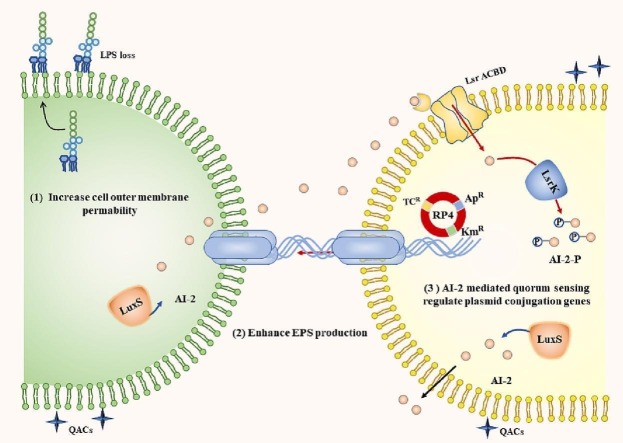
Abstract
During the pandemic of COVID-19, the amounts of quaternary ammonium compounds (QACs) used to inactivate the virus in public facilities, hospitals and households increased, which raised concerns about the evolution and transmission of antimicrobial resistance (AMR). Although QACs may play an important role in the propagation of antibiotic resistance gene (ARGs), the potential contribution and mechanism remains unclear. Here, the results showed that benzyl dodecyl dimethyl ammonium chloride (DDBAC) and didecyl dimethyl ammonium chloride (DDAC) significantly promoted plasmid RP4-mediated ARGs transfer within and across genera at environmental relevant concentrations (0.0004–0.4 mg/L). Low concentrations of QACs did not contribute to the permeability of the cell plasma membrane, but significantly increased the permeability of the cell outer membrane due to the decrease in content of lipopolysaccharides. QACs altered the composition and content of extracellular polymeric substances (EPS) and were positively correlated with the conjugation frequency. Furthermore, transcriptional expression levels of genes encode for mating pairing formation (trbB), DNA replication and translocation (trfA), and global regulators (korA, korB, trbA) are regulated by QACs. And we demonstrate for the first time that QACs decreased the concentration of extracellular AI-2 signals, which was verified to be involved in regulating conjugative transfer genes (trbB, trfA). Collectively, our findings underscore the risk of increased disinfectant concentrations of QACs on the ARGs transfer and provide new mechanisms of plasmid conjugation.
Keywords: Quaternary ammonium compounds, Plasmid conjugation, Cell outer membrane permeability, Quorum sensing, AI-2 signals, Antimicrobial resistance
Graphical abstract
1. Introduction
During the COVID-19 pandemic, indoor and outdoor disinfectants have been used extensively to limit the transmission of the SARS-CoV-2 virus (Dewey et al., 2021). The new regulations and habits for disinfection will likely be more routine in future, and these may, in turn, result in low but persistent levels of disinfectants remaining in the environment. Recently, concerns have been raised about the evolution and propagation of antibiotic resistance caused by these residual disinfectants (Hora et al., 2020; Lu and Guo, 2021; Mahoney et al., 2021). Conjugation is the primary mechanism of horizontal gene transfer (Amos et al., 2018; Gaze et al., 2011; Thomas and Nielsen, 2005). The plasmid-mediated ARGs transfer begins with cell-to-cell contact, connecting donor and recipient cells through the pilus (Thomas and Nielsen, 2005). Plasmid conjugation universally occurs in natural environments, such as soil (Xu et al., 2021), aquatic environments (Huang et al., 2022) and biofilms (Abe et al., 2020; Guo et al., 2018).
Quaternary ammonium compounds (QACs) are cationic surfactants. Because of their broad-spectrum antimicrobial properties, they are widely used in household, food processing, agricultural and clinical applications to control the spread of pathogens (Gosling et al., 2017; Levinson, 1999; Min-Jeong et al., 2010; Ruan et al., 2014). QACs effectively inactivate viruses, and half of the disinfectants formulations containing QACs are recommended by the U.S. Environmental Protection Agency for inactivating SARS-CoV-2 (Hora et al., 2020). Major classes of QACs include BACs (benzyl alkydimethyl ammonium compounds), ATMACs (alkytrimethyl ammonium compounds) and DADMACs (dialkyldiethyl ammonium compounds) (Ruan et al., 2014). As membrane active agents, positively charged quaternary nitrogen of QACs interact with the negatively charged acidic phospholipids on the cell membrane, and the hydrophobic carbon chains are integrated into the cell, leading to the disruption of cell membrane integrity and leakage of cellular contents (Buffet-Bataillon et al., 2012).
QACs have been detected widely in wastewater sludge (Ruan et al., 2014), surface water (Zhang et al., 2015) and soil (Mulder et al., 2017). Approximately 25 % of QACs is directly discharged into surface water and the marine environment through surface runoff, and 75 % of QACs is collected into wastewater treatment plants with sewage (Mulder et al., 2017). The concentration level of QACs in sewage influent is μg/L (Clara et al., 2007; Pati and Arnold, 2020). QACs with hydrophobic alkyl chains are easily adsorb to biosolids, leading to the transfer of QACs to anaerobic digesters, which is a long-term oxygen-limited environment that is unfavorable for the degradation of QACs (Garcia et al., 1999; Hora et al., 2020; Zhang et al., 2015). Didecyl dimethyl ammonium chloride (DDAC) and dodecyl dimethyl benzyl ammonium chloride (DDBAC) are the highest concentration detected in sewage among the DADMACs and BACs homologues (Clara et al., 2007). The concentration of DDAC in sediment gradually increased from 2005 to 2010 (Pati and Arnold, 2020). During the COVID-19, the mass load of DDBAC increased 441 % in sewage (Mohapatra et al., 2022). Alygizakis et al. (2021) showed that compared with non-COVID conditions in 2019, the QACs mass load in wastewater treatment plants increased by 331 % in 2020.
Wastewater treatment plants are a common pool for mobile genetic elements to be shared between environmental bacteria and pathogens. ARGs-carrying plasmids, especially broad-host plasmids, can spread across genera (Xu et al., 2021). Moreover, several emerging pollutants, such as nanoparticles (Yu et al., 2020), ionic liquid (Wang et al., 2015) and nonnutritive sweeteners (Yu et al., 2021b), can promote plasmid transfer by inducing oxidative stress and increasing the permeability of cell membranes. Zhao et al. (2023) reported a significant positive correlation between ARGs and integrons under exposure of dodecyl trimethyl ammonium chloride (ATMAC-C12), which indicated that QACs may promote horizontal gene transfer, thereby increasing the propagation of ARGs. Han et al. (2019) showed that five QACs (ATMAC-C12, BACs-C12–16, DADMAC-C12) promoted ARGs conjugative transfer, and the possible mechanisms were attributed to the enhanced cell plasma membrane permeability and stimulated bacteria to produce ROS. Although the previous studies reported the transmission of ARGs induced by several QACs, the mechanism remains unclear.
In this study, intra- and intergenera conjugation models were established to reveal the effect of QACs on plasmid conjugation. Model I was an intragenera conjugation process consisted of E. coli HB101 and E. coli MG1655 under exposure of QACs. Model II involves the intergenera conjugation from E. coli to Pseudomonas putida. DDAC and DDBAC were selected as the typical QACs. In order to reveal the mechanisms of plasmid transfer, the cell membrane permeability was determined first, followed by cell to cell contact, cell communication, energy supply and conjugative machinery. RNA- sequencing was used to analyze the differential expression genes of E. coli HB101 under stress of DDBAC and DDAC. Based on our study, we reported for the first time that QACs regulated plasmid conjugation by altering the AI-2 mediated quorum sensing system. This study provided new insights into the effects and mechanisms of QACs on the conjugative transfer of ARGs.
2. Material and methods
2.1. The establishment of plasmid conjugation models
In order to know the appropriate antibiotic concentrations to screen the transconjugants, the minimum inhibitory concentration (MIC) of the antibiotics were determined (Table S1). Meanwhile, the MIC of the QACs were also determined, and MIC90 was used in the experiment (Table S2) (Yu et al., 2021b). The determination method of MIC is detailed in Text S1.
To determine the effect of QACs on the plasmid conjugation, two conjugative transfer models were established, including intra- and intergenera conjugation. The intragenera conjugation consisted of the donor strain E. coli HB101, harboring plasmid RP4, encoding resistance to ampicillin (Amp), kanamycin (Km), and tetracycline (Tc), and the recipient strain E. coli MG1655, which was resistant to nalidixic acid (Nal). The intergenera conjugation was donor E. coli HB101 and the recipient Pseudomonas putida, which is isolated from seawater and resistant to cefoxitin (cefo). A single colony was selected from LB plates and then grown overnight in LB medium with corresponding antibiotic concentrations (Table S3). Then, the bacteria were washed by phosphate-buffered saline (PBS) twice The cell density of donor and recipient bacteria were adjusted to 108 CFU/mL. In the intragenera mating paired system, donors and recipients were mixed at a ratio of 1:1. For intergenera conjugation model, our previous result showed that E. coli almost accounted for 10 % of the total Pseudomonas spp. and E. coli isolates in sewage effluents (Haller et al., 2018). According to the bacteria composition in actual sewage, the donor (E. coli HB101) and recipient (Pseudomonas putida) were mixed at a ratio of 1:9 for intergenera conjugation model. QACs were added to the mating paired systems, and the final concentrations were as follows: 0, 0.0004, 0.004, 0.04, 0.4 mg/L, which is typical of concentrations observed in wastewater treatment plants (Table S4). After conjugation at 37 °C for 20 h, the mixtures were properly diluted and plated onto LB plates containing the corresponding concentrations to screen for transconjugants, donors and recipients (Table S3). Conjugation frequency was calculated as the formula: the cell number of transconjugants/the cell number of recipients. In order to verify the role of lipopolysaccharide (LPS) loss of cell outer membrane on plasmid conjugation, donors and recipients were pretreated with EDTA at a final concentration of 0.5 mM for 30 min, then EDTA was removed by centrifugation and the cells were resuspended in PBS (Huang et al., 2022). The donor and recipient were mixed for plasmid conjugation experiment at 37 °C for 4 h. 4,5-dihydroxy-2,3-pentanedione (DPD) (0.8, 4 μM), the precursor of AI-2, was added to the intragenera conjugation system to verify the role of AI-2 molecules on the plasmid conjugation. Transconjugants were verified by gel electrophoresis (Text S2).
2.2. Measurement of cell membrane permeability
Permeability of the cell outer membrane was measured with the hydrophobic probe 1-N-phenylnaphthylamine (NPN), which strictly accesses the outer membrane of E. coli, and its fluorescence is enhanced in a hydrophobic environment such as within the cell outer membrane (Loh et al., 1984). The bacteria were treated with QACs for 1 h and NPN was added at a final concentration of 10 μM. Fluorescence intensity was measured with a microplate reader (infinite M200 pro, Tecan, Switzerland) with excitation at 350 nm and emission at 420 nm. Cell cytoplasmic membrane permeability was also determined by propidium iodide dye (Text S3).
2.3. Extraction, purification and determination of lipopolysaccharide
Lipopolysaccharide (LPS) was extracted by the hot-phenol-water method (Rezania et al., 2011) and quantitatively determined by the Purpald method (Lee and Tsai, 1999). Details of the extraction, purification and quantification of LPS can be found in Text S4.
2.4. Determination of DPD concentration by LC-MS/MS in presence of QACs
The concentrations of 4,5-dihydroxy-2,3-pentanedione (DPD) in the intragenera conjugation system were measured to explore the effect of QACs on intracellular communication. In brief, donor and recipient were cultured for 4 h to logarithmic phase. After centrifugation to remove LB, an intragenera plasmid conjugation system was constructed and exposed to QACs at 0.4 mg/L for 3 h. Then, the cell suspension was filtered through a 0.45 μm filter (Sartorius). DPD was determined by LC-MS/MS based on a previous method with some modifications (Xu et al., 2022). The sample was subjected to a derivatization reaction with 2,3- diaminonaphthalene (DAN) (Sigma Aldrich, ≥ 98.0 %) to form a DPD-DAN complex before analysis by LC-MS/MS. Detailed parameters regarding derivatization and LC-MS/MS are given in Text S5.
2.5. RNA extraction, genome-wide RNA sequencing and transcriptomic analysis
E. coli HB101 were treated with DDAC and DDBAC for 4 h and harvested. Cell pellets were resuspended in 100 μL lysozyme (1 mg/mL) and incubated for 10 s. Total RNA was extracted by the RNeasy mini kit (Qiagen) according to the manufacturer's protocol (Cardinale et al., 2013) and sent to Novogene company (Singapore) for sequencing. All RNA samples were used to construct the sequencing library. Fragments Per Kilobases Per Millionreads (FPKM) was used to quantify gene expression. Differentially expressed genes (DEGs) were determined by using the DESeq2 packages and gene functions were described using Swiss-prot, Pfam, NR, COG and GO annotation databases. DEGs were selected by comparing the control and QACs-treated groups (0.4 mg/L) as |log1.2 | > 1, P < 0.05.
2.6. mRNA expression analysis
Transcriptional levels of genes related to conjugative transfer were determined by reverse transcription quantitative PCR (RT-qPCR). The isolated total RNA from E. coli intragenera mating mixture was transcribed to cDNA using ImProm-II™ Reverse Transcription System (Promega), followed by quantification via qPCR using the SYBRGreen dye (FastStart Universal SYBR Green Master (ROX), Roche). The qPCR reaction system was 20 μL, including 10 μL SYBRGreen dye, 0.5 μL (10 mM, IDT) of forward and reverse primers, 1 μL cDNA and 8 μL double-distilled water. The procedure was 50 °C for 2 min, 95 °C pre-denaturation for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. The melting curve acquisition temperature range was from 60 °C to 95 °C, and the signal was read for every 0.3 °C increase. A blank control was set to filter false positive amplification results. The mRNA expression levels were normalized to 16S rRNA. The detailed information of primers are listed in Table S5 (Qiu et al., 2012; Yu et al., 2021a).
2.7. EPS extraction and the determination of the polysaccharides and proteins
Overnight cultured E. coli HB101 was washed twice with PBS and adjusted to OD600 = 1.0. The cell suspension was treated by QACs for 4 h. Then, 15 mL of cell suspension was centrifuged and the supernatant was discarded. 5 mL of 0.05 % NaCl that has been preheated to 70 °C was added, vortexed for 1 min and centrifuged at 4500 g for 5 min. Supernatant was collected as loose EPS. 5 mL of 0.05 % NaCl was added again, and put into a 60 °C water bath for 30 min, centrifuged at 4500 g for 5 min and the supernatant collected as tight EPS (Liao et al., 2019). The content of polysaccharide was determined by the phenol‑sulfuric acid method, see details in Text S6. The protein content of EPS was determined by the Bradford method according to the instructions of the Protein Quantitation kit (Abcam, USA).
2.8. Other chemical analysis methods
ATP content were quantified by the ATP assay kit (Sigma). The overnight cultured E. coli HB101were washed twice with PBS and the OD600 was adjusted to 1.0. Then, QACs were added for 4 h, 1 mL of bacteria was centrifuged to collect the cell pellet following the instructions of the kit, and the absorbance was measured at 570 nm by colorimetry. Cell hydrophobicity was determined by Campanac's method (Campanac et al., 2002). All experiments in this study were performed at three biological replicates. Independent sample t-tests were used to evaluate the differences between groups. P < 0.05, p < 0.01 and p < 0.001 are marked as *, **, and ***, respectively.
3. Results and discussion
3.1. QACs promoted conjugative transfer of ARGs
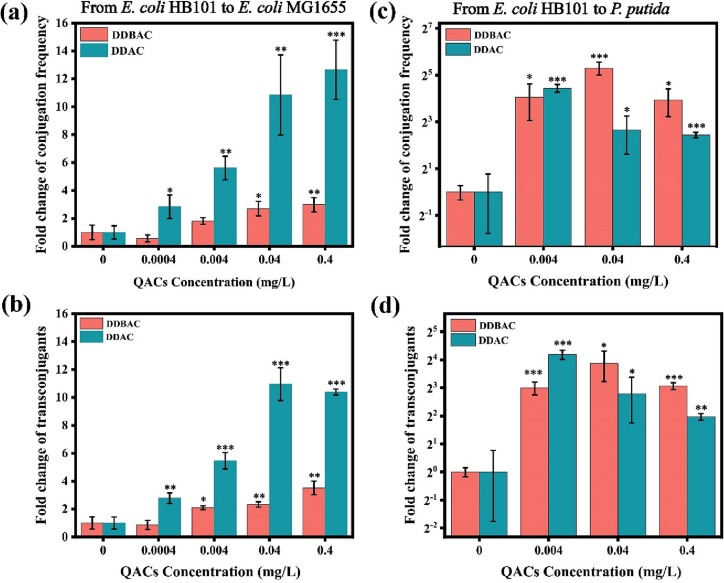
During the epidemic of COVID-19, we hypothesized that the increased use of QACs would drive the proliferation of antibiotic resistance genes. Two commonly used QACs (DDBAC and DDAC) were selected, and two conjugative models were established to verify the hypothesis. As shown in Fig. 1 , QACs significantly promoted the transfer of ARGs via plasmid conjugation in both intra- and intergenera conjugative transfer systems. The spontaneous intragenera conjugation frequency (from donor E. coli HB101 to recipients E. coli MG1655) was (1.8 ± 0.9) × 10−5, and DDBAC and DDAC significantly enhanced the conjugation frequency by 3.0-, 12.7-fold at 0.4 mg/L, respectively (Fig. 1a). Consistent with the increasing trend of conjugation frequency, the cell number of transconjugants also increased exposure to QACs. At 0.4 mg/L, the cell number of transconjugants increased by 3.5 and 10.4 times under DDBAC and DDAC, respectively (Fig. 1b). Notably, even at the lowest dose of DDAC at 0.0004 mg/L, the conjugation frequency was significantly increased by 2.8- fold (Fig. 1a). In order to predict the intragenera conjugation frequency, the correlation between the tested QACs concentration and the conjugation frequency was also analyzed. QACs exposure concentration had a good linear relationship with the intragenera plasmid conjugation frequency within the tested concentration range (Fig. S1a, b), which can be used to predict the conjugation frequency when the QACs concentration in the environment is already known.
Fig. 1.
QACs promoted the ARGs transfer via plasmid conjugation. (a) and (b) Fold change of conjugation frequency and cell number of transconjugants as a function of QACs concentration in the intragenera conjugation system. (c) and (d) Fold change of conjugation frequency and cell number of transconjugants as a function of QACs concentration in the intergenera conjugative system. Significant differences between the QACs-treated group and the control group were determined by independent samples t-test and are marked as * for p < 0.05, ** for p < 0.01 and *** for p < 0.001.
In the intergenera conjugation mating system, the recipient was P. putida, and the spontaneous interspecific conjugation frequency was (6.9 ± 0.2) × 10−7, which was lower than that of the intragenera conjugation system. DDBAC and DDAC significantly promoted the conjugative transfer frequency of plasmid RP4 and the cell number of transconjugants within the concentration range of 0.004–0.4 mg/L (Fig. 1c, d). For example, the intergenera conjugation frequency increased by 15.3- and 3.9- fold at 0.4 mg/L under DDBAC and DDAC, respectively. To avoid the negative effect of QACs on the density of donors and recipients, the cell number of both the donor and recipient were counted. The results clearly showed that the cell numbers of donor and recipients were not significantly changed compared to the blank group (Fig. S2). Intragenera transconjugants were also verified by gel electrophoresis (Fig. S3). In general, if the QACs concentration in wastewater treatment plants increased, it will promote the spread of ARGs, and the increased cell number of transconjugants may spread ARGs by vertical gene transfer or secondary conjugation (Li et al., 2018).
3.2. QACs increased the cell outer membrane permeability
The cell membrane acts as a barrier that controls the inlet and outlet of substances, including DNA material via plasmid conjugation (Samuels et al., 2000). Studies have shown that increased cell membrane permeability, especially in donor cells (Yu et al., 2021b), can facilitate plasmid conjugative transfer. As membrane-active agents, QACs promote the permeability of the cytoplasmic membrane, causing the leakage of intracellular substances and eventually leading to cell death (Gilbert and Moore, 2005). Hence, the PI dye was first used to measure the permeability of the cytoplasmic membrane. However, within the tested concentration range of QACs in this study, the fluorescence intensity of PI dyes did not significantly change in the QACs-treated group, suggesting that QACs did not increase the permeability of the cytoplasmic membrane (Fig. S4, p > 0.05). Furthermore, NPN was used to measure the permeability of the cell outer membrane. As shown in Fig. 2a, the cell outer membrane permeability was significantly enhanced under the treatment of QACs at 0.4 mg/L. The cell outer membrane is composed of lipopolysaccharides, phospholipids and proteins, usually carrying a net negative charge, and is stabilized by divalent cation ions (Mg2+ and Ca2+) (Gilbert and Moore, 2005). In order to reveal which component of the cell outer cell membrane is altered, the LPS of cells in the control and QACs-treated group were extracted. The results showed that the concentration of LPS was significantly decreased to 59.7 % and 40.7 % in DDBAC and DDAC-treated group, respectively (Fig. 2b). The proposed mechanism is that QACs interact with the cell outer membrane, involving displacement of Mg2+, resulting in the loss of cell outer membrane components such as LPS and outer membrane protein (Kwasniewska et al., 2020). In divalent cation ions solution, LPS can decrease the bactericidal ability of QACs by forming an enhanced potential electrostatic barrier against the invasion of cationic antimicrobial peptides (Strauss et al., 2009). Other studies also reported that low concentrations of the biosurfactant, rhamnolipids, caused the release of LPS of Pseudomonas aeruginosa (Miller, 1994).
Fig. 2.
QACs enhanced cell outer membrane permeability. (a) Relative fluorescence intensity of NPN and (b) relative content of LPS under QACs exposure at 0.4 mg/L. (c) The illustration of lipopolysaccharide synthesis pathway and gene expression level related to LPS synthesis. (d) Gene expression levels associated with lipoprotein. (e) Cell number of transconjugants of intragenera and (f) intergenera mating system. Donor and recipients were treated by EDTA at 0.5 mM or QACs at 0.4 mg/L. Independent-sample t-test (a, b, e and f) was used to compare the differences between QACs-treated and the control group, and are indicated by * P < 0.05 and **P < 0.01.
LPS consist of three moieties: lipid A, a core oligosaccharide (core-OS) and a long polysaccharide called O antigen. Since E. coli HB101 lacks O-antigen (Ruiz et al., 2009; Strauss et al., 2009), only lipid A and core oligosaccharide process were involved in LPS biosynthesis. Lipid A is synthesized through a group of Lpx* enzymes following a ligation of the core oligosaccharide, which is synthesized by a group of Waa* enzymes, and forming rough LPS. Rough LPS is flipped across the inner membrane by the MsbA enzyme. The assembled rough LPS is extracted from the inner membrane and transported to the outer membrane by the Lpt system. RNA transcriptome data were analyzed in detail with regard to the LPS synthesis pathway. Among these key genes related to LPS biosynthesis and transport, the lptD gene was significantly upregulated (p < 0.05) in the donor under DDBAC exposure (Fig. 2c, Table S6). The results indicated that the LPS transport pathway was enhanced to cope with the DDBAC exposure. Lipoprotein contributes to the formation and maintenance of cell shape and is essential for the integrity of the cell membrane (Gao et al., 2018; Narita and Tokuda, 2017). The expression levels of genes related to lipoprotein were significantly regulated under DDBAC exposure (p < 0.05), which indicted that a major disruption of the outer membrane induced by QACs (Fig. 2d, Table S7).
To confirm whether the release of LPS play a role in plasmid conjugation, EDTA was used to pretreat donor and recipients to increase cell outer membrane permeability. EDTA-treated Pseudomonas aeruginosa can release about 50 % LPS of the outer membrane (Russell and Chopra, 1996). As shown in Fig. 2e and f, EDTA significantly increased the cell number of intragenera and intergenera transconjugants compared with the control group, increasing by 2.7- and 4.9-fold, respectively. In total, QACs promoted plasmid conjugation by increasing cell outer membrane permeability.
3.3. Cell to cell contact
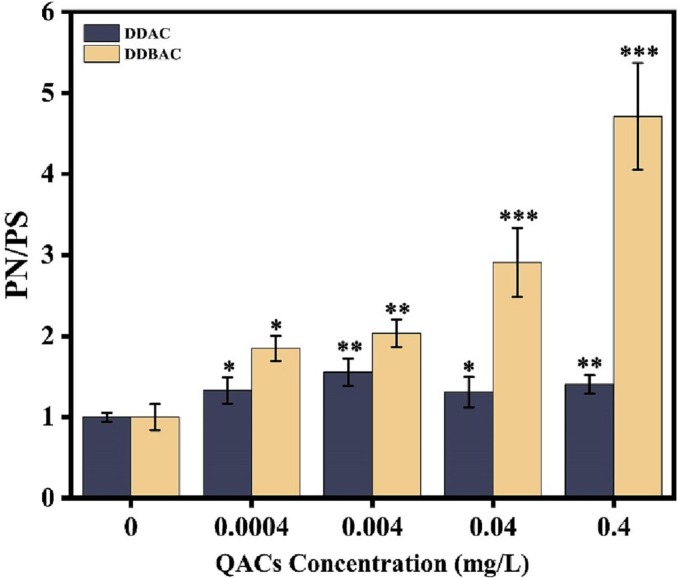
Cell-to-cell contact is the prerequisite for plasmid conjugation to occur. Several studies reported that EPS can strengthen the intercellular interactions, including the formation and function of cell aggregates, cell adhesion, and horizontal gene transfer (Czaczyk and Myszka, 2007; Flemming and Wingender, 2010). Extracellular polymeric substance (EPS) is mainly composed of extracellular polysaccharides (PS) and proteins (PN) (More et al., 2014). Therefore, the content and composition of EPS was determined. As shown in Fig. 3 , the ratio of PN/PS exhibited a concentration-dependent increase under exposure of DDAC and DDBAC. For example, the ratio of PN/PS increased by 1.4- and 4.7-fold when exposed to DDAC and DDBAC at 0.4 mg/L, respectively. The results indicated that E. coli can alter the composition of EPS to alleviate the stress of QACs. Previous studies also reported that sub-inhibitory concentrations of QACs enhanced biofilm formation of E. coli (Pagedar et al., 2012) and altered the EPS composition and content of Chlorella (Li et al., 2019). Pearson correlation analysis showed that plasmid conjugation frequency and the ratio of PN/PS were strong positively correlated (Table S8), indicating that QACs promoted plasmid-mediated transfer of ARGs that were associated with the altered extracellular EPS. Yu et al. (2020) demonstrated that that CeO2 nanoparticle altered the EPS content of E. coli, which played an important role in regulating plasmid conjugation.
Fig. 3.
QAC promoted cell to cell contact by improving the composition of the EPS.
The EPS covering on a cell surface influences the physiochemical characteristics such as cell hydrophobicity (Tsuneda et al., 2003). For biofilms, exopolysaccharides have hydrophilicity property due to a large number of hydrophilic groups (-COOH, -OH) and exoproteins show strong hydrophobicity (Campanac et al., 2002). Therefore, cell surface hydrophobicity of donors was determined by using cell adhesion to hydrocarbon. As shown in Fig. S5, DDAC did not affect cell surface hydrophobicity within the tested concentration range. However, DDBAC significantly increased cell surface hydrophobicity with increasing concentration (0.04, 0.4 mg/L). Increased cell hydrophobicity contributed to cell adhesion, thereby promoting the intercellular contact between donors and recipients (Araujo et al., 2010; Liao et al., 2019). Overall, sub-inhibitory concentrations of QACs mediated intercellular interactions by altering the composition of EPS.
3.4. Effects of QACs on mRNA expression levels of conjugative apparatus
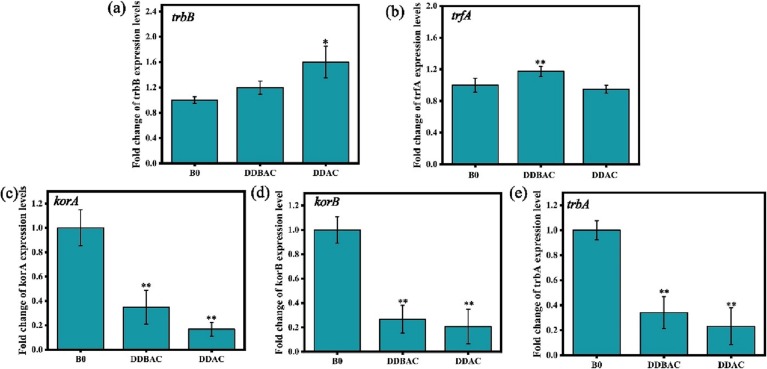
Bacterial conjugation is the transfer of genetic material from a host to the recipient through cell-to-cell contact. The establishment of initial physical contact between cells has been defined as the mating pair formation (Mpf). Gene trbB is involved in the mating pair formation during the plasmid conjugation (Zatyka et al., 2001). Gene trfA encoding the replication initiator protein, which is the second step of conjugative transfer and needs energy supply for the conjugation process (Kostelidou et al., 1988). The results showed that the expression level of trbB and trfA increased significantly under exposure of DDAC and DDBAC at 0.4 mg/L, respectively (Fig. 4a, b). Simultaneously, QACs also significantly repressed the expression level of the global regulator genes (korA, korB, trbA) (Fig. 4c-e). Compared to the untreated control group, korA gene expression levels were down-regulated by nearly 83 % and 65 % under DDAC and DDBAC treatments, respectively. TrbB promoter (trbBp) is repressed by korB and trbA (Shingler and Thomas, 1984). The trfA promoter (trfAp) is repressed by global regulatory genes (korA, korB trbA) (Jagura-Burdzy et al., 1992). Hence, the down-regulation of expression levels of korB and trbA are very important for activating the transcription of mating pair formation trbB gene. Due to the significant down-regulation of transcriptional levels of korA, korB and trbA by QACs, the expression of trfA gene was alleviated, thereby enhancing the plasmid transfer.
Fig. 4.
mRNA expression levels of conjugative transfer genes. (a) QACs significantly regulate the genes related to mating pair formation system trbB, (b) plasmid transfer and replication system gene trfA and global regulatory genes (c) korA, (d) korB and (e) trbA gene. Independent-sample t-test (a-e) was used to compare the differences between QACs-treated and the control group, and are indicated by * P < 0.05 and **P < 0.01.
Plasmid conjugation is known to be energy expensive and requires three plasmid-encoded ATPase genes (Ilangovan et al., 2015). In order to constrain the cost fitness, plasmids strictly control the expression of the conjugation machinary, and only a few bacteria in the microbiota can express the genes associated with the conjugation system when they are close to the recipients (San Millan and MacLean, 2017). The fitness costs of plasmid conjugation are from high ATP requirements for the Mpf and plasmid translocation (Ilangovan et al., 2015; San Millan and MacLean, 2017). Thus, the increased translation and expression of conjugative machinery of the donor cell requires additional ATP. Therefore, the upregulated expression of trbB and trfA was ascribed to the increased ATP content (Fig. S6a). We further analyzed the gene expression related to ATP production of the RNA-sequencing data. The generation of ATP came from a series of electron transport processes in the electron transport chain. Electron flow utilizes the transmembrane protein complex 1-IV, which is involved in generating a proton gradient and ultimately drives ATP production by complex V (Zhao et al., 2019). The NuoA gene, encoding NADH:quinone oxidoreductase in electron transport complex I, is the main entry point for electrons from NADH into the respiratory chain (Oppermann et al., 2022). Under the treatment of DDBAC, the expression level of NuoA gene was significantly up-regulated (p < 0.05), indicating that the increased ATP content was related to the activated electron transport chain (Fig. S6b). Other studies also showed that the changes of intracellular ATP content may be regulated by the gene expression levels of the electronic respiratory chain (Li et al., 2021; Yu et al., 2020). In total, QACs regulated RP4 plasmid-mediated conjugative transfer machinery under exposure to QACs, which was supported by an increase in ATP production.
3.5. AI-2 mediated quorum sensing regulates the plasmid transfer under QACs
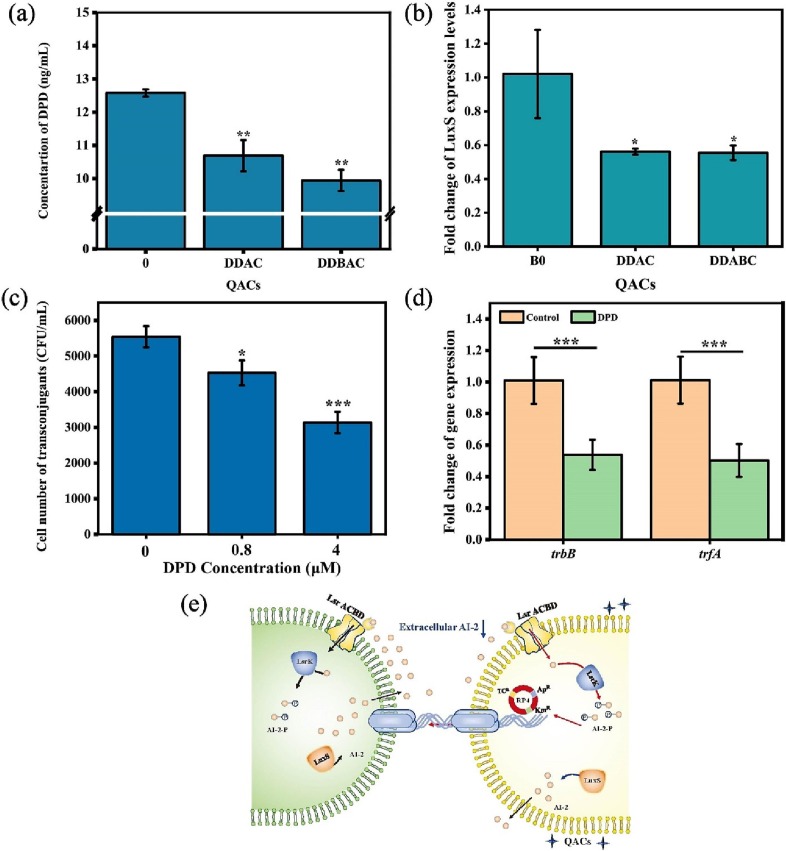
Quorum sensing plays a very important role in cell-to-cell communication, enabling bacteria to respond to environmental stress by producing, secreting and detecting signaling molecules, and regulating cellular behavior (Zheng et al., 2018; Zhu et al., 2020). The LuxS/AI-2 system has been proposed to involve bacteria phenotypes, including biofilm formation, motility and horizontal gene transfer (Pereira et al., 2013). Vibrio cholerae responds to autoinducer (AI) molecules produced by multispecies biofilm activating the ComEA expression related to DNA uptake and promoting plasmid horizontal gene transfer (Antonova and Hammer, 2011). Several studies have shown that exposure to disinfectants affects AI-2 activity and the gene expression level of gene luxS (Castillo et al., 2015; Hongmei et al., 2014; Yu et al., 2021a). Therefore, it is reasonable to speculate that QACs may control the plasmid conjugation process by regulating AI-2-mediated quorum sensing. In this study, the extracellular concentration of DPD, as a precursor of multiple AI-2 signals, was first determined by LC-MS/MS in a E. coli intragenera plasmid conjugation system under exposure to QACs. As shown in Fig. 5a, QACs significantly reduced the extracellular concentration of AI-2 signals. The extracellular concentrations of DPD were reduced by 15 % and 21 % in the DDAC and DDBAC-treated groups, respectively. Also, QACs significantly inhibited the expression level of LuxS (Fig. 5b). Transcriptome data showed that QACs significantly upregulated the expression levels of genes related to the uptake of AI-2 signals (lsrA) and the phosphorylation of AI-2 signals (lsrK) (Fig. S7). In summary, the results indicated that QACs inhibited the synthesis of AI-2 and promoted AI-2 uptake and phosphorylation of E. coli, thus reducing the extracellular concentration of DPD.
Fig. 5.
AI-2 signals involved in the plasmid transfer under QACs in the intragenera conjugation model. (a) Concentration of DPD and (b) The gene expression level of LuxS in the mating pair system under exposure of QACs at 0.4 mg/L. (c) The effects of exogenous DPD (0.8, 4 μM) on the cell number of transconjugants. (d) DPD (4 μM) regulated the gene expression level of trbB and trfA. (e) The schematic diagram of AI-2 signals regulated the plasmid conjugation under QACs exposure. The Independent-sample t-test was used to compare the differences between QACs-treated and the control group, and are indicated by * P < 0.05, **P < 0.01 and **P < 0.001.
To confirm the role of AI-2 in regulating the plasmid conjugation, exogenous DPD (0.8, 4 μM) was added to the plasmid conjugation system. DPD significantly reduced the cell number of transconjugants from 5541 to 4529 CFU/mL, resulting in a significant 19 % decrease compared to the blank treatment group at 0.8 μM. When the AI-2 signals were at 4 μM, the cell number decreased by 43 % (Fig. 5c). Also, exogenous DPD (4 μM) significantly decreased the transcriptional expression levels of trfA and trbB (Fig. 5d). The results revealed that increased extracellular concentration of AI-2 signals were unfavorable for the plasmid transfer. Several antibiotics can promote intergenera conjugation of RP4 by inhibiting AHLs signals mediated quorum sensing (Lu et al., 2017). Taken together, QACs apparently affect AI-2 signals in the E. coli conjugation system and thereby regulating the expression of intracellular plasmid-related conjugation genes (Fig. 5e). However, some research gaps still need to be filled such as how the AI-2 uptake process regulates plasmid conjugation under QACs treatment.
4. Conclusion
This study provides important insights into the effects and mechanisms of low concentrations QACs on the spread of antibiotic resistance genes via plasmid conjugation. The environmental concentrations of QACs promoted conjugation frequency and increased linearly with increasing concentrations, indicating increased disinfectant use during the pandemic of COVID-19 may promote AMR. QACs facilitated the plasmid conjugation by regulating cellular processes, including cell membrane permeability, EPS production, and genes expression associated with plasmid conjugation. This study also demonstrated for the first time that AI-2 signals were also involved in regulating plasmid conjugation under QACs exposure. However, there are some limitations in the research. The study explored the transmission risk of QACs on ARGs conjugative transfer only in artificially constructed conjugation models where intercellular contact between the donors and recipients were maximized. Future studies are necessary to evaluate the effects of QACs on the spread of ARGs among WWTP and natural ecosystem microbiota. Further efforts are also needed to understand how the uptake and detection of AI-2 signals in E. coli regulates plasmid conjugation. Considering that the increased use of QACs during the COVID-19 pandemic, we recommend that future studies pay more attention to evaluate and control the risk of QACs as the recommended disinfectant based on the development and evolution of antibiotic resistance.
CRediT authorship contribution statement
Congcong Liu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing - original draft.
Shin Giek Goh, Luhua You, Qiyi Yuan and Sanjeeb Mohapatra: Investigation, Writing -review & editing.
Baoliang Chen, Karina Yew-Hoong Gin: Conceptualization, Funding acquisition, Data curation, Writing- Reviewing and Editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant 22136004), funded by the National Research Foundation (NRF), Prime Minister's Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program, and administered by the NUS Environmental Research Institute (NERI), Singapore.
Editor: Fang Wang
Footnotes
Determination of the minimal inhibition concentration (MIC) towards antibiotics and QACs (Text S1), gel electrophoresis for the identification of transconjugants (Text S2), determination of plasma membrane permeability by propidium iodide dye (Text S3), the extraction, purification and quantitative of LPS (Text S4), quantification of DPD by LC-MS/MS (Text S5) and determination of polysaccharides in EPS (Text S6) were presented. Minimal inhibition concentration (90 %) of bacteria towards antibiotics (Table S1), minimal inhibition concentration (90 %) of bacteria towards QACs (Table S2), antibiotic concentration of cultivation and screening for donors, recipients and transconjugants (Table S3), QACs concentrations of the wastewater scenarios (Table S4), primers for qPCR (Table S5), gene expression level related to LPS (Table S6), gene expression level related to lipoprotein (Table S7) and pearson correlation analysis between conjugation frequency and the ratio of PN/PS (Table S8). Linear relationship between QACs concentration and conjugation frequency in intragenera conjugation system (Fig. S1), Cell number of donor, recipient and transconjugant in the intra- and intergenera conjugative transfer system (Fig. S2), The transconjugants verification by the gel electrophoresis (Fig. S3), fold change of cell plasma membrane permeability of E. coli HB101 (Fig. S4), effects of QACs on cell hydrophobicity of the donor (Fig. S5), QACs enhanced the production of ATP (Fig. S6) and transcriptional expression level of genes related to AI-2 uptake (Fig. S7) were presented. Supplementary data to this article can be found online at doi:https://doi.org/10.1016/j.scitotenv.2023.163781.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- Abe K., Nomura N., Suzuki S. Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiol. Ecol. 2020;96(5) doi: 10.1093/femsec/fiaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alygizakis N., Galani A., Rousis N.I., Aalizadeh R., Dimopoulos M.A., Thomaidis N.S. Change in the chemical content of untreated wastewater of Athens, Greece under COVID-19 pandemic. Sci. Total Environ. 2021;799 doi: 10.1016/j.scitotenv.2021.149230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos G.C.A., Ploumakis S., Zhang L., Hawkey P.M., Gaze W.H., Wellington E.M.H. The widespread dissemination of integrons throughout bacterial communities in a riverine system. ISME J. 2018;12(3):681–691. doi: 10.1038/s41396-017-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova E.S., Hammer B.K. Quorum-sensing autoinducer molecules produced by members of a multispecies biofilm promote horizontal gene transfer to Vibrio cholerae. FEMS Microbiol. Lett. 2011;322(1):68–76. doi: 10.1111/j.1574-6968.2011.02328.x. [DOI] [PubMed] [Google Scholar]
- Araujo E.A., de Andrade N.J., Mendes da Silva L.H., de Carvalho A.F., de Sa Silva C.A., Ramos A.M. Control of microbial adhesion as a strategy for food and bioprocess technology. Food Bioprocess Technol. 2010;3(3):321–332. [Google Scholar]
- Buffet-Bataillon S., Tattevin P., Bonnaure-Mallet M., Jolivet-Gougeon A. Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds–a critical review. Int. J. Antimicrob. Agents. 2012;39(5):381–389. doi: 10.1016/j.ijantimicag.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Campanac C., Pineau L., Payard A., Baziard-Mouysset G., Roques C. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob. Agents Chemother. 2002;46(5):1469–1474. doi: 10.1128/AAC.46.5.1469-1474.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale S., Joachimiak M.P., Arkin A.P. Effects of genetic variation on the E. coli host-circuit interface. Cell Rep. 2013;4(2):231–237. doi: 10.1016/j.celrep.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Castillo S., Heredia N., Garcia S. 2(5H)-furanone, epigallocatechin gallate, and a citric-based disinfectant disturb quorum-sensing activity and reduce motility and biofilm formation of Campylobacter jejuni. Folia Microbiol. 2015;60(1):89–95. doi: 10.1007/s12223-014-0344-0. [DOI] [PubMed] [Google Scholar]
- Clara M., Scharf S., Scheffknecht C., Gans O. Occurrence of selected surfactants in untreated and treated sewage. Water Res. 2007;41(19):4339–4348. doi: 10.1016/j.watres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Czaczyk K., Myszka K. Biosynthesis of extracellular polymeric substances (EPS) and its role in microbial biofilm formation. Pol. J. Environ. Stud. 2007;16(6):799–806. [Google Scholar]
- Dewey H.M., Jones J.M., Keating M.R., Budhathoki-Uprety J. Increased use of disinfectants during the COVID-19 pandemic and its potential impacts on health and safety. ACS Chem. Health Saf. 2021;29(1):27–38. [Google Scholar]
- Flemming H.C., Wingender J. The biofilm matrix. Nat. Rev. Microbiol. 2010;8(9):623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Gao W., Yin J., Bao L., Wang Q., Hou S., Yue Y., Yao W., Gao X. Engineering extracellular expression systems in Escherichia coli based on transcriptome analysis and cell growth state. ACS Synth. Biol. 2018;7(5):1291–1302. doi: 10.1021/acssynbio.7b00400. [DOI] [PubMed] [Google Scholar]
- Garcia M.T., Campos E., Sanchez-Leal J., Ribosa I. Effect of the alkyl chain length on the anaerobic biodegradability and toxicity of quaternary ammonium based surfactants. Chemosphere. 1999;38(15):3473–3483. doi: 10.1016/s0045-6535(98)00576-1. [DOI] [PubMed] [Google Scholar]
- Gaze W.H., Zhang L., Abdouslam N.A., Hawkey P.M., Calvo-Bado L., Royle J., Brown H., Davis S., Kay P., Boxall A.B.A., Wellington E.M.H. Impacts of anthropogenic activity on the ecology of class 1 integrons and integron-associated genes in the environment. ISME J. 2011;5(8):1253–1261. doi: 10.1038/ismej.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Moore L.E. Cationic antiseptics: diversity of action under a common epithet. J. Appl. Microbiol. 2005;99(4):703–715. doi: 10.1111/j.1365-2672.2005.02664.x. [DOI] [PubMed] [Google Scholar]
- Gosling R.J., Mawhinney I., Vaughan K., Davies R.H., Smith R.P. Efficacy of disinfectants and detergents intended for a pig farm environment where Salmonella is present. Vet. Microbiol. 2017;204:46–53. doi: 10.1016/j.vetmic.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Guo X.-P., Yang Y., Lu D.-P., Niu Z.-S., Feng J.-N., Chen Y.-R., Tou F.-Y., Garner E., Xu J., Liu M., Hochella M.F., Jr. Biofilms as a sink for antibiotic resistance genes (ARGs) in the Yangtze Estuary. Water Res. 2018;129:277–286. doi: 10.1016/j.watres.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Haller L., Chen H., Ng C., Le T.H., Koh T.H., Barkham T., Sobsey M., Gin K.Y. Occurrence and characteristics of extended-spectrum beta-lactamase- and carbapenemase- producing bacteria from hospital effluents in Singapore. Sci. Total Environ. 2018;615:1119–1125. doi: 10.1016/j.scitotenv.2017.09.217. [DOI] [PubMed] [Google Scholar]
- Han Y., Zhou Z.C., Zhu L., Wei Y.Y., Feng W.Q., Xu L., Liu Y., Lin Z.J., Shuai X.Y., Zhang Z.J., Chen H. The impact and mechanism of quaternary ammonium compounds on the transmission of antibiotic resistance genes. Environ. Sci. Pollut. Res. 2019;26(27):28352–28360. doi: 10.1007/s11356-019-05673-2. [DOI] [PubMed] [Google Scholar]
- Hongmei Z., Wenyuan Z., Wenyan Z., Anlin Y., Yanlan L., Yan J., Shaosong H., Jianyu S. Inhibitory effects of citral, cinnamaldehyde, and tea polyphenols on mixed biofilm formation by foodborne Staphylococcus aureus and Salmonella enteritidis. J. Food Prot. 2014;77(6):927–933. doi: 10.4315/0362-028X.JFP-13-497. [DOI] [PubMed] [Google Scholar]
- Hora P.I., Pati S.G., McNamara P.J., Arnold W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ. Sci. Technol. Lett. 2020;7(9):622–631. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- Huang H., Feng G., Wang M., Liu C., Wu Y., Dong L., Feng L., Zheng X., Chen Y. Nitric oxide: a neglected driver for the conjugative transfer of antibiotic resistance genes among wastewater microbiota. Environ. Sci. Technol. 2022;56(10):6466–6478. doi: 10.1021/acs.est.2c01889. [DOI] [PubMed] [Google Scholar]
- Ilangovan A., Connery S., Waksman G. Structural biology of the Gram-negative bacterial conjugation systems. Trends Microbiol. 2015;23(5):301–310. doi: 10.1016/j.tim.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Jagura-Burdzy G., Khanim F., Smith C.A., Thomas C.M. Crosstalk between plasmid vegetative replication and conjugative transfer: repression of the trfA operon by trbA of broad host range plasmid RK2. Nucleic Acids Res. 1992;20(15):3939–3944. doi: 10.1093/nar/20.15.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostelidou K., Jagura-Burdzy G., Thomas C.M. Mutational analysis of the Global Regulator KorA of Broad-host-range Plasmid RK2. J. Mol. Biol. 1988;281:453–463. doi: 10.1006/jmbi.1998.1956. [DOI] [PubMed] [Google Scholar]
- Kwasniewska D., Chen Y.L., Wieczorek D. Biological activity of quaternary ammonium salts and their derivatives. Pathogens. 2020;9(6):459. doi: 10.3390/pathogens9060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.-H., Tsai C.-M. Quantification of bacterial lipopolysaccharides by the purpald assay: measuring formaldehyde generated from 2-keto-3-deoxyoctonate and heptose at the inner core by periodate oxidation. Anal. Biochem. 1999;267(1):161–168. doi: 10.1006/abio.1998.2961. [DOI] [PubMed] [Google Scholar]
- Levinson M.I. Rinse-added fabric softener technology at the close of the twentieth century. J. Surfactant Deterg. 1999;2:223–235. [Google Scholar]
- Li B., Qiu Y., Zhang J., Huang X., Shi H., Yin H. Real-time study of rapid spread of antibiotic resistance plasmid in biofilm using microfluidics. Environ. Sci. Technol. 2018;52(19):11132–11141. doi: 10.1021/acs.est.8b03281. [DOI] [PubMed] [Google Scholar]
- Li F., Kuang Y., Liu N., Ge F. Extracellular polymeric substrates of Chlorella vulgaris F1068 weaken stress of cetyltrimethyl ammonium chloride on ammonium uptake. Sci. Total Environ. 2019;661:678–684. doi: 10.1016/j.scitotenv.2018.12.472. [DOI] [PubMed] [Google Scholar]
- Li H., Song R., Wang Y., Zhong R., Wang T., Jia H., Zhu L. Environmental free radicals efficiently inhibit the conjugative transfer of antibiotic resistance by altering cellular metabolism and plasmid transfer. Water Res. 2021;209 doi: 10.1016/j.watres.2021.117946. [DOI] [PubMed] [Google Scholar]
- Liao J., Huang H., Chen Y. CO2 promotes the conjugative transfer of multiresistance genes by facilitating cellular contact and plasmid transfer. Environ. Int. 2019;129:333–342. doi: 10.1016/j.envint.2019.05.060. [DOI] [PubMed] [Google Scholar]
- Loh B., Grant C., Hancock R.E.W. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1984;26(4):546–551. doi: 10.1128/aac.26.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Guo J. Disinfection spreads antimicrobial resistance. Science. 2021;371(6528) doi: 10.1126/science.abg4380. 474-474. [DOI] [PubMed] [Google Scholar]
- Lu Y., Zeng J., Wang L., Lan K., E S., Wang L., Xiao Q., Luo Q., Huang X., Huang B. Antibiotics promote Escherichia coli-Pseudomonas aeruginosa conjugation through inhibiting quorum sensing. Antimicrob. Agents Chemother. 2017;61(12) doi: 10.1128/AAC.01284-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney A.R., Safaee M.M., Wuest W.M., Furst A.L. The silent pandemic: emergent antibiotic resistances following the global response to SARS-CoV-2. iScience. 2021;24(4) doi: 10.1016/j.isci.2021.102304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller Y.Z.R.M. Effects of a Pseudomona rhamnolipid biosurfactans on cell hydrophobicity and biodegradation of octadecane. Appl. Environ. Microbiol. 1994;60(6):2101–2106. doi: 10.1128/aem.60.6.2101-2106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min-Jeong L., Ji-Hyoung H., Yong-Su K., Jee-Hoon R., Sang-Do H. Reduction of Bacillus cereus contamination in biofilms on stainless steel surfaces by application of sanitizers and commercial detergent. J. Korean Soc. Appl. Biol. Chem. 2010;53(1):89–93. [Google Scholar]
- Mohapatra S., Yutao L., Goh S.G., Ng C., Luhua Y., Tran N.H., Gin K.Y.-H. Quaternary ammonium compounds of emerging concern: classification, occurrence, fate, toxicity and antimicrobial resistance. J. Hazard. Mater. 2022;445 doi: 10.1016/j.jhazmat.2022.130393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More T.T., Yadav J.S., Yan S., Tyagi R.D., Surampalli R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014;144:1–25. doi: 10.1016/j.jenvman.2014.05.010. [DOI] [PubMed] [Google Scholar]
- Mulder I., Siemens J., Sentek V., Amelung W., Smalla K., Jechalke S. Quaternary ammonium compounds in soil: implications for antibiotic resistance development. Rev. Environ. Sci. Biotechnol. 2017;17(1):159–185. [Google Scholar]
- Narita S.I., Tokuda H. Bacterial lipoproteins; biogenesis, sorting and quality control. Biochim. Biophys. Acta. 2017;1862(11):1414–1423. doi: 10.1016/j.bbalip.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Oppermann S., Seng K., Shweich L., Friedrich T. The gene order in the nuo-operon is not essential for the assembly of E. coli complex I. Biochim. Biophys. ActaBioenerg. 2022;1863(7) doi: 10.1016/j.bbabio.2022.148592. [DOI] [PubMed] [Google Scholar]
- Pagedar A., Singh J., Batish V.K. Adaptation to benzalkonium chloride and ciprofloxacin affects biofilm formation potential, efflux pump and haemolysin activity of Escherichia coli of dairy origin. J. Dairy Res. 2012;79(4):383–389. doi: 10.1017/S0022029912000295. [DOI] [PubMed] [Google Scholar]
- Pati S.G., Arnold W.A. Comprehensive screening of quaternary ammonium surfactants and ionic liquids in wastewater effluents and lake sediments. Environ. Sci.Process. Impacts. 2020;22(2):430–441. doi: 10.1039/c9em00554d. [DOI] [PubMed] [Google Scholar]
- Pereira C.S., Thompson J.A., Xavier K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013;37(2):156–181. doi: 10.1111/j.1574-6976.2012.00345.x. [DOI] [PubMed] [Google Scholar]
- Qiu Z., Yu Y., Chen Z., Jin M., Yang D., Zhao Z., Wang J., Shen Z., Wang X., Qian D., Huang A., Zhang B., Li J.-W. Nanoalumina promotes the horizontal transfer of multiresistance genes mediated by plasmids across genera. Proc. Natl. Acad. Sci. U. S. A. 2012;109(13):4944–4949. doi: 10.1073/pnas.1107254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezania S., Amirmozaffari N., Tabarraei B., Jeddi-Tehrani M., Zarei O., Alizadeh R., Masjedian F., Zarnani A.H. Extraction, purification and characterization of lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna J.Med.Biotechnol. 2011;3(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- Ruan T., Song S., Wang T., Liu R., Lin Y., Jiang G. Identification and composition of emerging quaternary ammonium compounds in municipal sewage sludge in China. Environ. Sci. Technol. 2014;48(8):4289–4297. doi: 10.1021/es4050314. [DOI] [PubMed] [Google Scholar]
- Ruiz N., Kahne D., Silhavy T.J. Transport of lipopolysaccharide across the cell envelope: the long road of discovery. Nat. Rev. Microbiol. 2009;7(9):677–683. doi: 10.1038/nrmicro2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A.D., Chopra I. Second edition. 1996. Understanding Antibacterial Action And Resistance. [Google Scholar]
- Samuels A.L., Lanka E., Davies J.E. Conjugative junctions in RP4-mediated mating of Escherichia coli. J. Bacteriol. 2000;182(10):2709–2715. doi: 10.1128/jb.182.10.2709-2715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Millan A., MacLean R.C. Fitness costs of plasmids: a limit to plasmid transmission. Microbiol. Spectr. 2017;5(5):1–12. doi: 10.1128/microbiolspec.MTBP-0016-2017. [DOI] [PubMed] [Google Scholar]
- Shingler V., Thomas C.M. Transcription in the trfA region of broad host range plasmid RK2 is regulated by trfB and korB. Mol. Gen. Genet. MGG. 1984;195(3):523–529. doi: 10.1007/BF00341457. [DOI] [PubMed] [Google Scholar]
- Strauss J., Burnham N.A., Camesano T.A. Atomic force microscopy study of the role of LPS O-antigen on adhesion of E. coli. J. Mol. Recognit. 2009;22(5):347–355. doi: 10.1002/jmr.955. [DOI] [PubMed] [Google Scholar]
- Thomas C.M., Nielsen K.M. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat. Rev. Microbiol. 2005;3(9):711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- Tsuneda S., Aikawa H., Hayashi H., Yuasa A., Hirata A. Extracellular polymeric substances responsible for bacterial adhesion onto solid surface. FEMS Microbiol. Lett. 2003;223(2):287–292. doi: 10.1016/S0378-1097(03)00399-9. [DOI] [PubMed] [Google Scholar]
- Wang Q., Mao D., Luo Y. Ionic liquid facilitates the conjugative transfer of antibiotic resistance genes mediated by plasmid RP4. Environ. Sci. Technol. 2015;49(14):8731–8740. doi: 10.1021/acs.est.5b01129. [DOI] [PubMed] [Google Scholar]
- Xu B., Cho Q.A.C., Ng T.C.A., Huang S., Ng H.Y. Enriched autoinducer-2 (AI-2)-based quorum quenching consortium in a ceramic anaerobic membrane bioreactor (AnMBR) for biofouling retardation. Water Res. 2022;214 doi: 10.1016/j.watres.2022.118203. [DOI] [PubMed] [Google Scholar]
- Xu H., Chen Z., Huang R., Cui Y., Li Q., Zhao Y., Wang X., Mao D., Luo Y., Ren H. Antibiotic resistance gene-carrying plasmid spreads into the plant endophytic bacteria using soil bacteria as carriers. Environ. Sci. Technol. 2021;55(15):10462–10470. doi: 10.1021/acs.est.1c01615. [DOI] [PubMed] [Google Scholar]
- Yu K., Chen F., Yue L., Luo Y., Wang Z., Xing B. CeO2 nanoparticles regulate the propagation of antibiotic resistance genes by altering cellular contact and plasmid transfer. Environ. Sci. Technol. 2020;54(16):10012–10021. doi: 10.1021/acs.est.0c01870. [DOI] [PubMed] [Google Scholar]
- Yu T., Ma M., Sun Y., Xu X., Qiu S., Yin J., Chen L. The effect of sublethal concentrations of benzalkonium chloride on the LuxS/AI-2 quorum sensing system, biofilm formation and motility of Escherichia coli. Int. J. Food Microbiol. 2021;353 doi: 10.1016/j.ijfoodmicro.2021.109313. [DOI] [PubMed] [Google Scholar]
- Yu Z., Wang Y., Lu J., Bond P.L., Guo J. Nonnutritive sweeteners can promote the dissemination of antibiotic resistance through conjugative gene transfer. ISME J. 2021;15(7):2117–2130. doi: 10.1038/s41396-021-00909-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatyka M., Bingle L., Jones A.C., Thomas C.M. Cooperativity between KorB and TrbA repressors of broad-host-range plasmid RK2. J. Bacteriol. 2001;183(3):1022–1031. doi: 10.1128/JB.183.3.1022-1031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Cui F., Zeng G.M., Jiang M., Yang Z.Z., Yu Z.G., Zhu M.Y., Shen L.Q. Quaternary ammonium compounds (QACs): a review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015;518–519:352–362. doi: 10.1016/j.scitotenv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Zhao M., Gao J., Zhang H., Cui Y., Wang Z., Zhao Y., Zhang Y., Liu Y. Quaternary ammonium compounds promoted anoxic sludge granulation and altered propagation risk of intracellular and extracellular antibiotic resistance genes. J. Hazard. Mater. 2023;445 doi: 10.1016/j.jhazmat.2022.130464. [DOI] [PubMed] [Google Scholar]
- Zhao R.-Z., Jiang S., Zhang L., Yu Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (review) Int. J. Mol. Med. 2019;44(1):3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J., Chen T., Chen H. Antibiotic resistome promotion in drinking water during biological activated carbon treatment: is it influenced by quorum sensing? Sci. Total Environ. 2018;612:1–8. doi: 10.1016/j.scitotenv.2017.08.072. [DOI] [PubMed] [Google Scholar]
- Zhu L., Chen T., Xu L., Zhou Z., Feng W., Liu Y., Chen H. Effect and mechanism of quorum sensing on horizontal transfer of multidrug plasmid RP4 in BAC biofilm. Sci. Total Environ. 2020;698 doi: 10.1016/j.scitotenv.2019.134236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.