Abstract
The tumor microenvironment is one of the important drivers of tumor development. Cancer-associated fibroblasts (CAFs) are a major component of the tumor stroma and actively participate in tumor development, invasion, metastasis, drug resistance, and other biological behaviors. CAFs are a highly heterogeneous group of cells, a reflection of the diversity of their origin, biomarkers, and functions. The diversity of CAF origin determines the complexity of CAF biomarkers, and CAF subpopulations expressing different biomarkers may play contrasting roles in tumor progression. In this review, we provide an overview of these emerging CAF biomarkers and the biological functions that they suggest, which may give a better understanding of the relationship between CAFs and tumor cells and be of great significance for breakthroughs in precision targeted therapy for tumors.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-023-01125-0.
Keywords: Cancer-associated fibroblasts, Biomarker, Heterogeneity, Targeted therapy
Tumor tissue comprises tumor cells and the extracellular stroma. Since Paget described the importance of the surrounding environment to cancer development as the “seed and soil” theory in 1889, the role of the tumor microenvironment (TME) in understanding tumorigenesis and progression has become more important. The TME refers to the interstitial environment around tumor cells, which includes not only the extracellular matrix (ECM), blood vessels, and factors such as cytokines and growth factors that affect tumor development, but also many kinds of cells, such as fibroblasts, immune cells, endothelial cells, and adipocytes. Accordingly, the TME is closely related to the diverse biological behaviors of tumors [1–3].
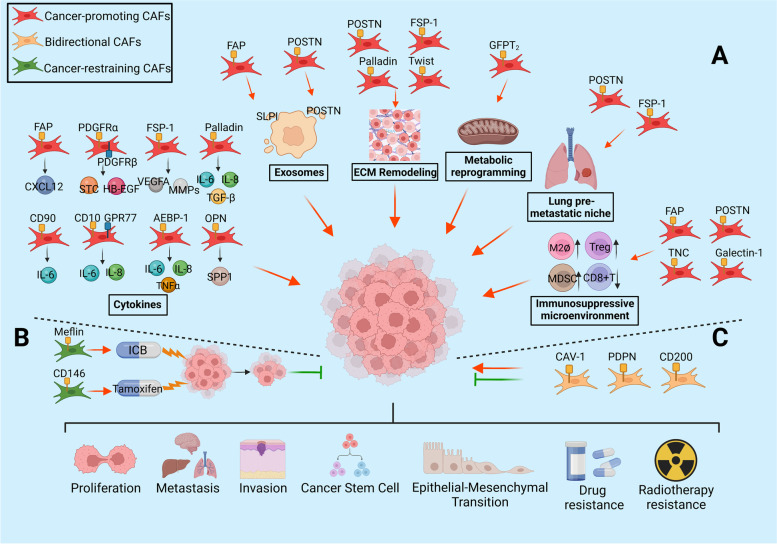
Cancer-associated fibroblasts (CAFs) are an important component of the tumor stroma. CAFs interact with tumor cells in various ways, such as by remodeling the ECM and secreting cytokines and exosomes and via metabolic reprogramming, and actively participate in a variety of biological behaviors, including tumor development, invasion, metastasis, and drug resistance (Fig. 1). Recent studies have found that the role of CAFs in tumors is bidirectional. CAFs can facilitate tumor development through metabolic interactions, thereby promoting angiogenesis and immunosuppression; CAFs can also effectively impair tumor invasiveness and increase drug sensitivity and be positively associated with a better prognosis, indicating their tumor-suppressive effects.
Fig. 1.
CAF biomarkers and functions. CAF subgroups identified by different biomarkers play distinct roles in tumor microenvironment. A Biomarkers suggesting cancer-promoting functions. Most biomarkers represent cancer-promoting CAFs including traditional CAF biomarkers (FAP, POSTN, PDGFRα/β, and FSP-1) as well as some emerging markers (CD90, Palladin, OPN, AEBP1, Twist, TNC, Gallectin1, CD10 and GPR77). They promote cancer progression though secreting cytokines and exosomes, remolding ECM, metabolic reprogramming, forming anterior niche in lung metastasis and immunosuppressive microenvironment, etc. B Biomarkers indicating cancer-restraining functions. Meflin+ CAFs are associated with favorable therapeutic response to ICB; CD146+CAF maintains sensitivity to tamoxifen of ER positive breast cancer. Both are related to better pathological histological features and prognosis of patients. C Biomarkers showing bidirectional functions. Cav-1, PDPN and CD200 labeled CAFs have both tumor-promoting and tumor-suppressing functions
The reason why CAFs have such a complex function is that CAFs are a highly heterogeneous group of cells, which is mainly due to their diverse origins. CAFs can be derived from a variety of cells, including normal fibroblasts (NFs) in the peritumoral stroma, bone marrow-derived mesenchymal stem cells (MSCs), stellate cells, epithelial cells, and endothelial cells. Other precursors of CAFs include vascular smooth muscle cells, perivascular cells, adipocytes, and adipose stem cells [4]. CAFs of different cell sources can express different biomarkers, and different markers can reflect the different biological properties of CAFs. The diversity of CAF cell origins determines the complexity of CAF markers. In recent years, a multitude of clinical observations have indicated that CAF subgroups expressing different markers play distinct roles in the tumor development process of various cancers, such as cancer-promoting (Table 1), cancer-restraining (Table 2), and bidirectional function (Table 3). Therefore, the study of CAF markers is of great importance for achieving breakthroughs in precision targeted therapy. In recent years, with the emphasis on tumor immune microenvironment and the development of molecular techniques, such as single-cell sequencing, there has been a great progress in the study of CAF markers. In this review, we incorporate some new CAF markers including Meflin and CD200, and also delve into new mechanisms of the so-called “old” CAF markers acting on tumor cells, such as leading to immunosuppressive TME status. We will discuss all these markers by origin and function, which will enable us to better understand the role of CAF markers in tumorigenesis and tumor-targeted therapy.
Table 1.
The role of CAFs labeled by cancer-promoting markers in various kinds of cancer
|
Bio marker |
Main origin |
Description | Roles in cancers | |||
|---|---|---|---|---|---|---|
| Pancreatic cancer | Breast cancer | Colorectal cancer | Lung cancer | |||
| FAP | NFs | Type II intact membrane protein of serine protease family, including two subunits, α and β | Regulating accumulation of regulatory T cells, inhibition T cell activity, secreting CXCL12 and marginalized T cells through CXCL12-CXCR4 signaling, leading to tumor immunosuppression; Tumor promoting | Enriched in HER2-positive and triple-negative breast cancer stroma; expressing PD-1/2, which can bind to PD-1 expressed by T cells and inhibiting T cell activity |
Interaction of FAP+ CAFs and SPP1+ macrophages contributing to desmoplastic tumor microenvironment and correlating with immunotherapy resistance |
Correlating with the immunosuppressive TME status; predictive biomarker of resistance to PD-1 blockade; poor prognosis marker |
| α-SMA | Cytoskeleton protein | α-SMAhighFAPhigh -myCAF α-SMAlowFAPlow -iCAF; relationship with survival is contradictory; inhibition IL-6 from α-SMA+CAF improving gemcitabine efficacy | Improving tumor growth via secretion of osteopontin; poor prognosis | Poor survival rates | MYH11+ α-SMA+ CAF and FAP+ α-SMA+ CAF driving T cell marginalization | |
| POSTN | Stromal cell protein | Associated with highly cellular tumors and macrophage infiltrates and shorter overall survival | Playing an important role in carcinogenesis in situ and perhaps beyond for those cancers that become invasive | Correlated with tumor progression, lymph node and distant metastases, and poor clinical outcomes | Prognostic marker | |
| PDGFRα/β | Tyrosine kinase receptor | Lymphatic invasion and lymph node metastasis; poor prognosis | PDGFRβ interacting with integrin A11 and leading to more aggressive behavior by activating JNK signaling |
PDGFRβ+CAFs increasing the invasion and metastasis ability in a secreted glycoprotein stanniocalcin-1 dependent manner |
Promoting recruitment of fibroblasts to the tumor infiltration front | |
| FSP-1 | Small calcium binding protein | Identifying a unique population of fibroblasts with minimal overlap with markers for αSMA and PDGFRβ | Positive in primary breast cancer and in matched LNMs; inducing metastasis; associated with poor patient survival | Associated with poor patient survival | Lung premetastasis niche formation | |
| Palladin | Actin binding protein | Secreting immunosuppressive cytokines to maintain the characteristics of iCAFs; producing functional desmoplastic ECMs to support cancer cell survival and proliferation; facilitating the invasion of cancer cells by remodeling ECM though regulating the small GTPase Cdc42; independent poor prognostic factor | As Akt1-specific substrate and regulating breast cancer cell migration | Correlated with reduced survival and relapse | Affecting the behavior of CAFs, leading to a pro-invasive henotype | |
| Twist1 | A basic helix-loop-helix transcription factor | - | Tumor promoting; related to shorter survival | Promoting matrix stiffness | - | |
| GFPT2 | Glutamine-Fructose-6-Phosphate Transaminase 2 | - | - | Regulating immunosuppression through JAK/STAT signaling pathway; associated with poorer pathological characteristics and OS | Regulating metabolic reprogramming and negatively correlates with prognosis | |
| Vim | MSCs | Cytoskeletal protein | Poor survival; pro-tumoural profile | Associated with indices of poor prognosis | Independent prognostic factor for recurrence; related to shorter survival and chemoresistance | Promoting lung cancer metastasis by surrounding the cancer cells sprouting from primary tumor |
| TNC | Extracellular matrix protein | Perineural invasion, high loco-regional recurrence, proliferation and invasion; poor prognosis | Related to LN metastasis and poorer outcome | Promoting EMT and proliferation; involved in tumor growth and metastasis via Hedgehog signaling; poor prognosis | Poorer clinical outcome biomarker | |
| CD90 | GPI-anchored glycoprotein | Promoting pancreatic cancer development | - | Supporting the stemness of tumor cells and inducing an immune adaptive inflammatory response | Promoting tumor cell invasion; poor prognosis | |
| CD10 | Metalloendoprotease | - | Supplying paracrine IL-8 and IL-6 through NF-κB signaling, forming a niche that protects CSC,leading to chemotherapy resistance and poor survival | - | Same to breast cancer | |
| GPR77 | Non-G protein coupled receptor | - | - | - | ||
| Galectin 1 | Stellate cells | β-galactoside binding protein | Tumor cell proliferation, angiogenesis, invasion, metastasis and inflammation | Immunosuppressive microenvironment; associated with high tumor grade and lymph node involvement; poor prognosis | Promoting CIC features and disease dissemination | Poorer clinical outcome biomarker |
| AEBP1 | Epithelial cells | Multifunctional protein | - | - | Promoting proliferation, invasion, migration and metastasis by activating NF-κB signaling | An independent poor prognostic factor |
| OPN | Not clear | secretory phosphoprotein | Promoting cancer stemness via SPP1-CD44 axis | Poor prognosis | Contributing to tumorigenesis by activation of the STAT3/PPARg pathway;poor prognosis | Promoting invasiveness and proliferation;poor prognosis |
Table 2.
The role of CAFs labeled by cancer-restraining markers in various kinds of cancer
|
Bio marker |
Main origin |
Description | Roles in cancers | |||
|---|---|---|---|---|---|---|
| Pancreatic cancer | Breast cancer | Colorectal cancer | Lung cancer | |||
| Meflin | Not clear | Glycosylated phosphatidylinositol anchored protein | Attenuating tumor aggressiveness; positively correlated with higher differentiated pathological histological features and better prognosis | - | - | Sensitive to ICB treatment |
| CD146 | Vascular endothelial cells | Cell adhesion molecule | Related to low histological grade and high-grade pancreatic intraepithelial neoplasia; inhibiting invasion and migration of cancer cells; better prognosis marker | Maintaining ER expression, estrogen-dependent proliferation and sensitivity to tamoxifen | - | - |
Table 3.
The role of CAFs labeled by bidirectional function markers in various kinds of cancer
|
Bio marker |
Main origin |
Description | Roles in cancers | |||
|---|---|---|---|---|---|---|
| Pancreatic cancer | Breast cancer | Colorectal cancer | Lung cancer | |||
| Cav-1 | NFs | A scaffold protein | Higher CA19-9 level and rates of advanced tumor stage; lower DFS and OS | Cav-1 deficiency is associated with early recurrence, late tumor stage, lymph node metastasis, triamcinolone resistance and poor prognosis; Cav-1 upregulation hardening TME to promote tumor cell invasion and metastasis | Tumor-promoting | A marker of good efficacy for nab-paclitaxel treatment |
| PDPN | Vascular endothelial cells | Mucin-type salivary glycoprotein | Associated with immune-related signatures and recruitment of dendritic cells; enhancing the progression, and serving as an independent predictor of poor outcome | Good independent prognostic marker | PNPDhigh CAFs are protective against cell invasion; associated with favorable clinicopathological parameters and prolonged DFS | PDPN+CAFs are related to immunosuppressive microenvironment; leading to primary resistance to EGFR-TKI by activating MAPK signaling; a risk factor for recurrence |
| CD200 | Not clear | OX2 membrane glycoprotein | No clear correlation with PFS and OS | - | - | Increasing sensitivity of EGFR mutation lung cancer to the EGFR-TKI |
CAF markers with cancer promotion
Markers of CAFs derived from NFs
Fibroblast activation protein
Fibroblast activation protein (FAP) is a type II membrane protein of the membrane-binding serine protease family comprising α and β subunits. Although it normally exists as a homodimer, when activated, it assembles into a heterodimer composed of the α and β subunits and participates in the migration of fibroblasts to the collagen matrix. FAP is highly expressed in several cancerous myofibroblasts and is considered an important marker of CAFs [5]. FAP+ CAFs mainly originate from NFs [6].
FAP promotes the progression of various cancers. It boosts the metastasis of phyllodes tumor [7], the invasive behavior of bladder high-grade urothelial carcinoma (UC) [8], prostate cancer cell invasion and proliferation [9], and cancer development by epithelial-mesenchymal transition (EMT) via the Wnt/β-catenin signaling pathway in gastric cancer [10]. FAP correlates with adverse clinicopathological factors, such as invasion depth in esophageal adenocarcinoma (EAC) [11], large tumor diameter (> 7 cm), advanced stage (pT3/4), high grade (G3/4), sarcomatoid transformation, tumor necrosis, and early lymph node metastasis of clear cell renal cell carcinoma (CCRCC) [12]. In ovarian cancer, secretory leukocyte protease inhibitor (SLPI) protein derived from FAPhigh α-SMAlow CAFs can be encapsulated in extracellular vesicles (EVs) and delivered to cancer cells, promoting cell proliferation, adhesion, invasion, and migration via the PI3K/AKT signaling pathway [13]. It is associated with poor prognosis of NSCLC [14], gastric cancer [10], CCRCC [12], ovarian cancer [13], and bladder high-grade UC [8], but not cervical cancer [15]. FAP+ CAFs are independent predictive factors to worse tumor regression grade (TRG), that means chemoresistance in gastric cancer [16].
FAP is correlated with immunosuppressive TME status and leads to immunotherapy resistance. The reciprocity of FAP+ fibroblasts and SPP1+ macrophages has been revealed through single-cell and spatial analysis in colorectal cancer (CRC). The reciprocity might be adjusted by TGF-β, IL-1, and chemerin, correlate with desmoplastic TME, and lead to immunotherapy resistance [17]. In gastric cancer, FAP expression is related to immunosuppressive cell infiltration, such as that of M2 macrophages (CD163+) and myeloid-derived suppressor cells (MDSCs) (CD11b+/CD33+). In advanced non-small cell lung cancer (NSCLC), FAP is related to a reduced density of CD8+ T cells and immunosuppressive TME status [18, 19]. In HER2+ and triple-negative breast cancer, the CAF subpopulation with high FAP expresses programmed death ligand-1/2 (PD-L1/2), which can bind to PD-1 expressed by T cells and directly inhibit T cell activity [20]. In a mouse model of pancreatic cancer, FAP+ CAFs are the main source of CXCL12 that combines with CXCR4 on the surface of cancer cells and marginalized T cells through a CXCL12–CXCR4 signaling mechanism, leading to tumor immunosuppression [21]. FAP also promotes the growth of intrahepatic cholangiocarcinoma cells by attracting MDSCs through CCL2 [22]. In summary, FAPs can be used as a biomarker to predict immunosuppressant resistance [14].
All of the above indicates that FAP+ CAFs are potential therapeutic targets for solid tumors. The vast majority of therapeutic approaches targeting FAP+ CAFs involve their depletion. The strategies include FAP antibodies [23–25], genetic deletion [26], pharmacological inhibition (PT630, PT-100) [27, 28], conditional ablation using diphtheria toxin [21] or FAP-PE38 [25], and novel FAP-targeting immunotherapies such as DNA vaccination [29] and chimeric antigen receptor (CAR) T cells [30, 31].
α-Smooth muscle actin
α-Smooth muscle actin (α-SMA) belongs to the actin family of cytoskeletal proteins and is widely known for its role in wound healing. α-SMA is frequently expressed in CAFs and is one of the preferred markers for identifying CAFs [32, 33]. α-SMA+ CAFs are mainly derived from NFs. Other sources include stellate cells, epithelial cells, vascular endothelial cells, vascular smooth muscle cells, and MSCs [4, 6, 33, 34].α-SMA+ CAFs participate in cancer progression. α-SMA-expressing CAFs can enhance the colony formation, proliferation, and invasiveness of pancreatic cancer cells [35]. α-SMA+ CAFs also promote the proliferation of bile duct epithelial cells and trigger their entry into the S + G2/M phase, resulting in tumor promotion of cholangiocarcinoma [36]. α-SMA+ CAFs secreting OPN promote luminal breast cancer growth [37]. However, studies of a pancreatic ductal adenocarcinoma (PDAC) mouse model have also identified tumor-restraining α-SMA+ CAFs (rCAFs) and tumor-promoting FAP+ CAFs (pCAFs), which are involved in the aggregation of regulatory T cells [38], suggesting the complexity of α-SMA+ CAF function.
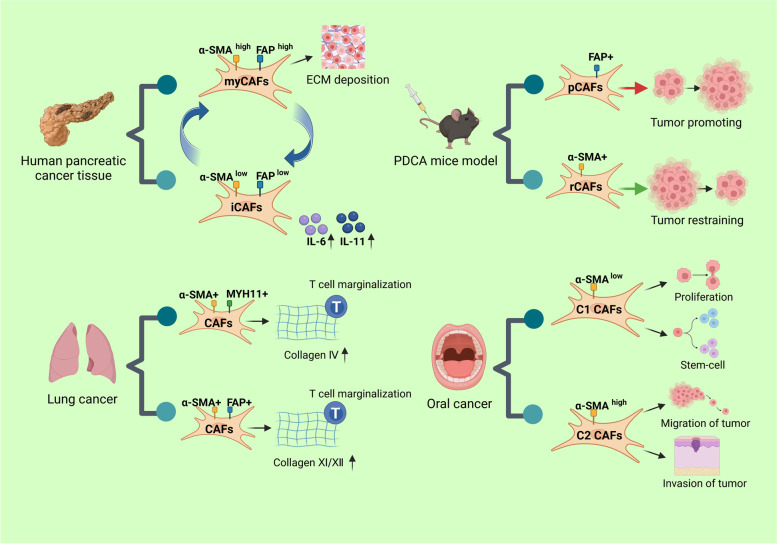
In human pancreatic cancer tissues, Ohlund et al. have found two major CAFs, one called myofibroblastic CAF (myCAF), which is located close to cancer cells and expresses high levels of α-SMA and FAP, has high levels of ECM-related genes, and is enriched in the TGF driver pathway. The other type of CAF is called inflammatory CAF (iCAF), which is distant from cancer cells and expresses low levels of α-SMA and FAP but secretes high levels of inflammatory mediators, including IL-6. By secreting cytokines and chemokines, iCAFs have more of a tumor-improving effect than myCAFs, indicating a more malignant biological behavior of pancreatic cancer. In contrast, myCAFs extensively deposit ECM to hinder drug delivery. The two can interconvert through paracrine factors [39, 40]. Grout et al. examined lung tumors via single-cell RNA sequencing combined with multiple imaging techniques and identified two CAF populations related to T cell marginalization, namely (i) myosin heavy chain 11 (MYH11)+SMA+ CAFs, which are present in early-stage tumors and form around aggregated cancer cells in a single cell layer; and (ii) FAP+SMA+ CAFs, which are present in more advanced tumors and are distributed in sheets in the stroma or in several layers around tumor clusters. Targeting of these CAF subpopulations may increase immunotherapy efficacy for tumors with marginalized T cells [41]. CAFs in oral cancer can be classified into C1 and C2 types by a low or high expression of α-SMA. C1-type CAFs regulate the proliferation and stemness of oral cancer cells; they are more supportive of cell proliferation but are not conducive to the self-renewal growth of stem-like cancer cells. C2-type CAFs are associated with the invasion and metastasis of oral cancer [42]. The above CAFs subgroups are showed in Fig. 2.
Fig. 2.
Subgroups of CAFs. An outline of different subgroups of CAFs found in human pancreatic cancer, PDCA mouse model, lung and oral cancer
α-SMA+ CAFs are associated with worse prognosis in oral tongue squamous cell carcinoma (OTSCC) [43], head and neck squamous cell carcinoma (HNSCC), EAC, colon cancer [44], prostate cancer [45], breast cancer [37], and bile duct cancer [36, 46]. The findings of α-SMA and pancreatic prognostic correlation are inconsistent. Fujita et al. found that patients with high SMA expression have a shorter survival [35], whereas Maehira and Yuzawa concluded that SMA+ CAFs are not associated with pancreatic cancer prognosis [47, 48].
In terms of treatment, the TGF-β and IL-1/JAK/STAT signaling pathways are related to the formation and interconversion of these two CAF subtypes. Patients can benefit from the combination of JAK inhibitors and TGFBR inhibitors.
Periostin
Periostin (POSTN) was originally isolated from a mouse osteoblast cell line as osteoblast-specific factor 2 and belongs to the matricellular protein family. In humans, POSTN expression can be increased by interleukins and TGF-β [49, 50]. The POSTN molecule comprises four fascia I domains, a cysteine-rich domain in the N-terminal region, and an alternative splicing domain in the C-terminal region [51–56]. POSTN binds to both type I collagen and fibronectin [56, 57], is involved in collagen fibrosis, and is also related to Th2-dependent immune responses and inflammation [58]. POSTN+ CAFs are mainly derived from NFs [59].
POSTN is overexpressed in a variety of cancer CAFs and promotes various biological processes, including invasion and metastasis, by binding to appropriate integrin receptors or affecting the microenvironment [60–62]. In EAC and ovarian cancer, CAF-derived POSTN acts as a ligand for integrins, activates the PI3K/Akt pathway, and induces the EMT, providing the impetus for cancer cell migration and invasion [63, 64]. CAFs promote lymph node metastasis by activating the integrin FAK/Src-VE-cadherin signaling pathway in lymphoendothelial cells and damaging the lymphatic endothelial barrier in cervical squamous carcinoma [65]. In addition to binding to integrins, POSTN can also affect the TME to promote tumor metastasis. Single-cell sequencing of gastric cancer tissues revealed that POSTN-expressing CAFs promote gastric cancer invasion and metastasis by degrading the ECM and attracting tumor-associated M2-like macrophages to form a niche conducive to metastasis [66]. In salivary gland adenoid cystic carcinoma, POSTN is a potential biomarker for EV-induced anterior niches in CAFs in lung metastasis [67].
POSTN+ CAFs are involved in maintaining tumor cell stemness. POSTN+ CAF-derived POSTN promotes the cancer stem cell (CSC) phenotype of HNSCC by activating protein tyrosine kinase 7 (PTK7)-Wnt/β-Catenin signaling through binding to PTK7 in cancer cells [68]. Evidence suggests that POSTN is a major part of the CSC niche that maintains stemness and the metastatic colonization of CRC [69].
POSTN+ CAFs participate in drug resistance. In melanoma, BRAF inhibitors activate B-linked proteins in CAFs, stimulating the secretion of POSTN, which activates PI3K/AKT signaling and then reactivates the ERK pathway inhibited by BRAFi/Meki, promoting drug resistance [70]. POSTN+ CAFs are associated with epithelial ovarian cancer primary chemoresistance and predict shortened progression-free survival after first-line chemotherapy [71].
POSTN overexpression in CAFs is associated with many adverse clinicopathological factors, such as higher T and clinical stages and a larger tumor volume in NSCLC [72]; highly cell-rich and macrophage-infiltrated pancreatic cancer [73]; and lymph node metastasis and distant metastasis in pancreatic cancer and CRC [69, 73]. POSTN+ CAFs are also related to poor prognosis in PDAC [73], CRC [69], NSCLC [72], EAC [64], gastric cancer [66], and ovarian cancer [63].
In terms of treatment, neutralization of POSTN with appropriate antibodies has been shown to reduce breast cancer metastasis to the lung [74, 75]. In addition, a DNA inducer that binds to POSTN has been shown to inhibit the growth and metastasis of breast cancer and may be used as a therapeutic tool for breast cancers with POSTNhigh CAFs [76]. Inhibition of POSTN may also weaken resistance to chemotherapeutic agents in breast cancer [77].
Platelet-derived growth factor receptor
Platelet-derived growth factor receptor (PDGFR) is a tyrosine kinase receptor that is located on the surface of fibroblasts, neural precursor cells, astrocytes, and pericytes. There are two types: PDGFRα and PDGFRβ. By binding to PDGF family members, it initiates a coagulation-forming response, stimulates angiogenesis, and promotes tumor growth and metastasis [78, 79]. PDGFRα/β+ CAFs are mainly derived from NFs and, to a lesser extent, from pericytes, and,vascular smooth muscle cells [6].
PDGFRα/β+ CAFs support malignant biological behaviors of tumors. PDGFRβ and integrin A11 (ITGA11) strongly colocalize and lead to more aggressive breast cancer cells by activating JNK signaling [78]. PDGFR interacts with integrin α5β1 to promote cell contraction and thus organize the ECM, leading to the directional migration of prostate cancer cells [80]. PDGFRβ in CAFs can interact with PDGF-BB expressed by cervical cancer cells and promote cancer cell growth by upregulating the expression of heparin-binding epidermal growth factor (HB-EGF) and activating the EGFR signaling pathway [81]. In CRC, PDGFRβ-labeled CAFs increase the metastatic and invasive ability of cancer cells in a secreted glycoprotein stanniocalcin-1-dependent manner [82]. PDGFRβ is a novel marker of stromal activation in oral squamous cell carcinoma [83]. In a xenograft mouse model of lung cancer, PDGFRα has been shown to promote the recruitment of fibroblasts to the tumor infiltration front. PDGFRα/βhigh CAFs have also been linked to lymph node metastasis and lymphovascular invasion in ovarian cancer [84] and pancreatic cancer [48] and lead to poorer prognosis in breast cancer [78], ovarian cancer [84], pancreatic cancer [48], and lobular breast tumors [85]. Inhibition of PDGFR signaling can restrain cervical cancer angiogenesis and cell proliferation [86]. In addition, a PDGFR inhibitor, dasatinib, partially reverses cancer-promoting CAFs to resting-state fibroblasts in lung adenocarcinoma (LUAC) and is a potential therapeutic strategy for LUAC [87].
Fibroblast-specific protein 1
Fibroblast-specific protein 1 (FSP-1) belongs to the S100 small calcium-binding protein family, also known as S100A4. Studies have demonstrated that FSP-1+ CAFs induce angiogenesis and promote cell motility and metastasis by producing ECM proteins and secreting cytokines, including Tenascin C (TNC), matrix metalloproteinases (MMPs), and vascular endothelial growth factor-A (VEGF-A) [88–90].
The main sources of FSP-1+ CAFs are NFs, vascular endothelial cells, epithelial cells, and adipocytes [33, 91–93].
FSP-1 is correlated with lymph node metastasis in cholangiocarcinoma [46] and breast cancer [94] patients. FSP-1 is positive in CAFs of primary breast cancer and in matched lymph node metastases but negative in fibroblasts of cancer-free lymph nodes. This suggests that the lymph node stroma imitates the microenvironment of primary breast cancer [94].
In addition to lymph node metastasis, FSP-1+ CAFs are also involved in lung metastasis. In HNSCC, knockdown of FSP-1 in CAFs reduces the invasion and lung metastasis of cancer cells in xenograft tumor animal models by decreasing MMP2 expression, secretion, and activity [95]. FSP-1 induces lung metastasis in rodent models of breast cancer by increasing cell motility and invasion, which is not dependent on ECM degradation [96]. In a transgenic mouse model, FSP-1+/+ fibroblasts induce a massive infiltration of T cells and release of specific cytokines in the pre-metastatic lung, providing a favorable microenvironment for metastasis formation and leading to increased lung metastasis [97].
Because FSP-1+ CAFs are involved in lymph node metastasis and lung metastasis, elevated levels of FSP-1 in CAFs have been related to poor survival in breast cancer, CRC, and intrahepatic cholangiocarcinoma patients [46, 98, 99].
FSP-1+ CAFs drive metastasis by affecting the TME, making them an appealing target for anticancer therapy. Anti-FSP-1 monoclonal antibody effectively reduces T cell infiltration in fibroblast monolayers, inhibits the invasive growth of mouse and human fibroblasts, and reduces the metastatic burden in the lungs of experimental animals. Therefore, we hypothesize that FSP-1 expressed in CAFs could be a target for anti-metastatic therapy [100].
Palladin
Palladin is an actin-binding protein that serves as a scaffold to connect actin bundles, stress fibers, focal adhesions, Z-discs, and subcellular structures that play a critical role in normal cell motility [101, 102]. Palladin+ CAFs are mainly derived from NFs and stellate cells [103, 104].
There are nine isoforms of palladin. Of these, isoforms 3 and 4 (ISO3/ISO4) are highly expressed in CAFs of quantities of malignancies, such as pancreatic, lung, colon, and gastric cancer. Palladin regulates the expression of various genes encoding ECM proteins, which functionally affect the behavior of CAFs, leading to a pro-invasive phenotype [105].
In PDAC, CAFs with high expression of ISO3/ISO4 can effectively secrete immunosuppressive cytokines (TGF-β1, IL-8, and IL-6) to maintain the characteristics of inflammatory CAFs and produce functional desmoplastic ECMs (d-ECMs) that support tumor cell survival and proliferation [106]. In addition, high expression of palladin in CAFs facilitates the invasion of cancer cells by remodeling the ECM through regulation of the small GTPase Cdc42, which is considered to be the main regulator of core actin polymerization in invasive pseudopodia [104].
In breast cancer, palladin is an Akt1-specific substrate [107]. Downregulation of palladin by miR-96/miR-182 in CAFs reduces breast cancer cell migration and invasion [108]. In CRC [109], PDAC [110], and renal cell carcinoma [111], palladinhigh CAFs are correlated with reduced survival.
Twist
Twist1 is a basic helix-loop-helix transcription factor that recruits nucleosome remodeling deacetylase (NuRD) to modify target gene-bound histones and participate in epigenetic processes in cells [112]. The main source of Twist1+ CAFs is NFs [113].
Twist1 expressed in CAFs is a marker of tumor progression. Twist1 expression in NFs can transdifferentiate them into CAFs via STAT3 phosphorylation and is sufficient and necessary for CAF transdifferentiation [113]. Twist1 is more commonly expressed in gastric CAFs than in those of other cancer species and is rarely expressed in non-cancerous tissues [114]. Increased expression of Twist1 in gastric cancer CAFs contributes to gastric cancer progression and worse patient survival while its increased expression in NFs can drive CAF marker expression and invasive features of gastric cancer cells both in vivo and in vitro. Inversely, silencing of Twist1 expression in CAFs abolishes their tumor-promoting characteristics [113]. Twist1 expression in CAFs is related to tumor size, lymph node metastasis, and depth of invasion, as well as poor survival in patients with gastric cancer, especially in patients with diffuse-type gastric cancer. Besides gastric cancer, Twist can be expressed in both cancer cells and CAFs of invasive ductal breast carcinoma and both are related to shorter patient survival, indicating Twist as a potential useful prognostic marker in invasive ductal breast carcinoma [115]. In CRC, Twist1 expression is mostly limited to the tumor stroma. It improves matrix stiffness by upregulating collagen α1 and palladin and promotes cell migration and invasion [109].
Glutamine-fructose-6-phosphate transaminase 2
Glutamine-fructose-6-phosphate transaminase 2 (GFPT2) is the rate-limiting enzyme of the hexosamine biosynthesis pathway (HBP), responsible for glycosylation [116]. Activation of HBP leads to altered O-GlcNAcylation and N-/O-glycosylation of transcription factors and kinases in various types of cancer. This process will stabilize proteins involved in tumorigenic processes, such as c-Myc and β-catenin, leading to proliferation, invasion, metastasis and drug resistance of tumor cells [117–122]. The main source of GFPT2+ CAFs is NFs [116].
In LUAC, the GFPT2 gene is upregulated after transformation of NFs into CAF-like cells by TGF-β, leading to increased glucose uptake. GFPT2 is predominantly expressed in invasive-edge CAFs and is a key regulator of tumor metabolic reprogramming. Its high expression in CAFs is negatively correlated with LUAC patient prognosis [116].
In CRC, GFPT2 is highly expressed, and CAFs are the main cells expressing GFPT2. GFPT2+CAFs positively correlates with immunosuppressive cells and T-cell exhaustion, implying that GFPT2 may be involved in immune escape of tumor cells. It regulates immunosuppression mainly through the JAK/STAT signaling pathway. Its high expression in CAFs is significantly associated with poorer pathological characteristics and OS in patients with CRC [123].
In addition to being expressed in CAFs, GFPT2 is also expressed in tumor parenchymal cells. The high expression of GFPT2 is closely related to invasion, metastasis, drug sensitivity, and poor prognosis of a variety of tumors including lung cancer [124, 125], epithelial ovarian cancer [126–128], CRC [129], breast cancer [130, 131], GC [132] and leiomyosarcoma [133].
Markers of CAFs derived from MSCs
Vimentin
Vimentin is a type III intermediate filament protein that acts as a cytoskeletal protein and is localized to the cytoplasm. It is an EMT biomarker that maintains cell structure and motility during cell migration. In the TME, it is expressed not only in CAFs, but also in epithelial cells undergoing EMT, vascular endothelial cells, and mesenchymal-derived cells such as adipocytes and myocytes [134]. Vimentin+ CAFs are mainly derived from MSCs [34], although other sources include vascular endothelial cells and epithelial cells [6].
Vimentin+ CAFs are related to tumorigenesis, metastasis, recurrence, drug resistance and poor prognosis in patients of several cancers.
In CRC, vimentin expression in CAFs may reflect the higher malignant potential of the tumor. Vimentin+CAFs are significantly associated with a higher rate of disease recurrence, regardless of lymph node status, and is therefore an independent prognostic factor for CRC recurrence. High expression of vimentin in CAFs is also associated with shorter survival in patient with CRC [135]. Another study on CRC has found that stromal vimentin expression is significantly correlated with T stage, suggesting its possible involvement in tumor invasion. Stromal vimentin expression is an independent prognostic factor for CRC-specific survival (CSS) and disease-free survival (DFS) of high-risk stage II patients. Moreover, high-risk stage II patients with how stromal vimentin expression benefit less from standard adjuvant chemotherapy than those with low stromal vimentin expression [136].
In PDAC, Maehira H et al. used double immunofluorescence staining to divide the vimentin-positive CAFs into two subgroups: α-SMA co-expression and α-SMA no co-expression. Vimentin+ CAFs without co-expression of α-SMA are an independent predictor of poor survival in PDAC patients [47]. Through molecular and functional analysis of primary cultures of CAF derived from PDAC patients, Neuzillet C et al. have found that subtype A CAF cultures displayed low expression of vimentin and α-SMA, and may be associated with a less pro-tumoural (less pro-proliferative and chemoprotective to cancer cells) profile than other non-subtype A CAFs [137].
In gastric cancer, strong immunoreactivity of vimentin is detected only in stroma cells, most of which are CAFs [138, 139]. Vimentin+ CAFs are mainly related to higher T stage, Lauren classification(diffuse type), and higher serum carcinoembryonic antigen level [138, 139]. They can promote EMT in cancer cells by secreting elastin fibers, leading to distant metastasis. Therefore, vimentin+CAFs are an independent prognostic factor, and associated with recurrence, distant metastasis and reduced survival in patients with gastric cancer [138, 139].
During the progression of esophageal cancer initiated by chronic inflammation, vimentin+ α-SMA+ myofibroblasts migrate to sites of intestinal metaplasia and dysplasia, and may become an innocent bystander in tumor progression by directly enhancing proliferation of nearby epithelial cells in an NF-κB-dependent manner as well as indirectly by recruiting and polarizing cells of the adaptive and innate immune system toward a tumor-promoting phenotype [140].
In breast cancer, vimentin is conveniently abundant in connective tissue septa, especialy CAFs, but usually absent from the tumour parenchyma. It is associated with indices of poor prognosis, such as high growth fraction/S-phase, lack of oestrogen receptor, and poor nuclear grade [141].
In a genetic engineering mouse model of lung cancer, vimentin+ CAFs have been found to surround the cancer cells sprouting from the primary tumor to improve lung cancer metastasis [142]. As a result, vimentin may be used as a target for anti-metastasis therapy [142].
In oropharyngeal squamous cell carcinoma (OPSCC), the vimentin expression in CAFs is stronger among HPV-positive tumors than HPV-negative tumors. That suggest vimentin+ CAFs may be involved in the development of OPSCC due to HPV infection [143].
Tenascin C
TNC is an ECM protein that assembles into a hexamer via a disulfide bond at the N terminus. TNC comprises several functional structural domains, including a C-terminal globular structural domain, a heptapeptide repeat sequence, an epidermal growth factor-like repeat sequence, and a fibronectin type III structural domain. The latter domain can combine with other cell surface receptors and ECM molecules such as integrins and fibronectin [144]. TNC+ CAFs are mainly derived from MSCs [34].
In pancreatic cancer, high expression of TNC in CAFs activates downstream signaling through the Annexin II receptor, promotes EMT, and leads to distant metastasis [145, 146]. In addition, TNC+ CAFs are significantly related to lymph node metastasis in breast cancer [147] and prostate cancer [45] and associated with an advanced clinical stage and an elevated microvessel density and tumor-associated macrophages (TAMs) population in prostate cancer [45].
Based on the ability of TNC to promote tumor development, the high expression of TNC in CAFs is an independent prognostic marker for multiple cancers, including breast cancer [147], bladder cancer [148], prostate cancer [45], CRC (especially stage II and III CRC) [149, 150], and pancreatic cancer [151]. TNC combined with other TME components, such as low stromal caveolin-1 and CD8+ T cell number, could be used as significant prognostic markers in patients with NSCLC [152].
CD90
CD90, encoded by THY1, is a glycosylated phosphatidylinositol-anchored glycoprotein expressed on a variety of cells, including activated microvascular endothelial cells, blood stem cells, neurons, and fibroblasts [153–155]. CD90 is a notable regulator of cell–matrix and cell–cell interactions and plays a major role in neural regeneration, cell migration, adhesion, and fibrosis [156]. The main source of CD90+ CAFs is MSCs [34] while stellate cells are another source [157].
CD90+ CAFs promote the malignant biological behavior of tumors. They are the primary source of IL-6, which maintains the stemness of CRC cells and induces an adaptive immune inflammatory response favoring tumor growth [158]. CD90+ CAFs promotes tumor cell invasion by enhancing tumor cell–endothelial cell attachment in a KrasG12D-driven LUAC (KrasLA1) mouse model [159]. In hepatocellular carcinoma (HCC), a significant number of CAFs highly express CD90, which correlates with angiogenesis markers (CD105, CD34, and CD31) and is related to poor prognosis in HCC patients [160]. As a marker of activated stellate cells in pancreatic cancer, CD90+ CAFs may participate in the tumor–stromal interaction and promote cancer progression [157]. It could serve as a prostate cancer biomarker for CD90+ CAFs and distinguish prostate cancer-related stroma from benign stroma [161].
CD10 and GPR77
CD10, also known as common acute lymphoblastic leukemia antigen (CALLA), is a 90–110-kDa protein belonging to the zinc-dependent type II metalloproteinase family, an endopeptidase that degrades various bioactive peptides in the ECM [162]. Bone marrow-derived MSCs are the main source of CD10+ CAFs [163]. In addition, pre-B lymphocytes can also differentiate into CD10+ CAFs [6].
In breast cancer, CD10+ CAFs are related to estrogen receptor (ER)-negative invasive breast cancer, whereas CD10− CAFs are associated with luminal-type invasive breast cancer [162].
In UC, CD10 expression in CAFs is significantly associated with poor prognostic clinicopathological factors, such as squamous differentiation, lymph node metastasis, and tumor necrosis. CD10 expression in cancer cells and CAFs are both related to a high tumor grade and shorter overall survival [164].
GPR77 belongs to the non-G protein-coupled receptor family [165]. Bone marrow-derived MSCs and polymorphonuclear neutrophils [6] are the main sources of GPR77+ CAFs. It has been found that CD10 and GPR77 can define human CAF subpopulations, and CAF subsets with high expression of CD10 and GPR77 are related to chemotherapy resistance and poor prognosis in patients with lung and breast cancer. CD10+GPR77+ CAFs supply consistent paracrine IL-8 and IL-6 through continuous nuclear factor-kappa B (NF-κB) signal transduction maintained by p65 acetylation and phosphorylation, forming a niche that protects CSCs from chemotherapy-induced cell death [163].
In a breast cancer patient-derived xenograft model, blockade of GPR77 with neutralizing monoclonal antibody markedly reduced the infiltration of CD10+GPR77+ CAFs and the proportion of ALDH1+ CSCs, resulting in decreased tumorigenesis and increased chemosensitivity [163].
Markers of CAFs derived from stellate cells
Galectin 1
Galectin 1 (Gal1) is a β-galactoside-binding protein that bidirectionally regulates cell proliferation in a cell-specific and dose-dependent manner. It improves angiogenesis via the vascular endothelial growth factor receptor 2 (VEGFR2) pathway and participates in immune regulation by inducing T cell apoptosis and suppressing T cell proliferation and antigen-presenting cell activation [166]. Stellate cells can differentiate into Gal1+ CAFs with TGF-β induction [167].
In breast cancer, CAF-derived Gal1 helps to delimitate the immunosuppressive microenvironment by shifting it to a Th2 cytokine profile and increasing the frequency of Treg cells [168, 169]. Gal1+ CAFs participate in different events in PDAC, including tumor cell proliferation, angiogenesis, invasion, metastasis, and inflammation [103, 167]. Stromal Gal-1 promotes the cancer-initiating cell trait and disease dissemination in CRC via β-catenin and SOX9 [170]. High Gal-1 expression in CAFs is related to a worse clinical outcome in lung and breast cancer patients [168, 169, 171].
Markers of CAFs derived from epithelial cells
Adipocyte enhancer-binding protein 1
Adipocyte enhancer-binding protein 1 (AEBP1) is a widely expressed multifunctional protein, especially in preadipocytes and macrophages, that produces a variety of inflammatory mediators, including tumor necrosis factor α (TNFα), monocyte chemotactic protein 1 (MCP-1), and IL-6 [172, 173]. AEBP1 has been found to be intimately related to EMT, suggesting that epithelial cells may be the main source of AEBP1+ CAFs [173].
Recent studies suggest that AEBP1 in the stroma may play a major role in cancer promotion. AEBP1 is related to numerous human malignancies, including gastric cancer, colon cancer, glioblastoma, and melanoma [174].
AEBP1 overexpression was recently found in CRC CAFs. It promotes proliferation, invasion, migration, and metastasis by activating NF-κB signaling. In melanoma and CRC, high expression of AEBP1 in CAFs is positively correlated with both fibroblast biomarker expression and EMT meta scores, suggesting that AEBP1 is responsible for EMT and that AEBP1-mediated EMT may promote fibroblast activity [174, 175]. AEBP1 is upregulated in CAFs of lung squamous carcinoma patients and is an independent prognostic factor in both univariate and multivariate analyses [176].
Cancer-promoting CAF markers of unclear origin
Osteopontin
Osteopontin (OPN) is a secretory phosphoprotein. As a cell attachment protein and cytokine, it signals through two kinds of cell adhesion molecules—integrin V-3 and CD44—to participate in tumorigenesis [177–180]. The source of OPN+ CAFs is not clear at present.
CAF-secreted OPN promotes cancer stemness via the secreted phosphoprotein 1(SPP1)–CD44 axis in pancreatic cancer [181]. It combines with avb3 and CD44, which activates the STAT3/PPARγ pathway and promotes macrophage M2 polarization and finally contributes to CRC tumorigenesis [182]. In lung cancer, OPN expressed by CAFs acts as a potential biomarker of invasiveness and proliferation [183]. Anti-OPN monoclonal antibody (AOM1) alone or coupled with carboplatin markedly suppresses the growth of lung metastases, thereby demonstrating the important function of OPN in tumor metastasis and progression [184]. The expression of OPN is related to poor survival in patients of various tumor types such as breast, lung, and colorectal cancers [185–188].
CAF markers with cancer-restraining function
Meflin
Meflin is a glycosylphosphatidylinositol-anchored protein encoded by the ISLR (immunoglobulin superfamily leucine-rich repeat sequence) gene [189, 190]. It can also be secreted into the culture medium of fibroblasts and specifically expressed in CAFs of human and mouse PDAC [190–193].
Meflin expression in CAFs of early pancreatic cancer effectively attenuates tumor aggressiveness. Patients with high levels of meflin-expressing CAFs are more likely to have highly differentiated pathological histological features and better prognosis, and meflin deficiency in CAFs can lead to more aggressive behavior and resistance to chemotherapeutic agents in PDAC. This suggests that meflin is a functional marker of rCAFs in PDAC [192, 194].
Meflin+ CAFs are associated with a favorable therapeutic response to immune checkpoint blockade in patients with NSCLC. In NSCLC patients and mouse syngeneic tumor models, large numbers of meflin+ CAFs attracted greater CD4+ T cell infiltration and promoted tumor angiogenesis, which in turn enhanced the response of NSCLC to immune checkpoint blockade therapy [195].
Interestingly, meflin+ fibroblasts are found in the mesenchyme of non-neoplastic fibrotic diseases, such as cardiac fibrosis, idiopathic pulmonary fibrosis, and renal fibrosis. They play an essential role in tissue repair and in inhibiting fibrosis [196–198]. To date, two proteins have been identified to interact with meflin. One is bone morphogenetic protein 7 (BMP7), a cytokine to which meflin binds, activating downstream Smad1/5 and functionally counteracting the profibrotic function of TGF-β [196, 199, 200]. Another meflin ligand is lysyl oxidase (Lox), a cross-linking agent of collagen fibers that promotes fibrosis and tissue sclerosis [194, 201–203]. Meflin interacts with Lox and inhibits its collagen cross-linking agent activity, which in turn inhibits fibrosis formation [194].
Meflin has been found to act as a marker of reversed CAFs with elevated drug sensitivity. A lineage tracing experiment has shown that meflin+ rCAFs differentiate into α-SMA+ meflin− CAFs, known as pCAFs, in the course of cancer progression. AM80, a synthetic unnatural retinoid, is an effective agent for the conversion of meflin− pCAFs to meflin+ rCAFs, enhancing drug distribution and chemosensitivity via the conversion of CAFs [204].
CD146
CD146 is a cell adhesion molecule originally considered a melanoma-specific cell adhesion molecule [205]. It participates in the immune response, MSC differentiation, cell development, cell migration, signal transduction, and angiogenesis [206]. The main source of CD146+ CAFs is vascular endothelial cells [6].
In ER+ breast cancers, CD146 distinguishes two CAF subgroups: CD146+ CAFs maintain ER expression, estrogen-dependent proliferation, and sensitivity to tamoxifen, whereas CD146− CAFs play the opposite role [207]. The expression of CD146 is higher in PDAC CAFs than in cancer cells, and CD146 expression in CAFs is related to low-histological grade PDAC and high-grade pancreatic intraepithelial neoplasia. Patients with CD146low CAFs have a worse prognosis. In vitro experiments using primary cultures of CAFs isolated from PDAC tissues have shown that blockade of CD146 expression in CAFs significantly increases the invasion and migration of cancer cells. Furthermore, knockdown of CD146 promotes CAF activation by inhibiting the NF-κB pathway and upregulating the secretion of CAF chemokines and cytokines, such as hepatocyte growth factor (HGF), CXCL1, SDF1A, COX2, and CCL5. In conclusion, the decreased expression of CD146 in CAFs promotes pancreatic cancer progression [208].
CAF markers with bidirectional functions
Caveolin-1
Caveolin-1 (Cav-1) is a scaffolding protein and a major component of cell surface depressions [209]. It participates in numerous physiological functions, such as intracellular cholesterol transport, endocytosis, and cell surface signaling [210–214], and plays a significant role in cellular senescence [215, 216]. Cav-1+ CAFs can be derived from NFs, vascular endothelial cells, and adipocytes [6].
In cancer, Cav-1 mainly participates in various processes, including cell transformation, tumor growth, invasion and metastasis, multidrug resistance, and angiogenesis [217, 218]. Downregulation of Cav-1 in the TME promotes the transformation of adjacent NFs into CAFs [219]. Promoter hypermethylation is the main cause of the silencing of Cav-1 gene expression [220].
Cav-1 in CAFs has been found to have tumor-suppressive properties, and deletion of Cav-1 predicts poor prognosis. In breast cancer, Cav-1 deletion from CAFs is related to a more advanced tumor stage, early cancer recurrence, lymph node metastasis, and poor prognosis. In patients selected for triamcinolone therapy, Cav-1 deletion in CAFs is a strong predictor of a poor clinical prognosis and can indicate triamcinolone resistance [217, 221–223]. In addition to the findings from breast cancer, Cav-1low CAFs in prostate and gastric cancers also predict a poor prognosis [217, 223]. In a phase II clinical trial of NSCLC patients treated with nab-paclitaxel, Cav-1 expression in CAFs was related to prolonged survival and increased response rates, indicating that Cav-1 is a marker of nab-paclitaxel treatment efficacy [224].
The tumor-suppressive properties of Cav-1 expressed in CAFs can be achieved via several mechanisms. First, Cav-1 deficiency can cause EMT. Pavlides et al. have found that CAFs with Cav-1 deficiency overexpress multiple growth factors, such as PDGF, VEGF, TGF-β, and IL-6, and stimulate normal mammary epithelial cells to generate EMT in a paracrine manner [209]. Second, Cav-1 deficiency also promotes the malignant phenotype of tumors by affecting tumor metabolism. Several studies have shown that, based on the reverse Warburg effect theory, the upregulation of TGF-β caused by Cav-1 deletion can induce fibroblasts to undergo aerobic glycolysis and produce lactic acid while promoting the transformation of fibroblasts to myofibroblasts, which exhibit more pronounced activity in oxygenated glycolysis and autophagy, providing energy material to neighboring cancer cells. This metabolic symbiosis also contributes to the malignant phenotype of the cancer, and the activation of autophagy in the tumor stroma leads to chemoresistance [225, 226]. Third, a progressive reduction in Cav-1 expression is involved in radioresistance in prostate cancer through upregulation of TRIAP1 (TP53-regulated inhibitor of apoptosis 1) [227].
However, some studies have come to the opposite conclusion, suggesting that Cav-1 expression in CAFs is a potential indicator of cancer progression [33, 228, 229]. Goetz JG et al. have found that Cav-1 expression is significantly higher in breast cancer CAFs than in NFs and that the 10-year risk of death is 2.5-fold higher in patients with Cav-1high than in controls, suggesting that Cav-1 in CAFs has the potential to improve tumor cell invasion and metastasis by regulating Rho-mediated cell contractility in a p190-dependent manner, remodeling the tumor stroma and intra-tumor microenvironment and hardening the TME [228]. In pancreatic cancer, the CA19-9 level and rates of an advanced tumor stage are markedly higher in CAV1high patients than in controls. Moreover, the disease-free survival and overall survival are markedly lower in patients with high CAV1 than in controls. The same conclusion was also reached for renal cell carcinoma, prostate cancer, colon cancer, and melanoma [33, 228, 229].
The above results indicate that Cav-1+ CAFs play a distinct role in tumor progression. Treatments targeting Cav-1 may require a completely different strategy.
Podoplanin
The mucous sialoglycoprotein podoplanin (PDPN) is widely used as a histopathological marker to differentiate lymphatic vessels from blood vessels due to its expression on lymphatic vessel endothelial cells [230, 231]. However, recent studies have reported that PDPN is also expressed in cancer cells, dendritic cells, and inflammatory macrophages, especially CAFs [232–234]. A previous study showed that PDPN is present in extracellular and intracellular regions [235]. The binding of its intracellular regions to Ezrin, Radioxin, and Moesin (ERM) proteins is thought to lead to cell morphological changes and cytoskeleton reorganization [236]. PDPN+ CAFs are mainly derived from vascular endothelial cells [6].
PDPN+ CAFs exert tumor-promoting functions by facilitating the invasion of cancer cells [237], remodeling of the ECM [234, 238, 239], and promoting an immunosuppressive microenvironment [240].
In LUAC, the CD204+ TAM population is significantly larger and the CD8/FOXP3 T cell ratio markedly lower in PDPN+ CAF patients than in PDPN− CAF patients, indicating that PDPN induces the polarization of M2 macrophages and suppresses the gene expression levels of cytokines of immune-related lymphocytes and thereby allowing LUAC to be characterized by an immunosuppressive microenvironment [240]. In stage I lung squamous cell cancer, PDPN+ CAFs highly express TGF-β1 and are associated with the infiltration of CD204+ TAMs, further demonstrating that PDPN+ CAFs can lead to an immunosuppressive microenvironment [241]. In addition, PDPN+ CAFs induce primary resistance to EGFR tyrosine kinase inhibitor (EGFR-TKI) in LUAC with EGFR mutation by activating the MAPK signaling pathway in cancer cells [242].
PDPN+ CAFs are associated with various adverse clinicopathological factors and poor prognosis. They are related to lymphatic vessel invasion and/or lymph node metastasis in EAC [243], perihilar cholangiocarcinoma [244], PDAC [245, 246], and melanoma [247]; an advanced tumor stage, including TNM stage in perihilar cholangiocarcinoma [244] and T stage in EAC [244] and PDAC [245, 246]; a high histological grade in PDAC [245, 246]; and a shorter survival time in EAC [243], PDAC [245, 246], melanoma [247], lung squamous cell cancer [248], and LUAC [240].
However, in breast and colorectal cancers, the opposite findings have been obtained. In invasive breast cancer, PDPN-expressing CAFs are a favorable independent prognostic factor [249]. In CRC, high PNPD expression in CAFs is protective against CRC cell invasion and is a major sign of good prognosis in patients with advanced CRC [250]. Another study has found that PDPN+CAFs are abundantly present in the tumor center rather than the infiltrative margins, and they are associated with favorable clinicopathological parameters and prolonged tumor-free survival [251]. As a mucin-type glycoproteins, PDPN has an extended brush-like conformation due to their extensive O-glycosylation [252]. This highly negatively charged structure is relatively resistant to proteases and provides a physical barrier protecting cells from environmental agents. In addition, the PDPN+ CAFs locate mainly in the superficial to deep area of the tumor, sparing the invasive front where tumor budding is often observed. The above reasons support the idea that podoplanin may play an important protective role against cancer invasion [215, 216].
The above results suggest that cancer cells respond differently to PDPN+ CAFs in different cancers or that the distinct functions of PDPN+ CAF in various organs may account for the different roles of PDPN in different cancers.
CD200
CD200 (OX2), an OX2 membrane glycoprotein, is normally expressed in hair follicle epithelial cells, neurons, lymphocytes, MSCs, and monocytes [253]. CD200 interacts with the CD200 receptor (CD200R1) to trigger an immunosuppressive response [254].
Recent studies have shown that CD200 can be expressed in CAFs. Analysis of samples from LUAC patients with an EGFR gene mutation treated with the EGFR-TKI gefitinib after surgery showed that those with CD200+ CAFs in resected LUAC tended to have longer progression-free survival in terms of postoperative recurrence. Further studies showed that a subpopulation of CD200+ CAFs exhibited increased gefitinib sensitivity due to an enhanced pro-apoptotic effect of gefitinib on cancer cells and that downregulation of CD200 expression deprived CAFs of their sensitizing ability. The above results indicated that CD200+ CAFs may play a major role in the therapeutic application of EGFR-TKIs [255].
In pancreatic cancer, CD200 is positively expressed in CAFs and negatively expressed in cancer cells, but no clear correlation is seen with either progression-free survival or overall survival [256]. However, as a regulator of myeloid cell activity, CD200 expression in PDAC CAFs limits the response of cancer cells to PD-1 immune checkpoint inhibitors by enhancing myeloid-derived suppressor cell activity. Blockage of CD200 significantly improves the effect of immunotherapy [257].
Samalizumab, a CD200 immune checkpoint inhibitor, showed favorable results in a phase I clinical trial of patients with chronic lymphocytic leukemia [258].
Other CAF markers
Several other markers have been found to be highly expressed in CAFs. Microfibril-associated protein 5 (MFAP5) is highly expressed in CAFs of tongue squamous cell carcinoma and is associated with the activation of multiple pro-growth signaling pathways, such as MAPK signaling. This indicates that MFAP5 may play a role in the identification of key oncogenic CAF subpopulations [259]. A new class of CAFs expressing MHC class II and CD74 has the ability to deliver antigens to CD4+ T cells and may regulate the immune response of PDAC and has been named the “antigen-presenting CAFs” [260]. CD70, which is more abundantly expressed in regulatory T cells, is highly expressed in CAFs of CRC and is negatively correlated with survival [261]. In addition, two markers, Transgelin (TAGLN) [59] and collagen type XI alpha I chain (COL11A1) [262], are also considered to be highly specific CAF markers. Their use in research is still limited, and further studies are needed to clarify the behavioral characteristics of these CAF markers in different cancers.
Summary
In summary, there are many CAF markers and, in addition to the traditional known markers, a variety of new markers have been discovered in recent years. However, there is still no clear marker to identify CAFs, and even the most commonly used FAP and α-SMA still have some unavoidable inaccuracies when used alone. First, the selection of markers is limited by cancer species, and even the same marker has different classification criteria among different cancer species. Second, multiple markers are required in combination to refine the specific functions of different CAF subtypes. The use of new methods, such as spatiotemporal single-cell transcriptome and proteome analyses and lineage tracing, will reveal a more exhaustive landscape of CAF differentiation and diversity in various cancers, allow us to better comprehend the behavior and function of CAFs and shed light on the crosstalk between genetic mutations in cancer cells and CAF heterogeneity. These approaches have implications for the identification of targets for tumor therapy. Third, we can reverse the pCAFs into rCAFs by targeting the appropriate CAF marker, which can compensate for the current deficiencies of cancer therapy by only targeting the cancer cells themselves. Finally, expansion of the definition of the TME by linking CAFs with other non-tumor cells in the TME, such as immune cells, can also make up for the deficiencies of CAFs themselves.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Liaoning Province (2020-ZLLH-45).
Authors’ contributions
• Zehua Zhao performed the literature search, wrote the first draft of the manuscript, authored or reviewed drafts of the paper, and approved the final draft. • Tianming Li drew the figure, and approved the final draft. • Yanmei Zhu conceived and critically revised drafts of the paper, and approved the final draft. • Yuan Yuan critically revised drafts of the paper, and approved the final draft.
Availability of data and materials
All data included in this study are available upon request by contact with the corresponding author.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare there are no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuan Yuan, Email: yuanyuan@cmu.edu.cn.
Yanmei Zhu, Email: zhuyanmei@cancerhosp-ln-cmu.com.
References
- 1.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 2.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganguly D, Chandra R, Karalis J, Teke M, Aguilera T, Maddipati R, Wachsmann MB, Ghersi D, Siravegna G, Zeh HJ, Brekken R, Ting DVT, Ligorio M. Cancer-associated fibroblasts: versatile players in the tumor microenvironment. Cancers. 2020;12(9):34. doi: 10.3390/cancers12092652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madar S, Goldstein I, Rotter V. 'Cancer associated fibroblasts' - more than meets the eye. Trends Mol Med. 2013;19(8):447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Peltier A, Seban R-D, Buvat I, Bidard F-C, Mechta-Grigoriou F. Fibroblast heterogeneity in solid tumors: From single cell analysis to whole-body imaging. Semin Cancer Biol. 2022;86(Pt 3):262–272. doi: 10.1016/j.semcancer.2022.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Han CC, Liu TY, Yin R. Biomarkers for cancer-associated fibroblasts. Biomark Res. 2020;8(1):8. doi: 10.1186/s40364-020-00245-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong C, Nie Y, Qu SH, Liao JY, Cui XY, Yao HR, Zeng YJ, Su FX, Song EW, Liu Q. miR-21 induces myofibroblast differentiation and promotes the malignant progression of breast phyllodes tumors. Can Res. 2014;74(16):4341–4352. doi: 10.1158/0008-5472.CAN-14-0125. [DOI] [PubMed] [Google Scholar]
- 8.Calvete J, Larrinaga G, Errarte P, Martin AM, Dotor A, Esquinas C, Nunes-Xavier CE, Pulido R, Lopez JI, Angulo JC. The coexpression of fibroblast activation protein (FAP) and basal-type markers (CK 5/6 and CD44) predicts prognosis in high-grade invasive urothelial carcinoma of the bladder. Hum Pathol. 2019;91:61–68. doi: 10.1016/j.humpath.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 9.An JL, Hou DK, Wang L, Wang LL, Yang YY, Wang HT. Fibroblast activation protein-alpha knockdown suppresses prostate cancer cell invasion and proliferation. Histol Histopath. 2022;37(6):597–607. doi: 10.14670/HH-18-430. [DOI] [PubMed] [Google Scholar]
- 10.Liu JY, Huang CQ, Peng CW, Xu F, Li Y, Yutaka Y, Xiong B, Yang XJ. Stromal fibroblast activation protein alpha promotes gastric cancer progression via epithelial-mesenchymal transition through Wnt/beta-catenin pathway. BMC Cancer. 2018;18:10. doi: 10.1186/s12885-018-5035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goscinski MA, Suo Z, Florenes VA, Vlatkovic L, Nesland JM, Giercksky KE. FAP-alpha and uPA show different expression patterns in premalignant and malignant esophageal lesions. Ultrastruct Pathol. 2008;32(3):89–96. doi: 10.1080/01913120802034934. [DOI] [PubMed] [Google Scholar]
- 12.Errarte P, Guarch R, Pulido R, Blanco L, Nunes-Xavier CE, Beitia M, Gil J, Angulo JC, Lopez JI, Larrinaga G. The expression of fibroblast activation protein in clear cell renal cell carcinomas is associated with synchronous lymph node metastases. PLoS ONE. 2016;11(12):13. doi: 10.1371/journal.pone.0169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun LY, Ke ML, Wang X, Yin MY, Wei JN, Xu L, Tian X, Wang F, Zhang H, Fu SB, Zhang CY. FAP(high) alpha-SMA(low) cancer-associated fibroblast-derived SLPI protein encapsulated in extracellular vesicles promotes ovarian cancer development via activation of PI3K/AKT and downstream signaling pathways. Mol Carcinog. 2022;61(10):910–923. doi: 10.1002/mc.23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Liu Y, Jia Y, Wang X, He J, Zhen S, Wang J, Liu L. Fibroblast activation protein in the tumor microenvironment predicts outcomes of PD-1 blockade therapy in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2022. 10.1007/s00432-022-04250-4. [DOI] [PMC free article] [PubMed]
- 15.Sun LL, Schroeder MC, Hagemann IS, Pfeifer JD, Schwarz JK, Grigsby PW, Markovina S, Lin AJ. Expression of potential biomarker targets by immunohistochemistry in cervical carcinomas. Int J Gynecol Pathol. 2022;41(6):628–635. doi: 10.1097/PGP.0000000000000853. [DOI] [PubMed] [Google Scholar]
- 16.Tong Y, Zhao Z, Zhang J, Wang W, Zhu Y. High expressions of CD10, FAP and GPR77 in CAFs are associated with chemoresistance and worse prognosis in gastric cancer. Front Oncol. 2022;12:984817. doi: 10.3389/fonc.2022.984817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi JJ, Sun HX, Zhang Y, Wang ZT, Xun ZZ, Li ZY, Ding XY, Bao RJ, Hong LW, Jia WQ, Fang F, Liu HZ, Chen L, Zhong J, Zou DW, Liu LX, Han L, Ginhoux F, Liu YB, Ye YQ, Su B. Single-cell and spatial analysis reveal interaction of FAP(+) fibroblasts and SPP1(+) macrophages in colorectal cancer. Nat Commun. 2022;13(1):20. doi: 10.1038/s41467-022-29366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen XY, He XP, Jiao F, Wang CH, Sun Y, Ren XQ, Li QW. Fibroblast activation protein-alpha-positive fibroblasts promote gastric cancer progression and resistance to immune checkpoint blockade. Oncol Res. 2017;25(4):629–640. doi: 10.3727/096504016X14768383625385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rong XX, Lv JY, Liu YT, Wang ZJ, Zeng DQ, Li YD, Li SW, Wu JH, Shen ZY, Shi M, Liao WJ, Wu ZZ, Wang CL. PET/Ct imaging of activated cancer-associated fibroblasts predict response to PD-1 blockade in gastric cancer patients (vol 11, 802257, 2022) Front Oncol. 2022;12:1. doi: 10.3389/fonc.2022.895938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, Bonneau C, Kondratova M, Kuperstein I, Zinovyev A, Givel AM, Parrini MC, Soumelis V, Vincent-Salomon A, Mechta-Grigoriou F. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33(3):463. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Feig C, Jones JO, Kraman M, Wells RJB, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinomaassociated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA. 2013;110(50):20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin YL, Li BJ, Yang XG, Cai Q, Liu WR, Tian MX, Luo HY, Yin W, Song Y, Shi YH, He R. Fibroblastic FAP promotes intrahepatic cholangiocarcinoma growth via MDSCs recruitment. Neoplasia. 2019;21(12):1133–1142. doi: 10.1016/j.neo.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, Divgi CR, Hanson LH, Mitchell P, Gansen DN, Larson SM, Ingle JN, Hoffman EW, Tanswell P, Ritter G, Cohen LS, Bette P, Arvay L, Amelsberg A, Vlock D, Rettig WJ, Old LJ. A phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res. 2003;9(5):1639–1647. [PubMed] [Google Scholar]
- 24.Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, Carswell EA, Cohen A, Larson SM, Old LJ. Antibody targeting in metastatic colon cancer: a phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol. 1994;12(6):1193–1203. doi: 10.1200/JCO.1994.12.6.1193. [DOI] [PubMed] [Google Scholar]
- 25.Fang JX, Xiao L, Joo KI, Liu YR, Zhang CP, Liu SL, Conti PS, Li ZB, Wang P. A potent immunotoxin targeting fibroblast activation protein for treatment of breast cancer in mice. Int J Cancer. 2016;138(4):1013–1023. doi: 10.1002/ijc.29831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330(6005):827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 27.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119(12):3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams S, Miller GT, Jesson MI, Watanabe T, Jones B, Wallner BP. PT-100, a small molecule dipeptidyl peptidase inhibitorg has potent antitumor effects and augments antibody-mediated cytotoxicity via a novel immune mechanism. Can Res. 2004;64(15):5471–5480. doi: 10.1158/0008-5472.CAN-04-0447. [DOI] [PubMed] [Google Scholar]
- 29.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116(7):1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang LCS, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, Antzis M, Cotner CE, Johnson LA, Durham AC, Solomides CC, June CH, Pure E, Albelda SM. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2(2):154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kakarla S, Chow KKH, Mata M, Shaffer DR, Song XT, Wu MF, Liu H, Wang LL, Rowley DR, Pfizenmaier K, Gottschalk S. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21(8):1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mezawa Y, Orimo A. The roles of tumor- and metastasis-promoting carcinoma-associated fibroblasts in human carcinomas. Cell Tissue Res. 2016;365(3):675–689. doi: 10.1007/s00441-016-2471-1. [DOI] [PubMed] [Google Scholar]
- 33.Bu L, Baba H, Yoshida N, Miyake K, Yasuda T, Uchihara T, Tan P, Ishimoto T. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene. 2019;38(25):4887–4901. doi: 10.1038/s41388-019-0765-y. [DOI] [PubMed] [Google Scholar]
- 34.Weber CE, Kothari AN, Wai PY, Li NY, Driver J, Zapf MAC, Franzen CA, Gupta GN, Osipo C, Zlobin A, Syn WK, Zhang J, Kuo PC, Mi Z. Osteopontin mediates an MZF1-TGF-beta 1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene. 2015;34(37):4821–4833. doi: 10.1038/onc.2014.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita H, Ohuchida K, Mizumoto K, Nakata K, Yu J, Kayashima T, Cui L, Manabe T, Ohtsuka T, Tanaka M. alpha-smooth muscle actin expressing stroma promotes an aggressive tumor biology in pancreatic ductal adenocarcinoma. Pancreas. 2010;39(8):1254–1262. doi: 10.1097/MPA.0b013e3181dbf647. [DOI] [PubMed] [Google Scholar]
- 36.Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21(4):957–969. doi: 10.3892/or_00000309. [DOI] [PubMed] [Google Scholar]
- 37.Muchlinska A, Nagel A, Popeda M, Szade J, Niemira M, Zielinski J, Skokowski J, Bednarz-Knoll N, Zaczek AJ. Alpha-smooth muscle actin-positive cancer-associated fibroblasts secreting osteopontin promote growth of luminal breast cancer. Cell Mol Biol Lett. 2022;27(1):14. doi: 10.1186/s11658-022-00351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAndrews KM, Chen Y, Darpolor JK, Zheng XF, Yang SJ, Carstens JL, Li BR, Wang HM, Miyake T, de Sampaio PC, Kirtley ML, Natale M, Wu CC, Sugimoto H, LeBleu VS, Kalluri R. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-mediated therapy resistance in pancreatic cancer. Cancer Discov. 2022;12(6):1580–1597. doi: 10.1158/2159-8290.CD-20-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartoschek M, Oskolkov N, Bocci M, Lovrot J, Larsson C, Sommarin M, Madsen CD, Lindgren D, Pekar G, Karlsson G, Ringner M, Bergh J, Bjorklund A, Pietras K. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9:13. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio IIC, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grout JA, Sirven P, Leader AM, Maskey S, Hector E, Puisieux I, Steffan F, Cheng EV, Tung NV, Maurin M, Vaineau R, Karpf L, Plaud M, Begue AL, Ganesh K, Mesple J, Casanova-Acebes M, Tabachnikova A, Keerthivasan S, Lansky A, Le Berichel J, Walker L, Rahman AH, Gnjatic S, Girard N, Lefevre M, Damotte D, Adam J, Martin JC, Wolf A, Flores RM, Beasley MB, Pradhan R, Muller S, Marron TU, Turley SJ, Merad M, Kenigsberg E, Salmon H. Spatial positioning and matrix programs of cancer-associated fibroblasts promote T-cell exclusion in human lung tumor. Cancer Discov. 2022;12(11):2606–2625. doi: 10.1158/2159-8290.CD-21-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel AK, Vipparthi K, Thatikonda V, Arun I, Bhattacharjee S, Sharan R, Arun P, Singh S. A subtype of cancer-associated fibroblasts with lower expression of alpha-smooth muscle actin suppresses stemness through BMP4 in oral carcinoma. Oncogenesis. 2018;7:15. doi: 10.1038/s41389-018-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okuyama K, Suzuki K, Yanamoto S, Naruse T, Tsuchihashi H, Yamashita S, Umeda M. Anaplastic transition within the cancer microenvironment in early-stage oral tongue squamous cell carcinoma is associated with local recurrence. Int J Oncol. 2018;53(4):1713–1720. doi: 10.3892/ijo.2018.4515. [DOI] [PubMed] [Google Scholar]
- 44.Hanley CJ, Noble F, Ward M, Bullock M, Drifka C, Mellone M, Manousopoulou A, Johnston HE, Johnston HE, Hayden A, Thirdborough S, Liu YM, Smith DM, Mellows T, Kao WJ, Garbis SD, Mirnezami A, Underwood TJ, Eliceiri KW, Thomas GJ. A subset of myofibroblastic cancer-associated fibroblasts regulate collagen fiber elongation, which is prognostic in multiple cancers. Oncotarget. 2016;7(5):6159–6174. doi: 10.18632/oncotarget.6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ni WD, Yang ZT, Cui CA, Cui Y, Fang LY, Xuan YH. Tenascin-C is a potential cancer-associated fibroblasts marker and predicts poor prognosis in prostate cancer. Biochem Biophys Res Commun. 2017;486(3):607–612. doi: 10.1016/j.bbrc.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Zhang XF, Dong M, Pan YH, Chen JN, Huang XQ, Jin Y, Shao CK. Expression pattern of cancer-associated fibroblast and its clinical relevance in intrahepatic cholangiocarcinoma. Hum Pathol. 2017;65:92–100. doi: 10.1016/j.humpath.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Maehira H, Miyake T, Iida H, Tokuda A, Mori H, Yasukawa D, Mukaisho KI, Shimizu T, Tani M. Vimentin expression in tumor microenvironment predicts survival in pancreatic ductal adenocarcinoma: heterogeneity in fibroblast population. Ann Surg Oncol. 2019;26(13):4791–4804. doi: 10.1245/s10434-019-07891-x. [DOI] [PubMed] [Google Scholar]
- 48.Yuzawa S, Kano MR, Einama T, Nishihara H. PDGFR beta expression in tumor stroma of pancreatic adenocarcinoma as a reliable prognostic marker. Med Oncol. 2012;29(4):2824–2830. doi: 10.1007/s12032-012-0193-0. [DOI] [PubMed] [Google Scholar]
- 49.Mecham RP. Overview of extracellular matrix. Curr Protoc Cell Biol. 2012;Chapter 10:Unit 10.1. doi: 10.1002/0471143030.cb1001s00. [DOI] [PubMed] [Google Scholar]
- 50.Aronson KJ, Miller AB, Woolcott CG, Sterns EE, McCready DR, Lickley LA, Fish EB, Hiraki GY, Holloway C, Ross T, Hanna WM, SenGupta SK, Weber JP. Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9(1):55–63. [PubMed] [Google Scholar]
- 51.Morra L, Rechsteiner M, Casagrande S, von Teichman A, Schraml P, Moch H, Soltermann A. Characterization of periostin isoform pattern in non-small cell lung cancer. Lung Cancer. 2012;76(2):183–190. doi: 10.1016/j.lungcan.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 52.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie ANJ, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118(1):98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 53.Ashley SL, Wilke CA, Kim KK, Moore BB. Periostin regulates fibrocyte function to promote myofibroblast differentiation and lung fibrosis. Mucosal Immunol. 2017;10(2):341–351. doi: 10.1038/mi.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14(7):1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 55.Izuhara K, Conway SJ, Moore BB, Matsumoto H, Holweg CTJ, Matthews JG, Arron JR. Roles of periostin in respiratory disorders. Am J Respir Crit Care Med. 2016;193(9):949–956. doi: 10.1164/rccm.201510-2032PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Dwyer DN, Moore BB. The role of periostin in lung fibrosis and airway remodeling. Cell Mol Life Sci. 2017;74(23):4305–4314. doi: 10.1007/s00018-017-2649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68(19):3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu L, Wang JS, Liu K. Role of periostin in ECRS. Eur Arch Oto-Rhino-Laryn. 2021;278(8):2665–2672. doi: 10.1007/s00405-020-06369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Planche A, Bacac M, Provero P, Fusco C, Delorenzi M, Stehle JC, Stamenkovic I. Identification of prognostic molecular features in the reactive stroma of human breast and prostate cancer. PLoS ONE. 2011;6(5):12. doi: 10.1371/journal.pone.0018640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bao SD, Ouyang G, Bai XF, Huang Z, Ma CY, Liu M, Shoo R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5(4):329–339. doi: 10.1016/S1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 61.Kikuchi Y, Kunita A, Iwata C, Komura D, Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita Y, Yashiro M, Hirakawa K, Miyazono K, Kudo A, Fukayama M, Kashima TG. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am J Pathol. 2014;184(3):859–870. doi: 10.1016/j.ajpath.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 62.Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY, Chang DD. Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(v)/beta(3) and alpha(v)beta(5) integrins and promotes cell motility. Can Res. 2002;62(18):5358–5364. [PubMed] [Google Scholar]
- 63.Yue HR, Li WZ, Chen RF, Wang JY, Lu X, Li J. Stromal POSTN induced by TGF-beta 1 facilitates the migration and invasion of ovarian cancer. Gynecol Oncol. 2021;160(2):530–538. doi: 10.1016/j.ygyno.2020.11.026. [DOI] [PubMed] [Google Scholar]
- 64.Underwood TJ, Hayden AL, Derouet M, Garcia E, Noble F, White MJ, Thirdborough S, Mead A, Clemons N, Mellone M, Uzoho C, Primrose JN, Blaydes JP, Thomas GJ. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol. 2015;235(3):466–477. doi: 10.1002/path.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei WF, Chen XJ, Liang LJ, Yu L, Wu XG, Zhou CF, Wang ZC, Fan LS, Hu Z, Liang L, Wang W. Periostin(+)cancer-associated fibroblasts promote lymph node metastasis by impairing the lymphatic endothelial barriers in cervical squamous cell carcinoma. Mol Oncol. 2021;15(1):210–227. doi: 10.1002/1878-0261.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li XC, Sun Z, Peng GX, Xiao Y, Guo JC, Wu B, Li XY, Zhou WX, Li JR, Li Z, Bai CM, Zhao L, Han Q, Zhao RC, Wang XY. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. 2022;12(2):620–638. doi: 10.7150/thno.60540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kong J, Tian HZ, Zhang FY, Zhang ZB, Li J, Liu X, Li XC, Liu J, Li XJ, Jin D, Yang XS, Sun B, Guo T, Luo Y, Lu Y, Lin BC, Liu TJ. Extracellular vesicles of carcinoma-associated fibroblasts creates a pre-metastatic niche in the lung through activating fibroblasts. Mol Cancer. 2019;18(1):16. doi: 10.1186/s12943-019-1101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu BB, Wu KL, Wang X, Zhang JJ, Wang LZ, Jiang YY, Zhu XQ, Chen WT, Yan M. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis. 2018;9:18. doi: 10.1038/s41419-018-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Deng XM, Ao S, Hou JN, Li ZF, Lei YP, Lyu GQ. Prognostic significance of periostin in colorectal cancer. Chin J Cancer Res. 2019;31(3):547–556. doi: 10.21147/j.issn.1000-9604.2019.03.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu TY, Zhou LL, Xiao Y, Andl T, Zhang YH. BRAF inhibitors reprogram cancer-associated fibroblasts to drive matrix remodeling and therapeutic escape in melanoma. Can Res. 2022;82(3):419–432. doi: 10.1158/0008-5472.CAN-21-0614. [DOI] [PubMed] [Google Scholar]
- 71.Ryner L, Guan YH, Firestein R, Xiao YY, Choi YJ, Rabe C, Lu S, Fuentes E, Huw LY, Lackner MR, Fu L, Amler LC, Bais C, Wang YL. Upregulation of periostin and reactive stroma is associated with primary chemoresistance and predicts clinical outcomes in epithelial ovarian cancer. Clin Cancer Res. 2015;21(13):2941–2951. doi: 10.1158/1078-0432.CCR-14-3111. [DOI] [PubMed] [Google Scholar]
- 72.Soltermann A, Tischler V, Arbogast S, Braun J, Probst-Hensch N, Weder W, Moch H, Kristiansen G. Prognostic significance of epithelial-mesenchymal and mesenchymal-epithelial transition protein expression in non-small cell lung cancer. Clin Cancer Res. 2008;14(22):7430–7437. doi: 10.1158/1078-0432.CCR-08-0935. [DOI] [PubMed] [Google Scholar]
- 73.Neuzillet C, Nicolle R, Raffenne J, Tijeras-Raballand A, Brunel A, Astorgues-Xerri L, Vacher S, Arbateraz F, Fanjul M, Hilmi M, Samain R, Klein C, Perraud A, Rebours V, Mathonnet M, Bieche I, Kocher H, Cros J, Bousquet C. Periostin- and podoplanin-positive cancer-associated fibroblast subtypes cooperate to shape the inflamed tumor microenvironment in aggressive pancreatic adenocarcinoma. J Pathol. 2022;258(4):408–425. doi: 10.1002/path.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Insua-Rodriguez J, Oskarsson T. The extracellular matrix in breast cancer. Adv Drug Deliv Rev. 2016;97:41–55. doi: 10.1016/j.addr.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 75.Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast. 2013;22:S66–S72. doi: 10.1016/j.breast.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 76.Lee YJ, Kim IS, Park SA, Kim Y, Lee JE, Noh DY, Kim KT, Ryu SH, Suh PG. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol Ther. 2013;21(5):1004–1013. doi: 10.1038/mt.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakazawa Y, Taniyama Y, Sanada F, Morishita R, Nakamori S, Morimoto K, Yeung KT, Yang J. Periostin blockade overcomes chemoresistance via restricting the expansion of mesenchymal tumor subpopulations in breast cancer. Sci Rep. 2018;8:14. doi: 10.1038/s41598-018-22340-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Primac I, Maquoi E, Blacher S, Heljasvaara R, Van Deun J, Smeland HYH, Canale A, Louis T, Stuhr L, Sounni NE, Cataldo D, Pihlajaniemi T, Pequeux C, De Wever O, Gullberg D, Noel A. Stromal integrin alpha 11 regulates PDGFR beta signaling and promotes breast cancer progression. J Clin Invest. 2019;129(11):4509–4528. doi: 10.1172/JCI125890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Papadopoulos N, Lennartsson J. The PDGF/PDGFR pathway as a drug target. Mol Asp Med. 2018;62:75–88. doi: 10.1016/j.mam.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 80.Erdogan B, Ao MF, White LM, Means AL, Brewer BM, Yang LJ, Washington MK, Shi CJ, Franco OE, Weaver AM, Hayward SW, Li DY, Webb DJ. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216(11):3799–3816. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Nola R, Menga A, Castegna A, Loizzi V, Ranieri G, Cicinelli E, Cormio G. The crowded crosstalk between cancer cells and stromal microenvironment in gynecological malignancies: biological pathways and therapeutic implication. Int J Mol Sci. 2019;20(10):29. doi: 10.3390/ijms20102401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pena C, Cespedes MV, Lindh MB, Kiflemariam S, Mezheyeuski A, Edqvist PH, Hagglof C, Birgisson H, Bojmar L, Jirstrom K, Sandstrom P, Olsson E, Veerla S, Gallardo A, Sjoblom T, Chang ACM, Reddel RR, Mangues R, Augsten M, Ostman A. STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Can Res. 2013;73(4):1287–1297. doi: 10.1158/0008-5472.CAN-12-1875. [DOI] [PubMed] [Google Scholar]
- 83.Kartha VK, Stawski L, Han R, Haines P, Gallagher G, Noonan V, Kukuruzinska M, Monti S, Trojanowska M. PDGFR beta is a novel marker of stromal activation in oral squamous cell carcinomas. PLoS ONE. 2016;11(4):15. doi: 10.1371/journal.pone.0154645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li JJ, Zhi XL, Sun YT, Chen M, Yao LQ. The PDGF family is associated with activated tumor stroma and poor prognosis in ovarian cancer. Dis Markers. 2022;2022:19. doi: 10.1155/2022/5940049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim H, Lee YK, Koo JS. Expression of CAF-related proteins is associated with histologic grade of breast phyllodes tumor. Dis Markers. 2016;2016:1–10. doi: 10.1155/2016/4218989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLos Med. 2008;5(1):123–138. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haubeiss S, Schmid JO, Murdter TE, Sonnenberg M, Friedel G, van der Kuip H, Aulitzky WE. Dasatinib reverses Cancer-associated Fibroblasts (CAFs) from primary lung carcinomas to a phenotype comparable to that of normal fibroblasts. Mol Cancer. 2010;9:8. doi: 10.1186/1476-4598-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schmidt-Hansen B, Ornås D, Grigorian M, Klingelhöfer J, Tulchinsky E, Lukanidin E, Ambartsumian N. Extracellular S100A4(mts1) stimulates invasive growth of mouse endothelial cells and modulates MMP-13 matrix metalloproteinase activity. Oncogene. 2004;23(32):5487–5495. doi: 10.1038/sj.onc.1207720. [DOI] [PubMed] [Google Scholar]
- 89.Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK, Vonderheide RH, Leach SD, Stanger BZ. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148(1–2):349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S, Couderc B, Escourrou G, Valet P, Muller C. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73(18):5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 91.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67(21):10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 92.Schmidt-Hansen B, Klingelhöfer J, Grum-Schwensen B, Christensen A, Andresen S, Kruse C, Hansen T, Ambartsumian N, Lukanidin E, Grigorian M. Functional significance of metastasis-inducing S100A4(Mts1) in tumor-stroma interplay. J Biol Chem. 2004;279(23):24498–24504. doi: 10.1074/jbc.M400441200. [DOI] [PubMed] [Google Scholar]
- 93.Cabezón T, Celis JE, Skibshøj I, Klingelhöfer J, Grigorian M, Gromov P, Rank F, Myklebust JH, Maelandsmo GM, Lukanidin E, Ambartsumian N. Expression of S100A4 by a variety of cell types present in the tumor microenvironment of human breast cancer. Int J Cancer. 2007;121(7):1433–1444. doi: 10.1002/ijc.22850. [DOI] [PubMed] [Google Scholar]
- 94.Mundim FGL, Pasini FS, Nonogaki S, Rocha RM, Soares FA, Brentani MM, Logullo AF. Breast carcinoma-associated fibroblasts share similar biomarker profiles in matched lymph node metastasis. Appl Immunohistochem. 2016;24(10):712–720. doi: 10.1097/PAI.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 95.Rasanen K, Sriswasdi S, Valiga A, Tang HY, Zhang G, Perego M, Somasundaram R, Li L, Speicher K, Klein-Szanto AJ, Basu D, Rustgi AK, Speicher DW, Herlyn M. Comparative secretome analysis of epithelial and mesenchymal subpopulations of head and neck squamous cell carcinoma identifies S100A4 as a potential therapeutic target. Mol Cell Proteomics. 2013;12(12):3778–3792. doi: 10.1074/mcp.M113.029587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jenkinson SR, Barraclough R, West CR, Rudland PS. S100A4 regulates cell motility and invasion in an in vitro model for breast cancer metastasis. Br J Cancer. 2004;90(1):253–262. doi: 10.1038/sj.bjc.6601483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grum-Schwensen B, Klingelhofer J, Grigorian M, Almholt K, Nielsen BS, Lukanidin E, Ambartsumian N. Lung metastasis fails in MMTV-PyMT oncomice lacking S100A4 due to a T-Cell deficiency in primary tumors. Can Res. 2010;70(3):936–947. doi: 10.1158/0008-5472.CAN-09-3220. [DOI] [PubMed] [Google Scholar]
- 98.Ferrer-Mayorga G, Gomez-Lopez G, Barbachano A, Fernandez-Barral A, Pena C, Pisano DG, Cantero R, Rojo F, Munoz A, Larriba MJ. Vitamin D receptor expression and associated gene signature in tumour stromal fibroblasts predict clinical outcome in colorectal cancer. Gut. 2017;66(8):1449–1462. doi: 10.1136/gutjnl-2015-310977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ismail NI, Kaur G, Hashim H, Hassan MS. S100A4 overexpression proves to be independent marker for breast cancer progression. Cancer Cell Int. 2008;8:6. doi: 10.1186/1475-2867-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Klingelhofer J, Grum-Schwensen B, Beck MK, Knudsen RSP, Grigorian M, Lukanidin E, Ambartsumian N. Anti-S100A4 antibody suppresses metastasis formation by blocking stroma cell invasion. Neoplasia. 2012;14(12):1260. doi: 10.1593/neo.121554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goicoechea SM, Arneman D, Otey CA. The role of palladin in actin organization and cell motility. Eur J Cell Biol. 2008;87(8–9):517–525. doi: 10.1016/j.ejcb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150(3):643–655. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Orozco CA, Martinez-Bosch N, Guerrero PE, Vinaixa J, Dalotto-Moreno T, Iglesias M, Moreno M, Djurec M, Poirier F, Gabius HJ, Fernandez-Zapico ME, Hwang RF, Guerra C, Rabinovich GA, Navarro P. Targeting galectin-1 inhibits pancreatic cancer progression by modulating tumor-stroma crosstalk. Proc Natl Acad Sci USA. 2018;115(16):E3769–E3778. doi: 10.1073/pnas.1722434115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goicoechea SM, Garcia-Mata R, Staub J, Valdivia A, Sharek L, McCulloch CG, Hwang RF, Urrutia R, Yeh JJ, Kim HJ, Otey CA. Palladin promotes invasion of pancreatic cancer cells by enhancing invadopodia formation in cancer-associated fibroblasts. Oncogene. 2014;33(10):1265–1273. doi: 10.1038/onc.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cannon AR, Owen MK, Guerrero MS, Kerber ML, Goicoechea SM, Hemstreet KC, Klazynski B, Hollyfield J, Chang EH, Hwang RF, Otey CA, Kim HJ. Palladin expression is a conserved characteristic of the desmoplastic tumor microenvironment and contributes to altered gene expression. Cytoskeleton. 2015;72(8):402–411. doi: 10.1002/cm.21239. [DOI] [PubMed] [Google Scholar]
- 106.Alexander JI, Vendramini-Costa DB, Francescone R, Luong T, Franco-Barraza J, Shah N, Gardiner JC, Nicolas E, Raghavan KS, Cukierman E. Palladin isoforms 3 and 4 regulate cancer-associated fibroblast pro-tumor functions in pancreatic ductal adenocarcinoma. Sci Rep. 2021;11(1):19. doi: 10.1038/s41598-021-82937-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell. 2010;38(3):333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gilam A, Conde J, Weissglas-Volkov D, Oliva N, Friedman E, Artzi N, Shomron N. Local microRNA delivery targets Palladin and prevents metastatic breast cancer. Nat Commun. 2016;7:14. doi: 10.1038/ncomms12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Garcia-Palmero I, Torres S, Bartolome RA, Pelaez-Garcia A, Larriba MJ, Lopez-Lucendo M, Pena C, Escudero-Paniagua B, Munoz A, Casal JI. Twist1-induced activation of human fibroblasts promotes matrix stiffness by upregulating palladin and collagen alpha 1(VI) Oncogene. 2016;35(40):5224–5236. doi: 10.1038/onc.2016.57. [DOI] [PubMed] [Google Scholar]
- 110.Sato D, Tsuchikawa T, Mitsuhashi T, Hatanaka Y, Marukawa K, Morooka A, Nakamura T, Shichinohe T, Matsuno Y, Hirano S. Stromal palladin expression is an independent prognostic factor in pancreatic ductal adenocarcinoma. PLoS ONE. 2016;11(3):14. doi: 10.1371/journal.pone.0152523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gupta V, Bassi DE, Simons JD, Devarajan K, Al-Saleem T, Uzzo RG, Cukierman E. Elevated expression of stromal palladin predicts poor clinical outcome in renal cell carcinoma. PLoS ONE. 2011;6(6):12. doi: 10.1371/journal.pone.0021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu JJ, Zhang LM, He T, Xiao XL, Liu XY, Wang L, Yang LQ, Yang MM, Zhang TD, Chen R, Xu JM. TWIST represses estrogen receptor-alpha expression by recruiting the NuRD protein complex in breast cancer cells. Int J Biol Sci. 2012;8(4):522–532. doi: 10.7150/ijbs.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee KW, Yeo SY, Sung CO, Kim SH. Twist1 is a key regulator of cancer-associated fibroblasts. Can Res. 2015;75(1):73–85. doi: 10.1158/0008-5472.CAN-14-0350. [DOI] [PubMed] [Google Scholar]
- 114.Sung CO, Lee KW, Han S, Kim SH. Twist1 is up-regulated in gastric cancer-associated fibroblasts with poor clinical outcomes. Am J Pathol. 2011;179(4):1827–1838. doi: 10.1016/j.ajpath.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grzegrzolka J, Wojtyra P, Biala M, Piotrowska A, Gomulkiewicz A, Rys J, Podhorska-Okolow M, Dziegiel P. Correlation between expression of twist and podoplanin in ductal breast carcinoma. Anticancer Res. 2017;37(10):5485–5493. doi: 10.21873/anticanres.11978. [DOI] [PubMed] [Google Scholar]
- 116.Zhang WR, Bouchard G, Yu A, Shafiq M, Jamali M, Shrager JB, Ayers K, Bakr S, Gentles AJ, Diehn M, Quon A, West RB, Nair V, van de Rijn MV, Napel S, Plevritis SK. GFPT2-expressing cancer-associated fibroblasts mediate metabolic reprogramming in human lung adenocarcinoma. Can Res. 2018;78(13):3445–3457. doi: 10.1158/0008-5472.CAN-17-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hanover JA, Chen W, Bond MR. O-GlcNAc in cancer: an oncometabolism-fueled vicious cycle. J Bioenerg Biomembr. 2018;50(3):155–173. doi: 10.1007/s10863-018-9751-2. [DOI] [PubMed] [Google Scholar]
- 118.de Queiroz RM, Carvalho E, Dias WB. O-GlcNAcylation: the sweet side of the cancer. Front Oncol. 2014;4:132. doi: 10.3389/fonc.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akella NM, Ciraku L, Reginato MJ. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019;17(1):52. doi: 10.1186/s12915-019-0671-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: a metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35(10):547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olivier-Van Stichelen S, Guinez C, Mir AM, Perez-Cervera Y, Liu C, Michalski JC, Lefebvre T. The hexosamine biosynthetic pathway and O-GlcNAcylation drive the expression of β-catenin and cell proliferation. Am J Physiology Endocrinol Metab. 2012;302(4):E417–E424. doi: 10.1152/ajpendo.00390.2011. [DOI] [PubMed] [Google Scholar]
- 122.Vasconcelos-Dos-Santos A, Oliveira IA, Lucena MC, Mantuano NR, Whelan SA, Dias WB, Todeschini AR. Biosynthetic machinery involved in aberrant glycosylation: promising targets for developing of drugs against cancer. Front Oncol. 2015;5:138. doi: 10.3389/fonc.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ding X, Liu H, Yuan Y, Zhong Q, Zhong X. Roles of GFPT2 expression levels on the prognosis and tumor microenvironment of colon cancer. Front Oncol. 2022;12:811559. doi: 10.3389/fonc.2022.811559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szymura SJ, Zaemes JP, Allison DF, Clift SH, D'Innocenzi JM, Gray LG, McKenna BD, Morris BB, Bekiranov S, LeGallo RD, Jones DR, Mayo MW. NF-κB upregulates glutamine-fructose-6-phosphate transaminase 2 to promote migration in non-small cell lung cancer. Cell Commun Signal. 2019;17(1):24. doi: 10.1186/s12964-019-0335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kim J, Lee HM, Cai F, Ko B, Yang C, Lieu EL, Muhammad N, Rhyne S, Li K, Haloul M, Gu W, Faubert B, Kaushik AK, Cai L, Kasiri S, Marriam U, Nham K, Girard L, Wang H, Sun X, Kim J, Minna JD, Unsal-Kacmaz K, DeBerardinis RJ. The hexosamine biosynthesis pathway is a targetable liability in KRAS/LKB1 mutant lung cancer. Nat Metab. 2020;2(12):1401–1412. doi: 10.1038/s42255-020-00316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang L, Sun W, Ren W, Zhang J, Xu G. Predicting panel of metabolism and immune-related genes for the prognosis of human ovarian cancer. Front Cell Dev Biol. 2021;9:690542. doi: 10.3389/fcell.2021.690542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Leung D, Price ZK, Lokman NA, Wang W, Goonetilleke L, Kadife E, Oehler MK, Ricciardelli C, Kannourakis G, Ahmed N. Platinum-resistance in epithelial ovarian cancer: an interplay of epithelial-mesenchymal transition interlinked with reprogrammed metabolism. J Transl Med. 2022;20(1):556. doi: 10.1186/s12967-022-03776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou L, Luo M, Cheng LJ, Li RN, Liu B, Linghu H. Glutamine-fructose-6-phosphate transaminase 2 (GFPT2) promotes the EMT of serous ovarian cancer by activating the hexosamine biosynthetic pathway to increase the nuclear location of β-catenin. Pathol Res Pract. 2019;215(12):152681. doi: 10.1016/j.prp.2019.152681. [DOI] [PubMed] [Google Scholar]
- 129.Liu L, Pan Y, Ren X, Zeng Z, Sun J, Zhou K, Liang Y, Wang F, Yan Y, Liao W, Ding Y, Liu X, Liang L. GFPT2 promotes metastasis and forms a positive feedback loop with p65 in colorectal cancer. Am J Cancer Res. 2020;10(8):2510–2522. [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Q, Karvelsson ST, Kotronoulas A, Gudjonsson T, Halldorsson S, Rolfsson O. Glutamine-Fructose-6-Phosphate Transaminase 2 (GFPT2) is upregulated in breast epithelial-mesenchymal transition and responds to oxidative stress. Mol Cell Proteomics. 2022;21(2):100185. doi: 10.1016/j.mcpro.2021.100185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simpson NE, Tryndyak VP, Beland FA, Pogribny IP. An in vitro investigation of metabolically sensitive biomarkers in breast cancer progression. Breast Cancer Res Treat. 2012;133(3):959–968. doi: 10.1007/s10549-011-1871-x. [DOI] [PubMed] [Google Scholar]
- 132.Deng H, Lin Y, Gan F, Li B, Mou Z, Qin X, He X, Meng Y. Prognostic model and immune infiltration of ferroptosis subcluster-related modular genes in gastric cancer. J Oncol. 2022;2022:5813522. doi: 10.1155/2022/5813522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tolwani A, Matusiak M, Bui N, Forgó E, Varma S, Baratto L, Iagaru A, Lazar AJ, van de Rijn M, Przybyl J. Prognostic relevance of the hexosamine biosynthesis pathway activation in leiomyosarcoma. NPJ Genom Med. 2021;6(1):30. doi: 10.1038/s41525-021-00193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ridge KM, Eriksson JE, Pekny M, Goldman RD. Roles of vimentin in health and disease. Genes Dev. 2022;36(7–8):391–407. doi: 10.1101/gad.349358.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda JI, Konishi K, Takemasa I, Ikeda M, Sekimoto M, Matsuura N, Monden M. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007;96(6):986–992. doi: 10.1038/sj.bjc.6603651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu LG, Yan XB, Xie RT, Jin ZM, Yang Y. Stromal expression of vimentin predicts the clinical outcome of stage II colorectal cancer for high-risk patients. Med Sci Monit. 2017;23:2897–2905. doi: 10.12659/MSM.904486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, Erkan M, Kleeff J, Wilson J, Apte M, Tosolini M, Wilson AS, Delvecchio FR, Bousquet C, Paradis V, Hammel P, Sadanandam A, Kocher HM. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248(1):51–65. doi: 10.1002/path.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Otsuki S, Inokuchi M, Enjoji M, Ishikawa T, Takagi Y, Kato K, Yamada H, Kojima K, Sugihara K. Vimentin expression is associated with decreased survival in gastric cancer. Oncol Rep. 2011;25(5):1235–1242. doi: 10.3892/or.2011.1185. [DOI] [PubMed] [Google Scholar]
- 139.Fang T, Zhang L, Yin X, Wang Y, Zhang X, Bian X, Jiang X, Yang S, Xue Y. The prognostic marker elastin correlates with epithelial-mesenchymal transition and vimentin-positive fibroblasts in gastric cancer. J Pathol Clin Res. 2023;9(1):56–72. doi: 10.1002/cjp2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anand A, Fang HY, Mohammad-Shahi D, Ingermann J, Baumeister T, Strangmann J, Schmid RM, Wang TC, Quante M. Elimination of NF-κB signaling in Vimentin+ stromal cells attenuates tumorigenesis in a mouse model of Barrett's Esophagus. Carcinogenesis. 2021;42(3):405–413. doi: 10.1093/carcin/bgaa109. [DOI] [PubMed] [Google Scholar]
- 141.Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer–observations in vitro and in vivo. Cells Tissues Organs. 2007;185(1–3):191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 142.Richardson AM, Havel LS, Koyen AE, Konen JM, Shupe J, Wiles WG, Martin WD, Grossniklaus HE, Sica G, Gilbert-Ross M, Marcus AI. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell-cancer associated fibroblast interactions during collective invasion. Clin Cancer Res. 2018;24(2):420–432. doi: 10.1158/1078-0432.CCR-17-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mohamed H, Haglund C, Jouhi L, Atula T, Hagström J, Mäkitie A. Expression and role of E-Cadherin, β-Catenin, and vimentin in human papillomavirus-positive and human papillomavirus-negative oropharyngeal squamous cell carcinoma. J Histochem Cytochem. 2020;68(9):595–606. doi: 10.1369/0022155420950841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Giblin SP, Midwood KS. Tenascin-C: form versus function. Celll Adhes Migr. 2015;9(1–2):48–82. doi: 10.4161/19336918.2014.987587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leppanen J, Lindholm V, Isohookana J, Haapasaari KM, Karihtala P, Lehenkari PP, Saarnio J, Kauppila JH, Karttunen TJ, Helminen O, Huhta H. Tenascin C, fibronectin, and tumor-stroma ratio in pancreatic ductal adenocarcinoma. Pancreas. 2019;48(1):43–48. doi: 10.1097/MPA.0000000000001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yoneura N, Takano S, Yoshitomi H, Nakata Y, Shimazaki R, Kagawa S, Furukawa K, Takayashiki T, Kuboki S, Miyazaki M, Ohtsuka M. Expression of annexin II and stromal tenascin C promotes epithelial to mesenchymal transition and correlates with distant metastasis in pancreatic cancer. Int J Mol Med. 2018;42(2):821–830. doi: 10.3892/ijmm.2018.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ishihara A, Yoshida T, Tamaki H, Sakakura T. Tenascin expression in cancer cells and stroma of human breast cancer and its prognostic significance. Clin Cancer Res. 1995;1(9):1035–1041. [PubMed] [Google Scholar]
- 148.Brunner A, Mayerl C, Tzankov A, Verdorfer I, Tschorner I, Rogatsch H, Mikuz G. Prognostic significance of tenascin-C expression in superficial and invasive bladder cancer. J Clin Pathol. 2004;57(9):927–931. doi: 10.1136/jcp.2004.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hashimoto M, Uesugi N, Osakabe M, Yanagawa N, Otsuka K, Kajiwara Y, Ueno H, Sasaki A, Sugai T. Expression patterns of microenvironmental factors and Tenascin-C at the invasive front of stage II and III colorectal cancer: novel tumor prognostic markers. Front Oncol. 2021;11:12. doi: 10.3389/fonc.2021.690816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang ZT, Zhang CY, Qi WB, Cui CA, Cui Y, Xuan YH. Tenascin-C as a prognostic determinant of colorectal cancer through induction of epithelial-to-mesenchymal transition and proliferation. Exp Mol Pathol. 2018;105(2):216–222. doi: 10.1016/j.yexmp.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 151.Hagiwara K, Harimoto N, Yokobori T, Muranushi R, Hoshino K, Gantumur D, Yamanaka T, Ishii N, Tsukagoshi M, Igarashi T, Tanaka H, Watanabe A, Kubo N, Araki K, Hosouchi Y, Shirabe K. High co-expression of large Tenascin C splice variants in stromal tissue and Annexin A2 in cancer cell membranes is associated with poor prognosis in pancreatic cancer. Ann Surg Oncol. 2020;27(3):924–930. doi: 10.1245/s10434-019-07708-x. [DOI] [PubMed] [Google Scholar]
- 152.Onion D, Isherwood M, Shridhar N, Xenophontos M, Craze ML, Day LJ, Garcia-Marquez MA, Pineda RG, Reece-Smith AM, Saunders JH, Duffy JP, Argent RH, Grabowska AM. Multicomponent analysis of the tumour microenvironment reveals low CD8 T cell number, low stromal caveolin-1 and high tenascin-C and their combination as significant prognostic markers in non-small cell lung cancer. Oncotarget. 2018;9(2):1760–1771. doi: 10.18632/oncotarget.18880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Craig W, Kay R, Cutler RL, Lansdorp PM. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177(5):1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saalbach A, Wetzig T, Haustein UF, Anderegg U. Detection of human soluble Thy-1 in serum by ELISA. Fibroblasts and activated endothelial cells are a possible source of soluble Thy-1 in serum. Cell Tissue Res. 1999;298(2):307–315. doi: 10.1007/s004419900079. [DOI] [PubMed] [Google Scholar]
- 155.Vidal M, Morris R, Grosveld F, Spanopoulou E. Tissue-specific control elements of the Thy-1 gene. EMBO J. 1990;9(3):833–840. doi: 10.1002/j.1460-2075.1990.tb08180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. Faseb J. 2006;20(8):1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- 157.Zhu JH, Thakolwiboon S, Liu XH, Zhang M, Lubman DM. Overexpression of CD90 (Thy-1) in pancreatic adenocarcinoma present in the tumor microenvironment. PLoS ONE. 2014;9(12):20. doi: 10.1371/journal.pone.0115507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huynh PT, Beswick EJ, Coronado YA, Johnson P, O'Connell MR, Watts T, Singh P, Qiu SM, Morris K, Powell DW, Pinchuk IV. CD90(+) stromal cells are the major source of IL-6, which supports cancer stem-like cells and inflammation in colorectal cancer. Int J Cancer. 2016;138(8):1971–1981. doi: 10.1002/ijc.29939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Schliekelman MJ, Creighton CJ, Baird BN, Chen YL, Banerjee P, Bota-Rabassedas N, Ahn YH, Roybal JD, Chen FJ, Zhang YQ, Mishra DK, Kim MP, Liu X, Mino B, Villalobos P, Rodriguez-Canales J, Behrens C, Wistuba II, Hanash SM, Kurie JM. Thy-1(+) cancer-associated fibroblasts adversely impact lung cancer prognosis. Sci Rep. 2017;7:12. doi: 10.1038/s41598-017-06922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu ZJ, Chen MM, Zhao RC, Huang Y, Liu F, Li B, Qin Y. CAF-induced placental growth factor facilitates neoangiogenesis in hepatocellular carcinoma. Acta Biochim Biophys Sin. 2020;52(1):18–25. doi: 10.1093/abbs/gmz134. [DOI] [PubMed] [Google Scholar]
- 161.True LD, Zhang H, Ye ML, Huang CY, Nelson PS, von Haller PD, Tjoelker LW, Kim JS, Qian WJ, Smith RD, Ellis WJ, Liebeskind ES, Liu AY. CD90/THY1 is overexpressed in prostate cancer-associated fibroblasts and could serve as a cancer biomarker. Mod Pathol. 2010;23(10):1346–1356. doi: 10.1038/modpathol.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kamarudin NA, Abd Shukor N, Farouk WI, Hanapi NAM, Mohammed F. Stromal expression of CD10 in invasive breast carcinoma and its association with tumour stage, grade, ER, PR and HER2 status. Malay J Pathol. 2021;43(3):389–396. [PubMed] [Google Scholar]
- 163.Su SC, Chen JN, Yao HR, Liu J, Yu SB, Lao LY, Wang MH, Luo ML, Xing Y, Chen F, Huang D, Zhao JH, Yang LB, Liao D, Su FX, Li MF, Liu Q, Song EW. CD10(+) GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172(4):841. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 164.Hussien MT, Helmy E, Elsaba TM, Elkady A, Alrefai H, Hetta HF. Assessing CD 10 expression level and its prognostic impact in Egyptian patients with urothelial carcinoma. Asian Pac J Cancer Prev. 2020;21(6):1573–1583. doi: 10.31557/APJCP.2020.21.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li R, Coulthard LG, Wu MCL, Taylor SM, Woodruff TM. C5L2: a controversial receptor of complement anaphylatoxin, C5a. Faseb J. 2013;27(3):855–864. doi: 10.1096/fj.12-220509. [DOI] [PubMed] [Google Scholar]
- 166.Huang YY, Wang HC, Zhao JW, Wu MH, Shih TC. Immunosuppressive roles of galectin-1 in the tumor microenvironment. Biomolecules. 2021;11(10):13. doi: 10.3390/biom11101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Martinez-Bosch N, Navarro P. Targeting galectin-1 in pancreatic cancer: immune surveillance on guard. OncoImmunology. 2014;3(8):3. doi: 10.4161/21624011.2014.952201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dalotto-Moreno T, Croci DO, Cerliani JP, Martinez-Allo VC, Dergan-Dylon S, Mendez-Huergo SP, Stupirski JC, Mazal D, Osinaga E, Toscano MA, Sundblad V, Rabinovich GA, Salatino M. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Can Res. 2013;73(3):1107–1117. doi: 10.1158/0008-5472.CAN-12-2418. [DOI] [PubMed] [Google Scholar]
- 169.Jung EJ, Moon HG, Cho BI, Jeong CY, Joo YT, Lee YJ, Hong SC, Choi SK, Ha WS, Kim JW, Lee CW, Lee JS, Park ST. Galectin-1 expression in cancer-associated stromal cells correlates tumor invasiveness and tumor progression in breast cancer. Int J Cancer. 2007;120(11):2331–2338. doi: 10.1002/ijc.22434. [DOI] [PubMed] [Google Scholar]
- 170.Peng KY, Jiang SS, Lee YW, Tsai FY, Chang CC, Chen LT, Yen BL. Stromal galectin-1 promotes colorectal cancer cancer-initiating cell features and disease dissemination through SOX9 and beta-catenin: development of niche-based biomarkers. Front Oncol. 2021;11:15. doi: 10.3389/fonc.2021.716055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carlini MJ, Roitman P, Nunez M, Pallotta MG, Boggio G, Smith D, Salatino M, Joffe E, Rabinovich GA, Puricelli LI. Clinical relevance of galectin-1 expression in non-small cell lung cancer patients. Lung Cancer. 2014;84(1):73–78. doi: 10.1016/j.lungcan.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 172.Majdalawieh A, Zhang L, Fuki IV, Rader DJ, Ro HS. Adipocyte enhancer-binding protein 1 is a potential novel atherogenic factor involved in macrophage cholesterol homeostasis and inflammation. Proc Natl Acad Sci USA. 2006;103(7):2346–2351. doi: 10.1073/pnas.0508139103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yorozu A, Yamamoto E, Niinuma T, Tsuyada A, Maruyama R, Kitajima H, Numata Y, Kai MS, Sudo G, Kubo T, Nishidate T, Okita K, Takemasa I, Nakase H, Sugai T, Takano K, Suzuki H. Upregulation of adipocyte enhancer-binding protein 1 in endothelial cells promotes tumor angiogenesis in colorectal cancer. Cancer Sci. 2020;111(5):1631–1644. doi: 10.1111/cas.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Li DD, Liu Z, Ding XR, Qin ZS. AEBP1 is one of the epithelial-mesenchymal transition regulatory genes in colon adenocarcinoma. Biomed Res Int. 2021;2021:16. doi: 10.1155/2021/3108933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Majdalawieh AF, Massri M, Ro HS. AEBP1 is a novel oncogene: mechanisms of action and signaling pathways. J Oncol. 2020;2020:20. doi: 10.1155/2020/8097872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sugai M, Yanagawa N, Shikanai S, Hashimoto M, Saikawa H, Osakabe M, Saito H, Maemondo M, Sugai T. Correlation of tumor microenvironment-related markers with clinical outcomes in patients with squamous cell carcinoma of the lung. Transl Lung Cancer Res. 2022;11(6):975. doi: 10.21037/tlcr-22-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhao HL, Chen Q, Alam A, Cui J, Suen KC, Soo AP, Eguchi S, Gu JT, Ma DQ. The role of osteopontin in the progression of solid organ tumour. Cell Death Dis. 2018;9:15. doi: 10.1038/s41419-018-0391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hao CC, Cui YX, Owen SE, Li WB, Cheng S, Jiang WG. Human osteopontin: potential clinical applications in cancer. Int J Mol Med. 2017;39(6):1327–1337. doi: 10.3892/ijmm.2017.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Weber GF, Ashkar S, Cantor H. Interaction between CD44 and osteopontin as a potential basis for metastasis formation. Proc Assoc Am Physicians. 1997;109(1):1–9. [PubMed] [Google Scholar]
- 180.Denhardt DT, Giachelli CM, Rittling SR. Role of osteopontin in cellular signaling and toxicant injury. Annu Rev Pharmacol Toxicol. 2001;41:723–749. doi: 10.1146/annurev.pharmtox.41.1.723. [DOI] [PubMed] [Google Scholar]
- 181.Nallasamy P, Nimmakayala RK, Karmakar S, Leon F, Seshacharyulu P, Lakshmanan I, Rachagani S, Mallya K, Zhang CM, Ly QP, Myers MS, Josh L, Grabow CE, Gautam SK, Kumar S, Lele SM, Jain M, Batra SK, Ponnusamy MP. Pancreatic tumor microenvironment factor promotes cancer stemness via SPP1-CD44 axis. Gastroenterology. 2021;161(6):1998. doi: 10.1053/j.gastro.2021.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yuan Q, Gu JC, Zhang J, Liu S, Wang QC, Tian T, Chen ZN, Zhang JH. MyD88 in myofibroblasts enhances colitis-associated tumorigenesis via promoting macrophage M2 polarization. Cell Rep. 2021;34(5):18. doi: 10.1016/j.celrep.2021.108724. [DOI] [PubMed] [Google Scholar]
- 183.Zhao B, Sun TM, Meng FJ, Qu AB, Li CL, Shen H, Jin Y, Li WX. Osteopontin as a potential biomarker of proliferation and invasiveness for lung cancer. J Cancer Res Clin Oncol. 2011;137(7):1061–1070. doi: 10.1007/s00432-010-0968-7. [DOI] [PubMed] [Google Scholar]
- 184.Shojaei F, Scott N, Kang XL, Lappin PB, Fitzgerald AA, Karlicek S, Simmons BH, Wu AD, Lee JH, Bergqvist S, Kraynov E. Osteopontin induces growth of metastatic tumors in a preclinical model of non-small lung cancer. J Exp Clin Cancer Res. 2012;31:12. doi: 10.1186/1756-9966-31-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chambers AF, Wilson SM, Kerkvliet N, O'Malley FP, Harris JF, Casson AG. Osteopontin expression in lung cancer. Lung cancer (Amsterdam, Netherlands) 1996;15(3):311–323. doi: 10.1016/0169-5002(95)00595-1. [DOI] [PubMed] [Google Scholar]
- 186.Coppola D, Szabo M, Boulware D, Muraca P, Alsarraj M, Chambers AF, Yeatman TJ. Correlation of osteopontin protein expression and pathological stage across a wide variety of tumor histologies. Clin Cancer Res. 2004;10(1):184–190. doi: 10.1158/1078-0432.CCR-1405-2. [DOI] [PubMed] [Google Scholar]
- 187.Agrawal D, Chen TG, Irby R, Quackenbush J, Chambers AF, Szabo M, Cantor A, Coppola D, Yeatman TJ. Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst. 2002;94(7):513–521. doi: 10.1093/jnci/94.7.513. [DOI] [PubMed] [Google Scholar]
- 188.Mirza M, Shaughnessy E, Hurley JK, Vanpatten KA, Pestano GA, He B, Weber GF. Osteopontin-c is a selective marker of breast cancer. Int J Cancer. 2008;122(4):889–897. doi: 10.1002/ijc.23204. [DOI] [PubMed] [Google Scholar]
- 189.Nagasawa A, Kubota R, Imamura Y, Nagamine K, Wang Y, Asakawa S, Kudoh J, Minoshima S, Mashima Y, Oguchi Y, Shimizu N. Cloning of the cDNA for a new member of the immunoglobulin superfamily (ISLR) containing leucine-rich repeat (LRR) Genomics. 1997;44(3):273–279. doi: 10.1006/geno.1997.4889. [DOI] [PubMed] [Google Scholar]
- 190.Maeda K, Enomoto A, Hara A, Asai N, Kobayashi T, Horinouchi A, Maruyama S, Ishikawa Y, Nishiyama T, Kiyoi H, Kato T, Ando K, Weng L, Mii S, Asai M, Mizutani Y, Watanabe O, Hirooka Y, Goto H, Takahashi M. Identification of meflin as a potential marker for mesenchymal stromal cells. Sci Rep. 2016;6:15. doi: 10.1038/srep22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Takahashi M, Kobayashi H, Mizutani Y, Hara A, Iida T, Miyai Y, Asai N, Enomoto A. Roles of the mesenchymal stromal/stem cell marker Meflin/Islr in cancer fibrosis. Front Cell Dev Biol. 2021;9:9. doi: 10.3389/fcell.2021.749924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mizutani Y, Kobayashi H, Iida T, Asai N, Masamune A, Hara A, Esaki N, Ushida K, Mii S, Shiraki Y, Ando K, Weng L, Ishihara S, Ponik SM, Conklin MW, Haga H, Nagasaka A, Miyata T, Matsuyama M, Kobayashi T, Fujii T, Yamada S, Yamaguchi J, Wang TT, Woods SL, Worthley D, Shimamura T, Fujishiro M, Hirooka Y, Enomoto A, Takahashi M. Meflin-positive cancer-associated fibroblasts inhibit pancreatic carcinogenesis. Can Res. 2019;79(20):5367–5381. doi: 10.1158/0008-5472.CAN-19-0454. [DOI] [PubMed] [Google Scholar]
- 193.Miyai Y, Esaki N, Takahashi M, Enomoto A. Cancer-associated fibroblasts that restrain cancer progression: hypotheses and perspectives. Cancer Sci. 2020;111(4):1047–1057. doi: 10.1111/cas.14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Iida T, Mizutani Y, Esaki N, Ponik SM, Burkel BM, Weng L, Kuwata K, Masamune A, Ishihara S, Haga H, Kataoka K, Mii S, Shiraki Y, Ishikawa T, Ohno E, Kawashima H, Hirooka Y, Fujishiro M, Takahashi M, Enomoto A. Pharmacologic conversion of cancer-associated fibroblasts from a protumor phenotype to an antitumor phenotype improves the sensitivity of pancreatic cancer to chemotherapeutics. Oncogene. 2022;41(19):2764–2777. doi: 10.1038/s41388-022-02288-9. [DOI] [PubMed] [Google Scholar]
- 195.Miyai Y, Sugiyama D, Hase T, Asai N, Taki T, Nishida K, Fukui T, Chen-Yoshikawa TF, Kobayashi H, Mii S, Shiraki Y, Hasegawa Y, Nishikawa H, Ando Y, Takahashi M, Enomoto A. Meflin-positive cancer-associated fibroblasts enhance tumor response to immune checkpoint blockade. Life Sci Alliance. 2022;5(6):18. doi: 10.26508/lsa.202101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hara A, Kobayashi H, Asai N, Saito S, Higuchi T, Kato K, Okumura T, Bando YK, Takefuji M, Mizutani Y, Miyai Y, Saito S, Maruyama S, Maeda K, Ouchi N, Nagasaka A, Miyata T, Mii S, Kioka N, Worthley DL, Murohara T, Takahashi M, Enomoto A. Roles of the mesenchymal stromal/stem cell marker meflin in cardiac tissue repair and the development of diastolic dysfunction. Circ Res. 2019;125(4):414–430. doi: 10.1161/CIRCRESAHA.119.314806. [DOI] [PubMed] [Google Scholar]
- 197.Minatoguchi S, Saito S, Furuhashi K, Sawa Y, Okazaki M, Shimamura Y, Kaihan AB, Hashimoto Y, Yasuda Y, Hara A, Mizutani Y, Ando R, Kato N, Ishimoto T, Tsuboi N, Esaki N, Matsuyama M, Shiraki Y, Kobayashi H, Asai N, Enomoto A, Maruyama S. A novel renal perivascular mesenchymal cell subset gives rise to fibroblasts distinct from classic myofibroblasts. Sci Rep. 2022;12(1):20. doi: 10.1038/s41598-022-09331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Nakahara Y, Hashimoto N, Sakamoto K, Enomoto A, Adams TS, Yokoi T, Omote N, Poli S, Ando A, Wakahara K, Suzuki A, Inoue M, Hara A, Mizutani Y, Imaizumi K, Kawabe T, Rosas IO, Takahashi M, Kaminski N, Hasegawa Y. Fibroblasts positive for meflin have anti-fibrotic properties in pulmonary fibrosis. Eur Resp J. 2021;58(6):15. doi: 10.1183/13993003.03397-2020. [DOI] [PubMed] [Google Scholar]
- 199.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-beta 1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9(7):964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 200.Kobayashi H, Gieniec KA, Wright JA, Wang TT, Asai N, Mizutani Y, Lida T, Ando R, Suzuki N, Lannagan TRM, Ng JQ, Hara A, Shiraki Y, Mii S, Ichinose M, Vrbanac L, Lawrence MJ, Sammour T, Uehara K, Davies G, Lisowski L, Alexander IE, Hayakawa Y, Butler LM, Zannettino ACW, Din MO, Hasty J, Burt AD, Leedham SJ, Rustgi AK, Mukherjee S, Wang TC, Enomoto A, Takahashi M, Worthley DL, Woods SL. The balance of stromal BMP signaling mediated by GREM1 and ISLR drives colorectal carcinogenesis. Gastroenterology. 2021;160(4):1224. doi: 10.1053/j.gastro.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Le Calve B, Griveau A, Vindrieux D, Marechal R, Wiel C, Svrcek M, Gout J, Azzi L, Payen L, Cros J, de la Fouchardiere C, Dubus P, Guitton J, Bartholin L, Bachet JB, Bernard D. Lysyl oxidase family activity promotes resistance of pancreatic ductal adenocarcinoma to chemotherapy by limiting the intratumoral anticancer drug distribution. Oncotarget. 2016;7(22):32100–32112. doi: 10.18632/oncotarget.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;12(8):540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 203.Cox TR. The matrix in cancer. Nat Rev Cancer. 2021;21(4):217–238. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
- 204.Mizutani Y, Iida T, Ohno E, Ishikawa T, Kinoshita F, Kuwatsuka Y, Imai M, Shimizu S, Tsuruta T, Enomoto A, Kawashima H, Fujishiro M. Safety and efficacy of MIKE-1 in patients with advanced pancreatic cancer: a study protocol for an open-label phase I/II investigator-initiated clinical trial based on a drug repositioning approach that reprograms the tumour stroma. BMC Cancer. 2022;22(1):11. doi: 10.1186/s12885-022-09272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lehmann JM, Riethmuller G, Johnson JP. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci USA. 1989;86(24):9891–9895. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang ZQ, Yan XY. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330(2):150–162. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 207.Brechbuhl HM, Finlay-Schultz J, Yamamoto TM, Gillen AE, Cittelly DM, Tan AC, Sams SB, Pillai MM, Elias AD, Robinson WA, Sartorius CA, Kabos P. Fibroblast subtypes regulate responsiveness of luminal breast cancer to estrogen. Clin Cancer Res. 2017;23(7):1710–1721. doi: 10.1158/1078-0432.CCR-15-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zheng B, Ohuchida K, Chijiiwa Y, Zhao M, Mizuuchi Y, Cui L, Horioka K, Ohtsuka T, Mizumoto K, Oda Y, Hashizume M, Nakamura M, Tanaka M. CD146 attenuation in cancer-associated fibroblasts promotes pancreatic cancer progression. Mol Carcinog. 2016;55(11):1560–1572. doi: 10.1002/mc.22409. [DOI] [PubMed] [Google Scholar]
- 209.Sotgia F, Martinez-Outschoorn UE, Howell A, Pestell RG, Pavlides S, Lisanti MP. Caveolin-1 and Cancer Metabolism in the Tumor Microenvironment: Markers, Models, and Mechanisms. In: Abbas AK, Galli SJ, Howley PM, editors. Annual Review of Pathology: Mechanisms of Disease, Vol 7. Palo Alto: Annual Reviews; 2012. pp. 423–467. [DOI] [PubMed] [Google Scholar]
- 210.Fujimoto T, Kogo H, Nomura R, Une T. Isoforms of caveolin-1 and caveolar structure. J Cell Sci. 2000;113(19):3509–3517. doi: 10.1242/jcs.113.19.3509. [DOI] [PubMed] [Google Scholar]
- 211.Yamaguchi T, Lu C, Ida L, Yanagisawa K, Usukura J, Cheng JL, Hotta N, Shimada Y, Isomura H, Suzuki M, Fujimoto T, Takahashi T. ROR1 sustains caveolae and survival signalling as a scaffold of cavin-1 and caveolin-1. Nat Commun. 2016;7:13. doi: 10.1038/ncomms10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Parton RG, del Pozo MA. Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14(2):98–112. doi: 10.1038/nrm3512. [DOI] [PubMed] [Google Scholar]
- 213.Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol. 2010;20(4):177–186. doi: 10.1016/j.tcb.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 214.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer. 2015;15(4):225–237. doi: 10.1038/nrc3915. [DOI] [PubMed] [Google Scholar]
- 215.Volonte D, Vyas AR, Chen C, Dacic S, Stabile LP, Kurland BF, Abberbock SR, Burns TF, Herman JG, Di YP, Galbiati F. Caveolin-1 promotes the tumor suppressor properties of oncogene-induced cellular senescence. J Biol Chem. 2018;293(5):1794–1809. doi: 10.1074/jbc.M117.815902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zou HF, Stoppani E, Volonte D, Galbiati F. Caveolin-1, cellular senescence and age-related diseases. Mech Ageing Dev. 2011;132(11–12):533–542. doi: 10.1016/j.mad.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Zhao XD, He YY, Gao J, Fan LF, Li ZH, Yang GF, Chen HL. Caveolin-1 expression level in cancer associated fibroblasts predicts outcome in gastric cancer. PLoS ONE. 2013;8(3):11. doi: 10.1371/journal.pone.0059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27(4):715–735. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 219.Martinez-Outschoorn UE, Pavlides S, Whitaker-Menezes D, Daumer KM, Milliman JN, Chiavarina B, Migneco G, Witkiewicz AK, Martinez-Cantarin MP, Flomenberg N, Howell A, Pestell RG, Lisanti MP, Sotgia F. Tumor cells induce the cancer associated fibroblast phenotype via caveolin-1 degradation Implications for breast cancer and DCIS therapy with autophagy inhibitors. Cell Cycle. 2010;9(12):2423–2433. doi: 10.4161/cc.9.12.12048. [DOI] [PubMed] [Google Scholar]
- 220.Elsheikh SE, Green AR, Rakha EA, Samaka RM, Ammar AA, Powe D, Reis-Filho JS, Ellis IO. Caveolin 1 and caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotype. Br J Cancer. 2008;99(2):327–334. doi: 10.1038/sj.bjc.6604463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sloan EK, Ciocca DR, Pouliot N, Natoli A, Restall C, Henderson MA, Fanelli MA, Cuello-Carrion FD, Gago FE, Anderson RL. Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol. 2009;174(6):2035–2043. doi: 10.2353/ajpath.2009.080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Witkiewicz AK, Dasgupta A, Sotgia F, Mercier I, Pestell RG, Sabel M, Kleer CG, Brody JR, Lisanti MP. An absence of stromal caveolin-1 expression predicts early tumor recurrence and poor clinical outcome in human breast cancers. Am J Pathol. 2009;174(6):2023–2034. doi: 10.2353/ajpath.2009.080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8(15):2420–2424. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Bertino EM, Williams TM, Nana-Sinkam SP, Shilo K, Chatterjee M, Mo XK, Rahmani M, Phillips GS, Villalona-Calero MA, Otterson GA. Stromal caveolin-1 is associated with response and survival in a phase II trial of nab-paclitaxel with carboplatin for advanced NSCLC patients. Clin Lung Cancer. 2015;16(6):466. doi: 10.1016/j.cllc.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 226.Sazeides C, Le A. Metabolic relationship between cancer-associated fibroblasts and cancer cells. Adv Exp Med Biol. 2021;1311:189–204. doi: 10.1007/978-3-030-65768-0_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Thompson TC, Tahir SA, Li L, Watanabe M, Naruishi K, Yang G, Kadmon D, Logothetis CJ, Troncoso P, Ren C, Goltsov A, Park S. The role of caveolin-1 in prostate cancer: clinical implications. Prostate Cancer Prostatic Dis. 2010;13(1):6–11. doi: 10.1038/pcan.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Goetz JG, Minguet S, Navarro-Lerida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibanez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJP, Garcia R, Keely PJ, Sanchez-Mateos P, Cukierman E, Del Pozo MA. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146(1):148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yamao T, Yamashita Y, Yamamura K, Nakao Y, Tsukamoto M, Nakagawa S, Okabe H, Hayashi H, Imai K, Baba H. Cellular senescence, represented by expression of caveolin-1, in cancer-associated fibroblasts promotes tumor invasion in pancreatic cancer. Ann Surg Oncol. 2019;26(5):1552–1559. doi: 10.1245/s10434-019-07266-2. [DOI] [PubMed] [Google Scholar]
- 230.Schoppmann SF, Birner P, Studer P, Breiteneder-Geleff S. Lymphatic microvessel density and lymphovascular invasion assessed by anti-podoplanin immunostaining in human breast cancer. Anticancer Res. 2001;21(4A):2351–2355. [PubMed] [Google Scholar]
- 231.Breiteneder-Geleff S, Soleiman A, Horvat R, Amann G, Kowalski H, Kerjaschki D. Podoplanin–a specific marker for lymphatic endothelium expressed in angiosarcoma. Verh Dtsch Ges Pathol. 1999;83:270–275. [PubMed] [Google Scholar]
- 232.Kerrigan AM, Navarro-Nunez L, Pyz E, Finney BA, Willment JA, Watson SP, Brown GD. Podoplanin-expressing inflammatory macrophages activate murine platelets via CLEC-2. J Thromb Haemost. 2012;10(3):484–486. doi: 10.1111/j.1538-7836.2011.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kreppel M, Scheer M, Drebber U, Ritter L, Zoller JE. Impact of podoplanin expression in oral squamous cell carcinoma: clinical and histopathologic correlations. Virchows Arch. 2010;456(5):473–482. doi: 10.1007/s00428-010-0915-7. [DOI] [PubMed] [Google Scholar]
- 234.Hoshino A, Ishii G, Ito T, Aoyagi K, Ohtaki Y, Nagai K, Sasaki H, Ochiai A. Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Can Res. 2011;71(14):4769–4779. doi: 10.1158/0008-5472.CAN-10-3228. [DOI] [PubMed] [Google Scholar]
- 235.Martin-Villar E, Scholl FG, Gamallo C, Yurrita MM, Munoz-Guerra M, Cruces J, Quintanilla M. Characterization of human PA2.26 antigen (T1 alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113(6):899–910. doi: 10.1002/ijc.20656. [DOI] [PubMed] [Google Scholar]
- 236.Smith SM, Melrose J. Podoplanin is expressed by a sub-population of human foetal rib and knee joint rudiment chondrocytes. Tissue Cell. 2011;43(1):39–44. doi: 10.1016/j.tice.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 237.Neri S, Ishii G, Hashimoto H, Kuwata T, Nagai K, Date H, Ochiai A. Podoplanin-expressing cancer-associated fibroblasts lead and enhance the local invasion of cancer cells in lung adenocarcinoma. Int J Cancer. 2015;137(4):784–796. doi: 10.1002/ijc.29464. [DOI] [PubMed] [Google Scholar]
- 238.Ito S, Ishii G, Hoshino A, Hashimoto H, Neri S, Kuwata T, Higashi M, Nagai K, Ochiai A. Tumor promoting effect of podoplanin-positive fibroblasts is mediated by enhanced RhoA activity. Biochem Biophys Res Commun. 2012;422(1):194–199. doi: 10.1016/j.bbrc.2012.04.158. [DOI] [PubMed] [Google Scholar]
- 239.Wicki A, Christofori G. The potential role of podoplanin in tumour invasion. Br J Cancer. 2007;96(1):1–5. doi: 10.1038/sj.bjc.6603518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sakai T, Aokage K, Neri S, Nakamura H, Nomura S, Tane K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Iyoda A, Tsuboi M, Ishii G. Link between tumor-promoting fibrous microenvironment and an immunosuppressive microenvironment in stage I lung adenocarcinoma. Lung Cancer. 2018;126:64–71. doi: 10.1016/j.lungcan.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 241.Suzuki J, Aokage K, Neri S, Sakai T, Hashimoto H, Su YH, Yamazaki S, Nakamura H, Tane K, Miyoshi T, Sugano M, Kojima M, Fujii S, Kuwata T, Ochiai A, Tsuboi M, Ishii G. Relationship between podoplanin-expressing cancer-associated fibroblasts and the immune microenvironment of early lung squamous cell carcinoma. Lung Cancer. 2021;153:1–10. doi: 10.1016/j.lungcan.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 242.Yoshida T, Ishii G, Goto K, Neri S, Hashimoto H, Yoh K, Niho S, Umemura S, Matsumoto S, Ohmatsu H, Iida S, Niimi A, Nagai K, Ohe Y, Ochiai A. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin Cancer Res. 2015;21(3):642–651. doi: 10.1158/1078-0432.CCR-14-0846. [DOI] [PubMed] [Google Scholar]
- 243.Schoppmann SF, Jesch B, Riegler MF, Maroske F, Schwameis K, Jomrich G, Birner P. Podoplanin expressing cancer associated fibroblasts are associated with unfavourable prognosis in adenocarcinoma of the esophagus. Clin Exp Metastasis. 2013;30(4):441–446. doi: 10.1007/s10585-012-9549-2. [DOI] [PubMed] [Google Scholar]
- 244.Obulkasim H, Shi XL, Wang J, Li J, Dai B, Wu PW, Wang S, Wang X, Ding YT. Podoplanin is an important stromal prognostic marker in perihilar cholangiocarcinoma. Oncol Lett. 2018;15(1):137–146. doi: 10.3892/ol.2017.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Hirayama K, Kono H, Nakata Y, Akazawa Y, Wakana H, Fukushima H, Fujii H. Expression of podoplanin in stromal fibroblasts plays a pivotal role in the prognosis of patients with pancreatic cancer. Surg Today. 2018;48(1):110–118. doi: 10.1007/s00595-017-1559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shindo K, Aishima S, Ohuchida K, Fujiwara K, Fujino M, Mizuuchi Y, Hattori M, Mizumoto K, Tanaka M, Oda Y. Podoplanin expression in cancer-associated fibroblasts enhances tumor progression of invasive ductal carcinoma of the pancreas. Mol Cancer. 2013;12:16. doi: 10.1186/1476-4598-12-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kan S, Konishi E, Arita T, Ikemoto C, Takenaka H, Yanagisawa A, Katoh N, Asai J. Podoplanin expression in cancer-associated fibroblasts predicts aggressive behavior in melanoma. J Cutan Pathol. 2014;41(7):561–567. doi: 10.1111/cup.12322. [DOI] [PubMed] [Google Scholar]
- 248.Irvine AF, Waise S, Green EW, Stuart B, Thomas GJ. Characterising cancer-associated fibroblast heterogeneity in non-small cell lung cancer: a systematic review and meta-analysis. Sci Rep. 2021;11(1):15. doi: 10.1038/s41598-021-81796-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Cai DY, Wu XH, Hong TT, Mao Y, Ge XS, Hua D. CD61+and CAF+ were found to be good prognosis factors for invasive breast cancer patients. Pathol Res Pract. 2017;213(10):1296–1301. doi: 10.1016/j.prp.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 250.Yamanashi T, Nakanishi Y, Fujii G, Akishima-Fukasawa Y, Moriya Y, Kanai Y, Watanabe M, Hirohashi S. Podoplanin expression identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology. 2009;77(1):53–62. doi: 10.1159/000226112. [DOI] [PubMed] [Google Scholar]
- 251.Choi SY, Sung R, Lee SJ, Lee TG, Kim N, Yoon SM, Lee EJ, Chae HB, Youn SJ, Park SM. Podoplanin, alpha-smooth muscle actin or S100A4 expressing cancer-associated fibroblasts are associated with different prognosis in colorectal cancers. J Korean Med Sci. 2013;28(9):1293–1301. doi: 10.3346/jkms.2013.28.9.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992;17(9):359–363. doi: 10.1016/0968-0004(92)90315-Z. [DOI] [PubMed] [Google Scholar]
- 253.Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102(2):173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290(5497):1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 255.Ishibashi M, Neri S, Hashimoto H, Miyashita T, Yoshida T, Nakamura Y, Udagawa H, Kirita K, Matsumoto S, Umemura S, Yoh K, Niho S, Tsuboi M, Masutomi K, Goto K, Ochiai A, Ishii G. CD200-positive cancer associated fibroblasts augment the sensitivity of Epidermal Growth Factor Receptor mutation-positive lung adenocarcinomas to EGFR tyrosine kinase inhibitors. Sci Rep. 2017;7:13. doi: 10.1038/srep46662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.MacNeil T, Vathiotis IA, Shafi S, Aung TN, Zugazagoitia J, Gruver AM, Driscoll K, Rimm DL. Multiplex quantitative analysis of tumor-infiltrating lymphocytes, cancer-associated fibroblasts, and CD200 in pancreatic cancer. Cancers. 2021;13(21):14. doi: 10.3390/cancers13215501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Choueiry F, Torok M, Shakya R, Agrawal K, Deems A, Benner B, Hinton A, Shaffer J, Blaser BW, Noonan AM, Williams TM, Dillhoff M, Conwell DL, Hart PA, Cruz-Monserrate Z, Bai XF, Carson WE, Mace TA. CD200 promotes immunosuppression in the pancreatic tumor microenvironment. J Immunother Cancer. 2020;8(1):12. doi: 10.1136/jitc-2019-000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mahadevan D, Lanasa MC, Farber C, Pandey M, Whelden M, Faas SJ, Ulery T, Kukreja A, Li L, Bedrosian CL, Zhang XP, Heffner LT. Phase I study of samalizumab in chronic lymphocytic leukemia and multiple myeloma: blockade of the immune checkpoint CD200. J Immunother Cancer. 2019;7(1):13. doi: 10.1186/s40425-019-0710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes Dev. 2016;30(9):1002–1019. doi: 10.1101/gad.279737.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 2019;9(8):1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Jacobs J, Deschoolmeester V, Zwaenepoel K, Flieswasser T, Deben C, Van den Bossche J, Hermans C, Rolfo C, Peeters M, De Wever O, Lardon F, Siozopoulou V, Smits E, Pauwels P. Unveiling a CD70-positive subset of cancer-associated fibroblasts marked by pro-migratory activity and thriving regulatory T cell accumulation. OncoImmunology. 2018;7(7):11. doi: 10.1080/2162402X.2018.1440167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Jia DY, Liu ZQ, Deng N, Tan TZ, Huang RYJ, Taylor-Harding B, Cheon DJ, Lawrenson K, Wiedemeyer WR, Walts AE, Karlan BY, Orsulic S. A COL11A1-correlated pan-cancer gene signature of activated fibroblasts for the prioritization of therapeutic targets. Cancer Lett. 2016;382(2):203–214. doi: 10.1016/j.canlet.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.