ABSTRACT
Background
No single study contrasts the extent and consequences of inequity of kidney care across the clinical course of kidney disease.
Methods
This population study of Grampian (UK) followed incident presentations of acute kidney injury (AKI) and incident estimated glomerular filtration rate (eGFR) thresholds of <60, <45 and <30 mL/min/1.73 m2 in separate cohorts (2011–2021). The key exposure was area-level deprivation (lowest quintile of the Scottish Index of Multiple Deprivation). Outcomes were care processes (monitoring, prescribing, appointments, unscheduled care), long-term mortality and kidney failure. Modelling involved multivariable logistic regression, negative binomial regression and cause-specific Cox models with and without adjustment of comorbidities.
Results
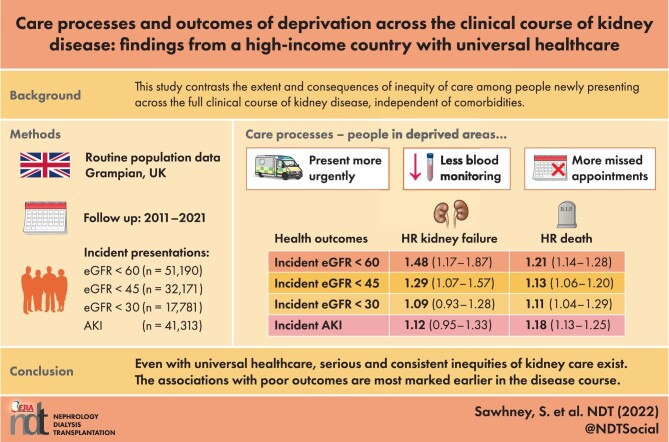
There were 41 313, 51 190, 32 171 and 17 781 new presentations of AKI and eGFR thresholds <60, <45 and <30 mL/min/1.73 m2. A total of 6.1–7.8% of the population was from deprived areas and (versus all others) presented on average 5 years younger, with more diabetes and pulmonary and liver disease. Those from deprived areas were more likely to present initially in hospital, less likely to receive community monitoring, less likely to attend appointments and more likely to have an unplanned emergency department or hospital admission episode. Deprivation had the greatest association with long-term kidney failure at the eGFR <60 mL/min/1.73 m2 threshold {adjusted hazard ratio [HR] 1.48 [95% confidence interval (CI) 1.17–1.87]} and this association decreased with advancing disease severity [HR 1.09 (95% CI 0.93–1.28) at eGFR <30 mL/min/1.73 m2), with a similar pattern for mortality. Across all analyses the most detrimental associations of deprivation were an eGFR threshold <60 mL/min/1.73 m2, AKI, males and those <65 years of age.
Conclusions
Even in a high-income country with universal healthcare, serious and consistent inequities in kidney care exist. The poorer care and outcomes with area-level deprivation were greater earlier in the disease course.
Keywords: AKI, care processes, CKD, epidemiology, health inequalities, prognosis
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
There is a recognised lack of evidence for inequity of kidney care that covers a full population across a full clinical course of kidney disease.
Previous studies have shown associations between deprivation and adverse kidney health in snapshots of health datasets or have shown poorer outcomes in specific disease subsets only (e.g. reduced access to transplantation for kidney failure).
What this study adds?
Across all incident patients in the Grampian population, healthcare in deprived areas was more reactive (more acute initial presentations and emergency hospital visits) and less proactive (less blood monitoring and outpatient attendances, less preventative prescribing).
Associations with mortality and kidney failure were most severe earlier in the clinical course (newly presenting eGFR <60 mL/min/1.73 m2 or AKI), in those of working age, and men.
Age- and sex-adjusted associations were not substantially affected by any further adjustment of known medical factors such as comorbidities.
What impact this may have on practice or policy?
Despite free universal healthcare in the UK, inequity of kidney care exists for people presenting at every stage of illness, but with particularly large inequity in the initial access to preventative care at early stages in the clinical course.
An overly medical approach to addressing these inequities (without focusing on social needs, health literacy, behaviour or access) is likely to be insufficient to close this inequity gap.
INTRODUCTION
Equitable health service design for people with long-term illnesses such as kidney disease requires evidence on how inequalities vary across the full clinical course [1]. Time points of greatest relevance are those when improvements in preventative healthcare could still feasibly modify the disease course [2]. Addressing inequalities in integrated care is a core objective of primary care services [3, 4], but the administrative data used to inform policies are typically coarse, providing limited insights into disease severity or progression [5]. This limitation poses a challenge for determining where the most pressing missed opportunities in care exist [3, 4].
Kidney disease, represented by chronic kidney disease (CKD) stages and intervening episodes of acute kidney injury (AKI), is a long-term condition where clinical course and severity can be readily followed through serial blood tests. The population burden of kidney disease is substantial, including associations with multimorbidity, older age and a major effect on mortality, morbidity and healthcare costs [6, 7]. As <10% of those with CKD or AKI receive follow-up by a nephrologist [8–13], prioritisation must occur, with coordination primarily in the community. This means kidney care may be prone to inequitable population-level coordination, but unlike other long-term conditions, a nuanced study across its full clinical course and severity may be objectively possible using blood test data.
As described in a report into kidney care equity in the UK [14] and consistent with reports from other high-income countries [15–18], ‘people from lower socio-economic groups are more likely to develop CKD … and die earlier’. Nevertheless, existing literature has been limited to isolated snapshots of kidney disease focusing on disease subsets or recruited cohorts rather than following a whole population over time [19–22]. Examples are studies focusing on ethnicity and the prevalence of CKD or kidney failure and of access to transplantation [20, 21, 23–28]. The UK report concluded that there was insufficient evidence to make specific recommendations for intervention studies or policy [14, 29]. For instance, without a lens on the full clinical course within a single study, it would be hard to compare the extent of inequitable care of those newly presenting with mild impairment [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2] with those developing severe disease (eGFR <30 mL/min/1.73 m2). Even in a country with free universal healthcare, such as the UK, recommended kidney care varies by severity: those with severe disease are prioritised for specialist nephrology care, whereas those with mild disease are often exclusively followed up in primary care. In addition, the relative contributions of social and medical factors towards overall health may shift as disease progresses, with medical factors predominating among those with more severe illness. Thus social deprivation may have a ‘ceiling effect’ among those with advancing kidney disease, but this has not previously been assessed.
The aim of this study was to assess, within the same population-level analysis, the extent to which a socio-economic gradient in care changes across the spectrum of kidney health and the implications for subsequent health outcomes and unscheduled care use. Specifically, we evaluated the care processes and outcomes of people from deprived and non-deprived backgrounds at different stages on a spectrum from mild to severe kidney impairment, independent of comorbidities. We hypothesised that inequitable care and outcomes for those from deprived areas would exist across all stages of kidney disease, but due to a ceiling effect, the magnitude of this inequity would be greater for those at earlier stages of kidney disease when the clinical course may also still be more modifiable.
MATERIALS AND METHODS
Modelling assumptions
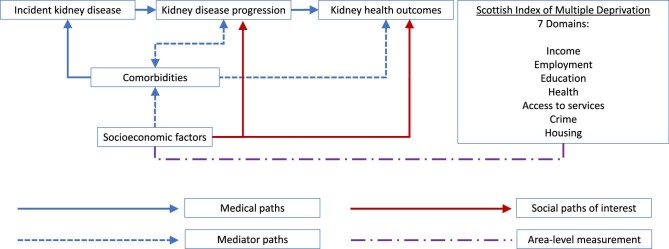
Based on broader (non-kidney) chronic disease literature, a complex role exists for socio-economic factors (e.g. income, employment, education, housing, access to care) in health and healthcare, with food insecurity, financial strain and housing instability highlighted as priorities [30]. For this study, socio-economic metrics were obtained from the Scottish Index of Multiple Deprivation (SIMD), which covers seven area-level domains of income, employment, education, health, access to services, crime and housing. Socio-economic factors may associate with worse subsequent kidney disease and other comorbidities, but bidirectionally also represent consequences of ill health (Figure 1). For this analysis we assumed the former predominated (forwards directional arrows). We tested this association at different levels of kidney disease severity with and without adjustment of comorbidities as a mediator. We addressed the analysis in stages by assessing the variation in 1-year mortality by area; 1-year healthcare processes, kidney failure and mortality by lowest quintile of Scotland for area-level deprivation versus all other levels; long-term kidney failure and mortality by lowest quintile for area-level deprivation versus other levels and, in sensitivity analyses, we evaluated outcomes across all quintiles of area-level deprivation and tested for effect modification by age, sex and acuity of presentation (i.e. evidence of prior or concurrent AKI within the prior 90 days).
FIGURE 1:
Logic model of assumptions for the bidirectional relationships between kidney disease clinical course, comorbidities and socio-economic factors.
Population
The Grampian Laboratory Outcomes Morbidity and Mortality Study provides routine health data for all adult residents in Grampian (adult population 473 226) [9, 31–33]. The study includes complete capture and data linkage (2009–2021 for this analysis) of all hospital admission episodes [Scottish Morbidity Record 01 (SMR01)] [34], emergency department visits (Accident and Emergency Data Mart) [35], outpatient clinic episodes [Scottish Morbidity Record 00 (SMR00)] [34], blood and urine tests from all locations [inpatient, community including primary care or outpatient, National Health Service (NHS), private practice] and prescribed medications [Prescribing Information System (PIS)] [36].
This analysis involved four subset cohorts nested within the Grampian population containing all new presentations of kidney disease in Grampian between January 2011 and December 2018. In the main analysis the four cohorts represented the first (incident) instance when the eGFR crossed thresholds of above to below 60, 45, 30 ml/min/1.73 m2, respectively, and the first (incident) instance of AKI among those with evidence of no prior presentation in 2009–2010. To reflect the real world in which high-risk patients may deteriorate and cross thresholds quickly, these cohort criteria were enabled to be non-mutually exclusive—a person presenting with either a rapid or unmonitored decrease in eGFR to 40 ml/min/1.73 m2 could belong to both the <60 and <45 eGFR cohorts, or only the <45 cohort if there was previous evidence of an eGFR of 45–60 ml/min/1.73 m2. Similarly, in the main analysis, co-presentation with AKI was allowed. In a sensitivity analysis, cohorts were made mutually exclusive by excluding those presenting concurrently with AKI from all other groups and by excluding those progressing rapidly (or unmonitored) through higher eGFR thresholds by adding lower boundaries (45–60, 30–44, <30 ml/min/1.73 m2).
To ensure we studied only new (incident) presentations of kidney impairment without a ‘prevalent pool effect’ (i.e. inadvertent mixing of prevalent and incident patients) we excluded patients with prior evidence of kidney impairment at the respective subset threshold in 2009 and 2010. We also recognised a priori that a narrow interpretation of Kidney Disease: Improving Global Outcomes (KDIGO) criteria with repeat testing over at least 90 days could lead to underascertainment of kidney disease among those with poor access to blood testing (a care process outcome of study that would thus lead to selection bias). To mitigate against this potential selection bias of testing access, we focused on first presentation of kidney impairment at the eGFR thresholds outlined above without requirement for confirmatory testing.
Exposure and comparator
Area-level deprivation was determined based on people living in the lowest quintile of Scotland for the SIMD from 2016 [37]. In statistical analyses, we compared people from deprived areas (lowest quintile of the SIMD) with those from all other non-deprived areas (all other quintiles of the SIMD). Specifically for data visualisation, we grouped people by residential areas, distinguishing areas containing the lowest quintile of the SIMD as ‘most deprived’, areas of affluence containing the top two quintiles of the SIMD as ‘most affluent’ and the remaining areas as neither deprivation nor affluence.
Covariates of interest
To determine eGFR, we used the Chronic Kidney Disease Epidemiology Collaboration equation excluding the coefficient for black race [38]. Additionally, the first (incident) instance of AKI was determined using a KDIGO-based AKI algorithm (changes in creatinine of 0.3 mg/dL within 48 hours, a 50% increase within 7 days or a 50% increase from a baseline creatinine estimated from the median of creatinine levels over the previous 90 and 365 days), as described in greater detail elsewhere [9, 31–32]. Additional covariates adjusted in models were age, sex and comorbidities (cardiovascular disease, coronary heart disease, heart failure, stroke, atrial fibrillation, peripheral arterial disease, diabetes mellitus, hypertension, cancer, liver disease and chronic pulmonary disease) based on previously recorded International Classification of Diseases, 10th Revision hospital episode codes using validated coding dictionaries as listed elsewhere [9, 39–41].
Outcomes
We studied kidney failure and mortality for all people and care processes for surviving at least 1 year from initial presentation. We evaluated mortality at 1 and 3 years and over the long term with the last follow-up on 1 July 2021 (up to 10 years). Onset of kidney failure was either sustained eGFR <15 mL/min/1.73 m2 for at least 90 days on at least two blood tests and all intervening tests or onset of chronic kidney replacement therapy (dialysis or transplant).
Care processes assessed were the context of the initial presentation (inpatient or community, from SMR01 episode dates), outpatient serum creatinine test within 90 days, urine protein assessment within 1 year for those with a history of diabetes mellitus (in accordance with screening guidelines) [42], renin–aldosterone–angiotensin system (RAAS) blocker prescription (British National Formulary subsection code 020505 in the PIS) within 1 year among those with a history of myocardial infarction or heart failure (as per UK clinical guidelines) [43, 44], kidney care clinic referral within 1 year (SMR00 specialty code AG for renal medicine) [34], non-attendance at the clinic among those referred to a clinic, emergency department use within 1 year and unscheduled (emergency) hospital admission within 1 year of presentation (SMR01) [34].
Statistical analysis
We reported the number and characteristics of people from deprived areas versus all other areas. To visualise the overall extent of area-level variation and potential contribution of deprivation (and affluence) on mortality at each point in the clinical course, we plotted age- and sex-adjusted 1-year mortality estimates across geographic areas in each kidney subset (AKI and eGFR 45–60, 30–44 and <30 mL/min/1.73 m2). We calculated estimates using a multilevel logistic regression model with random intercepts for resident area and age and sex as fixed effects using the ‘melogit’ package in Stata/SE 16 (StataCorp, College Station, TX, USA) [45].
Next, to quantify the influence of deprivation (worst quintile of SIMD versus all other areas) on early outcomes, we used multivariable logistic regression to calculate odds ratios (ORs) for the association of deprivation on mortality adjusting for age and sex and listed comorbidities for all patients. We used the same approach for each of the care processes listed in the outcomes section above. For processes reported as counts (number of emergency department attendances, total unscheduled inpatient care days), data were overdispersed and analysed using negative binomial regression with confidence intervals (CIs) derived from robust standard errors.
Finally, to assess the long-term risks of kidney failure and death (competing outcomes), we fitted long-term cause-specific Cox models for those with and without deprivation. Based on our a priori logic model (Figure 1), we theorised that comorbidities were a mediator of poor health outcomes. Accordingly, we fitted two models: one with age and sex adjustment and one with additional adjustment for comorbidities (to determine the age- and sex-adjusted role of deprivation, independent of known existing comorbidities).
We recognise that the influence of socio-economic factors may span the full spectrum from deprivation to affluence; however, for clarity we focused on deprived areas versus all other areas in the main analysis. In sensitivity analyses we included deprivation within five categories of SIMD quintiles to determine whether any association of deprivation with health outcomes was present only for those in the deprived quintile versus all others or if there was a continuum of association from deprivation to affluence. We used likelihood ratio tests of nested models to evaluate interactions with age (working age <65 years versus elderly ≥65 years) and sex. Finally, as incident presentations could arise from both gradual and rapid deteriorations, we tested for an interaction with prior/concurrent AKI. Where the interaction P-value was <.1, we reported results separately in a supplementary table. All analyses were performed using Stata/SE 16 (StataCorp) [45].
RESULTS
Characteristics
Table 1 reports characteristics of people newly presenting between January 2011 and December 2018 with AKI and eGFR 45–60, 30–44 and <30 mL/min/1.73 m2. Within these four subset cohorts there were 41 313, 51 190, 32 171 and 17 781 people, respectively. The respective proportions of people from deprived areas were 7.8%, 6.0%, 6.5% and 7.1%. Across cohorts, 54.4–57.7% were female and those from deprived areas presented on average 5 years younger. A greater proportion of those from deprived areas had diabetes, liver disease and pulmonary disease (Table 1).
Table 1.
Characteristics of people with new-onset kidney disease according to each of the four subset cohort definitions
| Characteristics | eGFR <60 ml/min/1.73 m2 (n = 51 190) | eGFR <45 ml/min/1.73 m2 (n = 32 171) | eGFR <30 ml/min/1.73 m2 (n = 17 781) | AKI (n = 41 313) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deprived areas | All other areas | Deprived areas | All other areas | Deprived areas | All other areas | Deprived areas | All other areas | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Total | 3135 | 6.1 | 48 055 | 93.9 | 2080 | 6.5 | 30 091 | 93.5 | 1257 | 7.1 | 16 524 | 92.9 | 3210 | 7.8 | 38 103 | 92.2 |
| Female | 1693 | 54.0 | 26 363 | 54.9 | 1125 | 54.1 | 16 468 | 54.7 | 655 | 52.1 | 8876 | 53.7 | 1724 | 53.7 | 20 619 | 54.1 |
| Ethnicity | ||||||||||||||||
| White | 861 | 27.5 | 13 512 | 28.1 | 546 | 26.3 | 8167 | 27.1 | 311 | 24.7 | 4365 | 26.4 | 825 | 25.7 | 10 454 | 27.4 |
| Non-white | 33 | 1.1 | 267 | 0.6 | 15 | 0.7 | 130 | 0.4 | 10 | 0.8 | 65 | 0.4 | 46 | 1.4 | 296 | 0.8 |
| Unknown | 2241 | 71.5 | 24 276 | 71.3 | 1519 | 73.0 | 21 794 | 72.4 | 936 | 74.5 | 12 094 | 73.2 | 2339 | 72.9 | 27 353 | 71.8 |
| Age (years), mean (95% CI) | 67 | 66.4–67.6 | 71.2 | 71.1–71.4 | 70.3 | 69.7–71.0 | 75 | 74.8–75.1 | 72.4 | 71.5–73.2 | 76.7 | 76.5–76.9 | 63.1 | 62.4–63.7 | 69 | 68.8–69.1 |
| AKI on presenting date | 405 | 12.9 | 4746 | 9.9 | 435 | 20.9 | 5617 | 18.7 | 380 | 30.2 | 4802 | 29.1 | 3210 | 100 | 38 103 | 100 |
| Atrial fibrillation | 254 | 8.1 | 3968 | 8.3 | 265 | 12.7 | 4335 | 14.4 | 229 | 18.2 | 3223 | 19.5 | 421 | 13.1 | 5825 | 15.3 |
| Cancer | 352 | 11.2 | 6399 | 13.3 | 321 | 15.4 | 5282 | 17.6 | 216 | 17.2 | 3328 | 20.1 | 545 | 17.0 | 8135 | 21.4 |
| Chronic obstructive pulmonary disease | 563 | 18.0 | 5509 | 11.5 | 464 | 22.3 | 4438 | 14.7 | 295 | 23.5 | 2798 | 16.9 | 795 | 24.8 | 6700 | 17.6 |
| Coronary heart disease | 319 | 10.2 | 4171 | 8.7 | 302 | 14.5 | 3933 | 13.1 | 235 | 18.7 | 2852 | 17.3 | 446 | 13.9 | 5472 | 14.4 |
| Diabetes | 407 | 13.0 | 4575 | 9.5 | 381 | 18.3 | 4715 | 15.7 | 306 | 24.3 | 3507 | 21.2 | 587 | 18.3 | 6757 | 17.7 |
| Heart failure | 178 | 5.7 | 2249 | 4.7 | 209 | 10.0 | 2759 | 9.2 | 205 | 16.3 | 2534 | 15.3 | 316 | 9.8 | 4121 | 10.8 |
| Hypertension | 884 | 28.2 | 12 862 | 26.8 | 827 | 39.8 | 12 020 | 39.9 | 614 | 48.8 | 8043 | 48.7 | 1159 | 36.1 | 15 327 | 40.2 |
| Liver disease | 206 | 6.6 | 1469 | 3.1 | 168 | 8.1 | 1296 | 4.3 | 133 | 10.6 | 947 | 5.7 | 284 | 8.8 | 2029 | 5.3 |
| Peripheral arterial disease | 170 | 5.4 | 1949 | 4.1 | 173 | 8.3 | 2055 | 6.8 | 147 | 11.7 | 1596 | 9.7 | 277 | 8.6 | 3028 | 7.9 |
| Stroke | 179 | 5.7 | 2491 | 5.2 | 178 | 8.6 | 2531 | 8.4 | 135 | 10.7 | 1786 | 10.8 | 287 | 8.9 | 3648 | 9.6 |
Care processes
Table 2 reports the association of deprivation with care processes within each subset cohort. Across the spectrum of kidney disease, those from deprived areas (versus other areas) were more likely to first present in an inpatient setting rather than through outpatient monitoring. Those from deprived areas were less likely to have subsequent blood test monitoring to evaluate for chronicity. While there were similar levels of renal outpatient referral, those from deprived settings attended their planned appointments less often, but were more likely to attend the emergency department or have an unplanned admission over the following year.
Table 2.
Healthcare processes for new-onset kidney disease in each of the subset cohorts
| Deprived areas versus all other areas (case mix adjusted) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFR <60 ml/min/1.73 m2 (n =51 190) | eGFR <45 ml/min/1.73 m2 (n = 32 171) | eGFR <30 ml/min/1.73 m2 (n = 17 781) | AKI (n = 41 313) | ||||||||||
| Healthcare processes (among 1-year survivors unless otherwise stated) | Events/cases (deprived), n/N | Events/cases (not deprived), n/N | OR (95% CI) | Events/cases (deprived), n/N | Events/cases (not deprived), n/N | OR (95% CI) | Events/cases (deprived), n/N | Events/cases (not deprived), n/N | OR (95% CI) | Events/cases (deprived), n/N | Events/cases (not deprived), n/N | OR (95% CI) | |
| Context | Presents first as an inpatient (among all cases) | 1232/3135 | 15 055/48 055 | 1.25 (1.16–1.36) | 1075/2080 | 13 318 /30 091 |
1.22 (1.11–1.34) | 800/1257 | 9271/16 524 | 1.27 (1.12–1.43) | 2096/3210 | 24 564/38 103 | 1.15 (1.06–1.25) |
| Monitoring | Outpatient blood monitoring within 90 days | 1596/2636 | 24 460/41 660 | 0.97 (0.89–1.05) | 1046/1542 | 15 757 /22 914 |
0.87 (0.87–0.98) | 562/769 | 7890/10 435 | 0.80 (0.68–0.95) | 1370/2346 | 17 383/27 241 | 0.84 (0.77–0.92) |
| Diabetes urine protein screen within 1 year | 223/331 | 2603/3692 | 0.87 (0.68–1.11) | 208/285 | 2472 /3488 |
1.07 (0.82–1.41) | 145/189 | 1617/2247 | 1.18 (0.83–1.68) | 268/412 | 3110/4671 | 0.95 (0.77–1.18) | |
| RAAS prescription after MI or heart failure within 1 year | 220/315 | 2939/4264 | 0.91 (0.71–1.18) | 167/267 | 2409 /3720 |
0.79 (0.61–1.03) | 95/170 | 1288/2300 | 0.92 (0.67–1.27) | 211/371 | 2691/4649 | 0.81 (0.65–1.01) | |
| Referrals | Specialty renal clinic referral within 1 year | 93/2636 | 1199/41 660 | 0.88 (0.70–1.10) | 122/1542 | 1289 /22 914 |
1.04 (0.84–1.28) | 131769 | 1461/10 435 | 0.92 (0.74–1.14) | 135/2346 | 1429/27 241 | 1.06 (0.88–1.28) |
| At least one specialty clinic non-attendance within 1 year | 400/2636 | 3169/41 660 | 1.74 (1.55–1.95) | 272/1542 | 2203 /22 914 |
1.60 (1.38–1.85) | 156/769 | 1316/10 435 | 1.40 (1.15–1.70) | 540/2346 | 3909/27 241 | 1.49 (1.34–1.66) | |
| Health care use | Emergency department use within 1 year | 414/1772 | 2511/27 521 | 1.44 (1.28–1.62) | 252/1019 | 2963 /14 858 |
1.26 (1.09–1.47) | 132/506 | 1585/6628 | 1.09 (0.89–1.35) | 467/1518 | 4007/17 547 | 1.44 (1.28–1.62) |
| Unscheduled hospitalisation within 1 year | 776/2636 | 9027/41 660 | 1.38 (1.26–1.51) | 581/1542 | 7099 /22 914 |
1.25 (1.12–1.40) | 350/769 | 4394/10 435 | 1.08 (0.93–1.26) | 966/2346 | 9557/27 241 | 1.34 (1.23–1.46) | |
| Emergency department days within 1 year, mean (SD) | 0.40 (1.08) | 0.25 (0.83) | RR 1.45 (1.28–1.64) | 0.40 (1.00) | 0.30 (0.82) | RR 1.21 (1.04–1.41) | 0.44 (1.11) | 0.37 (0.87) | RR 1.10 (0.89–1.36) | 0.61 (1.49) | 0.41 (1.30) | RR 1.34 (1.17–1.52) | |
| Unscheduled acute hospital days within 1 year, mean (SD) | 5.1 (17.1) | 3.7 (14.5) | RR 1.31 (1.13–1.51) | 6.8 (19.1) | 6.1 (18.6) | RR 1.11 (0.96–1.30) | 10.2 (26.2) | 9.1 (22.2) | RR 1.13 (0.94–1.35) | 8.3 (23.2) | 7.0 (19.7) | RR 1.28 (1.13–1.45) | |
MI, myocardial infarction; OR, odds ratio (from logistic regression); RR, rate ratio (from negative binomial regression with robust CIs).
Emergency department data available from 2014 onwards
Statistically significant values are in bold.
Mortality
Table 3 reports the association of deprivation with 1-year and long-term health outcomes. For those presenting with an eGFR <60 mL/min/1.73 m2 there was a significant (age- and sex-adjusted) excess risk of death among those from deprived areas at 1 year [OR 1.33 (CI 1.20–1.47)], which remained after additional adjustment for comorbidities [adjusted OR 1.28 (CI 1.15–1.42)]. Excess mortality was also present over a long-term follow-up to 10 years [Cox model adjusted hazard ratio (HR) 1.21 (CI 1.14–1.28)]. At each time horizon the excess mortality was attenuated for eGFR <30 mL/min/1.73 m2 [1-year adjusted OR 1.19 (CI 1.05–1.35); Cox model long-term adjusted mortality HR 1.11 (CI 1.04–1.19)].
Table 3.
Mortality and kidney failure outcomes for new-onset kidney disease in each of the subset cohorts
| Deprived areas versus all other areas (case mix adjusted) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| eGFR <60 ml/min/1.73 m2 (n = 51 190) | eGFR <45 ml/min/1.73 m2 (n = 32 171) | eGFR <30 ml/min/1.73 m2 (n = 17 781) | AKI (n = 41 313) | ||||||||||
| Health outcomes (among all patients) | Events/cases (deprived), n/N | Events/cases (not deprived), n/N | Estimate (95% CI) | Events/cases (deprived), n/N | Events/cases (not deprived), n/N | Estimate (95% CI) | Events/cases (deprived), n/N | Events/cases (not deprived) | Estimate (95% CI) | Events/cases (deprived), n/N | Events/cases (not deprived), n/N | Estimate (95% CI) | |
| 1-year mortality OR (logistic regression model) | Age and sex adjusted | 499/3135 | 6395/48 055 | 1.33 (1.20–1.47) | 538/2080 | 7177/30 091 | 1.22 (1.10–1.35) | 488/1257 | 6089/16 524 | 1.21 (1.07–1.37) | 864/3210 | 10 862/38 103 | 1.18 (1.09–1.29) |
| Fully adjusted | 499/3135 | 6395/48 055 | 1.28 (1.15–1.43) | 538/2080 | 7177/30 091 | 1.19 (1.06–1.32) | 488/1257 | 6089/16 524 | 1.19 (1.05–1.35) | 864/3210 | 10 862/38 103 | 1.20 (1.09–1.31) | |
| Long-term mortality HR (Cox model) | Age and sex adjusted | 1167/3135 | 16 432/48 055 | 1.27 (1.20–1.35) | 1094/2080 | 15 712/30 091 | 1.18 (1.11–1.26) | 823/1257 | 11 044/16 524 | 1.15 (1.07–1.23) | 1644/3210 | 19 964/38 103 | 1.22 (1.16–1.28) |
| Fully adjusted | 1167/3135 | 16 432/48 055 | 1.21 (1.14–1.28) | 1094/2080 | 15 712/30 091 | 1.13 (1.06–1.20) | 823/1257 | 11 044/16 524 | 1.11 (1.04–1.19) | 1644/3210 | 19 964/38 103 | 1.18 (1.13–1.25) | |
| Long-term kidney failure HR (cause-specific Cox model) | Age and sex adjusted | 80/3135 | 716/48 055 | 1.50 (1.19–1.90) | 115/2080 | 1111/30 091 | 1.28 (1.06–1.56) | 170/1257 | 1854/16 524 | 1.07 (0.91–1.26) | 153/3210 | 1854/38 103 | 1.12 (0.95–1.32) |
| Fully adjusted | 80/3135 | 716/48 055 | 1.48 (1.17–1.87) | 115/2080 | 1111/30 091 | 1.29 (1.07–1.57) | 170/1257 | 1854/16 524 | 1.09 (0.93–1.28) | 153/3210 | 1854/38 103 | 1.12 (0.95–1.33) | |
Covariates adjusted in models were age at first presentation, sex and comorbidities (cancer, coronary heart disease, heart failure, stroke, atrial fibrillation, peripheral arterial disease, diabetes mellitus, hypertension, liver disease and chronic pulmonary disease).
Significant values is; bold type where statistically significant.
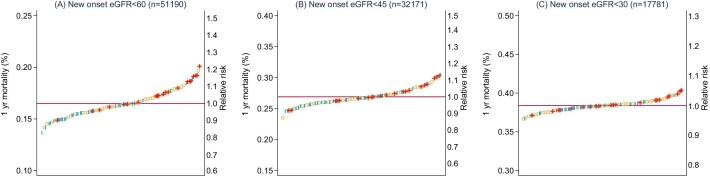
This pattern was also notable in Figure 2A–C of area-level variation in 1-year mortality, with greater variation in mortality among those presenting with eGFR <60 mL/min/1.73 m2 (Figure 2A) than eGFR <30 mL/min/1.73 m2 (Figure 2C). The separation in estimated mortalities between deprived (red) and affluent (blue) areas was also most evident in eGFR <60 mL/min/1.73 m2. The same pattern remained evident at 3 years (Supplementary Figure 1a–c) and with AKI (Supplementary Figure 2a, b).
FIGURE 2:
Age- and sex-standardised 1-year mortality risk of people with new-onset (A) eGFR <60 ml/min/1.73 m2, (B) eGFR <45 ml/min/1.73 m2 and (C) eGFR <30 ml/min/1.73 m2 grouped by localities of deprivation (red ``+''), affluence (blue ``I''), and all other areas (yellow ``o'').
Kidney failure
Table 3 also reports the long-term HRs of deprived areas (versus other areas) for kidney failure up to 10 years after initial presentation. The greatest excess of kidney failure was among those presenting with eGFR <60 mL/min/1.73 m2 [HR 1.48 (CI 1.17–1.87)] and the association was attenuated among those presenting with an eGFR <30 mL/min/1.73 m2 [HR 1.09 (CI 0.93–1.28)].
Sensitivity analyses
We evaluated associations of mortality and kidney failure with deprivation across all quintiles of the SIMD (Supplementary Table 1). Findings were materially consistent, with the greatest rate of kidney failure and death in the most deprived quintile and the lowest rate in the most affluent quintile. We detected no interactions with prior/recurrent AKI (all P-values >.1), but did detect significant interactions with age and sex. As reported in Supplementary Table 2, the associations between deprivation and kidney failure and death were greater among males than females and greater among those of working age (<65 years) than the elderly (≥65 years). Finally, we also created mutually exclusive cohorts by excluding patients with AKI from eGFR threshold cohorts and excluding high-risk individuals with rapid eGFR decreases from a higher eGFR threshold cohort. This led to smaller cohort sizes but not materially different findings, with poorer outcomes for those from deprived areas and the greatest impact in the eGFR 45–60 mL/min/1.73 m2 group (Supplementary Table 3).
DISCUSSION
Health service design is most successful when health conditions of greatest burden can be targeted at time points that enable prevention or modification of the disease course [1]. Beyond focusing solely on snapshots of disease prevalence, a detailed whole population view over the clinical course is needed but is difficult to obtain. Here we provide population-level insights into the care and long-term outcomes attributable to socio-economic deprivation across the spectrum of the AKI/CKD clinical course. Those from deprived backgrounds developed kidney impairment on average 5 years younger and had a higher burden of comorbidities. For all people newly presenting with AKI or CKD at any severity threshold, we found greater mortality, kidney failure and use of unscheduled healthcare among those from deprived versus non-deprived areas. Even though healthcare in the UK is free at the point of use, uptake of healthcare in deprived areas was more reactive (more likely to present in an acute setting, more emergency hospital visits) and less proactive (e.g. less outpatient blood monitoring, less preventative prescribing). Despite the poorer health outcomes and greater comorbidities, there was no difference in renal outpatient clinic referral and those referred were substantially less likely to attend appointments.
Of particular importance, the greatest associations between deprivation and clinical outcomes (especially kidney failure) were early in the clinical course at first presentation with an eGFR <60 ml/min/1.73 m2 or AKI. This is plausible because those with poorer kidney function may already have recognised advanced disease, whereby prevention may be less of an option. In addition, adjustment of comorbidities (a potential mediator of poor outcomes) made little difference to risk estimates. This implies that an overly medical approach to addressing inequities (without focusing on social needs, health literacy, behaviour or access) may be insufficient to close the inequity gap. One process we report here was the substantially increased clinic non-attendance in deprived areas. Adverse prognostic implications of clinic non-attendance have previously been reported for those attending diabetes clinics and driven by multifactorial reasons [46, 47]. Evidence of inequity in access to transplantation is also recognised [28], and poor health literacy has been associated with poorer kidney health outcomes in research mainly limited to kidney failure [48]. We also found the poorest outcomes among men and those of working age, which supports the need for further work understanding barriers to accessing kidney care. The extent to which these factors are causal and could be modified by an intervention is uncertain and the current lack of qualitative research is an acknowledged limiting factor [24].
Our broad finding of inequity of kidney care and outcomes is consistent with literature covering kidney diseases, cardiovascular disease and diabetes [14, 17, 21, 24]. The novel clinical course perspective of this analysis addresses a gap of existing literature [24] and spotlights a possible need for greater attention early (upstream) in the disease course and to non-medical factors. To restate a core public health principle, ‘so long as we continue to fight the battle downstream…we are doomed to frustration, repeated failure, and perhaps ultimately to a sicker society’ [2]. To determine the potential for interventions, further granular assessment of contributing non-medical factors should be guided by qualitative work to understand the lived experiences of those with early CKD and AKI. For instance, more than one-third of people in our analysis did not receive timely blood and urine monitoring in outpatient or community settings, even though this is advocated in clinical guidelines for both groups [49, 50]. Other potential factors may include the implications of health literacy, lifestyle, trust, stigmatisation and how high-risk individuals engage with healthcare along potentially complex individual care pathways. A first step at presentation of CKD/AKI is the initial recognition and communication of its existence. Tensions exist here between early communication to promote self-management versus concerns about causing unnecessary anxiety or unnecessary disruption [51–53]. Collectively these factors will need to be considered within the context of existing practice [54] and primary care stakeholders planning the integration of community and outpatient care of those with long-term conditions and multimorbidity [3, 4].
Strengths of this study include a population approach with data capture of all in the region, in all contexts and with long recruitment and follow-up periods. The analysis included both outcomes and care processes to contrast outcomes at all levels of kidney severity, including AKI. Our analysis focused on care in a high-income country with integrated universal healthcare. Thus the stark findings are despite the availability of care in Scotland, which is based on clinical need rather than the ability to pay. Healthcare has evolved in Scotland, England, Wales and Northern Ireland, and each faces similar challenges, with rising demand, tight budget limits and clinicians in each nation following the National Institute for Health and Care Excellence referral guidelines for AKI and CKD. Thus our analysis can be expected to be generalisable for care in England, Wales and Northern Ireland [55]. However, it can be assumed that the challenges for countries with fragmented or private healthcare provision may be even greater.
Of note, in 2004 the Quality and Outcomes Framework (QOF), a pay-for-performance scheme, was introduced across the UK, which used a range of quality indicators to incentivise general practices to improve long-term condition management, practice organisation and patient experience of care [56]. Our analysis predates the coronavirus 2019 pandemic and the introduction of new policies in the new primary care contract in Scotland (in 2018) [4]. This included removal of the QOF in Scotland and a shift to investment in general practitioner clusters, professional groupings of general practices focused on improving quality of care to improve ‘wellbeing, health and reduce health inequalities’ [4]. Accordingly additional UK-wide analyses are warranted in the future.
A further limitation is the use of an area-level rather than individual-level deprivation measure. This may have led to underestimation of the full extent of the influence of deprivation because areas may contain residents from a mixture of backgrounds and social statuses, leading to bias towards the null. We also note that while we focused on socio-economic differences, we did not evaluate ethnic differences. As reported in Table 1, ethnicity is poorly recorded in administrative health datasets in Scotland, unlikely to be missing at random, and therefore not imputable. The importance of ethnicity is reported in detail in other studies, whereas in Grampian an overwhelming (>95% in the previous census) white European population predominates. Similarly, health literacy, migrant status, body weight, obesity, smoking and alcohol intake are poorly recorded in hospital episode morbidity data and therefore not included in this analysis, but all would be relevant to understand health behaviour. Finally, our study used real-world data captured for clinical reasons rather than in a protocolised fashion. This is a strength because it reflects existing clinical practice, but it is not possible to interpret the clinical context and indications for blood test monitoring (which would be needed to more precisely understand and separate acute from chronic presentations and respective care requirements) nor disentangle the bidirectional relationship between kidney disease and accumulating non-kidney comorbidities, for which routine health data sources can provide only limited longitudinal information about disease severity at different time points.
In conclusion, by following the clinical course of kidney disease in a high-income country, we noted serious and consistent inequities of care and outcomes that were independent of comorbidities and greatest early in the disease course among those newly presenting with mild CKD and AKI. Greater understanding is now needed about how people initially present and how they access the support they require. This should include non-medical factors and incorporate lived experiences of those with early CKD or affected by AKI.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Audrey Hughes, on behalf of Grampian Kidney Patient Association, for her input into the design and interpretation of the analysis. We acknowledge the support of the Grampian Data Safe Haven (DaSH) facility within the Aberdeen Centre for Health Data Science and the associated financial support of the University of Aberdeen and NHS Research Scotland (through NHS Grampian investment in DaSH). For more information, visit the DaSH website: http://www.abdn.ac.uk/iahs/facilities/grampian-data-safe-haven.php
Contributor Information
Simon Sawhney, Aberdeen Centre for Health Data Science, University of Aberdeen, Aberdeen, UK; NHS Grampian, Aberdeen, UK.
Tom Blakeman, School of Community Based Medicine, University of Manchester, Manchester, UK.
Dimitra Blana, Aberdeen Centre for Health Data Science, University of Aberdeen, Aberdeen, UK.
Dwayne Boyers, Aberdeen Centre for Health Data Science, University of Aberdeen, Aberdeen, UK.
Nick Fluck, Aberdeen Centre for Health Data Science, University of Aberdeen, Aberdeen, UK; NHS Grampian, Aberdeen, UK.
Mintu Nath, Aberdeen Centre for Health Data Science, University of Aberdeen, Aberdeen, UK.
Shona Methven, NHS Grampian, Aberdeen, UK.
Magdalena Rzewuska, Aberdeen Centre for Health Data Science, University of Aberdeen, Aberdeen, UK.
Corri Black, Aberdeen Centre for Health Data Science, University of Aberdeen, Aberdeen, UK; NHS Grampian, Aberdeen, UK.
FUNDING
S.S. is supported by a Starter Grant for Clinical Lecturers from the Academy of Medical Sciences, Wellcome Trust, Medical Research Council, British Heart Foundation, Arthritis Research UK, Royal College of Physicians and Diabetes UK (SGL020\1076). C. B. is supported by Health Data Research UK, which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and the Wellcome Trust. The work was also supported in part by a grant from NHS Grampian Endowments (RG15955).
AUTHORS’ CONTRIBUTIONS
S.S. was responsible for the research idea and study design. S.S. and C.B. were responsible for data acquisition. S.S., D.Bl., N.F., S.M., M.R. and C.B. were responsible for data analysis/interpretation. S.S., D.Bo. and M.N. were responsible for statistical analysis. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author's own contributions and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
DATA AVAILABILITY STATEMENT
Datasets cannot be made available to other researchers due to ethical considerations. Information on how researchers may make requests to obtain similar datasets from the health research dataset custodians will be provided upon request.
CONFLICT OF INTEREST STATEMENT
All the authors have declared no competing interests.
REFERENCES
- 1. Bodenheimer T, Grumbach K.. Understanding Health Policy: A Clinical Approach. 7th ed.New York: McGraw-Hill Education; 2016. [Google Scholar]
- 2. McKinlay JB. A case for refocusing upstream: the political economy of illness. In: Enelow AJ, Henderson JB (ed). Applying behavioral science to cardiovascular risk. Washington, DC: American Heart Association; 1975:7–17. [Google Scholar]
- 3. NHS England . GP contract. https://www.england.nhs.uk/gp/investment/gp-contract/ (15 August 2022, date last accessed). [Google Scholar]
- 4. Scottish Government . The 2018 General Medical Services Contract in Scotland. https://publicinformationonline.com/download/155421 (2 February 2021, date last accessed). [Google Scholar]
- 5. Muggah E, Graves E, Bennett Cet al. Ascertainment of chronic diseases using population health data: a comparison of health administrative data and patient self-report. BMC Public Health 2013;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kerr M, Bray B, Medcalf Jet al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012;27(Suppl 3):iii73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kerr M, Bedford M, Matthews Bet al. The economic impact of acute kidney injury in England. Nephrol Dial Transplant 2014;29:1362–8. [DOI] [PubMed] [Google Scholar]
- 8. Brück K, Stel VS, Gambaro Get al. CKD prevalence varies across the European general population. J Am Soc Nephrol 2016;27:2135–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sawhney S, Marks A, Fluck Net al. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis 2017;69:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silver SA, Goldstein SL, Harel Zet al. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis 2015;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marks A, MacLeod C, McAteer Aet al. Chronic kidney disease, a useful trigger for proactive primary care? Mortality results from a large UK cohort. Fam Pract 2013;30:282–9. [DOI] [PubMed] [Google Scholar]
- 12. Siew ED, Peterson JF, Eden SKet al. Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol 2012;23:305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silver SA, Sawhney S.. Biomarkers to predict CKD after acute kidney injury: news or noise? Am J Kidney Dis 2022;79:620–2. [DOI] [PubMed] [Google Scholar]
- 14. Kidney Research UK . Kidney health inequalities in the United Kingdom. Reflecting on the past, reducing in the future. https://kidneyresearchuk.org/wp-content/uploads/2019/02/Health_Inequalities_Report_Complete_FINAL_Web_20181017.pdf (1 March 2022, date last accessed). [Google Scholar]
- 15. Centers for Medicare and Medicaid Services . Chronic kidney disease disparities: educational guide for primary care. https://www.cms.gov/files/document/chronic-kidney-disease-disparities-educational-guide-primary-care.pdf (1 March 2022, date last accessed). [Google Scholar]
- 16. Norris K, Nissenson AR.. Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 2008;19:1261–70. [DOI] [PubMed] [Google Scholar]
- 17. Australian Institute of Health and Welfare . Indicators of socioeconomic inequalities in cardiovascular disease, diabetes and chronic kidney disease. https://www.aihw.gov.au/getmedia/01c5bb07-592e-432e-9fba-d242e0f7e27e/aihw-cdk-12.pdf.aspx (1 March 2022, date last accessed). [Google Scholar]
- 18. Hemmelgarn BR, Pannu N, Ahmed SBet al. Determining the research priorities for patients with chronic kidney disease not on dialysis. Nephrol Dial Transplant 2016;32:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Boynton SA, Matheson MB, Ng DKet al. The relationship between neighborhood disadvantage and kidney disease progression in the Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis 2022;80:207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicholas SB, Kalantar-Zadeh K, Norris KC.. Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis 2015;22:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hounkpatin HO, Fraser SDS, Johnson MJet al. The association of socioeconomic status with incidence and outcomes of acute kidney injury. Clin Kidney J 2020;13:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Molokhia M, Okoli GN, Redmond Pet al. Uncoded chronic kidney disease in primary care: a cross-sectional study of inequalities and cardiovascular disease risk management. Br J Gen Pract 2020;70:e785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clark-Cutaia MN, Rivera E, Iroegbu Cet al. Disparities in chronic kidney disease-the state of the evidence. Curr Opin Nephrol Hypertens 2021;30:208–14. [DOI] [PubMed] [Google Scholar]
- 24. Wilkinson E, Brettle A, Waqar Met al. Inequalities and outcomes: end stage kidney disease in ethnic minorities. BMC Nephrol 2019;20:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norton JM, Moxey-Mims MM, Eggers PWet al. Social determinants of racial disparities in CKD. J Am Soc Nephrol 2016;27:2576–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kihal-Talantikite W, Vigneau C, Deguen Set al. Influence of socio-economic inequalities on access to renal transplantation and survival of patients with end-stage renal disease. PLoS One 2016;11:e0153431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu DA, Robb ML, Watson CJEet al. Barriers to living donor kidney transplantation in the United Kingdom: a national observational study. Nephrol Dial Transplant 2017;32:890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pruthi R, Robb ML, Oniscu GCet al. Inequity in access to transplantation in the United Kingdom. Clin J Am Soc Nephrol 2020;15:830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kidney Research UK . Kidney health inequalities in the UK. An agenda for change. https://kidneyresearchuk.org/wp-content/uploads/2019/09/Health_Inequalities_lay_report_FINAL_WEB_20190311.pdf (1 March 2022, date last accessed). [Google Scholar]
- 30. Davidson KW, Krist AH, Tseng Cet al. Incorporation of social risk in US preventive services task force recommendations and identification of key challenges for primary care. JAMA 2021;326:1410–5. [DOI] [PubMed] [Google Scholar]
- 31. Sawhney S, Robinson HA, van der Veer SNet al. Acute kidney injury in the UK: a replication cohort study of the variation across three regional populations. BMJ Open 2018;8:e019435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sawhney S, Bell S, Black Cet al. Harmonization of epidemiology of acute kidney injury and acute kidney disease produces comparable findings across four geographic populations. Kidney Int 2022;101:1271–81. [DOI] [PubMed] [Google Scholar]
- 33. Sawhney S, Marks A, Fluck Net al. Post-discharge kidney function is associated with subsequent ten-year renal progression risk among survivors of acute kidney injury. Kidney Int 2017;92:440–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Public Health Scotland . SMR Crib sheets. https://www.ndc.scot.nhs.uk/Data-Dictionary/SMR-Crib-Sheets/index.asp (1 March 2022, date last accessed). [Google Scholar]
- 35. Public Health Scotland . Accident and Emergency Data mart. https://www.isdscotland.org/Health-Topics/Emergency-Care/Accident-and-Emergency-Data-Mart/ (1 March 2022, date last accessed). [Google Scholar]
- 36. Alvarez-Madrazo S, McTaggart S, Nangle Cet al. Data resource profile: the Scottish National Prescribing Information System (PIS). Int J Epidemiol 2016;45:714–5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Public Health Scotland . The Scottish Index of Multiple Deprivation (SIMD). https://www.isdscotland.org/products-and-services/gpd-support/deprivation/simd/ (10 February 2022, date last accessed). [Google Scholar]
- 38. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tonelli M, Wiebe N, Fortin Met al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Making 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toi M, Wiebe N, Fortin Met al. Correction to: Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Making 2019;19:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Interdisciplinary Chronic Disease Collaboration . Database programming resources. https://cumming.ucalgary.ca/research/icdc/health-tools/codes. (2 February 2022, date last accessed). [Google Scholar]
- 42. Scottish Intercollegiate Guidelines Network . Management of diabetes: a national clinical guideline. P: ublication 116. https://www.sign.ac.uk/assets/sign116.pdf (1 March 2022, date last accessed). [Google Scholar]
- 43. National Institute for Health and Care Excellence . Chronic heart failure in adults: diagnosis and management. NG106. https://www.nice.org.uk/guidance/ng106 (1 March 2022, date last accessed). [PubMed] [Google Scholar]
- 44. National Institute for Health and Care Excellence . Acute coronary syndromes. NG185. https://www.nice.org.uk/guidance/ng185 (1 March 2022, date last accessed). [PubMed] [Google Scholar]
- 45. StataCorp . Stata statistical software: release 16. College Station, TX: StataCorp, 2019. [Google Scholar]
- 46. Brewster S, Bartholomew J, Holt RIGet al. Non-attendance at diabetes outpatient appointments: a systematic review. Diabet Med 2020;37:1427–42. [DOI] [PubMed] [Google Scholar]
- 47. Salemeh E, Olsen S, Howard D.. Nonattendance with clinic follow-up appointments: diabetes as exemplar. J Nurse Pract 2012;8:797–803. [Google Scholar]
- 48. Taylor DM, Fraser S, Dudley Cet al. Health literacy and patient outcomes in chronic kidney disease: a systematic review. Nephrol Dial Transplant 2018;33:1545–58. [DOI] [PubMed] [Google Scholar]
- 49. Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int 2012;2:1–138. [Google Scholar]
- 50. Kidney Disease: Improving Global Outcomes CKD Work Group . KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3:1–150. [Google Scholar]
- 51. May C, Montori VM, Mair FS.. We need minimally disruptive medicine. BMJ 2009;339:b2803. [DOI] [PubMed] [Google Scholar]
- 52. Blakeman T, Protheroe J, Chew-Graham Cet al. Understanding the management of early-stage chronic kidney disease in primary care: a qualitative study. Br J Gen Pract 2012;62:e233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Daker-White G, Rogers A, Kennedy Aet al. Non-disclosure of chronic kidney disease in primary care and the limits of instrumental rationality in chronic illness self-management. Soc Sci Med 2015;131:31–9. [DOI] [PubMed] [Google Scholar]
- 54. Tsang JY, Blakeman T, Hegarty Jet al. Understanding the implementation of interventions to improve the management of chronic kidney disease in primary care: a rapid realist review. Implement Sci. 2015;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. European Observatory on Health Systems and Policies . United Kingdom: health system review 2022. https://eurohealthobservatory.who.int/publications/i/united-kingdom-health-system-review-2022 6 June 2022, date last accessed). [Google Scholar]
- 56. Campbell SM, Reeves D, Kontopantelis Eet al. Effects of pay for performance on the quality of primary care in England. N Engl J Med 2009;361:368–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets cannot be made available to other researchers due to ethical considerations. Information on how researchers may make requests to obtain similar datasets from the health research dataset custodians will be provided upon request.