ABSTRACT
Cancer is the second leading cause of death in people with chronic kidney disease (CKD) after cardiovascular disease. The incidence of CKD in patients with cancer is higher than in the non-cancer population. Across various populations, CKD is associated with an elevated risk of cancer incidence and cancer death compared with people without CKD, although the risks are cancer site-specific. Higher risk of cancer is detectable in mild CKD [estimated glomerular filtration rate (eGFR) 60–89 mL/min/1.73 m2], although this risk is more obvious if sensitive markers of kidney disease are used, such as cystatin C. Independent of eGFR, albuminuria is associated with increased risk of site-specific cancer incidence and death. Here, we explore the potential mechanisms for the increased risk of cancer observed in CKD, including patient factors (shared risks such as cardiometabolic disease, obesity, smoking, diet, lifestyle and environment), disease (genetic, inflammatory and infective) and treatment factors. In particular, we discuss the ways in which renal adverse events associated with conventional chemotherapies and newer systemic anti-cancer therapies (including targeted and immunotherapies) may contribute to worse cancer outcomes in people with CKD. Finally, we review the potential benefits of acknowledging increased risk of cancer in risk prediction tools used for the management of CKD.
Keywords: cancer, CKD, creatinine, cystatin C, GFR
RISK OF CANCER INCIDENCE AND DEATH IN CKD
Chronic kidney disease (CKD) is a significant and growing health problem, with CKD stage 3–5 affecting 11–13% of the general population globally. Understanding the relationship between CKD and multimorbidity is crucial to improve outcomes for patients. There is clear overlap of the risk factors for CKD and cancer, but the incidence of CKD in patients with cancer is higher than in the general population and can have major impacts on treatment options and outcomes. In this review, we examine the potential processes underlying the multifactorial association between CKD and cancer.
Incident cancer—which eGFR equation?
The vast majority of studies report cancer risk using estimated glomerular filtration rate (eGFR) based on serum creatinine alone (eGFRcr) [1–11]. However, eGFR based on a combination of creatinine and cystatin C (eGFRcr-cys) has been shown to be more accurate than eGFRcr in comparison with measured GFR [12]. eGFR based on cystatin C alone (eGFRcys) is more sensitive in detecting early changes in kidney function and increased risk of cardiovascular disease [13, 14].
Two studies have reported cancer risk in CKD by eGFR measures that incorporate cystatin C, with conflicting results. In the Atherosclerosis Risk in Communities (ARIC) cohort in the USA (n = 8935) [15], there was no clear association between lower eGFRcr-cys and overall cancer incidence after adjustment for other relevant baseline demographics. However, in 431 263 participants from the UK Biobank, cancer incidence and cancer death were compared by eGFRcr, eGFRcys and eGFRcr-cys. eGFR <90 mL/min/1.73 m2 by eGFRcys and eGFRcr-cys (but not eGFRcr) was associated with an increased risk of incident cancer, with a graded increase in risk with progressive decline in eGFR [16]. The risk was most strongly associated with eGFRcys {hazard ratio (HR) 1.04 [95% confidence interval (CI) 1.03–1.04]} [16]. There was also an increased risk in site-specific incidence of haematological, respiratory and abdominal solid organ cancers in people with eGFR <90 mL/min/1.73 m2, but only when kidney function was estimated using eGFRcys, and not eGFRcr [16].
Incident cancer in all CKD
Amongst people diagnosed with cancer, CKD has increased prevalence. In a French observational study of 4684 patients (Renal Insufficiency and Cancer Medications: IRMA), more than half of participants diagnosed with cancer had mild reduction in kidney function [17] <90 mL/min/1.73 m2 [57% by creatinine clearance; 53% by Modified Diet in Renal Disease [18] (MDRD) eGFR], and 12–20% had creatinine clearance or MDRD eGFR <60 mL/min/1.73 m2.
Cancer event rates are higher amongst people with CKD than in the general population [16]. Population cohorts from Taiwan (n = 405 878 [1] and n = 123 717 [2]), China (n = 11 508) [4], Korea (n = 242 583) [5], Japan (n = 961) [6], Sweden (n = 719 033) [7], Australia (n = 4077) [8] and the UK (n = 431 263) [16] suggest that there is up to 108% increased risk of overall cancer in patients with moderate CKD (eGFRcr <60 mL/min/1.73 m2), even after accounting for shared risk factors for CKD and cancer.
Albuminuria is independently and additively associated with cancer risk, specifically 9–66% increase in risk of incident cancer [1, 5, 15, 16]. This is observed whether proteinuria is identified semi-quantitatively on dipstick testing [1, 5, 9, 19] or quantitatively by urinary albumin-to-creatinine ratio (uACR) [15, 16, 20], demonstrating a dose–response relationship [1, 5, 16].
Incident cancer in non-dialysis CKD
In non-dialysis CKD, several population cohorts [3, 7, 9, 11, 21] and one meta-analysis [10] describe no difference in risk of overall cancer incidence or death, but do report associations between lower eGFRcr and elevated risk of specific cancer subtypes [7, 9, 10, 15, 19]. Associations between lower eGFRcr and risk of incident cancer exist for site-specific cancers of the renal tract (kidney, ureter and bladder) [7, 10, 16], oropharyngeal [19], respiratory [4, 16], haematologic [7, 16] (including myeloma [9], leukaemia [9]), skin [7] and abdominal solid organs [16]. Lower eGFR predominantly is not associated with increased risk of sex-specific prostate [7, 9, 10] and breast [7, 9, 10] cancers. The presence of albuminuria has been associated with site-specific incidence from cancers of the renal tract [16, 19, 20], lung [15, 16, 19, 20], stomach [19], abdominal solid organs [16] (including liver [19] and pancreas [19]) and haematological [16] (including myeloma [19] and non-myeloma [16]) malignancies, although not prostate [16, 20], breast [16, 19, 20] or digestive tract (including colorectal [19, 20]) cancers.
Incident cancer in end-stage kidney disease
It is well established that patients with end-stage kidney disease are at a higher risk of cancer than the general and CKD populations. Kidney transplant recipients have around 3-fold increased risk of developing cancer than the general population [22, 23], particularly in association with infections (especially viruses) [23]. Malignancy (21%) and infection (23.6%) are now the most common causes of death in kidney transplant recipients in the UK, outside of the first year after transplantation [24]. The risk of cancer increases with time post-transplantation [23, 24]. There are also reasonable data to support increased risk of cancer incidence and cancer death in people on dialysis [10, 23]. In a meta-analysis including data from five clinical trials and one population cohort (n = 32 057 individuals), there was a trend towards increased risk of cancer incidence and a significant increase in cancer death across all cancer subtypes among patients on dialysis [10]. In particular, there was more than double the risk of incident urinary tract cancers, more than 11 times the risk of endocrine cancers and more than twice the risk of death from digestive cancers. However, this was balanced by a relative reduction in risk of other site-specific cancers such as prostate.
Cancer outcomes
Perhaps more importantly than its association with incident cancer, CKD is associated with reduced cancer survival.
Death from cancer is common in people with CKD, accounting for 14–39% of deaths in people with eGFR <60 mL/min/1.73 m2 [19, 21, 25]. Across all cancer subtypes, CKD is associated with a 20–48% increase in cancer death [1, 2, 8, 16]. This is most pronounced when eGFR is estimated using cystatin C [HR for death from cancer 1.10 (95% CI 1.08–1.11) per 10 mL/min/1.73 m2 decline in eGFRcys] [16]. Relative to people who do not have CKD, people with moderate-to-severe CKD (G3–G5) have a 50–74% increase in risk of cancer death for abdominal solid organs [16] including liver [2], more than 3 times the risk of death from kidney [2] cancers, up to 7 times the risk of death from urinary tract cancers [2, 16] and 3–7 times the risk of cancer death from haematological cancers including lymphoma.
Similarly, albuminuria is associated with a 17–53% increase in risk of cancer death overall [1, 16, 19], but a more pronounced association with cancer death in certain site-specific cancers. In particular, albuminuria is associated with 2–4 times the risk of death from urinary tract cancers [16, 19], nearly 4 times the risk of death from myeloma [19] and smaller increases in risk of death from cancers of the abdominal solid organs [16] (including liver [19] and pancreas [19]) and digestive tract [16] (including stomach [19]). This association may be stronger in younger (<65 years) people [16], but remains for older (≥65 years) people, suggesting that it is not solely due to a confounding effect of age. Transient dipstick proteinuria does not appear to be associated with increased risk of cancer death, although it is associated with an increased risk of cardiovascular death [26].
WHY MIGHT CKD BE ASSOCIATED WITH CANCER?
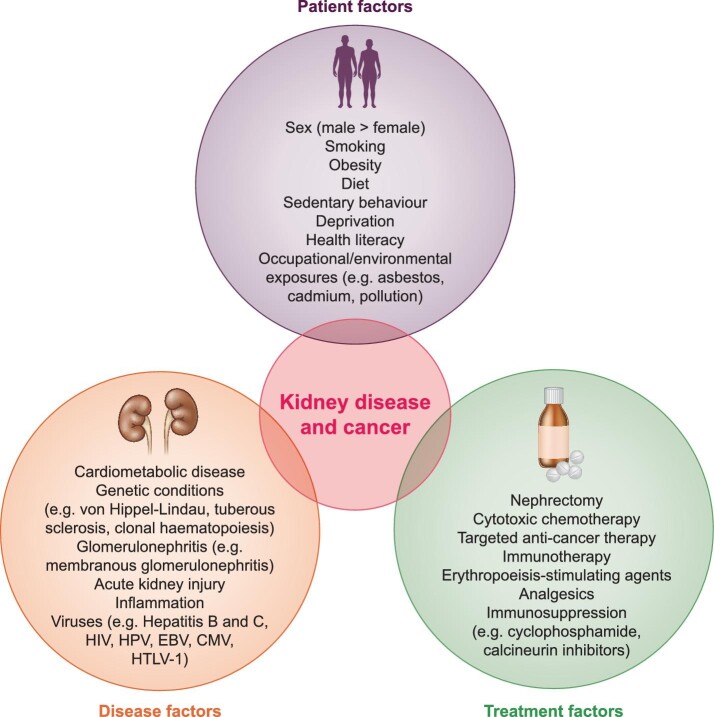
Patient, disease and treatment factors that may influence cancer risk in CKD are summarized in Figure 1.
FIGURE 1:
Patient, disease and treatment factors associated with kidney disease and cancer. HIV, human immunodeficiency virus; HPV, human papillomavirus; EBV, Epstein-Barr virus; CMV, cytomegalovirus; HTLV-1, human T-cell leukemia virus type 1.
Patient factors
In addition to shared risk factors including smoking, obesity and cardiometabolic disease, unhealthy lifestyle factors, such as poor diet and sedentary behaviour, are associated with increased risk of cancer mortality in patients with and without CKD [27], and increased risk of both incident [28] and progressive CKD [29]. The mechanisms behind the association between lifestyle and cancer have not been fully elucidated, but include a direct association with other cardiometabolic risk factors, increased inflammation leading to oxidative DNA damage or ingestion of carcinogens (such as nitrites contained in many preserved foods) [30]. Similarly, deprivation (reducing access to healthcare), health literacy (reducing understanding of the significance of symptoms or how to engage with healthcare services), and occupational or environmental exposures (such as asbestos, pesticides, dusts, hydrocarbons and other pollutants) may also contribute. Inadequate adjustment in observational models for these confounders may partly explain the observed associations. Exploration of causal pathways and the impact of shared genetic risks—e.g. using Mendelian randomization—could be a means of improving our understanding of these mechanisms.
Disease factors
Genetic conditions
Von Hippel-Lindau (VHL) disease is a rare genetic condition with an autosomal dominant inheritance pattern, characterized by mutations in the VHL tumour suppressor gene. This leads to abnormal tumour and cyst growth that may be benign and/or malignant, and, in particular, increases the risk of clear cell renal cell carcinoma. Resection of (recurrent) renal cell carcinoma in VHL disease often leads to progressive CKD and/or end-stage kidney disease requiring renal replacement therapy.
Tuberous sclerosis is another rare, autosomal dominant condition caused by inactivating mutations in tuberous sclerosis complex (TSC) tumour suppressor genes TSC1 or TSC2 [31]. Renal angiomyolipomata develop in the majority of patients with tuberous sclerosis. A smaller proportion of patients will develop large numbers of renal cysts and, more rarely, renal cell carcinoma. Ultimately, any of these abnormal growths can lead to kidney failure.
Clonal haematopoiesis is an age-related phenomenon characterized by somatic mutations in haematopoietic stem cells leading to clonal expansion of mutant leucocytes. People with evidence of clonal haematopoiesis are at substantially increased risk of haematologic cancer [32] (along with cardiovascular disease and premature death). More recently, myeloid clonal haematopoiesis has been shown to be causally associated with development of CKD (defined by eGFRcys) [33] and increases the risk of adverse outcomes (cardiovascular disease, kidney failure and myeloid cancers) in people with CKD [33].
Acute kidney injury, which causes CKD and is more common in pre-existing CKD, can promote the development of papillary renal cell carcinoma by stimulating DNA damage and repair processes, triggering renal progenitor cell proliferation and aberrant mitosis, and thus driving tumorigenesis [34].
Inflammatory and immune disorders
The most notable glomerular disorder is membranous glomerulonephritis, where 5–20% of patients have cancer. However, various other glomerulonephritides have been described in association with solid organ malignancies, most commonly minimal change disease and mesangio-proliferative glomerulonephritis, but also including immunoglobulin A nephropathy, focal segmental glomerulosclerosis, crescentic glomerulonephritis—including anti-glomerular basement membrane (GBM) disease and anti-neutrophil cystoplasmic antibody (ANCA)-associated vasculitis—and thrombotic microangiopathies [35].
CKD, albuminuria and cancer are associated with inflammation. Cystatin C is ubiquitously expressed and freely filtered at the glomerulus, functioning as a marker of glomerular filtration. As cystatin C is not impacted by age, gender and muscle mass, it may be a more sensitive marker of early changes in kidney function compared with serum creatinine. However, cystatin C may also be elevated in inflammatory states including some cancers. Cystatin C is an important endogenous inhibitor of regulatory enzymes involved in tumorigenesis, including cysteine proteases and tissue growth factor-β (TGF-β), but has been implicated both as a promoter [36] and as a suppressor [37, 38] of disease progression in certain cancer subtypes. It is not clear to what degree cystatin C as a marker both of inflammation and of glomerular filtration is confounded by the presence of cancer. To our knowledge, no study has investigated this specifically. Further discussion on this topic is beyond the scope of this review, but this may partially explain the stronger associations observed between eGFRcys and site-specific cancer risk.
Infections
Infections, and particularly viruses, can directly cause cancer and kidney disease, including Epstein–Barr virus, cytomegalovirus, human papillomavirus, human T-cell lymphotropic virus and hepatitis B and C. Human immunodeficiency virus can cause cancer and kidney disease on its own or exacerbate the carcinogenic effect of coinfection with other viruses. This partly explains the substantial increase in cancer risk, where the effects of immunosuppression appear to increase risk of cancer associated with infection, and the risk of incident cancer may be attenuated by withdrawal of immunosuppression [39].
Detection bias
Increased incidence of some renal-related cancers such as prostate and some haematologic cancers may be reported more frequently among people with CKD. Patients with CKD under specialty care have more frequent interactions with healthcare providers where ‘screening’ tests with urinalysis for haematuria by urine dipstick, blood testing for plasma cell dyscrasias and or investigation of urinary symptoms are undertaken as a part of routine clinical work up. Alternatively, kidney disease may be detected while undergoing investigation for an unrelated cancer diagnosis.
Reinforcing the concern about detection bias and/or reverse causality, one study reports a dissipation of the risk associated with CKD and specific cancer subtypes including prostate and haematological cancers after an observation period of 12 months [7]. However, landmark analyses in other populations have not shown significant attenuation of the association between eGFR [16] or albuminuria [16, 19] and cancer risk, suggesting that there may be a genuine association beyond the ability to detect pre-symptomatic cancer.
Treatment factors
Kidney disease treatments may cause cancer
Medications used in the treatment of CKD and associated conditions may result in cancer development. The obvious example is cyclophosphamide, used in the treatment of glomerulonephritis (such as membranous nephropathy) and haematological disorders (including lymphoma and myeloma), which is associated with the development of malignancy, particularly bladder cancer. Erythropoiesis-stimulating agents, used in the treatment of renal anaemia, may exacerbate pre-existing cancers [40]. Immunosuppression for inflammatory or immune glomerulonephritis, or after kidney transplantation, increases the risk of certain cancers, particularly, but not limited to, those driven by infections (especially viruses) [41].
Cancer treatments may cause kidney disease
The original platinum-based chemotherapy drug, cisplatin, was introduced in the 1970s and was a highly effective treatment against several solid organ malignancies including testicular, ovarian, lung, cervical and bladder cancers. However, it is renally excreted and highly nephrotoxic, causing a decrease in glomerular filtration (in a dose-dependent manner) within hours of administration [42]. Intravenous hydration can mitigate the risk of cisplatin-induced nephrotoxicity and is now considered an essential co-treatment, but cisplatin is routinely avoided in patients with pre-existing CKD (stages G3–G5). By comparison, carboplatin exhibits very low nephrotoxicity and may be selected as an alternative treatment in people with moderate CKD.
Many antimetabolites, used in the treatment of varied cancers, are at least partly renally excreted. At higher doses, methotrexate may be associated with intra-tubular precipitation, resulting in acute kidney injury and GFR decline [43]. Dose reductions are generally advised in those with pre-existing CKD G3–G5 or those on dialysis. Small studies suggest that there may be a similar, dose-dependent nephrotoxicity associated with pemetrexed therapy (used in the treatment of lung cancer), particularly when patients received a prolonged course of treatment of 10 cycles or more [44].
The alkylating agent ifosfamide is substantially more nephrotoxic than its cousin, cyclophosphamide, although the evidence for this mostly comes from the paediatric literature. Ifosfamide typically causes tubular dysfunction, or in some cases may result in a (usually mild) reduction in GFR [45], although the long-term effects are uncertain. The risk of renal side effects increases with higher doses and cumulative exposure, co-administration with cisplatin and in patients with pre-existing kidney disease.
More recent advances in treatment have been enabled by a better understanding of cancer biology and its immune microenvironment, leading to novel immune-based therapies with remarkable improvements in patient outcomes [46, 47]. The use of these new immune agents continues to grow, with use in increasingly early stages of disease [48]. Accordingly, we will continue to witness a shift from traditional cytotoxic chemotherapeutic agents to targeted therapies and immunotherapies. With this changing cancer therapy environment, the spectrum of adverse side effects will continue to diversify. This may be the case especially when these new anti-cancer agents are used in combination with conventional chemotherapies.
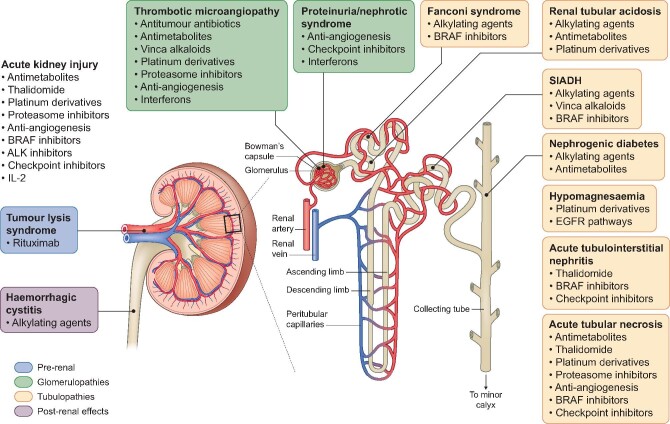
The use of systemic anti-cancer therapies (SACT) that are not renally excreted (e.g. immune checkpoints inhibitors; ICI) does not completely prevent off-target renal side effects [49]. SACT can affect any segment of the nephron leading to clinical manifestations including proteinuria, hypertension, electrolyte disturbances, glomerulopathy, acute or chronic interstitial nephritis, and repeated episodes of acute tubular injury leading to CKD [50]. Furthermore, patients even with mild CKD may have reduced excretion and metabolism of SACT, potentiating the risk of systemic toxicity, even when the drug is minimally renally excreted [51]. This potentially limits the dose or duration of SACT that can be delivered to patients with CKD. It therefore remains important to be aware of potential side effects of cancer agents to try to identify patients at higher risk to allow prevention or early management of kidney injury in order to avoid severe and permanent functional impairment. If severe injury is already established, providing adequate support to improve care for this cancer population is paramount. A summary of kidney side effects of common SACT are illustrated in Figure 2.
FIGURE 2:
Common renal adverse events associated with SACT by site of action. SIADH, syndrome of inappropriate antidiuretic hormone secretion; EGFR, epidermal growth factor receptor.
As a specific example, ICI treatment is associated with de novo CKD in around 13% of recipients with previously normal kidney function, especially at older age and with concomitant use of proton pump inhibitors, and is associated with more rapid eGFR decline that is more noticeable in people with pre-existing CKD [52]. Importantly, ICI are amongst only a few SACT that have been used in patients with advanced CKD or on dialysis [53]. Limited data from the literature suggest that patients on dialysis experience adverse events at a similar rate to the general population [53], but this cannot be regarded as conclusive and prospective work is warranted.
Around one-third of malignant cancers are treated with SACT, although trial evidence for use of specific drugs in people with CKD is often sparse. In a targeted search, Kitchlu et al. [54] assessed randomized controlled trials conducted in the five most common solid organ cancers (bladder, breast, colorectal, lung and prostate) from a selection of six high-impact medical and oncology journals from 2012 to 2017 inclusive. Of the 310 trials (292 889 patients) identified, 85% specifically excluded patients with markers of CKD. The exclusion criteria varied, but included elevated serum creatinine above normal limits (49% of trials), reduced creatinine clearance (highest threshold <60 mL/min; 44%), reduced eGFR (highest threshold <60 mL/min/1.73 m2; 5%), proteinuria (12% of trials), unspecified CKD (16% of trials) and/or multiple exclusion criteria for CKD (34% of trials). This report did not comment on the number of potential participants who were screened and then excluded from clinical cancer trials on the basis of CKD. Acknowledging that as many as half of patients with cancer may have evidence of CKD at diagnosis [17], it is likely that participants in clinical cancer trials are not representative of the patients who require treatment for cancer in the ‘real world’. Furthermore, the majority of SACT are licensed without safety information in patients with moderate-to-severe CKD, and almost none report safety information in patients on dialysis [51].
Dosing difficulties
Accurate estimation of GFR in people with cancer is essential to ensure optimal dosing of traditional cytotoxic chemotherapies and newer SACT. There are some concerns about the use of eGFR amongst people who have been diagnosed with cancer, particularly with respect to medication dosing. The consequences of inaccurate estimation of GFR include exposure of some patients to increased drug toxicity, whilst other patients may be denied treatments (or adequate doses of treatments), which are in fact safe.
Cisplatin is routinely avoided in people with eGFR <60 mL/min/1.73 m2. Although carboplatin is better tolerated and can be used in people with moderate CKD, it is purely renally excreted and dosing decisions are based on eGFR at the time of administration (using Calvert's formula). In people with cancer, GFR is generally overestimated by Cockcroft and Gault creatinine clearance and the CKD Epidemiology Collaboration, whereas GFR is generally underestimated by MDRD (although body surface area adjustment may improve accuracy) [55]. Compared with measured GFR, CamGFR—a model developed and validated in patients with cancer—has been shown to be more accurate than other published creatinine-based models and improves precision of carboplatin dosing to within clinically acceptable limits [56].
More evidence is needed to support dosing decisions and to advise on treatment efficacy and toxicity of SACT in patients with pre-existing CKD. Furthermore, different eGFR measures may be recommended for medication dosing decisions from those for CKD monitoring or cancer risk assessment.
CANCER RISK PREDICTION
As for cardiovascular disease, a variety of risk prediction tools have been developed and validated to assist in the prediction of cancer both in the presence of suggestive clinical scenarios and in asymptomatic individuals [57].
The risk assessment tools were designed for 15 cancer subtypes and display positive predictive value (PPV) ranges based on a patient's symptoms. National Institute for Health and Care Excellence (NICE) guidelines recommend referral for further investigation with a PPV of 3% or higher. The bladder cancer risk assessment tool incorporates ‘raised serum creatinine’; however, despite repeated studies showing association between eGFR, albuminuria and/or a categorical diagnosis of CKD, kidney function measures are not incorporated into risk assessment tools for lung, kidney, Hodgkin lymphoma and multiple myeloma. Furthermore, kidney function measures were not considered for inclusion in QCancer, designed to estimate the risk of undiagnosed cancer across all subtypes, based on existing risk factors, and/or current symptoms in men [58] and women [59].
We have illustrated that eGFRcr is inconsistently associated with risk of cancer overall, whilst eGFRcys may be more closely associated with cancer risk. Unlike serum creatinine, cystatin C is not influenced by age, sex, muscle mass or ethnicity, and demonstrates a negative, linear relationship with cardiovascular [13, 14] and cancer risk [16] below 90 mL/min/1.73 m2. By contrast, eGFRcr is not associated with increased cardiovascular or cancer risk until eGFRcr falls below ∼75 mL/min/1.73 m2 and has a U-shaped relationship with risk of cardiovascular disease and cancer [7, 13, 16].
By incorporating more sensitive measures of cancer risk relevant to CKD such as eGFRcys and albuminuria into cancer risk prediction tools, the PPV of the presenting symptoms in the setting of CKD may be raised above the threshold for further investigation, encouraging earlier referral, diagnosis and treatment. The utility of non-directed cancer screening in people with chronic disease (including CKD) is controversial, due to uncertainties around cost-effectiveness, accuracy and safety, particularly in a group at a higher risk of competing non-cancer illness and death. However, cancer risk prediction tools could be developed specifically for people with CKD to capture the elevated risk profile seen in some, but not all, cancer subtypes. Such tools may then permit targeted screening for those at the highest risk of specific cancers.
RELEVANCE AND FUTURE WORK
We summarize the relevance to the nephrologist of the relationship between CKD and cancer in Table 1. We have made some suggestions for future work to advance our understanding of cancer incidence, treatment and outcomes in people with CKD in Table 2.
Table 1.
Relevance to the nephrologist
| What is known already? | Cancer incidence is higher, though site-specific, in non-dialysis CKD, dialysis and after transplantation. |
| Cancer survival is reduced in people with CKD. | |
| Many or most cancer treatments have not been tested in clinical trials in people with advanced CKD (including dialysis and transplantation). | |
| SACT are associated with renal adverse events, including both acute and chronic glomerular and tubular injury. | |
| What do we not yet know? | Is there a causative relationship between CKD and site-specific cancers? |
| What is the value of cancer screening in people with CKD? Is this equivalent to the general population? Should screening be enhanced, relaxed or targeted? | |
| Are there differences in cancer treatment choices in people with CKD (e.g. reduced dose or duration of treatment)? Are systemic anti-cancer treatments associated with a worse side-effect profile in people with CKD? | |
| Why is survival reduced in people with CKD? Possibilities include delayed cancer diagnosis and more invasive disease at presentation, more aggressive disease course, less effective treatment or greater risk of complications (treatment side effects or increased propensity to infections, cardiovascular events, etc.) | |
| What can the nephrologist do? | Encourage participation of patients with CKD in general population cancer screening programmes (pending advice to the contrary). |
| Advocate for inclusion of patients with CKD in clinical cancer trials. | |
| Work with oncologists to establish standards for drug dosing and adverse event monitoring for SACT in patients with CKD, on dialysis and after transplantation. |
Table 2.
Suggested future directions to clarify the observed relationship between non-dialysis CKD and risk of cancer incidence and death
| Patient factors | Assess whether modifiable lifestyle factors (e.g. weight loss, increased exercise and smoking cessation) reduce the risk of cancer in CKD. |
| Disease factors | Exploration of causal pathways between CKD and cancer, and the impact of shared risks including behavioural, lifestyle, disease and genetic factors (e.g., using Mendelian randomization). |
| Identify whether patients with CKD present with more invasive cancer stage. | |
| Clarify the role of cystatin C in tumorigenesis and whether this may be a mechanism by which patients with CKD (thus increased circulating cystatin C) experience a higher incidence of cancer or more rapidly progressive disease. | |
| Investigation and treatment factors | Widespread assessment of the uptake and utility of cancer screening amongst people with CKD, including dialysis and transplantation, the association with outcome and cost-effectiveness. |
| Assess whether inclusion of eGFR or albuminuria into cancer risk prediction tools would facilitate earlier diagnosis, treatment and improve outcome. | |
| Assess whether there are differences in treatment response to SACT in people with CKD, including those on dialysis or with a kidney transplant. | |
| Explore whether renal adverse abbreviate the dose or duration of SACT in people with CKD. |
CONCLUSIONS
Cancer incidence and mortality is higher, though site-specific, in people with CKD. This risk is under-recognized: kidney function markers such as eGFR and albuminuria are not acknowledged in cancer risk calculators (e.g. rate assessment tools and QCancer). Cancer risk is not consistently detected until CKD is more advanced, perhaps confounded by the fact that lower eGFRcr and cancer are both more commonly observed in older people. More sensitive and linear markers of glomerular filtration like cystatin C detect an association with increased risk of cancer incidence and mortality at an earlier stage. More widespread use of eGFRcys may enhance risk stratification; however, there are contradictory data regarding its accuracy compared with measured kidney function in patients diagnosed with cancer, and alternative methods may be required for eGFR-based medication dosing. Increased incidence of and death associated with cancer in CKD is likely related to a combination of patient, disease and treatment factors. A clearer understanding of the mechanisms will aid in devising strategies to reduce cancer risk among people with CKD.
Contributor Information
Jennifer S Lees, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Benjamin M P Elyan, Department of Renal Medicine, University Hospital Monklands, Airdrie, UK.
Sandra M Herrmann, Department of Medicine, Mayo Clinic, Rochester, MN, USA.
Ninian N Lang, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
Robert J Jones, Institute of Cancer Sciences, University of Glasgow, Glasgow, UK.
Patrick B Mark, Institute of Cardiovascular and Medical Sciences, University of Glasgow, Glasgow, UK.
FUNDING
J.S.L. is funded by a Chief Scientist Office (Scotland) Postdoctoral Lectureship (PCL/20/10). S.M.H is supported by NIH grant K08 DK118120 and Mayo Clinic K2R award.
CONFLICT OF INTEREST STATEMENT
Outside the submitted work, J.S.L. reports personal fees from Pfizer, AstraZeneca and Bristol-Myers Squibb; P.B.M. reports personal fees and/or non-financial support from Vifor, Napp, Pharmacosmos, AstraZeneca, Astellas and Novartis, and grants from Boehringer Ingelheim; N.N.L. reports personal fees and non-financial support from Roche, Pfizer, Novartis, AstraZeneca, Pharmacosmos and Vifor Pharma, and grant support from Roche Diagnostics, AstraZeneca and Boehringer (all paid to the University of Glasgow, his employing institution). R.J.J. reports research support from Clovis, Astellas, Exelixis, AstraZeneca and Roche, and honoraria from Clovis, Astellas, AstraZeneca, Roche, Ipsen, Bristol-Myers-Squibb, Pfizer, Merck Serono, Merck Sharp Dohme, Janssen, Bayer and Novartis.
REFERENCES
- 1. Tu H, Wen CP, Tsai SPet al. Cancer risk associated with chronic diseases and disease markers: prospective cohort study. BMJ 2018; 360: k134. doi: 10.1136/bmj.k134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weng PH, Hung KY, Huang HLet al. Cancer-specific mortality in chronic kidney disease: longitudinal follow-up of a large cohort. Clin J Am Soc Nephrol 2011; 6: 1121–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lowrance WT, Ordoñez J, Udaltsova Net al. CKD and the risk of incident cancer. J Am Soc Nephrol 2014; 25: 2327–2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu L, Zhu M, Meng Qet al. Association between kidney function and the risk of cancer: results from the China Health and Retirement Longitudinal Study (CHARLS). J Cancer 2020; 11: 6429–6436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mok Y, Matsushita K, Ballew SHet al. Kidney function, proteinuria, and cancer incidence: the Korean Heart Study. Am J Kidney Dis 2017; 70: 512–521 [DOI] [PubMed] [Google Scholar]
- 6. Ishii T, Fujimaru T, Nakano Eet al. Association between chronic kidney disease and mortality in stage IV cancer. Int J Clin Oncol 2020; 25: 1587–1595 [DOI] [PubMed] [Google Scholar]
- 7. Xu H, Matsushita K, Su Get al. Estimated glomerular filtration rate and the risk of cancer. Clin J Am Soc Nephrol 2019; 14: 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Iff S, Craig JC, Turner Ret al. Reduced estimated GFR and cancer mortality. Am J Kidney Dis 2014; 63: 23–30 [DOI] [PubMed] [Google Scholar]
- 9. Park S, Lee S, Kim Yet al. Risk of cancer in pre-dialysis chronic kidney disease: a nationwide population-based study with a matched control group. Kidney Res Clin Pract 2019; 38: 60–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wong G, Staplin N, Emberson Jet al. Chronic kidney disease and the risk of cancer: an individual patient data meta-analysis of 32,057 participants from six prospective studies. BMC Cancer 2016; 16: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christensson A, Savage C, Sjoberg DDet al. Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years. Int J Cancer 2013; 133: 1452–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inker LA, Schmid CH, Tighiouart Het al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012; 367: 20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lees JS, Welsh CE, Celis-Morales CAet al. Glomerular filtration rate by differing measures, albuminuria and prediction of cardiovascular disease, mortality and end-stage kidney disease. Nat Med 2019; 25: 1753–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shlipak MG, Matsushita K, Ärnlöv Jet al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013; 369: 932–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mok Y, Ballew SH, Sang Yet al. Albuminuria, kidney function, and cancer risk in the community. Am J Epidemiol 2020; 189: 942–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lees JS, Ho F, Parra-Soto Set al. Kidney function and cancer risk: an analysis using creatinine and cystatin C in a cohort study. EClinicalMedicine 2021; 38: 101030. 10.1016/j.eclinm.2021.101030 (2 February 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Launay-Vacher V, Oudard S, Janus Net al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer 2007; 110: 1376–1384 [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Coresh J, Greene Tet al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254 [DOI] [PubMed] [Google Scholar]
- 19. Mok Y, Matsushita K, Sang Yet al. Association of kidney disease measures with cause-specific mortality: the Korean Heart Study. PLoS One 2016; 11: e0153429. 10.1371/journal.pone.0153429 (2 February 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jørgensen L, Heuch I, Jenssen Tet al. Association of albuminuria and cancer incidence. J Am Soc Nephrol 2008; 19: 992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navaneethan SD, Schold JD, Arrigain Set al. Cause-specific deaths in non-dialysis-dependent CKD. J Am Soc Nephrol 2015; 26: 2512–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheung CY, Lam MF, Chu KHet al. Malignancies after kidney transplantation: Hong Kong Renal Registry. Am J Transplant 2012; 12: 3039–3046 [DOI] [PubMed] [Google Scholar]
- 23. Vajdic CM, McDonald SP, McCredie MREet al. Cancer incidence before and after kidney transplantation. JAMA 2006; 296: 2823–2831 [DOI] [PubMed] [Google Scholar]
- 24. UK Renal Registry . UK Renal Registry 22nd Annual Report—data to 31/12/2018. 2020. https://ukkidney.org/audit-research/annual-report/22nd-annual-report-data-31122018 (2 February 2022, date last accessed) [Google Scholar]
- 25. Runesson B, Qureshi AR, Xu Het al. Causes of death across categories of estimated glomerular filtration rate: the Stockholm CREAtinine Measurements (SCREAM) project. PLoS One 2019; 14: e0209440. 10.1371/journal.pone.0209440 (2 February 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagai K, Yamagata K, Iseki Ket al. Cause-specific mortality in the general population with transient dipstick-proteinuria. PLoS One 2019; 14: e0223005. 10.1371/journal.pone.0223005 (2 February 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wakasugi M, Narita I, Iseki Ket al. The effect of CKD on associations between lifestyle factors and all-cause, cancer, and cardiovascular mortality: a population-based cohort study. Intern Med 2021; 60: 2189–2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kelly JT, Su G, Zhang Let al. Modifiable lifestyle factors for primary prevention of CKD: a systematic review and meta-analysis. J Am Soc Nephrol 2021; 32: 239–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu EA, Coresh J, Anderson CAMet al. Adherence to healthy dietary patterns and risk of CKD progression and all-cause mortality: findings from the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis 2021; 77: 235–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Key T, Bradbury K, Perez-Cornago Aet al. Diet, nutrition, and cancer risk: what do we know and what is the way forward? BMJ 2020; 368: m511. 10.1136/bmj.m511 (2 February 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lam HC, Siroky BJ, Henske EP.. Renal disease in tuberous sclerosis complex: pathogenesis and therapy. Nat Rev Nephrol 2018; 14: 704–716 [DOI] [PubMed] [Google Scholar]
- 32. Genovese G, Kähler AK, Handsaker REet al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014; 371: 2477–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dawoud AAZ, Gilbert RD, Tapper WJet al. Clonal myelopoiesis promotes adverse outcomes in chronic kidney disease. Leukemia 2022: 507–515. 10.1038/s41375-021-01382-3 (2 February 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peired AJ, Antonelli G, Angelotti MLet al. Acute kidney injury promotes development of papillary renal cell adenoma and carcinoma from renal progenitor cells. Sci Transl Med 2020; 12: eeaw6003. 10.1126/scitranslmed.aaw6003 (2 February 2022, date last accessed) [DOI] [PubMed] [Google Scholar]
- 35. Jhaveri KD, Shah HH, Calderon Ket al. Glomerular diseases seen with cancer and chemotherapy: a narrative review. Kidney Int 2013; 84: 34–44 [DOI] [PubMed] [Google Scholar]
- 36. Kos J, Krašovec M, Cimerman Net al. Cysteine proteinase inhibitors stefin A, stefin B, and cystatin C in sera from patients with colorectal cancer: relation to prognosis. Clin Cancer Res 2000; 6: 505–511 [PubMed] [Google Scholar]
- 37. Mori J, Tanikawa C, Funauchi Yet al. Cystatin C as a p53-inducible apoptotic mediator that regulates cathepsin L activity. Cancer Sci 2016; 107: 298–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wegiel B, Jiborn T, Abrahamson Met al. Cystatin C is downregulated in prostate cancer and modulates invasion of prostate cancer cells via MAPK/Erk and androgen receptor pathways. PLoS One 2009; 4: e7953. 10.1371/journal.pone.0007953 (2 February 2022, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Leeuwen MT, Webster AC, McCredie MREet al. Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ 2010; 340: c570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Magee C. Kidney disease and death from cancer. Am J Kidney Dis 2014; 63: 7–9 [DOI] [PubMed] [Google Scholar]
- 41. Rosales BM, De La Mata N, Vajdic CMet al. Cancer mortality in kidney transplant recipients: an Australian and New Zealand population-based cohort study, 1980–2013. Int J Cancer 2019; 146: 2703–2711 [DOI] [PubMed] [Google Scholar]
- 42. Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol 2012; 7: 1713–1721 [DOI] [PubMed] [Google Scholar]
- 43. Widemann BC, Adamson PC.. Understanding and managing methotrexate nephrotoxicity. Oncologist 2006; 11: 694–703 [DOI] [PubMed] [Google Scholar]
- 44. de Rouw N, Boosman RJ, van de Bruinhorst Het al. Cumulative pemetrexed dose increases the risk of nephrotoxicity. Lung Cancer 2020; 146: 30–35 [DOI] [PubMed] [Google Scholar]
- 45. Skinner R, Cotterill SJ, Stevens MC.. Risk factors for nephrotoxicity after ifosfamide treatment in children: a UKCCSG Late Effects Group Study. United Kingdom Children's Cancer Study Group. Br J Cancer 2000; 82: 1636–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hodi FS, O'Day SJ, McDermott DFet al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Motzer RJ, Tannir NM, McDermott DFet al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018; 378: 1277–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ninomiya K, Hotta K.. Pembrolizumab for the first-line treatment of non-small cell lung cancer. Expert Opin Biol Ther 2018; 18: 1015–1021 [DOI] [PubMed] [Google Scholar]
- 49. Herrmann SM, Perazella MA.. Immune checkpoint inhibitors and immune-related adverse renal events. Kidney Int Rep 2020; 5: 1139–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Malyszko J, Tesarova P, Capasso Get al. The link between kidney disease and cancer: complications and treatment. Lancet North Am Ed 2020; 396: 277–287 [DOI] [PubMed] [Google Scholar]
- 51. Krens SD, Lassche G, Jansman FGAet al. Dose recommendations for anticancer drugs in patients with renal or hepatic impairment. Lancet Oncol 2019; 20: e200–e207 [DOI] [PubMed] [Google Scholar]
- 52. Chute DF, Zhao S, Strohbehn IAet al. Incidence and predictors of CKD and estimated GFR decline in patients receiving immune checkpoint inhibitors. Am J Kidney Dis 2021; 79: 134–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kitchlu A, Jhaveri KD, Sprangers Bet al. Immune checkpoint inhibitor use in patients with end-stage kidney disease: an analysis of reported cases and literature review. Clin Kidney J 2021; 14: 2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kitchlu A, Shapiro J, Amir Eet al. Representation of patients with chronic kidney disease in trials of cancer therapy. JAMA 2018; 319: 2437–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shepherd STC, Gillen G, Morrison Pet al. Performance of formulae based estimates of glomerular filtration rate for carboplatin dosing in stage 1 seminoma. Eur J Cancer 2014; 50: 944–952 [DOI] [PubMed] [Google Scholar]
- 56. Janowitz T, Williams EH, Marshall Aet al. New model for estimating glomerular filtration rate in patients with cancer. J Clin Oncol 2017; 35: 2798–2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Usher-Smith J, Emery J, Hamilton Wet al. Risk prediction tools for cancer in primary care. Br J Cancer 2015; 113: 1645–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hippisley-Cox J, Coupland C.. Symptoms and risk factors to identify men with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2013; 63: e1–e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hippisley-Cox J, Coupland C.. Symptoms and risk factors to identify women with suspected cancer in primary care: derivation and validation of an algorithm. Br J Gen Pract 2013; 63: e11–e21 [DOI] [PMC free article] [PubMed] [Google Scholar]