Abstract
Objective To evaluate the association between sleep parameters and hypovitaminosis D in rotating shift drivers.
Material and Methods We conducted a cross-sectional study on 82 male rotating shift workers (24–57 years old) with at least one cardiovascular risk factor (such as hyperglycemia, dyslipidemia, abdominal obesity, physical inactivity, hypertension, and smoking). Polysomnography was used to evaluate sleep parameters. Logistic regression was used to model the association between hypovitaminosis D and sleep parameters after adjustment for relevant covariates.
Results Hypovitaminosis D (< 20 ng/mL) was seen in 30.5% of the workers. Shift workers with hypovitaminosis D had lower sleep efficiency (odds ratio [OR]: 3.68; 95% confidence interval [CI]: 1.95–5.53), lower arterial oxygen saturation (OR: 5.35; 95% CI: 3.37–6.12), and increased microarousal index (OR: 3.85; 95% CI: 1.26–5.63) after adjusting.
Conclusion We suggest that hypovitaminosis D is associated with greater sleep disturbances in rotating shift workers.
Keywords: sleep stages, sleep deprivation, circadian rhythm, sleep apnea syndromes, polysomnography, occupational health
Introduction
In the United States, sleep disorders are associated with seven of the fifteen leading causes of death. 1 Rotating shift workers tend to have a higher prevalence of sleep disorders because they must reverse their biological rhythms and perform activities at night, a time when there is a greater propensity for sleepiness. 2 Thus, they are often deprived of sleep quantity and quality. 2 3 Besides risk factors that have been well described in the literature, such as the male sex, older age, and being overweight, 4 there is a strong association between vitamin D levels and an increased risk for sleep disorders. 5
Hypovitaminosis D is a global health problem that affects more than one billion people worldwide. 6 The most described risk groups are the elderly, pregnant women, as well as patients with osteomalacia, osteoporosis, inflammatory diseases, chronic kidney disease, and obesity. 6 Yet, few studies have addressed occupational aspects, such as work shift schedules, as a risk factor. Shift work is classified into permanent or rotating. In permanent shift work, the individual works the same shift every day, for example, only the morning shift, or only the evening shift. On the rotating shift, individuals change their work schedule, working some days of the week on the morning shift and other days on the evening shift. 7 Rotating shifts are a predominant factor that promotes the discontinuation of a worker's normal feeding and sleeping patterns, inducing changes in the circadian rhythm, hormonal imbalances, and disruption of normal sleep architecture. 8 Besides, shift workers are at high risk for hypovitaminosis D, reaching up to 80% prevalence. 9 A contributing factor to this higher risk is the unconventional work schedule related to less physical activity and less time outdoors, which may be associated with less sun exposure and lower vitamin D synthesis. 9 10
Current evidence indicates that the pleiotropic effects of vitamin D and its metabolites go far beyond bone-mineral metabolism and parathyroid gland activity, as these effects are connected to other potential areas linked to sleep. 5 11 Therefore, it appears that workers on rotating shifts present risk factors for both hypovitaminosis D and changes in sleep quality. 9 12 The population evaluated was truck drivers, who present a higher risk of traffic crashes due to occupational characteristics, 13 especially as sleeping disorders are among the predictors of traffic accidents in this population. 14 The aim of this study was to evaluate the association between hypovitaminosis D and sleep parameters in alternating shift truck drivers. To our knowledge, there are no studies that have evaluated this association. Therefore, this study hypothesizes that shift workers with hypovitaminosis D have a higher prevalence of sleep pattern changes than shift workers with sufficient vitamin D levels.
Material and Methods
Design and Participants
A cross-sectional study was performed in 2012, with male off-road truck operators (aged 24–57 years) who had rotating shifts at an iron ore extraction company located in the Iron Quadrangle, Minas Gerais, Brazil. The inclusion criteria for the study were being male, working on alternating shifts, and having at least one cardiovascular risk factor.
These individuals worked alternate shifts, with 6 hours per shift and a 12-hour rest period between shifts. The initial shift was from 7 pm to 1 am; the following one was from 1 am to 7 am; the subsequent shift was from 7 am to 1 pm; ending the cycle of alternating shifts with the time from 1 pm to 7 pm. On the next day, after 36 hours of rest, which characterizes the day off, the worker restarts the cycle of shifts, from 7 pm to 1 am.
The participants were previously evaluated in a screening study entitled “Metabolic syndrome in mining workers in the state of Minas Gerais, Brazil,” conducted by the Federal University of Ouro Preto. The study aimed to perform a screening of all alternating shift workers with cardiovascular risk factors, to support employers in further interventions for the control of these predefined risk factors. The study was approved by the Research Ethics Committee of the Federal University of Ouro Preto (CAAE: 39682014.7.0000.5150).
The cardiovascular risk factors evaluated were: hypertension (systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg), 15 hyperglycemia (fasting blood glucose ≥ 100 mg/dL), 16 high total cholesterol (≥ 200 mg/dL), high triglycerides (≥ 150 mg/dL), high low-density lipoprotein cholesterol (LDL) (≥ 160 mg/dL), low high-density lipoprotein cholesterol (HDL) (<40 mg/dL), 17 abdominal obesity (waist circumference ≥ 90 cm), 15 tobacco use, and low level of physical activity (< 600 measured energy total - min/week). 18
Therefore, considering the inclusion criteria, 524 workers were invited to undergo polysomnography (PSG). Of these, 119 showed up for PSG, and 37 workers did not get their vitamin D levels measured. Therefore, at the end of the study, 82 workers underwent a PSG examination and had their vitamin D measured ( Figure 1 ). This study followed reported guidelines dictated by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE).
Fig. 1.
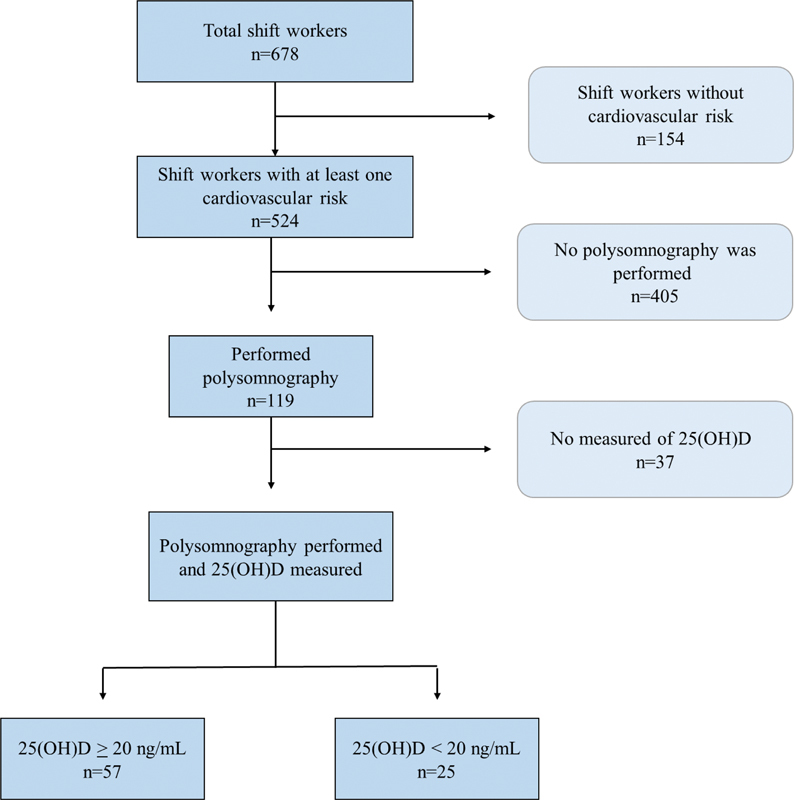
Flowchart of study participants.
Data Collection
The social, demographic, and economic variables evaluated were sex, age, self-reported skin color, and shift work time.
Polysomnography
All workers underwent a PSG examination at night (10 pm to 6 am) registered using the Alice 5 PSG system (Philips Respironics, Inc., Murrysville, PA, USA) and conducted in a hospital in the city of Ouro Preto.
The sleep parameters evaluated were sleep efficiency, sleep latency, stages of sleep, N1, N2, N3, rapid eye movement (REM) sleep, REM sleep latency, mean and minimum arterial oxygen saturation (SaO2), microarousal index (MAI), and apnea-hypopnea index (AHI). Short sleep duration was considered as a PSG-measured total sleep time lower than 6 hours (360 min). 19 Sleep onset latency was classified as increased when greater than 30 minutes. 19 Sleep efficiency (%) was calculated as total sleep time divided by total recording time on PSG, and sleep efficiency was classified as reduced if less than 85%. 19 The severity of obstructive sleep apnea (OSA) was categorized as no OSA (AHI 0–4/h), mild OSA (AHI 5–14/h), moderate OSA (AHI 15–30/h), or severe OSA (AHI > 30/h). 20 The nocturnal hypoxic episode was defined as SaO2 values < 90% at least once during sleep. 21 There is still no cut-off in the literature for the MAI, therefore, we classified it according to the sample 50th percentile (p50).
Anthropometric Data
Weight, body fat, and visceral fat area (VFA) were analyzed using bioelectrical impedance analysis (BIA) on the body composition monitor, InBody 720 (Biospace Co. Ltd., Urbandale, IA, USA). The percentage of body fat was classified as high if values were above the following in different age groups: 20% in 18 to 35 years old, 27% in 35 to 45 years old, and 28% in 46 to 65 years old. 22 The VFA greater than 130 cm 3 was classified as excess visceral adipose tissue. 23 Height was measured using the AlturExata (AlturExata, Belo Horizonte, Minas Gerais, Brazil) portable stadiometer that had a centimeter-scale with a 1 mm accuracy. The body mass index (BMI) was calculated using the following formula: weight (kg) / height (m), 2 and classified as overweight if BMI ≥ 25.0 kg/m 2 or obese if BMI ≥ 30.0 kg/m 2 .
Biochemical
The enzymatic colorimetric method measured fasting glucose, triglycerides, total cholesterol, and HDL cholesterol levels. The LDL cholesterol level was calculated using the Friedewald equation (1972) for triglyceride levels less than 400 mg/dL. 24 We classified blood glucose as hyperglycemia when the fasting glucose level was ≥ 100 mg/dL. 25 Lipid profile was classified as dyslipidemia when total cholesterol ≥ 190 mg/dL, LDL ≥ 130 mg/dL and/or HDL < 40 mg/dL, and/or triglycerides ≥ 150 mg/dL. 26
The concentration of vitamin D, 25(OH)D, was measured using a Liaison chemiluminescence immune analyzer (DiaSorin, Saluggia, Italy). The participants were classified as having hypovitaminosis D when 25(OH)D levels were less than 20 ng/mL. 27 The seasonality of blood sample collection was classified as summer (December 21 to March 19), autumn (March 20 to June 20), or spring (September 21 to December 20).
Statistical Analysis
Statistical analyses were performed using the Stata (StataCorp LLC., College Station, TX, US) software, version 15.0, with a significance level of 5%. The Shapiro-Wilk test was performed to assess data distribution. Continuous data are presented as medians and interquartile ranges (IQR) (p25–p75). The data were compared using the Chi-square analyses and the U Mann-Whitney test with the Bonferroni correction. Logistic regression analysis was performed to investigate whether hypovitaminosis D was associated with sleep parameters. Based on the results of the univariate analysis, variables were entered into multiple logistical models in descending order of statistical significance (stepwise technique). Only variables with p ≤ 0.20, biological plausibility and epidemiological relevance were considered. The adjusted model included variables with p < 0.05, including total body fat, age, and the seasonality of blood collection.
Sampling power (a posteriori) was performed on proportion and sample size data of similar studies using the OpenEpi program, version 3.01. The analysis was performed with an alpha level of 0.05 (using a two-tailed test). For sleep characteristics evaluated, only total sleep time and OSA had an estimated power of less than 0.80.
Results
A total of 82 shift workers underwent PSG and vitamin D level measurements. Of these, 30.5% had hypovitaminosis D (25(OH)D < 20 ng/mL). Most participants were 20 to 39 years old (70.7%), with the youngest at 24.4 and the oldest at 56.2 years. Most of the workers had previously worked for more than 5 years on a rotating shift (80.5%). Among those who were evaluated (n = 82), 75.6% were overweight, 61.0% had high body fat, and 53.7% had a high VFA ( Table 1 ).
Table 1. Characteristics of rotating shift workers according to vitamin D levels.
| Characteristics | All workers ( n = 82) |
25(OH)D (ng/mL) | p * | |
|---|---|---|---|---|
| ≥ 20 ( n = 57) | < 20 ( n = 25) | |||
| Age | ||||
| 20–39 years, n (%) | 58 (70.7) | 43 (75.4) | 15 (60.0) | 0.157 |
| 40–59 years, n (%) | 24 (29.3) | 14 (24.6) | 10 (40.0) | |
| Shift work | ||||
| < 5 years | 16 (19.5) | 11 (19.3) | 5 (20.0) | 0.941 |
| ≥ 5 years | ||||
| 66 (80.5) | 46 (80.7) | 20 (80.0) | ||
| Race/Skin color | ||||
| Not white, n (%) | 57 (69.5) | 39 (68.4) | 18 (72.0) | 0.746 |
| Anthropometric data | ||||
| Excess weight, n (%) | 62 (75.6) | 42 (73.7) | 20 (80.0) | 0.540 |
| Obesity, n (%) | 22 (26.8) | 13 (22.8) | 9 (36.0) | 0.215 |
| High body fat, n (%) | 50 (61.0) | 33 (57.9) | 17 (68.0) | 0.127 |
| VFA > 130 cm 3 , n (%) | 44 (53.7) | 28 (49.1) | 16 (64.0) | 0.214 |
| Clinical and behavioral variables | ||||
| Hypertension, n (%) | 58 (70.7) | 20 (80.0) | 38 (66.7) | 0.222 |
| a Dyslipidemia, n (%) | 45 (54.9) | 28 (49.1) | 17 (68.0) | 0.114 |
| b Tobacco consumption, n (%) | 19 (23.2) | 12 (21.0) | 7 (29.2) | 0.484 |
| Low physical activity, n (%) | 21 (25.6) | 13 (22.8) | 8 (32.0) | 0.380 |
| c Hiperglycemia, n (%) | 4 (4.9) | 2 (3.51) | 2 (8.0) | 0.385 |
| Seasonality | ||||
| Spring, n (%) | 54 (65.8) | 32 (56.1) | 22 (88.0) | 0.003 |
| Summer, n (%) | 27 (33.1) | 25 (43.9) | 3 (12.0) | |
p * value for Pearson's chi-square test or Fisher's exact test;
Dyslipidemia: total cholesterol ≥ 190 mg/dL, triglycerides 150 ≥ mg/dL, LDL ≥ 130 mg/dL, HDL < 40 mg/dL.
Current smokers or who quit less than six months ago.
Hyperglycemia: fasting glucose ≥ 100 mg/dL. Excess weight: BMI ≥ 25.0 kg/m 2 . Obesity: BMI ≥ 30.0 kg/m 2 .
Abbreviations: VFA: visceral fat area; BMI: body mass index; LDL: low-density lipoprotein; HDL: high-density lipoprotein cholesterol.
Regarding cardiovascular risk factors, 70.7% had hypertension, 54.9% had dyslipidemia, 23.2% had medium to high-risk tobacco intake, 25.6% had a low level of physical activity, and 4.9% had hyperglycemia. Furthermore, vitamin D levels showed seasonal variability, with higher hypovitaminosis D presence during spring (88.0%) than during summer (8.0%) ( p < 0.05) ( Table 1 ).
Workers with hypovitaminosis D had a longer sleep latency [(23.0 min; IQR: 20.0) versus (11.5 min; IQR: 11.0); p = 0.016)] and a lower sleep efficiency [(80.9%; IQR: 80.9) versus (86.5%; IQR: 10.0; p = 0.017)] when compared to workers who had higher levels of vitamin D ( Table 2 ).
Table 2. Sleep parameters of shift workers according to vitamin D levels.
| Characteristics | All workers ( n = 82) |
25(OH)D (ng/mL) | p * | |
|---|---|---|---|---|
| ≥ 20 ( n = 57) | < 20 ( n = 25) | |||
| Sleep features | ||||
| Total sleep time (hours) | 6.5 (5.8–6.9) | 6.6 (5.9–6.9) | 6.2 (5.4–6.8) | 0.095 |
| Sleep latency (min) | 13.2 (8.5–24.0) | 11.5 (8.5–19.5) | 23.0 (8.5–28.5) | 0.016 |
| REM sleep latency (min) | 99.7 (79.0–147.0) | 100.5 (79.0–136.0) | 99.0 (80.0–147.0) | 0.705 |
| Sleep efficiency (%) | 85.3 (76.9–89.2) | 86.5 (80.0–90.0) | 80.9 (73.8–86.6) | 0.017 |
| AHI (events/hour) | 13.7 (7.6–22.9) | 13.6 (7.9–22.3) | 16.3 (6.5–26.1) | 0.687 |
| MAI (events/hour) | 8.6 (5.5–12.4) | 7.7 (5.3–11.7) | 11.3 (7.6–13.2) | 0.103 |
| Arterial oxygen saturation | ||||
| SaO2 average (%) | 95.0 (94.0–96.0) | 95.0 (94.0–96.0) | 96.0 (95.0–96.0) | 0.221 |
| SaO2 minimum (%) | 87.0 (83.0–89.0) | 87.0 (84.0–89.0) | 86.0 (83.0–88.0) | 0.634 |
| Time with SaO2 < 90%, min | 1.4 (0.1–4.8) | 1.4 (0.0–7.0) | 1.4 (0.2–4.7) | 0.776 |
| Sleep stages | ||||
| N1 (% TST) | 5.7 (4.4–7.4) | 5.7 (4.3–7.2) | 5.3 (4.6–7.4) | 0.919 |
| N2 (%TST) | 59.1 (54.0–63.7) | 59.7 (53.6–64.3) | 56.8 (54.1–61.6) | 0.308 |
| N3 (%TST) | 15.8 (11.5–19.8) | 14.7 (11.2–19.0) | 18.1 (14.7–20.3) | 0.323 |
| REM (%TST) | 17.7 (14.5–24.6) | 18.5 (13.8–24.6) | 17.3 (15.8–23.0) | 0.875 |
Results are presented as median (interquartile range);
p * value for Mann Whitney test.
Abrevviations: MAI: microarousal index; AHI, apnea-hypopnea index; SaO2: arterial oxygen saturation; REM: rapid eye movement; TST: total sleep time.
Table 3 shows the association of hypovitaminosis D with the sleep parameters evaluated in univariate and multivariate analysis adjusted for seasonality, age, and total body fat. In the adjusted model, workers with hypovitaminosis D had 3.68 times the chance of having reduced sleep efficiency ( p = 0.016), 5.35 times the chance of having a nocturnal hypoxic episode ( p = 0.020), and 3.85 times the chance of having increased MAI ( p = 0.018) ( Table 3 ).
Table 3. Multiple logistic regression analysis of hypovitaminosis D associated with sleep characteristics in shift workers.
| Outcomes | Category | n (%) | 25(OH)D < 20 ng/mL | |||
|---|---|---|---|---|---|---|
| OR crude | p | OR adjusted | p * | |||
| Total sleep time | > 6 hours | 56 (68.3) | 1.00 | 1.00 | 0.358 | |
| ≤ 6 hours | 26 (31.7) | 1.71 (0.89–2.45) | 0.288 | 1.63 (0.74–2.13) | ||
| Sleep latency | < 30 min | 70 (85.4) | 70 (85.4) | 1.00 | 0.098 | |
| ≥ 30 min | 23 (14.6) | 2.68 (0.77–3.35) | 0.121 | 2.94 (0.80–3.70) | ||
| Sleep efficiency | > 85% | 56 (68.3) | 1.00 | 1.00 | 0.016 | |
| ≤ 85% | 26 (31.7) | 4.41 (2.83–7.62) | 0.005 | 3.68 (1.95–5.53) | ||
| Sleep apnea a | AHI < 5/h | 11 (13.4) | 1.00 | 1.00 | ||
| AHI 5–14/h | 34 (41.5) | 0.30 (0.08–1.11) | 0.073 | 0.25 (0.06–1.71) | 0.25 (0.06–1.71) | |
| AHI 15–30/h | 27 (32.9) | 1.48 (0.43–2.38) | 0.407 | 1.56 (0.58–2.23) | 0.295 | |
| AHI > 30/h | 10 (12.2) | 0.97 (0.39–2.13) | 0.971 | 0.98 (0.53–1.59) | 0.239 | |
| Arterial oxygen saturation | > 90% | 20 (24.4) | 1.00 | 1.00 | 0.020 | |
| < 90% | 62 (75.6) | 4.18 (2.87–5.36) | 0.041 | 5.35 (3.37–6.12) | ||
| Microarousal index | < P50 | 42 (51.2) | 1.00 | 1.00 | 0.018 | |
| > P50 | 40 (48.8) | 2.72 (1.03–4.56) | 0.048 | 3.85 (1.26–5.63) | ||
Multinomial logistic regression performed; p *: value for the Wald test.
OR crude : value estimated by the univariate analysis; OR adjusted : value adjusted for age, body fat, and seasonality.
Abbreviations: OR: odds ratio; P50: 50th percentile; AHI: apnea-hypopnea index
Discussion
In this study with male rotating shift workers, more specifically drivers of heavy off-road machinery from an iron ore extraction company (6 h of work followed by 12 h of rest), we found a high prevalence of hypovitaminosis D and a significant association with lower sleep efficiency, increased MAI, and nocturnal hypoxic episode during sleep.
The prevalence of hypovitaminosis D found in the study was 30.5%, lower than that found in a systematic review by Sowah et al. (2017), where 80% of the shift workers had a 25(OH)D value less than 20 ng/mL. 9 However, out of all 71 articles evaluated in the same review, only 2 were from Brazil. Both articles did not evaluate drivers or truck operators, or workers on rotating shifts, which may make it difficult to compare the data. Previous studies have suggested a higher prevalence of hypovitaminosis D due to the lack of sunlight exposure in shift workers. 10 In contrast, a rotating work schedule allows the worker to also have day shift periods (e.g., from 7 am to 1 pm and from 1 am to 7 am), or time off, which may increase their sun exposure at these times.
Another important issue to highlight is the high incidence of sunlight in Brazil during almost all seasons of the year, contributing to a higher cutaneous synthesis of vitamin D. 28 However, it is important to note that these workers were evaluated during the spring and summer seasons, with a higher incidence of sunlight. It is possible that if they were evaluated during the fall and winter, we would find a higher prevalence of hypovitaminosis D. Although Brazil has a high incidence of sunlight since it's a tropical country, the Iron Quadrangle is a colder region, with altitudes between 1,200 and 2,000 m. Due to the cold weather, the workers may increase the amount of clothing covering more parts of the body to keep warm, thus reducing the sunlight exposure on the epidermis during autumn and winter.
In this study, sleep assessment was conducted overnight using PSG. Rotating shift workers with hypovitaminosis D were 3.68 times more likely to have a sleep efficiency lower than 85% when compared to those with higher levels of the vitamin. A similar result was found by Massa et al. (2015), who evaluated 3,048 men, with lower vitamin D levels (20–30 ng/mL) being associated with a higher likelihood of low sleep efficiency (< 70%), as measured by actigraphy (OR: 1.45; p = 0.004). 29 However, the results in the literature remain controversial, as demonstrated by Lee et al. (2020), who evaluated the sleep of night and day workers by actigraphy and did not find any association between hypovitaminosis D and reduced sleep efficiency. 30 However, vitamin D levels < 10 ng/mL were used as a cutoff point, which was different from our study (vitamin D levels < 20 ng/mL). Moreover, workers who were on rotating shifts were not evaluated, a factor that is more associated with poor sleep quality when compared to other shifts. 30 One possible reason is the greater exposure to sunlight by workers with higher vitamin D levels, as exposure to sunlight comprises more than 80% of the daily need for this vitamin. 31 Furthermore, exposure to high levels of sunlight during the day is the most important stimulus for circadian rhythm adjustment, being associated with reduced sleep latency and increased sleep quality. 32
Another sleep disorder that is prevalent in the population is OSA. 4 Among the rotating shift workers, 86.6% had OSA to some degree but had no association with hypovitaminosis D. The sample size may have interfered, as the sample power was less than 80%, not having the minimum sampling necessary to find statistically significant differences. Furthermore, the prevalence of OSA found in this study was higher than that found in a systematic review article by Sakamoto et al. (2018), in which the prevalence in shift workers ranged from 14.3 to 38.1%. 33 However, it is important to note that our sample was composed entirely of male workers on rotating shifts with at least one cardiovascular risk factor, which is associated with a higher prevalence of OSA. Similar values were found in other studies with individuals at high risk for OSA, 83% in patients with suspected apnea, 34 and 76% in patients at risk for coronary heart disease. 35
Although we found no association between hypovitaminosis D and OSA, we did find that workers with vitamin D levels less than 20 ng/mL were more likely to have a higher MAI (OR: 3.85; 95% CI: 1.26–5.63) and nocturnal hypoxic episode during sleep (OR: 5.35; 95% CI: 3.37–6.12). Both MAI and nocturnal hypoxic episodes during sleep are known to be directly related to OSA. During sleep, the ventilatory control system is subjected to instabilities, increasing the occurrence of apnea or hypopnea, both of which occur when there is a complete or partial respiratory arrest. This results in a reduction of respiratory flow and a decrease in oxyhemoglobin saturation, either accompanied or not by microarousals. 4 These results are supported by the study of Goswami et al. (2016), wherein after adjusting for risk factors (age, BMI, neck circumference, and hypertension), there were no associations between vitamin D levels and OSA (OR: 1.05; 95% CI: 0.72–1.52). 36 However, these results remain divergent in the literature. A systematic review of 14 studies with 4937 individuals by Neighbors et al. (2018) demonstrated that serum vitamin D levels were lower in individuals with OSA than those without it (mean differences were 2.7% for mild OSA, 10.1% for moderate, and 17.4% for severe). 37 Alternatively, Archontogeorgis et al. (2018) found lower levels of vitamin D in patients with OSA and negatively correlated with oxygen desaturation index (r = −0.234, p = 0.011) and percentage of time with oxyhemoglobin saturation < 90% (r = −0.172, p = 0.041). 38 These results were also found in a cohort study, where chronic pulmonary disease patients had a higher risk of having hypovitaminosis D (OR: 2.32; 95% CI: 1.43–3.75), while the relationship between lung function and systemic vitamin D levels was almost linear even after adjustment for numerous confounders. 39
We found hypovitaminosis D to be related to several sleep parameters (lower sleep efficiency, increased MAI, and nocturnal hypoxic episode) in our sample of rotating shift workers, which remained significant after adjusting for confounding variables. This association may be explained by the intracellular distribution of vitamin D receptors in areas of the brain that regulate the sleep-wake cycle or through pro-inflammatory mediators. Experimental studies have shown that sleep regulatory substances, such as the tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 (IL-1), are inversely related to vitamin D levels. 40 Furthermore, vitamin D can negatively regulate cyclooxygenase-2, an enzyme that controls the production rate of prostaglandin D2 (PGD2) – which is an important regulator of the sleep-wake cycle – implying that hypovitaminosis D may increase circulating PGD2, contributing to the occurrence of sleep disorders. 41 Vitamin D is also involved in the production of melatonin, an essential hormone in the regulation of circadian rhythms and sleep. Melatonin synthesis is controlled by the active form of vitamin D, 1.25(OH)D, from the enzyme tryptophan hydroxylase. 42 This suggests a likely role of vitamin D deficiency in sleep disturbances. 40
To our knowledge, this is the first study to show that alternating shift workers with hypovitaminosis D are more likely to be at risk for sleep problems. Changes in sleep in these workers can lead to increased daytime sleepiness, impairing the performance of work activities, 43 social relationships, 44 45 and cognitive performance. These and a wide variety of other factors can contribute to increased perceived fatigue, and increase the chances of work-related accidents. 13 14 46
However, it is important to consider the results with caution, as some limitations should be considered. Our small sample size may have interfered with the results of the study. Furthermore, the nonrandom choice of the sample and the low attendance at the PSG exam should also be considered. However, these are common limitations in studies with similar methodology. 33 36 37 These limitations can be explained by the procedures for performing the method (sleeping one night in the hospital), performed during an individual's day off, which may be an inconvenience or infeasible for personal reasons. Furthermore, the evaluation of only one night's sleep in an unusual environment can be considered a limitation, since it may not reflect their actual sleep pattern. Another limitation is the fact that all the individuals were working at the same company.
The strengths of this study include the assessment of sleep from PSG, the gold standard test for sleep assessment. Also, the topic of vitamin D and sleep parameters is very poorly explored in the literature. Alternating shift workers are a population at risk for various comorbidities 2 47 and we encourage that similar studies be conducted with this population.
Conclusion
The sample for this study was composed of rotating shift workers, specifically drivers of heavy off-road machinery. It was discovered that they have a relatively high prevalence of hypovitaminosis D with a significant association with lower sleep efficiency, increased MAI, and lower oxygen saturation during sleep.
Acknowledgments
This study was funded by the Brazilian Council for Scientific and Technological Development (CNPq, Distrito Federal, Brazil) and Coordination for the Improvement of Higher Education Personnel-Brazil (CAPES), finance code 001 for Ph.D. student scholarship, and provided financial support for the conduct of the research. Furthermore, we would acknowledge the Federal University of Ouro Preto (UFOP) and the Research and Education Group in Nutrition and Collective Health (GPENSC).
All authors made significant contributions in conducting the study. RMNN, SNF, GLLMC, FAPP, and FLPO contributed to the conception and design of the work, to the acquisition, analysis, and interpretation of data, and review of the manuscript. LAAMJ, VCF contributed to the acquisition, analysis, and interpretation of data, preparation of the manuscript, and review of the manuscript. ALM contributed to the analysis and interpretation of data, preparation of the manuscript, and review of the manuscript.
Footnotes
Conflict of Interests The authors have no conflict of interests to declare.
References
- 1.Kochanek K D, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief. 2017;(293):1–8. [PubMed] [Google Scholar]
- 2.James S M, Honn K A, Gaddameedhi S, Van Dongen H PA. Shift Work: Disrupted Circadian Rhythms and Sleep-Implications for Health and Well-Being. Curr Sleep Med Rep. 2017;3(02):104–112. doi: 10.1007/s40675-017-0071-6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida C M, Malheiro A. Sleep, immunity and shift workers: A review. Sleep Sci. 2016;9(03):164–168. doi: 10.1016/j.slsci.2016.10.007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee W, Nagubadi S, Kryger M H, Mokhlesi B. Epidemiology of obstructive sleep apnea: A population-based perspective. Expert Rev Respir Med. 2008;2(03):349–364. doi: 10.1586/17476348.2.3.349.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Q, Kou T, Zhuang B, Ren Y, Dong X, Wang Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients. 2018;10(10):1395. doi: 10.3390/nu10101395.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossein-nezhad A, Holick M F. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(07):720–755. doi: 10.1016/j.mayocp.2013.05.011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simões M RL, Marques F C, Rocha A de M. Work in rotating shifts and its effects on the daily life of grain processing workers. Rev Lat Am Enfermagem. 2010;18(06):1070–1075. doi: 10.1590/S0104-11692010000600005.. [DOI] [PubMed] [Google Scholar]
- 8.Johnston J D, Ordovás J M, Scheer F A, Turek F W. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv Nutr. 2016;7(02):399–406. doi: 10.3945/an.115.010777.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowah D, Fan X, Dennett L, Hagtvedt R, Straube S. Vitamin D levels and deficiency with different occupations: a systematic review. BMC Public Health. 2017;17(01):519. doi: 10.1186/s12889-017-4436-z.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coppeta L, Papa F, Magrini A. Are shiftwork and indoor work related to D3 Vitamin deficiency? A systematic review of current evidences. J Environ Public Health. 2018;2018:8.468742E6. doi: 10.1155/2018/8468742.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai Y-H, Fang T-C. The pleiotropic effect of vitamin d. ISRN Nephrol. 2013;2013:898125. doi: 10.5402/2013/898125.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boivin D B, Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris) 2014;62(05):292–301. doi: 10.1016/J.PATBIO.2014.08.001.. [DOI] [PubMed] [Google Scholar]
- 13.Oliveira L G, Almeida C V, Barroso L P, Gouvea M JC, Muñoz D R, Leyton V. Truck drivers' traffic accidents in the State of São Paulo: prevalence and predictors. Cien Saude Colet. 2016;21(12):3757–3767. doi: 10.1590/1413-812320152112.11182015.. [DOI] [PubMed] [Google Scholar]
- 14.Catarino R, Spratley J, Catarino I, Lunet N, Pais-Clemente M. Sleepiness and sleep-disordered breathing in truck drivers : risk analysis of road accidents. Sleep Breath. 2014;18(01):59–68. doi: 10.1007/s11325-013-0848-x.. [DOI] [PubMed] [Google Scholar]
- 15.Alberti K GMM, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(05):469–480. doi: 10.1111/j.1464-5491.2006.01858.x.. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31 01:S55–S60. doi: 10.2337/dc08-S055.. [DOI] [PubMed] [Google Scholar]
- 17.Xavier H T, Izar M C, Faria Neto J R.V Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose Arq Bras Cardiol 2013101(4, Suppl 1)1–20. 10.5935/abc.2013S010. [DOI] [PubMed] [Google Scholar]
- 18.IPAQ RC . Guideline for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ): short and long forms. 2005. pp. 1–15.
- 19.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(05):487–504. doi: 10.5664/jcsm.27286.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine . Epstein L J, Kristo D, Strollo P J., Jr Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(03):263–276. doi: 10.5664/JCSM.27497.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorpy M. 4th ed. Springer New York; 2017. International classification of sleep disorders. Sleep Disorders Medicine: Basic Science, Technical Considerations and Clinical Aspects; pp. 475–484. [DOI] [Google Scholar]
- 22.Pollock M L, Wilmore J H.Exercício na Saúde e na Doença: Avaliação e Prescrição para Prevenção e Reabilitação 2nd ed.São Paulo; 1993. [Google Scholar]
- 23.Després J-P, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev. 1993;6(01):137–159. doi: 10.1079/nrr19930010.. [DOI] [PubMed] [Google Scholar]
- 24.Friedewald W T, Levy R I, Fredrickson D S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(06):499–502. doi: 10.1093/clinchem/18.6.499.. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira J EP, Montenegro R M, Junior, Vencio S.Diretrizes Sociedade Brasileira de Diabetes 2017–2018 2017.
- 26.Précoma D B, Oliveira G MM, Simão A F. Updated cardiovascular prevention guideline of the Brazilian society of cardiology – 2019. Arq Bras Cardiol. 2019;113(04):787–891. doi: 10.5935/abc.20190204.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Moraes A CF, Maeda S S, Batista M C. Posicionamento Oficial da Sociedade Brasileira de Patologia Clínica/ Medicina Laboratorial e da Sociedade Brasileira de Endocrinologia e Metabologia. J Bras Patol Med Lab. 2018;53:377–381. [Google Scholar]
- 28.Mendes M M, Hart K H, Botelho P B, Lanham-New S A. Vitamin D status in the tropics: Is sunlight exposure the main determinant? Nutr Bull. 2018;43:428–434. doi: 10.1111/nbu.12349.. [DOI] [Google Scholar]
- 29.Massa J, Stone K L, Wei E K. Vitamin D and actigraphic sleep outcomes in older community-dwelling men: the MrOS sleep study. Sleep. 2015;38(02):251–257. doi: 10.5665/sleep.4408.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niu S-F, Miao N-F, Liao Y-M, Chi M-J, Chung M-H, Chou K-R. Sleep Quality Associated With Different Work Schedules: A Longitudinal Study of Nursing Staff. Biol Res Nurs. 2017;19(04):375–381. doi: 10.1177/1099800417695483.. [DOI] [PubMed] [Google Scholar]
- 31.Holick M F, Vitamin D. Vitamin D deficiency. N Engl J Med. 2007;357(03):266–281. doi: 10.1056/NEJMra070553.. [DOI] [PubMed] [Google Scholar]
- 32.Düzgün G, Durmaz Akyol A. Effect of Natural Sunlight on Sleep Problems and Sleep Quality of the Elderly Staying in the Nursing Home. Holist Nurs Pract. 2017;31(05):295–302. doi: 10.1097/HNP.0000000000000206.. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto Y S, Porto-Sousa F, Salles C. [Prevalence of obstructive sleep apnea in shift workers: a systematic review] Cien Saude Colet. 2018;23(10):3381–3392. doi: 10.1590/1413-812320182310.21362018.. [DOI] [PubMed] [Google Scholar]
- 34.Vaz A P, Drummond M, Mota P C, Severo M, Almeida J, Winck J C. Translation of Berlin Questionnaire to Portuguese language and its application in OSA identification in a sleep disordered breathing clinic. Rev Port Pneumol. 2011;17(02):59–65. doi: 10.1016/S0873-2159(11)70015-0.. [DOI] [PubMed] [Google Scholar]
- 35.Sorajja D, Gami A S, Somers V K, Behrenbeck T R, Garcia-Touchard A, Lopez-Jimenez F. Independent association between obstructive sleep apnea and subclinical coronary artery disease. Chest. 2008;133(04):927–933. doi: 10.1378/chest.07-2544.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osteoporotic Fractures in Men Study Research Group . Goswami U, Ensrud K E, Paudel M L. Vitamin D concentrations and obstructive sleep apnea in a multicenter cohort of older males. Ann Am Thorac Soc. 2016;13(05):712–718. doi: 10.1513/AnnalsATS.201507-440OC.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neighbors C LP, Noller M W, Song S A. Vitamin D and obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med. 2018;43:100–108. doi: 10.1016/j.sleep.2017.10.016.. [DOI] [PubMed] [Google Scholar]
- 38.Archontogeorgis K, Nena E, Papanas N. Vitamin D Levels in Middle-Aged Patients with Obstructive Sleep Apnoea Syndrome. Curr Vasc Pharmacol. 2018;16(03):289–297. doi: 10.2174/1570161115666170529085708.. [DOI] [PubMed] [Google Scholar]
- 39.Persson L JP, Aanerud M, Hiemstra P S, Hardie J A, Bakke P S, Eagan T ML. Chronic obstructive pulmonary disease is associated with low levels of vitamin D. PLoS One. 2012;7(06):e38934. doi: 10.1371/journal.pone.0038934.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellia A, Garcovich C, D'Adamo M. Serum 25-hydroxyvitamin D levels are inversely associated with systemic inflammation in severe obese subjects. Intern Emerg Med. 2013;8(01):33–40. doi: 10.1007/s11739-011-0559-x.. [DOI] [PubMed] [Google Scholar]
- 41.Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med Rev. 2011;15(06):411–418. doi: 10.1016/j.smrv.2011.08.003.. [DOI] [PubMed] [Google Scholar]
- 42.Romano F, Muscogiuri G, Di Benedetto E. Vitamin D and Sleep Regulation: Is there a Role for Vitamin D? Curr Pharm Des. 2020;26(21):2492–2496. doi: 10.2174/1381612826666200310145935.. [DOI] [PubMed] [Google Scholar]
- 43.Costa G. Shift work and health: current problems and preventive actions. Saf Health Work. 2010;1(02):112–123. doi: 10.5491/SHAW.2010.1.2.112.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa D, Silva I S. Impactos Na Vida Social E Familiar Do Trabalho Por Turnos Na Perspectiva Dos Familiares. Revista de Administração de Empresas. 2019;59:108–120. doi: 10.1590/s0034-759020190204.. [DOI] [Google Scholar]
- 45.Pimenta F AP, Alves R L, de Oliveira F LP, do Nascimento Neto R M, Coelho G LLM, de Freitas S N. Qualidade de vida e excesso de peso em trabalhadores em turnos alternantes. Revista Brasileira de Saúde Ocupacional. 2019:44. doi: 10.1590/2317-6369000002417.. [DOI] [Google Scholar]
- 46.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ. 2016;355:i5210. doi: 10.1136/bmj.i5210.. [DOI] [PubMed] [Google Scholar]
- 47.Nascimento R A, Fajardo V C, Menezes Junior L AA.Work hours as a risk factor for SARS-CoV-2 infections: cardiometabolic and sleep characteristics in rotating shift workers Sleep Sci 202215(Spec 2):380–387. 10.5935/1984-0063.20210013. [DOI] [PMC free article] [PubMed] [Google Scholar]


