Abstract
N6-methyladenosine (m6A) is the most abundant RNA modification in eukaryotes, and it participates in the regulation of pathophysiological processes in various diseases, including malignant tumors, by regulating the expression and function of both coding and non-coding RNAs (ncRNAs). More and more studies demonstrated that m6A modification regulates the production, stability, and degradation of ncRNAs and that ncRNAs also regulate the expression of m6A-related proteins. Tumor microenvironment (TME) refers to the internal and external environment of tumor cells, which is composed of numerous tumor stromal cells, immune cells, immune factors, and inflammatory factors that are closely related to tumors occurrence and development. Recent studies have suggested that crosstalk between m6A modifications and ncRNAs plays an important role in the biological regulation of TME. In this review, we summarized and analyzed the effects of m6A modification-associated ncRNAs on TME from various perspectives, including tumor proliferation, angiogenesis, invasion and metastasis, and immune escape. Herein, we showed that m6A-related ncRNAs can not only be expected to become detection markers of tumor tissue samples, but can also be wrapped into exosomes and secreted into body fluids, thus exhibiting potential as markers for liquid biopsy. This review provides a deeper understanding of the relationship between m6A-related ncRNAs and TME, which is of great significance to the development of a new strategy for precise tumor therapy.
Keywords: m6A, ncRNA, exosome, tumor metastasis, tumor microenvironment, biomarker, targeted therapy
Introduction
Since Stephen Paget proposed the hypothesis of "seed and soil" theory of tumors in 1889, the relationship between tumors and tumor microenvironment (TME) has attracted extensive research attention. TME refers to the environment in which tumor cells grow. During the occurrence and development of tumor, TME can interact with tumor cells and play a key role in tumor proliferation, inflammation, immune evasion, metastasis and drug resistance. TME is a dynamic regulatory network involving multiple signals, in which a variety of cellular and molecular pathways can be potential therapeutic targets. Important anti-tumor therapeutic strategies involve blocking tumor-associated pathways in TME, inhibiting the activity of cells with tumor-enhancing effects, and promoting anti-tumor immunity, in combination with traditional treatments, such as surgery, chemotherapy, and radiotherapy. The molecular substances in TME are closely related to the pathogenesis and development of tumor and are potentially sensitive and specific tumor markers.
There are more than 100 chemical modifications of RNA, with methylation being the main form of modification in all types of RNA 1. RNA methylation accounts for > 60% of all RNA modifications. N6-methyladenosine (m6A) is the most abundant RNA modification in eukaryotes, and regulates the post-transcriptional expression of genes. M6A is mainly distributed in the protein coding sequence of message RNA (mRNA), 3' untranslated region (UTR), region around the stop codon, and long exon region 2, 3. The role of m6A modification in the regulation of gene expression is closely related to various normal physiological processes, including cell differentiation, DNA damage response, biological clock, and sex determination, and the occurrence and development of diseases, such as tumors. During tumor development, m6A modification can regulate the expression of oncogenes and tumor suppressor genes in tumor cells, thereby regulating tumor angiogenesis, extracellular matrix remodeling, epithelial-mesenchymal transition (EMT), and the immune microenvironment to promote tumorigenesis. Recent research has found that m6A modification regulates the transcription level of mRNA encoding genes and also affects the transcription and generation of a variety of non-coding RNAs (ncRNAs) (such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs)). Therefore, m6A modification participates in the regulation of various life processes in cells at multiple levels and plays a regulatory role in many diseases, including tumors 4.
Both m6A modification and ncRNAs can act as regulators of intracellular activities and affect the occurrence and development of tumors. The interaction between them influences the formation and stability of the fine regulatory network in the TME, further enhancing development of tumors. The interrelationship between m6A modification and ncRNAs and their role in TME is of great significance for a comprehensive understanding of the biological regulation of tumors, which is a field full of surprises. This review aimed to summarize the biological characteristics of ncRNAs related to m6A modification and their role in TME formation, explore the application prospects of ncRNAs related to m6A modification in clinical treatment, and provide new strategies for the accurate diagnosis and treatment of tumors.
Tumor Microenvironment
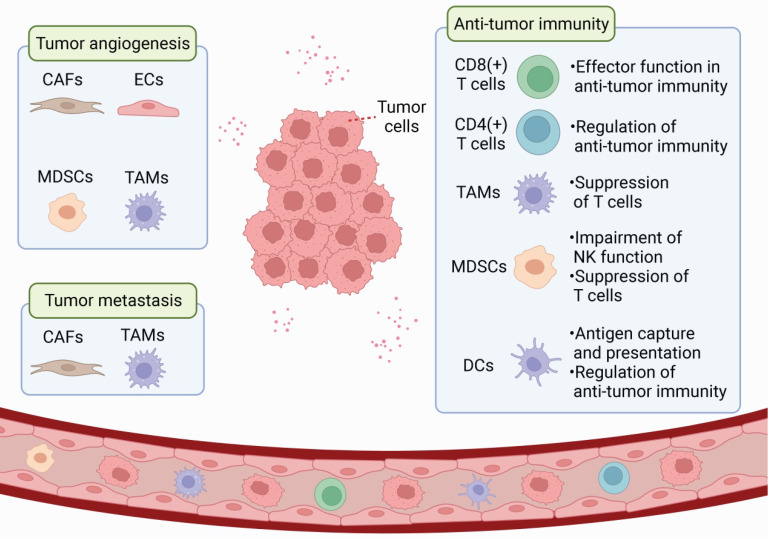
The tumor microenvironment (TME) includes the tumor cells, surrounding stromal cells (such as cancer-associated fibroblasts and tumor-related endothelial cells), immune and inflammatory cells (such as myeloid-derived suppressor cells, tumor-associated macrophages, dendritic cells, and tumor-infiltrating lymphocytes), as well as the extracellular matrix (ECM), microvessels and biomolecules in the nearby area 5, 6. In contrast to the normal microenvironment, TME is characterized by low oxygen content, acid accumulation, and abnormal local immune status. TME is a complex integrated system, and different cells in TME can communicate in several ways to promote tumor growth, angiogenesis, immune escape, and metastasis (Figure 1). As an important epigenetic regulatory mechanism, ncRNAs can regulate gene expression at the genomic and chromosomal levels 7, 8, participate widely in signaling, and be transmitted through exosomes to influence recipient cells and promote TME formation.
Figure 1.
Schematic representation of TME. TME is a complex and comprehensive system. In addition to tumor cells, stromal cells (eg. CAF and tumor-associated endothelial cells) and immune cells (eg. MDSC, TAM, DC, and TILs) are also important components of TME.
1
2
Stromal cells in tumor microenvironment
Stromal cells in TME, including cancer-associated fibroblasts (CAFs), tumor-related endothelial cells, pericytes, adipocytes and mesenchymal stem cells, which can be induced by signal factors secreted by tumor cells, and their gene expression and functional status are different from those of stromal cells in normal tissue 9. Stromal cells promote tumor progression by participating in the proliferation and migration of tumor cells, extracellular matrix remodeling, immune cell recruitment, and tumor angiogenesis.
There are numerous CAFs in TME. CAF can be derived from local normal fibroblasts, differentiated from bone marrow-derived mesenchymal cells, or generated by transdifferentiation of pericytes and other cell types 10, 11. Transforming growth factor β (TGF-β), platelet-derived growth factors (PDGFs), and fibroblast growth factor (FGF) 2 are the main inducers of CAF activation 12. Stromal derived factor 1 (SDF-1) secreted by CAF binds to C-X-C chemokine receptor (CXCR) 4 on the surface of tumor cells and vascular endothelial cells (ECs), increasing tumor growth and malignancy as well as promoting angiogenesis 13. Hepatocyte growth factor secreted by CAF enhances tumor invasion and metastasis by activating c-Met 14. CAF can also induce the transcription of long non-coding RNA (lncRNA) HOTAIR by secreting TGF-β1, thereby promoting EMT and the metastasis of breast cancer cells 15. In ovarian cancer, CAF upregulates lncRNA LINC00092 by secreting C-X-C motif ligand (CXCL) 14 and promotes cancer metastasis by altering glycolysis 16.
Angiogenesis plays an important role in tumor growth and metastasis and EC proliferation is the core process for neovascularization. CXCR7 expression is upregulated in tumor-associated EC, which promotes angiogenesis in TME through ERK1/2 phosphorylation 17. Exosomes containing miR-23a secreted by tumor cells promote tumor microvasculogenesis by acting on sirtuin1 (SIRT1) in EC 18. The vascular endothelial growth factor (VEGF)/vascular endothelial growth factor reporter (VEGFR) signaling pathway is important in tumor angiogenesis. VEGF upregulates Bcl-2 in vascular ECs and promotes tumor angiogenesis 19. miR-134 inhibits angiogenesis in osteosarcoma by targeting the VEGF/VEGFR1 pathway 20. In neuroblastoma, lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) promotes EC migration and angiogenesis through the upregulation of FGF2 21. In addition, tumor cells and ECs directly interact to promote tumor angiogenesis through the mitogen-activated protein kinase (MAPK) and Notch pathways 22, 23. Currently, anti-angiogenesis therapy is a conventional treatment strategy for tumor in clinical practice.
Cross-talk between different stromal components supports the formation of TME. Mesenchymal stem cells, tumor-associated macrophages and CAF secrete a variety of vascular growth factors into TME to promote angiogenesis 24. CAFs also enhance recruitment of endothelial progenitor cells and macrophage 25. These interactions have a role in maintaining the homeostasis of TME.
Immune cells in tumor microenvironment
During tumor development, tumor cells can take advantage of the negative regulation mechanism of the immune system to alter the functional status of various infiltrated immune cells, forming a microenvironment with low anti-tumor immunity, leading to tumor immune tolerance and immune escape. Therefore, inducing and enhancing the anti-tumor immune response is an important strategy to improve anti-tumor therapy efficacy.
TME comprises numerous immune cells, including myeloid-derived suppressor cells (MDSCs) that are a group of heterogeneous cells that originate from the myeloid system. MDSCs continue to differentiate into dendritic cells, macrophages, and granulocytes under normal circumstances, while under pathological conditions, MDSCs cannot differentiate but they develop into a cell population that can inhibit immune function. Several cytokines in TME can induce the proliferation of MDSCs, including cyclooxygenase-2 (COX2), interleukin6 (IL-6), granulocyte macrophage colony stimulating factor (GM-CSF), and VEGF 26. Lnc-CHOP enhances the immunosuppressive function of MDSCs by promoting CATT enhancer-binding protein β (C/EBPβ) activation and the expression of molecules associated with MDSC immunosuppressive activity 27. However, lnc-C/EBPβ inhibits the activation of C/EBPβ and reduces MDSC function 28. The immunosuppressive function of MDSCs mainly manifestes as the suppression of the immune-killing effect of T cells and natural killer (NK) cells. MDSCs can inhibit T cells by promoting T cell apoptosis, consuming essential amino acids for T cell function, reducing T cell migration to lymph nodes, preventing T cell signaling, and inducing T cell differentiation imbalance 29, 30. MDSCs inhibit NK cells by inhibiting activation receptor expression, downregulating perforin secretion, and limiting response to IL-2 by NK cells 31. MDSCs are also involved in the regulation of tumor angiogenesis 32.
Macrophages are classified into M1 and M2 phenotypes 33. M1 macrophages can secrete pro-inflammatory factors, such as IL-1, IL-12, and tumor necrosis factor alpha (TNF-α), which are involved in defense against infections and tumoricidal activities 34. In contrast, M2 macrophages highly express the immunosuppressive factor IL-10, which exerts anti-inflammatory and tumor-promoting effects 35. Tumor-associated macrophages (TAMs) infiltrate the tumor area, showing a functional phenotype similar to that of M2 macrophages. A variety of chemokines, growth factors, and cytokines in TME recruit macrophages to infiltrate 36 and induce M2 phenotype transformation. TAM recruitment and polarization are regulated by ncRNAs. LncRNAs LNMAT1 37 and lnc-BM 38 promote macrophage recruitment, while lncRNA-MM2P regulates the expression of M2-related genes in macrophages 39. miR-21-3p, miR-125b-5p and miR-181d-5p can be delivered into TME by cancer-derived exosomes to promote M2 polarization of macrophages 40. In TME, TAM can secrete matrix metalloproteinases (MMP) to promote basement membrane degradation, thus promoting metastasis. TAM can also secrete basic FGF, VEGF, CXCL8, and other factors that promote tumor angiogenesis 41. TGF-β and IL-10 released by M2 macrophages can inhibit T cell immune responses and anti-tumor immunity.
Dendritic cells (DCs) have a strong ability for antigen uptake and processing, which can induce an anti-tumor immune response by presenting tumor antigens. DCs are divided into plasmacytoid dendritic cells (pDCs) and myeloid dendritic cells (mDCs). pDCs exert non-specific anti-infection and anti-tumor immunity by activating monocytes, macrophages, B cells, NK cells, and naive T cells 40. mDCs mainly activate the transformation of initial CD4 cells to T helper (Th) 1 cells and CD8 cells to cytotoxic T lymphocytes (CTL) through antigen presentation and exert specific anti-infection and anti-tumor immunity 42. Lnc-DC can bind to signal transducer and activator of transcription 3 (STAT3) to promote DC differentiation and improve the antigen presentation capacity 43. However, in TME, various cytokines can induce DC differentiation disorders, resulting in abnormal immunomodulatory functions.
In TME, tumor-infiltrating lymphocytes (TILs) are influenced by different cytokines and activation mechanisms to produce different immune responses. CD8+T cells can recognize tumor antigens or secrete cytokines and play an effector-killing role in anti-tumor immunity. Lnc-Tim3 interacts with Tim-3 to release Bat3, resulting in CD8+ T-cell depletion and immune evasion in hepatocellular carcinoma (HCC) 44. Activated CD8+T cells express cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) and programmed death-1 (PD-1) 45. Immunocheckpoint inhibitors (ICIs) targeting CTLA-4 and PD-1 have become important anti-tumor immunotherapies. The overexpression of miR-142-5p in tumor cells can block the programmed cell death ligand 1 (PD-L1)/PD-1 pathway and enhance anti-tumor immune function 46. CD4+T cells assist and regulate immunity and can proliferate and differentiate into a variety of cell subsets: Th1, Th2, Th17, and regulatory T cells (Tregs) 47. Interferon (IFN)-γ secreted by Th1 cells can activate cytotoxic CD8+T cells, DCs, and macrophages and play an anti-tumor role. Th2 cells secrete IL-4 to activate the tumor-promoting macrophages; moreover, Tregs can inhibit the proliferation and differentiation of T cells, hinder antigen presentation, mediate target cell death, and play an immunosuppressive part 48. Several chemokines in TME and DC differentiation disorder can promote the recruitment, proliferation and activation of Tregs 49, 50. The lncRNA SNHG1 regulates Treg cell differentiation through the miR-448/IDO pathway, affecting tumor immune escape 51, while specific Treg clearance can enhance the anti-tumor immune response 52, 53.
M6a Modifications and Noncoding RNAs
Molecular component of m6A RNA methylation
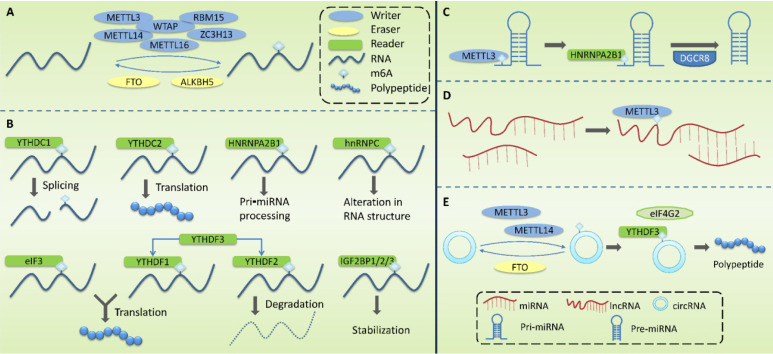
M6A methylation is a dynamic and reversible process 54, which is regulated by m6A methyltransferase complexes (m6A writers), m6A demethylases (m6A erasers), and m6A-binding proteins (m6A readers) (Figure 2).
Figure 2.
Molecular mechanism associated with the modification of m6A methylation. M6A methylation is regulated by m6A methyltransferase complexes (writers), m6A demethylases (erasers), and m6A-binding proteins (readers). (A) Writers are mainly composed of METTL3, METTL14, WTAP, METTL16, RBM15 and ZC3H13, mediating the modification of m6A methylation of RNA. Erasers mediate the process of m6A demethylation, mainly including FTO and ALKBH5. (B) Readers recognize m6A methylation, including YTHDF1-3, YTHDC1-2, HNRNPC, HNRNPA2B1, eIF3, and IGF2BP1/2/3. (C) m6A modification plays a regulatory role in primary miRNA (pri-miRNA) to regulate the processing and maturation of miRNAs. (D) m6A modification plays a regulatory role in lncRNA to affect the RNA-RNA interaction function of lncRNA. (E) m6A modification plays a regulatory role in circRNA to promote its' translation.
Writers take S⁃adenosylmethionine (SAM) as a methyl donor and mediate m6A methylation modification of RNA. These complexes are mainly composed of methyltransferase-like 3 (METTL3), methyltransferase-like 14 (METTL14), Wilms tumor1-associating protein (WTAP), methyltransferase-like 16 (METTL16), RNA binding motif protein 15 (RBM15), and zinc finger CCCH domain-containing protein 13 (ZC3H13). METTL3 contains a SAM-binding region 55 that identifies potential m6A modification sites and mediates the transfer of methyl from SAM to this site. METTL14 can form a heterodimer with METTL3 and specifically promote METTL3 recognizing RNA substrates. METTL3 and METTL14 are located on nuclear speckles and their localization is dependent on WTAP. By binding to the METTL3-METTL14 dimer, WTAP enables the methyltransferase complex to rapidly recognize potential m6A modification sites and activate the METTL3/METTL14 complex 56. It has been reported that ZC3H13 can bind to WTAP and anchor the METTL3/METTL14 complex to the nucleus 57.
Erasers mediate the process of m6A demethylation, including the modulation of fat mass and obesity-associated gene (FTO) and AlkB homolog 5(ALKBH5). FTO oxidizes N-methyl at the m6A site to form hydroxymethyl groups 54, 58. ALKBH5, co-locating with nuclear speckerae in an RNaseA-sensitive manner, can directly catalyze the removal of methyl from 6-methylated adenosine and tends to demethylate specific m6A-modified single-strand RNA 59.
Readers recognize m6A methylation and act by specifically binding to the m6A-modified region or altering the RNA secondary structure to facilitate protein binding to RNA. Readers include YTH domain family (YTHDF) 1-3, YTH domain-containing proteins (YTHDC) 1-2, heterogeneous nuclear ribonucleoproteins (HNRNP), HNRNPC and HNRNPA2B1, eukaryotic translation initiation factor 3(eIF3), and insulin-like growth factor 2 mRNA-binding protein 1/2/3 (IGF2BP1/2/3). The role of YTHDF1-3 is mainly in the cytoplasm. YTHDF1 promotes the translation of m6A-labeled transcripts 60 and YTHDF2 promotes the degradation of m6A mRNA 61. The combination of YTHDF3 with YTHDF1 enhanced its pro-translational ability, and the combination with YTHDF2 promoted its pro-degradation ability. The role of YTHDC1-2 is mainly in the nucleus. YTHDC1 may modulate precursor miRNAs (pre-miRNAs) splicing factors to regulate RNA splicing 62, and YTHDC2 promotes mRNA translation 63. The HNPNPC family regulates the selective splicing and structural alterations in mRNA 64. HNRNPA2B1 interacts with the Di George Cirtical Region 8 (DGCR8) protein to promote pri⁃miRNA processing 65. hnRNPC affects the local secondary structure of mRNA and lncRNA 64. eIF3 binds to the m6A site in the 5 'UTR of mRNA and promotes cap-independent mRNA translation 66. IGF2BP1/2/3 increase the stability and translation efficiency of mRNA 67.
In recent years, m6A detection technology has developed rapidly, and a large number of m6A methylation sites have been identified by combining immunoprecipitation and high-throughput sequencing (meRIP-seq 68 and m6Aseq 69). However, these two methods cannot identify m6A methylation sites that are very close to each other or accurately identify the m6A site. Photo-crosslinking co-immunoprecipitation techniques (such as PA-m6A-seq 70 and m6A-CLIP 71) can achieve more accurate identification of m6A sites on the individual bases of RNA. In addition, the site-specific cleavage and radioactive-labeling followed by ligation-assisted extraction and thin-layer chromatography (SCARLET) method can detect single m6A sites with high accuracy 72. However, it is expensive to detect m6A sites individually, and detection after bioinformatics prediction can greatly improve research efficiency. At present, multiple databases are available for researchers to use (Table 1), such as the WHISTLE database (http://whistle-epitranscriptome.com) 73, SRAMP database (http://www.cuilab.cn/sramp/) 74, and HSM6AP database (http://lab.malab.cn/~lijing/HSM6AP.html) 75, which can be used to predict m6A sites. Verified m6A targets and potential m6A targets can be queried in the M6A2Target database (http://m6a2target.canceromics.org) 76, and WITMSG database (http://rnamd.com/intron/) can be used to predict m6A sites of introns 77. M6A sites in specific cell lines or tissue types can be queried in the REPIC database (https://repicmod.uchicago.edu/repic) 78. RMBase v2.0 database (http://rna.sysu.edu.cn/rmbase) 79 and iMRM (http://www.bioml.cn/XG_iRNA/home) 80 have also been used to predict methylation modification sites other than m6A. As m6A modification can be regulated by m6A regulatory proteins and affects a variety of physiological and pathological processes, databases integrating m6A functional annotations have emerged. RMBase v2.0 database (http://rna.sysu.edu.cn/rmbase) contains abundant information for exploring the relationship between RNA modification/miRNA binding and disease-associated single nucleotide polymorphisms (SNPs) 79. Met-DB v2.0 database (http://compgenomics.utsa.edu/MeTDB/) integrates m6A "Writer,” "Eraser", and "Reader" database, which facilitates the understanding of the function of m6A modification 81. The development of technologies for m6A detection and prediction has greatly promoted in-depth research.
Table 1.
m6A modification-associated databases
| Database | Websites | Characteristic | References |
|---|---|---|---|
| WHISTLE | http://whistle-epitranscriptome.com | Predicted m6A sites | 73 |
| SRAMP | http://www.cuilab.cn/sramp/ | 74 | |
| HSM6AP | http://lab.malab.cn/~lijing/HSM6AP.html | 75 | |
| M6A2Target | http://m6a2target.canceromics.org | Searched verified m6A targets and potential m6A targets | 76 |
| WITMSG | http://rnamd.com/intron/ | Predicted m6A sites of introns | 77 |
| REPIC | https://repicmod.uchicago.edu/repic | Searched m6A sites in specific cell lines or tissue types | 78 |
| RMBase v2.0 | http://rna.sysu.edu.cn/rmbase | a. Predicted methylation modification sites other than m6A b. Explored the relationship between RNA modification/miRNA binding and disease-associated SNPs |
79 |
| iMRM | http://www.bioml.cn/XG_iRNA/home | Predicted methylation modification sites other than m6A | 80 |
| Met-DB v2.0 | http://compgenomics.utsa.edu/MeTDB/ | Integrated m6A "Writer,” "Eraser", and "Reader" database, which facilitates the understanding of the function of m6A modification | 81 |
Function and role of m6A RNA methylation
M6A modification occurs mainly in mRNA and it affects splicing, nuclear export, translation, and degradation of mRNA. Alterations in m6A modification can regulate mRNA processing (Table 2). FTO regulates exon splicing of the adipogenesis regulator RUNX1T1 by modulating m6A levels around the splicing site, thus regulating differentiation 82. SR proteins are important regulators of alternative splicing. YTHDC1 promotes the RNA-binding ability of splicing factors SR proteins 3 (SRSF3) and inhibits that of SRSF10 to regulate mRNA splicing 62. YTHDC1 also interacts with SRSF3 and RNA nuclear export factor 1 to regulate mRNA nuclear export 83. The regulation of m6A modification in the degradation and translation of mature mRNA is another way to regulate gene expression. METTL3-mediated m6A modification of SOX2 mRNA increases transcription stability 84. YTHDF1 60 and IGF2BP1/2/3 are involved in regulating the translation of m6A-modified mRNA 67. YTHDC2 enhances translation efficiency and reduces the stability of its target mRNA by interacting with translation and decay mechanisms 85.
Table 2.
Regulation of mRNAs by m6A modifications
| Function | M6A regulatory proteins | Mechanism | References |
|---|---|---|---|
| mRNA processing | FTO | regulate exon splicing of the adipogenesis regulator RUNX1T1 by modulating m6A levels around the splicing site, thus regulating differentiation | 82 |
| YTHDC1 | promote the RNA-binding ability of SRSF3 and inhibit that of SRSF10 to regulate mRNA splicing | 62 | |
| interact with SRSF3 and RNA nuclear export factor 1 to regulate mRNA nuclear export | 83 | ||
| Degradation and translation of mature mRNA | METTL3 | mediate m6A modification of SOX2 mRNA to increase transcription stability | 84 |
| YTHDF1 | enhance translation efficiency of its target RNA | 60 | |
| IGF2BP1/2/3 | regulate the translation of m6A-modified mRNA | 67 | |
| YTHDC2 | enhance translation efficiency and reduce the stability of its target mRNA by interacting with translation and decay mechanisms | 85 |
M6A is widely present in various cells and affects cell differentiation, apoptosis, and other biological processes, as well as tumor development and other pathological processes (Table 3). Cell differentiation is a process by which cells acquire different structure and function during ontogeny, and selective gene expression occurs during this stage. The regulation of m6A in mRNA enables m6A to regulate gene expression, thus affecting cell differentiation and ontogeny. There are multiple binding sites of METTL3 on the transcripts of pluripotent genes in mouse embryonic stem cells. It has been reported that METTL3 knockout decreases the m6A level of transcripts of pluripotent genes, which could inhibit the differentiation ability of embryonic stem cells 86. FTO can downregulate the expression of ASB2 and retinoic acid receptor alpha (RARA) by reducing m6A levels in UTRs of transcripts, resulting in the inhibition of all-trans retinoic acid (ATRA)-mediated acute myeloid leukemia (AML) cell differentiation 87.
Table 3.
Biological functions and role of m6A regulatory proteins in human diseases
| Functions | m6A regulatory proteins | Mechanisms | References |
|---|---|---|---|
| Spermatogenesis disorders | METTL3 | Altered splicing of spermatogenesis-related genes, thus regulating spermatogonial differentiation | 89 |
| METTL3 and METTL14 | Regulation of spermatogonial stem cells and spermatogenesis disorders | 88 | |
| ALKBH5 | Regulated nuclear export of RNA and sperm malformation in mice | 90 | |
| Central nervous system diseases | METTL3 | Regulated mice cerebellar development | 91 |
| METTL14 | Regulated mice cerebral cortex development | 92 | |
| FTO | Regulated mice memory | 94 | |
| Cardiovascular diseases | METTL3 | Contributed to cardiac hypertrophy | 97 |
| Promoted the maturation of miR-34a, which in turn inhibits SIRT1 and promotes the formation of abdominal aortic aneurysm | 99 | ||
| FTO | Participated in reduced cardiomyocyte contractile function during heart failure | 98 | |
| Tumors | METTL3 | Promoted the maturation of miR-25-3p, thus activating the Akt-P70S6K pathway and promoting the initiation and development of PDAC | 100 |
| FTO | Promoted glycolysis of breast cancer cells through the PI3K/AKT pathway | 101 | |
| Upregulated PKM2 exression through the demethylation of PKM2, thereby regulating glucose metabolism of HCC | 102 |
M6A is closely related to spermatogenesis as well as the development and function of the central nervous system. It has been reported that the knockout of METTL3 and METTL14 results in the loss of spermatogonial stem cells and impaired spermatogenesis 88. Moreover, METTL3 knockout causes decreased m6A levels and altered splicing of spermatogenesis-related genes, leading to the downregulation of gene expression and the regulation of spermatogonial differentiation 89. A previous study reported that ALKBH5 deficiency in mice increased m6A levels and nuclear export of RNA, ultimately causing sperm malformation in mice 90. During the development of the central nervous system, METTL3 knockout can lead to decreased m6A in the cerebellum of mice and increased apoptosis of new small brain cells, leading to severe cerebellar hypoplasia 91. METTL14 knockout resulted in impaired cerebral cortex development in mice 92. M6A modification in the brain is also involved in cognitive functions such as learning and memory 93. Artificial deficiency of FTO in the dorsal hippocampus enhanced memory in mice 94. Notably, abnormal m6A modifications can contribute to the development of Alzheimer's disease 95. In addition, m6A modifications can regulate the circadian rhythm of metabolism 96.
Abnormal regulation of m6A gene expression may promote the occurrence of certain diseases. Changes in the expression of m6A play a regulatory role in the occurrence and development of cardiovascular diseases, such as cardiac hypertrophy, heart failure, and aortic aneurysm. Hypertrophic stimulation leads to increased METTL3-mediated m6A modification, which contributes to cardiac hypertrophy 97. In heart failure, FTO expression is reduced, leading to increased m6A levels and reduced cardiomyocyte contractile function. Decreased cardiomyocyte contractile function induced by ischemia can be alleviated by increasing FTO expression 98. M6A modification promotes the maturation of miR-34a, which in turn inhibits SIRT1 expression and promotes the formation of abdominal aortic aneurysms 99. The regulation of m6A modifications in tumors involves several aspects. Cigarette smoke induces the overexpression of METTL3 and upregulation of m6A in pri-miR-25 to promote its processing and maturation. miR-25-3p inhibits PH domain leucine-rich repeat protein phosphatase 2 (PHLPP2) expression, which in turn activates the Akt-P70S6K pathway and promotes the initiation and development of pancreatic ductal adenocarcinoma (PDAC) 100. The energy metabolism of tumor cells differs from that of normal cells. Aerobic glycolysis promotes adaptation and rapid proliferation of tumor cells in the hypoxic microenvironment. FTO overexpression in breast cancer cell lines promotes glycolysis through the phosphatidylinositol‑4,5‑bisphosphate 3‑kinase (PI3K)/protein kinase B (Akt) pathway 101. FTO upregulates the expression of pyruvate kinase M2 (PKM2) through the demethylation of PKM2 mRNA in HCC, thus regulating glucose metabolism 102. Abnormal modification of m6A in TME can modulate tumor immune escape. A previous study analyzed the mRNA expression profiles of 1,938 gastric cancer (GC) samples and established an m6A scoring system based on the status of 21 m6A regulators 103. An increased mutation load and immune activation were observed with low m6A scores. Effective immune infiltration was not observed with a high m6A score. In fact, the modification of m6A is involved in the entire process of tumorigenesis and development, including the regulation of tumor cell proliferation, angiogenesis, metastasis and invasion, immune escape, and other aspects by promoting the formation of TME.
Regulation of ncRNAs by m6A modifications
M6A modification does not only occur in the mRNA that can encode proteins, but also in ncRNAs, and it plays an important regulatory role in the production and function of ncRNAs (Fig. 2C-E; Table 4). ncRNA refers to functional RNA molecules that cannot be translated into proteins, including miRNAs, circRNAs, lncRNAs, transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), small interfering RNAs (siRNAs), PIWI-interacting RNAs (piRNAs), small nuclear RNAs (snRNAs), and small nucleolar RNAs (snoRNAs) 4.
Table 4.
Regulation of ncRNAs by m6A modifications
| ncRNA | M6A regulatory proteins | Mechanisms | References |
|---|---|---|---|
| miRNAs | METTL3 | a. Marked pri-miRNA through m6A b. Increased Dicer splicing of pre-miRNAs |
106 |
| HNRNPA2B1 | Binded to m6A in pri-miRNA to recruit DGCR8, thereby promoting the maturation of miRNAs | 65 | |
| LncRNAs | HNRNPC and HNRNPG | Binded RNA sequences surrounding m6A in lncRNA MALAT1 | 64, 108 |
| — | Played a role in the lncRNA-miRNA interaction, thereby influencing miRNA level | 109 | |
| circRNAs | METTL3 and METTL14 | Enhanced m6A-driven circRNA translation | 110 |
| FTO | Inhibited m6A-driven circRNA translation | ||
| YTHDF3 and eIF4G2 | Played key roles in m6A-driven circRNA translation | ||
| YTHDF2 | Differentiated endogenous circRNA from exogenous circRNA and regulate innate immunity | 111, 112 |
miRNAs are non-coding single-stranded small RNAs (18-24 nucleotides in length), which regulate gene expression at the post-transcriptional level through the formation of an RNA-induced silencing complex (RISC) 104. The generation and maturation of miRNAs are regulated by m6A. In the nucleus, DNA is first transcribed into primary miRNAs (pri-miRNAs), which are then processed into precursor miRNAs (pre-miRNAs) by a microprocessor complex containing DGCR8 and DROSHA. Pre-miRNAs are then cleaved using Dicer into mature single-stranded miRNAs in the cytoplasm 105. In this process, METTL3 marks pri-miRNA through m6A, and HNRNPA2B1 binds to m6A in pri-miRNA to recruit DGCR8; this enables DGCR8 to recognize and bind to specific substrates, thereby promoting the maturation of miRNAs 65, 106. METTL3 can also promote miRNA biosynthesis by increasing Dicer splicing of pre-miRNAs.
LncRNAs are non-coding RNAs with more than 200 nucleotides that play a regulatory role in chromosomal inactivation and modification, transcription, shearing, translation, and protein activity. LncRNAs can also adsorb miRNAs through sequence complementation and inhibit the targeted regulatory effect of miRNAs on mRNA 107. M6A modification in lncRNAs may influence RNA-protein interactions. M6A in lncRNA may make the surrounding RNA sequences more likely to bind HNRNPC and HNRNPG 64, 108. It also plays a role in the lncRNA-miRNA interaction, thereby influencing miRNA level 109.
CircRNA is formed by the covalent binding of the 3 'end and 5' end of precursor mRNA after reverse splicing and can participate in biological processes, such as the regulation of miRNA expression and gene transcription. M6A can promote circRNA translation, and M6A in circRNA can act as an internal ribosomal entry site (IRES) for cap-independent translation 110. METTL3 and METTL14 enhance m6A-driven circRNA translation, whereas FTO-mediated m6A demethylation inhibits this translation process, with YTHDF3 and eIF4G2 playing key roles in this process. In addition, m6A also plays a role in circRNA immunity. Endogenous circRNA binding to YTHDF2 cannot activate retinoic acid inducible gene I (RIG-I), whereas exogenous circRNAs lacking m6A modification can activate the RIG-I pathway, leading to interferon production and induction of innate immunity 111, 112. This indicates that m6A can differentiate endogenous circRNAs from exogenous circRNAs and regulate innate immunity.
Regulation of m6A modifications by noncoding RNAs
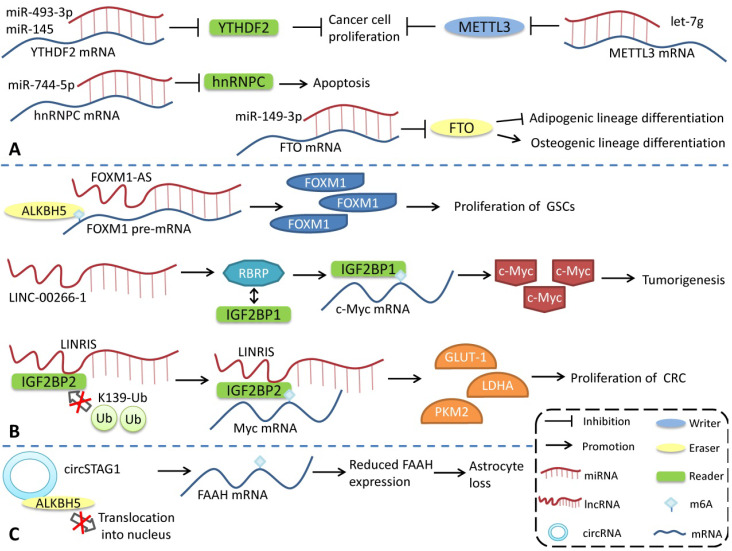
M6A modification can regulate the generation and function of ncRNAs. Conversely, ncRNAs, as important functional regulatory molecules, can also influence m6A modification by interacting with or regulating m6A regulatory proteins and participating in the regulation of various physiological and pathophysiological processes (Figure 3). miR-493-3p and miR-145 can downregulate YTHDF2 mRNA, alter the intracellular m6A level, and ultimately inhibit the proliferation of prostate 113 and liver cancer cells 114. However, miR-744-5p can silence HNRNPC, which influences miR-21 expression and Akt phosphorylation and ultimately promotes apoptosis 115. miR-149-3p binds to the 3ʹUTR of FTO mRNA to downregulate its expression, inhibit adipogenic lineage differentiation, and enhance osteogenic lineage differentiation 116. miR-141 regulates IGF2BP2 in PDAC, and IGF2BP2 activates the PI3K-Akt pathway in vivo and promotes pancreatic cancer growth 117. miRNA let-7g inhibits breast cancer development by targeting the 3'-UTR of METTL3 mRNA 118. miR-600 can also downregulate METTL3 and inhibit the proliferation and migration of lung cancer cells 119.
Figure 3.
Regulation of m6A modifications by ncRNAs. ncRNAs can influence m6A modification by interacting with or regulating m6A regulatory proteins. (A) miRNA regulates the expression of m6A regulatory proteins. (B) lncRNA affects m6A modification. (C) circRNA interacts with m6A regulatory proteins to regulate m6A modification.
In contrast to the way miRNAs directly bind to m6A mRNA to regulate its expression, lncRNAs modulates m6A by interacting with m6A regulatory proteins. LncRNA LINC-00266-1-encoded RNA-binding regulatory peptide (RBRP) interacts with IGF2BP1 to enhance the recognition of m6A on c-myc mRNA, enhance the stability and expression of c-myc mRNA, and promote tumorigenesis 120. The long intergenic non-coding RNA LINRIS maintains the stability of IGF2BP2 by blocking K139 ubiquitination and promoting colorectal cancer (CRC) proliferation 121. LINC01234 interacts with HNRNPA2B1, leading to the recruitment of DGCR8 and promoting the processing and maturation of miR-106b-5p, which inhibits CRY2 and promotes the growth of non-small cell lung cancer (NSCLC) cells 122. FOXM1-AS is an antisense lncRNA of Forkhead box M1 (FOXM1) that promotes the interaction of ALKBH5 with newborn FOXM1 transcripts, regulates m6A modification and FOXM1 expression, and regulates the proliferation of glioblastoma stem cell-like cells (GSCs) 123. LncRNA GAS5-AS, the antisense lncRNA of growth arrests specific transcript 5 (GAS5), can also interact with ALKBH5 to adjust the m6A modification of GAS5 to enhance its stability 124.
The regulation of circRNAs by m6A modification has also been reported. circSTAG1 can bind ALKBH5, inhibit its entry into the nucleus, upregulate the m6A modification level of fatty acid amide hydrolase (FAAH) mRNA, and affect its stability and expression, resulting in astrocyte dysfunction 125. However, compared with miRNA and lncRNA, the regulation of m6A modification by circRNA has a broader research scope. Notably, some m6A regulatory proteins have multiple functions, and the effects of m6A regulatory proteins are not necessarily entirely attributable to the alterations in the level of m6A modification. However, more detailed studies are needed to identify the role of different pathways in various physiological and pathological processes.
In addition, m6A modification can not only regulate the function of exosomes 126, but exosomal ncRNAs can also enter target cells and influence diseases occurrence by regulating the expression of m6A-related proteins 127-129. Yuan et al. demonstrated that human umbilical cord mesenchymal stem cell (hucMSC)-derived exosomal miR-26a-5p can enter the nucleus pulposus (NP) cells to inhibit pyroptotic NP cell death by targeting the METTL14/NLRP3 axis, thereby suppressing the progression of intervertebral disc degeneration (IVDD) 127.
Role Of m6A modification and ncRNAs in time
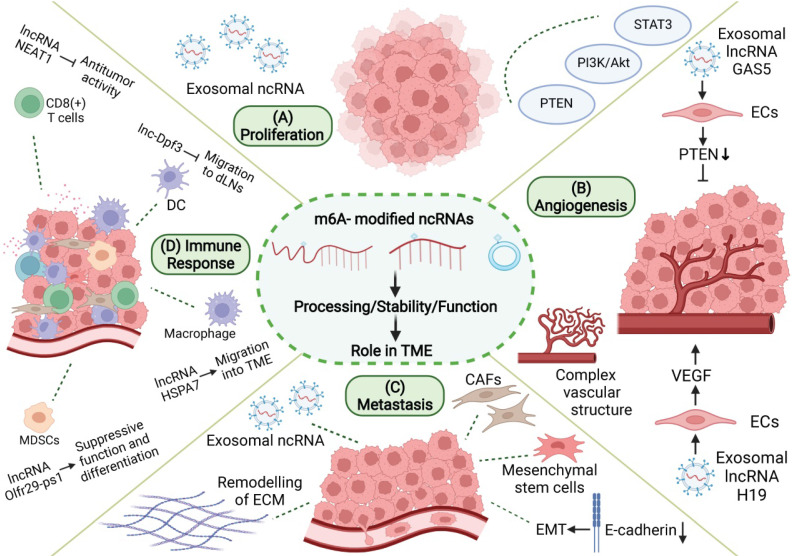
During tumorigenesis, tumor cells interact closely with stromal cells and immune cells in the microenvironment, jointly transforming the microenvironment into a site conducive to the growth, invasion, and metastasis of tumor. m6A-related ncRNA, as an important means of intracellular regulation of gene expression and functional status, as well as intercellular communication, can promote the formation and maturation of TME and play an important role in proliferation, angiogenesis, invasion, metastasis, immune escape, and other processes in tumor (Figure 4).
Figure 4.
Role of m6A modification and ncRNAs in TME. m6A-related ncRNAs are involved in the regulation of proliferation, angiogenesis, invasion and metastasis, and immune escape in TME. (A) M6A-related ncRNAs can affect tumor proliferation by regulating proliferation-related genes and signaling pathways, and can be transmitted in TME through exosomes. (B) M6A-related ncRNAs regulate tumor angiogenesis by regulating pro-angiogenic molecules, such as VEGF, and are involved in angiogenesis patterns of large vessels and complex vascular structures. (C) Tumor metastasis is related to EMT in tumor cells and the dissolution and remodeling of ECM. M6A-associated ncRNAs can also be delivered through exosomes, while regulating stromal cells in TME to promote metastasis. (D) M6A-related ncRNAs are involved in the recruitment, differentiation, and functional expression of immune cells in TME, and they promote the immune escape of tumor cells.
Mediation of tumor proliferation
Cell proliferation involves complex signal transduction. Uncontrolled proliferation of tumor cells is mostly related to the abnormality of proliferation-related genes and impaired signaling pathways involving cellular processes, such as oncogene activation, tumor suppressor gene inactivation, apoptosis resistance, and metabolic reprogramming 130, 131. However, the malignant proliferation of tumors is related to tumor cells, and TME can also enhance this abnormal proliferation. In various malignant tumors, CAF in TME can promote tumor proliferation through the synthesis and secretion of TGF-β, fibroblast secretory protein 1 (FSP1), SDF-1, and other growth factors 132. Mast cells can promote tumor proliferation through direct contact with tumor cells or the secretion of factors such as IL-17A 133.
M6A-related ncRNAs also play a regulatory role in tumor proliferation. M6A modification of ncRNAs can influence the expression of ncRNA by changing its stability or maturation process and regulating the expression of proliferation-related genes or signal pathways, thus promoting or inhibiting tumor proliferation. METTL3 promotes m6A-dependent miR-221/222 maturation and downregulates phosphatase and tensin homolog (PTEN), thereby promoting the proliferation of bladder cancer cells 134. Deoxycholic acid (DCA) levels are significantly decreased in gallbladder cancer (GBC). DCA can reduce the m6A level of pri-miR-92b, thereby reducing its expression and upregulating PTEN and consequently inhibiting tumor proliferation mediated by the PI3K/AKT pathway 135. Moreover, m6A-related ncRNAs also play an important regulatory role in TME, which may serve as a bridge between tumor proliferation and TME formation. The signals of abnormal proliferation in a single tumor cell may also be transmitted to other tumor cells by m6A-associated ncRNAs through TME, thereby promoting the spread of malignant phenotypes. The links between these three factors have not been elucidated. ALKBH5 can mediate m6A demethylation of lncRNA PVT1 in osteosarcoma, thus upregulating PVT1 and promoting osteosarcoma cell proliferation 136. PVT1 can also promote the transport and fusion of multivesicular bodies (MVB) to the plasma membrane, thus promoting exosome secretion by pancreatic cancer cells 137. Exosomes are important intercellular communication molecules that can promote information transmission between tumor cells, and between tumor cells and the tumor matrix, thus promoting the formation of TME and tumor development. These results suggest that m6A-related ncRNAs may be involved in tumor development by regulating the formation of tumor cells and TME. Adipocyte exosomal lncRNAs in TME of multiple myeloma (MM) inhibit MM cell apoptosis and support tumor growth 138. m6A modifications enhance the maturation of miR-181d. CAF-derived exosomal miR-181d-5p increases tumor growth in CRC 139. Breast cancer-derived exosomal miR-105 facilitates metabolic reprogramming of CAFs, which are induced to provide energy for adjacent cancer cells to support tumor growth 140. IGF2BP2 promotes the stability and expression of lncRNA ZNFX1 antisense RNA 1 (ZFAS1) in an m6A-dependent manner 141. In esophageal squamous cell carcinoma (ESCC), ZFAS1 can shuttle between tumor cells in the form of exosomes and act as competing endogenous RNAs (ceRNAs) to downregulate miR-124, thereby upregulating STAT3 and ultimately inhibiting cellular apoptosis and promoting proliferation, migration, and invasion 142. These results suggest that m6A-related ncRNA-mediated tumor proliferation may spread through exosomes in TME and increase tumor malignancy. In conclusion, tumor cells are closely related to other components of TME, and m6A-related ncRNAs can simultaneously regulate tumor proliferation and TME. Further studies are needed to confirm the potential crosstalk between the two factors.
Mediation of tumor angiogenesis
Tumor tissues require abundant blood flow to provide nutrients and excrete metabolites. In the process of tumor tissue growth and enlargement, the normal blood vessels of the original tissue are insufficient to support further growth. At this time, the components of TME can establish new blood circulation in the tumor tissue through complex signal communication. The rapid growth of tumor cells leads to a local anoxic microenvironment with metabolite accumulation, which promotes the formation of angiogenic mimicry and increases blood supply by promoting ECM remodeling and inducing the transformation of cancer stem cells (CSC) into endothelial phenotypes 143. In addition, CAFs and TAMs in TME can also secrete angiogenic factors, such as VEGF, and cytokines, such as interleukin and free ncRNAs, into TME to activate the angiogenesis signaling pathway and promote tumor angiogenesis 144, 145.
M6A-related ncRNA is one of the components released by tumor cells into TME to promote angiogenesis and plays a regulatory role in the tumor angiogenesis microenvironment. MiR-155 can target FTO and upregulate m6A levels in clear cell renal cell carcinoma (RCC) 146. However, melanoma-derived exosome miR-155 can be delivered to fibroblasts to downregulate SOCS1 and then activate the JAK2/STAT3 pathway, leading to upregulated VEGFa, FGF2, and MMP9 in the fibroblasts, enhanced reprogramming of fibroblasts into CAF, and improved angiogenesis promotion capacity 147. M6A modification can regulate the expression of H19 and miRNA675 148. H19 is the precursor lncRNA of miRNA675, both of which regulate angiogenesis. MiRNA675-5p is involved in angiogenesis in anoxic microenvironments 149. H19 is abundant in the exosomes secreted by CD90+ HCC cells. Through the adhesion of CD90 to human umbilical vein endothelial cells (HUVECs), exosomes can invade endothelial cells, deliver H19 to target cells, and stimulate angiogenesis through the synthesis and release of VEGF 150. Vir-like m6A methyltransferase associated (VIRMA) is a regulatory component of m6A, and its knockout can reduce the stability and expression of lncRNA CCAT1 through decreased m6A level 151. Pancreatic cancer (PC) cell-derived exosomes can transport CCAT1 to HUVEC. CCAT1 upregulates high mobility group A1 (HMGA1) expression through competitive binding of miR-138-5p and enhances angiogenesis in HUVEC 152. YTHDF3 promotes the degradation of lncRNA GAS5 in an m6A-dependent manner 153. GAS5 is expressed at low levels in human lung cancer tissues, lung cancer cells, and cell culture supernatant exosomes. The overexpression of GAS5 in lung cancer cells can inhibit proliferation and tube formation in HUVECs in the form of exosomes, thereby affecting angiogenesis. The mechanism involves the regulation of PTEN expression, as well as PI3K and AKT phosphorylation through the "sponging" of miR-29-3p 154. Therefore, tumor cells can increase the levels of pro-angiogenic m6A-related ncRNAs and decrease those of the anti-angiogenic m6A-related ncRNAs in secreted exosomes, thus regulating the angiogenesis process in TME. This provides a new research idea for anti-angiogenesis in tumor research. M6A modification of pri-miR-17-92 promotes its processing and maturation 155. MiR-20a, a member of the miR-17-92 cluster, is associated with angiogenesis patterns in large vessels and complex vascular structures in breast cancer 156. Tumor angiogenesis is an important event in TME, and changes in angiogenesis patterns may affect nutrient supply to various cells in TME, thus affecting their functional status. In addition, m6A modification can promote the splicing of the precursor miR-143-3p to promote its maturation. MiR-143-3p increases VEGFA expression in lung cancer cells by downregulating vasohibin-1 (VASH1) and promoting angiogenesis 157. miR-143-3p is highly expressed in the EVs of osteosarcoma cells and involved in the regulation of TME 158. The specific role of miR-143-3p in TME is still unclear, and further research is needed to determine if miR-143-3p can also affect tumor angiogenesis by regulating TME. In conclusion, the multiple roles of m6A-related ncRNAs in TME may involve a regulatory network that modulates angiogenesis and tumor progression through multiple pathways.
Mediation of tumor metastasis
Tumor metastasis is closely related to EMT as well as the adhesion, invasion, and migration of tumor cells 159, 160. The reduction in adhesion molecules on the surface of tumor cells allows cells to separate from each other, facilitating cell mobility. The ECM is a key regulator of tumor invasion and metastasis. Tumor or stromal cells can produce proteases (such as MMP) to dissolve ECM components and promote tumor cell migration. EMT is the transformation of tumor cells from an epithelial to a more aggressive mesenchymal phenotype, which promotes tumor metastasis. During EMT, E-cadherin and other cell adhesion proteins are gradually reduced, while N-cadherin, vimentin, and other mesenchymal marker proteins are enriched. Tumor metastasis is closely related to the enhanced invasion and metastatic ability of tumor cells as well as changes in TME. TME can promote tumor metastasis by promoting EMT and the invasion ability of tumor cells, promoting ECM remodeling and the formation of a premetastatic niche 161.
M6A-related ncRNAs play a significant regulatory role in many aspects of tumor metastasis. First, m6A-related ncRNAs can influence tumor invasion and metastasis by regulating the expression of metastasis-related genes. M6A modification can promote the splicing and maturation of miR-143-3p, while miR-143-3p can downregulate E-Cadherin and upregulate vimetin, MMP2, and MMP-9 in lung cancer cells to promote the occurrence of EMT 157. In nasopharyngeal carcinoma, m6A modification can enhance the stability of lncRNA FAM225A. As ceRNA, FAM225A inhibits the expression of miR-590-3p and miR-1275 and upregulates downstream integrin β3 (ITGB3), promoting proliferation, migration, and invasion of cancer cells through the FAK/PI3K/Akt pathway 162. In addition, future studies need to investigate if m6A-related ncRNAs regulate tumor metastasis by modulating TME. Small extracellular vesicles (sEV) derived from gastric cancer (GC) containing miR-151a-3p can be absorbed by hepatic Kupffer cells (KCs), and sEV-miR-151a-3p targets YTHDF3 and downregulates small ubiquitin-related modifiers 1 (SUMO1) translation in an m6A-dependent manner, thereby inhibiting SP3, inducing TGF-β1 expression, and promoting the formation of a local liver metastasis microenvironment through the activation of the SMAD2/3 pathway 163. METTL3 promotes the maturation of pri-miR-320b in an m6A-dependent manner, and miR-320b promotes EMT by downregulating PDCD4 and can be included in exosomes to promote lymphangiogenesis, thereby promoting ESCC metastasis 164. Breast cancer-derived exosomal miR-122 inhibits the glycolytic enzymes pyruvate-kinase-2 (PKM2) and GLUT1 to reduce glucose uptake, ultimately enhancing tumor migration 165. LncRNA X inactivation-specific transcript (XIST) is misexpressed in a variety of tumors and can regulate the malignant phenotype of tumors. YTHDF2 recognizes m6A modifications in XIST and mediates their degradation 166. The deletion of XIST in breast cancer can promote the phenotypic transformation of microglia through the transport of miR-503 exosomes to microglia, leading to local immunosuppression and promoting brain metastasis in breast cancer 167. ALKBH5 can upregulate lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) by mediating m6A demethylation, thereby influencing the expression of enhancer of zeste homolog 2 (EZH2) and inhibiting the invasion and metastasis of GC cells 168. Bone metastases from prostate cancer are typically osteogenic. NEAT1 can be transferred to human bone marrow-derived mesenchymal stem cells (hBMSCs) through exosomes secreted by prostate cancer cells, thereby upregulating RUNX2, promoting osteogenic differentiation 169. and participating in the formation of the local bone metastasis microenvironment. METTL3 and YTHDF3 increase the stability of MALAT1 in an m6A-dependent manner, and MALAT1 acts as ceRNA to "sponge" miR-1914-3p, thereby upregulating YAP and promoting NSCLC invasion and metastasis 170. In a GC study, MALAT1 increased the accumulation of sequestosome1 (SQSTM1) in tumor cells, which in turn activated NF-κB and increased the expression of IL-6. IL-6 promotes paracrine transformation of CAF 171. CAF secrete various cytokines to promote tumor metastasis. This suggests that m6A-related ncRNAs can not only change the gene expression of tumor cells to enhance their ability to invade and metastasize but also promote tumor metastasis by inducing the formation of TME. Tumor cells and TME carry out complex information transmission to synergistically promote tumor invasion and metastasis, and m6A-related ncRNAs play an important mediating role in this process. However, further research is needed to gain clearer understanding of the regulatory network of TME in different tumor types.
Mediation of tumor immune response
Under normal circumstances, the immune surveillance function of the immune system can promptly detect and eliminate tumor cells caused by gene mutations; however, the formation of TME can protect tumor cells from the immune system. In TME, the recruitment, differentiation, and functional expression of immune cells are precisely regulated, forming a low anti-tumor immune response microenvironment to promote the immune escape of tumor cells. M6A modification plays an important role in maintaining the normal function of immune cells, and changes in m6A modification can affect the formation of an immunosuppressive microenvironment in tumors 172. In DC, METTL3-mediated m6A modification promotes the expression of the costimulatory molecules, CD40 and CD80, and the proinflammatory cytokine IL-12, and promotes DC activation and maturation 173. YTHDF1 recognizes the m6A modification of lysosomal protease transcription and promotes its expression, which in turn blocks cross-presentation of tumor antigens by DC and antigen-specific activation of CD8+ T cells 174. METTL3 can also improve the stability of signal transducer and activator of transcription 1 (STAT1) mRNA by mediating m6A modifications, upregulating STAT1, and driving the polarization of M1 macrophages 175. FTO knockout inhibits NF-κB signaling and decreases the stability of STAT1 and PPAR-γ mRNA, thus impeding macrophage activation 176. In mouse models, METTL3 deficiency upregulated the mRNA expression of suppressor of cytokine signaling (SOCS) family by modulating mRNA m6A modification, thus inhibiting IL-7-mediated STAT5 activity and the homeostasis, proliferation, and differentiation of T cells 177. METTL14/YTHDF1 knockdown in GC cells downregulated IFN-α, -β, and-γ transcription levels 178. As m6A modification is ubiquitous in cells, its changes often involve alterations in multiple genes and signaling pathways, providing clues for subsequent in-depth studies.
M6A-related ncRNAs can regulate the migration, differentiation, and functional states of various immune cells, making the regulatory network of the tumor immune microenvironment more detailed and precise. The lncRNA Olfr29-ps1 can interact with miR-214-3p to inhibit its expression, thereby promoting the immunosuppressive function and differentiation of mononuclear MDSCs. In granulocyte-macrophage colony-stimulating factor (GM-CSF) combined IL6-induced MDSCs, Olfr29-ps1 was modified by m6A, which enhanced the regulation of Olfr29-ps1 on MDSCs 179. The lncRNA HSPA7 is significantly overexpressed in glioblastoma (GBM) tissues, and m6A modification of HSPA7 promotes its expression. HSPA7 promotes macrophage migration to the GBM TME by activating the YAP1-LOX axis and can promote an immunosuppressive phenotype and inhibit the anti-tumor immune response 180. In colorectal cancer, METTL3 upregulated the expression of miR-1246 by promoting the maturation of pri-miR-1246 through methylation 181. However, exosomes shed by mutant p53 colon cancer can carry miR-1246 to the microenvironment containing macrophages, where it reprogrammes macrophages and recruits immunosuppressive T cells to promote tumor development 182. DC migration to draining lymph nodes (DLNs) plays an important role in the initiation of anti-tumor T cells and adaptive immunity. The activation of chemokine receptor 7 (CCR7) can prevent lnc-Dpf3 degradation by removing its m6A modification. Lnc-Dpf3 is upregulated after recognition by YTHDF2. Upregulated lnc-Dpf3 can directly bind to HIF-1α protein and inhibit the transcription of Ldha, an HIF-1α dependent glycolysis gene, thus inhibiting glycolysis metabolism in DCs and CC7-mediated DC migration to DLNs, which plays an immunosuppressive role in tumors 183. In glioblastoma, ALKBH5 eliminates m6A-methylated lncRNA NEAT1 to stabilize transcripts and promote relocalization of SFPQ, thus facilitating the formation of immunosuppressive TME by upregulating CXCL8/IL8 184. M6A modification of circIGF2BP3 upregulates its expression by promoting its reverse splicing and cyclization. circIGF2BP3 upregulates PKP3 through competitive binding of miR-328-3p and miR-3173-5p, thereby inhibiting PD-L1 ubiquitination and promoting the immune escape of NSCLC cells 185. As immunotherapy has become a widely used anti-tumor therapy, these studies undoubtedly provide a new research direction for precise tumor therapy.
Potential clinical application of m6A-modified ncRNAs in cancers
m6A-related ncRNAs as potential biomarker
M6A-related ncRNAs are closely related to tumorigenesis and development, which makes them a biomarker for tumor diagnosis and prognosis evaluation and a new target for the development of anti-tumor drugs. M6A-related regulatory proteins and the level of m6A modification in peripheral blood have shown potential as tumor biomarkers.
The level of RNA m6A modification in the peripheral blood of patients with GC can be used as a marker for GC screening, and the diagnostic value of m6A can be improved by combining it with other tumor markers, such as carcinoembryonic antigen (CEA) or m6A demethylases, such as ALKBH5 and FTO. The downregulation of m6A after surgery makes it a possible marker for follow-up 186. YTHDF3 and VIRMA are significantly overexpressed in testicular seminoma and may be new markers for the identification of testicular germ cell tumor subtypes 187.
However, m6A regulatory proteins often simultaneously regulate the m6A modification of multiple target RNAs, and different target RNAs play different roles in tumor cells. Therefore, it may not be accurate enough to use m6A regulatory proteins as biomarkers to screen for or predict tumor prognosis. M6A-associated ncRNAs may represent a new class of tumor markers (Table 5). For example, YTHDC1 promotes the export of circNSUN2 from the nucleus to the cytoplasm in an m6A-dependent manner. In CRC, circRNA circNSUN2 is upregulated in the serum and metastatic liver tissue of patients with liver metastasis, and it is associated with poor prognosis 188. M6A level of lncRNA NEAT1-1 is a poor prognostic factor for prostate cancer, and high m6A levels are associated with bone metastasis 189. M6A modification can enhance the stability of lncRNA LNCAROD, and LNCAROD is associated with shortened overall survival of head and neck squamous cell carcinoma (HNSCC) 190. In epithelial ovarian cancer (EOC), m6A modification upregulates lncRNA RHPN1-AS1 by increasing its transcriptional stability, whereas high RHPN1-AS1 levels are significantly associated with distant metastasis and death 191. Combining multiple m6A-related ncRNAs to build risk models provides guidance for clinicians in making improved diagnosis and treatment decisions. A new prognostic index, m6AlRsPI, was constructed based on two m6A-modified hub lncRNAs in kidney renal clear cell carcinoma (KIRC). High m6AlRsPI is associated with poor prognosis, and its area under the receiver operating characteristic curve (AUC) for predicting 3-year and 5-year survival is 0.760 and 0.677, respectively 192. In metastatic cutaneous melanoma, an m6A-associated lncRNA model (m6A-LncM) containing 24 lncRNAs was constructed, most of which had potential m6A modification sites. The AUC for predicting 3-year, 5-year, and 10-year survival were 0.778, 0.813, and 0.828, respectively 193. m6A-LPS, which is composed of 12 m6A-associated lncRNAs, is an independent prognostic factor of breast cancer, with an AUC of 0.776, and these 12 lncRNAs are associated with clinicopathological features, such as age, sex, as well as T, M, and N stage of patients with breast cancer 194.
Table 5.
Correlation between m6A-related ncRNAs and clinical pathological characterizations of tumor patients
| Tumor types | Sample type | M6A-related ncRNAs | Expression | Biomarker type | References |
|---|---|---|---|---|---|
| CRC | Serum and tissues | circNSUN2 | Upregulated | CircNSUN2 is associated with poor prognosis. | 188 |
| Prostate cancer | Tissues | lncRNA NEAT1-1 | Upregulated | High m6A levels are associated with bone metastasis. | 189 |
| HNSCC | Tissues | lncRNA LNCARD | Upregulated | LNCAROD is associated with shortened overall survival of HNSCC. | 190 |
| EOC | Tissues | lncRNA RHPN1-AS1 | Upregulated | High RHPN1-AS1 levels are significantly associated with distant metastasis and death | 191 |
| KIRC | Tissues | LINC01820 and LINC02257 | Upregulated | High levels are associated with poor prognosis. | 192 |
| Pancreatic cancer | Serum | Serum miR-17-5p m6A level | Methylation level increased | Serum miR-17-5p m6A level is a potential marker for the early diagnosis of pancreatic cancer. | 195 |
| Glioma | Tissues | hsa_circ_0127664 and hsa_circ_0008362 | Methylation level increased | hsa_circ_0127664 and hsa_circ_0008362 could be used as potential diagnostic markers. | 196 |
| NSCLC | Tissues | Exsomal miR-4443 | Upregulated in CIS-R NSCLC | miR-4443 is expected to be a marker of cisplatin response in NSCLC. | 128 |
M6A-related ncRNAs can be used to predict tumor prognosis and also serve as markers for the early diagnosis of tumors and markers related to the tumor immune microenvironment. Serum miR-17-5p methylation levels increase in patients with early pancreatic cancer, compared with healthy controls, which makes it a potential marker for the early diagnosis of pancreatic cancer 195. The AUC of hsa_circ_0127664 was 0.8044 in diagnostic tests of patients with high-grade glioma (HGG) and low-grade glioma (LGG). The AUC of hsa_circ_0008362 in the diagnostic tests of HGG and normal tissues was 0.9467, suggesting that these circRNAs could be used as potential diagnostic markers. Hsa_circ_0127664 and hsa_circ_0008362 have relatively high levels of m6A modification, but their specific significance is unclear 196. M6A-related ncRNAs have also shown potential in the large-scale detection of cancer, and a serum m6A-miRNAs diagnostic signature has high accuracy and sensitivity, which should make it a low-cost, minimally invasive novel biomarker 197. In addition, because m6A-related ncRNAs are closely related to TME and participate in the formation and dynamic regulation of TME, some m6A-related ncRNAs are expected to become potential markers or therapeutic targets related to TME, opening a new path for the accurate diagnosis and effective treatment of tumors. Some studies constructed an m6A-related ncRNA model and combined it with the analysis of immune cells, immune-related molecules, or pathways, and some m6A-related lncRNAs that may be associated with the tumor immune microenvironment were predicted in colorectal adenocarcinoma 198, lung adenocarcinoma 199, renal clear cell carcinoma 200 and breast cancer 194, which can be used to predict immunotherapy response. Several m6A-associated lncRNAs, including LINC00342, are positively correlated with PD-1 expression in renal clear cell carcinoma 200 which could be a potential target for enhanced efficacy of immune checkpoint inhibitors against PD-1. As one of the important mediators of signal transduction in TME, m6A-related ncRNAs in exosomes are also expected to become markers of TME. MiR-4443 targets METTL3 in NSCLC, thereby upregulating ferroptosis suppressor protein 1 (FSP1) expression in an m6A-dependent manner, inhibiting cisplatin-induced iron death, and promoting tumor proliferation. Cisplatin-resistant (CIS-R) tumors release exosomes containing miR-4443, which are transferred to cisplatin-sensitive (CIS-S) cells to confer cisplatin resistance. The expression level of exosomal miR-4443 in CIS-S NSCLC is approximately one-third of that in normal lung tissue, while that in CIS-R NSCLC is approximately 1.5-fold higher than that in normal lung tissue. Therefore, miR-4443 is expected to be a marker of cisplatin response in NSCLC 128.
m6A-modified ncRNAs as therapeutic targets
The important role of m6A-related ncRNAs in TME makes them a new target for anti-tumor therapy. Because m6A methylation and demethylation are reversible processes, it is possible to change the expression of m6A-modified ncRNA by targeting m6A regulatory proteins, thus exerting an anti-tumor therapeutic effect. R-2-hydroxyglutarate (R-2HG) can inhibit FTO by increasing m6A levels and supressing leukemia cell proliferation. R-2HG combined with chemotherapy had a synergistic anticancer effect in a mouse leukemia model 201. Meclofenamic acid (MA) is another FTO inhibitor 202, and its derivative, MA2, upregulates m6A levels in glioblastoma stem cells. In a glioblastoma mouse model, intratumoral injection of MA2 inhibited tumor growth and extended survival 203. However, the unique advantages and disadvantages of ncRNA in animal and clinical trials can provide a reference for the development of m6A-related ncRNA-targeted drugs. The knockdown of lncRNA MALAT1 using an antisense oligonucleotide technique in a mouse breast cancer model resulted in decreased tumor growth and reduced metastasis 204. MRX34 is an analog of tumor suppressor miR-34a and significantly inhibits tumor growth in mouse models 205. In phase I clinical trials in patients with advanced solid tumors, partial responses (PR) or stable disease (SD) were achieved in some patients 206, 207. but further studies are needed to reduce immune-related toxicity of miRNA 207. Intratumoral injection of circNRIP1 siRNA inhibited tumor growth in a GC mouse model 208. In addition, a circRNA vaccine against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induced effective immune responses in both mouse and monkey models 209. However, it is not clear if circRNA vaccines can be used to induce anti-tumor immunity.
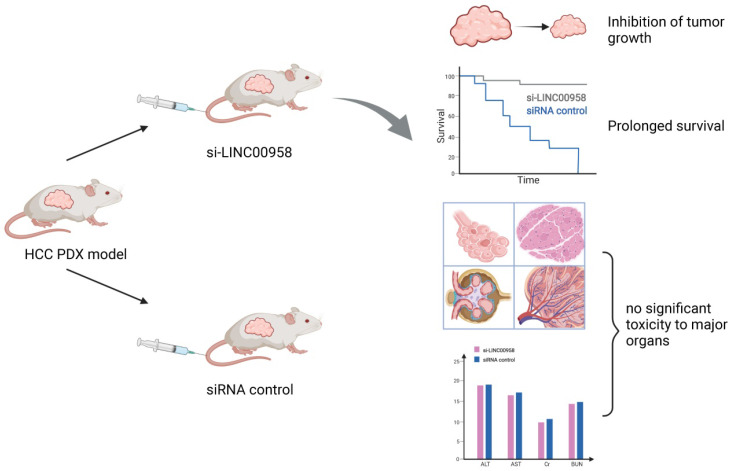
The regulation of m6A-related ncRNAs in TME makes them a potential therapeutic target for tumor growth inhibition and enhanced sensitivity to chemotherapy. M6A modification can upregulate LINC00958 by enhancing its stability, and LINC00958 can promote the proliferation, migration, invasion, and adipogenesis of HCC through the miR-3619-5p/hepatoma‑derived growth factor (HDGF) axis. PEGylated poly (lactic-co-glycolic acid) (PLGA) nanoparticles loaded with si-LINC00958 effectively inhibited tumor growth in a patient-derived xenograft (PDX) mouse model, significantly prolonged the overall survival of the mice, and showed no significant toxicity to the liver, kidney, lung, spleen, or heart 210 (Figure 5). The upregulation of m6A levels at specific sites of circRNA-SORE in HCC cells can enhance RNA stability, lead to upregulation of circRNA-SORE, and ultimately promote sorafenib resistance in cancer cells. In animal models, the local injection of circRNA-SORE interfering RNA significantly improved the efficacy of sorafenib 211. High-risk human papillomavirus produces m6A-modified circRNA circE7, which translates into the E7 oncoprotein and promotes cell transformation. After circE7 knockdown by shRNA, tumor growth was inhibited both in vitro and in a xenograft, mouse model 212. The practical application value of m6A-related ncRNAs as anti-tumor therapeutic targets remains to be further explored.
Figure 5.
Schematic diagram of a patient-derived xenograft mouse model of liver cancer treated with si-LINC00958. A drug system loaded with si-LINC00958 effectively inhibited tumor growth in the PDX mouse model and significantly prolonged the overall survival of the mice. The systemic toxicity of the drug was evaluated using hematoxylin and eosin staining and blood indexes, and there was no evident toxicity to the liver, kidney, lung, spleen, and heart.
Conclusions and Perspectives
In recent years, with in-depth research on the mechanism of tumor development and anti-tumor therapy, m6A-related ncRNAs have received increasing attention because of their key roles in tumor growth, invasion, angiogenesis, metastasis, and immune tolerance, which is mediated by TME. The interaction between ncRNA and m6A has enriched our understanding of the complex regulatory network in TME, providing a new direction for studies on the role of post-transcriptional regulation in tumors. This interaction is a potential target for tumor diagnosis, prognosis evaluation, and treatment. Currently, the understanding of the role of m6A-related ncRNAs in TME is very limited, and more studies are needed to further reveal its regulatory mechanism and biological effects. In addition, the following limitations still exist: (1) Although m6A modification is widely present in all types of RNAs, current studies mainly focus on mRNA m6A modification, and many m6A-related ncRNAs have not been identified. (2) Because the role of m6A-related ncRNAs in tumors is still an emerging field, the genetic/epigenetic heterogeneity of different tumor cell lines and samples may lead to different results, which needs to be clarified by conducting follow-up studies. M6A-related ncRNAs show different functions in different tumors, which may be related to different downstream pathways, requiring further verification. (3) There are other kinds of RNA modifications besides m6A, such as m5C (5-methylcytidine), m6Am (N6-2′-O-dimethyladenosine), pseudouridine, m1A (1-methyladenosine), m7G (7-methylguanosine), and hm5C (5-hydroxymethylcytidine). However, only a few of these have been studied; hence, it is necessary to consider the interaction between m6A and other modifications. Changes in specific phenotypes may not be entirely caused by alterations to a single type of RNA modification. (4) In terms of clinical applications, there is still a lack of specific methods for clinical diagnosis and treatment. As potential tumor markers, the diagnostic sensitivity and specificity of m6A-related ncRNAs need to be further validated, and their reference ranges in body fluids need to be determined. Sample handling and detection methods must be standardized. As a potential anti-tumor therapeutic target, more studies are needed to evaluate its efficacy and side effects and determine the appropriate administration method and dose. The role of m6A-related ncRNAs in TME is an exciting area of research. Clarifying the molecular mechanism of interaction between m6A-related ncRNA and the microenvironment, designing targeted drugs based on its complex and fine regulatory network, and combining it with traditional anti-tumor therapies to achieve improved efficacy and minimized side effects for individual precision medicine will be a major breakthrough in cancer research.
Acknowledgments
Funding
This work was supported by the National Natural Science Foundation of China (No. 81903032 and 81602167), the China Postdoctoral Science Foundation (No. 2020M672520), the Outstanding Youth Foundation of Hunan Provincial Natural Science Foundation of China (No. 2022JJ20098), the Hunan Provincial Natural Science Foundation of China (No. 2021JJ31100, 2021JJ41013 and 2022JJ40784), the Research Program of Hunan Health Commission, China (No. 202103030659), the Science and Technology Program Foundation of Changsha City (No. kq2004085), the Changsha Municipal Natural Science Foundation (No. kq2202374), and the Central South University Innovation-Driven Research Programme (No. 2023CXQD076).
Author Contributions
D.W. wrote the manuscript and created the figures. C.O., J.W., and X.H. provided direction and guidance throughout the preparation of this manuscript, and reviewed and made significant revision to the manuscript. Y.H., L.P., and T.H. collected and prepared the related papers. All authors read and approved the final manuscript.
Abbreviations
- C/EBPβ
CATT enhancer-binding protein β
- RARA
retinoic acid receptor alpha
- SIRT1
sirtuin1
- PI3K
Phosphatidylinositol‑4,5‑bisphosphate 3‑kinase
- PHLPP2
PH domain leucine-rich repeat protein phosphatase 2
- SRSF
splicing factors SR proteins
- FOXM1
Forkhead box M1
- GAS5
growth arrests specific transcript 5
- FAAH
fatty acid amide hydrolase
- STAT3
signal transducer and activator of transcription 3
- HMGA1
high mobility group A1
- PTEN
Phosphatase and tensin homolog
- ZFAS1
ZNFX1 antisense RNA 1
- ceRNAs
competing endogenous RNAs
- SUMO1
small ubiquitin-related modifiers 1
- XIST
X inactivation-specific transcript
- NEAT1
nuclear paraspeckle assembly transcript 1
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- CCR7
chemokine receptor 7
- MM
multiple myeloma
References
- 1.Cantara WA, Crain PF, Rozenski J, McCloskey JA, Harris KA, Zhang X, The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Research. 2011. 39D195-D201. [DOI] [PMC free article] [PubMed]
- 2.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive Analysis of mRNA Methylation Reveals Enrichment in 3 ' UTRs and near Stop Codons. Cell. 2012;149:1635–46. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S. et al. Topology of the human and mouse m(6)A RNA methylomes revealed by m(6)A-seq. Nature. 2012;485:201–U84. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 4.Ma S, Chen C, Ji X, Liu J, Zhou Q, Wang G. et al. The interplay between m6A RNA methylation and noncoding RNA in cancer. J Hematol Oncol. 2019;12:121. doi: 10.1186/s13045-019-0805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nie H, Liao Z, Wang Y, Zhou J, He X, Ou C. Exosomal long non-coding RNAs: Emerging players in cancer metastasis and potential diagnostic biomarkers for personalized oncology. Genes Dis. 2021;8:769–80. doi: 10.1016/j.gendis.2020.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q, Wang W, Zhou Q, Chen C, Yuan W, Liu J. et al. Roles of circRNAs in the tumour microenvironment. Mol Cancer. 2020;19:14. doi: 10.1186/s12943-019-1125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nie H, Wang Y, Liao Z, Zhou J, Ou C. The function and mechanism of circular RNAs in gastrointestinal tumours. Cell Prolif. 2020;53:e12815. doi: 10.1111/cpr.12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Nie H, He X, Liao Z, Zhou Y, Zhou J. et al. The emerging role of super enhancer-derived noncoding RNAs in human cancer. Theranostics. 2020;10:11049–62. doi: 10.7150/thno.49168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18:84. doi: 10.1186/s13058-016-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng L, Wang D, Han Y, Huang T, He X, Wang J, Emerging Role of Cancer-Associated Fibroblasts-Derived Exosomes in Tumorigenesis. Front Immunol. 2021. 12795372. [DOI] [PMC free article] [PubMed]
- 11.Zhou H, He X, He Y, Ou C, Cao P. Exosomal circRNAs: Emerging Players in Tumor Metastasis. Front Cell Dev Biol. 2021. 9786224. [DOI] [PMC free article] [PubMed]
- 12.Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–49. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 13.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R. et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Erez N, Glanz S, Raz Y, Avivi C, Barshack I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem Biophys Res Commun. 2013;437:397–402. doi: 10.1016/j.bbrc.2013.06.089. [DOI] [PubMed] [Google Scholar]
- 15.Ren Y, Jia HH, Xu YQ, Zhou X, Zhao XH, Wang YF. et al. Paracrine and epigenetic control of CAF-induced metastasis: the role of HOTAIR stimulated by TGF-ß1 secretion. Mol Cancer. 2018;17:5. doi: 10.1186/s12943-018-0758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Ji G, Le X, Wang C, Xu L, Feng M. et al. Long Noncoding RNA LINC00092 Acts in Cancer-Associated Fibroblasts to Drive Glycolysis and Progression of Ovarian Cancer. Cancer Res. 2017;77:1369–82. doi: 10.1158/0008-5472.CAN-16-1615. [DOI] [PubMed] [Google Scholar]
- 17.Yamada K, Maishi N, Akiyama K, Towfik Alam M, Ohga N, Kawamoto T. et al. CXCL12-CXCR7 axis is important for tumor endothelial cell angiogenic property. Int J Cancer. 2015;137:2825–36. doi: 10.1002/ijc.29655. [DOI] [PubMed] [Google Scholar]
- 18.Sruthi TV, Edatt L, Raji GR, Kunhiraman H, Shankar SS, Shankar V. et al. Horizontal transfer of miR-23a from hypoxic tumor cell colonies can induce angiogenesis. J Cell Physiol. 2018;233:3498–514. doi: 10.1002/jcp.26202. [DOI] [PubMed] [Google Scholar]
- 19.Yadav A, Kumar B, Yu JG, Old M, Teknos TN, Kumar P. Tumor-Associated Endothelial Cells Promote Tumor Metastasis by Chaperoning Circulating Tumor Cells and Protecting Them from Anoikis. PLoS One. 2015;10:e0141602. doi: 10.1371/journal.pone.0141602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Lv Z, Xu J, Chen C, Ge Q, Li P. et al. MicroRNA-134 inhibits osteosarcoma angiogenesis and proliferation by targeting the VEGFA/VEGFR1 pathway. Febs j. 2018;285:1359–71. doi: 10.1111/febs.14416. [DOI] [PubMed] [Google Scholar]
- 21.Zhou L, Zhu Y, Sun D, Zhang Q. Emerging Roles of Long non-coding RNAs in The Tumor Microenvironment. Int J Biol Sci. 2020;16:2094–103. doi: 10.7150/ijbs.44420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitulescu ME, Schmidt I, Giaimo BD, Antoine T, Berkenfeld F, Ferrante F. et al. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol. 2017;19:915–27. doi: 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- 23.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW. et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–7. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 26.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nature Reviews Cancer. 2013;13:739–U79. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y, Wang T, Li Y, Zhang Y, Yang R. Lnc-chop Promotes Immunosuppressive Function of Myeloid-Derived Suppressor Cells in Tumor and Inflammatory Environments. J Immunol. 2018;200:2603–14. doi: 10.4049/jimmunol.1701721. [DOI] [PubMed] [Google Scholar]
- 28.Gao Y, Sun W, Shang W, Li Y, Zhang D, Wang T. et al. Lnc-C/EBPβ Negatively Regulates the Suppressive Function of Myeloid-Derived Suppressor Cells. Cancer Immunol Res. 2018;6:1352–63. doi: 10.1158/2326-6066.CIR-18-0108. [DOI] [PubMed] [Google Scholar]
- 29.Umansky V, Sevko A. Tumor microenvironment and myeloid-derived suppressor cells. Cancer microenvironment: official journal of the International Cancer Microenvironment Society. 2013;6:169–77. doi: 10.1007/s12307-012-0126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Krueger C, Manns MP. et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 31.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer Immunology Research. 2017;5:3–8. doi: 10.1158/2326-6066.CIR-16-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–95. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–96. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, He W, Huang J, Wang B, Li H, Cai Q. et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun. 2018;9:3826. doi: 10.1038/s41467-018-06152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Liang K, Hu Q, Li P, Song J, Yang Y. et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J Clin Invest. 2017;127:4498–515. doi: 10.1172/JCI91553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao J, Dong R, Jiang L, Gong Y, Yuan M, You J. et al. LncRNA-MM2P Identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol Res. 2019;7:292–305. doi: 10.1158/2326-6066.CIR-18-0145. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018. pp. 43580–91. [DOI] [PubMed]
- 41.Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012. 2012948098. [DOI] [PMC free article] [PubMed]
- 42.Dubsky P, Ueno H, Piqueras B, Connolly J, Banchereau J, Palucka AK. Human dendritic cell subsets for vaccination. J Clin Immunol. 2005;25:551–72. doi: 10.1007/s10875-005-8216-7. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Xue Y, Han Y, Lin L, Wu C, Xu S. et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–3. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 44.Ji J, Yin Y, Ju H, Xu X, Liu W, Fu Q. et al. Long non-coding RNA Lnc-Tim3 exacerbates CD8 T cell exhaustion via binding to Tim-3 and inducing nuclear translocation of Bat3 in HCC. Cell Death Dis. 2018;9:478. doi: 10.1038/s41419-018-0528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Declerck S, Vansteenkiste J. Immunotherapy for lung cancer: ongoing clinical trials. Future Oncol. 2014;10:91–105. doi: 10.2217/fon.13.166. [DOI] [PubMed] [Google Scholar]
- 46.Jia L, Xi Q, Wang H, Zhang Z, Liu H, Cheng Y. et al. miR-142-5p regulates tumor cell PD-L1 expression and enhances anti-tumor immunity. Biochem Biophys Res Commun. 2017;488:425–31. doi: 10.1016/j.bbrc.2017.05.074. [DOI] [PubMed] [Google Scholar]
- 47.Raphael I, Nalawade S, Eagar TN, Forsthuber TG. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine. 2015;74:5–17. doi: 10.1016/j.cyto.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012. pp. 30531–64. [DOI] [PMC free article] [PubMed]
- 49.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 50.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–71. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pei X, Wang X, Li H. LncRNA SNHG1 regulates the differentiation of Treg cells and affects the immune escape of breast cancer via regulating miR-448/IDO. Int J Biol Macromol. 2018;118:24–30. doi: 10.1016/j.ijbiomac.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 52.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Telang S, Rasku MA, Clem AL, Carter K, Klarer AC, Badger WR, Phase II trial of the regulatory T cell-depleting agent, denileukin diftitox, in patients with unresectable stage IV melanoma. BMC Cancer. 2011. 11515. [DOI] [PMC free article] [PubMed]
- 54.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y. et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–7. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. Rna. 1997;3:1233–47. [PMC free article] [PubMed] [Google Scholar]
- 56.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L. et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–5. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen J, Lv R, Ma H, Shen H, He C, Wang J. et al. Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell. 2018;69:1028–38.e6. doi: 10.1016/j.molcel.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fedeles BI, Singh V, Delaney JC, Li D, Essigmann JM. The AlkB Family of Fe(II)/α-Ketoglutarate-dependent Dioxygenases: Repairing Nucleic Acid Alkylation Damage and Beyond. J Biol Chem. 2015;290:20734–42. doi: 10.1074/jbc.R115.656462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu C, Liu K, Tempel W, Demetriades M, Aik W, Schofield CJ. et al. Structures of human ALKBH5 demethylase reveal a unique binding mode for specific single-stranded N6-methyladenosine RNA demethylation. J Biol Chem. 2014;289:17299–311. doi: 10.1074/jbc.M114.550350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H. et al. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–99. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D. et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–20. doi: 10.1038/nature12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF. et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell. 2016;61:507–19. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 63.Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. Regulation of m(6)A Transcripts by the 3'→5' RNA Helicase YTHDC2 Is Essential for a Successful Meiotic Program in the Mammalian Germline. Mol Cell. 2017;68:374–87.e12. doi: 10.1016/j.molcel.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 64.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–4. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162:1299–308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O. et al. 5' UTR m(6)A Promotes Cap-Independent Translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang H, Weng H, Sun W, Qin X, Shi H, Wu H. et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol. 2018;20:285–95. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–46. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S. et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 70.Chen K, Lu Z, Wang X, Fu Y, Luo GZ, Liu N. et al. High-resolution N(6) -methyladenosine (m(6) A) map using photo-crosslinking-assisted m(6) A sequencing. Angew Chem Int Ed Engl. 2015;54:1587–90. doi: 10.1002/anie.201410647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A. et al. A majority of m6A residues are in the last exons, allowing the potential for 3' UTR regulation. Genes Dev. 2015;29:2037–53. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. Rna. 2013;19:1848–56. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen K, Wei Z, Zhang Q, Wu X, Rong R, Lu Z. et al. WHISTLE: a high-accuracy map of the human N6-methyladenosine (m6A) epitranscriptome predicted using a machine learning approach. Nucleic Acids Res. 2019;47:e41. doi: 10.1093/nar/gkz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Y, Zeng P, Li YH, Zhang Z, Cui Q. SRAMP: prediction of mammalian N6-methyladenosine (m6A) sites based on sequence-derived features. Nucleic Acids Res. 2016;44:e91. doi: 10.1093/nar/gkw104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, He S, Guo F, Zou Q. HSM6AP: a high-precision predictor for the Homo sapiens N6-methyladenosine (m^6 A) based on multiple weights and feature stitching. RNA Biol. 20211-11. [DOI] [PMC free article] [PubMed]
- 76.Deng S, Zhang H, Zhu K, Li X, Ye Y, Li R, M6A2Target: a comprehensive database for targets of m6A writers, erasers and readers. Brief Bioinform. 2021. 22. [DOI] [PubMed]
- 77.Liu L, Lei X, Meng J, Wei Z. WITMSG: Large-scale Prediction of Human Intronic m(6)A RNA Methylation Sites from Sequence and Genomic Features. Curr Genomics. 2020;21:67–76. doi: 10.2174/1389202921666200211104140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu S, Zhu A, He C, Chen M. REPIC: a database for exploring the N(6)-methyladenosine methylome. Genome Biol. 2020;21:100. doi: 10.1186/s13059-020-02012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xuan JJ, Sun WJ, Lin PH, Zhou KR, Liu S, Zheng LL. et al. RMBase v2.0: deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Res. 2018;46:D327–d34. doi: 10.1093/nar/gkx934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu K, Chen W. iMRM: a platform for simultaneously identifying multiple kinds of RNA modifications. Bioinformatics. 2020;36:3336–42. doi: 10.1093/bioinformatics/btaa155. [DOI] [PubMed] [Google Scholar]
- 81.Liu H, Wang H, Wei Z, Zhang S, Hua G, Zhang SW. et al. MeT-DB V2.0: elucidating context-specific functions of N6-methyl-adenosine methyltranscriptome. Nucleic Acids Res. 2018;46:D281–d7. doi: 10.1093/nar/gkx1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W. et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–19. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, Cui Y, YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife. 2017. 6. [DOI] [PMC free article] [PubMed]
- 84.Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V. et al. Essential role of METTL3-mediated m(6)A modification in glioma stem-like cells maintenance and radioresistance. Oncogene. 2018;37:522–33. doi: 10.1038/onc.2017.351. [DOI] [PubMed] [Google Scholar]
- 85.Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y. et al. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017;27:1115–27. doi: 10.1038/cr.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M. et al. Stem cells. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–6. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 87.Li Z, Weng H, Su R, Weng X, Zuo Z, Li C. et al. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N(6)-Methyladenosine RNA Demethylase. Cancer Cell. 2017;31:127–41. doi: 10.1016/j.ccell.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin Z, Hsu PJ, Xing X, Fang J, Lu Z, Zou Q. et al. Mettl3-/Mettl14-mediated mRNA N(6)-methyladenosine modulates murine spermatogenesis. Cell Res. 2017;27:1216–30. doi: 10.1038/cr.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu K, Yang Y, Feng GH, Sun BF, Chen JQ, Li YF. et al. Mettl3-mediated m(6)A regulates spermatogonial differentiation and meiosis initiation. Cell Res. 2017;27:1100–14. doi: 10.1038/cr.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ. et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang CX, Cui GS, Liu X, Xu K, Wang M, Zhang XX. et al. METTL3-mediated m6A modification is required for cerebellar development. PLoS Biol. 2018;16:e2004880. doi: 10.1371/journal.pbio.2004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, Jimenez-Cyrus D. et al. Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell. 2017;171:877–89.e17. doi: 10.1016/j.cell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nainar S, Marshall PR, Tyler CR, Spitale RC, Bredy TW. Evolving insights into RNA modifications and their functional diversity in the brain. Nat Neurosci. 2016;19:1292–8. doi: 10.1038/nn.4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walters BJ, Mercaldo V, Gillon CJ, Yip M, Neve RL, Boyce FM. et al. The Role of The RNA Demethylase FTO (Fat Mass and Obesity-Associated) and mRNA Methylation in Hippocampal Memory Formation. Neuropsychopharmacology. 2017;42:1502–10. doi: 10.1038/npp.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han M, Liu Z, Xu Y, Liu X, Wang D, Li F, Abnormality of m6A mRNA Methylation Is Involved in Alzheimer's Disease. Front Neurosci. 2020. 1498. [DOI] [PMC free article] [PubMed]
- 96.Zhong X, Yu J, Frazier K, Weng X, Li Y, Cham CM. et al. Circadian Clock Regulation of Hepatic Lipid Metabolism by Modulation of m(6)A mRNA Methylation. Cell Rep. 2018;25:1816–28.e4. doi: 10.1016/j.celrep.2018.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dorn LE, Lasman L, Chen J, Xu X, Hund TJ, Medvedovic M. et al. The N(6)-Methyladenosine mRNA Methylase METTL3 Controls Cardiac Homeostasis and Hypertrophy. Circulation. 2019;139:533–45. doi: 10.1161/CIRCULATIONAHA.118.036146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mathiyalagan P, Adamiak M, Mayourian J, Sassi Y, Liang Y, Agarwal N. et al. FTO-Dependent N(6)-Methyladenosine Regulates Cardiac Function During Remodeling and Repair. Circulation. 2019;139:518–32. doi: 10.1161/CIRCULATIONAHA.118.033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhong L, He X, Song H, Sun Y, Chen G, Si X, METTL3 Induces AAA Development and Progression by Modulating N6-Methyladenosine-Dependent Primary miR34a Processing. Mol Ther Nucleic Acids. 2020. pp. 21394–411. [DOI] [PMC free article] [PubMed]
- 100.Zhang J, Bai R, Li M, Ye H, Wu C, Wang C. et al. Excessive miR-25-3p maturation via N(6)-methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10:1858. doi: 10.1038/s41467-019-09712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, Wang R, Zhang L, Li J, Lou K, Shi B. The lipid metabolism gene FTO influences breast cancer cell energy metabolism via the PI3K/AKT signaling pathway. Oncol Lett. 2017;13:4685–90. doi: 10.3892/ol.2017.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J, Zhu L, Shi Y, Liu J, Lin L, Chen X. m6A demethylase FTO promotes hepatocellular carcinoma tumorigenesis via mediating PKM2 demethylation. Am J Transl Res. 2019;11(9):6084–92. [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang B, Wu Q, Li B, Wang D, Wang L, Zhou YL. m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer. 2020;19:53. doi: 10.1186/s12943-020-01170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Iwakawa HO, Tomari Y. The Functions of MicroRNAs: mRNA Decay and Translational Repression. Trends Cell Biol. 2015;25:651–65. doi: 10.1016/j.tcb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 105.Treiber T, Treiber N, Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nat Rev Mol Cell Biol. 2019;20(1):5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 106.Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–5. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Song J, Ye A, Jiang E, Yin X, Chen Z, Bai G. et al. Reconstruction and analysis of the aberrant lncRNA-miRNA-mRNA network based on competitive endogenous RNA in CESC. J Cell Biochem. 2018;119:6665–73. doi: 10.1002/jcb.26850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–63. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang D, Qiao J, Wang G, Lan Y, Li G, Guo X. et al. N6-Methyladenosine modification of lincRNA 1281 is critically required for mESC differentiation potential. Nucleic Acids Res. 2018;46:3906–20. doi: 10.1093/nar/gky130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y. et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27:626–41. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paramasivam A, Vijayashree Priyadharsini J. Novel insights into m6A modification in circular RNA and implications for immunity. Cell Mol Immunol. 2020;17:668–9. doi: 10.1038/s41423-020-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen YG, Chen R, Ahmad S, Verma R, Kasturi SP, Amaya L. et al. N6-Methyladenosine Modification Controls Circular RNA Immunity. Mol Cell. 2019;76:96–109.e9. doi: 10.1016/j.molcel.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J, Meng S, Xu M, Wang S, He L, Xu X. et al. Downregulation of N(6)-methyladenosine binding YTHDF2 protein mediated by miR-493-3p suppresses prostate cancer by elevating N(6)-methyladenosine levels. Oncotarget. 2018;9:3752–64. doi: 10.18632/oncotarget.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang Z, Li J, Feng G, Gao S, Wang Y, Zhang S. et al. MicroRNA-145 Modulates N(6)-Methyladenosine Levels by Targeting the 3'-Untranslated mRNA Region of the N(6)-Methyladenosine Binding YTH Domain Family 2 Protein. J Biol Chem. 2017;292:3614–23. doi: 10.1074/jbc.M116.749689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kleemann M, Schneider H, Unger K, Sander P, Schneider EM, Fischer-Posovszky P. et al. MiR-744-5p inducing cell death by directly targeting HNRNPC and NFIX in ovarian cancer cells. Sci Rep. 2018;8:9020. doi: 10.1038/s41598-018-27438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y, Yang F, Gao M, Gong R, Jin M, Liu T, miR-149-3p Regulates the Switch between Adipogenic and Osteogenic Differentiation of BMSCs by Targeting FTO. Mol Ther Nucleic Acids. 2019. pp. 17590–600. [DOI] [PMC free article] [PubMed]
- 117.Xu X, Yu Y, Zong K, Lv P, Gu Y. Up-regulation of IGF2BP2 by multiple mechanisms in pancreatic cancer promotes cancer proliferation by activating the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2019;38:497. doi: 10.1186/s13046-019-1470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cai X, Wang X, Cao C, Gao Y, Zhang S, Yang Z, HBXIP-elevated methyltransferase METTL3 promotes the progression of breast cancer via inhibiting tumor suppressor let-7g. Cancer Lett. 2018. pp. 41511–9. [DOI] [PubMed]
- 119.Wei W, Huo B, Shi X. miR-600 inhibits lung cancer via downregulating the expression of METTL3. Cancer Manag Res. 2019. pp. 111177–87. [DOI] [PMC free article] [PubMed]
- 120.Zhu S, Wang JZ, Chen D, He YT, Meng N, Chen M. et al. An oncopeptide regulates m(6)A recognition by the m(6)A reader IGF2BP1 and tumorigenesis. Nat Commun. 2020;11:1685. doi: 10.1038/s41467-020-15403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y, Lu JH, Wu QN, Jin Y, Wang DS, Chen YX. et al. LncRNA LINRIS stabilizes IGF2BP2 and promotes the aerobic glycolysis in colorectal cancer. Mol Cancer. 2019;18:174. doi: 10.1186/s12943-019-1105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Z, Chen X, Lei T, Gu Y, Gu J, Huang J. et al. Integrative Analysis of NSCLC Identifies LINC01234 as an Oncogenic lncRNA that Interacts with HNRNPA2B1 and Regulates miR-106b Biogenesis. Mol Ther. 2020;28:1479–93. doi: 10.1016/j.ymthe.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z. et al. m(6)A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell. 2017;31:591–606.e6. doi: 10.1016/j.ccell.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang X, Zhang J, Wang Y. Long noncoding RNA GAS5-AS1 suppresses growth and metastasis of cervical cancer by increasing GAS5 stability. Am J Transl Res. 2019;11:4909–21. [PMC free article] [PubMed] [Google Scholar]
- 125.Huang R, Zhang Y, Bai Y, Han B, Ju M, Chen B. et al. N(6)-Methyladenosine Modification of Fatty Acid Amide Hydrolase Messenger RNA in Circular RNA STAG1-Regulated Astrocyte Dysfunction and Depressive-like Behaviors. Biol Psychiatry. 2020;88:392–404. doi: 10.1016/j.biopsych.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 126.Nair L, Zhang W, Laffleur B, Jha MK, Lim J, Lee H. et al. Mechanism of noncoding RNA-associated N(6)-methyladenosine recognition by an RNA processing complex during IgH DNA recombination. Mol Cell. 2021;81:3949–64.e7. doi: 10.1016/j.molcel.2021.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yuan X, Li T, Shi L, Miao J, Guo Y, Chen Y. Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Mol Med. 2021;27:91. doi: 10.1186/s10020-021-00355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Song Z, Jia G, Ma P, Cang S. Exosomal miR-4443 promotes cisplatin resistance in non-small cell lung carcinoma by regulating FSP1 m6A modification-mediated ferroptosis. Life Sci. 2021. 276119399. [DOI] [PubMed]
- 129.Wang W, Qiao SC, Wu XB, Sun B, Yang JG, Li X. et al. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021;12:628. doi: 10.1038/s41419-021-03915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ou C, Sun Z, Li X, Li X, Ren W, Qin Z, MiR-590-5p, a density-sensitive microRNA, inhibits tumorigenesis by targeting YAP1 in colorectal cancer. Cancer Lett. 2017. pp. 39953–63. [DOI] [PubMed]
- 131.He X, Yu B, Kuang G, Wu Y, Zhang M, Cao P. et al. Long noncoding RNA DLEU2 affects the proliferative and invasive ability of colorectal cancer cells. J Cancer. 2021;12:428–37. doi: 10.7150/jca.48423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xing F, Saidou J, Watabe K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci (Landmark Ed) 2010. pp. 15166–79. [DOI] [PMC free article] [PubMed]
- 133.Aponte-López A, Muñoz-Cruz S. Mast Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020. pp. 1273159–73. [DOI] [PubMed]
- 134.Han J, Wang JZ, Yang X, Yu H, Zhou R, Lu HC. et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol Cancer. 2019;18:110. doi: 10.1186/s12943-019-1036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lin R, Zhan M, Yang L, Wang H, Shen H, Huang S. et al. Deoxycholic acid modulates the progression of gallbladder cancer through N(6)-methyladenosine-dependent microRNA maturation. Oncogene. 2020;39:4983–5000. doi: 10.1038/s41388-020-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen S, Zhou L, Wang Y. ALKBH5-mediated m(6)A demethylation of lncRNA PVT1 plays an oncogenic role in osteosarcoma. Cancer Cell Int. 2020. 2034. [DOI] [PMC free article] [PubMed]
- 137.Sun C, Wang P, Dong W, Liu H, Sun J, Zhao L. LncRNA PVT1 promotes exosome secretion through YKT6, RAB7, and VAMP3 in pancreatic cancer. Aging (Albany NY) 2020;12:10427–40. doi: 10.18632/aging.103268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Z, He J, Bach DH. et al. Induction of m(6)A methylation in adipocyte exosomal LncRNAs mediates myeloma drug resistance. J Exp Clin Cancer Res. 2022;41:4. doi: 10.1186/s13046-021-02209-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pan S, Deng Y, Fu J, Zhang Y, Zhang Z, Qin X. N6-methyladenosine upregulates miR-181d-5p in exosomes derived from cancer-associated fibroblasts to inhibit 5-FU sensitivity by targeting NCALD in colorectal cancer. Int J Oncol. 2022. 60. [DOI] [PMC free article] [PubMed]
- 140.Yan W, Wu X, Zhou W. et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20:597–609. doi: 10.1038/s41556-018-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lu S, Han L, Hu X, Sun T, Xu D, Li Y. et al. N6-methyladenosine reader IMP2 stabilizes the ZFAS1/OLA1 axis and activates the Warburg effect: implication in colorectal cancer. J Hematol Oncol. 2021;14:188. doi: 10.1186/s13045-021-01204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li Z, Qin X, Bian W, Li Y, Shan B, Yao Z. et al. Exosomal lncRNA ZFAS1 regulates esophageal squamous cell carcinoma cell proliferation, invasion, migration and apoptosis via microRNA-124/STAT3 axis. J Exp Clin Cancer Res. 2019;38:477. doi: 10.1186/s13046-019-1473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo Y. et al. Mechanisms of vasculogenic mimicry in hypoxic tumor microenvironments. Mol Cancer. 2021;20:7. doi: 10.1186/s12943-020-01288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang X, Wang J, Deng X, Xiong F, Zhang S, Gong Z. et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39:204. doi: 10.1186/s13046-020-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020. 353104119. [DOI] [PubMed]
- 146.Yang W, Xie L, Wang P, Zhuang C. MiR-155 regulates m(6)A level and cell progression by targeting FTO in clear cell renal cell carcinoma. Cell Signal. 2022. 91110217. [DOI] [PubMed]
- 147.Zhou X, Yan T, Huang C, Xu Z, Wang L, Jiang E. et al. Melanoma cell-secreted exosomal miR-155-5p induce proangiogenic switch of cancer-associated fibroblasts via SOCS1/JAK2/STAT3 signaling pathway. J Exp Clin Cancer Res. 2018;37:242. doi: 10.1186/s13046-018-0911-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hao J, Li C, Lin C, Hao Y, Yu X, Xia Y, Targeted point mutations of the m6A modification in miR675 using RNA-guided base editing induce cell apoptosis. Biosci Rep. 2020. 40. [DOI] [PMC free article] [PubMed]
- 149.Lo Dico A, Costa V, Martelli C, Diceglie C, Rajata F, Rizzo A. et al. MiR675-5p Acts on HIF-1α to Sustain Hypoxic Responses: A New Therapeutic Strategy for Glioma. Theranostics. 2016;6:1105–18. doi: 10.7150/thno.14700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015. 14155. [DOI] [PMC free article] [PubMed]
- 151.Barros-Silva D, Lobo J, Guimarães-Teixeira C, Carneiro I, Oliveira J, Martens-Uzunova ES, VIRMA-Dependent N6-Methyladenosine Modifications Regulate the Expression of Long Non-Coding RNAs CCAT1 and CCAT2 in Prostate Cancer. Cancers (Basel) 2020. 12. [DOI] [PMC free article] [PubMed]
- 152.Han W, Sulidankazha Q, Nie X, Yilidan R, Len K. Pancreatic cancer cells-derived exosomal long non-coding RNA CCAT1/microRNA-138-5p/HMGA1 axis promotes tumor angiogenesis. Life Sci. 2021. 278119495. [DOI] [PubMed]
- 153.Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A. et al. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m(6)A reader YTHDF3. Mol Cancer. 2019;18:143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cheng Y, Dai X, Yang T, Zhang N, Liu Z, Jiang Y. Low Long Noncoding RNA Growth Arrest-Specific Transcript 5 Expression in the Exosomes of Lung Cancer Cells Promotes Tumor Angiogenesis. J Oncol. 2019. 20192476175. [DOI] [PMC free article] [PubMed]
- 155.Sun Y, Li S, Yu W, Zhao Z, Gao J, Chen C. et al. N(6)-methyladenosine-dependent pri-miR-17-92 maturation suppresses PTEN/TMEM127 and promotes sensitivity to everolimus in gastric cancer. Cell Death Dis. 2020;11:836. doi: 10.1038/s41419-020-03049-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luengo-Gil G, Gonzalez-Billalabeitia E, Perez-Henarejos SA, Navarro Manzano E, Chaves-Benito A, Garcia-Martinez E. et al. Angiogenic role of miR-20a in breast cancer. PLoS One. 2018;13:e0194638. doi: 10.1371/journal.pone.0194638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang H, Deng Q, Lv Z, Ling Y, Hou X, Chen Z. et al. N6-methyladenosine induced miR-143-3p promotes the brain metastasis of lung cancer via regulation of VASH1. Mol Cancer. 2019;18:181. doi: 10.1186/s12943-019-1108-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 158.Jerez S, Araya H, Hevia D, Irarrázaval CE, Thaler R, van Wijnen AJ, Extracellular vesicles from osteosarcoma cell lines contain miRNAs associated with cell adhesion and apoptosis. Gene. 2019. pp. 710246–57. [DOI] [PMC free article] [PubMed]
- 159.Ou C, Sun Z, He X, Li X, Fan S, Zheng X. et al. Targeting YAP1/LINC00152/FSCN1 Signaling Axis Prevents the Progression of Colorectal Cancer. Adv Sci (Weinh) 2020;7:1901380. doi: 10.1002/advs.201901380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.He X, Li S, Yu B, Kuang G, Wu Y, Zhang M. et al. Up-regulation of LINC00467 promotes the tumourigenesis in colorectal cancer. J Cancer. 2019;10:6405–13. doi: 10.7150/jca.32216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ren B, Cui M, Yang G, Wang H, Feng M, You L. et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17:108. doi: 10.1186/s12943-018-0858-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zheng ZQ, Li ZX, Zhou GQ, Lin L, Zhang LL, Lv JW. et al. Long Noncoding RNA FAM225A Promotes Nasopharyngeal Carcinoma Tumorigenesis and Metastasis by Acting as ceRNA to Sponge miR-590-3p/miR-1275 and Upregulate ITGB3. Cancer Res. 2019;79:4612–26. doi: 10.1158/0008-5472.CAN-19-0799. [DOI] [PubMed] [Google Scholar]
- 163.Li B, Xia Y, Lv J, Wang W, Xuan Z, Chen C. et al. miR-151a-3p-rich small extracellular vesicles derived from gastric cancer accelerate liver metastasis via initiating a hepatic stemness-enhancing niche. Oncogene. 2021;40:6180–94. doi: 10.1038/s41388-021-02011-0. [DOI] [PubMed] [Google Scholar]
- 164.Liu T, Li P, Li J, Qi Q, Sun Z, Shi S, Exosomal and intracellular miR-320b promotes lymphatic metastasis in esophageal squamous cell carcinoma. Mol Ther Oncolytics. 2021. pp. 23163–80. [DOI] [PMC free article] [PubMed]
- 165.Fong MY, Zhou W, Liu L. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yang X, Zhang S, He C, Xue P, Zhang L, He Z. et al. METTL14 suppresses proliferation and metastasis of colorectal cancer by down-regulating oncogenic long non-coding RNA XIST. Mol Cancer. 2020;19:46. doi: 10.1186/s12943-020-1146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xing F, Liu Y, Wu SY, Wu K, Sharma S, Mo YY. et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal miRNA to Promote Brain Metastasis. Cancer Res. 2018;78:4316–30. doi: 10.1158/0008-5472.CAN-18-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhang J, Guo S, Piao HY, Wang Y, Wu Y, Meng XY. et al. ALKBH5 promotes invasion and metastasis of gastric cancer by decreasing methylation of the lncRNA NEAT1. J Physiol Biochem. 2019;75:379–89. doi: 10.1007/s13105-019-00690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mo C, Huang B, Zhuang J, Jiang S, Guo S, Mao X. LncRNA nuclear-enriched abundant transcript 1 shuttled by prostate cancer cells-secreted exosomes initiates osteoblastic phenotypes in the bone metastatic microenvironment via miR-205-5p/runt-related transcription factor 2/splicing factor proline- and glutamine-rich/polypyrimidine tract-binding protein 2 axis. Clin Transl Med. 2021;11:e493. doi: 10.1002/ctm2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jin D, Guo J, Wu Y, Du J, Yang L, Wang X. et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol. 2019;12:135. doi: 10.1186/s13045-019-0830-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 171.Wang Z, Wang X, Zhang T, Su L, Liu B, Zhu Z. et al. LncRNA MALAT1 promotes gastric cancer progression via inhibiting autophagic flux and inducing fibroblast activation. Cell Death Dis. 2021;12:368. doi: 10.1038/s41419-021-03645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shulman Z, Stern-Ginossar N. The RNA modification N(6)-methyladenosine as a novel regulator of the immune system. Nat Immunol. 2020;21:501–12. doi: 10.1038/s41590-020-0650-4. [DOI] [PubMed] [Google Scholar]
- 173.Wang H, Hu X, Huang M, Liu J, Gu Y, Ma L. et al. Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat Commun. 2019;10:1898. doi: 10.1038/s41467-019-09903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Han D, Liu J, Chen C, Dong L, Liu Y, Chang R. et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature. 2019;566:270–4. doi: 10.1038/s41586-019-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Y, Liu Z, Tang H, Shen Y, Gong Z, Xie N. et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 facilitates M1 macrophage polarization through the methylation of STAT1 mRNA. Am J Physiol Cell Physiol. 2019;317:C762–c75. doi: 10.1152/ajpcell.00212.2019. [DOI] [PubMed] [Google Scholar]
- 176.Gu X, Zhang Y, Li D, Cai H, Cai L, Xu Q. N6-methyladenosine demethylase FTO promotes M1 and M2 macrophage activation. Cell Signal. 2020. 69109553. [DOI] [PubMed]
- 177.Li HB, Tong J, Zhu S, Batista PJ, Duffy EE, Zhao J. et al. m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. 2017;548:338–42. doi: 10.1038/nature23450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang C, Zhang M, Ge S, Huang W, Lin X, Gao J. et al. Reduced m6A modification predicts malignant phenotypes and augmented Wnt/PI3K-Akt signaling in gastric cancer. Cancer Med. 2019;8:4766–81. doi: 10.1002/cam4.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shang W, Gao Y, Tang Z, Zhang Y, Yang R. The Pseudogene Olfr29-ps1 Promotes the Suppressive Function and Differentiation of Monocytic MDSCs. Cancer Immunol Res. 2019;7:813–27. doi: 10.1158/2326-6066.CIR-18-0443. [DOI] [PubMed] [Google Scholar]
- 180.Zhao R, Li B, Zhang S, He Z, Pan Z, Guo Q, The N(6)-Methyladenosine-Modified Pseudogene HSPA7 Correlates With the Tumor Microenvironment and Predicts the Response to Immune Checkpoint Therapy in Glioblastoma. Front Immunol. 2021. 12653711. [DOI] [PMC free article] [PubMed]
- 181.Peng W, Li J, Chen R, Gu Q, Yang P, Qian W. et al. Upregulated METTL3 promotes metastasis of colorectal Cancer via miR-1246/SPRED2/MAPK signaling pathway. J Exp Clin Cancer Res. 2019;38:393. doi: 10.1186/s13046-019-1408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J. et al. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9:771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu J, Zhang X, Chen K, Cheng Y, Liu S, Xia M. et al. CCR7 Chemokine Receptor-Inducible lnc-Dpf3 Restrains Dendritic Cell Migration by Inhibiting HIF-1α-Mediated Glycolysis. Immunity. 2019;50:600–15.e15. doi: 10.1016/j.immuni.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 184.Dong F, Qin X, Wang B. et al. ALKBH5 Facilitates Hypoxia-Induced Paraspeckle Assembly and IL8 Secretion to Generate an Immunosuppressive Tumor Microenvironment. Cancer Res. 2021;81:5876–5888. doi: 10.1158/0008-5472.CAN-21-1456. [DOI] [PubMed] [Google Scholar]
- 185.Liu Z, Wang T, She Y, Wu K, Gu S, Li L. et al. N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol Cancer. 2021;20:105. doi: 10.1186/s12943-021-01398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ge L, Zhang N, Chen Z, Song J, Wu Y, Li Z. et al. Level of N6-Methyladenosine in Peripheral Blood RNA: A Novel Predictive Biomarker for Gastric Cancer. Clin Chem. 2020;66:342–51. doi: 10.1093/clinchem/hvz004. [DOI] [PubMed] [Google Scholar]
- 187.Lobo J, Costa AL, Cantante M, Guimarães R, Lopes P, Antunes L. et al. m(6)A RNA modification and its writer/reader VIRMA/YTHDF3 in testicular germ cell tumors: a role in seminoma phenotype maintenance. J Transl Med. 2019;17:79. doi: 10.1186/s12967-019-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen RX, Chen X, Xia LP, Zhang JX, Pan ZZ, Ma XD. et al. N(6)-methyladenosine modification of circNSUN2 facilitates cytoplasmic export and stabilizes HMGA2 to promote colorectal liver metastasis. Nat Commun. 2019;10:4695. doi: 10.1038/s41467-019-12651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wen S, Wei Y, Zen C, Xiong W, Niu Y, Zhao Y. Long non-coding RNA NEAT1 promotes bone metastasis of prostate cancer through N6-methyladenosine. Mol Cancer. 2020;19:171. doi: 10.1186/s12943-020-01293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ban Y, Tan P, Cai J, Li J, Hu M, Zhou Y. et al. LNCAROD is stabilized by m6A methylation and promotes cancer progression via forming a ternary complex with HSPA1A and YBX1 in head and neck squamous cell carcinoma. Mol Oncol. 2020;14:1282–96. doi: 10.1002/1878-0261.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang J, Ding W, Xu Y, Tao E, Mo M, Xu W. et al. Long non-coding RNA RHPN1-AS1 promotes tumorigenesis and metastasis of ovarian cancer by acting as a ceRNA against miR-596 and upregulating LETM1. Aging (Albany NY) 2020;12:4558–72. doi: 10.18632/aging.102911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lin G, Wang H, Wu Y, Wang K, Li G. Hub Long Noncoding RNAs with m6A Modification for Signatures and Prognostic Values in Kidney Renal Clear Cell Carcinoma. Front Mol Biosci. 2021. 8682471. [DOI] [PMC free article] [PubMed]
- 193.Huang S, Lyu S, Gao Z, Zha W, Wang P, Shan Y, m6A-Related lncRNAs Are Potential Biomarkers for the Prognosis of Metastatic Skin Cutaneous Melanoma. Front Mol Biosci. 2021. 8687760. [DOI] [PMC free article] [PubMed]
- 194.Zhang X, Shen L, Cai R, Yu X, Yang J, Wu X, Comprehensive Analysis of the Immune-Oncology Targets and Immune Infiltrates of N (6)-Methyladenosine-Related Long Noncoding RNA Regulators in Breast Cancer. Front Cell Dev Biol. 2021. 9686675. [DOI] [PMC free article] [PubMed]
- 195.Konno M, Koseki J, Asai A, Yamagata A, Shimamura T, Motooka D. et al. Distinct methylation levels of mature microRNAs in gastrointestinal cancers. Nat Commun. 2019;10:3888. doi: 10.1038/s41467-019-11826-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Geng X, Zhang Y, Li Q, Xi W, Yu W, Shi L. et al. Screening and functional prediction of differentially expressed circular RNAs in human glioma of different grades. Aging (Albany NY) 2020;13:1989–2014. doi: 10.18632/aging.202192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang B, Chen Z, Tao B, Yi C, Lin Z, Li Y. et al. m(6)A target microRNAs in serum for cancer detection. Mol Cancer. 2021;20:170. doi: 10.1186/s12943-021-01477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang P, Liu G, Lu L. N6-Methylandenosine-Related lncRNA Signature Is a Novel Biomarkers of Prognosis and Immune Response in Colon Adenocarcinoma Patients. Front Cell Dev Biol. 2021. 9703629. [DOI] [PMC free article] [PubMed]
- 199.Xu F, Huang X, Li Y, Chen Y, Lin L. m(6)A-related lncRNAs are potential biomarkers for predicting prognoses and immune responses in patients with LUAD. Mol Ther Nucleic Acids. 2021. pp. 24780–91. [DOI] [PMC free article] [PubMed]
- 200.Qiu Y, Wang X, Fan Z, Zhan S, Jiang X, Huang J. Integrated analysis on the N6-methyladenosine-related long noncoding RNAs prognostic signature, immune checkpoints, and immune cell infiltration in clear cell renal cell carcinoma. Immun Inflamm Dis. 2021. [DOI] [PMC free article] [PubMed]
- 201.Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, Qing Y. et al. R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m(6)A/MYC/CEBPA Signaling. Cell. 2018;172:90–105.e23. doi: 10.1016/j.cell.2017.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Huang Y, Yan J, Li Q, Li J, Gong S, Zhou H. et al. Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res. 2015;43:373–84. doi: 10.1093/nar/gku1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G. et al. m(6)A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep. 2017;18:2622–34. doi: 10.1016/j.celrep.2017.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Arun G, Diermeier S, Akerman M, Chang KC, Wilkinson JE, Hearn S. et al. Differentiation of mammary tumors and reduction in metastasis upon Malat1 lncRNA loss. Genes Dev. 2016;30:34–51. doi: 10.1101/gad.270959.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Daige CL, Wiggins JF, Priddy L, Nelligan-Davis T, Zhao J, Brown D. Systemic delivery of a miR34a mimic as a potential therapeutic for liver cancer. Mol Cancer Ther. 2014;13:2352–60. doi: 10.1158/1535-7163.MCT-14-0209. [DOI] [PubMed] [Google Scholar]
- 206.Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J. et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35:180–8. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hong DS, Kang YK, Borad M, Sachdev J, Ejadi S, Lim HY. et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020;122:1630–7. doi: 10.1038/s41416-020-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang X, Wang S, Wang H, Cao J, Huang X, Chen Z. et al. Circular RNA circNRIP1 acts as a microRNA-149-5p sponge to promote gastric cancer progression via the AKT1/mTOR pathway. Mol Cancer. 2019;18:20. doi: 10.1186/s12943-018-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Qu L, Yi Z, Shen Y, Lin L, Chen F, Xu Y. et al. Circular RNA Vaccines against SARS-CoV-2 and Emerging Variants. Cell. 2022;185:1728–44. doi: 10.1016/j.cell.2022.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zuo X, Chen Z, Gao W, Zhang Y, Wang J, Wang J. et al. M6A-mediated upregulation of LINC00958 increases lipogenesis and acts as a nanotherapeutic target in hepatocellular carcinoma. J Hematol Oncol. 2020;13:5. doi: 10.1186/s13045-019-0839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Xu J, Wan Z, Tang M, Lin Z, Jiang S, Ji L. et al. N(6)-methyladenosine-modified CircRNA-SORE sustains sorafenib resistance in hepatocellular carcinoma by regulating β-catenin signaling. Mol Cancer. 2020;19:163. doi: 10.1186/s12943-020-01281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhao J, Lee EE, Kim J, Yang R, Chamseddin B, Ni C. et al. Transforming activity of an oncoprotein-encoding circular RNA from human papillomavirus. Nat Commun. 2019;10:2300. doi: 10.1038/s41467-019-10246-5. [DOI] [PMC free article] [PubMed] [Google Scholar]