Abstract
Background
Breast milk feedings are the optimal feeding choice for premature infants. Clinicians depend on accurate nutrient profiles of the breast milk in order to make informed decisions regarding the need for nutrient supplementation. Existing data for nutrient composition of preterm breast milk are dated and not representative of the current population of women delivering prematurely in the United States.
Objectives
The purpose of this prospective, longitudinal, single-center observational study was to measure the macronutrient and micronutrient composition of breast milk expressed by mothers, including women who self-identify as black, delivering preterm infants at ≤33 completed weeks of gestation.
Methods
We collected breast milk samples from mothers of preterm infants admitted to the neonatal intensive care unit at Augusta University Medical Center from January 2019 through November 2019. Mother’s milk samples were collected on postpartum days 7, 14, 21, and 28 and analyzed for macronutrients (energy, fat, protein, and carbohydrates) and micronutrients (sodium, potassium, chloride, calcium, phosphorus, magnesium, vitamin D, and zinc).
Results
Thirty-eight mothers, mean age 27 ± 5.1 y and majority black (66%), provided milk for the study. The mean estimated gestational age and birth weight were 28.2 ± 2.8 weeks of gestation and 1098 ± 347 g, respectively, with 42% of mothers in the cohort delivering before week 28 of pregnancy. Differences in protein, sodium, potassium, calcium, phosphorus, and zinc concentrations based on race, day, and milk volume were identified. Dilution effects for protein, sodium, chloride, and vitamin D concentrations over time were identified.
Conclusions
Our study is among the first to characterize breast milk composition from women who delivered extremely preterm infants and adds to the evidence that race, gestational age, and volume influence the composition of preterm mother’s milk. These factors should be considered when designing mother’s milk–based feeds for premature infants.
Keywords: premature infant, breast milk, human milk, nutrients, fortification, composition, African American
Introduction
Over the last 2 decades, increasing appreciation for the benefits of human milk for preterm infants has led to the widespread use of mother’s own milk or donor milk in neonatal intensive care units (NICUs). Human milk is associated with several short- and long-term benefits for preterm neonates including lower risk of necrotizing enterocolitis and infection (1, 2, 3, 4, 5, 6, 7, 8, 9). Mother’s milk is also associated with a reduction in hospitalizations before the first birthday and better cognitive outcomes that may persist out to adolescence (5, 6, 7, 8, 9, 10, 11, 12). The advantages of mother’s milk for the preterm infant are clear (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12); however, the preterm infant diet presents several obstacles to delivering sufficient energy and nutrients to support proper extrauterine growth. In particular, the preterm infant’s nutrient needs must be supplied in a weight-appropriate volume, which in isolation is insufficient to provide appropriate nutrition and often requires macronutrient and micronutrient supplementation (1, 13). Further, health care providers tasked with meeting the energy and nutrient needs of preterm infants, including the design and use of human milk fortifiers, must rely on data on mother’s milk composition that are extrapolated from term human milk or limited studies of preterm human milk. To date, few studies have examined the energy and nutrient composition of preterm human milk obtained from mothers who delivered before 28 completed weeks of gestation. Studies that include the earlier gestations have small sample sizes and either the numbers of mothers delivering before week 28 of pregnancy are limited or the mean estimated gestational age (EGA) was not reported (14, 15). Perhaps as importantly, the most comprehensive description of preterm human milk composition is >35 y old and does not include African-American or black mothers (16). In order to accurately meet the nutritional needs of preterm infants, a comprehensive analysis of mother’s milk that includes extremely preterm infants from a multiethnic population is warranted.
Objectives
The purpose of this prospective, longitudinal, single-center observational study was to measure the macronutrient (energy, fat, protein, and carbohydrates) and micronutrient (sodium, potassium, chloride, calcium, phosphorus, magnesium, vitamin D, and zinc) composition of breast milk expressed by mothers, including women who self-identify as black, delivering preterm infants at ≤33 completed weeks of gestation.
Methods
The study was approved by the Augusta University Institutional Review Board and written informed consent was obtained from all mothers before enrollment. The study collected samples from study participants who were mothers of preterm infants admitted to the NICU at Augusta University Medical Center (AUMC) from January 2019 through November 2019. Eligible mothers were identified in consultation with the health care team as women who delivered preterm infants before the completion of the 33rd week of gestation and whose preterm infant was admitted to the NICU at AUMC before day-of-life (DOL) 7. Mothers were excluded from the study if 1) removing the study volume (20 mL) of expressed breast milk 1 time/wk contributed to inadequate milk supply to meet the nutritional needs of the infant, 2) the mother identified as vegan or vegetarian, 3) she restricted her daily caloric intake to <1200 kcal/d, or 4) she was positive for HIV. Demographic data including maternal age, self-reported race, height, prepregnancy weight, BMI (in kg/m2), and preterm infant’s postmenstrual age, weight, and sex were extracted from the electronic medical record.
Milk collection
Expressed milk samples were collected on postpartum days 7, 14, 21, and 28. Mothers were provided with sterile single-use polypropylene bottles and access to a hospital-grade electric breast-pump throughout the study. Mothers were instructed to fully empty both breasts every 3 h and all bottles of expressed milk were labeled with the mother’s name, date, and time of each milk collection. Each collection of mother’s milk was processed by trained technicians in the AUMC Milk Laboratory and each 24-h collection was pooled for storage in a single-use container and total volume was recorded. Once pooled, a 20-mL sample of mother’s milk was extracted from the 24-h pool representing each time point. Study samples were stored in a sterile polypropylene bottle and frozen at −28.9°C for later analysis. The number of milk samples sent for analysis at each time point varied slightly based on the availability of adequate milk volume per subject. Forty-seven mothers consented to participate in the study; however, only 38 mothers had adequate milk supply to allow for nutrient analysis. By the fourth week of lactation, 10 mothers had ceased lactating, leaving 28 mothers for sample analysis at day 28. Tables 1 and 2 provide details of the sample size for each nutrient at each point in time.
TABLE 1.
Maternal and infant characteristics1
| Variable | Value | Range |
|---|---|---|
| Maternal age, y | 27 ± 5.1 | 18–37 |
| Gravida | 2.2 ± 1.6 | 1–6 |
| EGA, wk | 28.2 ± 2.8 | 22.9–33.0 |
| EGA < 28 wk | 16 (42) | |
| Prepregnancy BMI, kg/m2 | 31 ± 7.8 | 19–53.8 |
| Black | 32.5 ± 8.4 | |
| White | 28.8 ± 6.1 | |
| Infant birth weight, g | 1098 ± 347.3 | 545–2130 |
| Infant sex (male) | 20 (53) | |
| Race | ||
| Black | 25 (66) | |
| White | 13 (34) | |
| Mothers who delivered twins | 3 (8) | |
| Smoker | 2 (5) | |
| Prenatal vitamins | 33 (87) | |
n = 38. Values are n (%) or mean ± SD. Supplemental Tables 1–7 provide complete statistical results, interactions, and covariates for each nutrient at each point in time. EGA, estimated gestational age.
TABLE 2.
Sample size required to measure change in mean percentage meeting intake over time for 4 different nutrients at 4 different SD assumptions
| Assumed mean percentage meeting intake of nutrient at day | Sample size for time effect at SD | |||||||
|---|---|---|---|---|---|---|---|---|
| Nutrient | 7 d | 14 d | 21 d | 28 d | SD = 3% | SD = 5% | SD = 7% | SD = 10% |
| Magnesium | 6% | 4% | 13% | 15% | 6 | 8 | 11 | 17 |
| Sodium | 15% | 23% | 29% | 33% | 5 | 6 | 8 | 11 |
| Potassium | 7% | 9% | 13% | 15% | 7 | 11 | 18 | 31 |
| Zinc | 8% | 8% | 8% | 23% | 5 | 7 | 8 | 11 |
Milk analysis
Stored samples were shipped frozen every 4 wk to Eurofins S-F Laboratories (New Berlin, WI) for analysis. Upon arrival at the laboratory, milk samples were stored in the freezer at −10°F until analysis. All sample analysis met the standards outlined by the AOAC Official Methods of Analysis (17). Protein was determined by the Dumas combustion method which involves introducing the sample to a combustion furnace, followed by conversion of all nitrogen to N2 and detection by thermal conductivity (18). The nitrogen was then converted to protein using a factor of 6.38. Fat concentrations were determined using the Mojonnier method with AOAC official method 989.05 (19). A portion of the sample (tempered to 38°C) was transferred into a clean flask. Concentrated NH4OH was added to this 1.5 mL. To aid in visual differentiation of the interface, 3 drops of phenolphthalein indicator were added to the sample. The fat was extracted 3 times using 95% ethanol and petroleum ether. After extraction, the samples were centrifuged under conditions to promote phase separation, and subsequently decanted into a weighing dish. The ether was evaporated and the extracted fat was dried to a constant weight. Carbohydrate concentration was calculated using the Atwater Calculation using the analytically determined moisture, ash, fat, and protein content (17). Energy concentration was measured using bomb calorimetry (17). Vitamin D samples were saponified, extracted with petroleum ether, and quantified via reverse-phase HPLC using UV detection. Phosphorus, sodium, potassium, chloride, zinc, magnesium, and calcium were measured using inductively coupled plasma optical emission spectroscopy (ICP-OES) in accordance with Environmental Protection Agency (EPA) method SW-846 6010D (20). Each 0.5-mL sample was microwave digested with 4.0 mL nitric acid and 1.0 mL hydrochloric acid. Digested samples were analyzed for metals by ICP-OES. Chloride analysis was performed using ion chromatography in accordance with EPA method 300.0 Rev 2.1 (21). Samples were analyzed using a Dionex ICS-1100 and a Dionex IonPac AG22-Fast Analytical column with a mobile phase consisting of 4.5 mM sodium carbonate and 1.4 mM sodium bicarbonate in water at a flow rate of 1.2 mL/min.
Sample size determination
The sample size was determined assuming a mixed model to examine the change over time, the main effect, in the mean percentage of nutrient amounts meeting nutrient intake requirements in premature breast milk plus a fortifier. Other assumptions included an α level of 0.05, power of 80%, and a compound symmetric correlation structure between measurement days with a correlation of 0.6. Table 3 lists the assumed mean percentage meeting intake requirements at each measurement time for magnesium, sodium, potassium, and zinc. The sample sizes to examine the main effect of time for the assumed mean percentages meeting intake requirements for each nutrient for 4 different SDs of 3%, 5%, 7%, and 10% were calculated. For example, the required sample size for examining the change in the mean percentage meeting intake of magnesium requirements assumed at days 7, 14, 21, and 28 using an SD of 3% was a total of 6 subjects. The study required a total sample size of 31, corresponding to the change in mean percentage meeting potassium intake across the 4 measurement days assuming an SD of 10%.
TABLE 3.
Descriptive statistics for macronutrients by day1
| Macronutrient | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|
| Energy | ||||
| Concentration, kcal/dL | 66.3 ± 10.7 | 69.9 ± 11.4 | 70.4 ± 10.4 | 65.5 ± 9.6 |
| 95% CI | 62.7, 69.9 | 66.0, 73.8 | 66.7, 74.0 | 61.8, 69.2 |
| Observations, n | 36 | 35 | 34 | 28 |
| Fat | ||||
| Concentration, g/dL | 3.1 ± 1 | 3.5 ± 1.1 | 3.7 ± 1.1 | 3.2 ± 1.0 |
| 95% CI | 2.8, 3.4 | 3.1, 3.9 | 3.3, 4.0 | 2.9, 3.6 |
| Observations, n | 36 | 35 | 34 | 28 |
| Protein | ||||
| Concentration, g/dL | 2.2 ± 0.08 | 1.8 ± 0.08 | 1.8 ± 0.08 | 1.6 ± 0.10 |
| 95% CI | 2.0, 2.2 | 1.7, 2.1 | 1.5, 1.9 | 1.4, 1.8 |
| Observations, n | 36 | 35 | 34 | 28 |
| Carbohydrates | ||||
| Concentration, g/dL | 7.5 ± 0.6 | 7.7 ± 0.6 | 7.6 ± 0.7 | 7.5 ± 1.4 |
| 95% CI | 7.3, 7.7 | 7.5, 7.9 | 7.4, 7.9 | 7.0, 8.1 |
| Observations, n | 36 | 35 | 34 | 28 |
| Breast milk | ||||
| Volume, mL/d | 171.8 ± 148.5 | 198.5 ± 136.9 | 224.2 ± 159.9 | 210.3 ± 174.3 |
| 95% CI | 110.5, 233.1 | 140.7, 256.3 | 155.0, 293.3 | 126.3, 294.3 |
| Observations, n | 25 | 24 | 23 | 19 |
Values are mean ± SD unless otherwise noted. Supplemental Tables 1–7 provide complete statistical results, interactions, and covariates for each nutrient at each point in time.
Statistical analysis
All statistical analysis was performed using SAS version 9.4 (SAS Institute Inc.) and statistical significance was set at an α of 0.05. Descriptive statistics for all variables were determined using frequencies and percentages for categorical or ordinal variables and means ± SDs for continuous variables. To examine changes in breast-milk nutrients and other measures across multiple days, repeated-measures mixed models were used. The correlation structure between measurement days that provided the best fit to the data for each nutrient or sample parameter was used and was either unstructured, compound symmetric, or autoregressive order 1. The interaction of race or gestational age < 28 wk with volume of milk and day of collection was examined for each nutrient outcome and breast-milk parameter. If the 3-factor interaction did not meet statistical significance, the 2-factor interactions were examined. If the 2-factor interactions were not statistically significant, the main effects were examined. After any model reduction to a 2-factor interaction model or a main-effects model, various covariates were examined including age, race, vitamin use, BMI, gravida, infant birth weight, infant sex, and twin status. Nonsignificant covariates were removed 1 at a time starting with the least nonsignificant covariate and model fit criteria including Akaike’s Information Criterion (AIC) and the Bayes Information Criterion (BIC) were examined. If the AIC and BIC increased, the covariate was entered back into the model and the next least nonsignificant covariate was removed from the model. The final model contained any 3-factor or 2-factor interactions of race or gestational age < 28 wk with volume and day of collection along with any covariates that were statistically significant or needed in the model to improve fit. A Tukey–Kramer multiple comparison procedure was used to examine post hoc pairwise differences. For any interaction that involved volume, pairwise differences were examined at volumes of 65, 150, and 300 mL, corresponding to the 25th, 50th, and 75th percentiles for volume of mother’s milk from the cohort.
Results
Table 1 and Supplemental Table 1 detail participant characteristics for the 38 mothers (Figure 1) who provided milk for the study. The mean age of the study participants was 27 ± 5.1 y and the cohort were majority black (66%) and obese (mean BMI: 31 ± 7.8), which reflects the population in the local metropolitan area. There was no statistically significant difference (P = 0.0850) in mean BMI between the black (32.5 ± 8.4) and the white (28.8 ± 6.1) mothers. The mean EGA and birth weight were 28.2 ± 2.8 weeks of gestation and 1098 ± 347 g, respectively, with 42% of infants in the cohort delivered before the 28th completed week of pregnancy.
FIGURE 1.
Participant flowchart.
Macronutrients
Table 3 shows the data for each macronutrient at each time point. Supplemental Tables 1–7 provide the complete analysis with the final repeated-measures mixed model results controlling for various covariates. The final repeated-measures mixed model for energy and fat did not interact with race, volume, or EGA at birth. Energy varied considerably with a 95% CI of 62.7, 69.9 kcal/dL on day 7 and 66.0, 73.8 kcal/dL on day 14 (Figure 2A). Energy was significantly higher on day 7 than on day 28 and fat concentration was significantly lower on day 7 than it was on day 14, but there were no differences between the remaining days (Figure 2B).
FIGURE 2.
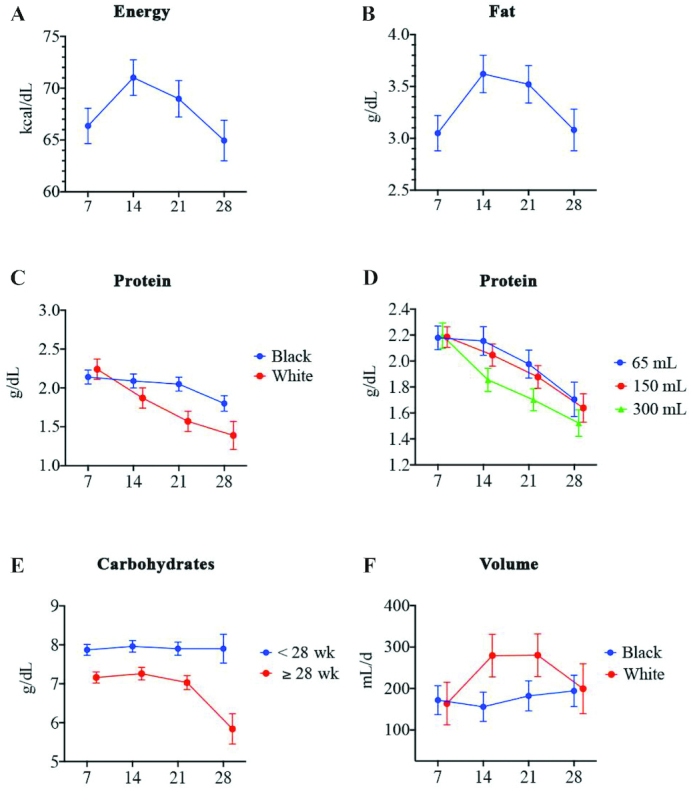
Macronutrient concentration in preterm breast milk on postpartum days 7 (n = 36), 14 (n = 35), 21 (n = 34), and 28 (n = 28). (A) Energy. (B) Fat. (C) Protein by race. (D) Protein by volume. At a volume of 65 mL, day 7 (P = 0.0028) and day 14 (P = 0.0066) had greater mean protein than day 28. At a volume of 150 mL, day 7 had a greater mean protein than day 21 (P = 0.0034) and day 28 (P < 0.0001), day 14 had a significantly greater mean protein than day 28 (P = 0.0019), and day 21 had a significantly greater mean protein than day 28 (P = 0.0400). At a volume of 300 mL, day 7 had a greater mean protein than day 14 (P = 0.0007), day 21 (P < 0.0001), and day 28 (P < 0.0001), and day 14 had a greater mean protein than day 28 (P = 0.0071). Among whites, day 7 had greater mean protein than day 21 (P = 0.0022) and day 28 (P = 0.0031). (E) Carbohydrates by gestational age. For those with a gestational age ≥28 wk, day 28 was lower than days 7 (P = 0.0187) and 14 (P = 0.0171). Within day 28, mean carbohydrate was lower among those with a gestational age ≥ 28 wk than among those with a gestational age < 28 wk. (F) Volume by race.
Post hoc pairwise comparisons found significant differences based on race and day (Figure 2C) and volume and day (Figure 2D) for protein concentration in mother’s milk over time. The protein concentration decreased by 36% from day 7 to day 28 for white participants and only 14% for blacks during the same period. Protein concentration was significantly different based on volume at several time points. For the lowest volume representing the 25th percentile (i.e., 65 mL), the protein concentration on days 7 (P = 0.0028) and 14 (P = 0.0066) was significantly greater than on day 28 (Figure 2D). At a volume of 150 mL (representing the 50th percentile), day 28 had a significantly lower protein concentration than days 7 (P < 0.0001), 14 (P = 0.0019), and 21 (P = 0.04). A similar trend for protein concentration over time was identified for the highest volume (i.e., 300 mL). No statistically significant differences for protein concentration based on EGA were identified.
Significant interactions between EGA and day, but not race or volume and day, were identified for carbohydrates. As seen in Figure 2E, carbohydrate concentration remained stable across all time points for mothers delivering at <28 weeks of gestation. Study subjects with an EGA ≥ 28 wk had a lower carbohydrate concentration in mother’s milk at day 28 than at days 7 (P = 0.0187) and 14 (P = 0.0171). The volume of mother’s milk over time was influenced by race (Figure 2F); however, no statistically significant differences between black and white study participants with respect to sample day or between sample days within each race were identified.
Micronutrients
Table 4 shows the data for each micronutrient at each time point. Supplemental Tables 1–7 present the complete analysis with the final repeated-measures mixed model results controlling for various covariates. Significant interactions between EGA, volume, and race and day were identified for sodium concentration in mother’s milk as seen in Figure 3A and B. Although the effect of EGA on sodium concentration over time was statistically significant, post hoc differences between time points within each EGA cohort or between EGA cohorts were not identified (Figure 3A).
TABLE 4.
Descriptive statistics for micronutrients by day1
| Micronutrients | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|
| Sodium | ||||
| Concentration, mg/dL | 36.1 ± 25 | 28.5 ± 21.2 | 30.5 ± 28.9 | 34.5 ± 37.6 |
| 95% CI | 27.6, 44.5 | 21.2, 35.8 | 20.4, 40.5 | 19.9, 49.1 |
| Observations, n | 36 | 35 | 34 | 28 |
| Potassium | ||||
| Concentration, mg/dL | 64.2 ± 11.4 | 55.5 ± 11.9 | 53.3 ± 11.7 | 50.4 ± 11.7 |
| 95% CI | 60.3, 68.0 | 51.4, 59.5 | 49.3, 57.4 | 45.9, 55.0 |
| Observations, n | 36 | 35 | 34 | 28 |
| Chloride | ||||
| Concentration, mg/dL | 69.5 ± 34.7 | 58.3 ± 31.9 | 58.2 ± 38.8 | 66.1 ± 51.3 |
| 95% CI | 57.7, 81.2 | 47.3, 69.3 | 44.6, 71.7 | 46.2, 86.0 |
| Observations, n | 36 | 35 | 34 | 28 |
| Calcium | ||||
| Concentration, mg/dL | 24.2 ± 8.9 | 22.3 ± 8.1 | 22.0 ± 5.9 | 21.2 ± 5.5 |
| 95% CI | 21.2, 27.2 | 19.5, 25.1 | 19.9, 24.0 | 19.1, 23.4 |
| Observations, n | 36 | 35 | 34 | 28 |
| Phosphorus | ||||
| Concentration, mg/dL | 15.0 ± 4.8 | 14.8 ± 3.7 | 14.0 ± 3.3 | 13.0 ± 3.8 |
| 95% CI | 13.4, 16.6 | 13.6, 16.1 | 12.9, 15.2 | 11.5, 14.5 |
| Observations, n | 36 | 35 | 34 | 28 |
| Magnesium | ||||
| Concentration, mg/dL | 3.2 ± 0.9 | 3.0 ± 0.8 | 2.8 ± 0.7 | 3.3 ± 3.4 |
| 95% CI | 2.9, 3.5 | 2.7, 3.3 | 2.6, 3.1 | 2.1, 4.6 |
| Observations, n | 36 | 35 | 34 | 29 |
| Vitamin D | ||||
| Concentration, IU/dL | 7.9 ± 11.8 | 8.1 ± 9.3 | 6.5 ± 7.7 | 5.0 ± 3.3 |
| 95% CI | 3.9, 11.9 | 4.9, 11.3 | 3.9, 9.2 | 3.7, 6.4 |
| Observations, n | 36 | 35 | 34 | 26 |
| Zinc | ||||
| Concentration, mg/dL | 0.5 ± 0.2 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.2 |
| 95% CI | 0.4, 0.5 | 0.4, 0.5 | 0.3, 0.4 | 0.3, 0.4 |
| Observations, n | 36 | 35 | 34 | 28 |
Values are mean ± SD unless otherwise noted. Supplemental Tables 1–7 provide complete statistical results, interactions, and covariates for each nutrient at each point in time.
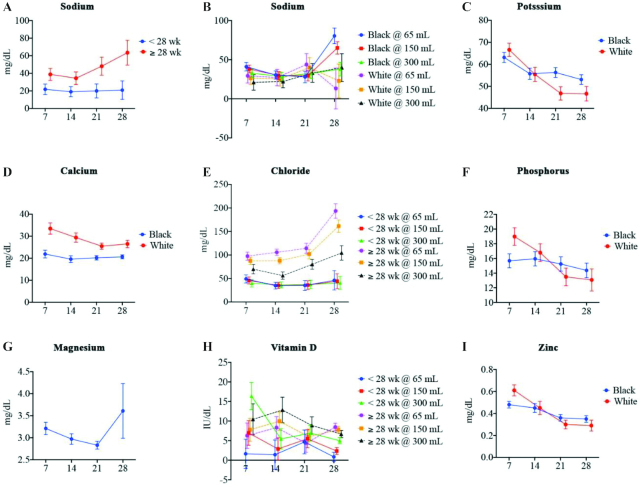
FIGURE 3.
Micronutrient concentration in preterm breast milk on postpartum days 7 (n = 36), 14 (n = 35), 21 (n = 34), and 28 (n = 28). (A) Sodium by gestational age. (B) Sodium by race and volume. Among blacks at a volume of 65 mL a significantly greater mean sodium was seen at day 7 than at day 14 (P = 0.0218) but was lower than at day 28 (P = 0.0004), day 14 was lower than day 28 (P < 0.0001), and day 21 was lower than day 28 (P = 0.0003). Among blacks at a volume of 150 mL a greater mean sodium was seen at day 7 than at day 14 (P = 0.0253) and lower than at day 28 (P = 0.0009). (C) Potassium by race. Among blacks, day 7 was greater than day 28 (P = 0.0099). Among whites, day 7 was greater than day 21 (P = 0.0001) and day 28 (P = 0.0001). (D) Calcium by race. Calcium was lower for blacks than for whites at day 7 (P = 0.0284) and day 14 (P = 0.0295). Among whites, day 7 calcium was greater than day 21 (P = 0.0102). (E) Chloride by gestational age and volume. Among those delivering at ≥28 weeks of gestation at a volume of 65 mL, day 7 was lower than day 21 (P = 0.0369) and day 28 (P < 0.0001), day 14 was lower than day 28 (P < 0.0001), and day 21 was lower than day 28 (P < 0.0001). Among those delivering at ≥28 weeks of gestation at a volume of 150 mL, day 7 was lower than day 21 (P = 0.0301) and day 28 (P = 0.0001), day 14 was lower than day 21 (P = 0.0083) and day 28 (P < 0.0001), and day 21 was lower than day 28 (P = 0.0002). Among those delivering at ≥28 weeks of gestation at a volume of 300 mL, day 14 was lower than day 21 (P = 0.0003) and day 28 (P = 0.0019). At a volume of 65 mL, those delivering at <28 weeks of gestation had lower mean chloride than those delivering at ≥28 weeks of gestation at day 7 (P = 0.0014), day 14 (P < 0.0001), day 21 (P < 0.0001), and day 28 (P < 0.0001). At a volume of 150 mL, those delivering at <28 weeks of gestation had lower mean chloride than those delivering at ≥28 weeks of gestation at day 7 (P = 0.0014), day 14 (P < 0.0001), day 21 (P < 0.0001), and day 28 (P < 0.0001). At a volume of 300 mL, those delivering at <28 weeks of gestation had lower mean chloride than those delivering at ≥28 weeks of gestation at day 7 (P = −0.0324), day 21 (P = 0.0070), and day 28 (P = 0.0057). (F) Phosphorus by race. (G) Magnesium. Day 7 was greater than day 21 (P = 0.0148). (H) Vitamin D by gestational age and volume. Among those delivering at <28 weeks of gestation at a volume of 300 mL, day 7 was greater than day 14 (P = 0.0005), day 21 (P = 0.0048), and day 28 (P = 0.0016). At a volume of 65 mL and day 28, those delivering at <28 weeks of gestation had lower mean vitamin D than those delivering at ≥28 weeks of gestation (P < 0.0001). At a volume of 150 mL and day 28, those delivering at <28 weeks of gestation had lower mean vitamin D than those delivering at ≥28 weeks of gestation (P = 0.0002). (I) Zinc by race. Among whites, day 7 was greater than day 21 (P = 0.0011) and day 28 (P = 0.0045), and day 14 was greater than day 21 (P = 0.0257).
Figure 3B demonstrates interactions involving both race and volume with day on sodium concentration in mother’s milk. At the lowest volume representing the 25th percentile (i.e., 65 mL), the sodium concentration in mother’s milk from black participants was significantly higher at day 28 than at days 7 (P = 0.0004), 14 (P < 0.0001), and 21 (P = 0.0003). Sodium concentration was also higher at day 7 than at days 14 (P = 0.022) and 21 (P = 0.028). Similar relations were identified for black participants at a volume of 150 mL (median) with sodium concentration significantly higher on day 28 than on days 7 (P = 0.0009), 14 (P < 0.0001), and 21 (P = 0.0009). No differences among black participants at a volume of 300 mL over time were identified.
For white participants, significant differences in sodium concentration by volume over time were identified. In comparison with black participants, white participants had significantly lower sodium concentrations in mother’s milk at volumes of 65 mL and 150 mL, but no differences between races at 300 mL were identified (Figure 3B).
A significant interaction between time and race was identified for potassium concentration in mother’s milk (Figure 3C). For both black and white participants, potassium concentration was significantly higher on day 7 than on day 28 (P = 0.0099 and P = 0.0001, respectively). A similar statistical relation between day 7 and day 21 for white participants was also identified (P < 0.0001). No differences between races at each time point were found.
Figure 3E demonstrates significant interactions involving both EGA and volume with day on chloride concentration in mother’s milk. Among participants with an EGA < 28 wk, no differences between days and volumes were identified. At each volume for participants with an EGA ≥ 28 wk, the chloride concentration was highest at 28 d when compared with days 7, 14, and 21. Similarly, the chloride concentration at each time point for all volumes of mother’s milk was significantly reduced in study participants with an EGA < 28 wk when compared with those with an EGA ≥ 28 wk.
A significant interaction between race and time was identified for calcium (Figure 3D) and phosphorus concentrations (Figure 3F) in mother’s milk. Black participants had significantly lower calcium concentrations than white participants on days 7 (P = 0.0284) and 14 (P = 0.0295). The calcium concentration in mother’s milk did not vary significantly over time for black participants, but a statistical difference for white participants was identified for day 7 compared with day 21 (P = 0.0102). Phosphorus concentration did not vary significantly over time between races, and between-day comparisons for black study participants were not statistically different. For white participants, phosphorus concentration on day 7 was significantly higher than on day 21 (P = 0.0013) and day 28 (P = 0.0140).
No interactions between race, EGA, or volume and time were identified for magnesium concentration (Figure 3G).
Figure 3H demonstrates significant interactions involving both EGA and volume with vitamin D concentration in mother’s milk. For study participants with an EGA ≥ 28 wk, no differences in vitamin D concentration between time points at any volume of mother’s milk were identified. Similarly, no differences in vitamin D concentration between time points and volumes of mother’s milk were identified in study participants with an EGA < 28 wk except at the highest volume (i.e., 300 mL), where vitamin D concentration was higher at day 7 than at days 14 (P = 0.0005), 21 (P = 0.0048), and 28 (P = 0.0016). In comparison with study participants with an EGA ≥ 28 wk, the vitamin D concentration in subjects with an EGA < 28 wk was lower at day 28 for the lowest (i.e., 65 mL; P < 0.0001) and middle (i.e., 150 mL; P = 0.0002) volumes.
A statistically significant interaction with race and time was found for zinc concentration in mother’s milk (Figure 3I). Among black participants, no differences in zinc concentration between time points were identified. For white study participants, zinc concentration at day 7 was significantly higher than at day 14 (P = 0.0045) and day 21 (P = 0.0011).
Discussion
Our report fills 2 major gaps in the current human milk literature. First, the mean EGA (28.2 ± 2.8 wk) and birth weight (1098 ± 347.3 g) in this report are lower than those in most previous publications and our report is novel in that we included a large proportion (42%) of mothers delivering before 28 completed weeks of gestation (14, 15, 16, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36). Second, our study was composed of a majority black (64%) population, which highlights the gross disparity in preterm births for African-American and black women and the under-representation of African-American and black women in the human milk literature. However, the causality of the differences in the nutrient composition based on race cannot be determined with our study and additional research in this area is needed. In our comprehensive analysis of longitudinal human milk samples across the first postnatal month, we identified several important differences in macronutrient and micronutrient composition in preterm mother’s milk that are related to race, gestational age, and volume of milk. These unique relations have not been identified in previous reports owing to an absence or under-representation of mothers who deliver before 28 completed weeks of gestation or the lack of inclusion of minorities or appreciation for the volume of milk in study samples.
Our findings shed new light on the content of preterm human milk and will help reveal potential dietary shortcomings in the current approach to preterm nutrition and human milk fortification. For example, protein balanced with fat and energy is an important factor in short-term outcomes including “return-to-birth-weight” and discharge weight as well as long-term developmental outcomes for the preterm infant (37, 38, 39). The amount of protein intake necessary to achieve adequate postnatal growth and development for a very-low-birth-weight (VLBW) preterm infant is 3 times greater than what is required for a newborn term infant. For the VLBW population, meeting the recommended 3.5–4.5 g · kg−1 · d−1 intake of protein with breast milk alone would not be possible within the recommended range of enteral volume and additional protein supplementation may be warranted (1, 13). The addition of protein supplements, either as multinutrient or single-nutrient fortification, assumes the protein concentration of the breast milk to be stagnant across the preterm lactation stages, race, gestational age, and milk volume. Our data show that by DOL 28 black mothers had 29% more protein in their milk than white mothers and that the protein concentration decreased as the volume of milk increased. At day 28, preterm infants of white mothers would need an additional 2–3 g · kg−1 · d−1 of protein supplementation, but infants of black mothers would need only 1–2 g · kg−1 · d−1 of supplementation. In addition, the dilution effect on protein must be considered for mothers who produce large volumes of breast milk per day (37, 38, 39, 40, 41).
Gestational age at the time of delivery had a significant impact on sodium concentration. We found that sodium was 3 times higher in mother’s milk from subjects delivering at ≥28th week of gestation than for those delivering before completion of the 28th week. Extremely preterm infants (i.e., <28 weeks of gestation) have less total body mass, increased fecal loss of sodium, lower tolerance for enteral volume, and are more likely to be exposed to diuretics (42, 43, 44). Combining these risk factors for sodium depletion with the lower sodium content in extremely preterm mother’s milk, the risk of sodium-related growth failure may be more prominent in extremely preterm infants. Human milk fortifiers add varying amounts of sodium to preterm human milk, but additional supplementation may be warranted for infants born before the 28th week of pregnancy. We found that chloride followed a pattern similar to sodium in that the mothers delivering before the 28th week had less chloride per deciliter at all volumes of mother’s milk. The chloride concentration rose from the first to the fourth week for mothers delivering at ≥28 wk, but the concentration did not rise for those delivering before 28 wk. As with protein, the dilution effect on sodium and chloride must be considered when prescribing sodium supplementation for this population.
Consistent with previous published reports, preterm mother’s milk was largely deficient in vitamin D across the first month after birth. Gestational age and volume influenced concentration; however, even at the highest concentration, the amount of vitamin D provided by mother’s milk alone fell well below the recommended intake. Hence, supplementation of 400–1000 IU vitamin D/d is necessary for all mother’s milk–fed preterm infants (1, 13, 45, 46, 47).
Similarly, the amount of zinc intake required to promote growth and prevent deficiency in the preterm infant cannot be met by mother’s milk alone at any stage of lactation and supplementation is required (1, 13, 45, 48, 49). Human milk fortifiers provide supplemental zinc but may not provide enough to meet the needs of the preterm infant. Prophylactic supplementation of zinc, in addition to what is provided by fortified mother’s milk, may be warranted for the infants of these mothers.
We acknowledge several limitations. First, most of the subjects were black or black and obese. Although representative of the population of the local metropolitan area, the over-representation of black women may not be generalizable to the general population where <15% of the US population identifies as black. Second, although our data are among the first to examine longitudinal mother’s milk samples from extremely preterm infants, we recognize that a larger cohort may provide additional insight into influences such as maternal adiposity (e.g., BMI), maternal diet, and other factors that were not included in this study (50, 51, 52, 53, 54). Third, our results focus on the first postnatal month and will not account for changes in mother’s milk composition after that time or as the volume of breast milk begins to decrease over time. Analysis of longitudinal samples after 28 weeks of gestation would be an important future consideration because this time period likely represents the largest volume of mother’s milk used in the preterm infant’s diet.
Mother’s milk offers a complex source of nutrients that represents the “gold standard” for the preterm infant diet (2, 3, 4, 8, 9, 10, 11, 12). However, the needs of nutritionally vulnerable preterm infants are difficult to meet with mother’s milk alone (1, 13). Supplementation and fortification strategies depend on an accurate representation of the nutrient profile in preterm mother’s milk. Our findings can be useful in the development of targeted fortification strategies for the human milk–fed preterm infant.
Acknowledgments
AG thanks Dr. Jatinder Bhatia for his continued mentorship and support. We also thank the AUMC NICU milk technicians, Alice Shoultz and Benita Perez, for their assistance in the breast milk collection.
The authors’ responsibilities were as follows—AG: conceptualized and designed the study, collected the data, and drafted the initial manuscript; TM, GDL, and BKS: reviewed and approved the study design; TM, GDL, BKS, and AG: reviewed and revised the manuscript; JLW: designed the statistical analysis plan, analyzed the data, and reviewed and revised the results; and all authors: agree to be accountable for all aspects of the work and read and approved the final manuscript. AG accepted a position as a Medical Science Liaison with Mead Johnson Nutrition in September 2020. She was not in this role at the time of the study or at the time of the original submission. AG maintains a faculty position at Augusta University. AG is a Mead Johnson Nutrition Speaker’s Bureau, Advisory Board grant recipient and a Medolac Laboratories in-kind grant recipient. TM reports funding from the Mead Johnson Nutrition Speaker’s Bureau. All other authors report no conflicts of interest.
Data Availability
Data from this article will be made available upon request pending application and approval.
Footnotes
Supported by an unrestricted grant from Mead Johnson Nutrition (to AG).
Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
SUPPORTING INFORMATION
nqab226_supplemental_file
References
- 1.American Academy of Pediatrics Committee on Nutrition . In: Pediatric nutrition. 8th ed. Kleinman RE, Greer FR, editors. American Academy of Pediatrics; Itasca, IL: 2020. Nutritional needs of the preterm infant; pp. 113–162. [Google Scholar]
- 2.Eidelman AI. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeed Med. 2012;7(5):323–324. doi: 10.1089/bfm.2012.0067. [DOI] [PubMed] [Google Scholar]
- 3.Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. 2014;(4):CD002971. doi: 10.1002/14651858.CD002971.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. J Perinatol. 2009;29(1):57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, Chan GM, Blanco CL, Abrams S, Cotten CM, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156(4):562–567. doi: 10.1016/j.jpeds.2009.10.040. e1. [DOI] [PubMed] [Google Scholar]
- 6.Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, Dudell G, Rechtman DJ, Lee ML, Lucas A, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163(6):1592–1595. doi: 10.1016/j.jpeds.2013.07.011. e1. [DOI] [PubMed] [Google Scholar]
- 7.Ghandehari H, Lee ML, Rechtman DJ. An exclusive human milk-based diet in extremely premature infants reduces the probability of remaining on total parenteral nutrition: a reanalysis of the data. BMC Res Notes. 2012;5:188. doi: 10.1186/1756-0500-5-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Victora CG, Bahl R, Barros AJ, Franca GVA, Horton A, Kraseve J, Murch S, Sankar MJ, Walker N, Rollins NC, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 10.Madore LS, Bora S, Erdei C, Jumani T, Dengos AR, Sen S. Effects of donor breastmilk feeding on growth and early neurodevelopmental outcomes in preterm infants: an observational study. Clin Ther. 2017;39(6):1210–1220. doi: 10.1016/j.clinthera.2017.05.341. [DOI] [PubMed] [Google Scholar]
- 11.Vohr BR, Poindexter BB, Dusick AM, McKinley LT, Wright LL, Langer JC, Poole WK, NICHD Neonatal Research Network Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118(1):e115–e123. doi: 10.1542/peds.2005-2382. [DOI] [PubMed] [Google Scholar]
- 12.Feldman R, Eidelman AI. Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Dev Psychobiol. 2003;43(2):109–119. doi: 10.1002/dev.10126. [DOI] [PubMed] [Google Scholar]
- 13.Koletzko B, Poindexter B, Uauy R. In: Nutritional care of preterm infants: scientific basis and practical guidelines. Koletzko B, Poindexter B, Uauy R, editors. Karger; Basel, Switzerland: 2014. Recommended nutrient intake levels for stable, fully enterally fed very low birth weight infants. pp. 297–299. [DOI] [PubMed] [Google Scholar]
- 14.Gates A, Marin T, De Leo G, Stansfield BK. Review of preterm human-milk nutrient composition. Nutr Clin Pract. 2020 doi: 10.1002/ncp.10570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14:216. doi: 10.1186/1471-2431-14-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross SJ, David RJ, Bauman L, Tomarelli RM. Nutritional composition of milk produced by mothers delivering preterm. J Pediatr. 1980;96(4):641–644. doi: 10.1016/s0022-3476(80)80729-3. [DOI] [PubMed] [Google Scholar]
- 17.Latimer GW. 21st ed. AOAC International; Rockville, MD: 2019. Official methods of analysis. [Google Scholar]
- 18.Wiles PG, Gray IK, Kissling RC. Routine analysis of proteins by Kjeldahl and Dumas methods: review and interlaboratory study using dairy products. J AOAC Int. 1998;81(3):620–632. [PubMed] [Google Scholar]
- 19.Lynch JM, Barbano DM, Fleming JR. Comparison of Babcock and ether extraction methods for determination of fat content of cream: collaborative study. J AOAC Int. 1996;79(4):907–916. [PubMed] [Google Scholar]
- 20.US Environmental Protection Agency (EPA) US EPA; Washington, DC: 2014. Method 6010D (SW-846): inductively coupled plasma-atomic emission spectrometry. Revision 4. [Google Scholar]
- 21.Pfaff JD. Environmental Monitoring Systems Laboratory, Office of Research and Development, US Environmental Protection Agency; Cincinnati, OH: 1993. Method 300.0: determination of inorganic anions by ion chromatography, rev. 2.1. pp. 1–28. [Google Scholar]
- 22.Anderson GH, Atkinson SA, Bryan MH. Energy and macronutrient content of human milk during early lactation from mothers giving birth prematurely and at term. Am J Clin Nutr. 1981;34(2):258–265. doi: 10.1093/ajcn/34.2.258. [DOI] [PubMed] [Google Scholar]
- 23.Guerrini P, Bosi G, Chierici R, Fabbri A. Human milk: relationship of fat content with gestational age. Early Hum Dev. 1981;5(2):187–194. doi: 10.1016/0378-3782(81)90051-7. [DOI] [PubMed] [Google Scholar]
- 24.Mendelson RA, Anderson GH, Bryan MH. Zinc, copper and iron content of milk from mothers of preterm and full-term infants. Early Hum Dev. 1982;6(2):145–151. doi: 10.1016/0378-3782(82)90101-3. [DOI] [PubMed] [Google Scholar]
- 25.Lemons JA, Moye L, Hall D, Simmons M. Differences in the composition of preterm and term human milk during early lactation. Pediatr Res. 1982;16(2):113–117. doi: 10.1203/00006450-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Anderson DM, Williams FH, Merkatz RB, Schulman PK, Kerr DS, Pittard WB., 3rd Length of gestation and nutritional composition of human milk. Am J Clin Nutr. 1983;37(5):810–814. doi: 10.1093/ajcn/37.5.810. [DOI] [PubMed] [Google Scholar]
- 27.Moran-Lev H, Mimouni FB, Ovental A, Mangel L, Mandel D, Lubetzky R. Circadian macronutrients variations over the first 7 weeks of human milk feeding of preterm infants. Breastfeed Med. 2015;10(7):366–370. doi: 10.1089/bfm.2015.0053. [DOI] [PubMed] [Google Scholar]
- 28.Butte NF, Garza C, Johnson CA, Smith EO, Nichols BL. Longitudinal changes in milk composition of mothers delivering preterm and term infants. Early Hum Dev. 1984;9(2):153–162. doi: 10.1016/0378-3782(84)90096-3. [DOI] [PubMed] [Google Scholar]
- 29.Ehrenkranz RA, Ackerman BA, Nelli CM. Total lipid content and fatty acid composition of preterm human milk. J Pediatr Gastroenterol Nutr. 1984;3(5):755–758. doi: 10.1097/00005176-198411000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Kumbhat MM, Khanna SA, Bijur AM, Jadhav CS. Breast milk composition in relation to gestation. Indian Pediatr. 1985;22(3):229–233. [PubMed] [Google Scholar]
- 31.Jitta JN, Musoke RN, Bwibo NO, Kioni J. Composition of early human milk of Kenyan mothers of preterm and term infants. East Afr Med J. 1986;63(11):693–698. [PubMed] [Google Scholar]
- 32.Kim S-Y, Park JH, Kim EA-R, Lee-Kim YC. Longitudinal study on trace mineral compositions (selenium, zinc, copper, manganese) in Korean human preterm milk. J Korean Med Sci. 2012;27(5):532–536. doi: 10.3346/jkms.2012.27.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutta S, Saini S, Prasad R. Changes in preterm human milk composition with particular reference to introduction of mixed feeding. Indian Pediatr. 2014;51(12):997–999. doi: 10.1007/s13312-014-0545-2. [DOI] [PubMed] [Google Scholar]
- 34.Hsu Y-C, Chen C-H, Lin M-C, Tsai C-R, Liang J-T, Wang T-M. Changes in preterm breast milk nutrient content in the first month. Pediatr Neonatol. 2014;55(6):449–454. doi: 10.1016/j.pedneo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Hascoët JM, Chauvin M, Pierret C, Skeweres S, Van Egroo AD, Rouge C, Franck P. Impact of maternal nutrition and perinatal factors on breast milk composition after premature delivery. Nutrients. 2019;11(2):366. doi: 10.3390/nu11020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faerk J, Skafte L, Petersen S, Peitersen B, Michaelsen KF. Macronutrients in milk from mothers delivering preterm. Adv Exp Med Biol. 2001;501:409–413. doi: 10.1007/978-1-4615-1371-1_51. [DOI] [PubMed] [Google Scholar]
- 37.Hay WW, Jr, Brown LD, Denne SC. In: Nutritional care of preterm infants: scientific basis and practical guidelines. Koletzko B, Poindexter B, Uauy R, editors. Karger; Basel, Switzerland: 2014. Energy requirements, protein-energy metabolism and balance, and carbohydrates in preterm infants. pp. 64–81. [DOI] [PubMed] [Google Scholar]
- 38.Coviello C, Keunen K, Kersbergen KJ, Groenendaal F, Leemans A, Peels B, Isgum I, Viergever MA, de Vries LS, Buonocore G, et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr Res. 2018;83(1):102–110. doi: 10.1038/pr.2017.227. [DOI] [PubMed] [Google Scholar]
- 39.Embleton ND, van den Akker CHP. Protein intakes to optimize outcomes for preterm infants. Semin Perinatol. 2019;43(7):151154. doi: 10.1053/j.semperi.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Osborn DA, Schindler T, Jones LJ, Sinn JK, Bolisetty S. Higher versus lower amino acid intake in parenteral nutrition for newborn infants. Cochrane Database Syst Rev. 2018;3(3):CD005949. doi: 10.1002/14651858.CD005949.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poindexter BB, Langer JC, Dusick AM, Ehrenkranz RA, National Institute of Child Health and Human Development Neonatal Research Network Early provision of parenteral amino acids in extremely low birth weight infants: relation to growth and neurodevelopmental outcome. J Pediatr. 2006;148(3):300–305. doi: 10.1016/j.jpeds.2005.10.038. e1. [DOI] [PubMed] [Google Scholar]
- 42.Fusch C, Jochum F. In: Nutritional care of preterm infants: scientific basis and practical guidelines. Koletzko B, Poindexter B, Uauy R, editors. Karger; Basel, Switzerland: 2014. Water, sodium, potassium, and chloride. pp. 99–120. [Google Scholar]
- 43.Verma RP, John E, Fornell L, Vidyasagar D. Fecal sodium and potassium losses in low birth weight infants. Indian J Pediatr. 1993;60(5):631–638. doi: 10.1007/BF02821723. [DOI] [PubMed] [Google Scholar]
- 44.Laughon MM, Chantala K, Aliaga S, Herring AH, Hornick CP, Hughes R, Clark RH, Smith PB. Diuretic exposure in premature infants from 1997 to 2011. Am J Perinatol. 2015;32(1):49–56. doi: 10.1055/s-0034-1373845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, Domellöf M, Embleton ND, Fusch C, Genzel-Borocivzeny O, et al. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2010;50(1):85–91. doi: 10.1097/MPG.0b013e3181adaee0. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed M, Kamleh M, Muzzy J, Groh-Wargo S, Abu-Shaweesh J. Association of protein and vitamin D intake with biochemical markers in premature osteopenic infants: a case-control study. Front Pediatr. 2020;8:546544. doi: 10.3389/fped.2020.546544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zung A, Topf-Olivestone C, Shinwell ES, Hofi L, Juster-Reicher A, Flidel-Rimon O. Reassessing vitamin D supplementation in preterm infants: a prospective study and review of the literature. J Pediatr Endocrinol Metab. 2020;33(10):1273–1281. doi: 10.1515/jpem-2020-0370. [DOI] [PubMed] [Google Scholar]
- 48.Terrin G, Boscarino G, Di Chiara M, Lacobelli S, Faccioli F, Greco C, Onesta E, Sabatini G, Pietravalle A, Oliva S, et al. Nutritional intake influences zinc levels in preterm newborns: an observational study. Nutrients. 2020;12(2):529. doi: 10.3390/nu12020529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harris T, Gardner F, Podany A, Kelleher SL, Doheny KK. Increased early enteral zinc intake improves weight gain in hospitalised preterm infants. Acta Paediatr. 2019;108(11):1978–1984. doi: 10.1111/apa.14828. [DOI] [PubMed] [Google Scholar]
- 50.Sims CR, Lipsmeyer ME, Turner DE, Andres A. Human milk composition differs by maternal BMI in the first 9 months postpartum. Am J Clin Nutr. 2020;112(3):548–557. doi: 10.1093/ajcn/nqaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Jackson RT, Khan SA, Ahuja J, Pehrsson PR. Human milk nutrient composition in the United States: current knowledge, challenges, and research needs. Curr Dev Nutr. 2018;2(7):nzy025. doi: 10.1093/cdn/nzy025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sánchez C, Fente C, Barreiro R, López-Racamonde O, Cepeda A, Regal P. Association between breast milk mineral content and maternal adherence to healthy dietary patterns in Spain: a transversal study. Foods. 2020;9(5):659. doi: 10.3390/foods9050659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao A, Ning Y, Zhang Y, Yang X, Wang J, Li W, Wang P. Mineral compositions in breast milk of healthy Chinese lactating women in urban areas and its associated factors. Chin Med J (Engl). 2014;127(14):2643–2648. [PubMed] [Google Scholar]
- 54.Karcz K, Królak-Olejnik B. Vegan or vegetarian diet and breast milk composition – a systematic review. Crit Rev Food Sci Nutr. 2021;61(7):1081–1098. doi: 10.1080/10408398.2020.1753650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
nqab226_supplemental_file
Data Availability Statement
Data from this article will be made available upon request pending application and approval.