Abstract
We have examined the properties of Streptococcus pneumoniae cultured in the murine peritoneal cavity and compared its virulence-associated characteristics to those of cultures grown in vitro. Analysis of mRNA levels for specific virulence factors demonstrated a 2.8-fold increase in ply expression and a 2.2-fold increase in capA3 expression during murine peritoneal culture (MPC). Two-dimensional gels and immunoblots using convalescent-phase patient sera and murine sera revealed distinct differences in protein production in vivo (MPC). MPC-grown pneumococci adhered to A549 epithelial cell lines at levels 10-fold greater than those cultured in vitro.
Streptococcus pneumoniae (pneumococcus) is a gram-positive, encapsulated diplococcus that is a significant cause of morbidity and mortality in humans and is capable of causing a wide spectrum of clinical diseases (21, 33). While most individuals colonized with pneumococci remain asymptomatic, young children and individuals with compromised immune systems are often affected (13, 22). S. pneumoniae has long been the subject of intense scrutiny, using both in vivo and in vitro models of infection. Studies to date have used an assortment of animal models and a variety of anatomical sites to identify vaccine candidates (27), assess antibiotics (1), and identify novel virulence factors necessary for bacterial survival in vivo (26). Generally, it is believed that identification and characterization of in vivo-expressed genes will provide insight into the mechanisms that underlie bacterial pathogenesis (12). Furthermore, in vivo-produced factors are likely necessary for bacterial adaptation and survival and disease progression. Examples of this include the Staphylococcus aureus autoinducer of virulence, RNA III-activating protein (3), and diphtheria toxin production by lysogenized Corynebacterium diphtheriae (37).
To date, relatively little information exists concerning the regulation of virulence gene expression in and protein production by gram-positive organisms in vivo. In part, this is because of the intrinsic difficulty of isolating sufficient quantities of bacteria from contaminating host tissues. In vitro models also fail to adequately mimic the in vivo environment. Using a novel murine peritoneal cavity model to grow S. pneumoniae, we obtained evidence showing that pneumococci respond to the in vivo murine peritoneal culture (MPC) environment. Our data demonstrate that pneumococci from MPCs increase expression of two virulence-associated genes and alter the production of specific proteins in comparison to those in in vitro-grown cultures. Moreover, pneumococci isolated from MPCs subsequently demonstrate an increased ability to adhere to respiratory epithelial cells in vitro.
To evaluate pneumococcal growth in the murine peritoneal cavity, S. pneumoniae, serotype 3, strain WU2 (7), was placed in a dialysis bag with a 25,000 molecular-weight cutoff limit (MWC). The dialysis bag was surgically implanted within the murine peritoneum. Briefly, overnight bacterial cultures were diluted to 105 to 106 CFU/ml in RPMI 1640 (Mediatech, Inc., Herndon, Va.) supplemented with glucose to a concentration of 0.4%. Dialysis tubing (Spectrum, Houston, Tex.) with a 25,000 MWC was sealed at one end, sterilized overnight with 0.1% sodium azide, and extensively rinsed with sterile RPMI 1640 prior to use. Aliquots (∼1.0 ml) of the bacterial suspension were added, the dialysis tubing was sealed, and the bags were rinsed with RPMI 1640. Swiss outbred mice weighing 25 to 30 g were anesthetized, and the dialysis bags were surgically implanted through a 1-cm abdominal incision. After insertion, incisions were closed using surgical staples. Unless stated otherwise, bags containing pneumococci were incubated for 8 h, after which mice were sacrificed, pneumococci were collected, and the bacteria were processed in the appropriate experiments. Parallel control cultures (CCs) were incubated in RPMI 1640 at 37°C in a candle extinction jar. The pneumococcal responses to MPC and CC were compared.
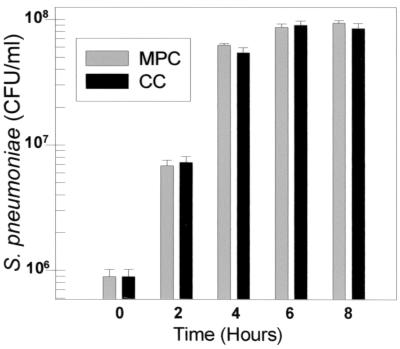
To exclude differences in gene expression or protein production arising from asynchrony of growth, growth rates for MPCs and CCs were shown to be the same over an 8-h incubation period (Fig. 1). At 8 h, MPCs and CCs had reached late logarithmic or early stationary phase with typical bacterial densities of 108 CFU/ml. Mean chain lengths were also similar, with isolated MPC pneumococci having a mean chain length of 2.2 pneumococci per CFU and CCs having a mean chain length of 1.9 (data not shown).
FIG. 1.
Growth profiles of S. pneumoniae WU2 in MPCs and CCs.
To analyze differences in the expression of known virulence-associated genes, Northern dot blot analysis was performed using total RNA isolated from pneumococci grown in MPCs versus those grown in CCs and DNA probes specific for known pneumococcal virulence genes. Total RNA was collected using RNAzol B (Tel-Test, Friendswood, Tex.) following lysis with sodium deoxycholate. Total RNA from in vivo-grown (MPC) and in vitro-grown (CC) pneumococci was blotted onto Hybond N+ nylon membranes (Amersham, Little Chalfont, Buckinghamshire, England) in parallel twofold serial dilutions. RNA was fixed to the membranes by soaking in 0.05 M NaOH for 20 min followed by rinsing with 5× SSC (5× SSC is 0.075 M sodium citrate and 0.75 M sodium chloride [pH 7.0]) and UV cross-linking. DNA probes specific for defined virulence gene sequences were prepared by PCR amplification using S. pneumoniae chromosomal DNA and primers designed from sequence data. Amplified fragments (between 0.3 and 0.6 kb) were TA cloned into pCR2.1 (Invitrogen, Carlsbad, Calif.). Subsequently, probes were PCR amplified from the vector with the same primers, radiolabeled using Ready-To-Go DNA labeling beads (dCTP) (Amersham), and used to probe membranes. After hybridization, membranes were washed at 65°C with 1× SSC for 10 min and 0.1× SSC twice for 30 min. Blot intensity was determined by densitometry using a densitometer (Applied Imaging Systems, Santa Clara, Calif.). Equalization of RNA load was verified by probing the same membrane with a 23S ribosomal probe and adjusted when necessary.
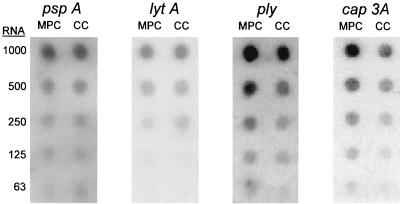
Virulence-related genes whose expression was characterized included ply (4, 5), the gene encoding pneumolysin; capA3 (also known as cps3S) (2, 9), a gene encoding one of the enzymes responsible for the synthesis of the type 3 polysaccharide capsule; pspA (14), a surface antigen recently shown to be involved in iron acquisition; and lytA (25), the major autolysin. These results are shown in Fig. 2. Of the four genes examined, only ply and capA3 showed changes in expression during growth in MPCs versus CCs. Expression of ply was increased 2.8-fold, whereas capA3 expression was increased 2.2-fold. No changes in expression were detected for pspA and lytA.
FIG. 2.
Northern dot blots showing the expression of S. pneumoniae pspA, lytA, ply, and capA3, following WU2 growth in MPCs and CCs. Expression of ply and capA3 was increased 2.8- and 2.2-fold during MPC and CC, respectively. No difference was observed in levels of expression for pspA and lytA.
Increased expression of ply and capA3 demonstrates that environmental factors present in MPCs are capable of altering virulence-related gene expression of the pneumococcus. Pneumolysin, the protein encoded by ply, is an intracellular, conserved, thiol-activated toxin that is released upon lysis of pneumococci (19, 29). Pneumolysin has also been shown to activate the classical complement pathway (20) and enhance the release of inflammatory cytokines (4, 16). High concentrations of pneumolysin are cytotoxic (6), whereas lower levels have a variety of effects, including arrest of the beating cilia on human respiratory epithelial cells (10), decreased migration and bactericidal activity of neutrophils (24), and inhibited lymphocyte proliferation and antibody synthesis (11).
The 2.2-fold increase in expression of capA3 is also notable. capA3 encodes a UDP-glucose dehydrogenase necessary for the synthesis of type 3 polysaccharide (2). Capsular polysaccharide (CPS) is the principal virulence determinant of S. pneumoniae, with increased levels of it being associated with enhanced resistance to phagocytosis and increased virulence (17, 28, 36). Increased levels of capA3 expression may or may not lead to increased levels of polysaccharide capsule in vivo. While increased levels of capsule have been correlated with reduced adherence (35), it is not currently known if increased capA3 mRNA levels lead to increased synthesis of CPS. If an increased level of capA3 mRNA following MPC does result in an increased level of CPS, this will have to be reconciled with the increased adherence activity (10-fold) observed in MPCs as discussed below.
We did not observe changes in pspA expression in MPC pneumococci. Recently, PspA has been associated with sequestration of iron (14). Since we did not take stringent precautions to remove contaminating traces of iron from the medium or from the dialysis membranes, levels of pspA expression may not reflect those during natural infection. Additional studies will therefore be needed to assess pspA expression in the in vivo environment. Moreover, expression of lytA also remained unchanged during MPC. This is of interest because lytA encodes the major autolysin, with bacterial cell lysis being responsible for the release of pneumolysin and other cell components (8, 32).
Differential two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) was used to characterize whole-cell proteins from MPCs and CCs. Briefly, MPCs and CCs were grown in parallel, the bacteria were collected, and the pneumococci were lysed with 0.5% sodium deoxycholate. Bacteria from five to eight individual mice were pooled for subsequent analyses. Duplicate 2D-PAGE gels were evaluated for each culture, with two independent samples collected from both MPCs and CCs. Protein profiles of pneumococcal whole-cell lysates were compared manually, with only proteins (spots) consistently showing differential production being considered. The 2D-PAGE was performed by Kendrick Laboratories (Madison, Wis.) according to the method of O'Farrell (23). Gels were standardized with molecular weight standards added to the agarose, which sealed the tube gel to the slab gel. Internal molecular mass standards included myosin, 220 kDa; phosphorylase A, 94 kDa; catalase, 60 kDa; actin, 43 kDa; carbonic anhydrase, 29 kDa; and lysozyme, 14 kDa. Additionally, an isoelectric focusing standard, tropomyosin, was added to the buffer bank on the onset of isoelectric focusing.
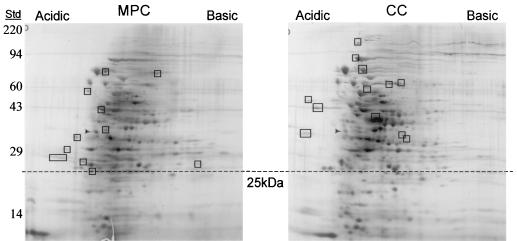
Analysis of silver-stained protein profiles revealed 11 and 12 proteins exclusively produced during growth in MPCs and CCs, respectively (Fig. 3). The approximate Mrs of these proteins are listed in the Fig. 3 legend. Proteins with molecular masses under 25 kDa were not considered, as they may have diffused into the dialysis membrane and would therefore represent murine contaminants.
FIG. 3.
2D-PAGE of WU2 whole-cell lysates following MPC and CC. Protein spots were detected by silver staining. Rectangles indicate proteins that are unique under each condition. Numbers to the left indicate the masses of protein standards (Std) in kilodaltons. The dashed line shows the boundary. Protein spots unique to MPCs have molecular masses of 73, 70, 64, 43, 34, 33, 32, 29, 27, 26, and 25 kDa. Protein spots unique to CCs have molecular masses of ∼115, 87, 74, 62, 60, 57, 52, 46, 42, 35, 35, and 32 kDa. Arrowheads indicate tropomyosin, an isoelectric focusing standard added to the buffer bank.
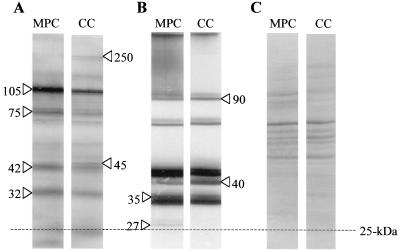
To further examine potential gene expression differences between growth in MPCs and growth in CCs, we considered whether there were differences in their immunoreactive-protein profiles. Western blot analysis (31) was performed using pooled convalescent-phase sera from four patients who had recently recovered from serologically confirmed pneumococcal pneumonia. Western blot analysis showed four reactive bands with increased production during growth in MPCs (Fig. 4A). The masses of these proteins were estimated to be ∼105, 75, 42, and 32 kDa. Additionally, two bands of 250 and 45 kDa with increased intensity were noted in the CC lane. Immunoblot analysis using murine antiserum to heat-killed pneumococci also exhibited differential protein production (Fig. 4B). These immunoblots showed increased production of two protein bands during MPC. These proteins were estimated to have masses of ∼35 and 27 kDa. Likewise, increased amounts of two proteins were also noted in the CC lane. These proteins were estimated to have masses of ∼90 and 40 kDa. These results were based on the equal loading of protein from the MPCs and CCs as demonstrated in Fig. 4C. Pooled sera from three adults with no history of pneumococcal pneumonia were also used to examine MPC and CC immunoreactive-protein profiles. While distinct bands of various molecular weights did appear on the Western blot, only faint bands were observed and they did not coincide with the MPC- or CC-induced bands described above (data not shown).
FIG. 4.
Immunogenic proteins from whole-cell lysates of WU2 following growth in MPCs and CCs. Immunogenic proteins were detected by using human convalescent-phase sera (A) or murine antisera to heat-killed pneumococci (B). Arrows indicate proteins that showed increased production. The masses, in kilodaltons, of the immunoreactive proteins are indicated. (C) Coomassie-stained gel showing that equal amounts of protein were loaded.
Two-dimensional whole-cell protein profiles and immunoblots from MPCs and CCs demonstrated that differential protein production occurs in response to environmental conditions. Moreover, these differences suggest that gene expression is also altered in response to MPC and CC environments.
Host environmental factors have long been recognized as being potent inducers of bacterial gene expression. Examples include factors such as temperature, oxygen, carbon dioxide, nitrogen, and iron (12). Incubation of S. pneumoniae within the implanted dialysis bag exposes the bacteria to inducing host factors while maintaining the homogeneity of the bacterial culture. Soluble factors present in the mouse peritoneum diffuse through the dialysis membrane and may induce genes that normally would be transcribed during peritoneal infection. These proteins include those that function in the adaptation of the bacteria to the host environment and may be involved in metabolic synthesis, transport, and virulence factors (15, 18, 26).
To further characterize differences between MPCs and CCs, in vitro adherence assays were performed using monolayers of A549 (a human lung carcinoma epithelial cell line) (30). Logarithmic-phase pneumococci from MPCs and CCs were pelleted, resuspended and diluted fivefold in RPMI 1640, and applied to confluent A549 monolayers (1.0 × 106 cells/well) previously established on 24-well tissue culture plates (Corning Inc., Corning, N.Y.). Monolayers were incubated at 37°C in a 5% CO2 humidified atmosphere for 1.5 h. Nonadherent bacteria were selected by aspiration during washing of the monolayer five times with RPMI 1640. After the final wash, monolayers were overlaid with 450 μl of tryptic soy agar supplemented with 5% sheep blood. Overlaid monolayers were incubated overnight at 37°C, and adherent pneumococci were determined by counting colonies. Adhesion assays were performed in triplicate. Statistical analysis was performed using a paired Student t test. Assays measuring bacterial adhesion to A549 cells demonstrated that pneumococci from MPCs adhered at levels 10-fold higher than those for pneumococci grown in vitro (CCs). MPCs adhered at approximately 4.0 × 106 CFU per 106 A549 cells, whereas CCs adhered at approximately 3.5 × 105 CFU per 106 A549 cells. The difference in adherence of MPCs versus that of CCs was found to be statistically significant (P = 9.0 × 10−3).
Intrastrain variation in pneumococcal adherence has previously been reported. Recently, phase variation-dependent changes in choline-binding-protein A production were shown to affect the capacity of pneumococci to bind nasopharyngeal cells (28). Phase variation in the pneumococcus occurs spontaneously with a frequency of 10−3 to 10−6 (36). We were unable to determine the phase of this highly encapsulated strain (WU2), consistent with a previous report (36) showing that high levels of capsule obscure recognition of variations in opacity. However, because of the nature of spontaneous variation, it is unlikely that WU2 phase variation is the source of the observed differences in adherence to A549 cells. It is possible, though, that MPC induces production of a known or previously described adhesin or decreases the quantity of CPS. Previously, CPS levels have been shown to be inversely related to adherence and invasion (35).
It is not known why some pneumococcal virulence genes respond to in vivo growth with increased expression while others do not. It is possible that not all virulence factors respond to soluble and/or physiological factors present during in vivo (MPC) conditions. Additionally, MPC does not exactly mimic the in vivo environment. In MPCs, bacterium-host cell contact is not permitted, nor is the interaction of bacteria with host proteins whose size exceeds the MWC of the membrane. It is likely that this model provides less than the full complement of inducing elements present during natural infection. Even so, this model represents an improvement over in vitro culture techniques to assess the virulence activities of S. pneumoniae.
Overall, use of the MPC model has allowed evaluation of pneumococcal gene and protein expression under in vivo conditions. Introduction of pneumococci into the peritoneal cavity has long been an accepted model for assaying S. pneumoniae virulence (34). We have used the MPC model to examine protein production, adherence, and expression of virulence genes by S. pneumoniae. Analysis of pneumococci following MPC growth represents a novel method and may therefore provide important insights into pneumococcal virulence.
Acknowledgments
We thank Dan Musher and Ashok Chopra for their helpful comments.
This work was supported by Graduate Research Fellowship PGE 9818286 from the National Science Foundation, grant 98-HEDS02-291 from NASA, and a grant from the Texas Advanced Research Program, 004952-0090.
REFERENCES
- 1.Ahmed A, Jafri H, Lutsar I, McCoig C C, Trujillo M, Wubbel L, Shelton S, McCracken G H., Jr Pharmacodynamics of vancomycin for the treatment of experimental penicillin- and cephalosporin-resistant pneumococcal meningitis. Antimicrob Agents Chemother. 1999;43:876–881. doi: 10.1128/aac.43.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrecubieta C, López R, García E. Molecular characterization of cap3A, a gene from the operon required for the synthesis of the capsule of Streptococcus pneumoniae type 3: sequencing of mutations responsible for the unencapsulated phenotype and localization of the capsular cluster on the pneumococcal chromosome. J Bacteriol. 1994;176:6375–6383. doi: 10.1128/jb.176.20.6375-6383.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban N, Goldkorn T, Nhan R T, Dang L B, Scott S, Ridgley R M, Rasooly A, Wright S C, Larrick J W, Rasooly R, Carlson J R. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcus aureus. Science. 1998;280:438–440. doi: 10.1126/science.280.5362.438. [DOI] [PubMed] [Google Scholar]
- 4.Berry A M, Ogunniyi A D, Miller D C, Paton J C. Comparative virulence of Streptococcus pneumoniae strains with insertion-duplication, point, and deletion mutations in the pneumolysin gene. Infect Immun. 1999;67:981–985. doi: 10.1128/iai.67.2.981-985.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry A M, Paton J C. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect Immun. 2000;68:133–140. doi: 10.1128/iai.68.1.133-140.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulnois G J. Pneumococcal proteins and the pathogenesis of disease caused by Streptococcus pneumoniae. J Gen Microbiol. 1992;138:249–259. doi: 10.1099/00221287-138-2-249. [DOI] [PubMed] [Google Scholar]
- 7.Briles D E, Nahm M, Schroer K, Davie J, Baker P, Kearney J, Barletta R. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981;153:694–705. doi: 10.1084/jem.153.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canvin J R, Marvin A P, Sivakumaran M, Paton J C, Boulnois G J, Andrew P W, Mitchell T J. The role of pneumolysin and autolysin in the pathology of pneumonia and septicemia in mice infected with a type 2 pneumococcus. J Infect Dis. 1995;172:119–123. doi: 10.1093/infdis/172.1.119. [DOI] [PubMed] [Google Scholar]
- 9.Cartee R T, Forsee W T, Schutzbach J S, Yother J. Mechanism of type 3 capsular polysaccharide synthesis in Streptococcus pneumoniae. J Biol Chem. 2000;275:3907–3914. doi: 10.1074/jbc.275.6.3907. [DOI] [PubMed] [Google Scholar]
- 10.Feldman C, Mitchell T J, Andrew P W, Boulnois G J, Read R C, Todd H C, Cole P J, Wilson R. The effect of Streptococcus pneumoniae pneumolysin on human respiratory epithelium in vitro. Microb Pathog. 1990;9:275–284. doi: 10.1016/0882-4010(90)90016-j. [DOI] [PubMed] [Google Scholar]
- 11.Ferrante A, Rowan-Kelly B, Paton J C. Inhibition of in vitro human lymphocyte response by the pneumococcal toxin pneumolysin. Infect Immun. 1984;46:585–589. doi: 10.1128/iai.46.2.585-589.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomez M, Alter S, Kumar M L, Murphy S, Rathore M H. Neonatal Streptococcus pneumoniae infection: case reports and review of the literature. Pediatr Infect Dis J. 1999;18:1014–1018. doi: 10.1097/00006454-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Hammerschmidt S, Bethe G, Remane P H, Chhatwal G S. Identification of pneumococcal surface protein A as a lactoferrin-binding protein of Streptococcus pneumoniae. Infect Immun. 1999;67:1683–1687. doi: 10.1128/iai.67.4.1683-1687.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heithoff D M, Conner C P, Hanna P C, Julio S M, Hentschel U, Mahan M J. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci USA. 1997;94:934–939. doi: 10.1073/pnas.94.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J O, Romero-Steiner S, Sorensen U B, Blom J, Carvalho M, Barnard S, Carlone G, Weiser J N. Relationship between cell surface carbohydrates and intrastrain variation on opsonophagocytosis of Streptococcus pneumoniae. Infect Immun. 1999;67:2327–2333. doi: 10.1128/iai.67.5.2327-2333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahan M J, Tobias J W, Slauch J M, Hanna P C, Collier R J, Mekalanos J J. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc Natl Acad Sci USA. 1995;92:669–673. doi: 10.1073/pnas.92.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell T J, Andrew P W. Biological properties of pneumolysin. In: Tomasz A, editor. Streptococcus pneumoniae. Larchmont, N.Y: Mary Ann Liebert Inc.; 2000. pp. 279–286. [Google Scholar]
- 20.Mitchell T J, Andrew P W, Saunders F K, Smith A N, Boulnois G J. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol Microbiol. 1991;5:1883–1888. doi: 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 21.Murray P R, Kobayashi G S, Pfaller M A, Rosenthal K S. Streptococcus and related gram-positive bacteria. In: Farrel R, editor. Medical microbiology. 2nd ed. St. Louis, Mo: Mosby-Year Book, Inc.; 1994. pp. 180–198. [Google Scholar]
- 22.Musher D M. Streptococcus pneumoniae. In: Mandell G L, Bennett J E, Dolin R, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 5th ed. Vol. 2. New York, N.Y: Churchill Livingstone; 2000. pp. 2128–2147. [Google Scholar]
- 23.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 24.Paton J C. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 25.Paton J C, Berry A M, Lock R A. Molecular analysis of putative pneumococcal virulence proteins. In: Tomasz A, editor. Streptococcus pneumoniae. Larchmont, N.Y: Mary Ann Liebert Inc.; 2000. pp. 261–270. [DOI] [PubMed] [Google Scholar]
- 26.Polissi A, Pontiggia A, Feger G, Altieri M, Mottl H, Ferrari L, Simon D. Large-scale identification of virulence genes from Streptococcus pneumoniae. Infect Immun. 1998;66:5620–5629. doi: 10.1128/iai.66.12.5620-5629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prellner K, Hermansson A, White P, Melhus A, Briles D. Immunization and protection in pneumococcal otitis media studied in a rat model. Microb Drug Resist. 1999;5:73–82. doi: 10.1089/mdr.1999.5.73. [DOI] [PubMed] [Google Scholar]
- 28.Rosenow C, Ryan P, Weiser J N, Johnson S, Fontan P, Ortqvist A, Masure H R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–829. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 29.Rossjohn J, Gilbert R J, Crane D, Morgan P J, Mitchell T J, Rowe A J, Andrew P W, Paton J C, Tweten R K, Parker M W. The molecular mechanism of pneumolysin, a virulence factor from Streptococcus pneumoniae. J Mol Biol. 1998;284:449–461. doi: 10.1006/jmbi.1998.2167. [DOI] [PubMed] [Google Scholar]
- 30.Rubens C E, Smith S, Hulse M, Chi E Y, van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect Immun. 1992;60:5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Sato K, Quartey M K, Liebeler C L, Le C T, Giebink G S. Roles of autolysin and pneumolysin in middle ear inflammation caused by a type 3 Streptococcus pneumoniae strain in the chinchilla otitis media model. Infect Immun. 1996;64:1140–1145. doi: 10.1128/iai.64.4.1140-1145.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sopena N, Sabria M, Pedro-Botet M L, Manterola J M, Matas L, Dominguez J, Modol J M, Tudela P, Ausina V, Foz M. Prospective study of community-acquired pneumonia of bacterial etiology in adults. Eur J Clin Microbiol Infect Dis. 1999;18:852–858. doi: 10.1007/s100960050419. [DOI] [PubMed] [Google Scholar]
- 34.Sternberg G M. A fatal form of septicaemia in the rabbit, produced by the subcutaneous injection of human saliva. Annu Rep Natl Board Health. 1881;3:87–108. [Google Scholar]
- 35.Talbot U M, Paton A W, Paton J C. Uptake of Streptococcus pneumoniae by respiratory epithelial cells. Infect Immun. 1996;64:3772–3777. doi: 10.1128/iai.64.9.3772-3777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welkos S L, Holmes R K. Regulation of toxinogenesis in Corynebacterium diphtheriae. I. Mutations in bacteriophage β that alter the effects of iron on toxin production. J Virol. 1981;37:936–945. doi: 10.1128/jvi.37.3.936-945.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]