Abstract
Objetive The aim of this systematic review is to analyze the recent scientific evidence of the clinical effects of altitude on breathing during sleep in healthy persons and sleep disordered patients.
Material and Methods A search was carried out in PubMed and Scopus looking for articles published between January 1, 2010 and December 31, 2021, in English and Spanish, with the following search terms: “sleep disorders breathing and altitude”. Investigations in adults and carried out at an altitude of 2000 meters above mean sea level (MAMSL) or higher were included. The correlation between altitude, apnea hypopnea index (AHI) and mean SpO2 during sleep was calculated.
Results 18 articles of the 112 identified were included. A good correlation was found between altitude and AHI (Rs = 0.66 P = 0.001), at the expense of an increase in the central apnea index. Altitude is inversely proportional to oxygenation during sleep (Rs = −0.93 P = 0.001), and an increase in the desaturation index was observed (3% and 4%). On the treatment of respiratory disorders of sleeping at altitude, oxygen is better than servoventilation to correct oxygenation during sleep in healthy subjects and acetazolamide controlled respiratory events and oxygenation during sleep in patients with obstructive sleep apnea under treatment with CPAP.
Conclusions Altitude increases AHI and decreases oxygenation during sleep; oxygen and acetazolamide could be an effective treatment for sleep-disordered breathing at altitude above 2000 MAMSL.
Keywords: altitude; sleep apnea, obstructive; sleep apnea, central; hypoxia; sleep apnea syndromes
Introduction
Around 140 million people reside at high altitudes over 2,500 meters above mean sea level (MAMSL), but an even greater number may live at moderate altitudes of 2,000 to 2,500 MAMSL, and some 35 million people travel to sites at these elevations each year for work or leisure activities. 1 A significant percentage of these populations live in Latin American countries like Bolivia, Colombia, Ecuador, Mexico, and Peru.
Because altitude is inversely proportional to barometric pressure, it reduces the partial pressure of inspired oxygen (PIO 2 ); as a result, ascending to high elevations generates a decrease in partial pressure of arterial oxygen (PaO 2 ), reduces arterial oxygen saturation (SaO 2 ), and causes ventilatory changes due to acclimatization characterized by increase in minute ventilation and respiratory alkalosis 2 3 ; these changes are especially important during sleep. 4
Sleep-related breathing disorders (SRBDs) comprise a heterogeneous group of conditions characterized by respiratory disturbances that occur or worsen during sleep. 5 The main SRBD associated with ascent to moderate/high altitudes is the central sleep apnea syndrome, 6 but exposure to altitude could also aggravate a preexisting disorder such as obstructive sleep apnea syndrome (OSAS), the latter being the most common worldwide affecting around one billion people and with prevalences that are on the rise . 7 8 The aim of this systematic review is to analyze the recent scientific evidence of the clinical effects of altitude on breathing during sleep in healthy persons and sleep disordered patients.
Material and Methods
A literature search was carried out in the PubMed and Scopus databases to identify articles published between January 1, 2010, and December 31, 2021, using the following search terms: sleep disorders breathing and altitude . Filters ensured that research in English and Spanish would be identified. Two independent reviewers (JLCA and SRC) analyzed all the information gathered, beginning with the titles and abstracts of all potentially eligible articles as a preliminary screening. A list of full-text articles was then organized. Subsequently, both reviewers read the complete texts of all those articles to apply the inclusion/exclusion criteria. Both observational and interventions studies were included as long as they were (i) carried out at altitudes of 2,000 MAMSL or higher, (ii) involved adults aged 18 years or older, and (iii) utilized objective measurements of breathing during sleep. Review articles, conference abstracts, comments, and editorials were excluded. The quality of the information was assessed using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) model 9 ; articles with very low quality of evidence were eliminated of the final analysis, and disagreements were resolved by consensus. In the final step, one author (SRC) extracted the data, and the second author (JLCA) reviewed it.
The data extracted focused on the following: the apnea hypopnea index (AHI), the central apnea index (CAI), the obstructive apnea index (OAI), the oxygen desaturation index (ODI), mean saturation during sleep (mean SpO 2 ), and minimum saturation during sleep (minimum SpO 2 ). The information was organized in three categories: the effect of altitude on the apnea hypopnea index (AHI); the effect of altitude on oxygenation during sleep; and the treatment of sleep-related breathing disorders at moderate and high altitudes. To evaluate the association between altitude and breathing alterations during sleep, a correlation was performed among altitude, the AHI, and mean SpO 2 ; due to skewness of data (evaluated with the Shapiro-Wilk test), the Spearman Rho test was used. All correlation analyses were run in the Stata 15 for MAC (StataCorp., LLC, College Station, TX, USA) program. Correlation plots were constructed using the Stata 15 for MAC program and edited in Microsoft PowerPoint (Microsoft Corp., Redmond, WA, USA).
Results
Some of the results of this research were presented previously as an abstract. Of the 112 articles identified in the initial search, 74 were eliminated after analyzing and discussing the title and abstract, leaving 38 to be evaluated by reading the full text. Of those 38 studies, 20 were eliminated due to very low quality of evidence, so the final selection included 18 articles ( Figure 1 ). 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Fifteen reports were based on real ascents to high or moderate elevations, while the other 3 presented studies of simulated altitudes. The data analyzed involved a total of 530 subjects and presented the results of 1,291 sleep studies. According to GRADE, the information evaluated was of moderate-to-low quality ( Table 1 ).
Fig. 1.
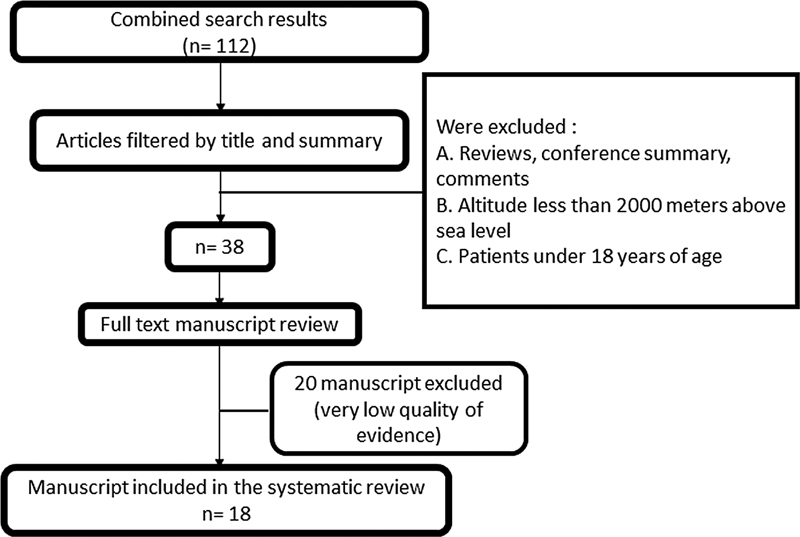
Flowchart of the article selection and evaluation process.
Table 1. Quality of the evidence according to the GRADE system.
| Items that lower the quality | Items that raise quality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Manuscripts | Year | A | B | C | D | E | F | G | H | I | Quality | Type of investigation |
| Bloch et al. 10 | 2010 | 1 | Moderate | Randomized | ||||||||
| Nussbaumer-Ochsner et al. 11 | 2010 | 1 | 1 | Low | Randomized | |||||||
| Pagel et al. 12 | 2011 | 1 | Low | Observational | ||||||||
| Latshang et al. 13 | 2012 | 1 | Low | Randomized | ||||||||
| Nussbaumer-Ochsner et al. 14 | 2012 | 1 | Moderate | Randomized | ||||||||
| Latshang et al. 15 | 2013 | 1 | Moderate | Randomized | ||||||||
| Lombardi et al. 16 | 2013 | 1 | 1 | Low | Observational | |||||||
| Ulrich et al. 17 | 2014 | 1 | 1 | Low | Randomized | |||||||
| Shogilev et al. 18 | 2015 | 1 | 1 | Low | Observational | |||||||
| Heinzer et al. 19 * | 2016 | 1 | 1 | Moderate | Randomized | |||||||
| Steier et al. 20 | 2017 | 1 | 1 | Low | Observational | |||||||
| Pramsohler et al. 21 * | 2017 | 1 | 1 | Low | Randomized | |||||||
| Orr et al. 22 | 2018 | 1 | 1 | Low | Randomized | |||||||
| Pramsohler et al. 23 * | 2019 | 1 | 1 | 1 | Low | Randomized | ||||||
| Tan et al. 24 | 2020 | 1 | Moderate | Randomized | ||||||||
| Frost et al. 25 | 2021 | 1 | 1 | Low | Observational | |||||||
| Ju et al. 26 | 2021 | 1 | 1 | Low | Observational | |||||||
| Bird et al. 27 | 2021 | 1 | 1 | 1 | Moderate | Observational | ||||||
GRADE ITEMS: A = Limitations in the design and execution of the study, B = Inconsistency of results, C = Uncertainty that the evidence is direct, D = Imprecision, E = Publication or notification bias, F = Association strength, G = Very strong association, H = Existence of the dose-response gradient, I = Evidence that all possible confounders could have reduced the observed effect.
Studies conducted with simulated altitude.
Effect of Altitude on the Apnea Hypopnea Index
A total of 14 studies evaluated the effect of altitude on the AHI: 9 with healthy individuals, 1 with patients with chronic obstructive pulmonary disease (COPD), and 4 with patients suffering from OSAS ( Table 2 ). A directly proportional relation was determined between altitude and AHI (Rs = 0.66, P = 0.001) ( Figure 2 ). This increase in AHI was secondary to an increase in the CAI, but it is important to note that in the studies by Pagel et al. (2011), Nussbaumer-Ochsner et al. (2012), and Ulrich et al. (2014), based on OSAS patients, the increase in the CAI did not exceed the proportion of 50% of total AHI; therefore, the most important disorder in this group of patients was found to be of the obstructive type.
Table 2. Effect of altitude on the apnea hypopnea index.
| Manuscripts | Altitude MAMSL | n | AHI h −1 mean/median ± SD/IR | CAI h −1 mean/median ± SD/IR | OAI h −1 mean/median ± SD/IR | Evaluation method | |
|---|---|---|---|---|---|---|---|
| Healthy persons | |||||||
| Bloch et al., 2010 10 | 490 (basal) | 34 | 4.0 (1.2–9.7) | NR | NR | RP | |
| 4,497 | 32 | 71.9 (37.2–96.2) * | NR | NR | |||
| 5,533 | 29 | 114.1 (81.1–130.3) * | NR | NR | |||
| 6,265 | 24 | 142.6 (120.4–154.2) * | NR | NR | |||
| 6,865 | 24 | 132.3 (103.3–157.4) * | NR | NR | |||
| Latshang et al., 2013 15 | 490 (Basal) | 51 | 4.6 (2.3–7.9) | 2.0 (1.2–3.7) | 1.3 (0.3–4.6) | PSG | |
| 2,590 (1 st night) | 13.1 (6.7–32.1) * | 8.9 (5.0–25.8) * | 1.8 (0.7–3.8) | ||||
| 2,590 (2 nd night) | 8.0 (4.4–23.1) * ^ | 5.8 (2.8–13.1) * ^ | 1.6 (0.7–3.4) | ||||
| Lombardi et al., 2013 16 | M | F | |||||
| Sea level (basal) | 37 | 0.03 ± 0.11 | 0.14 ± 0.21 | NR | NR | RP | |
| 3,400 | 36 | 2.4 ± 2.8 * | 40.3 ± 33.0 * & | NR | NR | ||
| 5,400 (1 st /2 nd night) | 24 | 41.1 ± 44 * | 87.5 ± 35.7 * & | NR | NR | ||
| 5,400 (10 th night) | 28 | 84.7 ± 22.5 * | 97.0 ± 30.3 * & | NR | NR | ||
| Heinzer et al., 2016 19 # | 485 (NN, basal) | 13 | 8.2 (3.9–8.8) | NR | NR | PSG | |
| 3,450 (NH) | 11.4 (5.0–65.4) * | NR | NR | ||||
| 3,450 (HH) | 20.5 (15.8–57.4) * | NR | NR | ||||
| Pramsohler et al., 2017 21 # | 5,500 | 11 | 143.1 (24.6–168) | NR | NR | PSG | |
| Pramsohler et al., 2019 23 # | 3,500 (basal) | 11 | 37.96 | NR | NR | PSG | |
| 4,500 | 68.55 * | NR | NR | ||||
| 5,500 | 93.44 * | NR | NR | ||||
| Frost et al., 2021 25 | 340 (basal) | 15 | 4.3 (4.5) | 0.5 (0.6) | 0.5 (1.1) | RP | |
| 3,800 (1 st night) | 35.3 (28.7) * | 14.0 (17.1) | 0.3 (0.8) | ||||
| 3,800 (2 nd night) | 16.0 (21.1) | 7.6 (15.2) | 0.1 (0.2) | ||||
| 3,800 (3 rd night) | 7.3 (5.3) | 0.8 (0.8) | 0.1 (0.2) | ||||
| Ju et al., 2021 26 | 154 (basal) | 10 | 1.4 (0.8–3.0) | NR | NR | RP | |
| 2,761 | 10.3 (5.7–15.4) * | NR | NR | ||||
| Bird et al., 2021 27 | Rapid ascent | RP | |||||
| 1,130 (basal) | 21 | 3.4 ± 3.5 | NR | NR | |||
| 3,800 (2 nd night) | 20 | 12.3 ± 14.5 * | NR | NR | |||
| 3,800 (9 th night) | 20 | 24.6 ± 23.7 * | NR | NR | |||
| Incremental ascent | |||||||
| 1,130/1,400 (basal) | 21 | 3.7 ± 4.1 | NR | NR | |||
| 3440 (2 nd /3 th night) | 19 | 10.8 ± 11.8 | NR | NR | |||
| 4240 (6/7 th night) | 14 | 26.7 ± 17.8 * | NR | NR | |||
| 5160 (9/10 th night) | 15 | 39.2 ± 32.6 * | NR | NR | |||
| Patients with chronic obstructive pulmonary disease | |||||||
| Tan et al., 2020 24 | 490 (basal) | 32 | 22.0 ± 15.8 | 1.6 ± 1.9 | 20.4 ± 15.2 | PSG | |
| 2048 | 44.0 ± 35.8 * | 32.4 ± 32.3 * | 11.6 ± 13.2 * | ||||
| Patients with obstructive sleep apnea syndrome | |||||||
| Nussbaumer-Ochsner et al., 2010 11 | 490 (basal) | 40 | 51.2 (31.7–74.4) | 2.4 (0.4–8.8) | 21.0 (16.4–24.9) | PSG | |
| 2590 (1 st night) | 90.0 (64.2–103.2) * | 51.3 (32.8–75.5) * | 32.0 (3.3–54.6) | ||||
| 2,590 (2 nd night) | 88.6 (62.4–108.4) * | 49.4 (22.6–57.8) * | 25.2 (6.5–58.8) | ||||
| Pagel et al., 2011 12 | 2,165 | 142 | 46.7 ± 26.8 | 15.8 | 30.9 | PSG | |
| Nussbaumer-Ochsner et al., 2012 14 | 490 (basal) | 45 | 51.2 (42.4– 72.2) | 1.6 (0.5– 3.2) | 49.4 (41.4– 67.6) | PSG | |
| 2590 | 86.2 (67.2–103.1) * | 23.4 (14.0–44.5) * | 55.5 (34–75.0) | ||||
| Ulrich et al., 2014 17 | 490 (Basal) | 18 | 57.3 (46.5–67.3) | 0.8 (0.2–1.8) | 49.6 (42.2–62.2) | PSG | |
| 2,590 | 86.5 (70–117) | 30.7 (21.2–48.2) * | 61.3 (33.9–75.0) | ||||
Abbreviations: AHI, apnea hypopnea index, CAI, central apnea index, COPD, chronic obstructive pulmonary disease, F, female, h-1, events per hour, HH, hypobaric hypoxia, IR, interquartile range, M, male, MAMSL, meters above sea level, NH, normobaric hypoxia, NN, normobaric normoxia, NR, not reported, OAI, obstructive apnea index, PSG, polysomnography, RP, respiratory polygraphy, SD, standard deviation.
p < 0.05 vs. Basal.
p < 0.05 vs 1 st night.
p < 0.05 males vs females.
Studies conducted with simulated altitude.
Fig. 2.
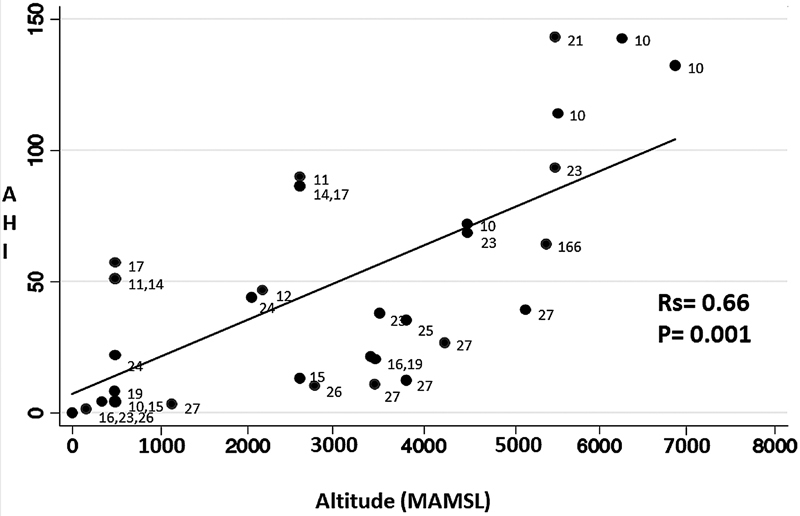
The directly proportional association (measured by Rho Spearman) between altitude in meters above mean sea level (MAMSL) and the apnea hypopnea index (AHI) in events per hour of sleep is presented.
The effect of altitude on the AHI may diminish over time; this would explain why the AHI decreased on the second night after the ascent, as in the report by Latshang et al. (2013) and Frost et al. (2021) on healthy individuals; however, this was not corroborated by Lombardi et al. (2013) and Bird et al. (2021) (in a rapid ascent to an altitude of 3,800 MAMSL) in their study of healthy individuals, or by Nussbaumer-Ochsner et al. (2010) in their work with OSAS patients. Lombardi et al. (2013) conducted an additional assessment on the 10 th night after an ascent to 5,400 MAMSL, but only made comparisons between biological sexes.
Effect of Altitude on Oxygenation During Sleep
We identified 16 articles with information on the impact of altitude on oxygenation during sleep: 11 in healthy persons, 1 in patients with COPD, and 4 in patients with OSAS ( Table 3 ). Altitude is inversely proportional to oxygenation during sleep, the correlation between altitude and the mean SpO 2 during sleep presented an Rs = −0.93 and a p = 0.001 ( Figure 3 ). In addition, an increase in ODI (3% and 4%) and a decrease in the minimum SpO 2 were observed ( Table 3 ). The work by Lombardi et al. (2013) stands out in this regard because it reported differences between biological sexes, which showed that altitude had a greater impact on men than women; also important is the study by Heinzer et al. (2016), who found differences between normobaric hypoxemia and hypobaric hypoxemia; and the work by Bird et al. (2021), which describes changes in respiration with 2 different patterns of ascent (rapid and incremental). ( Table 3 ).
Table 3. Effect of altitude on oxygenation during sleep.
| Manuscripts | Altitude MAMSL | n | ODI 3% h −1 mean/median ± SD/IR | ODI 4% h −1 mean/median ± SD/IR | Mean SpO 2% mean/median ± SD/IR | Minimun SpO 2% mean/median ± SD/IR | Evaluation method | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy individuals | ||||||||||
| Bloch et al., 2010 10 | 490 (Basal) | 34 | NR | NR | NR | 95 (94–96) | RP | |||
| 4,497 | 32 | NR | NR | NR | 80 (78–82) | |||||
| 5,533 | 29 | NR | NR | NR | 70 (68–72) | |||||
| 6,265 | 24 | NR | NR | NR | 62(60–66) | |||||
| 6,865 | 24 | NR | NR | NR | 60(55–63) | |||||
| Latshang et al., 2013 15 | 490 (Basal) | 51 | 0.3 (0.0–1.1) | NR | 96 (95–96) | NR | PSG | |||
| 2,590 (1° night) | 8.1 (3.3–30.9) * | NR | 90 (89–91) * | NR | ||||||
| 2,590 (2° night) | 5.4 (2.5–14.8) * | NR | 91 (90–92) * | NR | ||||||
| Lombardi et al., 2013 16 | M | F | M | F | M | F | ||||
| Sea level (basal) | 37 | 0.1 ± 0.2 | 0.1 ± 0.2 | NR | 97.4 ± 0.9 | 97.3 ± 0.8 | 92.9 ± 1.5 | 93.0 ± 1.0 | RP | |
| 3,400 | 36 | 8.6 ± 5.7 | 37.9 ± 25.8 | NR | 84.9 ± 3.0 | 82.1 ± 3.8 * | 75.5 ± 4.2 | 73.7 ± 5.1 * | ||
| 5,400 (1 st /2 nd night) | 24 | 55.8 ± 29.6 | 79.9 ± 25.6 * | NR | 72.5 ± 4.9 | 73.1 ± 4.2 * | 61.8 ± 6.2 | 62.2 ± 4.7 * | ||
| 5,400 (10 th night) | 28 | 45.3 ± 34 | 84.7 ± 22.4 * | NR | 78 ± 2.3 | 76.5 ± 2.9 * | 68.7 ± 3.8 | 66.1 ± 6.0 * | ||
| Shogilev et al., 2015 18 | 760 (basal) | 4 | NR | NR | 95.6 | NR | PR | |||
| 2,670 | NR | NR | 91 | NR | ||||||
| 3,200 | NR | NR | 88.4 | NR | ||||||
| 3,540 | NR | NR | 87.2 | NR | ||||||
| Heinzer et al., 2016 19 # | 485 (NN, basal) | 13 | 4.4 (2.2–4.8) | 0.9 (0.5–1.2) | 95.5 ± 0.9 | 92.0 ± 1.5 | PSG | |||
| 3,450 (NH) | 22.7 (13.1–73.8) * | 9.1 (5.7–59.2) * | 83.6 ± 1.9 * | 74.7 ± 7.0 * | ||||||
| 3,450 (HH) | 47.6 (22.1–82.2) * | 29.2 (8.8–57.1) * | 81.2 ± 3.1 * | 72.6 ± 4.2 * | ||||||
| Steier et al., 2017 20 | Sea level (basal) | 4 | NR | 0.8 ± 0.4 | 97.5 ± 1.3 | 95.3 ± 1.7 | PSG | |||
| 3,380 | NR | 22.0 ± 7.2 | 84.8 ± 0.5 | 68.1 ± 8.6 | ||||||
| 4,370 | NR | 61.4 ± 26.9 | 81.0 ± 4.1 | 67.4 ± 7.6 | ||||||
| 5,570 | 1 | NR | 144.9 | 68.5 | 50.4 | |||||
| Pramsohler et al., 2017 21 # | 5,500 | 11 | NR | NR | 65.6 ± 3.7 | NR | PSG | |||
| Pramsohler et al., 2019 23 # | 3,500 (basal) | 11 | NR | NR | NR | 66.0 ± 10.7 | PSG | |||
| 4,500 | NR | NR | NR | 56.8 ± 9.8 * | ||||||
| 5,500 | NR | NR | NR | 55.6 ± 4.03 * | ||||||
| Frost et al., 2021 25 | 340 (basal) | 15 | 3.1 (3.3) | NR | 94.7 (0.9) | 85.8 (4.4) | RP | |||
| 3,800 (1 st night) | 34.3 (22.6) * | NR | 77.0 (2.4) * | 65.3 (6.2) * | ||||||
| 3,800 (2 nd night) | 19.5 (22.9) | NR | 77.6 (2.9) | 68.0 (6.4) | ||||||
| 3,800 (3 rd night) | 7.2 (6.1) | NR | 78.5 (1.6) | 70.7 (3.6) | ||||||
| Ju et al., 2021 26 | 154 (basal) | 10 | NR | NR | 95.7 (95.1–96.2) | NR | RP | |||
| 2,761 | NR | NR | 86.9 (84.7–88.9) | NR | ||||||
| Bird et al., 2021 27 | Rapid ascent | Rapid ascent | Rapid ascent | Rapid ascent | ||||||
| 1,130 (basal) | 21 | 6.8 ± 5.3 | NR | 93.7 ± 2.1 | 86.0 ± 5.1 | RP | ||||
| 3,800 (2 nd night) | 20 | 26.1 ± 18.1 * | NR | 81.1 ± 3.6 * | 71.2 ± 6.2 * | |||||
| 3,800 (9th night) | 20 | 38.8 ± 26.7 * | NR | 84.0 ± 2.3 * | 74.3 ± 6.1 * | |||||
| Incremental ascent | Incremental ascent | Incremental ascent | Incremental ascent | |||||||
| 1,130/1,400 (basal) | 21 | 9.8 ± 7.8 | NR | 94.3 ± 1.6 | 87.1 ± 3.7 | |||||
| 3,440 (2 nd /3 rd night) | 19 | 28.5 ± 15.3 * | NR | 84.8 ± 5.5 * | 75.4 ± 6.7 * | |||||
| 4,240 (6/7 th night) | 14 | 43.7 ± 21.7 * | NR | 81.6 ± 3.1 * | 70.6 ± 5.0 * | |||||
| 5,160 (9/10 th night) | 15 | 54.4 ± 24.8 * | NR | 73.5 ± 4.2 * | 63.7 ± 6.6 * | |||||
| Patients with chronic obstructive pulmonary disease | ||||||||||
| Tan et al., 2020 24 | 490 (basal) | 32 | 0.8 ± 1.3 | NR | 92 ± 2 | NR | PSG | |||
| 2,048 | 4.2 ± 5.6 * | NR | 86 ± 3 * | NR | ||||||
| Patients with obstructive sleep apnea syndrome | ||||||||||
| Nussbaumer-Ochsner et al., 2010 11 | 490 (basal) | 40 | 37.3 (14.6 - 52.7) | NR | 94 (93–95) | NR | PSG | |||
| 2,590 (1 st night) | 80.6 (52.4–103.4) | NR | 86 (84–89) | NR | ||||||
| 2,590 (2 nd night) | 71.5 (43.4–98.6) | NR | 87(84–89) | NR | ||||||
| Pagel et al., 2011 12 | 1,421 (basal) | 150 | NR | NR | NR | 73.5 ± 11.3 | PSG | |||
| 2,165 | 142 | NR | NR | NR | 74.2 ± 9.6 | |||||
| Nussbaumer-Ochsner et al., 2012 14 | 490 (basal) | 45 | NR | NR | 93 (92–94) | NR | PSG | |||
| 2590 | NR | NR | 85 (83–88) * | NR | ||||||
| Ulrich et al., 2014 17 | 490 (basal) | 18 | NR | NR | 93 (92–94) | NR | PSG | |||
| 2,590 | NR | NR | 86 (84–87) * | NR | ||||||
Abbreviations: F, female, h-1, events per hour, HH, hypobaric hypoxia, IR, interquartile range, M, male, MAMSL, meters above mean sea level, NH, normobaric hypoxia, NN= normobaric normoxia, NR, not reported, ODI, oxygen desaturation index, PSG, polysomnography, RP, respiratory polygraphy, SD, standard deviation, SpO 2, pulse oximetry.
p < 0.05 vs. basal.
Studies conducted with simulated altitude.
Fig. 3.
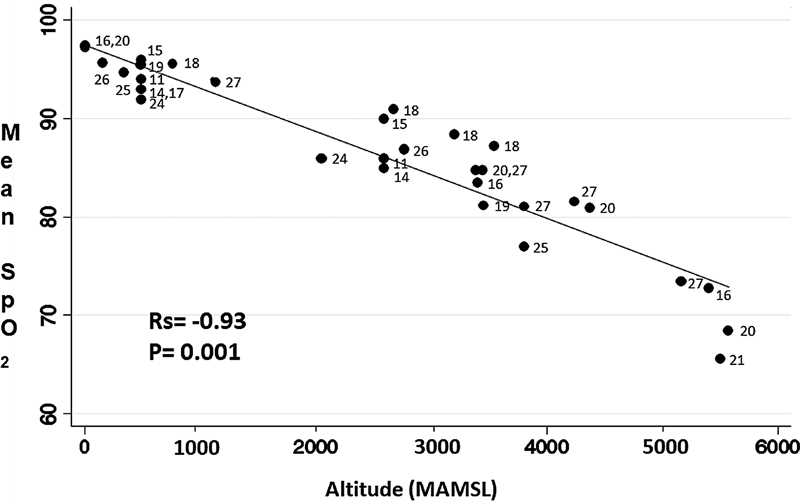
The inversely proportional association (measured by Rho Spearman) between altitude in meters above mean sea level (MAMSL) and average SpO2 during sleep (expressed as a percentage) is presented.
Treatment of Sleep-Related Breathing Disorders at High-to-Moderate Altitudes
Only two publications were identified for this aspect of our review; one involving healthy individuals, the second OSAS patients. Orr et al. (2018) analyzed a group of healthy individuals who ascended to 3,800 MAMSL; they found that oxygen therapy was more effective at reducing the ODI 3% and increasing mean SpO 2 than adaptive servoventilation. Latshang et al. (2012), meanwhile, working with a group of OSAS patients who were receiving CPAP treatment, reported that this therapy plus acetazolamide decreased the AHI by reducing the number of central events and improving oxygenation during sleep, compared to CPAP therapy plus placebo ( Table 4 ).
Table 4. Treatment of sleep-related breathing disorders at high-to-moderate altitudes.
| Manuscripts | Altitude MAMSL | Treatment | n | AHI h-1 median (IR) | CAI h −1 median (IR) | OAI h −1 median (IR) | ODI 3% h −1 mean ± SD | mean SpO 2% mean ± DE | TP cmH2O median (IR) | Evaluation method |
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy individuals | ||||||||||
| Orr et al., 2018 22 | 3,800 | ASV vs No Tx | 16 | NR | NR | NR | 10.7 ± 2.9 vs 17.1 ± 4.2 | 81 ± 1 vs. 79 ± 1 & | NR | PSG |
| O2 vs No Tx | 15 | NR | NR | NR | 0.5 ± 0.2 vs 16.5 ± 4.5 & | 96 ± 0 vs. 79 ± 1 & | NR | |||
| ASV vs O2 | 15 | NR | NR | NR | 8.8 ± 1.9 vs 0.5 ± 0.2 & | 80 ± 1 vs. 97 ± 0 & | NR | |||
| Patients with obstructive sleep apnea syndrome | ||||||||||
| Latshang et al., 2012 13 | 490 (basal) | CPAP | 25 | 6.6 (4.5–11.4) | 1.6 (0.5–4.3) | 3.5 (1.6–6.6) | 1.3 (0.5–2.5) | 95 (94–96) | 8.4 (7.5–10.9) | PSG |
| 2,590 | CPAP + placebo | 12 | 19.3 (9.3–29.0) * | 12.6 (5.6–23.0) * | 3.5 (1.6–7.9) | 16.2 (9.2–27.3) * | 89 (87–91) * | 10.0 (8.9–13.2) * | ||
| CPAP + acetazolamide | 13 | 6.8 (3.5–10.1) ^ | 4.0 (1.2–7.6) ^ | 2.3 (1.0–5.5) * ^ | 6.4 (2.6–11.9) * ^ | 91 (90–92) * ^ | 8.9 (7.1–10.8) ^ | |||
Abbreviations: AHI, apnea hypopnea index, ASV, adaptive servo ventilation, CAI, central apnea index, CPAP, continuous positive airway pressure, h −1 , events per hour, IR, interquartile range, MAMSL, meters above mean sea level, OAI, obstructive apnea index, ODI, oxygen desaturation index, PSG, polysomnography, RP, respiratory polygraphy, TP, therapeutic pressure, Tx, treatment.
p < 0.05 vs. CPAP 490 m.
p < 0.05 vs. CPAP + placebo.
p < 0.05.
Discussion
This systematic review identified recent and updated evidence obtained from observational and intervention studies regarding the effects of altitude on breathing during sleep in healthy persons, patients with COPD and OSAS and possible solutions to SRDB at altitude. People who travel to high altitudes often report symptoms that include low sleep quality, insomnia, and frequent awakenings with a sensation of suffocation secondary to alterations in breathing. Although idiosyncratic reactions may occur at altitudes above 1,500 MAMSL, periodic breathing and central apneas secondary to altitude typically appear with variable severity at elevations over 2,000 MAMSL, while at altitudes above 4,000 MAMSL, these disorders will be present in practically all individuals. 28 The decrease in barometric pressure secondary to altitude and the hypoxia that this produces generate a process of ventilatory acclimatization characterized by a progressive increase in ventilation (hyperventilation) until a partial restoration of PaO 2 is achieved with a concomitant decrease in PaCO 2 . 4 29 The precise mechanisms that control this process of acclimatization are not well understood because a large number of influencing factors may be involved, including: the sensitivity of central and peripheral chemoreceptors, cerebral blood flow, pulmonary artery pressure, the micro/macro architecture of sleep, and the complex interaction among these parameters. 4
Altitude was found to have a directly proportional relation to AHI; in both healthy persons and COPD patients. This increase in AHI was secondary to an increase in the number of central apneas; thus, hyperventilation secondary to ventilatory acclimatization intensifies over time and after 10 minutes of sleep tidal volume oscillates at increasing magnitudes, decreasing PaCO 2 even more; this alters loop gain, reaches the apneic threshold, and causes central apneas. 30 The results of OSAS patients show that while they had a considerable number of central apneas, the most important disorders affecting them were of the obstructive type, indicating that obstructive and central events are interrelated in this group of patients in such a way that central apneas could represent ventilatory instability secondary to altitude, and this instability could foster obstructive events. 31 32
It is important to note that in two investigations, a decrease in AHI was reported on subsequent nights compared to the first; although completing the ventilatory acclimatization process may take weeks, most of it is accomplished between days 1 and 2 of the ascent, so this phenomenon may be the reason behind this change 33 34 35 ; However, this finding was not consistent, so different altitude acclimatization phenotypes could be present among individuals.
Altitude was also associated with hypoxemia during sleep, as indicated by several markers: mean SpO 2 , minimum SpO 2 , and ODI. Though the decline in the PIO 2 secondary to the decrease in barometric pressure due to altitude alone might explain this phenomenon, the most important generating mechanism involves the ventilatory oscillations secondary to both central and obstructive respiratory events.
Given this correlation among altitude, apneas, and hypoxia, the most obvious form of treatment would consist simply in descending to lower altitudes, but this is not always possible. Although the information available for analysis is scarce, it appears that measures which stabilize ventilation by modifying respiratory control are more effective than positive pressure devices that function by directly regulating ventilation during sleep. Thus, increasing FIO 2 and thereby raising SaO 2 gradually decreases both hyperventilation and PaCO 2 . 36 Acetazolamide is known to inhibit central apneas by 50 to 80% by generating metabolic acidosis, stimulating ventilation, and favoring CO 2 retention, 37 38 and it is important to keep in mind that positive pressure devices can have a double antagonistic effect, which could decrease their effectiveness in eliminating respiratory events at higher altitudes. While they could increase ventilation and decrease PaCO 2 even more, they might also increase functional residual capacity, thereby increasing PaO 2 . 4 Unfortunately, the evidence available to date is so limited that we are unable to determine the ideal treatment for respiratory disorders associated with sleeping at high altitudes. Finally, we must evaluate the possibility that central apneas with periodic respiration could act as a compensatory mechanism rather than a pathological process, since this type of respiration reduces demand for O 2 by the respiratory muscles. 39
Other significant considerations are that most of the data analyzed was generated at high altitudes using healthy individuals, and that few studies have been carried out at moderate altitudes. It may be, however, that moderate altitudes have little clinical significance for healthy individuals; for example, Hernández-Zenteno et al. (2002) reported the results of a polysomnographic study of asymptomatic subjects conducted at 2,240 MAMSL, with the mean SpO 2 of those individuals being 93 ± 2%, the minimum SpO 2 was 86 ± 6, and the ODI 3% was 10 ± 22 h −1 . 40 Ascending to moderate altitudes, however, could have a greater impact on patients who have some chronic respiratory disorder, such as diffuse interstitial lung disease or chronic obstructive pulmonary disease (COPD), in which more severe hypoxemia during sleep has been reported. 41 42
Several limitations must be mentioned regarding interpretations of the data presented. First, several different study designs were included. Second, follow-up times were short. Third, distinct parameters were applied to classify respiratory events. Fourth, most of the data was generated with acute exposure to altitude during rapid ascent so a comparison with incremental ascent and/or high-altitude residents was not possible. Fifth, various reports did not employ the gold standard. Sixth, the information was reported in a very heterogeneous way, which made it difficult to group it into categories. Finally, because central hypopneas are difficult to identify by simplified monitoring, their frequency may has been underestimated. The strengths of this review, in contrast, include the substantial number of sleep studies and subjects involved, and the extensive evaluations conducted at a broad range of altitudes (from sea level to 6,865 MAMSL). The latter aspect made it possible to establish a dose-response gradient among altitude, respiratory events, and oxygenation during sleep. According to the GRADE scale, the quality of the evidence was moderate-to-low, but it is important to recognize that a parameter like exposure to altitude is difficult to randomize or study using blind approaches. For this reason, an assessment scale like GRADE, which favors information obtained from controlled clinical trials, tends to systematically indicate low scores.
Clearly, future research should include larger study populations with more patients who have preexisting sleep-related breathing disorders. They must also strive to produce evidence of higher quality and to assess such potential confounders as acclimatization, biological sex, and the acid-base balance.
Conclusion
Altitude increases the AHI at the expense of central events while decreasing oxygenation during sleep. In patients with OSAS, altitude aggravates the preexisting sleep-related breathing disorder. Administering oxygen to healthy persons and acetazolamide with CPAP to OSAS patients could prove to be more effective forms of treatment than adaptive servoventilation devices.
Funding Statement
Sources of Funding The authors declare that the article entitled “did not receive external funding and was carried out with the resources of the National Institute of Respiratory Diseases of Mexico.
Footnotes
Conflict of Interests The authors have no conflict of interest to declare.
References
- 1.Moore L G, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol. 1998;27 27:25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 2.Vázquez-García J C, Pérez-Padilla R. Valores gasométricos estimados para las principales poblaciones y sitios a mayor altitud en México. Rev Inst Nal Enf Resp Mex. 2000;13:6–13. [Google Scholar]
- 3.Dempsey J A, Powell F L, Bisgard G E, Blain G M, Poulin M J, Smith C A. Role of chemoreception in cardiorespiratory acclimatization to, and deacclimatization from, hypoxia. J Appl Physiol. 2014;116(07):858–866. doi: 10.1152/japplphysiol.01126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainslie P N, Lucas S JE, Burgess K R. Breathing and sleep at high altitude. Respir Physiol Neurobiol. 2013;188(03):233–256. doi: 10.1016/j.resp.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Matheus-Ramírez E P, Bello-Carrera R S, Torres-Fraga M G. Comentarios Clínicos a la 3 ra Clasificación Internacional de los Trastornos de Respiratorios del Dormir, Primera Parte: Síndromes de Apnea Obstructiva . Respirar. 2017;9:4–9. [Google Scholar]
- 6.Burgess K R, Ainslie P N. Central Sleep Apnea at High Altitude. Adv Exp Med Biol. 2016;903:275–283. doi: 10.1007/978-1-4899-7678-9_19. [DOI] [PubMed] [Google Scholar]
- 7.Benjafield A V, Ayas N T, Eastwood P R. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(08):687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons M M, Bhatt N Y, Pack A I, Magalang U J. Global burden of sleep-disordered breathing and its implications. Respirology. 2020;25(07):690–702. doi: 10.1111/resp.13838. [DOI] [PubMed] [Google Scholar]
- 9.Aguayo-Albasini J L, Flores-Pastor B, Soria-Aledo V. Sistema GRADE: clasificación de la calidad de la evidencia y graduación de la fuerza de la recomendación. Cir Esp. 2014;92(02):82–88. doi: 10.1016/j.ciresp.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Bloch K E, Latshang T D, Turk A J. Nocturnal periodic breathing during acclimatization at very high altitude at Mount Muztagh Ata (7,546 m) Am J Respir Crit Care Med. 2010;182(04):562–568. doi: 10.1164/rccm.200911-1694OC. [DOI] [PubMed] [Google Scholar]
- 11.Nussbaumer-Ochsner Y, Schuepfer N, Ulrich S, Bloch K E. Exacerbation of sleep apnoea by frequent central events in patients with the obstructive sleep apnoea syndrome at altitude: a randomised trial. Thorax. 2010;65(05):429–435. doi: 10.1136/thx.2009.125849. [DOI] [PubMed] [Google Scholar]
- 12.Pagel J F, Kwiatkowski C, Parnes B. The effects of altitude associated central apnea on the diagnosis and treatment of obstructive sleep apnea: comparative data from three different altitude locations in the mountain west. J Clin Sleep Med. 2011;7(06):610–5A.. doi: 10.5664/jcsm.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latshang T D, Nussbaumer-Ochsner Y, Henn R M. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390–2398. doi: 10.1001/jama.2012.94847. [DOI] [PubMed] [Google Scholar]
- 14.Nussbaumer-Ochsner Y, Latshang T D, Ulrich S, Kohler M, Thurnheer R, Bloch K E. Patients with obstructive sleep apnea syndrome benefit from acetazolamide during an altitude sojourn: a randomized, placebo-controlled, double-blind trial. Chest. 2012;141(01):131–138. doi: 10.1378/chest.11-0375. [DOI] [PubMed] [Google Scholar]
- 15.Latshang T D, Lo Cascio C M, Stöwhas A C. Are nocturnal breathing, sleep, and cognitive performance impaired at moderate altitude (1,630-2,590 m)? Sleep. 2013;36(12):1969–1976. doi: 10.5665/sleep.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.HIGHCARE Investigators . Lombardi C, Meriggi P, Agostoni P. High-altitude hypoxia and periodic breathing during sleep: gender-related differences. J Sleep Res. 2013;22(03):322–330. doi: 10.1111/jsr.12012. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich S, Nussbaumer-Ochsner Y, Vasic I. Cerebral oxygenation in patients with OSA: effects of hypoxia at altitude and impact of acetazolamide. Chest. 2014;146(02):299–308. doi: 10.1378/chest.13-2967. [DOI] [PubMed] [Google Scholar]
- 18.Shogilev D J, Tanner J B, Chang Y, Harris N S. Periodic Breathing and Behavioral Awakenings at High Altitude. Sleep Disord. 2015;2015:279263. doi: 10.1155/2015/279263.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinzer R, Saugy J J, Rupp T. Comparison of Sleep Disorders between Real and Simulated 3,450-m Altitude. Sleep. 2016;39(08):1517–1523. doi: 10.5665/sleep.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steier J, Cade N, Walker B, Moxham J, Jolley C. Observational Study of Neural Respiratory Drive During Sleep at High Altitude. High Alt Med Biol. 2017;18(03):242–248. doi: 10.1089/ham.2016.0097. [DOI] [PubMed] [Google Scholar]
- 21.Pramsohler S, Wimmer S, Kopp M. Normobaric hypoxia overnight impairs cognitive reaction time. BMC Neurosci. 2017;18(01):43. doi: 10.1186/s12868-017-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orr J E, Heinrich E C, Djokic M. Adaptive Servoventilation as Treatment for Central Sleep Apnea Due to High-Altitude Periodic Breathing in Nonacclimatized Healthy Individuals. High Alt Med Biol. 2018;19(02):178–184. doi: 10.1089/ham.2017.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pramsohler S, Schilz R, Patzak A, Rausch L, Netzer N C. Periodic breathing in healthy young adults in normobaric hypoxia equivalent to 3500 m, 4500 m, and 5500 m altitude. Sleep Breath. 2019;23(02):703–709. doi: 10.1007/s11325-019-01829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan L, Latshang T D, Aeschbacher S S. Effect of Nocturnal Oxygen Therapy on Nocturnal Hypoxemia and Sleep Apnea Among Patients With Chronic Obstructive Pulmonary Disease Traveling to 2048 Meters: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(06):e207940. doi: 10.1001/jamanetworkopen.2020.7940.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost S, Oeung B. Improvements in sleep-disordered breathing during acclimatization to 3800 m and the impact on cognitive function. Physiol Rep. 2021;9(09):e14827.. doi: 10.14814/phy2.14827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju J D, Zhang C, Sgambati F P. Acute Altitude Acclimatization in Young Healthy Volunteers: Nocturnal Oxygenation Increases Over Time, Whereas Periodic Breathing Persists. High Alt Med Biol. 2021;22(01):14–23. doi: 10.1089/ham.2020.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bird J D, Kalker A, Rimke A N. Severity of central sleep apnea does not affect sleeping oxygen saturation during ascent to high altitude. J Appl Physiol. 2021;131(05):1432–1443. doi: 10.1152/japplphysiol.00363.2021. [DOI] [PubMed] [Google Scholar]
- 28.American Academy of Sleep Medicine International Classification of Sleep Disorders 3rd ed.Darien Il.American Academy of Sleep Medicine; 2014. [Google Scholar]
- 29.Robbins P A.Role of the peripheral chemoreflex in the early stages of ventilatory acclimatization to altitude Respir Physiol Neurobiol 2007158(2-3):237–242. [DOI] [PubMed] [Google Scholar]
- 30.Salazar-Peña C M, Torres-Fraga M, Schalch-Ponce de León J M. Sobre el control central de la respiración: A propósito de una mujer con apnea obstructiva del sueño, enfermedad de Lyme y consumo crónico de opioides. Neumol Cir Torax. 2016;75:25–31. [Google Scholar]
- 31.Dempsey J A, Xie A, Patz D S, Wang D. Physiology in medicine: obstructive sleep apnea pathogenesis and treatment–considerations beyond airway anatomy. J Appl Physiol. 2014;116(01):3–12. doi: 10.1152/japplphysiol.01054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie A, Teodorescu M, Pegelow D F. Effects of stabilizing or increasing respiratory motor outputs on obstructive sleep apnea. J Appl Physiol. 2013;115(01):22–33. doi: 10.1152/japplphysiol.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiodi H. Respiratory adaptations to chronic high altitude hypoxia. J Appl Physiol. 1957;10(01):81–87. doi: 10.1152/jappl.1957.10.1.81. [DOI] [PubMed] [Google Scholar]
- 34.Rahn H, Otis A B. Man's respiratory response during and after acclimatization to high altitude. Am J Physiol. 1949;157(03):445–462. doi: 10.1152/ajplegacy.1949.157.3.445. [DOI] [PubMed] [Google Scholar]
- 35.West J B. Rate of ventilatory acclimatization to extreme altitude. Respir Physiol. 1988;74(03):323–333. doi: 10.1016/0034-5687(88)90040-0. [DOI] [PubMed] [Google Scholar]
- 36.Lahiri S, Barnard P. Role of arterial chemoreflex in breathing during sleep at high altitude. Prog Clin Biol Res. 1983;136:75–85. [PubMed] [Google Scholar]
- 37.Swenson E R, Leatham K L, Roach R C, Schoene R B, Mills W J, Jr, Hackett P H. Renal carbonic anhydrase inhibition reduces high altitude sleep periodic breathing. Respir Physiol. 1991;86(03):333–343. doi: 10.1016/0034-5687(91)90104-q. [DOI] [PubMed] [Google Scholar]
- 38.Teppema L J, Rochette F, Demedts M. Effects of acetazolamide on medullary extracellular pH and PCO2 and on ventilation in peripherally chemodenervated cats. Pflugers Arch. 1990;415(05):519–525. doi: 10.1007/BF02583501. [DOI] [PubMed] [Google Scholar]
- 39.Ghazanshahi S D, Khoo M C. Optimal ventilatory patterns in periodic breathing. Ann Biomed Eng. 1993;21(05):517–530. doi: 10.1007/BF02584334. [DOI] [PubMed] [Google Scholar]
- 40.Hernández-Zenteno R J, Pérez-Padilla R, Vázquez J C. Normal breathing during sleep at an altitude of 2240 meters. Arch Med Res. 2002;33(05):489–494. doi: 10.1016/s0188-4409(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 41.Vázquez J C, Pérez-Padilla R. Effect of oxygen on sleep and breathing in patients with interstitial lung disease at moderate altitude. Respiration. 2001;68(06):584–589. doi: 10.1159/000050576. [DOI] [PubMed] [Google Scholar]
- 42.Vázquez-García J C, Pérez-Padilla R. Respiración durante el sueño en pacientes con enfermedad pulmonar obstructiva crónica a una altitud de 2,240 metros. Rev Invest Clin. 2004;56(03):334–340. [PubMed] [Google Scholar]


