ABSTRACT
Supported by several high-quality randomized controlled trials and registry analyses, catheter-based renal denervation is becoming an important adjunctive treatment modality for the safe and efficacious treatment of hypertension besides lifestyle modifications and antihypertensive medication. Renal denervation is of particular interest to nephrologists as the intervention may provide additional benefits to hypertensive people with chronic kidney disease (CKD), a condition typically characterized by sympathetic hyperactivity. A growing body of clinical evidence supports the safety and efficacy of renal denervation in this difficult-to-control population. In addition, preclinical and clinical research works indicate potential nephroprotective effects in CKD patients. The current review examines recent research on renal denervation with a focus on renal disease and assesses the latest findings and their implications from a nephrologist's perspective.
Keywords: chronic kidney disease, hypertension, nephroprotection, renal denervation, sympathetic hyperactivity
INTRODUCTION
Chronic kidney disease (CKD) and hypertension are intrinsically linked. High blood pressure (BP) is present in four out of five patients with CKD [1]. In a vicious feedback loop, the presence of hypertension drives CKD severity: uncontrolled resistant hypertension is associated with a marked increase in the risk of developing the end-stage renal disease (ESRD) over a 5-year period [2]. As renal function declines, the incidence and severity of hypertension increase [1]. The consequences are severe: CKD and associated cardiovascular (CV) disease were responsible for 4.6% of global deaths in 2017 [3].
It is especially difficult to control elevated BP in patients with CKD, as shown by reports of apparently resistant hypertension in roughly two in five patients with CKD [4]. There are many reasons for this lack of success, e.g. physician inertia, patient non-adherence, lack of social support, depression and the complexity of polypharmacy regimens in more severe cases of hypertension [5].
Renal function in health and disease is at the core of treatment considerations for patients with hypertension. This review focuses on patients with CKD and discusses preclinical and clinical research around the role of interventional methods to reduce BP, such as catheter-based renal denervation using radiofrequency energy, ultrasound or perivascular injection of neurotoxic agents. Renal denervation is included as a therapeutic option in the latest European Society of Cardiology/European Society of Hypertension (ESC/ESH) guidelines and position statements [5–7]. Though these technologies are at different stages of development, several have been tested in sham-controlled trials designed towards US regulatory approval.
As will be seen further, currently available preclinical and clinical research works suggest that renal denervation may play a particularly beneficial role in patients with CKD and hypertension.
RATIONALE FOR RENAL DENERVATION IN PRIMARY HYPERTENSION
The aim of renal denervation is 2-fold: to reduce BP and at the same time reduce the progressive loss of renal function in hypertensive individuals. The rationale is to attenuate overactive mutual signalling between the kidney and the central nervous system (CNS). It is well established that efferent renal nerves contribute to the regulation of renin secretion, tubular sodium reabsorption and renal haemodynamics. Efferent nerve activation reduces renal blood flow and urinary excretion of salt and water, and increases renin release from the kidney, with the effect of increasing BP [8]. Renal afferent nerves also contribute, as increased afferent signalling to the brain leads to CNS-mediated arterial vasoconstriction. Increased sympathetic activity is a driver of elevated BP and end-organ damage, e.g. cardiac hypertrophy and reduced renal function [9]. Plasma noradrenaline has been identified as an independent predictor of mortality as well as fatal and nonfatal CV events in patients undergoing haemodialysis [10].
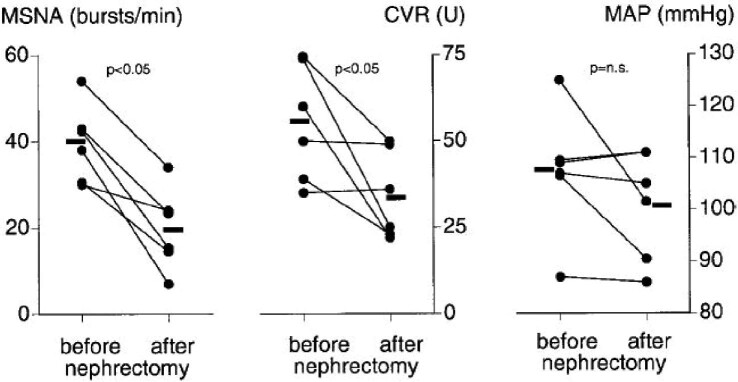
The role of the kidney in mediating sympathetic activity was demonstrated by early studies in patients with CKD. In this population, bilateral nephrectomy led to significant reductions in sympathetic nerve discharge, calf vascular resistance and mean arterial pressure (MAP) [11]. More recent studies in renal transplant patients have supported the concept of ailing kidneys mediating increased CNS activity. In transplant patients without nephrectomy, the native kidneys mediated increased SNS activity despite excellent graft function. In transplant patients who had undergone bilateral nephrectomy, muscle sympathetic nerve activity (MSNA) and calf vascular resistance were significantly lower (Fig. 1) [12].
FIGURE 1:
Effect of bilateral nephrectomy on SNS activity in renal transplant patients. From Hausberg et al [12]. MSNA, muscle sympathetic activity; CVR, calf vascular resistance; n.s., not significant.
MSNA, reflecting the systemic sympathetic activity, has also been shown to be significantly reduced after renal denervation in humans with hypertension [13]. The reduction grew with time, becoming statistically significant after 3 months. Both multi-unit MSNA and single-unit MSNA (measuring the firing properties of single sympathetic vasoconstrictive fibres) are reduced by renal denervation [14].
The above findings support the potential for renal denervation to achieve the dual effect of reducing efferent and afferent nerve activity, moderating the direct effect of renal signalling on BP and the indirect effect of afferent signalling on brain sympathetic activity outflow [15].
CLINICAL TRIAL EVIDENCE FOR THE EFFICACY OF RENAL DENERVATION
The development of renal denervation nearly stopped in 2014, when the sham-controlled SYMPLICITY-3 HTN trial failed to show significant BP reduction relative to sham-treated control [16]. Although office BP was reduced significantly from baseline in the active treatment group, to almost the same degree as in earlier single-arm trials, the difference to sham was not significant. This was later attributed to procedural factors, e.g. proximal (instead of distal) location of energy delivery and incomplete non-circumferential denervation, but also to a high rate of drug changes and lack of adherence to treatment. The latter two confounders would explain the substantial BP reductions in the sham group [17, 18].
Similarly, in the recently completed REQUIRE trial of ultrasound renal denervation in treated patients with resistant hypertension, systolic BP (SBP) reductions in the sham group were unexpectedly large (no difference to the actively treated group) [19]. A number of confounders were identified: lack of standardized medications, incomplete medication blinding of treating physicians and coordinators, and lack of objective measurement of adherence [19, 20].
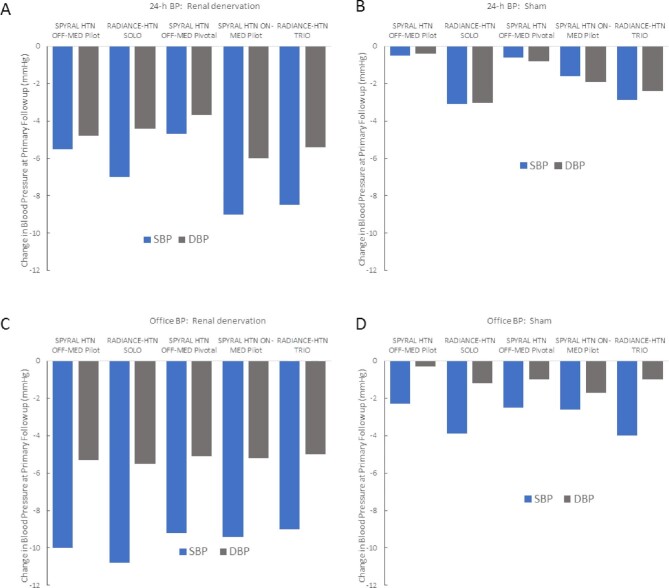
Since 2017, however, a number of rigorously designed and executed sham-controlled randomized controlled trials (RCTs) of second-generation renal denervation systems have been completed, providing a substantial and growing body of evidence for the efficacy and safety of the procedures. Whether radiofrequency (Spyral system) or ultrasound (Paradise system), five completed sham-controlled RCTs demonstrated significant BP reductions in the active treatment groups compared with sham-treated patients (Fig. 2) [21–25]. Renal denervation achieved clinically meaningful BP reductions in the presence as well as the absence of medication. Notably, two controlled trials in patients with resistant hypertension and high medication burden, the prospective, randomized, open-label blinded endpoint DENERHTN [26] and the two multicentre, single-blind, sham-controlled trials RADIANCE-HTN TRIO [24] and SPYRAL HTN-ON MED [27] have found that adherence to antihypertensive treatment had no relevant influence on the antihypertensive effect of renal denervation, as non-adherence rates were numerically greater in the renal denervation group.
FIGURE 2:
Changes in 24-h ambulatory BP (top) and office BP (bottom), respectively, after renal denervation (A and C) and sham (B and D) treatment in sham-controlled RCTs with second-generation technologies.
Other systems for renal denervation are in development, but with efficacy not yet proven in large-scale sham-controlled RCTs. Alcohol-based ablation achieved significant BP reductions in an open-label study of patients with resistant hypertension [28] and two Phase II studies with the technology (TARGET BP OFF-MED, NCT03503773, and TARGET BP I, NCT02910414) are ongoing. The REDUCE HTN:REINFORCE trial using bipolar radiofrequency, a technology that is not available at present, was prematurely stopped during recruitment [29].
All second-generation sham-controlled RCTs used the change in ambulatory BP as the primary endpoint (24 h mean with the Spyral system and daytime mean with the Paradise system) to enable a relevant assessment of the impact of elevated BP on CV, cerebrovascular and renal systems [7]. The ‘always on’ effect of the intervention has been highlighted [5], with sustained reductions relative to sham at all time points within a 24-h interval. This was recently confirmed in a meta-analysis of nine trials and 1555 patients undergoing renal denervation or sham [30].
Additional studies [SPYRAL HTN-ON MED Study (NCT02439775) RADIANCE II (NCT03614260)] are ongoing and will hopefully provide further evidence of efficacy as well as indications to the importance of confounders in RCTs with catheter interventions.
LONG-TERM EFFECTIVENESS OF RENAL DENERVATION
In contrast to drugs, which must be taken regularly and possibly for life, renal denervation is considered a single intervention [31]. Accordingly, the sustainability of the antihypertensive effect is a highly relevant question. A study in normotensive sheep in 2015 found no morphologically functional nerve regeneration 11 months after renal denervation, but the authors noted recovered functional afferent and efferent responses to electric stimulation [32].
Subsequent work with the hypertensive CKD sheep model by the same group found a marked difference between normotensive and hypertensive animals in renal nerve regrowth at 30 months after renal denervation, indicating that results from normotensive models may be of limited relevance to clinical renal denervation for hypertension [33]. Normotensive animals demonstrated renal nerve regrowth and neural control of renal function to pre-intervention levels. In contrast, in hypertensive-CKD animals, levels of renal tyrosine hydroxylase, calcitonin gene-related peptide and noradrenaline, as well as a vascular contraction to nerve stimulation and reflex activation of the sympathetic nerves were only partially restored at 30 months. The reduction in BP and improvements in renal function after renal denervation were all sustained. These results indicate a relevant difference between general reinnervation and the re-establishment of functional axons.
Other models support this distinction. There is good evidence from normotensive porcine models that a progressive regenerative response may occur as early as 7 days after renal denervation [34], but immunohistochemical observations have identified prominent fibrotic infiltration at the site of procedural injury, which attenuates the original delineation of the nerve and precludes re-creation of functional axonal connections [34]. More recently, Sharp et al., using the same model, found no recovery of nerve architecture 180 days post-ablation [35]. Neither partially nor fully destroyed nerves recovered additional function and the noradrenaline reduction post-renal denervation was maintained at 6 months. The relevance to humans of these observations can only be established by clinical data. However, the transplanted human kidney does not achieve functional reinnervation [36] and according to our current knowledge, the same appears to be true for denervated kidneys.
As the technology has only recently been adopted, published data cover modest durations of follow-up. The RCTs reported their primary endpoints at between 2 and 6 months, which may be too short for a meaningful conclusion. After unblinding, it may become difficult to attribute an antihypertensive effect to the intervention over a longer time frame. Primary care physicians managing patients after renal denervation may need to modify antihypertensive therapy as necessary to reduce BP further and achieve target levels.
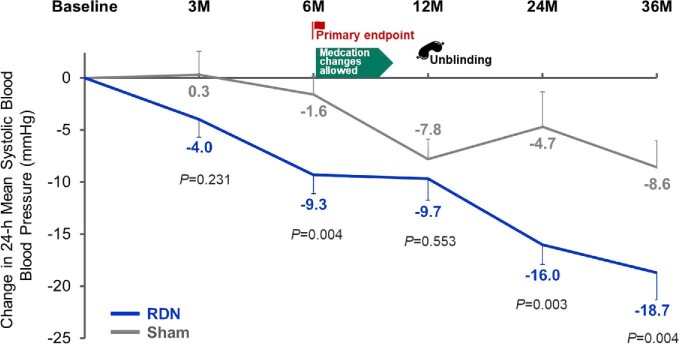
In the Global Symplicity Registry, the largest cohort followed currently, reductions in both office and ambulatory BP have been sustained over a 3-year period [37]. Three-year follow-up data from SPYRAL-ON MED were recently published [27]. Over the 36-months follow-up, reductions in 24-h BP remained significantly greater in patients who underwent renal denervation than in patients who had undergone a sham control procedure (Fig. 3). These differences persisted despite similar antihypertensive drug use in both treatment arms.
FIGURE 3:
Changes from baseline with renal denervation or sham up to 36 months in the randomized, sham-controlled SPYRAL-ON MED study. Medication changes were allowed after end of primary follow-up at 6 months and patients were unblinded to randomization status at 12 months. Adapted from Mahfoud et al [27].
Small single-centre studies also suggest sustained BP reduction. An analysis of 49 out of 73 patients treated for resistant hypertension with follow-up data at 48 months found a significant reduction from baseline in office and ambulatory BP [38]. The lack of control groups and the possibility of bias from losses to follow-up need to be taken into consideration when assessing such findings, but there is no indication that the antihypertensive effect abates over at least several years.
RENAL DENERVATION IN HYPERTENSIVE PATIENTS WITH CKD
Renal denervation trials initially focused on patients with severe treatment-resistant hypertension, often defined as SBP above 160 mmHg, despite being treated with at least three antihypertensive drugs including one diuretic [39]. However, the usefulness of the term ‘resistant hypertension’ has been queried [5]. Many patients with ‘resistant hypertension’ are non-adherent to prescribed medication and do not qualify for ‘true resistant’ hypertension [40]. A recent position statement advocated prioritizing hypertensive subjects with uncontrolled BP and at elevated CV risk, possibly with an established CV event or hypertension-mediated organ damage [5]. A relevant selection criterion for renal-denervation candidates may be high sympathetic activity accompanying elevated BP [41]. This situation is commonly observed in patients with CKD. In this population, the reduction in BP combined with possible nephroprotection from sympatholytic effects may be especially beneficial.
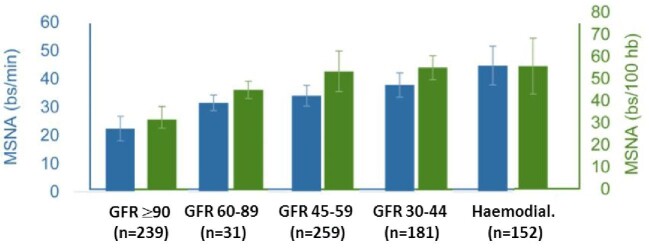
A significant increase in nerve endings in the internal area of renal artery adventitia has been found in patients with ESRD on haemodialysis, compared with patients with less severe CKD or normal renal function [42]. A recent meta-analysis found a significant reverse correlation between microneurographic sympathetic nerve activity and glomerular filtration rate (GFR), across the range from ≥90 mL/min per 1.73 m2 to haemodialysis patients (Fig. 4) [43].
FIGURE 4:
Muscle sympathetic nerve activity (MSNA) expressed as burst frequency (bs/min; blue bars; left-hand scale) and burst incidence (bs/100 heartbeats; green bars; right-hand scale) in patients and healthy individuals grouped according to progressively more severe degrees of renal dysfunction. Adapted from Grassi et al [43].
In addition to elevated BP and hyperactive sympathetic nervous system, CKD is associated with lower kidney renalase activity. As renalase degrades catecholamines the reduced activity exposes the kidney to increased levels of these molecules, with associated adverse effects on BP and cardiac function [44].
The renal denervation procedure is effective in hypertensive patients with CKD: in the meta-analysis in 2021 of single-centre studies on patients with CKD [45], office BP and 24-h ambulatory BP were significantly reduced from baseline to 1 month (P < .05) and sustained for at least 24 months. A recent analysis of 3-year follow-up data from the Global Symplicity Registry included 475 subjects with CKD [baseline estimated GFR (eGFR) <60 mL/min/1.73 m2 but ≥15 mL/min/1.73 m2] versus 1505 subjects without CKD (baseline eGFR ≥60 mL/min/1.73 m2) [46]. In subjects with data on both baseline and 36-month BP, similar reductions in office and 24-h ambulatory BP were found in both groups. Thus, BP reduction is achieved after renal denervation independently of renal function. A small randomized study in renal transplant recipients indicated that the intervention is also effective and safe in this population [47]. Possible nephroprotective effects are discussed as follows.
SAFETY OF RENAL DENERVATION
The procedural safety of renal denervation was well demonstrated in the clinical trials over the latest decade. The main concerns regarded potential procedural damage to the vessel endothelium from the applied energy, de novo renal artery stenosis or contrast-induced nephropathy, as well as possible eGFR loss in the long term.
However, the short-term risk from renal denervation has been shown to be limited: damaged endothelium recovers within a week [48], the arterial wall and soft tissue within 6 months [49]. Clinical evidence provides further assurance: no safety signal emerged in any of the sham-controlled RCTs, with similar rates of major adverse events in the renal denervation and control groups [19, 21–25, 29]. It should be noted that the pivotal RCTs used eGFR <45 mL/min/1.73 m² as an exclusion criterion and the conclusion is limited to this population. A meta-analysis of 50 trials, 5769 patients and 10 249 patient-years of data, estimated a 0.20 annual incidence of renal artery stenting following RF renal denervation [50]. This rate is comparable to the reported natural incidence of events in an untreated hypertensive population. The meta-analysis included a subgroup of 396 patients with CKD and found no reported adverse events in the distal arteries of this group [50].
As with efficacy, data on long-term safety with renal denervation are not available beyond a few years of follow-up. However, the available analyses are reassuring. A meta-analysis in 2019 of 977 patients from six trials found no significant difference between intervention and sham in the changes from baseline in eGFR, either in first- or second-generation trials [51]. Sanders [52] analysed 2381 patients for whom data were available in 2017 and found no statistically significant change in eGFR after on average 9.1 ± 7.0 months (pooled mean change 0.64 mL/min/1.73 m2; P = .26). In the presentation from RADIANCE-HTN SOLO 2-year follow-up [53], no adverse safety signals were observed. In the SPYRAL HTN-ON MED trial no safety signal emerged up to 3 years; there was no instance of renal artery stenosis and no re-intervention associated with renal denervation during the 36 months of follow-up. Up to 36 months, the renal denervation and the sham group did not differ in changes from baseline in eGFR (–4.9 ± 11.5 and –4.5 ± 11.7 mL/min/1.73 m2 with renal denervation and sham treatment, respectively), serum creatinine, sodium or potassium levels [27].
The safety of renal denervation is a key concern for hypertensive patients with CKD. Given the lack of RCT data, what is available comes mostly from small, isolated studies. A meta-analysis of procedure-related events in 238 patients from 11 single-centre, non-randomized, uncontrolled trials found low rates of haematoma (2.9%) and pseudoaneurysm (1.3%) [45]. In the retrospective analysis of 3-year follow-up data from the Global Symplicity Registry, there were no differences in safety outcomes between subjects with and without CKD, with rates of renal artery stenosis <0.5% [46].
These findings are clearly reassuring, as they indicate the overall safety of renal denervation, with the limitation that patients with an eGFR <45 mL/min/1.73 m² have not been thoroughly examined. Beyond antihypertensive efficacy and a favourable safety profile in hypertensive patients, a particularly salient question is whether renal denervation may have nephroprotective effects in the CKD population.
IS RENAL DENERVATION NEPHROPROTECTIVE?
It has long been discussed whether there is an additional effect from antihypertensive treatments beyond reducing the risk of kidney damage associated with high BP [54]. Accumulated evidence supports a nephroprotective effect from angiotensin-receptor blockers (ARBs) and sodium-glucose co-transporter-2 (SLGT2) inhibitors [55, 56]. However, to date sympatholytic drugs have not shown a nephroprotective effect in patients with CKD. A small study in 15 patients with type 1 diabetes in 2001 reported reduction in microalbuminuria with moxonidine, a sympatholytic agent [57], but the effect on renal function remains elusive. Renal denervation may be able to fill this gap, as it targets renal sympathetic activity directly.
Preclinical research provides indications that renal denervation might benefit the kidney beyond BP reduction. In a hypertensive sheep model, Singh et al. showed that although GFR declined by 22% over 30 months in sham-treated animals, it increased to a similar degree after renal denervation (26%; P < .0001 for the comparison) [33]. This increase in GFR was accompanied by a sustained reduction in MAP; conversely there was a continual increase in MAP with time in sham-treated sheep. The improvements in BP and renal function were associated with improvements in left ventricular (LV) mass and albuminuria, indicative of additional, cardioprotective effects from renal denervation in CKD. Urinary albumin levels at 11 and 30 months were almost 60% lower in animals who underwent renal denervation than in the sham-treated group, although levels remained significantly higher than in normotensive controls. LV mass increased with time only in the sham-treated hypertensive-CKD group while animals undergoing renal denervation did not experience increased LV mass up to 30 months.
In the same ovine model, the group recently found that urinary NOx (nitrate + nitrite) excretion was significantly increased 2 months after renal denervation and remained elevated at 30 months (Fig. 5) [58]. Renal haemodynamics also improved, as indicated by augmented renal vasoconstrictor responses to inhibition of endothelial NO synthase and greater eNOS kidney protein expression in treated relative to sham-treated CKD sheep.
FIGURE 5:
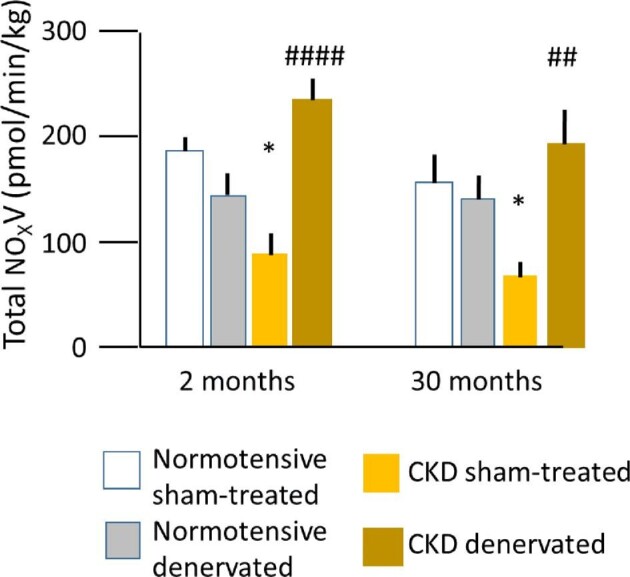
Mean basal total urinary NOx (nitrate + nitrite) excretion (V) in normotensive (control) and hypertensive sheep with CKD 2 and 30 months after renal denervation or sham procedures, respectively. Lines indicate standard error. *P < .05 comparing sham groups at follow-up. ##P < .05, ####P < .0001 comparing sham and treated groups at follow-up. From Singh et al [58].
The relevance to humans needs to be demonstrated but the findings are suggestive. It is known that basal NO activity of the renal vasculature is reduced in patients with chronic glomerular disease compared with age- and BP-matched control groups. This is at least in part due to increased oxidative stress in the renal vasculature [59]. The effects of renal denervation on eGFR have been reported to be greater in uncontrolled and in controlled hypertensive patients with CKD [60], which is in line with the reported greater effects in CKD sheep than in normotensive animals.
In patients with resistant hypertension and elevated urinary albumin-to-creatinine ratio, there is a significant reduction in albuminuria after catheter-based renal denervation [61]. There is also evidence of benefits on eGFR from a number of independent research groups. We have shown annual eGFR loss in 27 uncontrolled hypertensive patients with an eGFR 30–59 mL/min/1.73 m2 to be attenuated over at least 12 months by renal denervation [61]. Similar stabilisation of eGFR for up to 24 months after the intervention were shown in 46 patients with CKD Stage 3 [62]. A study of 30 patients with mild-to-moderate CKD (mean eGFR 61.9 ± 23.9 mL/min/1.73 m2) and refractory hypertension, eGFR even increased from baseline after renal denervation and the difference remained significant at all time points up to the end of follow-up at 24 months [63].
In the recent analysis of CKD patients in the Global Symplicity Registry [46], patients with CKD had a less steep decline in GFR from baseline to 1 year than those without CKD, and after the first year, GFR decline per year was in the expected range, without any clinically meaningful reduction in any of the groups [46].
More data on the effect of renal denervation in CKD patients will arrive over the next few years. The SPYRAL AFFIRM global study (NTC05198674) is a single-arm interventional study designed to evaluate the long-term safety, efficacy and durability of the Symplicity Spyral renal denervation system. In contrast to the RCTs, the exclusion criterion for renal function in SPYRAL AFFIRM is an eGFR of <30 mL/min/1.73 m2, which will enable the enrolment of a relevant number of patients with significantly reduced renal function. Another trial specifically designed to enrol patients with CKD stage 3a or 3b is the RDN-CKD Study (NTC04264403), a prospective, randomized, double-blind multicentre feasibility study investigating the effect of ultrasound renal denervation on 24-h ambulatory BP and eGFR over up to 12 months.
FUTURE PERSPECTIVES
Renal denervation is becoming a relevant treatment option for hypertension, as confirmed by the latest position statements and consensus documents from international [5, 6, 64] and national societies. These recommendations position interventional therapies as an important adjunct to pharmacotherapy, capitalizing on the safety, ‘always on’ effect and lack of adherence issues. Importantly, patients may express preference for renal denervation for different reasons to those of physicians and these perspectives need to be respected in a shared decision process [6].
In addition, the potential benefits from the sympatholytic effects of renal denervation need to be further explored and may point to a role for the intervention in particular patient groups such as people with CKD, who are characterized by increased sympathetic activity. The accumulating evidence for the safety of renal denervation justify trials in various CKD populations, as the early concerns and exclusion criteria for clinical trials lose their relevance.
Ongoing research is targeting a number of outstanding questions: which subpopulations may derive the greatest BP reductions from the intervention [65] long-term effectiveness and safety (beyond 3 years); predictors of response; appropriate markers of successful ablation; and, as shown in this review, the potential nephroprotection and benefits beyond BP reduction in CKD patients.
If the recent past is any guide, ongoing and planned clinical studies will provide the necessary reassurance to physicians and healthcare authorities for renal denervation to take its place as a safe, effective and protective antihypertensive intervention of particular interest to hypertensive patients with CKD.
ACKNOWLEDGEMENTS
Editorial support for this article was provided by Pelle Stolt PhD, Basel Switzerland, with support from Medtronic. The author had full control of the content and any views expressed are his own.
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.
REFERENCES
- 1. Muntner P, Anderson A, Charleston Jet al. . Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2010; 55: 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sim JJ, Bhandari SK, Shi Jet al. . Comparative risk of renal, cardiovascular, and mortality outcomes in controlled, uncontrolled resistant, and nonresistant hypertension. Kidney Int 2015; 88: 622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bikbov B, Purcell CA, Levey ASet al. . Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet North Am Ed 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas G, Xie D, Chen H-Yet al. . Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the chronic renal insufficiency cohort study. Hypertension 2016; 67: 387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kandzari DE, Townsend RR, Bakris Get al. . Renal denervation in hypertension patients: proceedings from an expert consensus roundtable cosponsored by SCAI and NKF. Catheter Cardiovasc Interv 2021; 98: 416–426 [DOI] [PubMed] [Google Scholar]
- 6. Schmieder RE, Mahfoud F, Mancia Get al. . European society of hypertension position paper on renal denervation 2021. J Hypertens 2021; 39: 1733–1741 [DOI] [PubMed] [Google Scholar]
- 7. Williams B, Mancia G, Spiering Wet al. . 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104 [DOI] [PubMed] [Google Scholar]
- 8. DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1517–R1524 [DOI] [PubMed] [Google Scholar]
- 9. Schmieder RE. Renal denervation: where do we stand and what is the relevance to the nephrologist? Nephrol Dial Transplant 2020; 37: 638–644 [DOI] [PubMed] [Google Scholar]
- 10. Zoccali C, Mallamaci F, Parlongo Set al. . Plasma norepinephrine predicts survival and incident cardiovascular events in patients with end-stage renal disease. Circulation 2002; 105: 1354–1359 [DOI] [PubMed] [Google Scholar]
- 11. Converse RL, Jacobsen TN, Toto RDet al. . Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 1992; 327: 1912–1918 [DOI] [PubMed] [Google Scholar]
- 12. Hausberg M, Kosch M, Harmelink Pet al. . Sympathetic nerve activity in end-stage renal disease. Circulation 2002; 106: 1974–1979 [DOI] [PubMed] [Google Scholar]
- 13. Grassi G, Seravalle G, Brambilla Get al. . Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension 2015; 65: 1209–1216 [DOI] [PubMed] [Google Scholar]
- 14. Hering D, Lambert EA, Marusic Pet al. . Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 2013; 61: 457–464 [DOI] [PubMed] [Google Scholar]
- 15. Osborn JW, Tyshynsky R, Vulchanova L. Function of renal nerves in kidney physiology and pathophysiology. Annu Rev Physiol 2021; 83: 429–450 [DOI] [PubMed] [Google Scholar]
- 16. Bhatt DL, Kandzari DE, O'Neill WWet al. . A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014; 370: 1393–1401 [DOI] [PubMed] [Google Scholar]
- 17. Kandzari DE, Bhatt DL, Brar Set al. . Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 2015; 36: 219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahfoud F, Böhm M, Azizi Met al. . Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015; 36: 2219–2227 [DOI] [PubMed] [Google Scholar]
- 19. Kario K, Yokoi Y, Okamura Ket al. . Catheter-based ultrasound renal denervation in patients with resistant hypertension: the randomized, controlled REQUIRE trial. Hypertens Res 2022; 45: 221–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schmieder RE, Bosch A. Editorial comment: renal denervation. Hypertens Res 2022; 45: 241–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Townsend RR, Mahfoud F, Kandzari DEet al. . Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017; 390: 2160–2170 [DOI] [PubMed] [Google Scholar]
- 22. Kandzari DE, Böhm M, Mahfoud Fet al. . Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018; 391: 2346–2355 [DOI] [PubMed] [Google Scholar]
- 23. Azizi M, Schmieder RE, Mahfoud Fet al. . Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018; 391: 2335–2345 [DOI] [PubMed] [Google Scholar]
- 24. Azizi M, Sanghvi K, Saxena Met al. . Ultrasound renal denervation for hypertension resistant to a triple medication pill (RADIANCE-HTN TRIO): a randomised, multicentre, single-blind, sham-controlled trial. Lancet 2021; 397: 2476–2486 [DOI] [PubMed] [Google Scholar]
- 25. Böhm M, Kario K, Kandzari DEet al. . Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED pivotal): a multicentre, randomised, sham-controlled trial. Lancet 2020; 395: 1444–1451 [DOI] [PubMed] [Google Scholar]
- 26. Azizi M, Pereira H, Hamdidouche Iet al. . Adherence to antihypertensive treatment and the blood pressure-lowering effects of renal denervation in the renal denervation for hypertension (DENERHTN) trial. Circulation 2016; 134: 847–857 [DOI] [PubMed] [Google Scholar]
- 27. Mahfoud F, Kandzari DE, Kario Ket al. . Long-term efficacy and safety of renal denervation in the presence of antihypertensive drugs (SPYRAL HTN-ON MED): a randomised, sham-controlled trial. Lancet North Am Ed 2022; 399: 1401–10. S014067362200455X [DOI] [PubMed] [Google Scholar]
- 28. Mahfoud F, Renkin J, Sievert Het al. . Alcohol-mediated renal denervation using the peregrine system infusion catheter for treatment of hypertension. JACC Cardiovasc Interv 2020; 13: 471–484 [DOI] [PubMed] [Google Scholar]
- 29. Weber MA, Kirtane AJ, Weir MRet al. . The REDUCE HTN: REINFORCE: randomized, sham-controlled trial of bipolar radiofrequency renal denervation for the treatment of hypertension. JACC Cardiovasc Interv 2020; 13: 461–470 [DOI] [PubMed] [Google Scholar]
- 30. Ogoyama Y, Tada K, Abe Met al. . Effects of renal denervation on blood pressures in patients with hypertension: a systematic review and meta-analysis of randomized sham-controlled trials. Hypertens Res 2022; 45: 210–220 [DOI] [PubMed] [Google Scholar]
- 31. Ott C, Schmieder RE. Diagnosis and treatment of arterial hypertension 2021. Kidney Int 2022; 101: 36–46 [DOI] [PubMed] [Google Scholar]
- 32. Booth LC, Nishi EE, Yao STet al. . Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radiofrequency renal denervation in sheep. Hypertension 2015; 65: 393–400 [DOI] [PubMed] [Google Scholar]
- 33. Singh RR, McArdle ZM, Iudica Met al. . Sustained decrease in blood pressure and reduced anatomical and functional reinnervation of renal nerves in hypertensive sheep 30 months after catheter-based renal denervation. Hypertension 2019; 73: 718–727 [DOI] [PubMed] [Google Scholar]
- 34. Rousselle SD, Brants IK, Sakaoka Aet al. . Neuromatous regeneration as a nerve response after catheter-based renal denervation therapy in a large animal model: immunohistochemical study. Circ Cardiovasc Interv 2015; 8: e002293. [DOI] [PubMed] [Google Scholar]
- 35. Sharp A, Tunev S, Schlaich MPet al. . Abstract P195: no functional nerve recovery to 6 months post renal denervation with rf energy in a normotensive pig model. Hypertension 2021; 78: AP195 [Google Scholar]
- 36. Hansen JM, Abildgaard U, Fogh-Andersen Net al. . The transplanted human kidney does not achieve functional reinnervation. Clin Sci 1994; 87: 13–20 [DOI] [PubMed] [Google Scholar]
- 37. Mahfoud F, Mancia G, Schmieder Ret al. . Three-year safety and efficacy in the Global Symplicity Registry: impact of anti-hypertensive medication burden on blood pressure reduction [Internet]. https://media.pcronline.com/diapos/PCReCourse2020/172-20200625_1611_Abstracts_and_Cases_Corner_Mahfoud_Felix_0000_(672)/Mahfoud_Felix_20200625_1600_Community_channel_4.pdf (2 April 2021, date last accessed) [Google Scholar]
- 38. Juknevičius V, Berūkštis A, Juknevičienė Ret al. . Long-term effects of renal artery denervation. Med Kaunas Lith 2021; 57: 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krum H, Schlaich M, Whitbourn Ret al. . Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet North Am Ed 2009; 373: 1275–1281 [DOI] [PubMed] [Google Scholar]
- 40. Carey RM, Sakhuja S, Calhoun DAet al. . Prevalence of apparent treatment-resistant hypertension in the United States: comparison of the 2008 and 2018 American Heart Association scientific statements on resistant hypertension. Hypertension 2019; 73: 424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahfoud F, Townsend RR, Kandzari DEet al. . Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol 2021; 77: 2909–2919 [DOI] [PubMed] [Google Scholar]
- 42. Mauriello A, Rovella V, Anemona Let al. . Increased sympathetic renal innervation in hemodialysis patients is the anatomical substrate of sympathetic hyperactivity in end-stage renal disease. J Am Heart Assoc 2015; 4: e002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grassi G, Biffi A, Seravalle Get al. . Sympathetic nerve traffic overactivity in chronic kidney disease: a systematic review and meta-analysis. J Hypertens 2021; 39: 408–416 [DOI] [PubMed] [Google Scholar]
- 44. Desir GV. Renalase deficiency in chronic kidney disease, and its contribution to hypertension and cardiovascular disease. Curr Opin Nephrol Hypertens 2008; 17: 181–185 [DOI] [PubMed] [Google Scholar]
- 45. Xia M, Liu T, Chen Det al. . Efficacy and safety of renal denervation for hypertension in patients with chronic kidney disease: a meta-analysis. Int J Hyperthermia 2021; 38: 732–742 [DOI] [PubMed] [Google Scholar]
- 46. Ott C, Mahfoud F, Mancia Get al. . Renal denervation in patients with versus without chronic kidney disease: results from the Global SYMPLICITY Registry with follow-up data of 3 years. Nephrol Dial Transplant 2021; 37: 304–310. [DOI] [PubMed] [Google Scholar]
- 47. Schneider S, Promny D, Sinnecker Det al. . Impact of sympathetic renal denervation: a randomized study in patients after renal transplantation (ISAR-denerve). Nephrol Dial Transplant 2015; 30: 1928–1936 [DOI] [PubMed] [Google Scholar]
- 48. Rippy MK, Zarins D, Barman NCet al. . Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol 2011; 100: 1095–1101 [DOI] [PubMed] [Google Scholar]
- 49. Sakakura K, Tunev S, Yahagi Ket al. . Comparison of histopathologic analysis following renal sympathetic denervation over multiple time points. Circ Cardiovasc Interv 2015; 8: e001813. [DOI] [PubMed] [Google Scholar]
- 50. Townsend RR, Walton A, Hettrick DAet al. . Review and meta-analysis of renal artery damage following percutaneous renal denervation with radiofrequency renal artery ablation. EuroIntervention 2020; 16: 89–96 [DOI] [PubMed] [Google Scholar]
- 51. Sardar P, Bhatt DL, Kirtane AJet al. . Sham-Controlled randomized trials of catheter-based renal denervation in patients with hypertension. J Am Coll Cardiol 2019; 73: 1633–1642 [DOI] [PubMed] [Google Scholar]
- 52. Sanders MF, Reitsma JB, Morpey Met al. . Renal safety of catheter-based renal denervation: systematic review and meta-analysis. Nephrol Dial Transplant 2017; 32: 1440–1447 [DOI] [PubMed] [Google Scholar]
- 53. Rader F, Kirtane A, Wang Yet al. . Durability of reduced office-measured blood pressure and antihypertensive medication use after ultrasound renal denervation: 24-month results from the RADIANCE-HTN SOLO trial. J Am Coll Cardiol 2021; 78: B152 [Google Scholar]
- 54. Schmieder RE. Nephroprotection by antihypertensive agents. J Cardiovasc Pharmacol 1994; 24 Suppl 2: S55–S64 [PubMed] [Google Scholar]
- 55. Piperidou A, Loutradis C, Sarafidis P.. SGLT-2 inhibitors and nephroprotection: current evidence and future perspectives. J Hum Hypertens 2021; 35: 12–25 [DOI] [PubMed] [Google Scholar]
- 56. Strippoli GFM, Craig M, Deeks JJet al. . Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ 2004; 329: 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Strojek K, Grzeszczak W, Górska Jet al. . Lowering of microalbuminuria in diabetic patients by a sympathicoplegic agent: novel approach to prevent progression of diabetic nephropathy? J Am Soc Nephrol 2001; 12: 602–605 [DOI] [PubMed] [Google Scholar]
- 58. Singh RR, McArdle ZM, Booth LCet al. . Increase in bioavailability of nitric oxide after renal denervation improves kidney function in sheep with hypertensive kidney disease. Hypertension 2021; 77: 1299–1310 [DOI] [PubMed] [Google Scholar]
- 59. Schäufele TG, Schlaich MP, Delles Cet al. . Impaired basal NO activity in patients with glomerular disease and the influence of oxidative stress. Kidney Int 2006; 70: 1177–1181 [DOI] [PubMed] [Google Scholar]
- 60. Kiuchi MG, Chen S.. Improvement of renal function after renal sympathetic denervation in CKD patients with controlled vs. uncontrolled hypertension . Int J Cardiol 2016; 223: 494–496 [DOI] [PubMed] [Google Scholar]
- 61. Ott C, Mahfoud F, Schmid Aet al. . Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens 2015; 33: 1261–1266 [DOI] [PubMed] [Google Scholar]
- 62. Hering D, Marusic P, Duval Jet al. . Effect of renal denervation on kidney function in patients with chronic kidney disease. Int J Cardiol 2017; 232: 93–97 [DOI] [PubMed] [Google Scholar]
- 63. Kiuchi MG, Graciano ML, Carreira MAM de Qet al. . Long-term effects of renal sympathetic denervation on hypertensive patients with mild to moderate chronic kidney disease. J Clin Hypertens 2016; 18: 190–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kandzari DE, Mahfoud F, Weber MAet al. . Clinical trial design principles and outcomes definitions for device-based therapies for hypertension: a consensus document from the hypertension academic research consortium. Circulation 2022; 145: 847–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mahfoud F, Mancia G, Schmieder Ret al. . Renal denervation in high-risk patients with hypertension. J Am Coll Cardiol 2020; 75: 2879–2888 [DOI] [PubMed] [Google Scholar]