Abstract
Cholera toxin (CT)-specific antibody responses of the immunoglobulin E (IgE) isotype in the sera of adult patients suffering from infection with either Vibrio cholerae O1, V. cholerae O139, or enterotoxigenic Escherichia coli (ETEC) were analyzed and compared with those in the sera of volunteers immunized with a bivalent B subunit O1/O139 whole-cell cholera vaccine. A significant IgE response to CT was observed in 90% of the patients with V. cholerae O1 infection (18 of 20; P = <0.001) and 95% of the patients with V. cholerae O139 infection (19 of 20; P = <0.001). Similarly, the majority of the patients with ETEC diarrhea (83%; 13 of 15) showed a positive IgE response to CT. Eight of 10 North American volunteers (80%) orally challenged with V. cholerae O1 showed CT-specific IgE responses (P = 0.004). In contrast, Swedish volunteers immunized with the oral cholera vaccine showed no IgE responses to CT (P value not significant). During the study period, total IgE levels in the sera of the diarrheal patients, the North American volunteers, and the Swedish cholera vaccinees alike remained unchanged. However, the total IgE levels in the sera of patients and healthy Bangladeshi controls were on average 89-fold higher than those in the sera of the healthy Swedish volunteers and 34-fold higher than those in the sera of the North American volunteers.
Cholera toxin (CT) is an extensively studied protein enterotoxin, produced by strains of Vibrio cholerae O1 (7) as well as by the more recently described serogroup O139 (4). Patients with cholera seroconvert to CT with antibodies of the immunoglobulin A (IgA) and IgG isotype. Experiments with mice indicated that when CT is administered as a mucosal adjuvant it stimulates a predominantly Th2-type immune response with increased interleukin 4 (IL-4) levels and associated increments in total and specific IgE antibody levels (18, 34). It has been shown that CT affects the release of IL-6 and tumor necrosis factor alpha but not histamine by rat peritoneal mast cells (16). Hitherto, increased levels of IgE antibodies have mainly been described for allergic disorders and parasitic infections, especially intestinal worm infections. However, a recent study demonstrated Haemophilus influenzae and Streptococcus pneumoniae-specific IgE responses in patients with chronic bronchitis (15). Further, immunization against diphtheria and tetanus in adults has been shown to result in specific IgE responses (2). In Helicobacter pylori-infected children, an increase in IgE antibody levels has also been observed (28). Patients infected with Mycobacterium tuberculosis had increased levels of total IgE in sera as well as ascaris-specific IgE responses (1). Whether IgE responses occur in humans exposed to enterotoxin during cholera and other secretory diarrheal diseases is not known. We have therefore investigated whether CT and the Escherichia coli heat-labile enterotoxin are able to induce IgE responses in patients suffering from cholera or diarrhea due to enterotoxigenic E. coli (ETEC). We have, in addition, studied North American volunteers challenged with live V. cholerae O1 and Swedish volunteers orally immunized with the bivalent B subunit O1/O139 whole-cell (B-O1/O139 WC) cholera vaccine and evaluated their CT-specific IgE responses.
For this purpose, 55 adult male Bangladeshi patients with acute watery diarrhea were recruited. Among these, 20 were found to be infected with V. cholerae O139, 20 were found to be infected with V. cholerae O1 El Tor (18 Ogawa and 2 Inaba strains), and 15 were found to be infected with ETEC strains. The patients were 18 to 45 years of age, had a history of 4 to 15 h (median, 8 h) of watery diarrhea prior to hospitalization, and suffered from moderate to severe dehydration. Venous blood was collected from the cholera patients at the acute stage of the disease, i.e., on the second day of hospitalization, which was considered to be approximately 2 days after the onset of diarrhea for the purpose of this study (day 2). Blood was also collected 5, 9, and 20 days later, during convalescence (that is 7, 11, and 22 days after the onset of diarrhea, respectively). From the 15 patients with ETEC diarrhea, samples could only be collected at the acute stage of infection (day 3 after the onset of diarrhea) and about 6 days later, at convalescence (day 9 after the onset of diarrhea); late-convalescence-stage samples could not be collected. Sera were separated from blood samples and stored in aliquots at −20°C until tested. Feces samples were also collected on each study day, and fecal extracts were prepared and stored in aliquots at −70°C (21). Sera from 10 adult North American volunteers orally challenged with 105 CFU of live V. cholerae O1, El Tor Inaba (24) were also analyzed. Samples collected prior to challenge (day 0) and 7, 10, and 14 days after challenge were tested. Sera from 20 Swedish volunteers orally immunized with two doses of the B-O1/O139 WC cholera vaccine were also analyzed in the study (12). Serum samples were collected prior to immunization (day 0) and around 7 days after intake of two doses of the vaccine (day 21 or 22). Twenty-six adult males of similar age as the patients (i.e., 18 to 40 years of age) and of similar socioeconomic background, but with no history of diarrhea during the previous 6 months, were included as controls and are referred to herein as Bangladeshi controls. The preimmunization (day 0) samples from North American and Swedish volunteers were considered control specimens. Informed consent was obtained from the patients and controls. The study was approved by the ethical review committees of the respective institutions.
Microbiological confirmation of strains was carried out using standard procedures as described earlier for V. cholerae O1 and O139 (22) and ETEC (32). All 15 ETEC strains produced both heat-labile and heat-stable enterotoxins (30, 31). Slide agglutination with specific monoclonal antibodies (17) further showed that all ETEC strains produced defined colonization factor (CF) antigens (6). One strain was positive for CFA/I, four strains were positive for CFA/II, and 10 strains were positive for CFA/IV.
The total IgE content in serum samples was determined by an enzyme-linked immunosorbent assay (ELISA) method (18) as follows. Wells of microtiter plates (Nunc Immuno, Roskilde, Denmark) were coated with 100 μl of goat anti-human IgE (1:1,000 dilution; Sigma, St. Louis, Mo.) in 10 mM phosphate-buffered saline (PBS), pH 7.2, and incubated overnight at 4°C. After being washed three times with PBS, wells were blocked with 10% goat serum (200 μl per well; Sigma) for 1 h at room temperature. Plates were washed three times with PBS containing 0.05% Tween 20 (PBS-Tween; Sigma), and then 100 μl of human standard IgE myeloma (starting concentration, 1 μg/ml; Calbiochem, La Jolla, Calif.) or sera from patients or controls was added to wells in duplicate and serial dilutions (in PBS-Tween) were carried out. The serum samples were serially diluted twofold starting at 1:50 for cholera patients and Bangladeshi controls and at 1:5 for non-Bangladeshi individuals. Incubation was carried out for 4 h at 37°C. After five washes with PBS-Tween, 100 μl of biotinylated mouse anti-human IgE (1:2,000 dilution in PBS-Tween; Pharmingen, San Diego, Calif.) was added and the plates were incubated overnight at 4°C. After five washes with PBS-Tween, streptavidin-conjugated horseradish peroxidase (100 μl per well, 1:4,000 dilution in PBS-Tween; Amersham, Buckinghamshire, United Kingdom) was added. Plates were incubated for 90 min at room temperature. After five washes with PBS-Tween the substrate 3,3′,5,5′-tetramethyl benzidine (Sigma) was added, and after 30 min, the color development was stopped by the addition of 1.0 M H2SO4 (50 μl/well) and absorbance was measured at 450 nm. Total IgE levels in serum samples were quantified (in micrograms per mililiter) by comparison with the IgE myeloma protein standard. IgE levels in fecal extracts prepared from stools (21) obtained from eight V. cholerae O1- and eight V. cholerae O139-infected patients (at acute stage, on day 2, and during convalescent stage, on day 22) were also determined.
Serum samples were tested for CT-specific antibodies of the IgE isotype (18) using a modified version of the previously described GM1 ELISA method (29) with the recombinant B-subunit of the cholera toxin (rCTB) as the coating antigen (25). Microtiter plates (Nunc Immuno) were coated with 100 μl of GM1 (Sigma) per well in PBS (1 nmol/ml) and incubated overnight at room temperature. After three washes with PBS, rCTB was added (100 μl/well, 2 μg/ml) and the plates were incubated at room temperature for 1 h. After three washes with PBS, blocking was carried out with 10% goat serum (200 μl per well) for 1 h at room temperature. After five washes with PBS-Tween, samples were applied at an initial dilution of 1:2 and then serial twofold dilutions were carried out. Plates were incubated for 4 h at 37°C. After five washes with PBS-Tween, biotinylated mouse anti-human IgE (1:500 dilution in PBS-Tween; Pharmingen) was added and the plates were incubated overnight at 4°C. After five washes, streptavidin-conjugated horseradish peroxidase (1:500 dilution in PBS-Tween) was added, the color reaction developed as described above, and endpoint titers were determined (21). A cholera patient, challenged volunteer, or vaccinee showing a twofold or greater increase in CT-specific IgE antibody response over the acute-stage level at any point during the convalescent stage was designated a responder. To confirm that the IgE anti-CT response observed in the cholera and ETEC-infected patients was specific, we assayed patient sera for responsiveness to an unrelated protein of parasite origin, namely, Entamoeba histolytica (extracts of axenic culture of strain HM1:1MSS). An ELISA procedure described earlier was employed (19, 20) using the protein extract (10 μg/ml) as the coating antigen. The remaining procedures were similar to those described above for detecting the CT-specific IgE responses.
For statistical analyses of results, the Wilcoxon signed-rank test and the Mann-Whitney U test were used where applicable; a P value of <0.05 was the criterion for a significant difference. Geometric means (GM) and the standard deviations or the standard errors of the means (SEM) were calculated for samples.
The concentration of total IgE in the sera of cholera and ETEC-infected patients from Bangladesh varied from about 0.2 μg/ml to a maximum of over 70 μg/ml. No increase in total IgE levels in sera was observed between the acute and convalescent stages of the disease in V. cholerae O1-, V. cholerae O139-, or ETEC-infected patients (P value was not significant [NS] in all cases) (Table 1). Furthermore, the total IgE levels in patients were not different from those seen in Bangladeshi controls. The total IgE levels in patients and healthy Bangladeshi controls were on average 34 times higher than those in the North American individuals and 89-fold higher than those in the Swedish individuals. However, as with the Bangladeshis, there were no increases (P value was NS) in the levels of total IgE after oral challenge or immunization in either North Americans or Swedes. In contrast, the levels of total IgE in the sera of North American volunteers and the Swedish vaccinees (Table 1) were significantly lower than those in the healthy Bangladeshi controls and in the patients (P = <0.001). No IgE could be detected in the fecal extracts of the cholera patients.
TABLE 1.
Total IgE antibody titers in serum
| Study group | Total IgE (μg/ml) on daysa:
|
|||
|---|---|---|---|---|
| 0–3 | 7–9 | 10–11 | 14–22 | |
| V. cholerae O1-infected patients | ||||
| GM | 4.11 | 4.72 | 4.18 | 5.46 |
| Rangeb | 2.98–5.68 | 3.53–6.31 | 3.17–5.51 | 3.94–7.55 |
| nc | 20 | 20 | 20 | 20 |
| V. cholerae O139-infected patients | ||||
| GM | 2.64 | 2.17 | 4.04 | 2.30 |
| Range | 1.89–3.70 | 1.69–2.80 | 3.09–5.37 | 1.74–3.05 |
| n | 20 | 20 | 20 | 20 |
| ETEC-infected patients | ||||
| GM | 5.0 | 5.02 | ||
| Range | 3.76–6.68 | 3.99–6.32 | ||
| n | 15 | 13 | ||
| North American volunteers challenged with V. cholerae O1 | ||||
| GM | 0.13 | 0.13 | 0.15 | 0.13 |
| Range | 0.08–0.20 | 0.08–0.22 | 0.08–0.25 | 0.08–0.22 |
| n | 10 | 10 | 10 | 10 |
| Swedish cholera vaccinees | ||||
| GM | 0.05 | 0.045 | ||
| Range | 0.03–0.07 | 0.03–0.08 | ||
| n | 20 | 20 | ||
Days after onset of diarrhea or after oral challenge with live V. cholerae O1 or immunization with the bivalent B-O1/O139 WC cholera vaccine. Day 0 levels are preimmune levels.
Range is GM of IgE level ± 1 SEM.
n = number of study persons. The GM titer of total IgE in sera of healthy Bangladeshi controls (n = 26) was 4.45 μg/ml, and the range was 3.32 to 5.6 μg/ml.
Our findings corroborate earlier studies in developing countries which have shown that the sera of both healthy individuals and subjects with symptomatic as well as asymptomatic parasitic infections have elevated levels of IgE compared to individuals residing in developing countries (1, 8, 13, 20). Since there is an association between helminthic infections and elevated levels of IgE and the parasite prevalence rate is high in healthy subjects in developing countries (14), we examined our patients for the presence of common parasites in the stool both at the acute stage and during convalescence. Stools were examined by direct microscopy to detect cyst and vegetative forms of parasites and ova of helminths. Neither helminths nor other common parasites were detected in the stools of the 40 cholera patients. Among the ETEC-infected patients, however, three had cysts of Giardia lamblia in their stool samples. In examinations of the stool samples from Bangladeshi controls, Trichuris trichiura was detected in two samples, Ascaris lumbricoides was detected in two samples, and hookworm was detected in one sample. Thus, in spite of the presence of G. lamblia in 3 of 15 ETEC-infected patients and the presence of other parasites in 5 of the 26 Bangladeshi controls, more than 90% of the Bangladeshi study subjects were found to be free of parasites; however, these persons may have harbored parasites a short time prior to the start of the study.
Elevated levels of IgE can also result from the presence of blood-borne parasites. For this reason, stained blood smears were also screened for the presence of malarial and filarial parasites (33). However, these parasites were not detected in the blood of either patients or healthy Bangladeshi controls.
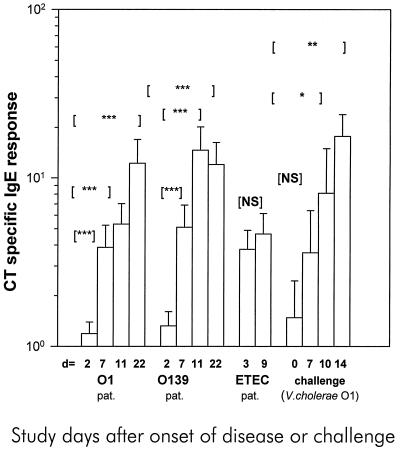
The majority of the cholera patients (18 of 20 infected with O1 serogroup strains and 19 of 20 infected with O139 serogroup strains) had significantly increased titers of IgE specific for CT in serum by day 22, i.e., 3 weeks after the onset of diarrhea (Fig. 1). The CT-specific IgE antibody response was very rapid, a fact reflected by the finding that a significant response was observed in more than half of the patients by day 7 after the onset of illness. The IgE response to CT in the cholera patients showed a wide variation (titer range, 1.1 to >209 arbitrary units) (Fig. 1). The CT-specific IgE response was also analyzed in relation to the total serum IgE concentration (specific IgE/total IgE in samples) in order to correct for differences in individual total IgE concentrations. The same responder frequency was observed using these calculations; significant CT-specific responses were found between the acute (day 2) and convalescent (day 7, 11 or 22) phases of infection in both O1 (P = <0.001) and O139 (P = <0.001) cholera patients. The maximal average increase in CT-specific IgE antibody titer was 11-fold on day 11 for O139 patients and 10-fold on day 22 for O1 patients compared to the acute-stage levels. No CT-specific IgE response could be detected in the fecal extracts obtained from any of the cholera patients.
FIG. 1.
The CT-specific IgE antibody response in sera from Bangladeshi patients infected either with V. cholerae serogroup O1 or O139 or with ETEC and North American volunteers challenged with V. cholerae O1. The antibody levels on different days (d) after onset of disease or challenge are shown. GM titers (+1 SEM [error bars]) are given. Asterisks denote statistically significant differences for comparisons between IgE levels at the acute stage and the convalescent stage in cholera and ETEC-infected patients; for challenged volunteers, comparisons between preimmune levels and levels after challenge were carried out. ∗, P was from <0.05 to 0.01; ∗∗, P was from <0.01 to 0.001; ∗∗∗, P was <0.001. NS, not significant; pat., patients.
For the ETEC-infected patients, a CT-specific IgE titer 2 standard deviations above the GM level recorded in controls, i.e., a CT-specific IgE titer of >1.00, was taken as a positive response. The reason for this was that serum could only be collected later after the onset of diarrhea (day 3 after hospitalization), when the CT-specific IgE levels were already elevated (Fig. 1). Of the 15 patients with ETEC diarrhea, 13 responded with IgE specific to CT at the acute phase (day 3), and of the 13 patients evaluated at the convalescent phase of infection, 11 showed a positive response. However, no significant difference in response was found between the acute and convalescent phases of infection (P = 0.547). This is probably because the acute- and the convalescent-stage samples were collected too early after the onset of diarrhea. A late-convalescent-stage sample (e.g., 3 weeks later) could not be collected as for the cholera patients. The response to CT at the acute stage of ETEC infection was more elevated than the response seen at the acute stage (day 2) in V. cholerae O1-infected (P = <0.001) and V. cholerae O139-infected (P = 0.002) patients.
The North American volunteers showed significantly increased CT-specific IgE responses by day 10 (5 of 10 responders, P = 0.01) and day 14 (8 of 10 responders, P = 0.004) after challenge with V. cholerae O1 (Fig. 1). The response on day 14 after challenge was in fact higher in the North American volunteers than the response recorded on day 22 after onset of illness in Bangladeshi patients with natural V. cholerae O1 infection (P = 0.037) but not that in patients with V. cholerae O139 infection. In contrast, no significant increase in CT-specific IgE antibody titer was detected in the 20 Swedish vaccinees after immunization with the killed oral cholera vaccine (P value was NS). Only two vaccinees showed a marginal increase in antibody titers after immunization with two doses of the cholera vaccine. The same volunteers developed IgA and IgG antibody responses to the toxin (12).
To confirm that the IgE anti-CT response observed in the cholera and ETEC-infected patients was specific, we assayed patient sera for responsiveness to an unrelated protein of parasite origin, namely, E. histolytica. Entamoeba infections are prevalent in populations of low socioeconomic status in the developing world and give rise to strong IgE responses in serum. None of the patients or controls in the study were infected, however. The diarrheal patients had low antibody titers to this antigen at both the acute (GM titer range, 0.35 to 0.47) and the convalescent phases of infection (GM titer range, 0.44 to 0.66). In summary, neither the patients nor the controls had significant responses to E. histolytica.
To further study the specificity of the CT-IgE response, we also tested serum samples from patients with an unrelated invasive diarrheal disease, shigellosis. Fourteen pairs of serum samples (23) from patients with either Shigella dysenteriae type 1 (n = 10) or Shigella flexneri (n = 4) infections were tested in the ELISA for responses to CT. None of the patients showed any serum antibody response to CT of the IgE isotype.
We also tested the response of the cholera and ETEC-infected patients to bacterial antigens other than CT. Serum samples from the V. cholerae O1- and O139-infected patients were tested for IgE response to homologous O1 or O139 lipopolysaccharide (LPS), and sera from ETEC-infected patients were examined for responses to homologous CF antigens. For LPS-specific responses, wells of microtiter plates were coated with purified LPS obtained from V. cholerae O1 or O139 strains (22) at a concentration of 2.5 μg/ml. For the ETEC-infected patients, the anti-CF responses to the homologous CF identified on the infecting ETEC strains were measured. For this purpose, purified antigens, e.g., CFA/I, CFA/II (CS1 and CS2 subcomponents), or CFA/IV (CS4 and CS5 subcomponents) were used at concentrations of 1 μg/ml according to a previously described ELISA method (3). The responses to these cell surface bacterial antigens were poor (titer, <0.5), and in no case was an increase in titer observed at convalescent stage. This showed that the observed serum IgE responses were elicited by CT and not by other bacterial antigens that are known to give rise to strong antibody responses of other isotypes.
All the cholera and ETEC-infected diarrheal patients in the present study also had increases in levels of IgA and IgG antibodies specific for the enterotoxin (22, 32). Although a majority of the patients in the present study showed responses to CT of the IgE isotype, the CT-specific antibody levels were much lower than those than usually observed (11, 22) for IgA (about 200-fold lower) and IgG (about 800-fold lower) isotypes at convalescent stage. An amplified ELISA detection technique had to be employed for the detection of IgE (18), which is not surprising, since, of all the isotypes and subclasses of the antibodies, IgE occurs at the lowest concentration in serum. Previously, it has been reported that intranasal immunization of adult Swedish volunteers with rCTB did not result in increases of either the total or the specific IgE responses in serum (5). In mice, the coadministration of rCTB as adjuvant with tetanus toxoid did not give rise to IgE-mediated responses suggesting that administration of the B subunit of CT as an adjuvant may avoid IgE-mediated allergic reactions. The North American volunteers challenged with live V. cholerae O1, which produces CT, showed significantly increased CT-specific IgE responses, whereas the Swedish volunteers immunized with the cholera vaccine containing the recombinant CTB responded poorly. These results suggest that the holotoxin but not the rCTB toxoid is capable of inducing IgE responses and are similar to results obtained in studies of mice (9, 18, 34).
The reason that both natural disease and experimental cholera induce CT-specific IgE responses and the function of these responses, whether protective or deleterious, can only be speculated upon. However, CT is known to modulate a Th2 type of cytokine response which promotes antibody switch to the IgE isotype (9, 18, 34). An IgE response is also indicative of an inflammatory response, which may now be applicable to ETEC- and V. cholerae-induced toxigenic diarrhea. Inflammatory mediators like nitric oxide (10) and lactoferrin have been found to increase during cholera (26). A possible function for IgE production during infection could be to increase vascular permeability by activating mast cells (27). Cholera toxin has been shown to modulate the activity of mast cells and induce IL-6 production (16). Increased vascular permeability could permit the accumulation of antibody-producing plasma cells and give highly localized protection at the gut surface to bacterial pathogens such as V. cholerae and ETEC. However, the role of the modulators in acute watery diarrhea needs to be better understood.
Acknowledgments
This research was supported by the European Union (EU) (contract DG12 HSMU), the Swedish Agency for Research Cooperation with Developing Countries (Sida-SAREC, grant 1998-05440), the Swedish Medical Research Council (grant 16X-3382), NIAID contract NO1-AI 45252, grant RR-00035 to the General Clinical Research Center (The Johns Hopkins Hospital, Baltimore, Md.), and the ICDDR, B:Centre for Health and Population Research, which is supported by agencies and countries which share its concern for the health problems of developing countries.
REFERENCES
- 1.Adams J F A, Schölvinck E H, Gie R P, Potter P C, Beyers N, Beyers A D. Decline in total serum IgE after treatment of tuberculosis. Lancet. 1999;353:2030–2033. doi: 10.1016/s0140-6736(98)08510-9. [DOI] [PubMed] [Google Scholar]
- 2.Aggerbeck H, Fenger C, Heron I. Booster vaccination against diphtheria and tetanus in man. Comparison of calcium phosphate and aluminium hydroxide as adjuvants II. Vaccine. 1995;13:1366–1374. doi: 10.1016/0264-410x(94)00082-x. [DOI] [PubMed] [Google Scholar]
- 3.Ahren C, Wenneras C, Holmgren J, Svennerholm A-M. Intestinal antibody response after oral immunization with a prototype cholera B subunit colonisation factor antigen enterotoxigenic Escherichia coli vaccine. Vaccine. 1993;11:929–934. doi: 10.1016/0264-410x(93)90380-g. [DOI] [PubMed] [Google Scholar]
- 4.Albert M J. Vibrio cholerae O139 Bengal. J Clin Microbiol. 1994;32:2345–2349. doi: 10.1128/jcm.32.10.2345-2349.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergquist C, Johansson E L, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaastra W, Svennerholm A-M. Colonization factors of human enterotoxigenic Escherichia coli (ETEC) Trends Microbiol. 1996;4:444–452. doi: 10.1016/0966-842x(96)10068-8. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren J. Actions of cholera toxin and the prevention and treatment of cholera. Nature. 1981;292:413–417. doi: 10.1038/292413a0. [DOI] [PubMed] [Google Scholar]
- 8.Hussain R, Hamilton R G, Kumaraswami H V, Adkinson N F, Jr, Ottesen D. IgE responses in human filariasis. J Immunol. 1981;127:1623–1629. [PubMed] [Google Scholar]
- 9.Isaka M, Yasuda Y, Kozuka S, Taniguchi T, Miura Y, Matano K, Goto N, Tochikubo K. Intranasal or subcutaneous co-administration of recombinant cholera toxin B subunit stimulates only a slight or no level of the specific IgE response in mice to tetanus toxoid. Vaccine. 1999;17:944–948. doi: 10.1016/s0264-410x(98)00280-1. [DOI] [PubMed] [Google Scholar]
- 10.Janoff E N, Hayakawa H, Taylor D N, Fasching C E, Kenner J R, Jaimes E, Raij L. Nitric oxide production during V. cholerae infection. Am J Physiol. 1997;273:G1160–G1167. doi: 10.1152/ajpgi.1997.273.5.G1160. [DOI] [PubMed] [Google Scholar]
- 11.Jertborn M, Svennerholm A-M, Holmgren J. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J Clin Microbiol. 1986;24:203–209. doi: 10.1128/jcm.24.2.203-209.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jertborn M, Svennerholm A M, Holmgren J. Intestinal and systemic immune responses in humans after oral immunization with a bivalent B subunit-O1/O139 whole cell cholera vaccine. Vaccine. 1996;14:1459–1465. doi: 10.1016/s0264-410x(96)00071-0. [DOI] [PubMed] [Google Scholar]
- 13.Johansson S G O, Mellbin T, Vahlquist B. Immunoglobulin levels in Ethiopian preschool children with special reference to high concentrations of immunoglobulin E (IgND) Lancet. 1968;i:1118–1121. doi: 10.1016/s0140-6736(68)90187-6. [DOI] [PubMed] [Google Scholar]
- 14.Khan M U, Alam A N, Rahman N, Shahidullah M, Begum T. Impact of acute diarrhoea on parasite loads. Trop Med Parasitol. 1990;41:163–164. [PubMed] [Google Scholar]
- 15.Kjaergard L L, Larsen F O, Norn S, Clementson P, Skov P S, Permin H. Basophil bound IgE and serum IgE directed against Haemophilus influenzae and Streptococcus pneumoniae in patients with chronic bronchitis during acute exacerbations. APMIS. 1996;104:61–67. [PubMed] [Google Scholar]
- 16.Leal Berumen I, Snider D P, Barajas-Lopez C, Marshall J S. Cholera toxin increases IL-6 synthesis and decreases TNF-α production by rat peritoneal mast cells. J Immunol. 1996;156:316–321. [PubMed] [Google Scholar]
- 17.Lopez-Vidal Y, Svennerholm A-M. Monoclonal antibodies against different subcomponents of colonization factor antigen II of enterotoxigenic Escherichia coli. J Clin Microbiol. 1990;28:1906–1912. doi: 10.1128/jcm.28.9.1906-1912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinaro M, Staats H F, Hiroi T, Jackson R J, Coste M, Boyaka P N, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 19.Merino E, Glender W, del Muro R, Ortiz-Ortiz L. Evaluation of the ELISA test for detection of Entamoeba histolytica in feces. J Clin Lab Anal. 1990;4:39–42. doi: 10.1002/jcla.1860040108. [DOI] [PubMed] [Google Scholar]
- 20.Palmer D R, Hall A, Haque R, Anwar K S. Antibody isotype responses to antigens of Ascaris lumbricoides in a case control study of persistently heavily infected Bangladeshi children. Parasitology. 1995;111:385–393. doi: 10.1017/s0031182000081944. [DOI] [PubMed] [Google Scholar]
- 21.Qadri F, Jonson G, Wenneras C, Begum Y A, Hossain J, Albert M J, Svennerholm A M. Immune response to the mannose sensitive hemagglutinin (MSHA) in patients with cholera due to Vibrio cholerae O1 and O139. Clin Diagn Lab Immunol. 1997;4:429–434. doi: 10.1128/cdli.4.4.429-434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qadri F, Wenneras C, Hossain J, Begum Y A, Mohi G, Salam M A, Sack R B, Albert M J, Svennerholm A M. Comparison of immune response in patients infected with Vibrio cholerae O139 and O1. Infect Immun. 1997;65:3571–3576. doi: 10.1128/iai.65.9.3571-3576.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raqib R, Tzipori S, Islam M, Lindberg A A. Immune response to Shigella dysenteriae 1 and Shigella flexneri lipopolysaccharide and polysaccharide antigens in Bangladeshi patients with shigellosis. Serodiagn Immunother Infect Dis. 1993;1:37–45. [Google Scholar]
- 24.Sack D A, Tacket C O, Cohen M B, Sack R B, Losonsky G A, Shimko J, Nataro J P, Edelman R, Levine M M, Giannella R A, Schiff G, Lang D. Validation of a volunteer model of cholera with frozen bacteria as the challenge. Infect Immun. 1998;66:1968–1972. doi: 10.1128/iai.66.5.1968-1972.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez J, Holmgren J. Recombinant system for overexpression of cholera toxin B subunit in Vibrio cholerae as a basis for vaccine development. Proc Natl Acad Sci USA. 1989;86:481–485. doi: 10.1073/pnas.86.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva T M J, Schleupner M A, Tacket C O, Steiner T S, Kaper J B, Edelman R, Guerrant R L. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect Immun. 1996;64:2362–2364. doi: 10.1128/iai.64.6.2362-2364.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinberg P, Ishizaka K, Norman P S. Possible role of IgE mediated reaction in immunity. J Allergy Clin Immunol. 1974;54:359–366. [Google Scholar]
- 28.Studenikin M, a., A. Y, Nijevitch A, Mutalov A G. Consideration of Helicobacter pylori infection in childhood: immune response, endoscopic and morphological findings. Acta Paediatr Jpn. 1995;37:551–556. doi: 10.1111/j.1442-200x.1995.tb03376.x. [DOI] [PubMed] [Google Scholar]
- 29.Svennerholm A-M, Holmgren J, Black R, Levine M, Merson M. Serologic differentiation between antitoxic response to infection with Vibrio cholerae and enterotoxin producing Escherichia coli. J Infect Dis. 1983;147:514–521. doi: 10.1093/infdis/147.3.514. [DOI] [PubMed] [Google Scholar]
- 30.Svennerholm A M, Holmgren J. Identification of Escherichia coli heat labile enterotoxin by means of ganglioside immunosorbent assay (GM1 ELISA) procedure. Curr Microbiol. 1978;1:9–23. [Google Scholar]
- 31.Svennerholm A-M, Wikström M, Lindblad M, Holmgren J. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J Clin Microbiol. 1986;24:585–590. doi: 10.1128/jcm.24.4.585-590.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenneräs C, Qadri F, Bardhan P K, Sack R B, Svennerholm A-M. Intestinal immune responses in patients infected with enterotoxigenic Escherichia coli and in vaccinees. Infect Immun. 1999;67:6234–6241. doi: 10.1128/iai.67.12.6234-6241.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Manual of basic techniques for a healthy laboratory. Geneva, Switzerland: World Health Organization; 1980. pp. 111–284. [Google Scholar]
- 34.Yamamoto S, Kiyono H, Yamamoto M, Imaoka K, Yamamoto M, Fujihashi K, Van Ginkel F W, Noda M, Takeda Y, McGhee J R. A nontoxic mutant of cholera toxin elicits Th-2 type responses for enhanced mucosal-immunity. Proc Natl Acad Sci USA. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]