed AF at the time of/during hospitalization occurred in 1 in 10 patients and was also independently linked with adverse post-discharge prognosis. The relationship between AF and clinical outcomes by HF subtype has varied in prior studies. While chronic AF-HFrEF was associated with a higher risk of mortality compared with chronic AF-HFpEF in the Framingham Heart Study,1 a larger, European registry of chronic HF demonstrated that AF was uniquely associated with increased risk of HF hospitalization and death in HFpEF, but not in HFmrEF or HFrEF.5 In a mixed cohort of chronic and hospitalized HF within the Swedish Heart Failure Registry, AF was consistently associated with worse outcomes across the LVEF spectrum.14 By leveraging a large cohort of only hospitalized HF linked to long-term outcome data, we demonstrate a stronger relationship between AF and several outcomes, including all-cause mortality, in HFpEF compared with HFmrEF. Through cause-specific readmission data, we further identify that AF may be more strongly associated with increased risk of HF readmissions in the HFpEF cohort than HFmrEF, but not readmissions due to stroke. These findings suggest that AF, as a result of progressive atrial cardiomyopathy, may contribute to promotion of congestion and progression of HF specifically in HFpEF, and are consistent with the overarching data that AF-predominant HFpEF is a distinct phenotype characterized by increased congestion and poor haemodynamic reserve.17–19
In light of the frequency and morbid prognosis of the AF-HFpEF syndrome, the 2022 AHA/ACC/Heart Failure Society of America support management of AF in HFpEF as a class 2a recommendation.10 Although dedicated clinical trials of rhythm control therapies in AF-HFpEF are lacking, there is suggestion of potential benefit of such therapies in certain clinical circumstances. A post-hoc analysis of the EAST-AFNET 4 trial (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) demonstrated that early comprehensive rhythm control reduced cardiovascular events compared with guideline-based symptom-directed rhythm control on top of rate control among 798 individuals with early AF and concomitant HF, of which 83% had HFmrEF or HFpEF.20 Notably, early AF was defined as AF diagnosed within 1 year of trial enrolment. Additional evidence of benefit was suggested in the ATHENA trial, in which dronedarone reduced risk of death or cardiovascular hospitalization compared with placebo among 534 patients with HFmrEF/HFpEF.21 Finally, among 778 patients with HF enrolled in the CABANA trial (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation), of which the majority had HFmrEF/HFpEF, catheter ablation of AF led to reduction in all-cause mortality and improvement in quality of life.22
In our study, newly diagnosed AF at time of HF hospitalization was significantly associated with high risk of long-term clinical outcomes and may serve to benefit most from early rhythm control strategies. However, overall care patterns suggest low rates of anti-arrhythmic drug prescription in AF patients post-hospitalization for HFpEF and HFmrEF. In an older analysis of GWTG-HF from 2008 to 2014, the prevalence of rhythm control was ~12% in AF-HFpEF.23 Our contemporary study demonstrates only a modest increase from 2014 to 2019, in which ~18% of patients with AF-HFpEF received anti-arrhythmic drug prescription. While amiodarone, dofetilide and flecainide prescriptions increased slightly during this time period, dronedarone, propafenone and sotalol use remained low over time. There are several potential reasons to explain the low use of anti-arrhythmic drugs in the AF-HFpEF population. Although early rhythm control of AF regardless of AF-specific symptoms was associated with reduction in cardiovascular events in the EAST-AFNET 4 trial, decisions for rhythm control therapy are traditionally based on the presence of AF-related symptoms, which may be difficult to distinguish from HFpEF-related symptoms. In clinical practice, the absence of definitive AF symptoms may result in deferral of rhythm control therapies. Despite the fact that dronedarone may be well-tolerated in HFmrEF/HFpEF,21 it is possible that its use has remained low due to results of the ANDROMEDA trial, in which dronedarone was associated with increased early mortality in unstable HF patients (recently hospitalized or New York Heart Association class IV), with severely reduced ejection fraction20, 24 and where AF was not an inclusion criteria. Some anti-arrhythmic drugs, including dofetilide and sotalol, have strict contraindications based upon renal function, which may have influenced their use, whereas flecainide and propafenone are not recommended in patients with AF and structural heart disease.25 Finally, many patients with AF in HFpEF have chronotropic incompetence,26 and the β-blocking effects of some class III anti-arrhythmic drugs may result in hesitancy to prescribe this class in HFpEF. Taken together, further investigation is required to understand clinical reasons for low use of anti-arrhythmic drug use in the AF-HFpEF population.
Our contemporary analysis demonstrated an overall increase in anticoagulant prescriptions over time. Reassuringly, the increase in factor Xa prescriptions was greater than temporal decrease in warfarin use, resulting in an overall larger proportion of patients receiving any anticoagulant. However, nearly 40% individuals with AF and HFmrEF/HFpEF did not receive a prescription for anticoagulation within 90 days of HF hospitalization in 2019. These findings contrast with a lower risk cohort of patients with AF alone, as a GWTG-AF registry analysis demonstrated that >96% of such treatment-eligible patients received therapy.27 The heightened stroke risk among individuals with HFmrEF/HFpEF calls for further investigation to account for this apparent risk-treatment gap and to identify strategies to improve guideline-concordant anticoagulant prescription in this population.28 These findings may be in part related to the older age of our cohort and resultant higher perceived bleeding risks.
Limitations
Ascertainment of AF was based upon case report forms filled out by trained study personnel, and electrocardiograms at admission were not adjudicated. AF type (paroxysmal, persistent, permanent) was not captured in GWTG-HF, which limits understanding of anti-arrhythmic drug prescription by AF type. Participation in GWTG-HF is voluntary, which may affect the generalizability of our findings to all treatment settings. Our analyses of post-discharge outcomes and prescriptions were limited to Medicare Fee-for-Service beneficiaries, and these findings may not be consistent across other populations of varying age, insurance status, and comorbidity burden. Dose of anti-arrhythmic drugs was not captured, and anti-arrhythmic drug prescription for ventricular arrhythmias in patients with AF not targeted for rhythm control could not be differentiated. Readmission data relied upon ICD coding and causes of hospitalizations were not independently adjudicated. However, data suggests that ICD codes for cause-specific hospitalizations may be valid in this setting and are currently used as the primary mechanism of event capture for a number of CMS programmes. Data regarding post-discharge AF ablation were not captured as part of this analysis. Finally, due to the deidentified nature of GWTG-HF, this analysis leveraged distinct hospitalizations rather than unique patients, and therefore some patients may have contributed to more than one hospitalization. The CMS linked analyses, however, represent unique patients.
Conclusion
In this contemporary cohort of hospitalized HFmrEF or HFpEF, AF was highly prevalent, and has increased slightly over time. Both history of AF and newly diagnosed AF were significantly associated with several long-term clinical outcomes, including all-cause mortality, particularly for HFpEF. Anti-arrhythmic drug use in patients with AF after HFmrEF/HFpEF hospitalization is relatively low, has increased only modestly over time, and is largely limited to amiodarone use. Although use of anticoagulants has increased over time, nearly 40% of individuals with AF and HFmrEF/HFpEF do not receive anticoagulation within 90 days of discharge. In aggregate, further efforts are required to mitigate risk among patients with AF and HFmrEF/HFpEF, who remain at high risk for adverse clinical outcomes, particularly after HF hospitalization.
Supplementary Material
Funding
This study received support from Sanofi (https://www.sanofi.com) in the form of an investigator-sponsored study (number GM-2021-13548) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study. The Get With The Guidelines®–Heart Failure (GWTG-HF) programme is provided by the American Heart Association. GWTG-HF is sponsored, in part, by Novartis, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Bayer, Tylenol and Alnylam Pharmaceuticals.
Footnotes
Conflict of interest: R.B.P. has received research support from the National Institutes of Health. S.J.G. has received research support from the Duke University Department of Medicine Chair’s Research Award, American Heart Association (#929502), National Heart Lung and Blood Institute, Amgen, AstraZeneca, Bristol Myers Squibb, Cytokinetics, Merck, Novartis, Pfizer, and Sanofi; has served on advisory boards for Amgen, AstraZeneca, Boehringer Ingelheim/Lilly, Bristol Myers Squibb, Cytokinetics, Roche Diagnostics, and Sanofi; serves as a consultant for Amgen, Bayer, Boehringer Ingelheim/Lilly, Bristol Myers Squibb, CSL Vifor, Merck, PharmaIN, Roche Diagnostics, Sanofi, Tricog Health, and Urovant Pharmaceuticals; and has received speaker fees from Boehringer Ingelheim and Cytokinetics. G.C.F. has consulted for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Eli Lilly, Jannsen, Medtronic, Merck, and Novartis. M.V. has received research grant support or served on advisory boards for American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health, speaker engagements with AstraZeneca, Novartis, and Roche Diagnostics, and participates in clinical trial committees for studies sponsored by Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics. All other authors have nothing to disclose.
References
- 1.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel RB, Vaduganathan M, Rikhi A, Chakraborty H, Greene SJ, Hernandez AF, et al. History of atrial fibrillation and trajectory of decongestion in acute heart failure. JACC Heart Fail. 2019;7:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cikes M, Planinc I, Claggett B, Cunningham J, Milicic D, Sweitzer N, et al. Atrial fibrillation in heart failure with preserved ejection fraction: the PARAGON-HF trial. JACC Heart Fail. 2022;10:336–46. [DOI] [PubMed] [Google Scholar]
- 4.Butt JH, Kondo T, Jhund PS, Comin-Colet J, de Boer RA, Desai AS, et al. Atrial fibrillation and dapagliflozin efficacy in patients with preserved or mildly reduced ejection fraction. J Am Coll Cardiol. 2022;80:1705–17. [DOI] [PubMed] [Google Scholar]
- 5.Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, et al. ; ESC-HFA HF Long-Term Registry Investigators. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J. 2018;39:4277–84. [DOI] [PubMed] [Google Scholar]
- 6.Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. 2013;128:1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cikes M, Claggett B, Shah AM, Desai AS, Lewis EF, Shah SJ, et al. Atrial fibrillation in heart failure with preserved ejection fraction: the TOPCAT trial. JACC Heart Fail. 2018;6:689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel RB, Lam CSP, Svedlund S, Saraste A, Hage C, Tan RS, et al. Disproportionate left atrial myopathy in heart failure with preserved ejection fraction among participants of the PROMIS-HFpEF study. Sci Rep. 2021;11:4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. 2020;76:1051–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e263–421. [DOI] [PubMed] [Google Scholar]
- 11.Hong Y, LaBresh KA. Overview of the American Heart Association “Get With The Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol. 2006;5:179–86. [DOI] [PubMed] [Google Scholar]
- 12.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. ; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N EnglJ Med. 2021;385:1451–61.34449189 [Google Scholar]
- 13.Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. ; PARAGON-HF Investigators and Committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–20. [DOI] [PubMed] [Google Scholar]
- 14.Sartipy U, Dahlstrom U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail. 2017;5:565–74. [DOI] [PubMed] [Google Scholar]
- 15.Goyal P, Almarzooq ZI, Cheung J, Kamel H, Krishnan U, Feldman DN, et al. Atrial fibrillation and heart failure with preserved ejection fraction: insights on a unique clinical phenotype from a nationally-representative United States cohort. Int J Cardiol. 2018;266:112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Son MK, Park JJ, Lim NK, Kim WH, Choi DJ. Impact of atrial fibrillation in patients with heart failure and reduced, mid-range or preserved ejection fraction. Heart. 2020;106:1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel RB, Shah SJ. Therapeutic targeting of left atrial myopathy in atrial fibrillation and heart failure with preserved ejection fraction. JAMA Cardiol. 2020;5:497–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Packer M, Lam CSP, Lund LH, Redfield MM. Interdependence of atrial fibrillation and heart failure with a preserved ejection fraction reflects a common underlying atrial and ventricular myopathy. Circulation. 2020;141:4–6. [DOI] [PubMed] [Google Scholar]
- 19.Lam CS, Rienstra M, Tay WT, Liu LCY, Hummel YM, van der Meer P, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail. 2017;5:92–8. [DOI] [PubMed] [Google Scholar]
- 20.Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt G, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. 2021;144:845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaduganathan M, Piccini JP, Camm AJ, Crijns HJGM, Anker SD, Butler J, et al. Dronedarone for the treatment of atrial fibrillation with concomitant heart failure with preserved and mildly reduced ejection fraction: a post-hoc analysis of the ATHENA trial. Eur J Heart Fail. 2022;24:1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Packer DL, Piccini JP, Monahan KH, al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. ; CABANA Investigators. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly JP, DeVore AD, Wu J, Hammill BG, Sharma A, Cooper LB, et al. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from Get With The Guidelines-Heart Failure. J Am Heart Assoc. 2019;8:e011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kober L, Torp-Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, et al. ; Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–87. [DOI] [PubMed] [Google Scholar]
- 25.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS Fuideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–76. [DOI] [PubMed] [Google Scholar]
- 26.Palau P, Seller J, Dominguez E, Sastre C, Ramón JM, de la Espriella R, et al. Effect of beta-blocker withdrawal on functional capacity in heart failure and preserved ejection fraction. J Am Coll Cardiol. 2021;78:2042–56. [DOI] [PubMed] [Google Scholar]
- 27.Piccini JP, Xu H, Cox M, Matsouaka RA, Fonarow GC, Butler J, et al. ; Get With The Guidelines-AFIB Clinical Working Group and Hospitals. Adherence to guideline-directed stroke prevention therapy for atrial fibrillation is achievable. Circulation. 2019;139:1497–506. [DOI] [PubMed] [Google Scholar]
- 28.Uhm JS, Kim J, Yu HT, Kim TH, Lee SR, Cha MJ, et al. Stroke and systemic embolism in patients with atrial fibrillation and heart failure according to heart failure type. ESC Heart Fail. 2021;8:1582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


