Abstract
Context
Tirzepatide is a glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist approved for treatment of type 2 diabetes (T2D). SURPASS-1, a phase 3 trial of tirzepatide monotherapy in people with early T2D, enables evaluating effects of tirzepatide on pancreatic beta-cell function and insulin sensitivity (IS) without other background antihyperglycemic medications.
Objective
Explore changes in biomarkers of beta-cell function and IS with tirzepatide monotherapy.
Design
Post hoc analyses of fasting biomarkers with analysis of variance and mixed model repeated measures.
Setting
Forty-seven sites in 4 countries.
Patients
Four hundred seventy-eight T2D participants.
Intervention
Tirzepatide (5, 10, 15 mg), placebo.
Main Outcome Measure(s)
Analyze biomarkers of beta-cell function and IS at 40 weeks.
Results
At 40 weeks, markers of beta-cell function improved with tirzepatide monotherapy vs placebo with reductions from baseline in fasting proinsulin levels (49-55% vs −0.6%) and in intact proinsulin/C-peptide ratios (47-49% vs −0.1%) (P < .001, all doses vs placebo). Increases from baseline in homeostatic model assessment for beta-cell function (computed with C-peptide) (77-92% vs −1.4%) and decreases in glucose-adjusted glucagon levels (37-44% vs +4.8%) were observed with tirzepatide vs placebo (P < .001, all doses vs placebo). IS improved as indicated by reductions from baseline in homeostatic model assessment for insulin resistance (9-23% vs +14.7%) and fasting insulin levels (2-12% vs +15%), and increases in total adiponectin (16-23% vs −0.2%) and insulin-like growth factor binding protein 2 (38-70% vs +4.1%) with tirzepatide vs placebo at 40 weeks (P ≤ .031, all doses vs placebo, except for fasting insulin levels with tirzepatide 10 mg).
Conclusions
As monotherapy for early T2D, tirzepatide achieved significant improvements in biomarkers of both pancreatic beta-cell function and IS.
Keywords: tirzepatide, beta-cell function, insulin sensitivity, type 2 diabetes, incretin, monotherapy
Tirzepatide is a novel glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that was recently approved for the treatment of type 2 diabetes (T2D). In the SURPASS trials, once-weekly treatment with tirzepatide (5, 10, and 15 mg) demonstrated robust improvements in glycosylated hemoglobin A1c (HbA1c; up to −2.59% reduction) and body weight [up to −12.9 kg (−13.9%) reduction] across a wide spectrum of people with T2D ranging from those treated with tirzepatide monotherapy to tirzepatide combined with oral antihyperglycemic medications with or without basal insulin [1‐5]. Notably, in studies comparing tirzepatide with selective GLP-1 receptor agonists, tirzepatide delivered superior glycemic control and weight reduction in people with T2D [2, 6, 7].
Although metformin is often used as an initial treatment for newly diagnosed T2D, more than 80% of people with early T2D treated with tirzepatide as monotherapy reached target HbA1c goals of <7.0% in the SURPASS-1 trial [4]. This study provides a unique opportunity to assess mechanisms by which tirzepatide improves glycemic function in the absence of potential confounding effects of other background antihyperglycemic medications.
Impaired insulin production from pancreatic beta cells and impaired insulin action both contribute to hyperglycemia in T2D [8]. To date, effects of tirzepatide monotherapy in people with T2D on pancreatic beta-cell function or insulin sensitivity have not been investigated. In preclinical studies, tirzepatide improved insulin sensitivity in GLP-1 receptor null mice, thus demonstrating the important role of GIP receptor agonism in insulin sensitization [9] in addition to contributions of both GLP-1 and GIP receptor agonism to glucose-dependent insulin secretion [10]. In this analysis, we explored changes in biomarkers of beta-cell function and insulin sensitivity after tirzepatide monotherapy in participants from the SURPASS-1 trial.
Methods
Trial Design and Participants
The study design, full inclusion and exclusion criteria, and primary results of the SURPASS-1 clinical trial have been previously reported [4, 11]. Briefly, participants in this 40-week, multicenter, randomized, double-blind, placebo-controlled, parallel group trial were randomly assigned (1:1:1:1) to receive once a week tirzepatide (5, 10, or 15 mg) or volume-matched placebo in a single-dose pen. Assignment to treatment group was determined by a computer-generated random sequence using the Eli Lilly and Company interactive web-response system. Key eligibility criteria included adults with T2D (HbA1c of ≥7.0% to ≤9.5% at screening), naïve to injectable therapy for T2D, no diabetes medication within 90 days of the screening visit, body mass index (BMI) of ≥23 kg/m2, and stable weight (±5%) during the previous 3 months with agreement to not initiate a diet or exercise program during the study with the intent of reducing body weight other than the lifestyle and dietary measures for diabetes treatment. The SURPASS-1 trial was conducted in accordance with the International Conference on Harmonisation Guidelines for Good Clinical Practice and the Declaration of Helsinki. All participants provided signed informed consent and protocols were approved by local ethical review boards. This study is registered with ClinicalTrials.gov (NCT03954834).
Fasting blood samples were collected for in-protocol and exploratory biomarker measures at baseline and at weeks 8, 16, 24, and 40 and during the safety follow-up. In-protocol biomarker analyses included fasting blood glucose, insulin, C-peptide, glucagon, and total adiponectin. Exploratory post hoc biomarker analyses included fasting intact proinsulin and insulin-like growth factor binding protein 2 (IGFBP-2). Homeostatic model assessment for beta-cell function (HOMA2-B), computed with fasting C-peptide levels, and homeostatic model assessment for insulin resistance (HOMA2-IR), computed with fasting insulin levels, indices were generated using the HOMA2 Calculator [12]. Fasting proinsulin levels enabled fasting proinsulin/C-peptide ratio calculations. All in-protocol biomarkers were measured at the central laboratory (Pacific Biomarkers Inc., Seattle, WA, USA). Post hoc exploratory biomarkers IGFBP-2 and intact proinsulin were measured using enzyme linked immunosorbent assays (Covance Laboratories Inc., Greenfield, IN, USA).
Statistical Analyses
All analyses were performed on the modified intent-to-treat population, comprised of all randomly assigned participants with at least 1 dose of study drug exposure. Participants who discontinued study drug due to inadvertent enrollment were excluded. Data collected after study drug discontinuation or rescue drug initiation were excluded from analysis. Overall, 478 participants were randomized and took at least 1 dose of the study drug, and 475 participants were included in this analysis (tirzepatide 5 mg, n = 121; 10 mg, n = 121; 15 mg, n = 120; placebo, n = 115). Analysis for baseline measures was performed using ANOVA, and analyses for change from baseline as well as percent change from baseline were conducted using mixed model repeated measures for postbaseline measures. For percent change from baseline analyses (except for fasting serum glucose), parameters of interest were analyzed on log scale and then converted back to original scale. There were no adjustments for multiplicity. Statistical test results were considered statistically significant at the two-sided alpha level of .05. Statistical analyses were performed using SAS version 9.4, unless otherwise specified.
Results
Baseline Demographics and Clinical Characteristics
Demographics and clinical characteristics were similar between groups (Table 1). The overall mean duration of diabetes was 4.7 years with a mean baseline HbA1c of 7.94% and BMI of 31.9 kg/m2. Fifty-four percent of participants had no prior use of oral antihyperglycemic medication, which was similarly distributed across groups.
Table 1.
Baseline demographics and patient characteristics
| Parameter | Tirzepatide 5 mg n = 121 | Tirzepatide 10 mg n = 121 | Tirzepatide 15 mg n = 121 | Placebon = 115 |
|---|---|---|---|---|
| Age, years | 54.1 ± 11.9 | 55.8 ± 10.4 | 52.9 ± 12.3 | 53.6 ± 12.8 |
| Female, n (%) | 65 (53.7) | 49 (40.5) | 58 (47.9) | 59 (51.3) |
| Duration of diabetes, years | 4.6 ± 5.08 | 4.9 ± 5.61 | 4.8 ± 4.99 | 4.5 ± 5.87 |
| HbA1c, % | 8.0 ± 0.84 | 7.9 ± 0.78 | 7.9 ± 1.02 | 8.1 ± 0.80 |
| Fasting serum glucose, mg/dL | 153.7 ± 37.28 | 152.6 ± 41.72 | 153.3 ± 40.40 | 154.8 ± 40.26 |
| Weight, kg | 87.0 ± 21.15 | 86.2 ± 19.50 | 85.4 ± 18.51 | 84.8 ± 20.01 |
| BMI, kg/m2 | 32.2 ± 6.98 | 32.2 ± 7.65 | 31.5 ± 5.48 | 31.7 ± 6.07 |
| Prior use of OAM, n (%) | 55 (45.5) | 53 (43.8) | 56 (46.3) | 55 (47.8) |
| Fasting insulin, pmol/L | 96.2 ± 6.09 | 90.7 ± 5.82 | 95.2 ± 6.19 | 84.8 ± 5.64 |
| HOMA2-B (computed with c-peptide) | 52.9 ± 2.67 | 51.8 ± 2.63 | 49.0 ± 2.54 | 49.4 ± 2.60 |
| HOMA2-IR (computed with insulin) | 2.0 ± 0.11 | 1.8 ± 0.10 | 2.0 ± 0.12 | 1.8 ± 0.10 |
| Proinsulin/C-peptide ratio | 11.1 ± 0.66 | 13.2 ± 0.80 | 12.8 ± 0.81 | 12.5 ± 0.84 |
| Fasting proinsulin, pmol/L | 8.2 ± 0.62 | 9.3 ± 0.72 | 8.9 ± 0.69 | 9.0 ± 0.71 |
| Fasting C-peptide, nmol/L | 0.8 ± 0.03 | 0.7 ± 0.03 | 0.7 ± 0.03 | 0.7 ± 0.03 |
| Adiponectin, mg/mL | 3.7 ± 0.21 | 3.8 ± 0.22 | 3.9 ± 0.23 | 3.8 ± 0.22 |
| IGFBP-2, ng/mL | 189.8 ± 10.1 | 195.3 ± 10.6 | 194.4 ± 10.5 | 207.5 ± 11.5 |
Data are mean ± SD or n (%) at baseline all randomized population (patient demographics) and estimate means (SE) from mITT population using log transformation then convert back to original scale (beta-cell function and insulin sensitivity parameters).
Abbreviations: HbA1c, glycosylated hemoglobin A1c; HOMA2-B, homeostatic model assessment for beta-cell function; HOMA2-IR, homeostatic model assessment for insulin resistance; IGFBP-2, insulin-like growth factor binding protein 2; mITT, modified intent-to-treat; OAM, oral antihyperglycemic medication.
Tirzepatide Improved Glycemic Control and Body Weight
Changes in HbA1c, fasting serum glucose, and body weight have been published [4]. Briefly, significant reductions in HbA1c, fasting serum glucose, and body weight were achieved with all doses of tirzepatide compared with placebo [11]. Glucose and weight reductions were observed as early as 4 to 8 weeks with dose escalation of tirzepatide to 5 mg [11].
Tirzepatide Improved Markers of Beta-cell Function
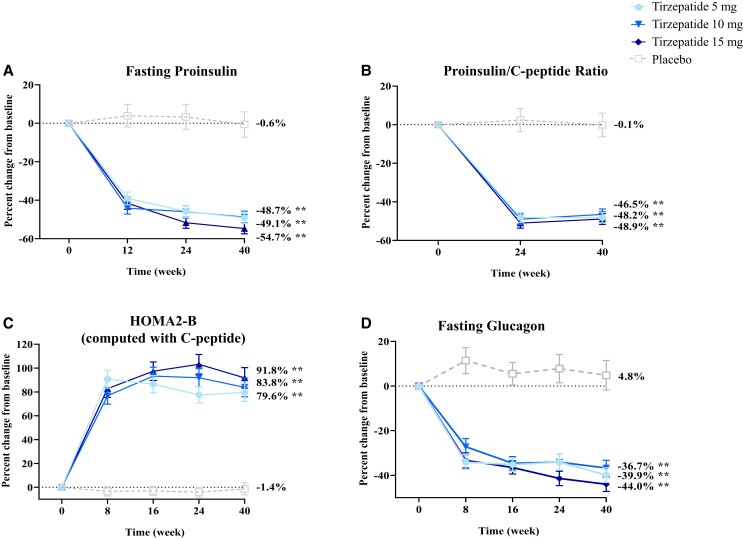
To assess effects of tirzepatide treatment on fasting biomarkers of pancreatic beta-cell function, we measured levels of intact proinsulin, a marker of pre-proinsulin processing indicative of beta-cell stress, and calculated proinsulin/C-peptide ratios. Intact proinsulin levels significantly decreased over time at all time points from baseline with tirzepatide 5, 10, and 15 mg (P < .001, all doses) (Fig. 1A). At 40 weeks, percent change from baseline of fasting proinsulin levels ranged from −55% to −49% with tirzepatide (P < .001, all doses) compared to −0.6% with placebo (P > .05) (Fig. 1A and Table 2). Estimated treatment differences in percent change from baseline in fasting proinsulin levels were significant between tirzepatide and placebo by 12 weeks when dose escalation of tirzepatide in higher dose cohorts had reached 7.5 mg and were sustained at 40 weeks (P < .001, all tirzepatide doses vs placebo) (Table 2).
Figure 1.
Percent change from baseline over time in markers of beta-cell function data are estimated percentage means (SE) overtime, mITT (efficacy analysis set) ANOVA analysis (week 0) and MMRM analysis (week 40) using log transformation then convert back to original scale. **P < .001 vs placebo at 40 weeks.
Abbreviations: HOMA2-B, homeostatic model assessment for beta-cell function; mITT, modified intent-to-treat; MMRM, mixed-model repeated measures.
Table 2.
Measures of biomarkers for beta-cell function and insulin sensitivity at week 40
| ITT-efficacy analysis seta, week 40 | Tirzepatide 5 m n = 121 |
Tirzepatide 10 mg n = 121 |
Tirzepatide 15 mg n = 120 |
Placebo n = 113 |
|---|---|---|---|---|
| Fasting serum glucose, mg/dL | ||||
| Baseline | 153.7 (3.65) | 152.6 (3.70) | 154.6 (3.73) | 155.2 (3.80) |
| Change from baseline | −43.6 (3.40) | −45.9 (3.45) | −49.3 (3.62) | 12.9 (4.00) |
| Difference vs placebo | −56.5 (−66.8, -46.1)** | −58.8 (−69.2, −48.4)** | −62.1 (−72.7, −51.5)** | — |
| Fasting insulin | ||||
| Baseline, pmol/L | 96.2 (6.09) | 90.7 (5.82) | 95.2 (6.19) | 84.8 (5.64) |
| Percent change from baseline | −4.5 (5.28) | −1.6 (5.50) | −11.8 (5.21) | 15.1 (7.60) |
| Difference vs placebo | −17.0 (−29.9, −1.7)* | −14.5 (−27.8, 1.4) | −23.3 (−35.6, −8.8)* | — |
| Fasting glucagon (adjusted for fasting glucose) | ||||
| Baseline, pmol/L | 83.1 (4.45) | 80.0 (4.29) | 77.1 (4.15) | 85.5 (4.73) |
| Percent change from baseline | −39.9 (3.28) | −36.7 (3.43) | −44.0 (3.20) | 4.8 (6.62) |
| Difference vs placebo | −42.7 (−51.3, −32.4)** | −39.6 (−48.7, −28.9)** | −46.6 (−54.8, −36.9)** | — |
| HOMA2-B (computed with C-peptide) | ||||
| Baseline | 52.9 (2.68) | 51.8 (2.63) | 49.0 (2.54) | 49.4 (2.60) |
| Percent change from baseline | 79.6 (7.57) | 83.8 (7.79) | 91.8 (8.56) | −1.4 (5.00) |
| Difference vs placebo | 82.2 (60.0, 107.4)** | 86.5 (63.7, 112.4)** | 94.6 (70.3, 122.3)** | — |
| HOMA2-IR (computed with insulin) | ||||
| Baseline | 1.98 (0.11) | 1.78 (0.10) | 1.98 (0.12) | 1.76 (0.10) |
| Percent change from baseline | −8.7 (4.82) | −8.5 (4.76) | −23.4 (4.36) | 14.7 (7.11) |
| Difference vs placebo | −20.4 (−32.2, −6.5)* | −20.2 (−31.9, −6.4)* | −33.2 (−43.4, −21.1)** | — |
| Fasting proinsulin | ||||
| Baseline, pmol/L | 8.22 (0.62) | 9.31 (0.72) | 8.93 (0.69) | 8.97 (0.71) |
| Percent change from baseline | −49.1 (2.93) | −48.7 (3.04) | −54.7 (2.75) | −0.6 (6.62) |
| Difference vs placebo | −48.9 (−57.0, −39.2)** | −48.4 (−56.7, −38.5)** | −54.5 (−61.9, −45.6)** | — |
| Fasting C-peptide | ||||
| Baseline, nmol/L | 0.75 (0.034) | 0.70 (0.032) | 0.68 (0.031) | 0.67 (0.032) |
| Percent change from baseline | −2.8 (3.69) | −3.1 (3.71) | −9.4 (3.65) | −0.7 (4.46) |
| Difference vs placebo | −2.2 (−12.9, 9.8) | −2.5 (−13.2, 9.5) | −8.8 (−19.0, 2.7) | — |
| Proinsulin/C-peptide ratio | ||||
| Baseline | 11.1 (0.66) | 13.2 (0.80) | 12.8 (0.81) | 12.5 (0.84) |
| Percent change from baseline | −48.2 (2.67) | −46.5 (2.81) | −48.9 (2.81) | −0.1 (6.13) |
| Difference vs placebo | −48.1 (−55.7, −39.3)** | −46.4 (−54.3, −37.2)** | −48.8 (−56.5, −39.8)** | — |
| Adiponectin | ||||
| Baseline, ug/mL | 3.68 (0.21) | 3.79 (0.22) | 3.93 (0.23) | 3.80 (0.22) |
| Percent change from baseline | 21.9 (5.25) | 15.7 (5.09) | 22.9 (5.64) | −0.2 (5.06) |
| Difference vs placebo | 22.2 (7.2, 39.2)* | 15.9 (1.6, 32.3)* | 23.2 (7.7, 40.9)* | — |
| IGFBP-2 | ||||
| Baseline, ng/mL | 189.8 (10.1) | 195.3 (10.6) | 194.4 (10.5) | 207.5 (11.5) |
| Percent change from baseline | 38.3 (6.74) | 60.5 (7.98) | 70.0 (8.82) | 4.1 (6.00) |
| Difference vs placebo | 32.8 (14.5, 54.1)** | 54.1 (32.7, 79.1)** | 63.3 (40.2, 90.2)** | — |
Data are LSM (SE) at baseline and change from baseline (fasting serum glucose) or estimate (SE) at baseline and percent change from baseline (all other biomarkers), and LSM (95% CI) (fasting serum glucose) or estimate (95% CI) (all other biomarkers) treatment difference vs placebo at week 40. Data were analyzed with MMRM analysis using the mITT efficacy analysis set (except for fasting serum glucose, analysis was performed on log-transformed data then converted back to the original scale). All markers were measured at the fasting state. Percent changes from baseline (and change from baseline for fasting serum glucose) at week 40 were significant for all biomarkers with tirzepatide, except fasting insulin, HOMA2-IR, and fasting C-peptide with the 5 and 10 mg dose. *P < .05 and **P < .001 vs placebo.
Abbreviations: BMI, body mass index; CI, confidence interval; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1; HbA1c, glycosylated hemoglobin; HOMA2-B, homeostasis model assessment of β-cell function; HOMA2-IR, homeostasis model assessment of insulin resistance; IGFBP-2, insulin-like growth factor binding protein 2; LSM, least squares mean; mITT, modified intent-to-treat (all randomized participants who took at least 1 dose of study drug); MMRM, mixed model repeated measures; RA, receptor agonist; T2D, type 2 diabetes.
mITT on treatment without rescue therapy and excluding participants who discontinued study drug due to inadvertent enrollment.
Intact proinsulin/C-peptide ratios significantly decreased at all time points from baseline with all tirzepatide doses, indicative of improved insulin processing within pancreatic beta cells. Decreases in percent change from baseline of intact proinsulin/C-peptide ratios ranged from 49% to 47% with tirzepatide (P < .001, all doses) compared to 0.1% with placebo (P > .05) at 40 weeks (Fig. 1B and Table 2). Estimated treatment differences in percent change from baseline in intact proinsulin/C-peptide ratios were significant between all tirzepatide doses and placebo by 24 weeks and maintained at 40 weeks (P < .001) (Table 2).
HOMA2-B index (computed with C-peptide), a marker of fasting beta-cell function, also significantly increased over time at all time points from baseline for all tirzepatide doses (P < .001, all doses). At 40 weeks, HOMA2-B (computed with C-peptide) significantly increased with all tirzepatide doses, ranging from 80% to 92% (P < .001, all doses) and decreased with placebo by −1.4% (P > .05) (Fig. 1C). Estimated treatment differences in percent change from baseline in HOMA2-B (computed with C-peptide) were significant between all tirzepatide doses and placebo by 8 weeks after dose escalation to 5 mg in all tirzepatide arms and maintained at 40 weeks (P < .001) (Table 2).
Glucose-adjusted glucagon levels significantly decreased over time at all time points from baseline for tirzepatide 5, 10, and 15 mg (P < .001, all doses) and increased with placebo at week 8 (P = .039) (Fig. 1D). At 40 weeks, decreases in percent change from baseline of glucose-adjusted glucagon levels ranged from 37% to 44% with tirzepatide (P < .001, all doses) compared to an increase of 4.8% with placebo (P = .461) (Fig. 1D). Estimated treatment differences in percent change from baseline in glucose-adjusted glucagon levels were significant between tirzepatide and placebo as early as 8 weeks after dose escalation to 5 mg in all tirzepatide arms, with further reductions at 40 weeks (P < .001, all tirzepatide doses vs placebo) (Table 2).
Tirzepatide Improved Markers of Insulin Sensitivity
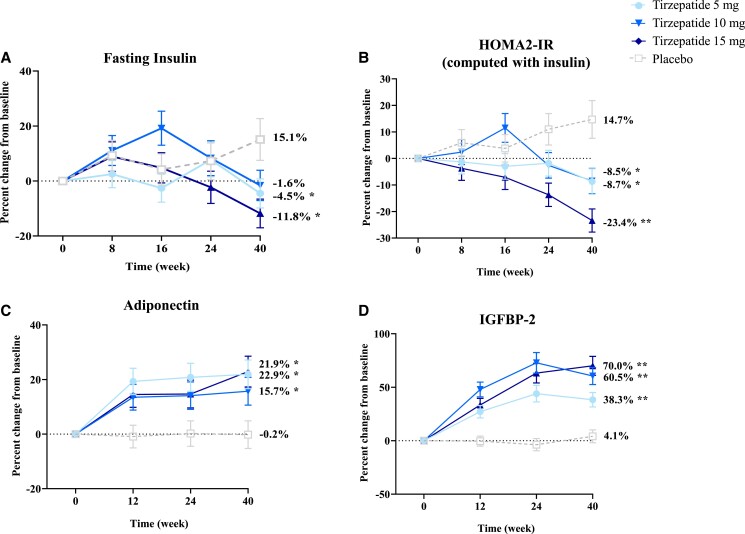
We measured fasting insulin levels as a reflection of insulin production from pancreatic beta cells in the context of changes in metabolic demand and insulin sensitivity. Fasting insulin levels significantly increased from baseline during the early stages of dose escalation at week 8 (after 4 weeks at 5 mg) and week 16 (after 4 weeks at 10 mg) in the tirzepatide 10 mg cohort (P ≤ .032), consistent with increases in insulin secretion. However, over the subsequent 24 weeks of the active treatment period, fasting insulin levels decreased toward baseline in the tirzepatide 10 mg group, likely reflecting reduced metabolic demand for insulin production as glycemic control improved. Fasting insulin also increased with a similar temporal pattern during the initial weeks of dose escalation to 5 mg in the tirzepatide 15 mg cohort but over time significantly decreased (P = .034) vs increased levels with placebo at week 40 (P = .034) (Fig. 2A). At 40 weeks, reductions in percent change from baseline of fasting insulin ranged from 2% to 12% with tirzepatide (P > .05 tirzepatide 5 and 10 mg; P = .034, tirzepatide 15 mg) compared to increases of 15% with placebo (P = .034) (Fig. 2A). Estimated treatment differences in percent change from baseline in fasting insulin levels were significant between tirzepatide 5 and 15 mg vs placebo at 40 weeks (P ≤ .031) (Table 2).
Figure 2.
Percent change from baseline over time in markers of insulin sensitivity data are estimated percentage means (SE) overtime, mITT (efficacy analysis set) ANOVA analysis (week 0), and MMRM analysis (week 40) using log transformation then convert back to original scale. *P < .05 and **P < .001 vs placebo at 40 weeks. Abbreviations: HOMA2-B, homeostatic model assessment for beta-cell function; IGFBP-2, insulin- like growth factor binding protein 2; mITT, modified intent-to-treat; MMRM, mixed-model repeated measures.
We assessed longitudinal changes in HOMA2-IR index, a fasting measure of insulin resistance. HOMA2-IR (computed with insulin) significantly increased from baseline in the tirzepatide 10 mg cohort early in dose escalation through week 16 (P = .025), with a temporal pattern similar to fasting insulin levels, followed by subsequent decreases over time. HOMA2-IR (computed with insulin) was significantly decreased by weeks 24 and 40 in the tirzepatide 15 mg cohort (P ≤ .004), indicative of improved insulin sensitivity (Fig. 2B). At 40 weeks, percent change from baseline of HOMA2-IR (computed with insulin) reductions with tirzepatide ranged from 9% to 23% (P < .001, tirzepatide 15 mg only) compared to a 14.7% increase with placebo (P = .028) (Fig. 2B and Table 2). Estimated treatment differences in percent change from baseline in HOMA2-IR (computed with insulin) were significant between tirzepatide and placebo at 40 weeks (P ≤ .006, all tirzepatide doses vs placebo) (Table 2).
To further assess fasting biomarkers associated with improvements in insulin sensitivity, we measured adiponectin and IGFBP-2 levels. Total adiponectin levels significantly increased over time at all time points from baseline with tirzepatide 5, 10, and 15 mg (P ≤ .003, all doses) (Fig. 2C), indicative of improved insulin sensitivity. At 40 weeks, percent increase from baseline of total adiponectin levels ranged from 16% to 23% with tirzepatide (P ≤ .001, all doses) compared to a decrease of .2% with placebo (P > .05) (Fig. 2C and Table 2). Estimated treatment differences in percent change from baseline in total adiponectin levels were significant between tirzepatide and placebo at 40 weeks (P ≤ .028, all tirzepatide doses vs placebo) (Table 2).
IGFBP-2 levels significantly increased at all time points from baseline with tirzepatide 5, 10, and 15 mg (P < .001, all doses) with a pattern consistent with increasing insulin sensitivity over time (Fig. 2D). At 40 weeks, percent increase from baseline of IGFBP-2 levels ranged from 38% to 70% with tirzepatide (P < .001, all doses) compared to 4.1% with placebo (P > .05) (Fig. 2D). Estimated treatment differences in percent change from baseline in IGFBP-2 levels were significant between tirzepatide and placebo at 40 weeks (P < .001, all tirzepatide doses vs placebo) (Table 2).
Discussion
This is the first study to investigate the effects of tirzepatide monotherapy in the absence of any other background antihyperglycemic medications on pancreatic beta-cell function and insulin sensitivity in people with early T2D. Tirzepatide monotherapy at doses of 5, 10, and 15 mg in the SURPASS-1 trial demonstrated significant and clinically relevant enhancements in markers of pancreatic beta-cell function and insulin sensitivity in conjunction with improved glycemic control and body weight reduction.
Tirzepatide significantly improved multiple markers of pancreatic beta-cell function including HOMA2-B (calculated with C-peptide), fasting intact proinsulin, and intact proinsulin/C-peptide ratio. HOMA2-B indices rapidly increased at week 8 and reached the peak up to 103% at week 24 in a dose-dependent manner with tirzepatide treatment. HOMA2-B improvements have also been observed with tirzepatide treatment combined with metformin in independent studies [13, 14]. Elevated levels of fasting proinsulin are associated with pancreatic beta-cell dysfunction in T2D related to the impaired proinsulin to insulin conversion in a setting of an increased demand for insulin production [15]. Elevated proinsulin levels and proinsulin/C-peptide ratios may lead to endoplasmic reticulum stress and failure of pancreatic beta cells [16]. With tirzepatide monotherapy treatment, we observed a rapid and significant reduction of elevated intact proinsulin levels and proinsulin/C-peptide ratios by approximately 50%, suggesting an improvement in pancreatic beta-cell stress and dysfunction. These findings are consistent with a 26-week, randomized, double-blind phase 2b clinical trial of tirzepatide with metformin background in most participants that showed improved beta-cell function with reductions in fasting proinsulin, intact proinsulin/C-peptide ratios, and dose-dependent increases in HOMA2-B indices compared with dulaglutide 1.5 mg [14].
Treatment with tirzepatide at all doses demonstrated significant and rapid improvement in hyperglucagonemia and related alpha-cell dysfunction, as indicated by reductions in fasting glucagon levels adjusted for fasting glucose ranging from 37% to 44%. This finding is consistent with studies that demonstrated greater reductions in glucagon levels with tirzepatide treatment compared with selective GLP-1 receptor agonists in adults with T2D and background metformin therapy [6, 14]. Hyperglucagonemia is a hallmark in T2D, promoting hepatic glucose output and exacerbating hyperglycemia [17, 18]. Similarly, in people with obesity, fasting and postprandial glucagon levels are higher compared to those with normal body weight [19]. The relatively rapid reductions observed at week 8 in fasting glucagon and glucose levels with tirzepatide treatment preceded a gradual and substantial weight loss observed throughout the 40-week treatment period in this study. This pattern indicates that early improvements in glycemic control, as reflected by the observed reductions in fasting glucagon and glucose, primarily occurred before substantial body weight reductions.
In a recently published mechanism of action study, once-weekly tirzepatide at the 15 mg dose added to background metformin demonstrated significant improvements in glycemic control through concurrent improvements in beta-cell function and insulin sensitivity and by reductions in glucagon secretion. Improvements in both beta-cell function and insulin sensitivity contributed to a 6-fold increase from baseline in clamp disposition index with tirzepatide, as measured in hyperglycemic and euglycemic hyperinsulinemic clamp studies [6].
Tirzepatide reduced markers of insulin resistance including fasting insulin levels by up to 12% and HOMA2-IR (calculated by insulin) by up to 23% in the absence of concomitant antihyperglycemic therapy. These observations are consistent with effects of tirzepatide in other T2D populations on concomitant antihyperglycemic medications including metformin [6, 13, 14]. In a phase 2b trial, only 21% of the variation in improvement HOMA2-IR was directly attributable to weight loss with tirzepatide 15 mg and 13% with tirzepatide 10 mg in multiple linear regression analysis, suggesting that the observed insulin-sensitizing effects of tirzepatide were only partially attributable to weight loss [14]. Similarly, in SURPASS-2 on background metformin therapy, tirzepatide treatment resulted in greater reductions in HOMA2-IR and improvements in HOMA2-B than active comparator semaglutide 1 mg in conjunction with substantial improvements in HbA1c and body weight [2, 13].
Moreover, tirzepatide treatment resulted in increases in levels of insulin sensitivity biomarkers, adiponectin, and IGFBP-2. Adiponectin is exclusively produced in adipocytes, and adiponectin levels are lower in T2D and obesity compared to the healthy state [20, 21]. Reduced levels of adiponectin have also been associated with metabolic syndrome, dyslipidemia, and cardiovascular disease [22]. In human adipocytes, GIP receptors are present and functionally active [23‐25]. The GIP and GLP-1 receptor agonist tirzepatide increased adiponectin levels as monotherapy in these subjects with early T2D consistent with phase 2 clinical studies in people with T2D on metformin [6, 14]. Increases in adiponectin have been demonstrated to improve insulin sensitivity through insulin-sensitizing effects in mice [20]. IGFBP-2 is a member of the insulin and IGF signaling pathway, and IGFBP-2 levels in humans are inversely associated with BMI, fat mass, insulin levels, and fatty liver index [26‐30]. Tirzepatide treatment substantially increased IGFBP-2 levels in a dose and time-dependent pattern. Together, these findings demonstrated significant improvements in insulin sensitivity with tirzepatide monotherapy.
A strength of this study includes the opportunity to characterize actions of tirzepatide when administered as monotherapy. In this study, participants were not taking any antihyperglycemic medication other than tirzepatide, enabling a better assessment of the role of tirzepatide treatment to improve insulin sensitivity and beta-cell function without the confounding influence of other background antihyperglycemic medications. Notably, in these patients with early T2D, tirzepatide monotherapy reduced both pancreatic beta-cell stress and insulin resistance. Limitations of this study include the exploratory nature of the post hoc biomarker analysis and that assessments were made under fasting conditions. Additional studies are warranted to further define mechanisms by which tirzepatide results in improved glucose control and insulin sensitivity.
In conclusion, substantial improvements in both pancreatic beta-cell function and insulin sensitivity were observed with tirzepatide monotherapy, which may contribute to the enhanced glycemic control achieved with tirzepatide treatment in individuals with early T2D.
Acknowledgments
The authors would like to thank Chrisanthi A. Karanikas (Eli Lilly and Company) for medical writing and editing assistance with an earlier draft of the manuscript. Partial data from this study were presented at the American Diabetes Association 82nd Scientific Sessions held June 3-7, 2022 in New Orleans, LA. Additional details of this phase 3 study, titled, “A Study of Tirzepatide (LY3298176) in Participants With Type 2 Diabetes Not Controlled With Diet and Exercise Alone (SURPASS-1)” can be found at http://clinicaltrials.gov as NCT03954834.
Contributor Information
Clare J Lee, Email: clare.lee@lilly.com, Eli Lilly and Company, Indianapolis, IN, 46285, USA.
Huzhang Mao, Eli Lilly and Company, Indianapolis, IN, 46285, USA.
Vivian T Thieu, Eli Lilly and Company, Indianapolis, IN, 46285, USA.
Laura Fernández Landó, Eli Lilly and Company, Indianapolis, IN, 46285, USA.
Melissa K Thomas, Eli Lilly and Company, Indianapolis, IN, 46285, USA.
Funding
Funding for this study was provided by Eli Lilly and Company.
Author Contributions
L.F.L., M.K.T., and V.T.T. contributed to the study design. C.J.L. and V.T.T. provided medical oversight during the trial. H.M. was responsible for the statistical analyses. All authors are the guarantors of this work and, as such, take responsibility for the integrity of the data and the accuracy of the data analysis. All authors participated in interpretation of the data and critical review of the manuscript, had full access to all the data in the study and approved of this manuscript to be submitted for publication.
Disclosures
All authors are employees and shareholders of Eli Lilly and Company.
Data Availability
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1. Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811‐1824. doi: 10.1016/S0140-6736(21)02188-7 [DOI] [PubMed] [Google Scholar]
- 2. Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503‐515. doi: 10.1056/NEJMoa2107519 [DOI] [PubMed] [Google Scholar]
- 3. Ludvik B, Giorgino F, Jodar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583‐598. doi: 10.1016/S0140-6736(21)01443-4 [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock J, Wysham C, Frias JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143‐155. doi: 10.1016/S0140-6736(21)01324-6 [DOI] [PubMed] [Google Scholar]
- 5. Dahl D, Onishi Y, Norwood P, Huh R, Patel H, Rodríguez A. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327(6):534‐545. doi: 10.1001/jama.2022.0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heise T, Mari A, DeVries JH, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022;10(6):418‐429. doi: 10.1016/S2213-8587(22)00085-7 [DOI] [PubMed] [Google Scholar]
- 7. Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180‐2193. doi: 10.1016/S0140-6736(18)32260-8 [DOI] [PubMed] [Google Scholar]
- 8. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804‐814. doi: 10.1038/nm.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Samms RJ, Christe ME, Collins KA, et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J Clin Invest. 2021;131(12):e146353. doi: 10.1172/JCI146353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coskun T, Sloop KW, Loghin C, et al. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3‐14. doi: 10.1016/j.molmet.2018.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee CJ, Mao H, Thieu VT, Fernández Landó, L, Thomas MK. Supplemental Appendix: Tirzepatide as monotherapy improved markers of beta-cell function and insulin sensitivity in people with type 2 diabetes (SURPASS-1).https://zenodo.org/deposit/7798863 [DOI] [PMC free article] [PubMed]
- 12. University of Oxford . HOMA calculator. Accessed September 4, 2022. https://www.dtu.ox.ac.uk/homacalculator
- 13. Brown K, Fernández Landó L, Bergman B, Thomas MK, Liu B, Lee CJ. Tirzepatide improved markers of islet-cell function (fasting glucagon and HOMA2-B) and insulin sensitivity (fasting insulin and HOMA2-IR) compared with semaglutide in people with type 2 diabetes. Diabetes. 2022;71(Suppl 1):337-OR. doi: 10.2337/db22-337-OR [DOI] [Google Scholar]
- 14. Thomas MK, Nikooienejad A, Bray R, et al. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2021;106(2):388‐396. doi: 10.1210/clinem/dgaa863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahrén B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med. 2005;5(3):275‐286. doi: 10.2174/1566524053766004 [DOI] [PubMed] [Google Scholar]
- 16. Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia. 2007;50(4):752‐763. doi: 10.1007/s00125-006-0590-z [DOI] [PubMed] [Google Scholar]
- 17. Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36(3):274‐283. doi: 10.2337/diab.36.3.274 [DOI] [PubMed] [Google Scholar]
- 18. Shah P, Vella A, Basu A, Basu R, Schwenk WF, Rizza RA. Lack of suppression of glucagon contributes to postprandial hyperglycemia in subjects with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2000;85(11):4053‐4059. doi: 10.1210/jcem.85.11.6993 [DOI] [PubMed] [Google Scholar]
- 19. Stern JH, Smith GI, Chen S, Unger RH, Klein S, Scherer PE. Obesity dysregulates fasting-induced changes in glucagon secretion. J Endocrinol. 2019;243(2):149‐160. doi: 10.1530/JOE-19-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941‐946. doi: 10.1038/90984 [DOI] [PubMed] [Google Scholar]
- 21. Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79‐83. doi: 10.1006/bbrc.1999.0255 [DOI] [PubMed] [Google Scholar]
- 22. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 2006;116(7):1784‐1792. doi: 10.1172/JCI29126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yip RG, Wolfe MM. GIP Biology and fat metabolism. Life Sci. 2000;66(2):91‐103. doi: 10.1016/s0024-3205(99)00314-8 [DOI] [PubMed] [Google Scholar]
- 24. Samms RJ, Coghlan MP, Sloop KW. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol Metab. 2020;31(6):410‐421. doi: 10.1016/j.tem.2020.02.006 [DOI] [PubMed] [Google Scholar]
- 25. Weaver RE, Donnelly D, Wabitsch M, Grant PJ, Balmforth AJ. Functional expression of glucose-dependent insulinotropic polypeptide receptors is coupled to differentiation in a human adipocyte model. Int J Obes (Lond). 2008;32(11):1705‐1711. doi: 10.1038/ijo.2008.148 [DOI] [PubMed] [Google Scholar]
- 26. Wittenbecher C, Ouni M, Kuxhaus O, et al. Insulin-like growth factor binding protein 2 (IGFBP-2) and the risk of developing type 2 diabetes. Diabetes. 2019;68(1):188‐197. doi: 10.2337/db18-0620 [DOI] [PubMed] [Google Scholar]
- 27. Ballerini MG, Ropelato MG, Domené HM, Pennisi P, Heinrich JJ, Jasper HG. Differential impact of simple childhood obesity on the components of the growth hormone-insulin-like growth factor (IGF)-IGF binding proteins axis. J Pediatr Endocrinol Metab. 2004;17(5):749‐757. doi: 10.1515/jpem.2004.17.5.749 [DOI] [PubMed] [Google Scholar]
- 28. Allen NE, Appleby PN, Kaaks R, Rinaldi S, Davey GK, Key TJ. Lifestyle determinants of serum insulin-like growth-factor-I (IGF-I), C-peptide and hormone binding protein levels in British women. Cancer Causes Control. 2003;14(1):65‐74. doi: 10.1023/a:1022518321634 [DOI] [PubMed] [Google Scholar]
- 29. Street ME, Smerieri A, Montanini L, et al. Interactions among pro-inflammatory cytokines, IGF system and thyroid function in pre-pubertal obese subjects. J Biol Regul Homeost Agents. 2013;27(1):259‐266. [PubMed] [Google Scholar]
- 30. Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GBJ. The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75(3):762‐767. doi: 10.1210/jcem.75.3.1381372 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the United States and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.