ABSTRACT
Background
The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial assessed dapagliflozin versus placebo, in addition to standard therapy, in patients with chronic kidney disease (CKD) and albuminuria, and was terminated prematurely due to overwhelming efficacy. The study objective was to model the long-term clinical outcomes of DAPA-CKD beyond the trial follow-up.
Methods
A Markov model extrapolated event incidence per 1000 patients and CKD progression rates for patients receiving dapagliflozin or placebo over a 10-year time horizon. We derived treatment-specific CKD stage transition matrices using DAPA-CKD trial data. We extrapolated relevant efficacy endpoints using parametric survival equations for all-cause mortality and generalized estimating equations for recurrent events.
Results
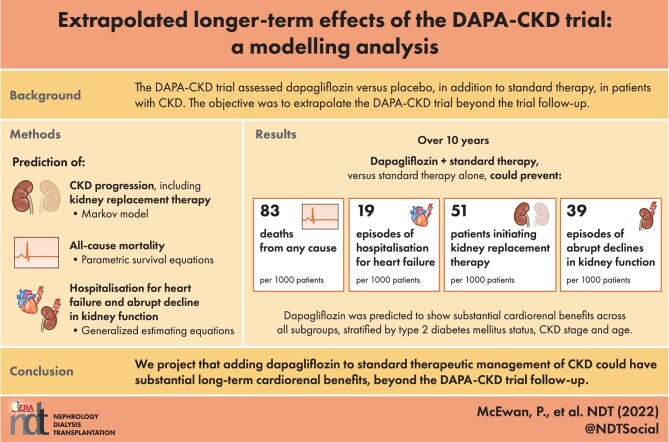
When extrapolated over a 10-year period, patients randomized to dapagliflozin spent more time in CKD stages 1–3 and less in stages 4–5 than placebo [0.65 (95% CrI 0.41, 0.90) and –0.23 (95% CrI -0.45, 0.00) years per patient, respectively]. Dapagliflozin prevented an estimated 83 deaths and 51 patients initiating kidney replacement therapy per 1000 patients over 10 years. Predicted rates of hospitalized heart failure and abrupt declines in kidney function were reduced (19 and 39 estimated events per 1000 patients, respectively).
Conclusions
Adding dapagliflozin to standard therapeutic management of CKD is expected to have long-term cardiorenal benefit beyond what has been demonstrated in the DAPA-CKD trial, with patients predicted to live longer with fewer complications.
Keywords: chronic kidney disease, dapagliflozin, dialysis, kidney transplantation, SGLT2 inhibitor
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
An unmet need exists for therapies providing cardiorenal protective efficacy in addition to standard regimens in chronic kidney disease (CKD). The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial demonstrated dapagliflozin is efficacious in slowing CKD progression and reducing adverse outcomes.
What this study adds?
Using a Markov model, we project over 10 years that patients treated with dapagliflozin and standard of care could experience slower rates of CKD progression, longer life expectancy and fewer adverse events beyond the DAPA-CKD trial follow-up period, versus patients on standard of care alone.
What impact this may have on practice or policy?
The projected cardiorenal benefits are likely to translate to slowing progression to dialysis and preventing adverse disease-related outcomes, which in turn may improve health-related quality of life, and lessen the burden of CKD on healthcare budgets and resources.
INTRODUCTION
The prevalence of chronic kidney disease (CKD) is considered to be 11%–14% worldwide [1, 2]. With ageing populations and the rising prevalence of comorbidities such as hypertension and type 2 diabetes mellitus (T2DM) [3], CKD is projected to affect 16% of adults by 2030 [4]. CKD is associated with numerous complications, including higher rates of atherosclerotic events (including myocardial infarction, ischaemic stroke and peripheral arterial events) and hospitalizations for heart failure. The risk of death and adverse cardiovascular (CV) events increases significantly as kidney function declines. Impaired kidney function also subsequently increases the risk of episodes of abrupt declines in kidney function which, in turn, can accelerate CKD progression [5]. Patients on dialysis have a life expectancy less than one-third that of patients without end-stage kidney disease (ESKD) [4], and the 5-year survival rate on dialysis is less than 50% in the USA [6]. Patients with CKD commonly experience impaired physical and cognitive function, along with diminished health-related quality of life (HRQoL), all of which tend to deteriorate upon progression to kidney failure despite, or perhaps related to, initiation of dialysis [7]. Hence, there are healthcare benefits to be gained from halting or delaying CKD progression.
CKD requires effective management of risk factors to avoid disease progression, including hypertension and albuminuria [8–10]. To date, angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers (ARBs) are standard of care and the most effective proven treatment option in the management of CKD [11]. However, even under optimal doses of these medications, many individuals will progress towards more advanced CKD [11]. Consequently, there is an unmet need for therapies demonstrating additive cardiorenal protective efficacy, to slow CKD progression and reduce the rate of disease-related adverse cardiorenal outcomes.
Sodium-glucose transport protein 2 (SGLT2) inhibitors are a well-established treatment option for patients with T2DM, with trials having demonstrated considerable additional therapeutic value to patients with heart failure [12, 13] and diabetic kidney disease [14–16]. Recent evidence from the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial demonstrated the cardiorenal protective effects of dapagliflozin in patients with CKD and albuminuria, showing the value of SGLT2 inhibitors in patients with and without T2DM [17].
In the DAPA-CKD trial, patients received either dapagliflozin or placebo, in addition to standard therapy (ACE inhibitors or ARBs in all patients if tolerated) [17]. The trial enrolled 4304 patients, showing a reduction in the primary composite endpoint of worsening kidney function (defined as a composite of a sustained ≥50% estimated glomerular filtration rate (eGFR) decline, onset of ESKD or death from CV or kidney-related cause) by 39% when treated with dapagliflozin compared with placebo [18]. Results were consistent in patients with and without T2DM [18]. Outcomes in DAPA-CKD were evaluated after a median follow up of 2.4 years; however, in clinical practice, patients are typically treated for a longer period.
The objective of this study was to estimate longer-term effects of treatment with dapagliflozin, in addition to standard therapy, by extrapolating DAPA-CKD clinical data using decision analytical modelling.
MATERIALS AND METHODS
DAPA-CKD (NCT03036150) was an international, event-driven, randomized, double-blind, parallel group, placebo-controlled study, conducted in patients with stages 2–4 CKD (with/without T2DM) and albuminuria, defined as urinary–albumin creatinine ratio (UACR) from 200 to 5000 mg/g. The trial evaluated the efficacy and safety of dapagliflozin, 10 mg daily, versus placebo when used in addition to standard therapy. The trial design, baseline characteristics and primary results have been previously published [17, 19, 20].
Patient population
The modelled cohort mirrored the DAPA-CKD clinical trial population: adult patients with eGFR ≥25 mL/min/1.73 m2 and ≤75 mL/min/1.73 m2, and UACR ≥200 mg/g and ≤5000 mg/g, receiving stable treatment with ACE inhibitors or ARBs if tolerated. The patient profile incorporated into the model is stated in the Supplementary data (Table S1).
Model structure
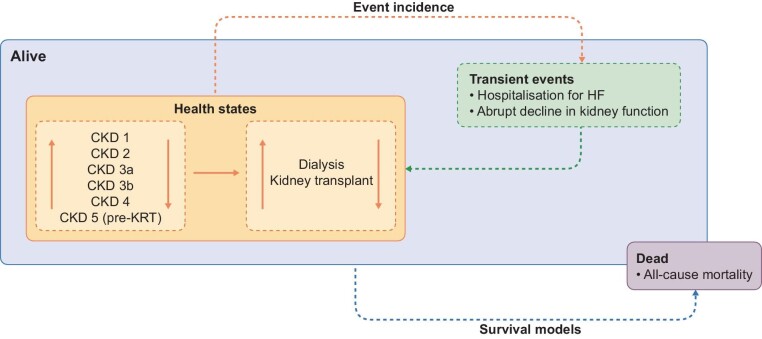
We used a Markov state transition model to extrapolate CKD progression and the incidence of adverse outcomes over 10 years for patients receiving treatment with dapagliflozin or placebo, based on observations from DAPA-CKD [21, 22]. Disease progression was modelled by the transition between discrete health states representing CKD stages (defined by eGFR) and on to kidney replacement therapy (KRT), comprised of dialysis or kidney transplant. Since CKD is associated with multiple comorbidities and reduced life-expectancy, we modelled the effect of dapagliflozin compared with placebo on adverse outcomes and mortality. These included all-cause mortality (ACM), hospitalization for heart failure (HHF) and abrupt decline in kidney function (pre-defined endpoint: doubling of serum creatinine between two visits), in addition to KRT incidence. We calculated event incidences and the mean time in each CKD stage for dapagliflozin and placebo groups. Figure 1 presents the structure of the model and full technical specifications of the model are published elsewhere [21, 22].
Figure 1:
Model schematic.
CKD progression
To demonstrate the treatment effect of dapagliflozin on CKD progression, we derived transition matrices for each treatment group, in which patients can move between CKD stages 1–5. We derived treatment-specific transition probabilities, as well as the incidence of KRT (dialysis or kidney transplantation), from the monthly transition count data collected in the DAPA-CKD trial. In the trial, there was an acute decrease in eGFR associated with the initiation of dapagliflozin treatment; as such, the first four months of follow-up in DAPA-CKD were used to generate independent transition matrices. After 4 months, we calculated the rate of CKD progression assuming a linear eGFR decline.
There were insufficient observed data in the DAPA-CKD trial to inform transition probabilities in post-KRT health states. Therefore, we incorporated published estimates of the probability of receiving a kidney transplant in patients receiving dialysis, the probability of transplant failure, and mortality in patients receiving KRT, derived in a recent systematic literature review [23].
All-cause mortality
We employed parametric survival equations to extrapolate ACM data beyond the end of trial follow-up, in accordance with guidelines set out by the National Institute for Health and Care Excellence (NICE) in the UK for the analysis of survival data alongside clinical trials [24]. The rationale for fitting parametric functions rather than using Kaplan–Meier data directly is to accommodate potential differences in follow-up time available across subgroups and, crucially, to allow extrapolation beyond the trial period [25, 26].
We adjusted models for baseline patient characteristics, current CKD stage (reflecting elevated risk upon disease progression) and randomized treatment. We conducted survival analysis from an intention-to-treat perspective. Within-trial goodness of fit of survival functions (exponential, gamma, Gompertz, log-logistic, lognormal and Weibull) were evaluated through minimizing the Akaike Information Criterion [21], in addition to clinical expert opinion [27]. A Gompertz distribution was considered the most appropriate for mortality and was incorporated into the base case analysis.
Incidence of HHF and abrupt declines in kidney function
The model is subject to the memoryless principle of a Markov model, i.e. a patient's simulated history prior to reaching their current health state is not considered in the calculation of subsequent transitions. However, patients can experience HHF or abrupt declines in kidney function more than once within the model simulation. Therefore, we employed negative binomial regression models to estimate the incidence of HHF and abrupt decline in kidney function, using generalized estimating equations adjusted for patient baseline characteristics, CKD stage and dapagliflozin use.
Model output
The cumulative incidence of KRT, ACM, HHF and abrupt decline in kidney function, per 1000 patients treated with standard therapy plus dapagliflozin or placebo over the 10-year period, were stratified by prognostic baseline characteristics, including CKD stage, age and presence of T2DM. We further stratified these incidences into a ‘heatmap’ grid of event incidences across subgroups defined by a combination of these prognostic factors.
Scenario analysis
To quantify additional interactions among prognostic characteristics on patient outcomes, we also stratified subgroups by prior history of heart failure (HF) at baseline into heatmap grids of event incidences in addition to what is outlined above.
Probabilistic sensitivity analysis
We conducted a probabilistic sensitivity analysis by random sampling of parameters (1000 replicates) according to appropriate distributions to generate 95% credibility intervals (95% CrI).
RESULTS
Predicted effects of dapagliflozin on adverse clinical outcomes
In the trial, dapagliflozin in addition to standard therapy delayed disease progression in participants randomized to DAPA-CKD, as compared with those randomized to placebo. Over the modelled 10-year time horizon, patients randomized to dapagliflozin were projected to remain in CKD stages 1–3b for a longer time [0.65 (95% CrI 0.41, 0.90) years per patient], and spend less time in stages 4 and above [–0.23 (95% CrI –0.45, 0.00) years per patient], compared with patients randomized to placebo (Table 1).
Table 1:
Time spent per patient at each CKD stage (mean years).
| Dapagliflozin (95% CrI) | Placebo (95% CrI) | Incremental (95% CrI) | ||
|---|---|---|---|---|
| CKD 1 | 0.04 (0.03, 0.06) | 0.03 (0.02, 0.05) | 0.01 (0.00, 0.03) | 0.65 (0.41, 0.90) |
| CKD 2 | 0.58 (0.52, 0.64) | 0.54 (0.47, 0.61) | 0.04 (–0.04, 0.12) | |
| CKD 3a | 1.58 (1.48, 1.68) | 1.42 (1.30, 1.53) | 0.17 (0.04, 0.29) | |
| CKD 3b | 3.07 (2.88, 3.21) | 2.64 (2.45, 2.80) | 0.43 (0.27, 0.60) | |
| CKD 4 | 2.48 (2.29, 2.63) | 2.53 (2.30, 2.72) | –0.06 (–0.24, 0.13) | –0.23 (–0.45, 0.00) |
| CKD 5 (pre-KRT) | 0.29 (0.24, 0.34) | 0.34 (0.27, 0.41) | –0.05 (–0.11, 0.02) | |
| Dialysis | 0.63 (0.54, 0.73) | 0.73 (0.61, 0.83) | –0.10 (–0.23, 0.03) | |
| Transplant | 0.16 (0.12, 0.20) | 0.19 (0.14, 0.24) | –0.03 (–0.08, 0.02) | |
The projected risks of ACM, HHF and abrupt decline in kidney function were reduced consistently by dapagliflozin over the modelled time horizon (Fig. 2). Overall, dapagliflozin was predicted to prevent 83 deaths per 1000 patients over 10 years, compared with placebo (Table 2). Dapagliflozin use compared with placebo was also associated with 19 fewer HHF events and 39 fewer abrupt decline in kidney function events per 1000 patient years. Furthermore, dapagliflozin was predicted to prevent 51 patients initiating KRT per 1000 patients compared with placebo over the modelled time horizon.
Figure 2:
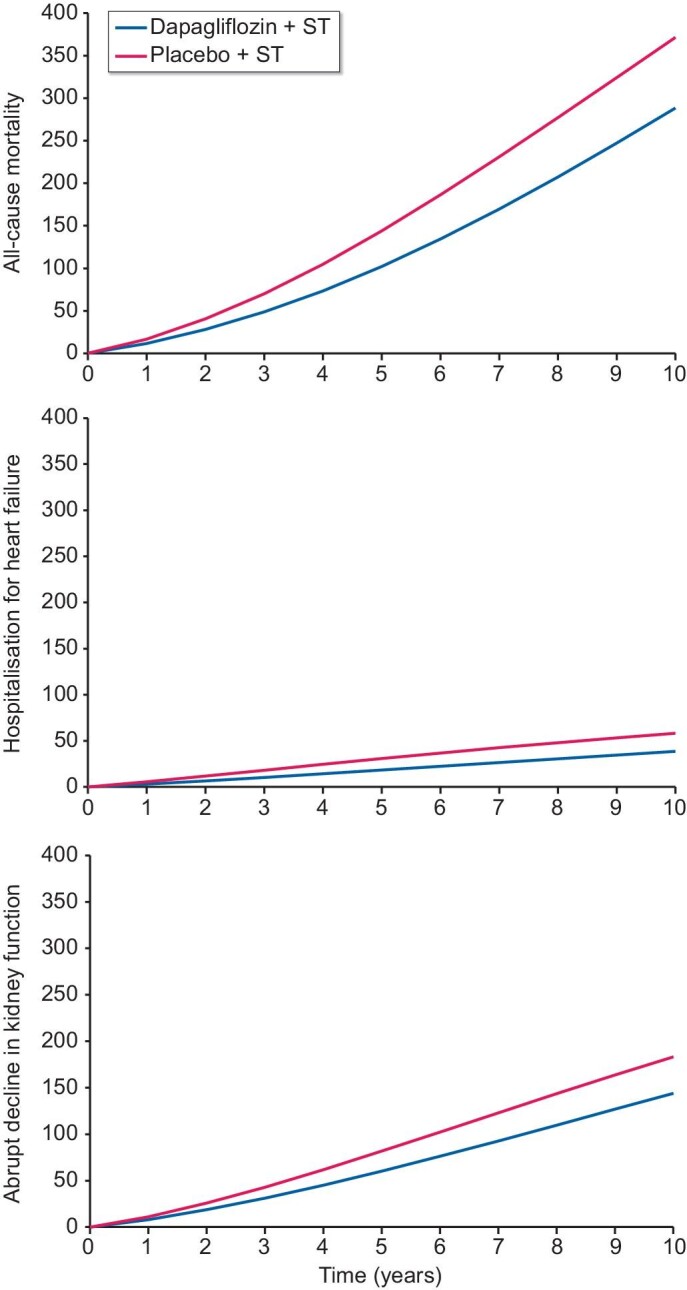
Cumulative incidence of ACM, HHF and abrupt decline in kidney function and (top to bottom) per 1000 patients treated with placebo versus dapagliflozin, in addition to standard therapy, over a 10-year horizon. ST: standard therapy.
Table 2:
Predicted number of events in the DAPA-CKD population and sub-populations per 1000 patients over 10 years.
| Subgroup | Event | Dapagliflozin | Placebo | Incremental |
|---|---|---|---|---|
| Overall population | HHF | 39 | 58 | –19 |
| Abrupt decline in kidney functiona | 144 | 183 | –39 | |
| KRT | 241 | 292 | –51 | |
| Dialysis | 200 | 245 | –45 | |
| Transplant | 41 | 47 | –6 | |
| ACM | 288 | 371 | –83 | |
| T2DM | HHF | 61 | 91 | –30 |
| Abrupt decline in kidney functiona | 162 | 205 | –43 | |
| KRT | 233 | 282 | –49 | |
| Dialysis | 193 | 236 | –43 | |
| Transplant | 40 | 46 | –6 | |
| ACM | 321 | 408 | –87 | |
| No T2DM | HHF | 15 | 23 | –8 |
| Abrupt decline in kidney functiona | 112 | 144 | –32 | |
| KRT | 253 | 312 | –59 | |
| Dialysis | 210 | 262 | –52 | |
| Transplant | 43 | 50 | –7 | |
| ACM | 236 | 311 | –75 | |
| eGFR <45 mL/min/1.73 m2 | HHF | 40 | 60 | –20 |
| Abrupt decline in kidney functiona | 159 | 204 | –45 | |
| KRT | 253 | 309 | –56 | |
| Dialysis | 210 | 259 | –49 | |
| Transplant | 43 | 50 | –7 | |
| ACM | 307 | 396 | –89 | |
| eGFR ≥45 mL/min/1.73 m2 | HHF | 37 | 56 | –19 |
| Abrupt decline in kidney functiona | 124 | 157 | –33 | |
| KRT | 224 | 269 | –45 | |
| Dialysis | 185 | 225 | –40 | |
| Transplant | 39 | 44 | –5 | |
| ACM | 263 | 337 | –74 | |
| Aged <65 years | HHF | 23 | 34 | –11 |
| Abrupt decline in kidney functiona | 132 | 169 | –37 | |
| KRT | 251 | 309 | –58 | |
| Dialysis | 208 | 259 | –51 | |
| Transplant | 43 | 50 | –7 | |
| ACM | 234 | 309 | –75 | |
| Aged ≥65 years | HHF | 70 | 104 | –34 |
| Abrupt decline in kidney functiona | 156 | 196 | –40 | |
| KRT | 222 | 267 | –45 | |
| Dialysis | 184 | 223 | –39 | |
| Transplant | 38 | 44 | –6 | |
| ACM | 376 | 469 | –94 | |
| Prior HF | HHF | 257 | 375 | –118 |
| Abrupt decline in kidney functiona | 306 | 373 | –67 | |
| KRT | 192 | 222 | –30 | |
| Dialysis | 158 | 185 | –27 | |
| Transplant | 34 | 37 | –3 | |
| ACM | 520 | 622 | –102 | |
| No prior HF | HHF | 30 | 46 | –15 |
| Abrupt decline in kidney functiona | 130 | 166 | –36 | |
| KRT | 245 | 298 | –53 | |
| Dialysis | 203 | 250 | –48 | |
| Transplant | 42 | 48 | –7 | |
| ACM | 270 | 350 | –80 |
Defined as a doubling of serum creatinine between two visits.
Predicted effect of dapagliflozin across subgroups
All-cause mortality
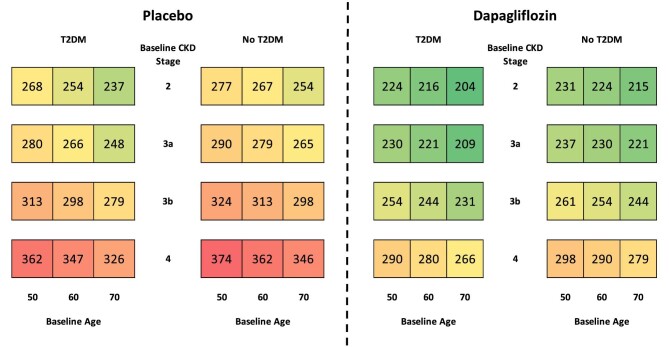
Within each modelled arm, ACM was more frequent in patients with T2DM, eGFR <45 mL/min/1.73 m2, aged ≥65 years and in patients with prior history of HF (Table 2). The anticipated number of deaths over the 10-year period in patients treated with dapagliflozin were reduced in all considered subgroups, compared with placebo. Treatment with dapagliflozin was also predicted to prevent more deaths per 1000 patients, depending on T2DM status (T2DM: 87; no T2DM: 75), baseline eGFR range (eGFR <45 mL/min/1.73 m2: 89; eGFR ≥45 mL/min/1.73 m2: 74), age at baseline (aged ≥65 years: 94; aged <65 years: 75) and prior HF (history of HF: 102; no history of HF: 80).
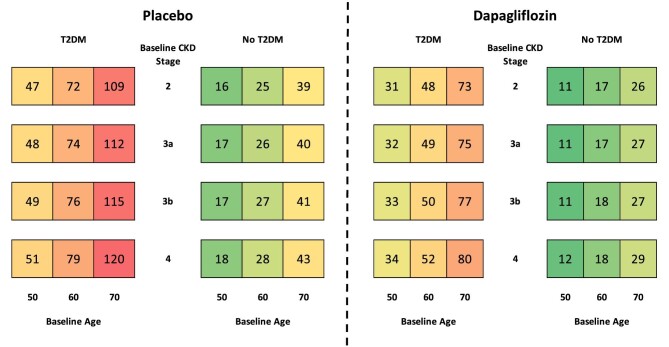
The extrapolated cumulative 10-year mortality incidence rates per 1000 patients, stratified by baseline CKD stage, T2DM status and age are presented in Fig. 3. Patients treated with dapagliflozin were projected to experience fewer deaths than those treated with dapagliflozin in all subgroups, with higher rates of mortality observed in patients with T2DM, higher age at baseline and more advanced CKD.
Figure 3:
Cumulative incidence of ACM per 1000 patients treated with placebo versus dapagliflozin, in addition to standard therapy, over a 10-year horizon.
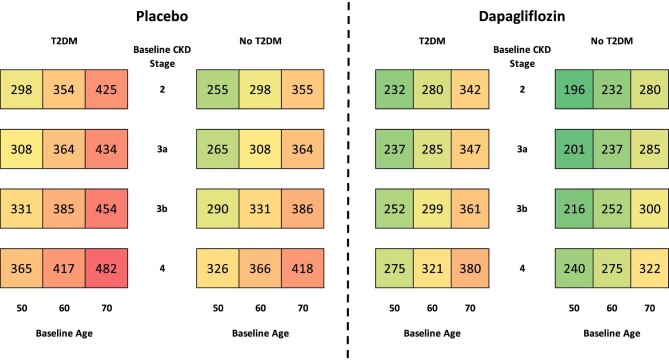
Initiation of KRT
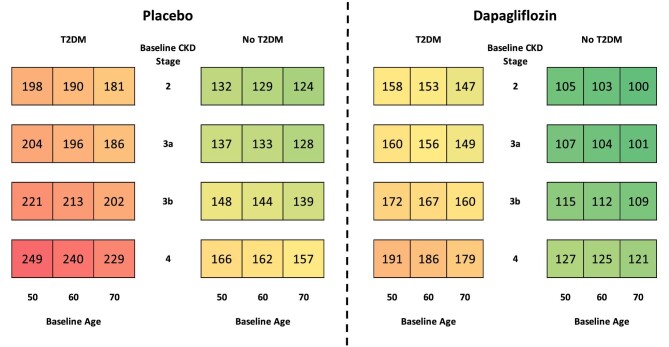
In both modelled arms, more patients initiated KRT over the 10-year period in patients with no T2DM, eGFR <45 mL/min/1.73 m2, aged <65 years or no prior HF (Table 2) than the overall population. Treatment with dapagliflozin was predicted to lead to fewer people initiating KRT in all subgroups, compared with placebo, over the 10-year period. The treatment effect of dapagliflozin prevented more patients initiating KRT in patients without T2DM (59 versus 49 with T2DM), eGFR <45 mL/min/1.73 m2 (56 versus 45 with eGFR ≥45 mL/min/1.73 m2), aged <65 years (58 versus 45 in people aged <65 years) and no prior HF (53 versus 30 in patients with prior HF).
Dapagliflozin reduced the cumulative incidence of KRT initiation per 1000 patients across all patient subgroups (stratified by CKD stage, T2DM status and age) in Fig. 4. Dialysis and transplantation are also shown separately in the Supplementary data (Figs S1 and S2). Patients with more severe CKD at baseline were predicted to be more likely to initiate KRT regardless of T2DM status, with both groups of patients displaying a similar predicted incidence of KRT over the 10-year time horizon. Randomization to dapagliflozin consistently reduced the expected rate of KRT initiation across all subgroups, with the most pronounced estimated effects predicted to occur in patients modelled at baseline with stage 4 disease versus placebo.
Figure 4:
Cumulative incidence of KRT initiation per 1000 patients treated with placebo versus dapagliflozin, in addition to standard therapy, over a 10-year horizon.
Hospitalization for heart failure
In both treatment groups, patients with T2DM, eGFR <45 mL/min/1.73 m2, aged ≥65 years, or prior history of HF were projected to have a higher incidence of HHF than the overall population. Dapagliflozin consistently reduced the rate of HHF across all subgroups considered. In patients treated with dapagliflozin, more hospitalizations for HF were prevented per 1000 patients, depending on T2DM status (T2DM: 30; no T2DM: 8), age at baseline (aged ≥65 years: 34; aged <65 years: 11) and prior HF (history of HF: 102; no history of HF: 80). However, the effect did not vary by categories of eGFR (<45 mL/min/1.73 m2: 20; ≥45 mL/min/1.73 m2: 19).
Patients in the dapagliflozin group were also predicted to suffer fewer episodes of HHF across all subgroups (defined by CKD stage, T2DM status and age) presented in Fig. 5. Comorbid T2DM and older age groups were associated with an increased risk of HHF, while advanced CKD stage was also associated, but to a lesser extent.
Figure 5:
Cumulative incidence of HHF per 1000 patients treated with placebo versus dapagliflozin, in addition to standard therapy, over a 10-year horizon.
Abrupt decline in kidney function
Abrupt declines in kidney function per 1000 patients were more frequent in patients with T2DM, eGFR <45 mL/min/1.73 m2, aged ≥65 years and in patients with prior history of HF (Table 2). The effect of dapagliflozin treatment led to fewer abrupt declines in kidney function in all subgroups. Over the 10-year period, more patients avoided an abrupt decline in kidney function as a result of dapagliflozin treatment depending on T2DM status (T2DM: 43; no T2DM: 32), eGFR (<45 mL/min/1.73 m2: 45; ≥45 mL/min/1.73 m2: 32), and particularly based on a prior history of HF (history of HF: 67; no history of HF: 36). Patients treated with dapagliflozin avoided a similar number of incidences depending on age at baseline (aged ≥65 years: 40; aged <65 years: 37).
Figure 6 displays the cumulative incidence per 1000 patients of abrupt decline in kidney function across subgroups stratified by CKD stage, T2DM status and age. Higher rates of abrupt declines in kidney function were more prevalent in higher CKD stages and in those with T2DM, while the rate of abrupt decline in kidney function was less affected by the baseline age of patients. Dapagliflozin consistently reduced the rate of abrupt decline in kidney function in all considered subgroups.
Figure 6:
Cumulative incidence of abrupt declines in kidney function per 1000 patients treated with placebo versus dapagliflozin, in addition to standard therapy, over a 10-year horizon.
Scenario analysis
The incidences of ACM, KRT, HHF and abrupt declines in kidney function were further stratified by a history of HF at baseline (Supplementary data, Figs S3–S6, respectively). Modelled patients with a history of HF at baseline are at high risk of mortality across all subgroups, with the highest risk in older patients with T2DM (Supplementary data, Fig. S3). Projections of ACM data showed dapagliflozin had a clear effect across all subgroups, with the most pronounced benefit in patients with more severe CKD.
Patients with a history of HF were predicted to initiate KRT at consistently lower rates than those without prior history of HF across all subgroups (Supplementary data, Fig. S4), but showed consistently higher rates of HHF and abrupt decline in kidney function across the considered subgroups (Supplementary data, Figs S5 and S6). Patients in all subgroups had lower predicted event rates when treated with dapagliflozin versus placebo across all considered endpoints.
DISCUSSION
This modelling analysis was conducted to estimate the cardiorenal protective efficacy associated with extended dapagliflozin treatment in addition to standard therapy, beyond the follow-up period of the DAPA-CKD trial. Our results demonstrate that dapagliflozin could further delay progression of CKD, including deferral of KRT initiation, into the longer term. Patients receiving dapagliflozin were less likely to die from any cause and adverse outcomes were also reduced, with fewer kidney and CV events occurring in patients treated with dapagliflozin.
Given that the scope of this analysis is limited to 10 years, the potential longer-term benefits are likely underestimated. Furthermore, SGLT2 inhibitors are associated with other benefits not considered in this study, including partial correction of anaemia [28, 29], reduced incidence of gout [30, 31] and improved control of hypertension [32], potentially reducing the need for other medications. In patients with T2DM and mild to moderate CKD, SGLT2 inhibitors also improve glycaemic control [33], as well as reducing new onset of T2DM in patients with CKD without diabetes [34]. In addition, given that most patients undergoing dialysis have significantly impaired HRQoL [7], the effect observed in this analysis could translate to HRQoL gains over the course of long-term treatment with dapagliflozin.
While the direct comparability of these studies is limited, our analysis is reminiscent of a cost-effectiveness analysis of early treatment with ACE inhibitors in patients with T2DM, hypertension and microalbuminuria [35]. Early treatment (initiating upon diagnosed microalbuminuria) with irbesartan was estimated to improve life expectancy (11.46 versus 10.50 undiscounted life years) and reduce incidence of ESKD (130 fewer events per 1000 patients) when compared with placebo over a 25-year time horizon. The estimated clinical benefits in this study were predicted to translate to cost savings of US$11.9 million per 1000 patients (cost year 2000) over the 25-year timeframe. Potential clinical benefits afforded to patients with CKD through treatment with dapagliflozin could also enable reallocation of finite budget and resources across healthcare systems.
Our analysis employs a robust method of extrapolating clinical outcomes beyond the final trial follow-up period, incorporating a more complex and clinically valid approach than incorporating extrapolation of eGFR trajectories in isolation. Here, we determine modelled outcomes across the spectrum of patient baseline characteristics, and incorporate multiple adverse events per patient over the model horizon. This analysis presents trial data in a manner that can inform on the longer-term risks to CKD based on the treatment provided and can assists policy makers in decision making.
It is also important to address that this modelling analysis is subject to a number of limitations. First, as with any analysis in which outcomes are extrapolated beyond the available trial follow-up data, the projections are subject to uncertainty. Adherence to therapy may wane over time, and it is unknown whether the effectiveness may be attenuated with long-term use, although we are unaware of any evidence suggesting diminished efficacy or effectiveness over time. Second, the model described assumes that heterogeneity among patients is effectively represented by the health states, stratifying by CKD stage, which does not completely capture differential risks. However, as clinical guidance for CKD management is generally aligned to these stage classifications, we consider this model structure to adequately capture patient characteristics and outcomes. Third, due to insufficient follow up from the DAPA-CKD trial, post-KRT outcomes had to be sourced from external literature, although the sources were derived by a systematic literature review of CKD modelling methodologies to ensure the most representative estimates were incorporated. Fourth, the DAPA-CKD trial was not designed to capture acute kidney injury as an adjudicated endpoint, thereby limiting the capability of the model to extrapolate acute kidney injury. However, abrupt decline in kidney function (defined by the adjudicated endpoint of a defined as a doubling of serum creatinine between two visits) was considered appropriate for reflecting acute episodes of decline that may be associated with acute kidney injury [36]. Finally, a Markov model simulates cohort level outcomes, so characterization of the interplay between the extrapolated events is inherently limited. For example, higher rates of ACM (such as in patients with a history of HF or in older patients) may coincide with lower incidence of other events than would be observed in the proportion of patients that do not die from any cause.
The results of this modelled Markov analysis predict that adding dapagliflozin to standard CKD treatment could offer a long-term cardiorenal protective efficacy beyond what was demonstrated in the DAPA-CKD trial, predicting slower progression to advanced kidney disease, lower prevalence of cardiovascular complications and extended survival.
Supplementary Material
ACKNOWLEDGEMENTS
Medical writing and editorial support were provided by Peter Gabb of Health Economics and Outcomes Research Ltd who received fees from AstraZeneca in relation to this study.
Contributor Information
Phil McEwan, Health Economics and Outcomes Research Ltd, Cardiff, UK.
Rebecca Boyce, Health Economics and Outcomes Research Ltd, Cardiff, UK.
Juan Jose Garcia Sanchez, Global Market Access and Pricing, BioPharmaceuticals, AstraZeneca, Cambridge, UK.
C David Sjöström, Late-Stage Development, Cardiovascular, Renal, and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Bergur Stefansson, Late-Stage Development, Cardiovascular, Renal, and Metabolism, BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden.
Stephen Nolan, Global Medical Affairs, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK.
Ricardo Correa-Rotter, Department of Nephrology and Mineral Metabolism, National Medical Science and Nutrition Institute Salvador Zubiran, Mexico City, Mexico.
Peter Rossing, Steno Diabetes Center Copenhagen, Herlev, Denmark; Department of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark.
Glenn M Chertow, Departments of Medicine and Epidemiology and Population Health, Stanford University School of Medicine, Stanford, CA, USA.
John J V McMurray, British Heart Foundation Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
David C Wheeler, Department of Renal Medicine, University College London, London, UK.
Hiddo J L Heerspink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands; The George Institute for Global Health, Sydney, Australia.
FUNDING
This work was supported by AstraZeneca. Author independence in study design, data interpretation, manuscript preparation for publication was ensured by the funding agreement.
AUTHORS’ CONTRIBUTIONS
P.M., R.B. and J.J.G.S. conceptualized and designed the study. R.B. was responsible for data analysis. All authors contributed to interpretation of the results, preparation and review of the manuscript, and approval of the final manuscript for publication.
DATA AVAILABILTY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
CONFLICT OF INTEREST STATEMENT
P.M. and R.B. are/were employees of Health Economics and Outcomes Research Ltd, Cardiff, UK. Health Economics and Outcomes Research Ltd received fees from AstraZeneca in relation to this study. J.J.G.S., C.D.S., B.S. and S.N. are employees of AstraZeneca. R.C.-R. has received honoraria as consultant from: AstraZeneca, Boehringer Ingelheim, Janssen, Bayer, Chinook, AbbVie and Novo Nordisk, and research support from AstraZeneca, GSK and Novo Nordisk. P.R. has received honoraria to Steno Diabetes Center Copenhagen for consultancy from AstraZeneca, Astellas, Bayer, Boehringer Ingelheim, Gilead, Novo Nordisk, Merck, Mundipharma, Sanofi and Vifor, and research support from AstraZeneca and Novo Nordisk. G.M.C. has received honoraria as consultant from: Akebia, Amgen, Ardelyx, AstraZeneca, Baxter, Cricket, DiaMedica, Gilead, Miromatrix, Outset, Reata, Sanifit and Vertex. He has received research support from Amgen and Janssen. He has served on data and safety monitoring boards for Angion, Bayer and Recor. J.J.V.M. reports non-financial support and other from AstraZeneca, during the conduct of the study; non-financial support and other from Cardiorentis, Amgen, Oxford University/Bayer, Theracos, AbbVie, other from DalCor, Pfizer, Novartis, GSK, Vifor-Fresenius, Kidney Research UK, Bayer, Merck and Bristol-Myers Squibb, outside the submitted work. D.C.W. provides ongoing consultancy services to AstraZeneca and has received honoraria and/or consultancy fees from Amgen, AstraZeneca, Boehringer Ingelheim, Bayer, GSK, Janssen, Napp, Mundipharma, Medscape, Merck Sharp and Dohme, Pharmacosmos, Reata, Takeda and Vifor-Fresenius. H.J.L.H. is a consultant for AstraZeneca, AbbVie, Boehringer Ingelheim, CSL Behring, Bayer, Chinook, Dimerix, Gilead, Goldfinch, Merck, Novo Nordisk, Janssen and Travere Pharmaceuticals. He received research support from AstraZeneca, Boehringer Ingelheim, Janssen and Novo Nordisk.
REFERENCES
- 1. Hill NR, Fatoba ST, Oke JLet al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 2016;11:e0158765. 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol 2019;1165:3–15. 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 3. Bikbov B, Purcell CA, Levey ASet al. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet North Am Ed 2020;395:709–33. 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ward F, Holian J, Murray PT. Drug therapies to delay the progression of chronic kidney disease. Clin Med 2015;15:550–7. 10.7861/clinmedicine.15-6-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tuegel C, Bansal N. Heart failure in patients with kidney disease. Heart 2017;103:1848–53. 10.1136/heartjnl-2016-310794. [DOI] [PubMed] [Google Scholar]
- 6. United States Renal Data System. Annual Report. End Stage Renal Disease: Chapter 5. 2020; updated 19 October 2021. Available from: https://adr.usrds.org/2020/end-stage-renal-disease/5-mortality (15 November 2021, date last accessed). [Google Scholar]
- 7. Pagels AA, Söderkvist BK, Medin Cet al. Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 2012;10:71. 10.1186/1477-7525-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Botdorf J, Chaudhary K, Whaley-Connell A. Hypertension in cardiovascular and kidney disease. Cardiorenal Med 2011;1:183–92. 10.1159/000329927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. MacIsaac RJ, Jerums G, Ekinci EI. Effects of glycaemic management on diabetic kidney disease. World J Diabetes 2017;8:172–86. 10.4239/wjd.v8.i5.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bilous R. Microvascular disease: what does the UKPDS tell us about diabetic nephropathy? Diabet Med 2008;25:25–9. 10.1111/j.1464-5491.2008.02496.x. [DOI] [PubMed] [Google Scholar]
- 11. Burnier M. Renin-angiotensin system blockade in advanced kidney disease: stop or continue? Kidney Med 2020;2:231–4. 10.1016/j.xkme.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McMurray JJV, Solomon SD, Inzucchi SEet al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 13. Packer M, Anker SD, Butler Jet al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–24. 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 14. Mosenzon O, Wiviott SD, Cahn Aet al. Effects of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes: an analysis from the DECLARE-TIMI 58 randomised trial. Lancet Diabetes Endocrinol 2019;7:606–17. 10.1016/S2213-8587(19)30180-9. [DOI] [PubMed] [Google Scholar]
- 15. Perkovic V, de Zeeuw D, Mahaffey KWet al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS program randomised clinical trials. Lancet Diabetes Endocrinol 2018;6:691–704. 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 16. Wanner C, Inzucchi SE, Lachin JMet al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016;375:323–34. 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 17. Heerspink HJL, Stefansson BV, Chertow GMet al. Rationale and protocol of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplantat 2020;35:274–82. 10.1093/ndt/gfz290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wheeler DC, Stefánsson BV, Jongs Net al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol 2021;9:22–31. [DOI] [PubMed] [Google Scholar]
- 19. Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–46. 10.1056/NEJMoa2024816. [DOI] [PubMed] [Google Scholar]
- 20. Wheeler DC, Stefansson BV, Batiushin Met al. The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics. Nephrol Dial Transplant 2020;35:1700–11. 10.1093/ndt/gfaa234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Institiute for Health and Care Excellence. Dapagliflozin for treating chronic kidney disease [TA775]. 2022. Available from: https://www.nice.org.uk/guidance/TA775 (12 December 2021, date last accessed). [Google Scholar]
- 22. McEwan P, Darlington O, Wheeler Det al. POS-335 Cost-effectiveness of dapagliflozin as a treatment for chronic kidney disease: a health-economic analysis of DAPA-CKD. Kidney Int Rep 2021;6:S145–6. 10.1016/j.ekir.2021.03.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sugrue DM, Ward T, Rai Set al. Economic modelling of chronic kidney disease: a systematic literature review to inform conceptual model design. Pharmacoeconomics 2019;37:1451–68. 10.1007/s40273-019-00835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. 2013; Updated 30 October 2020. Available from : https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781 (30 October 2020, date last accessed). [PubMed] [Google Scholar]
- 25. National Institiute for Health and Care Excellence. NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations Alongside Clinical Trial - Extrapolation with Patient-level Data. 2011; updated 12 April 2021. Available from : http://nicedsu.org.uk/wp-content/uploads/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf (12 April 2021, date last accessed). [PubMed] [Google Scholar]
- 26. May M, Sterne J, Egger M.. Parametric survival models may be more accurate than Kaplan-Meier estimates. BMJ 2003;326:822. 10.1136/bmj.326.7393.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Willigers B, Ouwens M, Briggs Aet al. MO516A structured expert elicitation to inform and validate mortality extrapolations for a cost-effectiveness analysis of dapagliflozin. Nephrol Dial Transplant 2021;36:i314–5. 10.1093/ndt/gfab087.0036. [DOI] [Google Scholar]
- 28. Oshima M, Neuen BL, Jardine MJet al. Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: a post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol 2020;8:903–14. [DOI] [PubMed] [Google Scholar]
- 29. Stefánsson BV, Heerspink HJL, Wheeler DCet al. Correction of anemia by dapagliflozin in patients with type 2 diabetes. J Diabetes Complications 2020;34:107729. 10.1016/j.jdiacomp.2020.107729. [DOI] [PubMed] [Google Scholar]
- 30. Li J, Badve SV, Zhou Zet al. The effects of canagliflozin on gout in type 2 diabetes: a post-hoc analysis of the CANVAS program. Lancet Rheumatol 2019;1:e220–8. 10.1016/S2665-9913(19)30078-5. [DOI] [PubMed] [Google Scholar]
- 31. Ferreira JP, Inzucchi SE, Mattheus Met al. Empagliflozin and uric acid metabolism in diabetes: a post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes Obes Metab 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinto LC, Rados DV, Remonti LRet al. Efficacy of SGLT2 inhibitors in glycemic control, weight loss and blood pressure reduction: a systematic review and meta-analysis. Diabetol Metab Syndr 2015;7:A58. [Google Scholar]
- 33. Kelly MS, Lewis J, Huntsberry AMet al. Efficacy and renal outcomes of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Postgrad Med 2019;131:31–42. 10.1080/00325481.2019.1549459. [DOI] [PubMed] [Google Scholar]
- 34. Rossing P, Vart P, Chertow GMet al. 130-LB: dapagliflozin and the incidence of type 2 diabetes in patients with chronic kidney disease. Diabetes 2021;70:130–LB. 10.2337/db21-130-LB. [DOI] [Google Scholar]
- 35. Palmer AJ, Annemans L, Roze Set al. Cost-Effectiveness of early irbesartan treatment versus control (standard antihypertensive medications excluding ACE inhibitors, other angiotensin-2 receptor antagonists, and dihydropyridine calcium channel blockers) or late irbesartan treatment in patients with type 2 diabetes, hypertension, and renal disease. Diabetes Care 2004;27:1897–903. [DOI] [PubMed] [Google Scholar]
- 36. Heerspink HJL, Cherney D, Postmus Det al. A pre-specified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) randomized controlled trial on the incidence of abrupt declines in kidney function. Kidney Int 2022;101:174–84. 10.1016/j.kint.2021.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.